Serum Complement C4 Levels Are a Useful Biomarker for Predicting End-Stage Renal Disease in Microscopic Polyangiitis
Abstract
:1. Introduction
2. Results
2.1. Patient Profiles
2.2. Comparison of the Baseline Clinical Characteristics and Disease Severity Classification of MPA Patients Who Have Progressed to ESRD and Those without ESRD
2.3. Selection of Risk Factor for ESRD in MPA
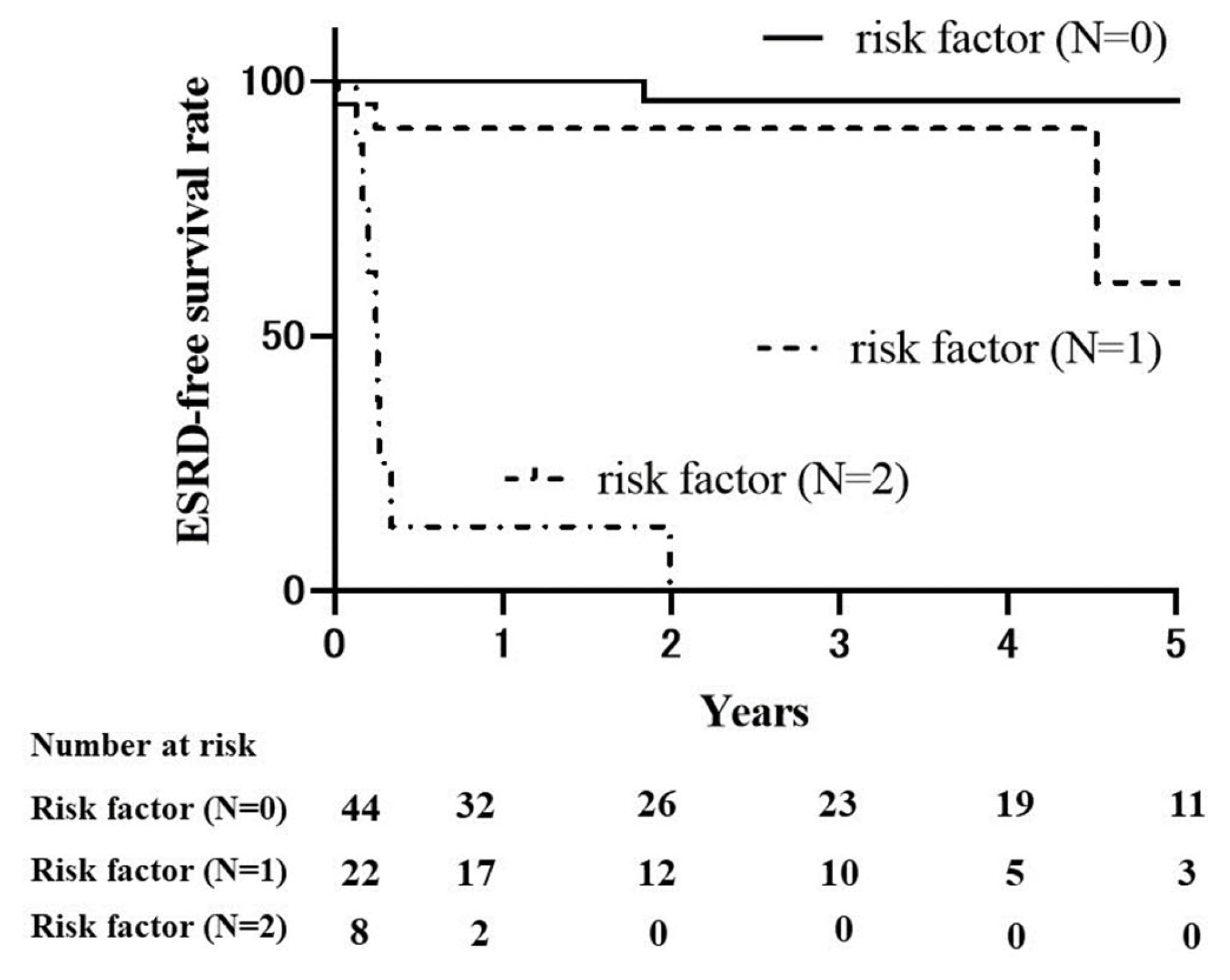
2.4. Renal Survival Rates in MPA
2.5. Comparison of the Baseline Clinical Characteristics and Disease Severity Classification of Patients with MPA with Serum C4 Levels above and below the Cut-Off Value
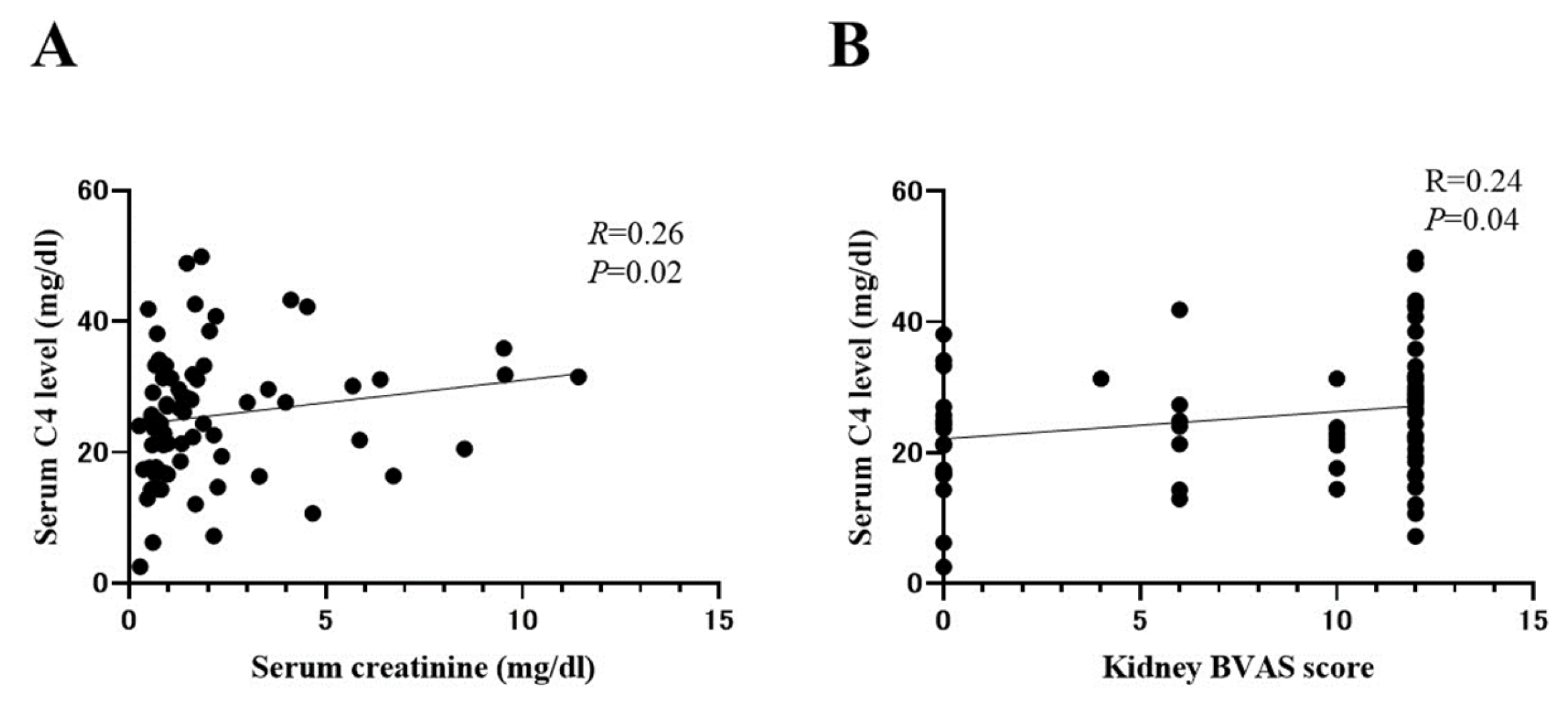
2.6. Correlation between Serum C4 and Indicators of AAV Disease Activity
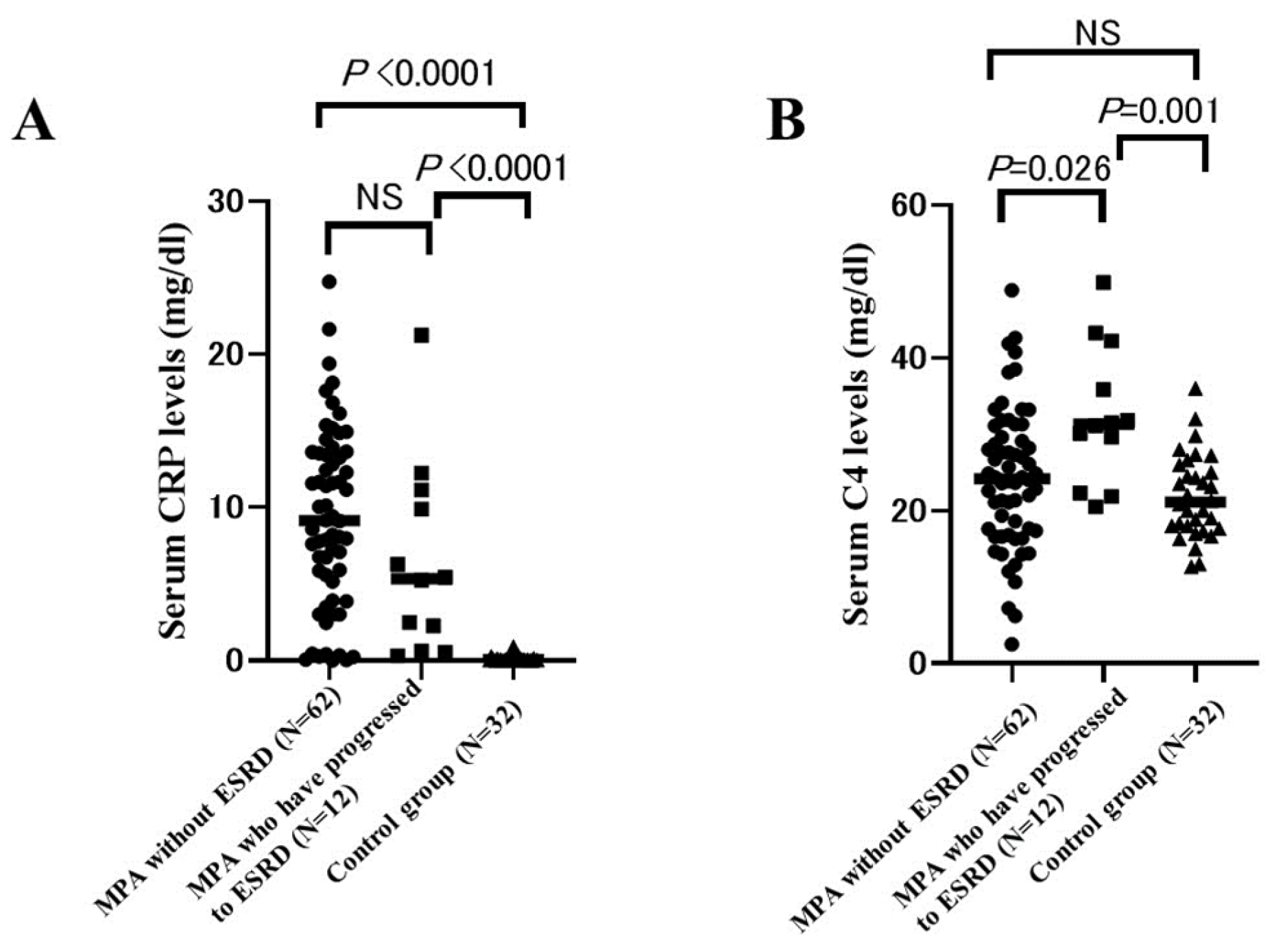
2.7. Comparison of Serum CRP Levels and C4 Levels between MPA Patients Who Have Progressed to ESRD and Those without ESRD and Control Group
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Measurement of Clinical Signs and Laboratory Parameters
4.3. Evaluation of Systemic Disease Severity
4.4. Renal Outcome
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kallenberg, C.G.M.; Stegeman, C.A.; Abdulahad, W.H.; Heeringa, P. Pathogenesis of ANCA-Associated Vasculitis: New Possibilities for Intervention. Am. J. Kidney Dis. 2013, 62, 1176–1187. [Google Scholar] [CrossRef]
- Matsuda, S.; Kotani, T.; Suzuka, T.; Kiboshi, T.; Fukui, K.; Wakama, M.; Ishida, T.; Fujiki, Y.; Shiba, H.; Nagai, K.; et al. Evaluation of Poor Prognostic Factors of Respiratory Related Death in Microscopic Polyangiitis Complicated by Interstitial Lung Disease. Sci. Rep. 2021, 11, 1490. [Google Scholar] [CrossRef]
- Matsuda, S.; Kotani, T.; Wakura, R.; Suzuka, T.; Kuwabara, H.; Kiboshi, T.; Wada, Y.; Shiba, H.; Hata, K.; Shoda, T.; et al. Examination of Nailfold Videocapillaroscopy Findings in ANCA-Associated Vasculitis. Rheumatology 2023, 62, 747–757. [Google Scholar] [CrossRef]
- Guillevin, L.; Durand-Gasselin, B.; Cevallos, R.; Gayraud, M.; Lhote, F.; Callard, P.; Amouroux, J.; Casassus, P.; Jarrousse, B. Microscopic Polyangiitis: Clinical and Laboratory Findings in Eighty-Five Patients. Arthritis Rheum. 1999, 42, 421–430. [Google Scholar] [CrossRef]
- Booth, A.D.; Almond, M.K.; Burns, A.; Ellis, P.; Gaskin, G.; Neild, G.H.; Plaisance, M.; Pusey, C.D.; Jayne, D.R.W. Outcome of ANCA-Associated Renal Vasculitis: A 5-Year Retrospective Study. Am. J. Kidney Dis. 2003, 41, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Flossmann, O.; Berden, A.; De Groot, K.; Hagen, C.; Harper, L.; Heijl, C.; Höglund, P.; Jayne, D.; Luqmani, R.; Mahr, A.; et al. Long-Term Patient Survival in ANCA-Associated Vasculitis. Ann. Rheum. Dis. 2011, 70, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Tanna, A.; Guarino, L.; Tam, F.W.K.; Rodriquez-Cubillo, B.; Levy, J.B.; Cairns, T.D.; Griffith, M.; Tarzi, R.M.; Caplin, B.; Salama, A.D.; et al. Long-Term Outcome of Anti-Neutrophil Cytoplasm Antibody-Associated Glomerulonephritis: Evaluation of the International Histological Classification and Other Prognostic Factors. Nephrol. Dial. Transplant. 2015, 30, 1185–1192. [Google Scholar] [CrossRef]
