A Comprehensive Genomic Analysis of Chinese Indigenous Ningxiang Pigs: Genomic Breed Compositions, Runs of Homozygosity, and Beyond
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of Reference Populations
2.2. Estimation of Genomic Breed Compositions of Purebred Ningxiang Pigs
2.3. Population Stratification and Identification of Crossbred Pigs
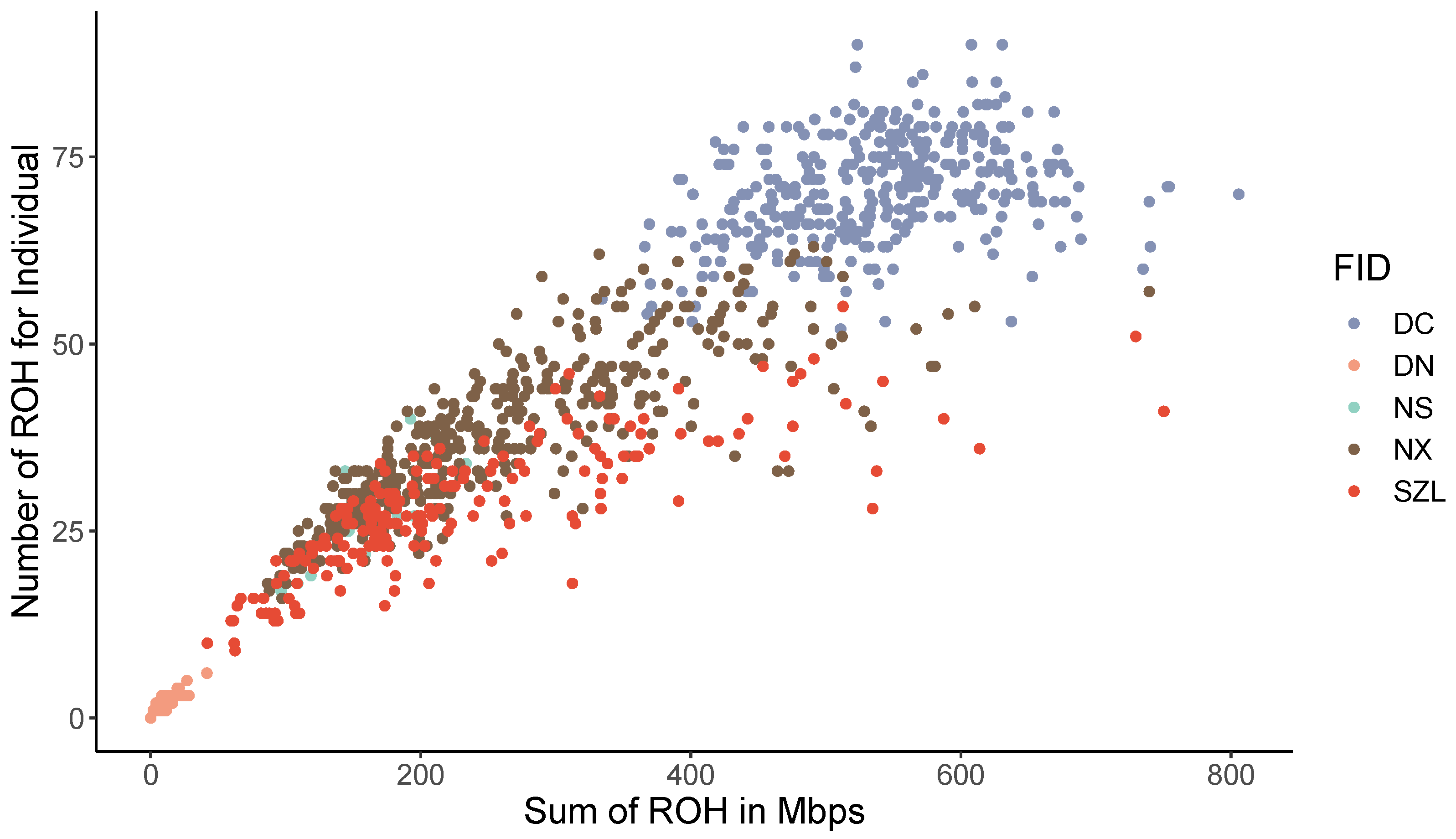
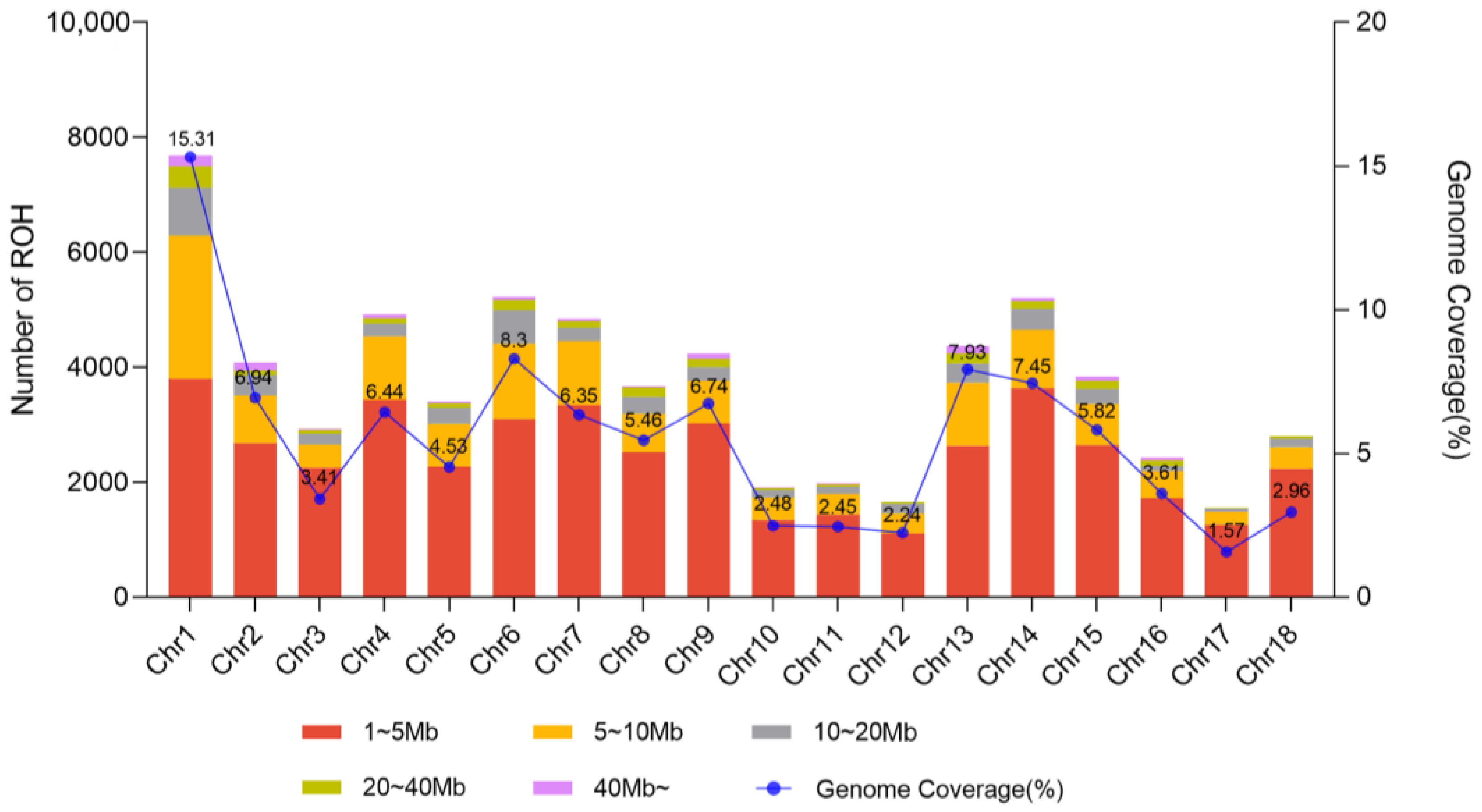
2.4. Genomic Characterization and Runs of Homozygosity
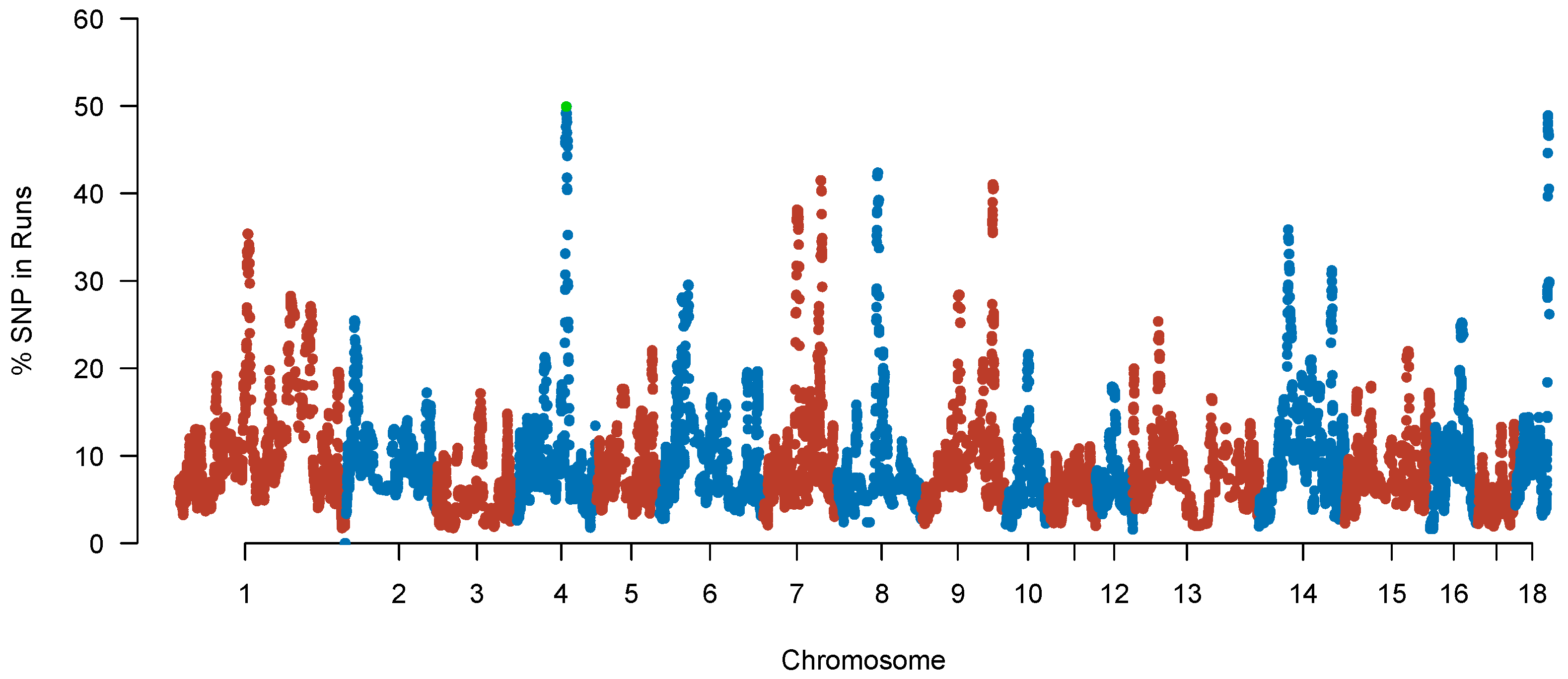
2.5. Identification of ROH Islands, Candidate Genes, and QTL
| Gene | Phenotype | Species | Sources |
|---|---|---|---|
| Reproduction Trait | |||
| SCAMP2, DPF3, SULT1E1 | Teat numbers | Pig | Lee et al. [51], Li et al. [52], Jiang et al. [25] |
| ALDH1A2, TCF12 | Litter traits | Pig | Tao et al. [53], Wu et al. [54] |
| NEDD4 | Sperm storage capacity | Human | Chu et al. [55] |
| CCPG1 | Unilateral cryptorchidism | Pig | Da Silve et al. [46] |
| SPIDR | Primary ovarian insufficiency | Human | Heddar et al. [56] |
| ATP1B1 | Sperm motility | Boar | Mańkowska et al. [47] |
| ADCY10 | Sperm quality | Boar, Human | Tate et al. [57], Akbari et al. [58] |
| CYP11A1 | Polycystic ovary Syndrome (PCOS) and Infertility | Human | Heidarzadehpilehrood et al. [59] |
| CABS1 | Sperm structure | Pig | Zhang et al. [16] |
| JCHAIN | Polycystic ovary syndrome | Human | Zou et al.[60] |
| EIF4ENIF1 | Premature ovarian insufficiency | Human | Zhao et al. [61] |
| ABHD2 | Sperm activation | Human | Bononi et al. [62] |
| Carcass and Growth Traits | |||
| ZNF280D PLIN1, PAPPA2, SLC5A4, CABIN1 | Backfat thickness | Pig | Lee et al. [41], Gandolfi et al. [42], Wang et al. [43], Fowler et al. [44], Zhao et al. [45] |
| POU6F2 | Intern organ weight | Pig | Li et al. [63] |
| ARID3B | Body fat thickness | Pig | Lee et al. [64] |
| Lipid and Energy Metabolism, and Fat Deposition | |||
| ZNF70 | Hepatic steatosis | Pig | Watanabe et al. [65] |
| AQP9 | Fat deposition | Pig | Kuriyama et al. [66] |
| PYGO1 | Steatosis | Human | Anstee et al. [67] |
| CEBPD | Adipogenesis | Pig | Óvilo et al. [49] |
| POUF21 | Adipocyte differentiation | Human | Currie et al. [68] |
| CREG1 | Intramuscular fatty acid content and composition | Pig | Puig-Oliveras et al. [69] |
| CSK | Browning of white adipose tissue (obesity) | Human | Huang et al. [70] |
| SFI1 | Obesity | Human | Goutzelas et al. [71] |
| YTHDC1 | Adipose tissue depot | Human | Rønningen et al. [72] |
| RGS6 | Fat distribution | Human | Norris et al. [73] |
| MMP11 | Fat accumulation | Human | Rockstroh et al. [74] |
| CHCHD10 | Adipocytes metabolism | Human | Ding et al. [75] |
| PTPN9 | Glucose uptake | Human | Kim et al. [76] |
| Skeletal Development | |||
| AMBN | Skeletal development | Human | Kawasaki et al. [77] |
| PISD | Skeletal dysplasia | human | Zhao et al. [78] |
| Immunity | |||
| CD247 | T-cell receptor zeta | Pig | Van Goor et al. [79] |
| MIF | Inflammation | Bama Minipig | Li et al. [80] |
2.6. Estimating Genomic Inbreeding Coefficient
3. Materials and Methods
3.1. Animals and Genotypes
3.1.1. Genotyping
3.1.2. Constructing SNP Panel, Genotype Imputation, and Quality Control
3.2. Statistical Methods
3.2.1. Admixture Model
3.2.2. Principal Component Analysis and Hierarchical Clustering
3.2.3. Runs of Homozygosity and Genomic Inbreeding Coefficients
- Genomic inbreeding coefficient
- ROH hotspots, gene annotation, enrichment analysis, and QTL screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, B.; Gao, H.; Yang, F.; Li, Y.; Yang, Q.; Liao, Y.; Guo, H.; Xu, K.; Tang, Z.; Gao, N.; et al. Comparative Characterization of Volatile Compounds of Ningxiang Pig, Duroc and Their Crosses (Duroc × Ningxiang) by Using SPME-GC-MS. Foods 2023, 12, 1059. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Wu, X.-L.; Zeng, Q.; Li, H.; Ma, H.; Jiang, J.; Rosa, G.J.M.; Gianola, D.; Tait, R.G., Jr.; Bauck, S. Genomic Mating as Sustainable Breeding for Chinese Indigenous Ningxiang Pigs. PLoS ONE 2020, 15, e0236629. [Google Scholar] [CrossRef] [PubMed]
