LOXL2 in Cancer: A Two-Decade Perspective
Abstract
1. Introduction
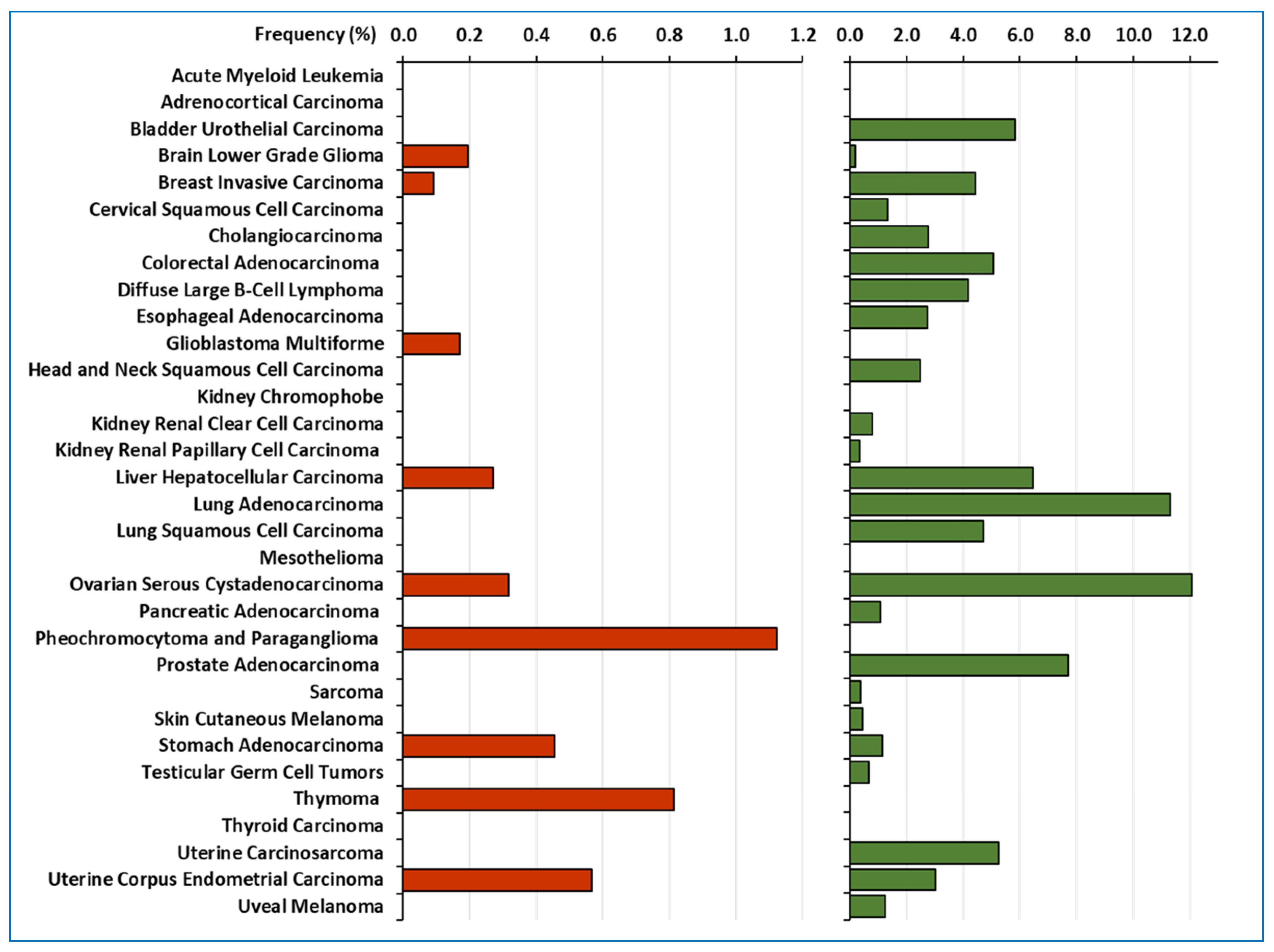
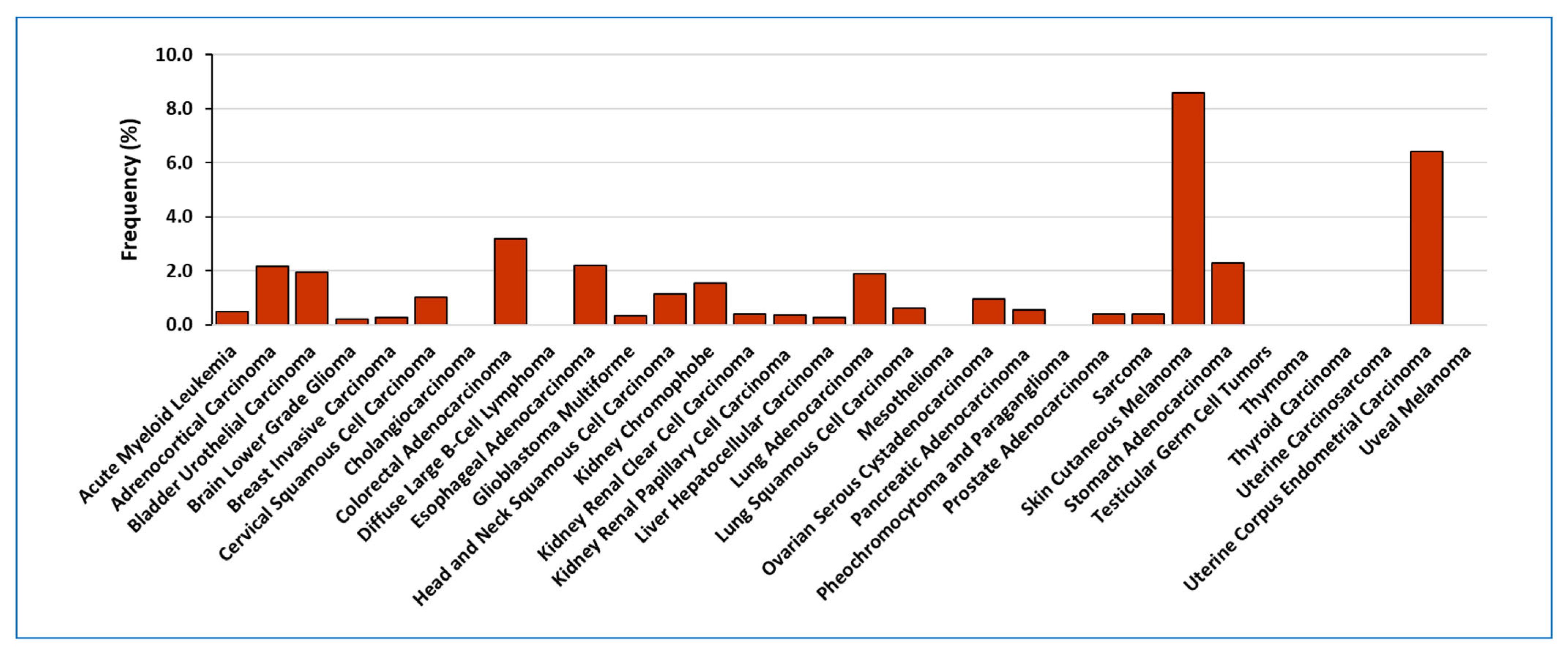
2. LOXL2 in Human Tumour Samples
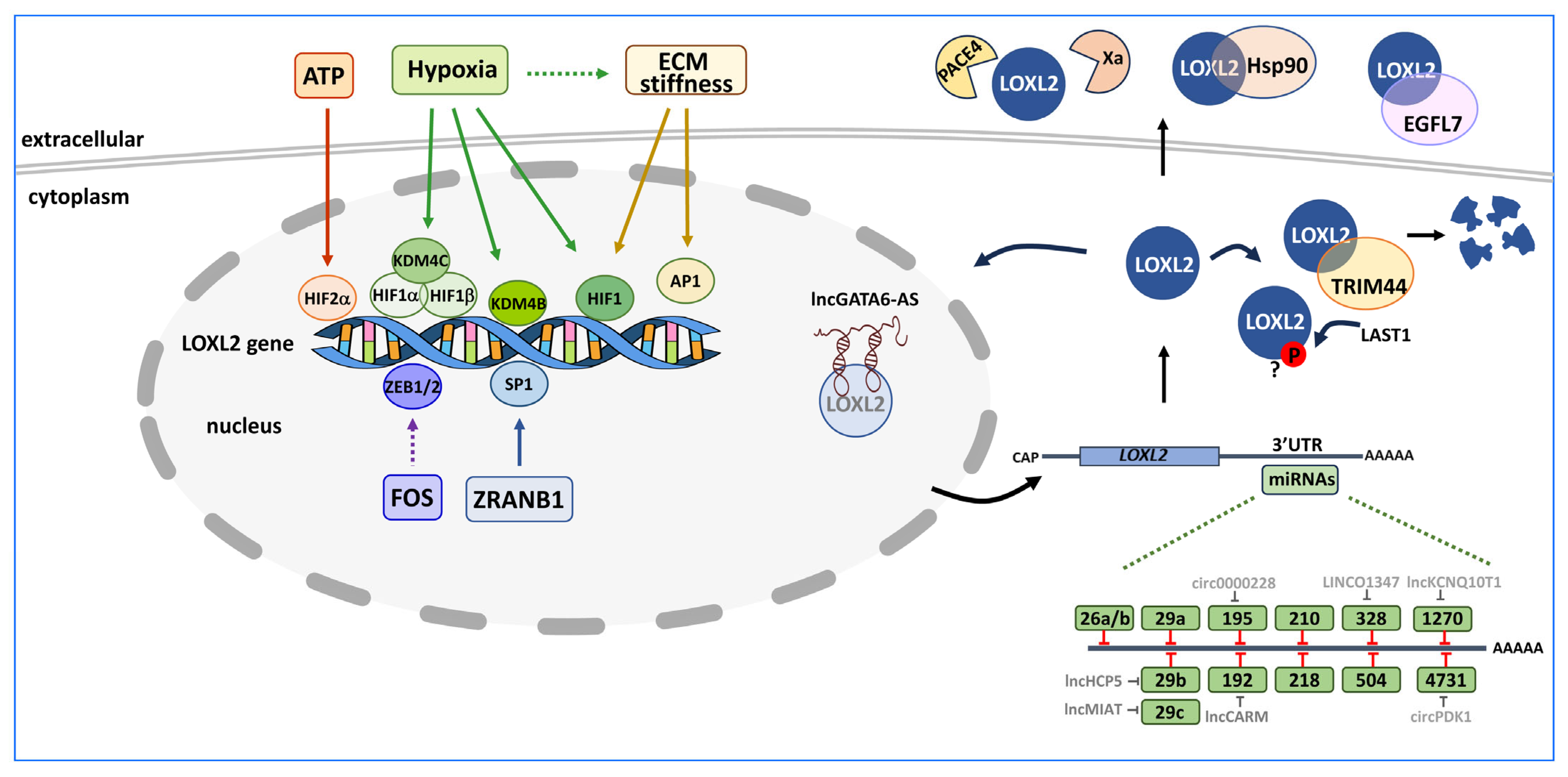
3. Control of LOXL2 Expression
3.1. Transcriptional Regulation of LOXL2
3.2. Post-Transcriptional Regulation of LOXL2
3.3. Post-Translational Regulation of LOXL2
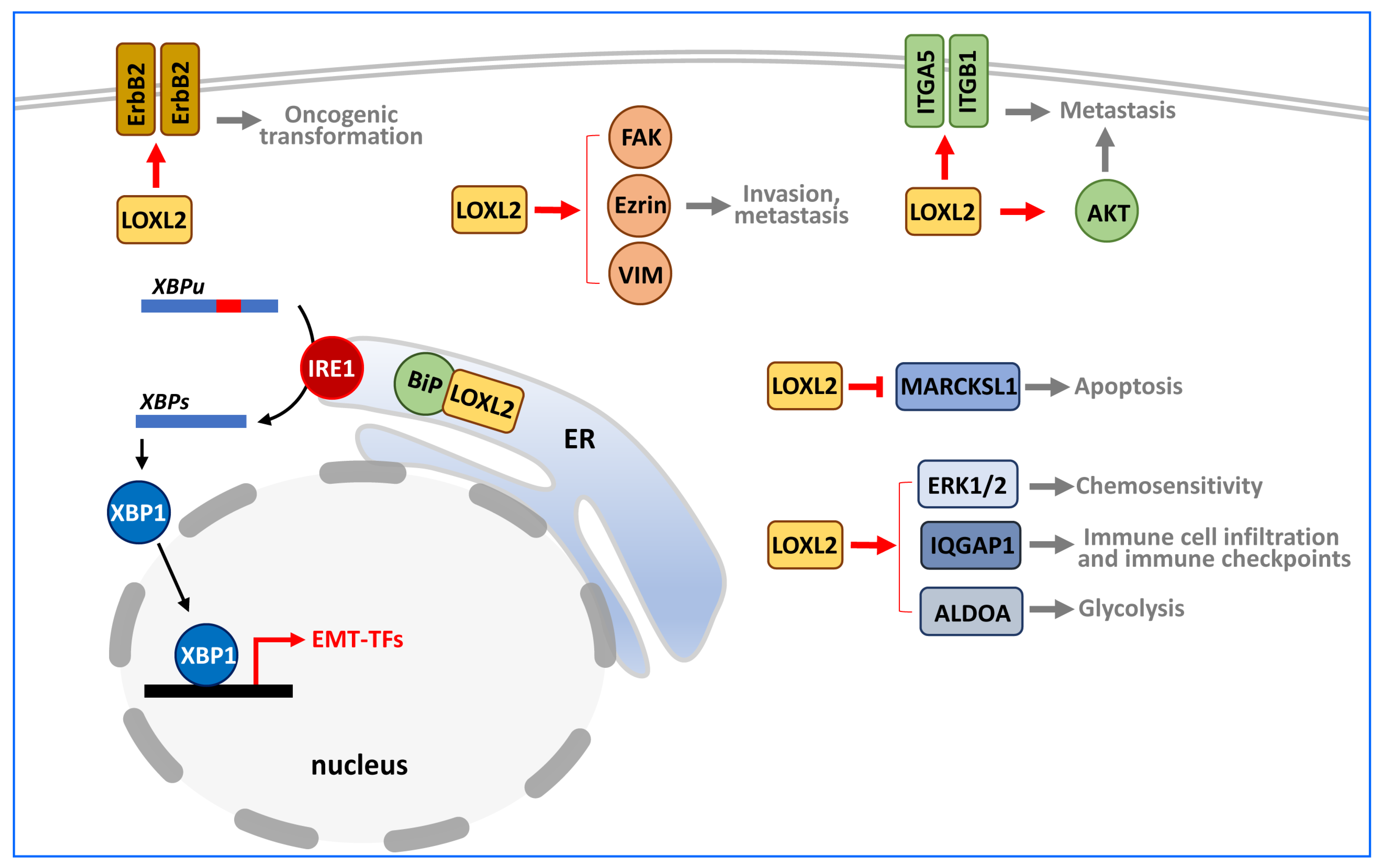
4. Targets of LOXL2 in Cancer
4.1. Extracellular Targets
4.2. Cytoplasmic Targets
4.3. Nuclear Targets
5. Genetically Modified Mouse Models
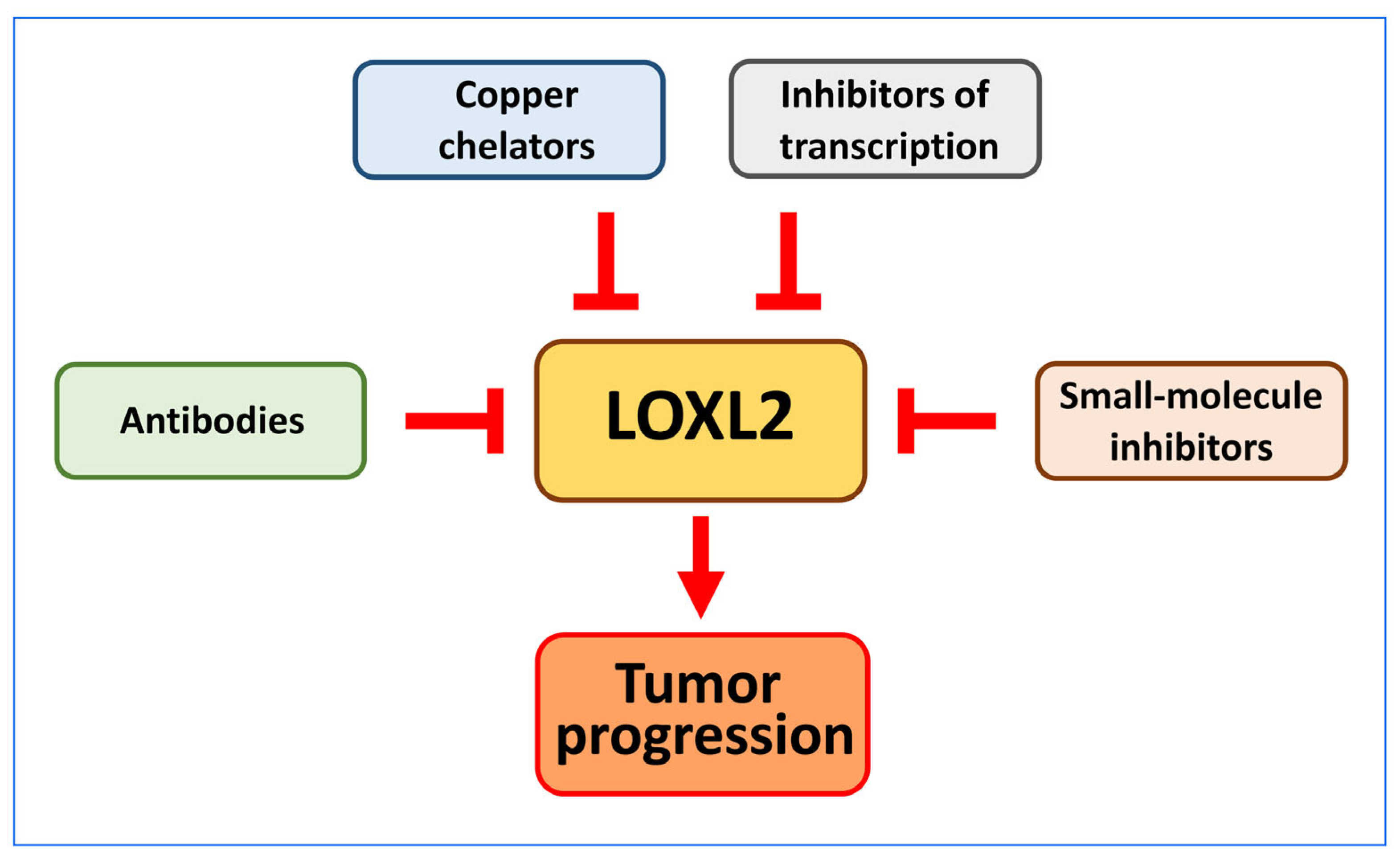
6. Prognostic and Therapeutic Value of LOXL2 in Cancer
6.1. Antibodies
6.2. Copper Chelators
6.3. Small Molecule Inhibitors of LOXL2
6.4. Inhibitors of LOXL2 Transcription
7. Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liburkin-Dan, T.; Toledano, S.; Neufeld, G. Lysyl Oxidase Family Enzymes and Their Role in Tumor Progression. Int. J. Mol. Sci. 2022, 23, 6249. [Google Scholar] [CrossRef] [PubMed]
- Grau-Bové, X.; Ruiz-Trillo, I.; Rodriguez-Pascual, F. Origin and Evolution of Lysyl Oxidases. Sci. Rep. 2015, 5, 10568. [Google Scholar] [CrossRef] [PubMed]
- Trackman, P.C. Lysyl Oxidase Isoforms and Potential Therapeutic Opportunities for Fibrosis and Cancer. Expert. Opin. Ther. Targets 2016, 20, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Hajdú, I.; Kardos, J.; Major, B.; Fabó, G.; Lőrincz, Z.; Cseh, S.; Dormán, G. Inhibition of the LOX Enzyme Family Members with Old and New Ligands. Selectivity Analysis Revisited. Bioorg. Med. Chem. Lett. 2018, 28, 3113–3118. [Google Scholar] [CrossRef] [PubMed]
- Finney, J.; Moon, H.-J.; Ronnebaum, T.; Lantz, M.; Mure, M. Human Copper-Dependent Amine Oxidases. Arch. Biochem. Biophys. 2014, 546, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.A.; Moon, H.-J.; Sabuncu, S.; Singh, P.; Ronnebaum, T.A.; Ou, S.; Douglas, J.T.; Jackson, T.A.; Moënne-Loccoz, P.; Mure, M. Insight into the Spatial Arrangement of the Lysine Tyrosylquinone and Cu2+ in the Active Site of Lysyl Oxidase-like 2. Int. J. Mol. Sci. 2022, 23, 13966. [Google Scholar] [CrossRef]