- Lionaki, S.; Hogan, S.L.; Jennette, C.E.; Hu, Y.; Hamra, J.B.; Jennette, J.C.; Falk, R.J.; Nachman, P.H. The Clinical Course of ANCA Small-Vessel Vasculitis on Chronic Dialysis. Kidney Int. 2009, 76, 644–651. [Google Scholar] [CrossRef]
- Moiseev, S.; Novikov, P.; Jayne, D.; Mukhin, N. End-Stage Renal Disease in ANCA-Associated Vasculitis. Nephrol. Dial. Transplant. 2017, 32, 248–253. [Google Scholar] [CrossRef]
- Berden, A.E.; Ferrario, F.; Hagen, E.C.; Jayne, D.R.; Jennette, J.C.; Joh, K.; Neumann, I.; Noël, L.H.; Pusey, C.D.; Waldherr, R.; et al. Histopathologic Classification of ANCA-Associated Glomerulonephritis. J. Am. Soc. Nephrol. 2010, 21, 1628–1636. [Google Scholar] [CrossRef]
- Manenti, L.; Vaglio, A.; Gnappi, E.; Maggiore, U.; Allegri, L.; Allinovi, M.; Urban, M.L.; Delsante, M.; Galetti, M.; Nicastro, M.; et al. Association of Serum C3 Concentration and Histologic Signs of Thrombotic Microangiopathy with Outcomes among Patients with ANCA-Associated Renal Vasculitis. Clin. J. Am. Soc. Nephrol. 2015, 10, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Shen, C.; Zhong, Y.; Ooi, J.D.; Eggenhuizen, P.; Zhou, Y.O.; Luo, H.; Huang, J.; Chen, J.B.; Wu, T.; et al. Glomerular Immune Deposition in MPO-ANCA Associated Glomerulonephritis Is Associated With Poor Renal Survival. Front. Immunol. 2021, 12, 625672. [Google Scholar] [CrossRef]
- Kim, M.K.; Pyo, J.Y.; Ahn, S.S.; Song, J.J.; Park, Y.B.; Lee, S.W. A retrospective analysis of antineutrophil cytoplasmic antibody-associated vasculitis aiming for an equation prediction end-stage renal disease. Clin. Rheumatol. 2022, 41, 773–781. [Google Scholar] [CrossRef]
- de Joode, A.A.E.; Sanders, J.S.F.; Stegeman, C.A. Renal Survival in Proteinase 3 and Myeloperoxidase ANCA-Associated Systemic Vasculitis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Sada, K.E.; Yamamura, M.; Harigai, M.; Fujii, T.; Takasaki, Y.; Amano, K.; Fujimoto, S.; Muso, E.; Murakawa, Y.; Arimura, Y.; et al. Different Responses to Treatment across Classified Diseases and Severities in Japanese Patients with Microscopic Polyangiitis and Granulomatosis with Polyangiitis: A Nationwide Prospective Inception Cohort Study. Arthritis Res. Ther. 2015, 17, 305. [Google Scholar] [CrossRef]
- Brix, S.R.; Noriega, M.; Tennstedt, P.; Vettorazzi, E.; Busch, M.; Nitschke, M.; Jabs, W.J.; Özcan, F.; Wendt, R.; Hausberg, M.; et al. Development and Validation of a Renal Risk Score in ANCA-Associated Glomerulonephritis. Kidney Int. 2018, 94, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Koyama, A.; Yamagata, K.; Makino, H.; Arimura, Y.; Wada, T.; Nitta, K.; Nihei, H.; Muso, E.; Taguma, Y.; Shigematsu, H.; et al. A Nationwide Survey of Rapidly Progressive Glomerulonephritis in Japan: Etiology, Prognosis and Treatment Diversity. Clin. Exp. Nephrol. 2009, 13, 633–650. [Google Scholar] [CrossRef]
