Serum Proteomic Profiles of Patients with High and Low Risk of Endometrial Cancer Recurrence
Abstract
:1. Introduction
2. Results
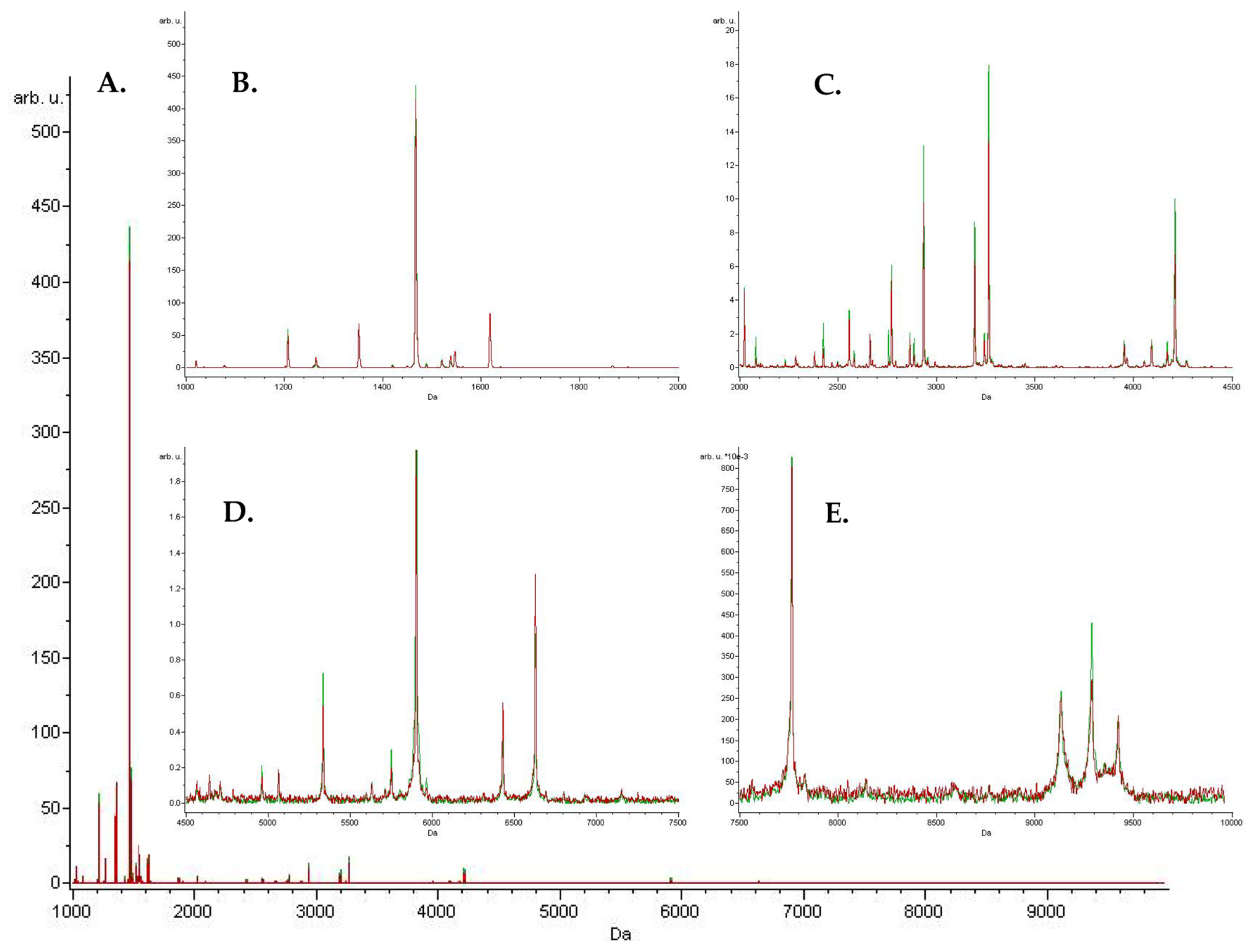
Peaks Identification
3. Discussion
4. Materials and Methods
4.1. Study Groups and Sample Collection
4.2. Serum Samples Pretreatment
4.3. MALDI-TOF Proteomic Profiling
4.4. Data Analysis
4.5. NanoLC-MALDI-TOF/TOF MS Discriminative Peaks Identification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Boer, S.M.; Powell, M.E.; Mileshkin, L.; Katsaros, D.; Bessette, P.; Haie-Meder, C.; Ottevanger, P.B.; Ledermann, J.A.; Khaw, P.; D’Amico, R.; et al. Adjuvant Chemoradiotherapy versus Radiotherapy Alone in Women with High-Risk Endometrial Cancer (PORTEC-3): Patterns of Recurrence and Post-Hoc Survival Analysis of a Randomised Phase 3 Trial. Lancet Oncol. 2019, 20, 1273–1285. [Google Scholar] [CrossRef]
- Seebacher, V.; Schmid, M.; Polterauer, S.; Hefler-Frischmuth, K.; Leipold, H.; Concin, N.; Reinthaller, A.; Hefler, L. The Presence of Postmenopausal Bleeding as Prognostic Parameter in Patients with Endometrial Cancer: A Retrospective Multi-Center Study. BMC Cancer 2009, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.A.; Long, B.J.; Del Mar Morillo, A.; Arbyn, M.; Bakkum-Gamez, J.N.; Wentzensen, N. Association of Endometrial Cancer Risk With Postmenopausal Bleeding in Women. JAMA Intern. Med. 2018, 178, 1210. [Google Scholar] [CrossRef]
- American Cancer Society. Endometrial Cancer Causes, Risk Factors, and Prevention Endometrial Cancer Risk Factors; American Cancer Society: Atlanta, GA, USA, 2019. [Google Scholar]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP Guidelines for the Management of Patients with Endometrial Carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Stelloo, E.; Bosse, T.; Nout, R.A.; MacKay, H.J.; Church, D.N.; Nijman, H.W.; Leary, A.; Edmondson, R.J.; Powell, M.E.; Crosbie, E.J.; et al. Refining Prognosis and Identifying Targetable Pathways for High-Risk Endometrial Cancer; a TransPORTEC Initiative. Mod. Pathol. 2015, 28, 836–844. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Available online: Https://Www.Iarc.Who.Int/News-Events/Publication-of-the-Who-Classification-of-Tumours-5th-Edition-Volume-4-Female-Genital-Tumours/ (accessed on 15 June 2023).
- Crosbie, E.J.; Kitson, S.J.; McAlpine, J.N.; Mukhopadhyay, A.; Powell, M.E.; Singh, N. Endometrial Cancer. Lancet 2022, 399, 1412–1428. [Google Scholar] [CrossRef]