- Herranz, N.; Dave, N.; Millanes-Romero, A.; Pascual-Reguant, L.; Morey, L.; Díaz, V.M.; Lórenz-Fonfría, V.; Gutierrez-Gallego, R.; Jerónimo, C.; Iturbide, A.; et al. Lysyl Oxidase-like 2 (LOXL2) Oxidizes Trimethylated Lysine 4 in Histone H3. FEBS J. 2016, 283, 4263–4273. [Google Scholar] [CrossRef]
- Iturbide, A.; Pascual-Reguant, L.; Fargas, L.; Cebrià, J.P.; Alsina, B.; García de Herreros, A.; Peiró, S. LOXL2 Oxidizes Methylated TAF10 and Controls TFIID-Dependent Genes during Neural Progenitor Differentiation. Mol. Cell 2015, 58, 755–766. [Google Scholar] [CrossRef]
- Rosell-García, T.; Rivas-Muñoz, S.; Colige, A.; Rodriguez-Pascual, F. Cleavage of LOXL1 by BMP1 and ADAMTS14 Proteases Suggests a Role for Proteolytic Processing in the Regulation of LOXL1 Function. Int. J. Mol. Sci. 2022, 23, 3285. [Google Scholar] [CrossRef]
- Uzel, M.I.; Scott, I.C.; Babakhanlou-Chase, H.; Palamakumbura, A.H.; Pappano, W.N.; Hong, H.-H.; Greenspan, D.S.; Trackman, P.C. Multiple Bone Morphogenetic Protein 1-Related Mammalian Metalloproteinases Process Pro-Lysyl Oxidase at the Correct Physiological Site and Control Lysyl Oxidase Activation in Mouse Embryo Fibroblast Cultures. J. Biol. Chem. 2001, 276, 22537–22543. [Google Scholar] [CrossRef]
- Sarrias, M.R.; Gronlund, J.; Padilla, O.; Madsen, J.; Holmskov, U.; Lozano, F. The Scavenger Receptor Cysteine-Rich (SRCR) Domain: An Ancient and Highly Conserved Protein Module of the Innate Immune System. Crit. Rev. Immunol. 2004, 24, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Reichhardt, M.P.; Loimaranta, V.; Lea, S.M.; Johnson, S. Structures of SALSA/DMBT1 SRCR Domains Reveal the Conserved Ligand-Binding Mechanism of the Ancient SRCR Fold. Life Sci. Alliance 2020, 3, e201900502. [Google Scholar] [CrossRef] [PubMed]
- Umana-Diaz, C.; Pichol-Thievend, C.; Marchand, M.F.; Atlas, Y.; Salza, R.; Malbouyres, M.; Barret, A.; Teillon, J.; Ardidie-Robouant, C.; Ruggiero, F.; et al. Scavenger Receptor Cysteine-Rich Domains of Lysyl Oxidase-Like2 Regulate Endothelial ECM and Angiogenesis through Non-Catalytic Scaffolding Mechanisms. Matrix Biol. 2020, 88, 33–52. [Google Scholar] [CrossRef] [PubMed]