- Sada, K.E.; Harigai, M.; Amano, K.; Atsumi, T.; Fujimoto, S.; Yuzawa, Y.; Takasaki, Y.; Banno, S.; Sugihara, T.; Kobayashi, M.; et al. Comparison of Severity Classification in Japanese Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis in a Nationwide, Prospective, Inception Cohort Study. Mod. Rheumatol. 2016, 26, 730–737. [Google Scholar] [CrossRef]
- Brilland, B.; Garnier, A.S.; Chevailler, A.; Jeannin, P.; Subra, J.F.; Augusto, J.F. Complement Alternative Pathway in ANCA-Associated Vasculitis: Two Decades from Bench to Bedside. Autoimmun. Rev. 2020, 19, 102424. [Google Scholar] [CrossRef]
- Xiao, H.; Schreiber, A.; Heeringa, P.; Falk, R.J.; Jennette, J.C. Alternative Complement Pathway in the Pathogenesis of Disease Mediated by Anti-Neutrophil Cytoplasmic Autoantibodies. Am. J. Pathol. 2007, 170, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xing, G.Q.; Yu, F.; Liu, G.; Zhao, M.H. Complement Deposition in Renal Histopathology of Patients with ANCA-Associated Pauci-Immune Glomerulonephritis. Nephrol. Dial. Transplant. 2009, 24, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Huugen, D.; Van Esch, A.; Xiao, H.; Peutz-Kootstra, C.J.; Buurman, W.A.; Tervaert, J.W.C.; Jennette, J.C.; Heeringa, P. Inhibition of Complement Factor C5 Protects against Anti-Myeloperoxidase Antibody-Mediated Glomerulonephritis in Mice. Kidney Int. 2007, 71, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Jayne, D.R.W.; Bruchfeld, A.N.; Harper, L.; Schaier, M.; Venning, M.C.; Hamilton, P.; Burst, V.; Grundmann, F.; Jadoul, M.; Szombati, I.; et al. Randomized Trial of C5a Receptor Inhibitor Avacopan in ANCA-Associated Vasculitis. J. Am. Soc. Nephrol. 2017, 28, 2756–2767. [Google Scholar] [CrossRef] [PubMed]
- Furuta, S.; Chaudhry, A.N.; Hamano, Y.; Fujimoto, S.; Nagafuchi, H.; Makino, H.; Matsuo, S.; Ozaki, S.; Endo, T.; Muso, E.; et al. Comparison of Phenotype and Outcome in Microscopic Polyangiitis between Europe and Japan. J. Rheumatol. 2014, 41, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.; Canepa, C.; Buettner, A.; Ryan, L.; Moloney, B.; Cormican, S.; Walsh, C.; White, A.; Salama, A.D.; Little, M.A. A Cohort Study to Investigate Sex-Specific Differences in ANCA-Associated Glomerulonephritis Outcomes. Sci. Rep. 2021, 11, 13080. [Google Scholar] [CrossRef]
- Mohammad, A.J.; Segelmark, M. A Population-Based Study Showing Better Renal Prognosis for Proteinase 3 Antineutrophil Cytoplasmic Antibody (ANCA)-Associated Nephritis versus Myeloperoxidase ANCA-Associated Nephritis. J. Rheumatol. 2014, 41, 1366–1373. [Google Scholar] [CrossRef]