- Mujibi, F.D.; Okoth, E.; Cheruiyot, E.K.; Onzere, C.; Bishop, R.P.; Fèvre, E.M.; Thomas, L.; Masembe, C.; Plastow, G.; Rothschild, M. Genetic Diversity, Breed Composition and Admixture of Kenyan Domestic Pigs. PLoS ONE 2018, 13, e0190080. [Google Scholar] [CrossRef] [PubMed]
- Babigumira, B.M.; Sölkner, J.; Mészáros, G.; Pfeiffer, C.; Lewis, C.R.G.; Ouma, E.; Wurzinger, M.; Marshall, K. A Mix of Old British and Modern European Breeds: Genomic Prediction of Breed Composition of Smallholder Pigs in Uganda. Front. Genet. 2021, 12, 676047. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.-L.; Zhao, S. Editorial: Advances in Genomics of Crossbred Farm Animals. Front. Genet. 2021, 12, 709483. [Google Scholar] [CrossRef]
- Vandenplas, J.; Calus, M.P.L.; Sevillano, C.A.; Windig, J.J.; Bastiaansen, J.W.M. Assigning Breed Origin to Alleles in Crossbred Animals. Genet. Sel. Evol. 2016, 48, 61. [Google Scholar] [CrossRef]
- Bansal, V.; Libiger, O. Fast Individual Ancestry Inference from DNA Sequence Data Leveraging Allele Frequencies for Multiple Populations. BMC Bioinform. 2015, 16, 4. [Google Scholar] [CrossRef]
- Chiang, C.W.K.; Gajdos, Z.K.Z.; Korn, J.M.; Kuruvilla, F.G.; Butler, J.L.; Hackett, R.; Guiducci, C.; Nguyen, T.T.; Wilks, R.; Forrester, T.; et al. Rapid Assessment of Genetic Ancestry in Populations of Unknown Origin by Genome-Wide Genotyping of Pooled Samples. PLoS Genet. 2010, 6, e1000866. [Google Scholar] [CrossRef]
- Kuehn, L.A.; Keele, J.W.; Bennett, G.L.; McDaneld, T.G.; Smith, T.P.L.; Snelling, W.M.; Sonstegard, T.S.; Thallman, R.M. Predicting Breed Composition Using Breed Frequencies of 50,000 Markers from the US Meat Animal Research Center 2,000 Bull Project1,2. J. Anim. Sci. 2011, 89, 1742–1750. [Google Scholar] [CrossRef]
- Akanno, E.C.; Chen, L.; Abo-Ismail, M.K.; Crowley, J.; Wang, Z.; Li, C.; Basarab, J.; MacNeil, M.; Plastow, G. Genomic Prediction of Breed Composition and Heterosis Effects in Angus, Charolais and Hereford Crosses Using 50K Genotypes. Can. J. Anim. Sci. 2017, 97, 431–438. [Google Scholar] [CrossRef]
- Li, Z.; Wu, X.-L.; Guo, W.; He, J.; Li, H.; Rosa, G.J.M.; Gianola, D.; Tait, R.G.; Parham, J.; Genho, J.; et al. Estimation of Genomic Breed Composition of Individual Animals in Composite Beef Cattle. Anim. Genet. 2020, 51, 457–460. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, H.; Ferretti, R.; Simpson, B.; Walker, J.; Parham, J.; Mastro, L.; Qiu, J.; Schultz, T.; Tait, R.G.; et al. A Unified Local Objective Function for Optimally Selecting SNPs on Arrays for Agricultural Genomics Applications. Anim. Genet. 2020, 51, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.; Morton, N.E.; Collins, A. Extended Tracts of Homozygosity in Outbred Human Populations. Hum. Mol. Genet. 2006, 15, 789–795. [Google Scholar] [CrossRef]
- Szpiech, Z.A.; Xu, J.; Pemberton, T.J.; Peng, W.; Zöllner, S.; Rosenberg, N.A.; Li, J.Z. Long Runs of Homozygosity Are Enriched for Deleterious Variation. Am. J. Hum. Genet. 2013, 93, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Sun, H.; Lu, S.; Gou, X.; Yan, D.; Xu, Z.; Zhang, Z.; Qadri, Q.R.; Zhang, Z.; Wang, Z.; et al. Genetic Diversity and Selection Signatures within Diannan Small-Ear Pigs Revealed by Next-Generation Sequencing. Front. Genet. 2020, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yang, M.; Zhou, M.; Wang, Y.; Wu, X.; Zhang, X.; Ding, Y.; Zhao, G.; Yin, Z.; Wang, C. Identification of Signatures of Selection by Whole-Genome Resequencing of a Chinese Native Pig. Front. Genet. 2020, 11, 566255. [Google Scholar] [CrossRef]
- Megens, H.-J.; Crooijmans, R.P.; Cristobal, M.S.; Hui, X.; Li, N.; Groenen, M.A. Biodiversity of Pig Breeds from China and Europe Estimated from Pooled DNA Samples: Differences in Microsatellite Variation between Two Areas of Domestication. Genet. Sel. Evol. 2008, 40, 103. [Google Scholar] [CrossRef]