- Eraso, P.; Mazón, M.J.; Jiménez, V.; Pizarro-García, P.; Cuevas, E.P.; Majuelos-Melguizo, J.; Morillo-Bernal, J.; Cano, A.; Portillo, F. New Functions of Intracellular LOXL2: Modulation of RNA-Binding Proteins. Molecules 2023, 28, 4433. [Google Scholar] [CrossRef]
- Ma, L.; Huang, C.; Wang, X.-J.; Xin, D.E.; Wang, L.; Zou, Q.C.; Zhang, Y.S.; Tan, M.; Wang, Y.; Zhao, T.C.; et al. Lysyl Oxidase 3 Is a Dual-Specificity Enzyme Involved in STAT3 Deacetylation and Deacetylimination Modulation. Mol. Cell 2017, 65, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.-W.; Zhan, X.-H.; Wang, J.-J.; He, L.-X.; Guo, Z.-C.; Xu, X.-E.; Liao, L.-D.; Huang, X.; Wen, B.; Xu, Y.-W.; et al. LOXL2-Dependent Deacetylation of Aldolase A Induces Metabolic Reprogramming and Tumor Progression. Redox Biol. 2022, 57, 102496. [Google Scholar] [CrossRef]
- Lu, X.; Xin, D.E.; Du, J.K.; Zou, Q.C.; Wu, Q.; Zhang, Y.S.; Deng, W.; Yue, J.; Fan, X.S.; Zeng, Y.; et al. Loss of LOXL2 Promotes Uterine Hypertrophy and Tumor Progression by Enhancing H3K36ac-Dependent Gene Expression. Cancer Res. 2022, 82, 4400–4413. [Google Scholar] [CrossRef]
- Kamiya, T.; Kadowaki, M.; Atobe, T.; Kunieda, K.; Morimoto, K.; Hara, H. Inhibition of N-glycosylation by Glucosamine Hydrochloride Inhibits TGF-β1-induced LOXL2 Secretion. J. Cell. Biochem. 2023, 124, 797–807. [Google Scholar] [CrossRef]
- Moon, H.-J.; Finney, J.; Xu, L.; Moore, D.; Welch, D.R.; Mure, M. MCF-7 Cells Expressing Nuclear Associated Lysyl Oxidase-like 2 (LOXL2) Exhibit an Epithelial-to-Mesenchymal Transition (EMT) Phenotype and Are Highly Invasive in Vitro. J. Biol. Chem. 2013, 288, 30000–30008. [Google Scholar] [CrossRef]
- Meier, A.A.; Go, E.P.; Moon, H.-J.; Desaire, H.; Mure, M. Mass Spectrometry-Based Disulfide Mapping of Lysyl Oxidase-like 2. Int. J. Mol. Sci. 2022, 23, 5879. [Google Scholar] [CrossRef]