- Church, D.N.; Stelloo, E.; Nout, R.A.; Valtcheva, N.; Depreeuw, J.; Ter Haar, N.; Noske, A.; Amant, F.; Tomlinson, I.P.M.; Wild, P.J.; et al. Prognostic Significance of POLE Proofreading Mutations in Endometrial Cancer. JNCI J. Natl. Cancer Inst. 2015, 107, dju402. [Google Scholar] [CrossRef]
- Mirza, M.R.; Chase, D.M.; Slomovitz, B.M.; dePont Christensen, R.; Novák, Z.; Black, D.; Gilbert, L.; Sharma, S.; Valabrega, G.; Landrum, L.M.; et al. Dostarlimab for Primary Advanced or Recurrent Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2145–2158. [Google Scholar] [CrossRef]
- León-Castillo, A.; de Boer, S.M.; Powell, M.E.; Mileshkin, L.R.; Mackay, H.J.; Leary, A.; Nijman, H.W.; Singh, N.; Pollock, P.M.; Bessette, P.; et al. Molecular Classification of the PORTEC-3 Trial for High-Risk Endometrial Cancer: Impact on Prognosis and Benefit From Adjuvant Therapy. J. Clin. Oncol. 2020, 38, 3388–3397. [Google Scholar] [CrossRef]
- Zong, L.; Mo, S.; Sun, Z.; Lu, Z.; Chen, J.; Yu, S.; Xiang, Y. Incorporating Molecular Classification When Stratifying the Survival Risk of Patients with High-Grade Endometrial Carcinomas. J. Clin. Med. 2023, 12, 530. [Google Scholar] [CrossRef]
- Abu-Rustum, N.R.; Alektiar, K.; Iasonos, A.; Lev, G.; Sonoda, Y.; Aghajanian, C.; Chi, D.S.; Barakat, R.R. The Incidence of Symptomatic Lower-Extremity Lymphedema Following Treatment of Uterine Corpus Malignancies: A 12-Year Experience at Memorial Sloan-Kettering Cancer Center. Gynecol. Oncol. 2006, 103, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Boliukh, I.; Rombel-Bryzek, A.; Radecka, B. Immunological Aspects of Heat Shock Protein Functions and Their Significance in the Development of Cancer Vaccines. Nowotw. J. Oncol. 2022, 72, 174–183. [Google Scholar] [CrossRef]
- Li, X.; Wang, J. Mechanical Tumor Microenvironment and Transduction: Cytoskeleton Mediates Cancer Cell Invasion and Metastasis. Int. J. Biol. Sci. 2020, 16, 2014–2028. [Google Scholar] [CrossRef] [PubMed]
- Ong, M.S.; Deng, S.; Halim, C.E.; Cai, W.; Tan, T.Z.; Huang, R.Y.-J.; Sethi, G.; Hooi, S.C.; Kumar, A.P.; Yap, C.T. Cytoskeletal Proteins in Cancer and Intracellular Stress: A Therapeutic Perspective. Cancers 2020, 12, 238. [Google Scholar] [CrossRef]
- Bateman, A.; Martin, M.-J.; Orchard, S.; Magrane, M.; Ahmad, S.; Alpi, E.; Bowler-Barnett, E.H.; Britto, R.; Bye-A-Jee, H.; Cukura, A.; et al. UniProt: The Universal Protein Knowledgebase in 2023. Nucleic Acids Res. 2023, 51, D523–D531. [Google Scholar] [CrossRef]
- Alshehri, S.; Pavlovič, T.; Farsinejad, S.; Behboodi, P.; Quan, L.; Centeno, D.; Kung, D.; Rezler, M.; Lee, W.; Jasiński, P.; et al. Extracellular Matrix Modulates Outgrowth Dynamics in Ovarian Cancer. Adv. Biol. 2022, 6, 2200197. [Google Scholar] [CrossRef] [PubMed]