- Sacri, A.S.; Chambaraud, T.; Ranchin, B.; Florkin, B.; Sée, H.; Decramer, S.; Flodrops, H.; Ulinski, T.; Allain-Launay, E.; Boyer, O.; et al. Clinical Characteristics and Outcomes of Childhood-Onset ANCA-Associated Vasculitis: A French Nationwide Study. Nephrol. Dial. Transplant. 2015, 30 (Suppl. S1), i104–i112. [Google Scholar] [CrossRef]
- Villacorta, J.; Diaz-Crespo, F.; Acevedo, M.; Cavero, T.; Guerrero, C.; Praga, M.; Fernandez-Juarez, G. Circulating C3 Levels Predict Renal and Global Outcome in Patients with Renal Vasculitis. Clin. Rheumatol. 2016, 35, 2733–2740. [Google Scholar] [CrossRef]
- Fukui, S.; Iwamoto, N.; Takatani, A.; Igawa, T.; Shimizu, T.; Umeda, M.; Nishino, A.; Koga, T.; Kawashiri, S.Y.; Tamai, M.; et al. Antineutrophilic Cytoplasmic Antibody-Associated Vasculitis with and without Renal Involvement: C3 Contributes to Prognosis, but Renal Involvement Does Not. Int. J. Rheum. Dis. 2019, 22, 789–796. [Google Scholar] [CrossRef]
- Xing, G.Q.; Chen, M.; Liu, G.; Heeringa, P.; Zhang, J.J.; Zheng, X.; Jie, E.; Kallenberg, C.G.M.; Zhao, M.H. Complement Activation Is Involved in Renal Damage in Human Antineutrophil Cytoplasmic Autoantibody Associated Pauci-Immune Vasculitis. J. Clin. Immunol. 2009, 29, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liu, M. Complement C4, Infections, and Autoimmune Diseases. Front. Immunol. 2021, 12, 694928. [Google Scholar] [CrossRef]
- Augusto, J.F.; Langs, V.; Demiselle, J.; Lavigne, C.; Brilland, B.; Duveau, A.; Poli, C.; Chevailler, A.; Croue, A.; Tollis, F.; et al. Low Serum Complement C3 Levels at Diagnosis of Renal ANCA-Associated Vasculitis Is Associated with Poor Prognosis. PLoS ONE 2016, 11, e0158871. [Google Scholar] [CrossRef] [PubMed]
- Deshayes, S.; Aouba, A.; Khoy, K.; Mariotte, D.; Lobbedez, T.; Silva, N.M. Hypocomplementemia Is Associated with Worse Renal Survival in ANCA-Positive Granulomatosis with Polyangiitis and Microscopic Polyangiitis. PLoS ONE 2018, 13, e0195680. [Google Scholar] [CrossRef] [PubMed]
- Moroni, G.; Binda, V.; Leoni, A.; Raffiotta, F.; Quaglini, S.; Banfi, G.; Messa, P. Predictors of Renal Survival in ANCA-Associated Vasculitis. Validation of a Histopatological Classification Schema and Review of the Literature. Clin. Exp. Rheumatol. 2015, 33, 56–63. [Google Scholar]
- Gou, S.J.; Yuan, J.; Wang, C.; Zhao, M.H.; Chen, M. Alternative Complement Pathway Activation Products in Urine and Kidneys of Patients with ANCA-Associated GN. Clin. J. Am. Soc. Nephrol. 2013, 8, 1884–1891. [Google Scholar] [CrossRef]
- Kojima, T.; Inoue, D.; Wajima, T.; Uchida, T.; Yamada, M.; Ohsawa, I.; Oda, T. Circulating Immune-Complexes and Complement Activation through the Classical Pathway in Myeloperoxidase-ANCA-Associated Glomerulonephritis. Ren. Fail. 2022, 44, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zha, Y.; Zhang, P.; He, P.; He, L. The Association Between Serum Complement 4 and Kidney Disease Progression in Idiopathic Membranous Nephropathy: A Multicenter Retrospective Cohort Study. Front. Immunol. 2022, 13, 896654. [Google Scholar] [CrossRef]