- Schmelzer, C.E.H.; Heinz, A.; Troilo, H.; Lockhart-Cairns, M.P.; Jowitt, T.A.; Marchand, M.F.; Bidault, L.; Bignon, M.; Hedtke, T.; Barret, A.; et al. Lysyl Oxidase-like 2 (LOXL2)-mediated Cross-linking of Tropoelastin. FASEB J. 2019, 33, 5468–5481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Q.; Wu, J.; Wang, J.; Shi, Y.; Liu, M. Crystal Structure of Human Lysyl Oxidase-like 2 (HLOXL2) in a Precursor State. Proc. Natl. Acad. Sci. USA 2018, 115, 3828–3833. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.A.; Kuczera, K.; Mure, M. A 3D–Predicted Structure of the Amine Oxidase Domain of Lysyl Oxidase–Like 2. Int. J. Mol. Sci. 2022, 23, 13385. [Google Scholar] [CrossRef] [PubMed]
- Meier, A.A.; Moon, H.-J.; Toth, R.; Folta-Stogniew, E.; Kuczera, K.; Middaugh, C.R.; Mure, M. Oligomeric States and Hydrodynamic Properties of Lysyl Oxidase-Like 2. Biomolecules 2021, 11, 1846. [Google Scholar] [CrossRef] [PubMed]
- Erasmus, M.; Samodien, E.; Lecour, S.; Cour, M.; Lorenzo, O.; Dludla, P.; Pheiffer, C.; Johnson, R. Linking LOXL2 to Cardiac Interstitial Fibrosis. Int. J. Mol. Sci. 2020, 21, 5913. [Google Scholar] [CrossRef]
- Chen, W.; Yang, A.; Jia, J.; Popov, Y.V.; Schuppan, D.; You, H. Lysyl Oxidase (LOX) Family Members: Rationale and Their Potential as Therapeutic Targets for Liver Fibrosis. Hepatology 2020, 72, 729–741. [Google Scholar] [CrossRef] [PubMed]
- Vallet, S.D.; Ricard-Blum, S. Lysyl Oxidases: From Enzyme Activity to Extracellular Matrix Cross-Links. Essays Biochem. 2019, 63, 349–364. [Google Scholar] [CrossRef]
- Puente, A.; Fortea, J.; Cabezas, J.; Arias Loste, M.; Iruzubieta, P.; Llerena, S.; Huelin, P.; Fábrega, E.; Crespo, J. LOXL2—A New Target in Antifibrogenic Therapy? Int. J. Mol. Sci. 2019, 20, 1634. [Google Scholar] [CrossRef]
- Poe, A.; Martinez Yus, M.; Wang, H.; Santhanam, L. Lysyl Oxidase Like 2 (LOXL2) in Fibrosis and Cardiovascular Disease. Am. J. Physiol. Cell Physiol. 2023, 325, C694–C707. [Google Scholar] [CrossRef]
- Akiri, G.; Sabo, E.; Dafni, H.; Vadasz, Z.; Kartvelishvily, Y.; Gan, N.; Kessler, O.; Cohen, T.; Resnick, M.; Neeman, M.; et al. Lysyl Oxidase-Related Protein-1 Promotes Tumor Fibrosis and Tumor Progression in Vivo. Cancer Res. 2003, 63, 1657–1666. [Google Scholar]