- Ai, H.; Huang, L.; Ren, J. Genetic Diversity, Linkage Disequilibrium and Selection Signatures in Chinese and Western Pigs Revealed by Genome-Wide SNP Markers. PLoS ONE 2013, 8, e56001. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.-L.; Li, Z.; Bao, Z.; Tait, R.G.; Bauck, S.; Rosa, G.J.M. Estimation of Genomic Breed Composition for Purebred and Crossbred Animals Using Sparsely Regularized Admixture Models. Front. Genet. 2020, 11, 576. [Google Scholar] [CrossRef]
- VanRaden, P.M.; Tooker, M.E.; Chud, T.C.S.; Norman, H.D.; Megonigal, J.H.; Haagen, I.W.; Wiggans, G.R. Genomic Predictions for Crossbred Dairy Cattle. J. Dairy Sci. 2020, 103, 1620–1631. [Google Scholar] [CrossRef]
- Howard, J.T.; Tiezzi, F.; Huang, Y.; Gray, K.A.; Maltecca, C. Characterization and Management of Long Runs of Homozygosity in Parental Nucleus Lines and Their Associated Crossbred Progeny. Genet. Sel. Evol. 2016, 48, 91. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Raya, L.; Dunkelberger, J.R.; Lunney, J.K.; Rowland, R.R.R.; Rauw, W.M.; Dekkers, J.C.M. Non-Random Distribution of Runs of Homozygosity across the Genome of Landrace × Large White Crossbreds. J. Anim. Sci. 2017, 95, 21. [Google Scholar] [CrossRef]
- Ganteil, A.; Rodriguez-Ramilo, S.T.; Ligonesche, B.; Larzul, C. Characterization of Autozygosity in Pigs in Three-Way Crossbreeding. Front. Genet. 2021, 11, 584556. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R. A fuller theory of “Junctions” in inbreeding. Heredity 1954, 8, 187–197. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, X.; Liu, J.; Zhang, W.; Zhou, M.; Wang, J.; Liu, L.; Su, S.; Zhao, F.; Chen, H.; et al. Genome-Wide Detection of Genetic Structure and Runs of Homozygosity Analysis in Anhui Indigenous and Western Commercial Pig Breeds Using PorcineSNP80k Data. BMC Genom. 2022, 23, 373. [Google Scholar] [CrossRef]
- Fang, Y.; Hao, X.; Xu, Z.; Sun, H.; Zhao, Q.; Cao, R.; Zhang, Z.; Ma, P.; Sun, Y.; Qi, Z.; et al. Genome-Wide Detection of Runs of Homozygosity in Laiwu Pigs Revealed by Sequencing Data. Front. Genet. 2021, 12, 629966. [Google Scholar] [CrossRef]
- Tong, S.; Zhu, M.; Xie, R.; Li, D.; Zhang, L.; Liu, Y. Genome-wide detection for runs of homozygosity analysis in three pig breeds from Chinese Taihu Basin and Landrace pigs by SLAF-seq data. J. Integr. Agric. 2022, 21, 3293–3301. [Google Scholar] [CrossRef]
- Falconer, D.; Mackat, T. Introduction to Quantitative Genetics, 4th ed.; Pearson Education Limited: Essex, UK, 1996. [Google Scholar]
- Meyermans, R.; Gorssen, W.; Buys, N.; Janssens, S. How to Study Runs of Homozygosity Using PLINK? A Guide for Analyzing Medium Density SNP Data in Livestock and Pet Species. BMC Genom. 2020, 21, 94. [Google Scholar] [CrossRef]
- Ceballos, F.C.; Joshi, P.K.; Clark, D.W.; Ramsay, M.; Wilson, J.F. Runs of Homozygosity: Windows into Population History and Trait Architecture. Nat. Rev. Genet. 2018, 19, 220–234. [Google Scholar] [CrossRef]
- Szmatoła, T.; Jasielczuk, I.; Semik-Gurgul, E.; Szyndler-Nędza, M.; Blicharski, T.; Szulc, K.; Skrzypczak, E.; Gurgul, A. Detection of Runs of Homozygosity in Conserved and Commercial Pig Breeds in Poland. J. Anim. Breed. Genet. 2020, 137, 571–580. [Google Scholar] [CrossRef]
- Yuan, J.; Zhou, X.; Xu, G.; Xu, S.; Liu, B. Genetic Diversity and Population Structure of Tongcheng Pigs in China Using Whole-Genome SNP Chip. Front. Genet. 2022, 13, 910521. [Google Scholar] [CrossRef] [PubMed]
- Purfield, D.C.; McParland, S.; Wall, E.; Berry, D.P. The Distribution of Runs of Homozygosity and Selection Signatures in Six Commercial Meat Sheep Breeds. PLoS ONE 2017, 12, e0176780. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Liu, Q.; Hou, X.; Liu, X.; Wang, L.; Zhao, F.; Gao, H.; Shi, L.; Wang, L.; Zhang, L. Genome-wide Association Study Revealed ABCD4 on SSC7 and GREB1L and MIB1 on SSC6 as Crucial Candidate Genes for Rib Number in Beijing Black Pigs. Anim. Genet. 2022, 53, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Horodyska, J. Hamill, R.; Varley P.; Reyer, H.; Wimmers, K.; Davoli R. Genome-wide association analysis and functional annotation of positional candidate genes for feed conversion efficiency and growth rate in pigs. PLoS ONE. 2017, 12, e0173482. [Google Scholar] [CrossRef]