- Pan, M.; Zhang, J.; Li, Z.; Jin, L.; Zheng, Y.; Zhou, Z.; Zhen, S.; Lu, G. Increased C4 and Decreased C3 Levels Are Associated with a Poor Prognosis in Patients with Immunoglobulin A Nephropathy: A Retrospective Study. BMC Nephrol. 2017, 18, 231. [Google Scholar] [CrossRef]
- Duan, S.; Sun, L.; Nie, G.; Chen, J.; Zhang, C.; Zhu, H.; Huang, Z.; Qian, J.; Zhao, X.; Xing, C.; et al. Association of Glomerular Complement C4c Deposition with the Progression of Diabetic Kidney Disease in Patients with Type 2 Diabetes. Front. Immunol. 2020, 11, 2073. [Google Scholar] [CrossRef] [PubMed]
- Crnogorac, M.; Horvatic, I.; Kacinari, P.; Ljubanovic, D.G.; Galesic, K. Serum C3 Complement Levels in ANCA Associated Vasculitis at Diagnosis Is a Predictor of Patient and Renal Outcome. J. Nephrol. 2018, 31, 257–262. [Google Scholar] [CrossRef]
- Gigante, A.; Salviani, C.; Giannakakis, K.; Rosato, E.; Barbano, B.; Amoroso, A.; Gasperini, M.L.; Nofroni, I.; Salsano, F.; Cianci, R.; et al. Clinical and Histological Outcome Predictors in Renal Limited Pauci-Immune Crescentic Glomerulonephritis: A Single Centre Experience. Int. J. Immunopathol. Pharmacol. 2012, 25, 287–292. [Google Scholar] [CrossRef]
- Sethi, S.; Zand, L.; de Vriese, A.S.; Specks, U.; Vrana, J.A.; Kanwar, S.; Kurtin, P.; Theis, J.D.; Angioi, A.; Cornell, L.; et al. Complement Activation in Pauci-Immune Necrotizing and Crescentic Glomerulonephritis: Results of a Proteomic Analysis. Nephrol. Dial. Transplant. 2017, 32, I139–I145. [Google Scholar] [CrossRef]
- Castellano, G.; Melchiorre, R.; Loverre, A.; Ditonno, P.; Montinaro, V.; Rossini, M.; Divella, C.; Battaglia, M.; Lucarelli, G.; Annunziata, G.; et al. Therapeutic Targeting of Classical and Lectin Pathways of Complement Protects from Ischemia-Reperfusion-Induced Renal Damage. Am. J. Pathol. 2010, 176, 1648–1659. [Google Scholar] [CrossRef]
- Gadjeva, M.; Verschoor, A.; Brockman, M.A.; Jezak, H.; Shen, L.M.; Knipe, D.M.; Carroll, M.C. Macrophage-Derived Complement Component C4 Can Restore Humoral Immunity in C4-Deficient Mice. J. Immunol. 2002, 169, 5489–5495. [Google Scholar] [CrossRef]
- Zhou, W.; Andrews, P.A.; Wang, Y.; Wolff, J.; Pratt, J.; Hartley, B.R.; Verroust, P.; Sacks, S.H. Evidence for Increased Synthesis of Complement C4 in the Renal Epithelium of Rats with Passive Heymann Nephritis. J. Am. Soc. Nephrol. 1997, 8, 214–222. [Google Scholar] [CrossRef]
- Shi, J.; Shen, Q.; Chen, X.M.; Du, X.G. Clinical Characteristics and Outcomes in Microscopic Polyangiitis Patients with Renal Involvement: A Study of 124 Chinese Patients. BMC Nephrol. 2019, 20, 339. [Google Scholar] [CrossRef]
- Chen, Y.; Bao, H.; Liu, Z.; Liu, X.; Gao, E.; Zeng, C.; Zhang, H.; Liu, Z.; Hu, W. Risk Factors for Renal Survival in Chinese Patients with Myeloperoxidase-ANCA-Associated GN. Clin. J. Am. Soc. Nephrol. 2017, 12, 417–425. [Google Scholar] [CrossRef]