- Zhang, X.; Huang, J.; You, F.; Li, W.; Zou, Z. Prognostic and Clinicopathological Significance of LOXL2 in Cancers: A Systematic Review and Meta-analysis. J. Cell. Physiol. 2019, 234, 21369–21379. [Google Scholar] [CrossRef]
- Almacellas-Rabaiget, O.; Monaco, P.; Huertas-Martinez, J.; García-Monclús, S.; Chicón-Bosch, M.; Maqueda-Marcos, S.; Fabra-Heredia, I.; Herrero-Martín, D.; Rello-Varona, S.; de Alava, E.; et al. LOXL2 Promotes Oncogenic Progression in Alveolar Rhabdomyosarcoma Independently of Its Catalytic Activity. Cancer Lett. 2020, 474, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.-H.; Jiao, J.-W.; Zhang, H.-F.; Xu, X.-E.; He, J.-Z.; Li, R.-L.; Zou, H.-Y.; Wu, Z.-Y.; Wang, S.-H.; Wu, J.-Y.; et al. LOXL2 Upregulates Phosphorylation of Ezrin to Promote Cytoskeletal Reorganization and Tumor Cell Invasion. Cancer Res. 2019, 79, 4951–4964. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, E.P.; Eraso, P.; Mazón, M.J.; Santos, V.; Moreno-Bueno, G.; Cano, A.; Portillo, F. LOXL2 Drives Epithelial-Mesenchymal Transition via Activation of IRE1-XBP1 Signalling Pathway. Sci. Rep. 2017, 7, 44988. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, E.P.; Moreno-Bueno, G.; Canesin, G.; Santos, V.; Portillo, F.; Cano, A. LOXL2 Catalytically Inactive Mutants Mediate Epithelial-to-Mesenchymal Transition. Biol. Open 2014, 3, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Lugassy, J.; Zaffryar-Eilot, S.; Soueid, S.; Mordoviz, A.; Smith, V.; Kessler, O.; Neufeld, G. The Enzymatic Activity of Lysyl Oxidas-like-2 (LOXL2) Is Not Required for LOXL2-Induced Inhibition of Keratinocyte Differentiation. J. Biol. Chem. 2012, 287, 3541–3549. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.R.; Mackay-Smith, A.; Mouri, K.; Garcia, M.F.; Dong, M.X.; Akers, J.F.; Noble, M.; Li, X.; Lindblad-Toh, K.; Karlsson, E.K.; et al. The Functional and Evolutionary Impacts of Human-Specific Deletions in Conserved Elements. Science 2023, 380, eabn2253. [Google Scholar] [CrossRef]