- Fabbri, M.C.; Zappaterra, M.; Davoli, R.; Zambonelli, P. Genome-wide Association Study Identifies Markers Associated with Carcass and Meat Quality Traits in Italian Large White Pigs. Anim. Genet. 2020, 51, 950–952. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhou, X.; Liu, Z.; Wang, T.; Liu, B. Genome-wide Association Study Reveals a Genomic Region on SSC7 Simultaneously Associated with Backfat Thickness, Skin Thickness and Carcass Length in a Large White × Tongcheng Advanced Generation Intercross Resource Population. Anim. Genet. 2023, 54, 216–219. [Google Scholar] [CrossRef]
- Zou, Y.; Ruan, S.; Jin, L.; Chen, Z.; Han, H.; Zhang, Y.; Jian, Z.; Lin, Y.; Shi, N.; Jin, H. CDK1, CCNB1, and CCNB2 Are Prognostic Biomarkers and Correlated with Immune Infiltration in Hepatocellular Carcinoma. Med. Sci. Monit. 2020, 26, 925289. [Google Scholar] [CrossRef]
- Li, T.-H.; Qin, C.; Zhao, B.-B.; Cao, H.-T.; Yang, X.-Y.; Wang, Y.-Y.; Li, Z.-R.; Zhou, X.-T.; Wang, W.-B. Identification METTL18 as a Potential Prognosis Biomarker and Associated with Immune Infiltrates in Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 665192. [Google Scholar] [CrossRef]
- Tervasmäki, A.; Mantere, T.; Hartikainen, J.M.; Kauppila, S.; Lee, H.-M.; Koivuluoma, S.; Grip, M.; Karihtala, P.; Jukkola-Vuorinen, A.; Mannermaa, A.; et al. Rare Missense Mutations in RECQL and POLG Associate with Inherited Predisposition to Breast Cancer: Breast Cancer Risk Alleles in RECQL & POLG. Int. J. Cancer 2018, 142, 2286–2292. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Shin, D. Genome-Wide Association Studies Associated with Backfat Thickness in Landrace and Yorkshire Pigs. Genom. Inf. 2018, 16, 59–64. [Google Scholar] [CrossRef]
- Gandolfi, G.; Mazzoni, M.; Zambonelli, P.; Lalatta-Costerbosa, G.; Tronca, A.; Russo, V.; Davoli, R. Perilipin 1 and Perilipin 2 Protein Localization and Gene Expression Study in Skeletal Muscles of European Cross-Breed Pigs with Different Intramuscular Fat Contents. Meat Sci. 2011, 88, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wu, P.; Wang, S.; Ji, X.; Chen, D.; Jiang, A.; Xiao, W.; Gu, Y.; Jiang, Y.; Zeng, Y.; et al. Genome-Wide DNA Methylation Analysis in Chinese Chenghua and Yorkshire Pigs. BMC Genom. Data 2021, 22, 21. [Google Scholar] [CrossRef] [PubMed]
- Fowler, K.E.; Pong-Wong, R.; Bauer, J.; Clemente, E.J.; Reitter, C.P.; Affara, N.A.; Waite, S.; Walling, G.A.; Griffin, D.K. Genome Wide Analysis Reveals Single Nucleotide Polymorphisms Associated with Fatness and Putative Novel Copy Number Variants in Three Pig Breeds. BMC Genom. 2013, 14, 784. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Mo, D.; Li, A.; Gong, W.; Xiao, S.; Zhang, Y.; Qin, L.; Niu, Y.; Guo, Y.; Liu, X.; et al. Comparative Analyses by Sequencing of Transcriptomes during Skeletal Muscle Development between Pig Breeds Differing in Muscle Growth Rate and Fatness. PLoS ONE 2011, 6, e19774. [Google Scholar] [CrossRef]
- Da Silva, A.N.; Ibelli, A.M.G.; Savoldi, I.R.; Cantão, M.E.; Zanella, E.L.; Marques, M.G.; Da Silva, M.V.G.B.; De Peixoto, J.O.; Ledur, M.C.; Lopes, J.S.; et al. Whole-Exome Sequencing Indicated New Candidate Genes Associated with Unilateral Cryptorchidism in Pigs. Sex Dev. 2023, 17, 56–66. [Google Scholar] [CrossRef]
- Mańkowska, A.; Gilun, P.; Zasiadczyk, Ł.; Sobiech, P.; Fraser, L. Expression of TXNRD1, HSPA4L and ATP1B1 Genes Associated with the Freezability of Boar Sperm. Int. J. Mol. Sci. 2022, 23, 9320. [Google Scholar] [CrossRef] [PubMed]
- Trukhachev, V.; Skripkin, V.; Kvochko, A.; Kulichenko, A.; Kovalev, D.; Pisarenko, S.; Volynkina, A.; Selionova, M.; Aybazov, M.; Golovanova, N.; et al. Associations between Newly Discovered Polymorphisms of the CEBPD GENE LOCUS and Body Parameters in Sheep. Anim. Biotechnol. 2016, 27, 217–222. [Google Scholar] [CrossRef]