- Bi, T.D.; Zheng, J.N.; Zhang, J.X.; Yang, L.S.; Liu, N.; Liu, L.L.; Yao, L. Serum Complement C4 Is an Important Prognostic Factor for IgA Nephropathy: A Retrospective Study. BMC Nephrol. 2019, 20, 244. [Google Scholar] [CrossRef]
- Mitsnefes, M.M.; Kathman, T.S.; Mishra, J.; Kartal, J.; Khoury, P.R.; Nickolas, T.L.; Barasch, J.; Devarajan, P. Serum Neutrophil Gelatinase-Associated Lipocalin as a Marker of Renal Function in Children with Chronic Kidney Disease. Pediatr. Nephrol. 2007, 22, 101–108. [Google Scholar] [CrossRef]
- Scullion, K.M.; Vliegenthart, A.D.B.; Rivoli, L.; Oosthuyzen, W.; Farrah, T.E.; Czopek, A.; Webb, D.J.; Hunter, R.W.; Bailey, M.A.; Dhaun, N.; et al. Circulating Argonaute-Bound MicroRNA-126 Reports Vascular Dysfunction and Treatment Response in Acute and Chronic Kidney Disease. iScience 2020, 24, 101937. [Google Scholar] [CrossRef]
- Lieberthal, J.G.; Cuthbertson, D.; Carette, S.; Hoffman, G.S.; Khalidi, N.A.; Koening, C.L.; Langford, C.A.; Maksimowicz-McKinnon, K.; Seo, P.; Specks, U.; et al. Urinary Biomarkers in Relapsing Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. J. Rheumatol. 2013, 40, 674–683. [Google Scholar] [CrossRef]
- Tam, F.W.K.; Sanders, J.S.; George, A.; Hammad, T.; Miller, C.; Dougan, T.; Cook, H.T.; Kallenberg, C.G.M.; Gaskin, G.; Levy, J.B.; et al. Urinary Monocyte Chemoattractant Protein-1 (MCP-1) Is a Marker of Active Renal Vasculitis. Nephrol. Dial. Transplant. 2004, 19, 2761–2768. [Google Scholar] [CrossRef]
- Park, P.G.; Pyo, J.Y.; Ahn, S.S.; Song, J.J.; Park, Y.B.; Lee, S.W. Serum Glycated Albumin as a Predictive Biomarker for Renal Involvement of Antineutrophil Cytoplasmic Antibody-Associated Vasculitis in Non-Diabetic Patients. BMC Nephrol. 2022, 23, 288. [Google Scholar] [CrossRef]
- Mukhtyar, C.; Lee, R.; Brown, D.; Carruthers, D.; Dasgupta, B.; Dubey, S.; Flossmann, O.; Hall, C.; Hollywood, J.; Jayne, D.; et al. Modification and Validation of the Birmingham Vasculitis Activity Score (Version 3). Ann. Rheum. Dis. 2009, 68, 1827–1832. [Google Scholar] [CrossRef]
- Watts, R.; Lane, S.; Hanslik, T.; Hauser, T.; Hellmich, B.; Koldingsnes, W.; Mahr, A.; Segelmark, M.; Cohen-Tervaert, J.W.; Scott, D. Development and Validation of a Consensus Methodology for the Classification of the ANCA-Associated Vasculitides and Polyarteritis Nodosa for Epidemiological Studies. Ann. Rheum. Dis. 2007, 66, 222–227. [Google Scholar] [CrossRef]
- Hellmich, B.; Flossmann, O.; Gross, W.L.; Bacon, P.; Cohen-Tervaert, J.W.; Guillevin, L.; Jayne, D.; Mahr, A.; Merkel, P.A.; Raspe, H.; et al. EULAR Recommendations for Conducting Clinical Studies and/or Clinical Trials in Systemic Vasculitis: Focus on Anti-Neutrophil Cytoplasm Antibody-Associated Vasculitis. Ann. Rheum. Dis. 2007, 66, 605–617. [Google Scholar] [CrossRef]