- Wen, B.; Xu, L.-Y.; Li, E.-M. LOXL2 in Cancer: Regulation, Downstream Effectors and Novel Roles. Biochim. Biophys. Acta Rev. Cancer 2020, 1874, 188435. [Google Scholar] [CrossRef]
- Alonso-Nocelo, M.; Ruiz-Cañas, L.; Sancho, P.; Görgülü, K.; Alcalá, S.; Pedrero, C.; Vallespinos, M.; López-Gil, J.C.; Ochando, M.; García-García, E.; et al. Macrophages Direct Cancer Cells through a LOXL2-Mediated Metastatic Cascade in Pancreatic Ductal Adenocarcinoma. Gut 2023, 72, 345–359. [Google Scholar] [CrossRef]
- Li, R.; Li, H.; Zhu, L.; Zhang, X.; Liu, D.; Li, Q.; Ni, B.; Hu, L.; Zhang, Z.; Zhang, Y.; et al. Reciprocal Regulation of LOXL2 and HIF1α Drives the Warburg Effect to Support Pancreatic Cancer Aggressiveness. Cell Death Dis. 2021, 12, 1106. [Google Scholar] [CrossRef]
- Jiang, H.; Torphy, R.J.; Steiger, K.; Hongo, H.; Ritchie, A.J.; Kriegsmann, M.; Horst, D.; Umetsu, S.E.; Joseph, N.M.; McGregor, K.; et al. Pancreatic Ductal Adenocarcinoma Progression Is Restrained by Stromal Matrix. J. Clin. Investig. 2020, 130, 4704–4709. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Lin, S.; Zhi, W.; Lazare, C.; Meng, Y.; Wu, P.; Gao, P.; Wei, J.; Wu, P. LOXL2 Expression Status Is Correlated with Molecular Characterizations of Cervical Carcinoma and Associated with Poor Cancer Survival via Epithelial-Mesenchymal Transition (EMT) Phenotype. Front. Oncol. 2020, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Lin, S.; Meng, Y.; Gao, P.; Wu, P.; Zhi, W.; Ding, W.; Cao, C.; Wu, P. LOXL2 Small Molecule Inhibitor Restrains Malignant Transformation of Cervical Cancer Cells by Repressing LOXL2-Induced Epithelial-Mesenchymal Transition (EMT). Cell Cycle 2022, 21, 1827–1841. [Google Scholar] [CrossRef]
- Matsuoka, K.; Bakiri, L.; Wolff, L.I.; Linder, M.; Mikels-Vigdal, A.; Patiño-García, A.; Lecanda, F.; Hartmann, C.; Sibilia, M.; Wagner, E.F. Wnt Signaling and Loxl2 Promote Aggressive Osteosarcoma. Cell Res. 2020, 30, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Chen, C.; Guo, Y.; Liu, T.; Che, N.; Zhang, D.; Liang, X.; Zhang, Y.; Zhao, X. LOXL2 Serves as a