- Óvilo, C.; Benítez, R.; Fernández, A.; Núñez, Y.; Ayuso, M.; Fernández, A.; Rodríguez, C.; Isabel, B.; Rey, A.; López-Bote, C.; et al. Longissimus Dorsi Transcriptome Analysis of Purebred and Crossbred Iberian Pigs Differing in Muscle Characteristics. BMC Genom. 2014, 15, 413. [Google Scholar] [CrossRef]
- Watanabe, K.; Stringer, S.; Frei, O.; Umićević Mirkov, M.; de Leeuw, C.; Polderman, T.J.C.; van der Sluis, S.; Andreassen, O.A.; Neale, B.M.; Posthuma, D. A Global Overview of Pleiotropy and Genetic Architecture in Complex Traits. Nat. Genet. 2019, 51, 1339–1348. [Google Scholar] [CrossRef]
- Lee, J.-B.; Jung, E.-J.; Park, H.-B.; Jin, S.; Seo, D.-W.; Ko, M.-S.; Cho, I.-C.; Lee, J.-H.; Lim, H.-T. Genome-Wide Association Analysis to Identify SNP Markers Affecting Teat Numbers in an F2 Intercross Population between Landrace and Korean Native Pigs. Mol. Biol. Rep. 2014, 41, 7167–7173. [Google Scholar] [CrossRef]
- Li, Y.; Pu, L.; Shi, L.; Gao, H.; Zhang, P.; Wang, L.; Zhao, F. Revealing New Candidate Genes for Teat Number Relevant Traits in Duroc Pigs Using Genome-Wide Association Studies. Animals 2021, 11, 806. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Mei, S.; Sun, X.; Peng, X.; Zhang, X.; Ma, C.; Wang, L.; Hua, L.; Li, F. Associations of TCF12, CTNNAL1 and WNT10B Gene Polymorphisms with Litter Size in Pigs. Anim. Reprod. Sci. 2013, 140, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Wang, K.; Yang, Q.; Zhou, J.; Chen, D.; Ma, J.; Tang, Q.; Jin, L.; Xiao, W.; Jiang, A.; et al. Identifying SNPs and Candidate Genes for Three Litter Traits Using Single-Step GWAS across Six Parities in Landrace and Large White Pigs. Physiol. Genom. 2018, 50, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Chu, J.; Ma, Y.; Song, H.; Zhao, Q.; Wei, X.; Yan, Y.; Fan, S.; Zhou, B.; Li, S.; Mou, C. The Genomic Characteristics Affect Phenotypic Diversity from the Perspective of Genetic Improvement of Economic Traits. iScience 2023, 26, 106426. [Google Scholar] [CrossRef]
- Heddar, A.; Guichoux, N.; Auger, N.; Misrahi, M. A SPIDR Homozygous Nonsense Pathogenic Variant in Isolated Primary Ovarian Insufficiency with Chromosomal Instability. Clin. Genet. 2022, 101, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Tate, S.; Nakamura, K.; Suzuki, C.; Noda, T.; Lee, J.; Harayama, H. Evidence of the Existence of Adenylyl Cyclase 10 (ADCY10) Ortholog Proteins in the Heads and Connecting Pieces of Boar Spermatozoa. J. Reprod. Dev. 2010, 56, 271–278. [Google Scholar] [CrossRef]
- Akbari, A.; Pipitone, G.B.; Anvar, Z.; Jaafarinia, M.; Ferrari, M.; Carrera, P.; Totonchi, M. ADCY10 Frameshift Variant Leading to Severe Recessive Asthenozoospermia and Segregating with Absorptive Hypercalciuria. Hum. Reprod. 2019, 34, 1155–1164. [Google Scholar] [CrossRef]
- Heidarzadehpilehrood, R.; Pirhoushiaran, M.; Abdollahzadeh, R.; Binti Osman, M.; Sakinah, M.; Nordin, N.; Abdul Hamid, H. A Review on CYP11A1, CYP17A1, and CYP19A1 Polymorphism Studies: Candidate Susceptibility Genes for Polycystic Ovary Syndrome (PCOS) and Infertility. Genes 2022, 13, 302. [Google Scholar] [CrossRef]
- Zou, J.; Li, Y.; Liao, N.; Liu, J.; Zhang, Q.; Luo, M.; Xiao, J.; Chen, Y.; Wang, M.; Chen, K.; et al. Identification of Key Genes Associated with Polycystic Ovary Syndrome (PCOS) and Ovarian Cancer Using an Integrated Bioinformatics Analysis. J. Ovarian Res. 2022, 15, 30. [Google Scholar] [CrossRef]
- Zhao, M.; Feng, F.; Chu, C.; Yue, W.; Li, L. A Novel EIF4ENIF1 Mutation Associated with a Diminished Ovarian Reserve and Premature Ovarian Insufficiency Identified by Whole-Exome Sequencing. J. Ovarian Res. 2019, 12, 119. [Google Scholar] [CrossRef]