- Guillevin, L.; Pagnoux, C.; Seror, R.; Mahr, A.; Mouthon, L.; Toumelin, P. Le The Five-Factor Score Revis ited: Assessment of Prognoses of Systemic Necrotizing Vasculitides Based on the French Vasculitis Study Group (FVSG) Cohort. Medicine 2011, 90, 19–27. [Google Scholar] [CrossRef]

| Characteristics | AAV (N= 74) |
|---|---|
| Age, years | 75 (70–79) |
| Female, n (%) | 37 (50) |
| Laboratory findings | |
| WBC,/mm3 | 11,315 (7980–14,705) |
| Hb, g/dL | 9.8 (8.7–11.8) |
| Alb, g/dL | 2.5 (2.1–3.1) |
| Cr, mg/dL | 1.3 (0.7–2.2) |
| CRP, mg/dL | 8.4 (3.8–13.3) |
| Serum C3 levels, mg/dL | 112 (93.8–132.3) |
| Serum C4 levels, mg/dL | 24.9 (18.4–31.4) |
| Positive, anti-MPO-ANCA, n (%) | 73 (98.6) |
| Positive, anti-PR3-ANCA, n (%) | 3 (4.1) |
| MPO-ANCA titer, U/mL | 128.6 (65.0–280.0) a |
| BVAS at onset | 18 (12–22) |
| Five factor score 2009 | |
| ≤1 | 15 (20.3) |
| 2 | 35 (47.3) |
| ≥3 | 24 (32.4) |
| EUVAS-defined disease activity | |
| Localized | 2 (2.7) |
| Early systemic | 16 (21.6) |
| Systemic | 42 (56.8) |
| Severe | 14 (18.9) |
| Characteristics | MPA Who Have Progressed to ESRD (N = 12) | MPA without ESRD (N = 62) | p-Value |
|---|---|---|---|
| Age, years | 72 (70–77) | 76 (70–79) | 0.52 |
| Female, n (%) | 4 (33.3) | 33 (53.2) | 0.34 |
| Laboratory findings | |||
| WBC,/mm3 | 8005 (5155–11,793) | 11,870 (8698–15,510) | 0.02 * |
| Hb, g/dL | 9.1 (7.5–9.5) | 10.4 (9.0–12) | 0.004 ** |
| Alb, g/dL | 2.8 (2.5–3.4) | 2.4 (2.1–3.0) | 0.06 |
| Cr, mg/dL | 5.8 (3.7–9.3) | 0.95 (0.69–1.68) | <0.0001 *** |
| CRP, mg/dL | 5.3 (1.0–10.8) | 9.1 (4.8–13.6) | 0.095 |
| Serum C3 level, mg/dL | 96.5 (79.3–115.8) | 114 (97.3–133) | 0.03 * |
| Serum C4 level, mg/dL | 31.3 (24.1–40.6) | 24.2 (17.3–30.0) | 0.009 ** |
| Positive, anti-MPO-ANCA, n (%) | 12 (100) | 61 (98.4) | 1.00 |
| Positive, anti-PR3-ANCA, n (%) | 0 (0) | 3 (4.9) | 1.00 |
| MPO-ANCA titer, U/mL | 94.4 (57.3–572.5) | 143(68.7–280) a | 0.78 |
| BVAS at onset | 19 (18–22.8) | 17 (11.8–21.0) | 0.09 |
| Five factor score 2009 | |||
| ≤1 | 0 (0) | 15 (24.2) | 0.11 |
| 2 | 3 (25.0) | 32 (51.6) | 0.12 |
| ≥3 | 9 (75.0) | 15 (24.2) | 0.001 ** |
| EUVAS-defined disease activity | |||
| Localized | 0 (0) | 2 (3.2) | 1.00 |
| Early systemic | 0 (0.0) | 16 (25.8) | 0.058 |
| Systemic | 4 (33.3) | 38 (61.3) | 0.11 |
| Severe | 8 (66.7) | 6 (9.7) | <0.0001 *** |
| Characteristics | ESRD (N= 12) | Non-ESRD (N= 62) | p-Value |
|---|---|---|---|
| Initial treatment | |||
| PDN, mg/day | 50 (40–60) | 50 (36.9–60) | 0.46 |
| MPDN pulse, n (%) | 7 (58.3) | 13 (21.0) | 0.013 * |
| Immunosuppressants | |||
| IVCY, n (%) | 8 (66.7) | 33 (53.2) | 0.53 |
| Total IVCY dose (g) | 1.2 (0.18–1.5) | 1.4 (0.65–2.1) | 0.09 |
| RTX, n (%) | 3 (25.0) | 7 (11.3) | 0.35 |
| IVIG, n (%) | 0 (0) | 2 (3.2) | 1.0 |
| AZA/MTX/TAC/MZB, n (%) | 6 (50.0)/0 (0)/0 (0)/0 (0) | 44 (71.0)/2 (3.2)/4 (6.5)/1 (1.6) | 0.19/1.0/1.0/1.0 |