Prognostic Biomarker for Hepatocellular Carcinoma by Mediating Immune Infiltration and Vasculogenic Mimicry. Dig. Liver Dis. 2023, 55, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zheng, L.; Lu, Y.; Xia, Q.; Zhou, P.; Liu, Z. Comprehensive Analysis on the Expression Levels and Prognostic Values of LOX Family Genes in Kidney Renal Clear Cell Carcinoma. Cancer Med. 2020, 9, 8624–8638. [Google Scholar] [CrossRef] [PubMed]
- Bharti, A.; Urs, A.; Kumar, P. Significance of HIF-1α Expression and LOXL-2 Localization in Progression of Oral Squamous Cell Carcinoma. Asian Pac. J. Cancer Prev. 2021, 22, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Cebrià-Costa, J.P.; Pascual-Reguant, L.; Gonzalez-Perez, A.; Serra-Bardenys, G.; Querol, J.; Cosín, M.; Verde, G.; Cigliano, R.A.; Sanseverino, W.; Segura-Bayona, S.; et al. LOXL2-Mediated H3K4 Oxidation Reduces Chromatin Accessibility in Triple-Negative Breast Cancer Cells. Oncogene 2020, 39, 79–121. [Google Scholar] [CrossRef]
- Ramos, S.; Ferreira, S.; Fernandes, A.S.; Saraiva, N. Lysyl Oxidases Expression and Breast Cancer Progression: A Bioinformatic Analysis. Front. Pharmacol. 2022, 13, 883998. [Google Scholar] [CrossRef]
- Wang, C.; Xu, S.; Tian, Y.; Ju, A.; Hou, Q.; Liu, J.; Fu, Y.; Luo, Y. Lysyl Oxidase-Like Protein 2 Promotes Tumor Lymphangiogenesis and Lymph Node Metastasis in Breast Cancer. Neoplasia 2019, 21, 413–427. [Google Scholar] [CrossRef]
- Weinstein, J.N.; Collisson, E.A.; Mills, G.B.; Shaw, K.R.M.; Ozenberger, B.A.; Ellrott, K.; Shmulevich, I.; Sander, C.; Stuart, J.M. The Cancer Genome Atlas Pan-Cancer Analysis Project. Nat. Genet. 2013, 45, 1113–1120. [Google Scholar] [CrossRef]
- Chalmers, Z.R.; Connelly, C.F.; Fabrizio, D.; Gay, L.; Ali, S.M.; Ennis, R.; Schrock, A.; Campbell, B.; Shlien, A.; Chmielecki, J.; et al. Analysis of 100,000 Human Cancer Genomes Reveals the Landscape of Tumor Mutational Burden. Genome Med. 2017, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Schietke, R.; Warnecke, C.; Wacker, I.; Schödel, J.; Mole, D.R.; Campean, V.; Amann, K.; Goppelt-Struebe, M.; Behrens, J.; Eckardt, K.