- Bononi, G.; Tuccinardi, T.; Rizzolio, F.; Granchi, C. α/β-Hydrolase Domain (ABHD) Inhibitors as New Potential Therapeutic Options against Lipid-Related Diseases. J. Med. Chem. 2021, 64, 9759–9785. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, J.; Zhuang, Z.; Ye, Y.; Zhou, S.; Qiu, Y.; Ruan, D.; Wang, S.; Yang, J.; Wu, Z.; et al. Integrated Single-Trait and Multi-Trait GWASs Reveal the Genetic Architecture of Internal Organ Weight in Pigs. Animals 2023, 13, 808. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kang, J.-H.; Kim, J.-M. Bayes Factor-Based Regulatory Gene Network Analysis of Genome-Wide Association Study of Economic Traits in a Purebred Swine Population. Genes 2019, 10, 293. [Google Scholar] [CrossRef]
- Watanabe, K.; Nakayama, K.; Ohta, S.; Tago, K.; Boonvisut, S.; Millings, E.J.; Fischer, S.G.; LeDuc, C.A.; Leibel, R.L.; Iwamoto, S. ZNF70, a Novel ILDR2-Interacting Protein, Contributes to the Regulation of HES1 Gene Expression. Biochem. Biophys. Res. Commun. 2016, 477, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Kuriyama, H.; Shimomura, I.; Kishida, K.; Kondo, H.; Furuyama, N.; Nishizawa, H.; Maeda, N.; Matsuda, M.; Nagaretani, H.; Kihara, S.; et al. Coordinated Regulation of Fat-Specific and Liver-Specific Glycerol Channels, Aquaporin Adipose and Aquaporin 9. Diabetes 2002, 51, 2915–2921. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Darlay, R.; Cockell, S.; Meroni, M.; Govaere, O.; Tiniakos, D.; Burt, A.D.; Bedossa, P.; Palmer, J.; Liu, Y.-L.; et al. Genome-Wide Association Study of Non-Alcoholic Fatty Liver and Steatohepatitis in a Histologically Characterised Cohort☆. J. Hepatol. 2020, 73, 505–515. [Google Scholar] [CrossRef]
- Currie, R.A.; Eckel, R.H. Characterization of a High Affinity Octamer Transcription Factor Binding Site in the Human Lipoprotein Lipase Promoter. Arch. Biochem. Biophys. 1992, 298, 630–639. [Google Scholar] [CrossRef]
- Puig-Oliveras, A.; Revilla, M.; Castelló, A.; Fernández, A.I.; Folch, J.M.; Ballester, M. Expression-Based GWAS Identifies Variants, Gene Interactions and Key Regulators Affecting Intramuscular Fatty Acid Content and Composition in Porcine Meat. Sci. Rep. 2016, 6, 31803. [Google Scholar] [CrossRef]
- Huang, L.O.; Rauch, A.; Mazzaferro, E.; Preuss, M.; Carobbio, S.; Bayrak, C.S.; Chami, N.; Wang, Z.; Schick, U.M.; Yang, N.; et al. Genome-Wide Discovery of Genetic Loci That Uncouple Excess Adiposity from Its Comorbidities. Nat. Metab. 2021, 3, 228–243. [Google Scholar] [CrossRef]
- Goutzelas, Y.; Kontou, P.; Mamuris, Z.; Bagos, P.; Sarafidou, T. Meta-Analysis of Gene Expression Data in Adipose Tissue Reveals New Obesity Associated Genes. Gene 2022, 818, 146223. [Google Scholar] [CrossRef]
- Rønningen, T.; Dahl, M.B.; Valderhaug, T.G.; Cayir, A.; Keller, M.; Tönjes, A.; Blüher, M.; Böttcher, Y. m6A Regulators in Human Adipose Tissue—Depot-Specificity and Correlation with Obesity. Front. Endocrinol. 2021, 12, 778875. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.M.; Langefeld, C.D.; Talbert, M.E.; Wing, M.R.; Haritunians, T.; Fingerlin, T.E.; Hanley, A.J.G.; Ziegler, J.T.; Taylor, K.D.; Haffner, S.M.; et al. Genome-Wide Association Study and Follow-up Analysis of Adiposity Traits in Hispanic Americans: The IRAS Family Study. Obesity 2009, 17, 1932–1941. [Google Scholar] [CrossRef] [PubMed]
- Rockstroh, D.; Löffler, D.; Kiess, W.; Landgraf, K.; Körner, A. Regulation of Human Adipogenesis by miR125b-5p. Adipocyte 2016, 5, 283–297. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ding, M.; Ma, Y.; Du, R.; Zhou, W.; Dou, X.; Yang, Q.; Tang, Y.; Qian, S.; Liu, Y.; Pan, D.; et al. CHCHD10 Modulates Thermogenesis of Adipocytes by Regulating Lipolysis. Diabetes 2022, 71, 1862–1879. [Google Scholar] [CrossRef]
- Kim, J.; Son, J.; Ahn, D.; Nam, G.; Zhao, X.; Park, H.; Jeong, W.; Chung, S.J. Structure–Activity Relationship of Synthetic Ginkgolic Acid Analogs for Treating Type 2 Diabetes by PTPN9 Inhibition. Int. J. Mol. Sci. 2022, 23, 3927. [Google Scholar] [CrossRef]
- Kawasaki, K.; Suzuki, T.; Weiss, K.M. Genetic basis for the evolution of vertebrate mineralized tissue. Proc. Natl. Acad. Sci. USA 2004, 101, 11356–11361. [Google Scholar] [CrossRef]