| Variable | Odds Ratio | Odds Ratio (95% CI) | p-Value |
|---|---|---|---|
| Serum C3 levels (for 1 mg/dL) | 0.98 | 0.92–1.05 | 0.65 |
| Serum C4 levels (for 1 mg/dL) | 1.24 | 1.03–1.49 | 0.02 * |
| Serum Cr levels (for 1 mg/dL) | 4.40 | 1.25–15.5 | 0.02 * |
| WBC (for 1/mm3) | 0.99 | 0.99–1.00 | 0.37 |
| Hb (for 1 g/dL) | 0.35 | 0.12–1.01 | 0.053 |
| Characteristics | C4 Levels < 29.6 mg/dL(N = 49) | C4 Levels ≥ 29.6 mg/dL(N = 25) | p-Value |
|---|---|---|---|
| Age, years | 73 (69–79) | 76 (71–79) | 0.55 |
| Female, n (%) | 27 (55.1) | 10 (40.0) | 0.33 |
| Laboratory findings | |||
| WBC,/mm3 | 12,270 (9005–15,845) | 8800 (6155–12,145) | 0.002 ** |
| Hb, g/dL | 9.7 (8.5–11.1) | 10 (9.1–12.3) | 0.16 |
| Alb, g/dL | 2.3 (2.0–2.9) | 2.8 (2.3–3.7) | 0.005 ** |
| Cr, mg/dL | 0.95 (0.66–1.79) | 1.73 (0.94–4.32) | 0.008 ** |
| CRP, mg/dL | 9.9 (6.3–13.5) | 5.2 (0.5–11.9) | 0.038 * |
| Serum C3 level, mg/dL | 113 (89.5–131.5) | 108 (96.5–132.5) | 0.87 |
| MPO-ANCA titer | 120.2 (61.2–256.0) a | 223 (72.0–292.6) | 0.20 |
| BVAS at onset | 18 (12–21.5) | 17 (12–22) | 0.60 |
| Five factor score 2009 | |||
| ≤1 | 13 (26.5) | 2 (8.0) | 0.07 |
| 2 | 22 (44.9) | 13 (52.0) | 0.63 |
| ≥3 | 14 (28.6) | 10 (40.0) | 0.43 |
| EUVAS-defined disease activity | |||
| Localized | 0 (0) | 2 (8.0) | 0.11 |
| Early systemic | 11 (22.5) | 5 (20.0) | 1.00 |
| Systemic | 30 (61.2) | 12 (48.0) | 0.33 |
| Severe | 8 (16.3) | 6 (24.0) | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuda, S.; Oe, K.; Kotani, T.; Okazaki, A.; Kiboshi, T.; Suzuka, T.; Wada, Y.; Shiba, H.; Hata, K.; Shoda, T.; et al. Serum Complement C4 Levels Are a Useful Biomarker for Predicting End-Stage Renal Disease in Microscopic Polyangiitis. Int. J. Mol. Sci. 2023, 24, 14436. https://doi.org/10.3390/ijms241914436
Matsuda S, Oe K, Kotani T, Okazaki A, Kiboshi T, Suzuka T, Wada Y, Shiba H, Hata K, Shoda T, et al. Serum Complement C4 Levels Are a Useful Biomarker for Predicting End-Stage Renal Disease in Microscopic Polyangiitis. International Journal of Molecular Sciences. 2023; 24(19):14436. https://doi.org/10.3390/ijms241914436
Chicago/Turabian StyleMatsuda, Shogo, Katsumasa Oe, Takuya Kotani, Ayana Okazaki, Takao Kiboshi, Takayasu Suzuka, Yumiko Wada, Hideyuki Shiba, Kenichiro Hata, Takeshi Shoda, and et al. 2023. "Serum Complement C4 Levels Are a Useful Biomarker for Predicting End-Stage Renal Disease in Microscopic Polyangiitis" International Journal of Molecular Sciences 24, no. 19: 14436. https://doi.org/10.3390/ijms241914436
APA StyleMatsuda, S., Oe, K., Kotani, T., Okazaki, A., Kiboshi, T., Suzuka, T., Wada, Y., Shiba, H., Hata, K., Shoda, T., & Takeuchi, T. (2023). Serum Complement C4 Levels Are a Useful Biomarker for Predicting End-Stage Renal Disease in Microscopic Polyangiitis. International Journal of Molecular Sciences, 24(19), 14436. https://doi.org/10.3390/ijms241914436