- Uccella, S.; Cromi, A.; Vigetti, D.; Cimetti, L.; Deleonibus, S.; Casarin, J.; Passi, A.; Riva, C.; Ghezzi, F. Endometrial Cancer Cells Can Express Fibrinogen: Immunohistochemistry and RT-PCR Analysis. J. Obstet. Gynaecol. 2016, 36, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Hamm, A.; Veeck, J.; Bektas, N.; Wild, P.J.; Hartmann, A.; Heindrichs, U.; Kristiansen, G.; Werbowetski-Ogilvie, T.; Del Maestro, R.; Knuechel, R.; et al. Frequent Expression Loss of Inter-Alpha-Trypsin Inhibitor Heavy Chain (ITIH) Genes in Multiple Human Solid Tumors: A Systematic Expression Analysis. BMC Cancer 2008, 8, 25. [Google Scholar] [CrossRef]
- Mittal, P.; Klingler-Hoffmann, M.; Arentz, G.; Winderbaum, L.; Kaur, G.; Anderson, L.; Scurry, J.; Leung, Y.; Stewart, C.J.; Carter, J.; et al. Annexin A2 and Alpha Actinin 4 Expression Correlates with Metastatic Potential of Primary Endometrial Cancer. Biochim. Biophys. Acta Proteins Proteom. 2017, 1865, 846–857. [Google Scholar] [CrossRef]
- Helfman, D.M.; Flynn, P.; Khan, P.; Saeed, A. Tropomyosin as a Regulator of Cancer Cell Transformation. In Tropomyosin; Springer: New York, NY, USA, 2008; pp. 124–131. [Google Scholar]
- Wang, J.; Tang, C.; Yang, C.; Zheng, Q.; Hou, Y. Tropomyosin-1 Functions as a Tumor Suppressor with Respect to Cell Proliferation, Angiogenesis and Metastasis in Renal Cell Carcinoma. J. Cancer 2019, 10, 2220–2228. [Google Scholar] [CrossRef]
- Xu, X.; Wang, Y.; Bryce, N.S.; Tang, K.; Meagher, N.S.; Kang, E.Y.; Kelemen, L.E.; Köbel, M.; Ramus, S.J.; Friedlander, M.; et al. Targeting the Actin/Tropomyosin Cytoskeleton in Epithelial Ovarian Cancer Reveals Multiple Mechanisms of Synergy with Anti-Microtubule Agents. Br. J. Cancer 2021, 125, 265–276. [Google Scholar] [CrossRef]
- Kim, H.-R.; Park, J.-S.; Karabulut, H.; Yasmin, F.; Jun, C.-D. Transgelin-2: A Double-Edged Sword in Immunity and Cancer Metastasis. Front. Cell Dev. Biol. 2021, 9, 606149. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, J.E.; Dechat, T.; Grin, B.; Helfand, B.; Mendez, M.; Pallari, H.-M.; Goldman, R.D. Introducing Intermediate Filaments: From Discovery to Disease. J. Clin. Investig. 2009, 119, 1763–1771. [Google Scholar] [CrossRef]
- Lu, W.; Kang, Y. Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis. Dev. Cell 2019, 49, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Usman, S.; Waseem, N.H.; Nguyen, T.K.N.; Mohsin, S.; Jamal, A.; Teh, M.-T.; Waseem, A. Vimentin Is at the Heart of Epithelial Mesenchymal Transition (EMT) Mediated Metastasis. Cancers 2021, 13, 4985. [Google Scholar] [CrossRef]