- Zhao, T.; Goedhart, C.M.; Sam, P.N.; Sabouny, R.; Lingrell, S.; Cornish, A.J.; Lamont, R.E.; Bernier, F.P.; Sinasac, D.; Parboosingh, J.S.; et al. PISD Is a Mitochondrial Disease Gene Causing Skeletal Dysplasia, Cataracts, and White Matter Changes. Life Sci. Alliance 2019, 2, e201900353. [Google Scholar] [CrossRef]
- Van Goor, A.; Pasternak, A.; Walugembe, M.; Chehab, N.; Hamonic, G.; Dekkers, J.C.M.; Harding, J.C.S.; Lunney, J.K. Genome Wide Association Study of Thyroid Hormone Levels Following Challenge with Porcine Reproductive and Respiratory Syndrome Virus. Front. Genet. 2023, 14, 1110463. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Z.; Xia, J.; Xin, L.; Chen, Y.; Yang, S.; Li, K. A Long-Term High-Fat/High-Sucrose Diet Promotes Kidney Lipid Deposition and Causes Apoptosis and Glomerular Hypertrophy in Bama Minipigs. PLoS ONE 2015, 10, e0142884. [Google Scholar] [CrossRef]
- Zhuang, Z.; Ding, R.; Peng, L.; Wu, J.; Ye, Y.; Zhou, S.; Wang, X.; Quan, J.; Zheng, E.; Cai, G.; et al. Genome-Wide Association Analyses Identify Known and Novel Loci for Teat Number in Duroc Pigs Using Single-Locus and Multi-Locus Models. BMC Genom. 2020, 21, 344. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Y.; Wang, L.; Liu, X.; Yan, H.; Gao, H.; Hou, X.; Zhang, Y.; Guo, H.; Yue, J.; et al. Genomic Variants Associated with the Number and Diameter of Muscle Fibers in Pigs as Revealed by a Genome-Wide Association Study. Animal 2020, 14, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhou, L.; Zhang, J.; Liu, X.; Zhang, Y.; Cai, L.; Zhang, W.; Cui, L.; Yang, J.; Ji, J.; et al. A Large-Scale Comparison of Meat Quality and Intramuscular Fatty Acid Composition among Three Chinese Indigenous Pig Breeds. Meat Sci. 2020, 168, 108182. [Google Scholar] [CrossRef] [PubMed]
- Schiavo, G.; Bovo, S.; Bertolini, F.; Tinarelli, S.; Dall’Olio, S.; Nanni Costa, L.; Gallo, M.; Fontanesi, L. Comparative Evaluation of Genomic Inbreeding Parameters in Seven Commercial and Autochthonous Pig Breeds. Animal 2020, 14, 910–920. [Google Scholar] [CrossRef]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- He, J.; Guo, Y.; Xu, J.; Li, H.; Fuller, A.; Tait, R.G.; Wu, X.-L.; Bauck, S. Comparing SNP Panels and Statistical Methods for Estimating Genomic Breed Composition of Individual Animals in Ten Cattle Breeds. BMC Genet. 2018, 19, 56. [Google Scholar] [CrossRef]
- Mo, J.; Li, Y.; Lu, Y.; Shi, Y.; Chen, C.; Qi, W.; Zhu, S.; Feng, L.; Liang, J.; Lan, G. Genetic Structure, Selection Signal Analysis, and ROH Detection of Guangxi Indigenous Pig Population. Chin. J. Anim. Sci. 2021, 57, 206–213. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, P.; Ma, Z.; Zhong, D.; Liu, Y.; Ni, N.; Wu, J. Genome Wide ROH Detection and Candidate Gene Identification of Fengjing Pig, Meishan Pig and Shawutou Pig. Jiangsu J. Agric. Sci. 2021, 37, 1234–1243. [Google Scholar]
- Browning, B.L.; Zhou, Y.; Browning, S.R. A One-Penny Imputed Genome from Next-Generation Reference Panels. Am. J. Hum. Genet. 2018, 103, 338–348. [Google Scholar] [CrossRef]
- Nawi, N.; Ransing, M.; Ransing, R. An Improved Learning Algorithm Based on The Broyden-Fletcher-Goldfarb-Shanno (BFGS) Method For Back Propagation Neural Networks. In Proceedings of the Sixth International Conference on Intelligent Systems Design and Applications, Jian, China, 16–18 October 2006; Volume 1, pp. 152–157. [Google Scholar]
- Patterson, N.; Price, A.L.; Reich, D. Population Structure and Eigenanalysis. PLoS Genet. 2006, 2, e190. [Google Scholar] [CrossRef]
- Xie, R.; Shi, L.; Liu, J.; Deng, T.; Wang, L.; Liu, Y.; Zhao, F. Genome-Wide Scan for Runs of Homozygosity Identifies Candidate Genes in Three Pig Breeds. Animals 2019, 9, 518. [Google Scholar] [CrossRef]
- McQuillan, R.; Leutenegger, A.-L.; Abdel-Rahman, R.; Franklin, C.S.; Pericic, M.; Barac-Lauc, L.; Smolej-Narancic, N.; Janicijevic, B.; Polasek, O.; Tenesa, A.; et al. Runs of Homozygosity in European Populations. Am. J. Hum. Genet. 2008, 83, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Mastrangelo, S.; Ciani, E.; Sardina, M.T.; Sottile, G.; Pilla, F.; Portolano, B.; the Bi.Ov. Ita Consortium. Runs of Homozygosity Reveal Genome-Wide Autozygosity in Italian Sheep Breeds. Anim. Genet. 2018, 49, 71–81. [Google Scholar] [CrossRef] [PubMed]
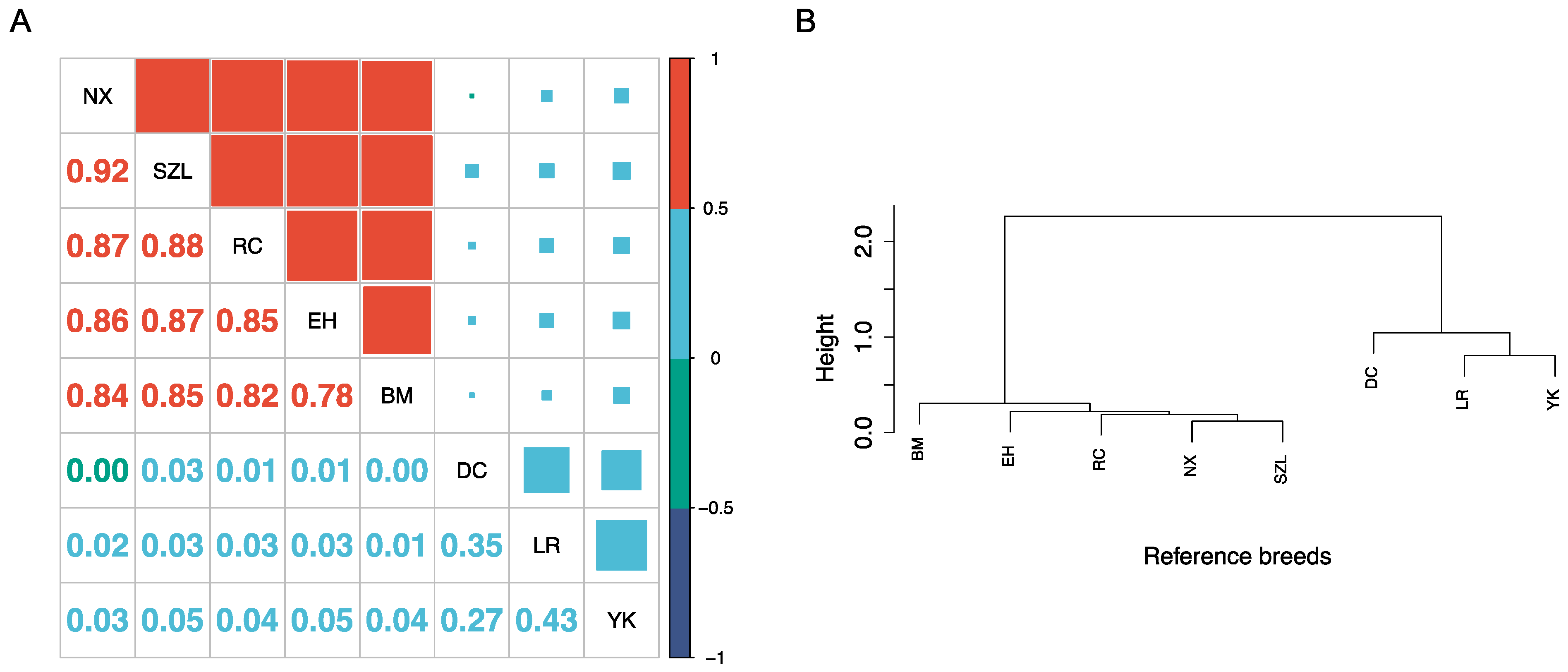
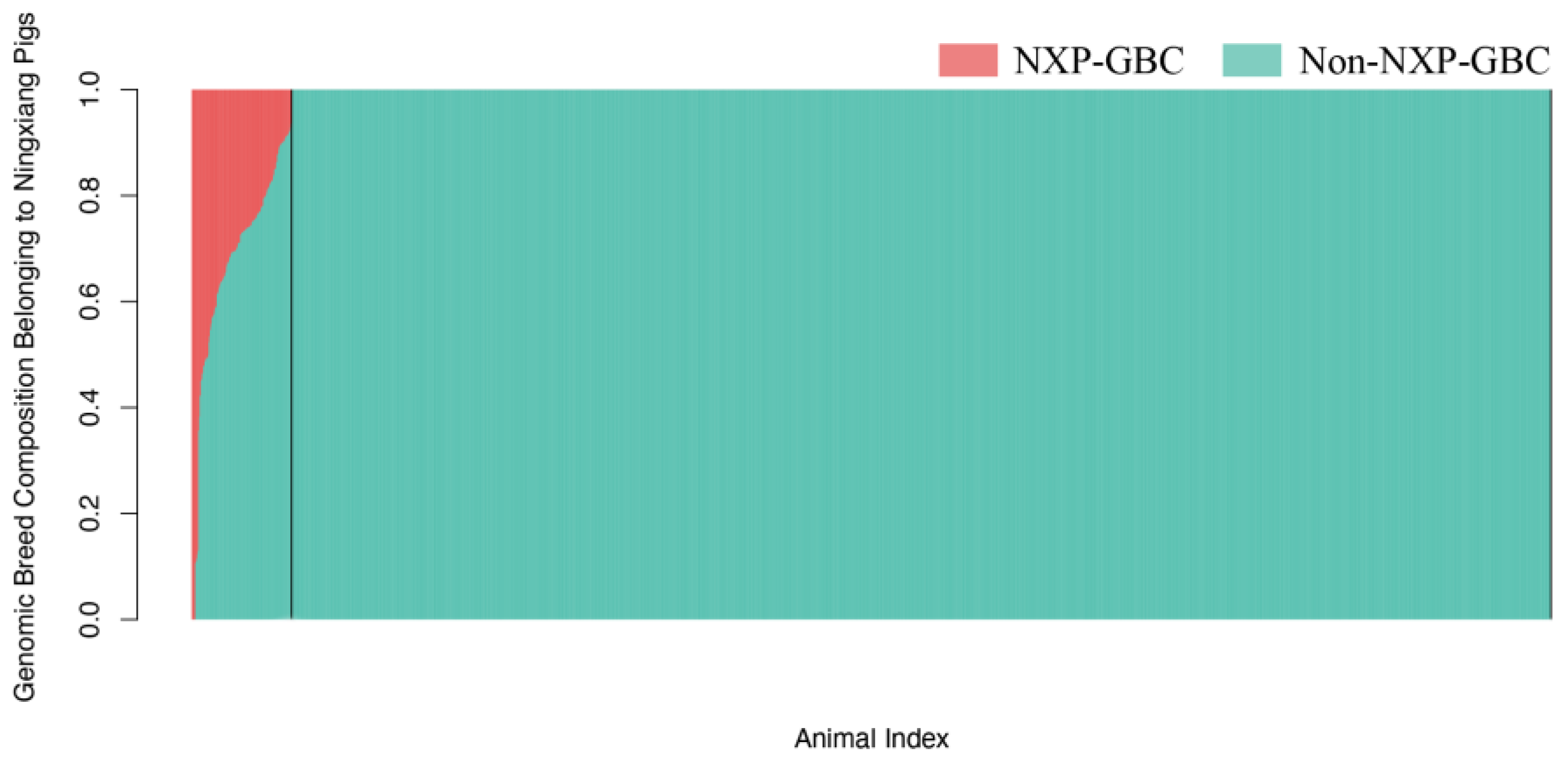

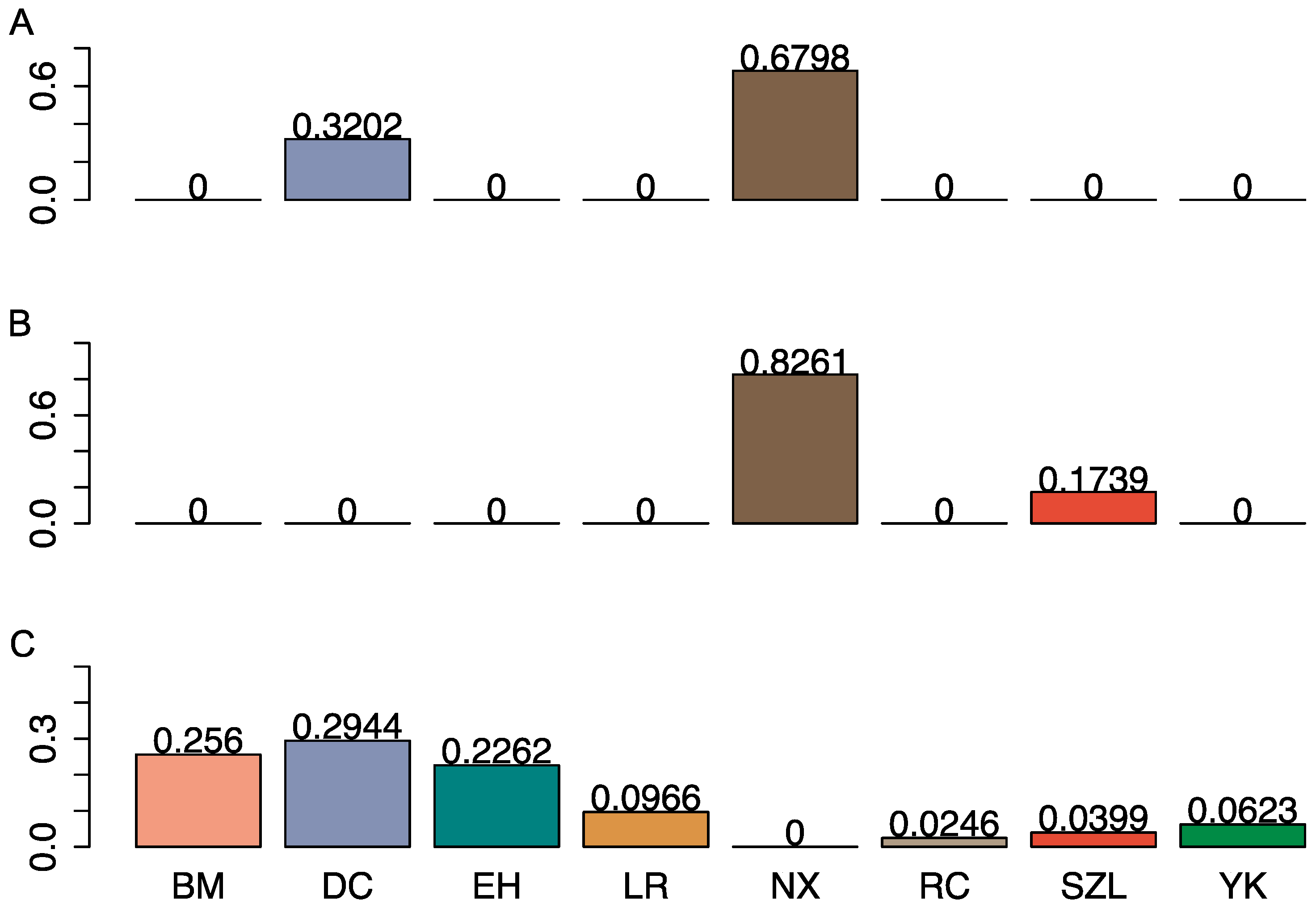
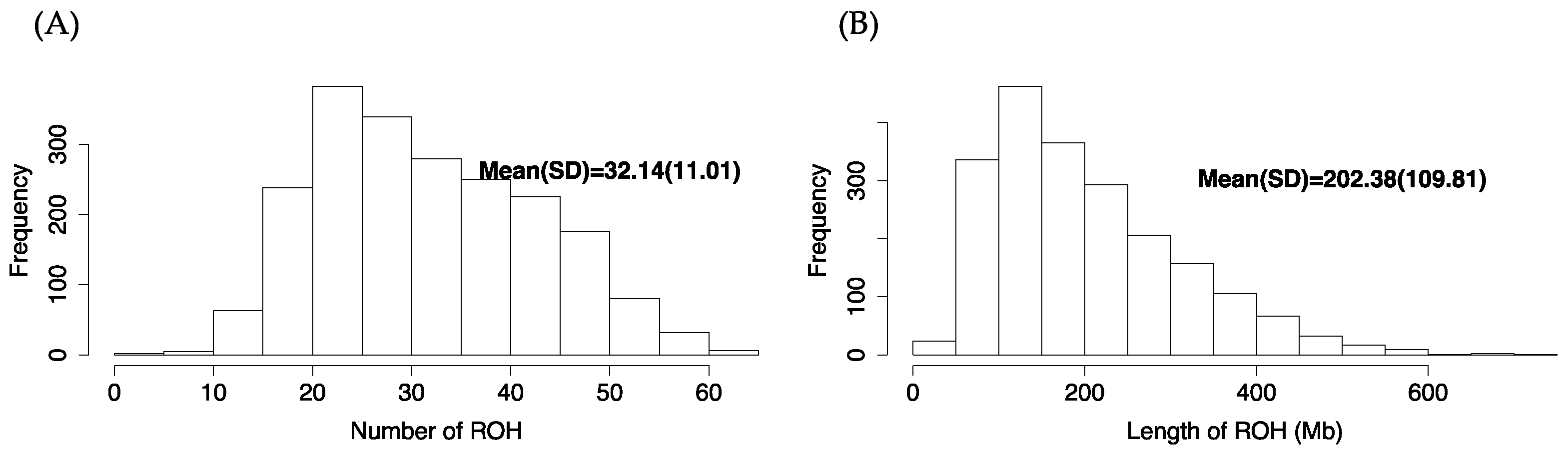

| Breed | N | SNP Chip | N | FreqA |
|---|---|---|---|---|
| Bama (BM) | 297 | Zhongxin-1 | 51,315 | 0.427 |
| Duroc (DC) | 1001 | GGP porcine 50K | 50,697 | 0.496 |
| Erhua (EH) | 52 | GGP porcine 50K | 51,315 | 0.430 |
| Landrace (LR) | 828 | GGP porcine 50K | 50,697 | 0.496 |
| Ningxiang (NX) | 384 | GGP porcine 50K | 50,697 | 0.428 |
| Rongchang (RC) | 139 | Illumina porcine 60K | 61,565 | 0.426 |
| Shaziling (SZL) | 190 | GGP porcine 50K | 50,697 | 0.431 |
| Yorkshire (YK) | 2711 | GGP porcine 50K | 50,697 | 0.495 |
| Test animals | 2242 | GGP porcine 50K | 50,697 | 0.423 |
| SNP Panel | GBC Cut-Off | Purebred | Crossbred | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| C12K | NYP-GBC = 1 | 2077 | 92.64 | 165 | 7.36 |
| NYP-GBC ≥ 0.94 | 2077 | 92.64 | 165 | 7.36 | |
| NYP-GBC ≥ 0.90 | 2092 | 93.31 | 150 | 6.69 | |
| NYP-GBC ≥ 0.80 | 2119 | 94.51 | 123 | 5.49 | |
| U10K | NYP-GBC = 1 | 2077 | 92.64 | 165 | 7.36 |
| NYP-GBC ≥ 0.94 | 2077 | 92.64 | 165 | 7.36 | |
| NYP-GBC ≥ 0.90 | 2092 | 93.31 | 150 | 6.69 | |
| NYP-GBC ≥ 0.80 | 2120 | 94.56 | 122 | 5.44 | |
| U5K | NYP-GBC = 1 | 2105 | 93.89 | 137 | 6.11 |
| NYP-GBC ≥ 0.94 | 2105 | 93.89 | 137 | 6.11 | |
| NYP-GBC ≥ 0.90 | 2107 | 93.98 | 135 | 6.02 | |
| NYP-GBC ≥ 0.80 | 2137 | 95.32 | 105 | 4.68 | |
| U1K | NYP-GBC = 1 | 2143 | 95.58 | 99 | 4.42 |
| NYP-GBC ≥ 0.94 | 2143 | 95.58 | 99 | 4.42 | |
| NYP-GBC ≥ 0.90 | 2143 | 95.58 | 99 | 4.42 | |
| NYP-GBC ≥ 0.80 | 2162 | 96.43 | 80 | 3.57 | |
| Panel Density (SNP Number) | Identified D×N Crosses |
|---|---|
| C12K (11,703) | 96 |
| U10K (10,031) | 96 |
| U5K (5007) | 95 |
| U1K (1013) | 67 |
| Known crossbred | 98 |
| ROH Range | N | N% | Mean_L (Mb) | TL% |
|---|---|---|---|---|
| 1~5 Mb | 44,415 | 66.54 | 3.09 | 32.61 |
| 5~10 Mb | 14,451 | 21.65 | 6.72 | 23.11 |
| 10~20 Mb | 4866 | 7.29 | 13.97 | 16.17 |
| 20~40 Mb | 2004 | 3.00 | 27.79 | 13.25 |
| 40~ Mb | 1010 | 1.51 | 61.84 | 14.86 |
| All | 66,746 | 100 | 6.30 | 100 |
| Chromosome | Region (Mb) | N_SNP | Length (Mb) | N_Gene |
|---|---|---|---|---|
| 1 | 112.87~117.35 | 50 | 4.48 | 31 |
| 4 | 77.43~83.74 | 62 | 4.30 | 34 |
| 7 | 54.56~59.34 | 54 | 4.78 | 72 |
| 7 | 94.49~97.05 | 52 | 2.55 | 13 |
| 8 | 65.43~68.86 | 47 | 3.42 | 27 |
| 9 | 116.73~119.25 | 59 | 2.51 | 12 |
| 14 | 48.06~50.38 | 35 | 2.31 | 46 |
| 14 | 119.63~120.11 | 15 | 0.48 | 0 |
| 18 | 53.05~55.77 | 64 | 2.71 | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, S.; Li, Z.; Yang, F.; Guo, H.; Zhao, Q.; Zhang, Y.; Yin, Y.; Wu, X.; He, J. A Comprehensive Genomic Analysis of Chinese Indigenous Ningxiang Pigs: Genomic Breed Compositions, Runs of Homozygosity, and Beyond. Int. J. Mol. Sci. 2023, 24, 14550. https://doi.org/10.3390/ijms241914550
Yin S, Li Z, Yang F, Guo H, Zhao Q, Zhang Y, Yin Y, Wu X, He J. A Comprehensive Genomic Analysis of Chinese Indigenous Ningxiang Pigs: Genomic Breed Compositions, Runs of Homozygosity, and Beyond. International Journal of Molecular Sciences. 2023; 24(19):14550. https://doi.org/10.3390/ijms241914550
Chicago/Turabian StyleYin, Shishu, Zhi Li, Fang Yang, Haimin Guo, Qinghua Zhao, Yuebo Zhang, Yulong Yin, Xiaolin Wu, and Jun He. 2023. "A Comprehensive Genomic Analysis of Chinese Indigenous Ningxiang Pigs: Genomic Breed Compositions, Runs of Homozygosity, and Beyond" International Journal of Molecular Sciences 24, no. 19: 14550. https://doi.org/10.3390/ijms241914550
APA StyleYin, S., Li, Z., Yang, F., Guo, H., Zhao, Q., Zhang, Y., Yin, Y., Wu, X., & He, J. (2023). A Comprehensive Genomic Analysis of Chinese Indigenous Ningxiang Pigs: Genomic Breed Compositions, Runs of Homozygosity, and Beyond. International Journal of Molecular Sciences, 24(19), 14550. https://doi.org/10.3390/ijms241914550