- Lien, H.E.; Berg, H.F.; Halle, M.K.; Trovik, J.; Haldorsen, I.S.; Akslen, L.A.; Krakstad, C. Single-Cell Profiling of Low-Stage Endometrial Cancers Identifies Low Epithelial Vimentin Expression as a Marker of Recurrent Disease. EBioMedicine 2023, 92, 104595. [Google Scholar] [CrossRef] [PubMed]
- Albakova, Z.; Mangasarova, Y. The HSP Immune Network in Cancer. Front. Immunol. 2021, 12, 796493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, Q.; Li, T.; Liao, Q.; Zhao, Y. Role of the Complement System in the Tumor Microenvironment. Cancer Cell Int. 2019, 19, 300. [Google Scholar] [CrossRef]
- Pastusiak, K.; Matuszewska, E.; Pietkiewicz, D.; Matysiak, J.; Bogdanski, P. MALDI-TOF MS Characterisation of the Serum Proteomic Profile in Insulin-Resistant Normal-Weight Individuals. Nutrients 2021, 13, 3853. [Google Scholar] [CrossRef]
- Swiatly, A.; Horala, A.; Hajduk, J.; Matysiak, J.; Nowak-Markwitz, E.; Kokot, Z.J. MALDI-TOF-MS Analysis in Discovery and Identification of Serum Proteomic Patterns of Ovarian Cancer. BMC Cancer 2017, 17, 472. [Google Scholar] [CrossRef]
| Group | Low Risk | High Risk |
|---|---|---|
| Number of patients | 67 | 52 |
| Age [years] | 64 (60–70.5) | 65 (61–72) |
| BMI [kg/m2] | 32.1 (28.2–37.2) | 28.9 (26.7–33.1) |
| FIGO stage | ||
| IA | 49 | 6 |
| IB | 18 | 7 |
| II | 0 | 20 |
| III | 0 | 16 |
| IV | 0 | 3 |
| Grade | ||
| 1 | 41 | 9 |
| 2 | 23 | 20 |
| 3 | 3 | 22 |
| Histology | ||
| Endometroid | 67 | 33 |
| Non-endometroid | 0 | 19 |
| GA | QC | SNN |
|---|---|---|
| 2661.15 2284.03 1073.18 6432.36 3278.62 1568.47 3241.62 1866.53 1202.48 1520.06 1373.53 2294.99 1537.99 4091.16 1993.93 | 1036.97 1073.18 1202.48 1520.06 1993.93 3192.65 3263.71 4209.97 5904.21 6432.36 6630.42 | 1207.39 1528.12 2080.94 1537.99 2933.38 3241.62 1546.67 3192.65 2884.59 1639.7 4209.97 2579.98 1419.82 4170.79 1510.74 3215.33 7765.27 6630.42 4149.28 1264.4 |
| GA | QC | SNN | |
|---|---|---|---|
| Cross-validation (%) | 83.07 | 58.11 | 67.58 |
| Recognition capability (%) | 96.93 | 63.72 | 56.87 |
| Precursor Ion m/z | Protein Fragmentation Sequence | UniProtKB-ID | Protein Name | Protein Expression in High-Risk Patients Compared to Low-Risk Patients |
|---|---|---|---|---|
| 2661.15 | K.ANQQFLVYCEIDGSGNGWTVFQK.R | FIBG_HUMAN | Fibrinogen gamma chain | ↑ |
| 2284.03 | R.EIEDPEDRKPEDWDERPK.I | CALX_HUMAN | Calnexin | ↑ |
| 1073.18 | K.LDKENAIDR.A | TPM2_HUMAN | Tropomyosin beta chain | ↑ |
| 1568.47 | H.GHEQQHGLGHGHKF.K | KNG1_HUMAN | Kininogen-1 | ↑ |
| 1866.53 | M.AGRLPACVVDCGTGYTK.L | ARP3_HUMAN | Actin-related protein 3 | ↑ |
| 1202.48 | K.NVIGLQMGTNR.G | TAGL2_HUMAN | Transgelin-2 | ↑ |
| 1520.06 | R.KTFTAWCNSHLR.K | ACTN4_HUMAN | Alpha-actinin-4 | ↓ |
| 2294.99 | R.IQEIIEQLDVTTSEYEKEK.L | CH60_HUMAN | 60 kDa heat shock protein, mitochondrial | ↑ |
| 1537.99 | R.FAIQDISVEETSAK.E | ACTN1_HUMAN | Alpha-actinin-1 | ↑ |
| 1993.93 | K.TGPPGPAGQDGRPGPPGPPGAR.G | CO1A1_HUMAN | Collagen alpha-1(I) chain | ↑ |
| 1036.97 | K.RLDGSVDFK.K | FIBG_HUMAN | Fibrinogen gamma chain | ↑ |
| 1528.12 | R.AKFEELNMDLFR.S | GRP78_HUMAN | 78 kDa glucose-regulated protein | ↓ |
| 2080.94 | K.SMEAEMIQLQEELAAAER.A | MYH9_HUMAN | Myosin-9 | ↓ |
| 1546.67 | K.LKECCEKPLLEK.S | ALBU_HUMAN | Serum albumin | ↑ |
| 1639.7 | R.LDLAGRDLTDYLMK.I | ACTB_HUMAN | Actin, cytoplasmic 1 | ↓ |
| 2579.98 | K.QKPDGVFQEDAPVIHQEMIGGLR.N | CO3_HUMAN | Complement C3 | ↓ |
| 1419.82 | K.VQFELHYQEVK.W | ITIH2_HUMAN | Inter-alpha-trypsin inhibitor heavy chain H2 | ↓ |
| 1510.74 | R.MFGGPGTASRPSSSR.S | VIME_HUMAN | Vimentin | ↓ |
| 1264.4 | R.RAPFDLFENR.K | HS90A_HUMAN | Heat shock protein HSP 90-alpha | ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietkiewicz, D.; Zaborowski, M.P.; Jaz, K.; Matuszewska, E.; Światły-Błaszkiewicz, A.; Kluz, T.; Kokot, Z.J.; Nowak-Markwitz, E.; Matysiak, J. Serum Proteomic Profiles of Patients with High and Low Risk of Endometrial Cancer Recurrence. Int. J. Mol. Sci. 2023, 24, 14528. https://doi.org/10.3390/ijms241914528
Pietkiewicz D, Zaborowski MP, Jaz K, Matuszewska E, Światły-Błaszkiewicz A, Kluz T, Kokot ZJ, Nowak-Markwitz E, Matysiak J. Serum Proteomic Profiles of Patients with High and Low Risk of Endometrial Cancer Recurrence. International Journal of Molecular Sciences. 2023; 24(19):14528. https://doi.org/10.3390/ijms241914528
Chicago/Turabian StylePietkiewicz, Dagmara, Mikołaj Piotr Zaborowski, Kamila Jaz, Eliza Matuszewska, Agata Światły-Błaszkiewicz, Tomasz Kluz, Zenon J. Kokot, Ewa Nowak-Markwitz, and Jan Matysiak. 2023. "Serum Proteomic Profiles of Patients with High and Low Risk of Endometrial Cancer Recurrence" International Journal of Molecular Sciences 24, no. 19: 14528. https://doi.org/10.3390/ijms241914528
APA StylePietkiewicz, D., Zaborowski, M. P., Jaz, K., Matuszewska, E., Światły-Błaszkiewicz, A., Kluz, T., Kokot, Z. J., Nowak-Markwitz, E., & Matysiak, J. (2023). Serum Proteomic Profiles of Patients with High and Low Risk of Endometrial Cancer Recurrence. International Journal of Molecular Sciences, 24(19), 14528. https://doi.org/10.3390/ijms241914528