-U.; et al. The Lysyl Oxidases LOX and LOXL2 Are Necessary and Sufficient to Repress E-Cadherin in Hypoxia. J. Biol. Chem. 2010, 285, 6658–6669. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Chang, R.; Zhong, J.; Pandey, A.; Semenza, G.L. Histone Demethylase JMJD2C Is a Coactivator for Hypoxia-Inducible Factor 1 That Is Required for Breast Cancer Progression. Proc. Natl. Acad. Sci. USA 2012, 109, E3367–E3376. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.; Qiu, L.; Hong, Y.; Karnik, T.; Tadros, G.; Mau, B.; Ma, T.; Mu, Y.; New, J.; Louie, R.J.; et al. The Histone Demethylase KDM4B Regulates Peritoneal Seeding of Ovarian Cancer. Oncogene 2017, 36, 2565–2576. [Google Scholar] [CrossRef]
- Yang, H.; Geng, Y.; Wang, P.; Zhou, Y.; Yang, H.; Huo, Y.; Zhang, H.; Li, Y.; He, H.; Tian, X.; et al. Extracellular ATP Promotes Breast Cancer Invasion and Epithelial-mesenchymal Transition via Hypoxia-inducible Factor 2α Signaling. Cancer Sci. 2019, 110, 2456–2470. [Google Scholar] [CrossRef] [PubMed]
- Dekker, Y.; Le Dévédec, S.E.; Danen, E.H.J.; Liu, Q. Crosstalk between Hypoxia and Extracellular Matrix in the Tumor Microenvironment in Breast Cancer. Genes 2022, 13, 1585. [Google Scholar] [CrossRef]
- Wu, S.; Zheng, Q.; Xing, X.; Dong, Y.; Wang, Y.; You, Y.; Chen, R.; Hu, C.; Chen, J.; Gao, D.; et al. Matrix Stiffness-Upregulated LOXL2 Promotes Fibronectin Production, MMP9 and CXCL12 Expression and BMDCs Recruitment to Assist Pre-Metastatic Niche Formation. J. Exp. Clin. Cancer Res. 2018, 37, 99. [Google Scholar] [CrossRef]
- Xing, X.; Wang, Y.; Zhang, X.; Gao, X.; Li, M.; Wu, S.; Zhao, Y.; Chen, J.; Gao, D.; Chen, R.; et al. Matrix Stiffness-mediated Effects on Macrophages Polarization and Their LOXL2 Expression. FEBS J. 2021, 288, 3465–3477. [Google Scholar] [CrossRef]
- Li, Q.; Chao, Q.; Liu, Y.; Fang, J.; Xie, J.; Zhen, J.; Ding, Y.; Fu, B.; Ke, Y.; Xiao, F.; et al. Deubiquitinase ZRANB1 Drives Hepatocellular Carcinoma Progression through SP1-LOXL2 Axis. Am. J. Cancer Res. 2021, 11, 4807–4825. [Google Scholar]
- Lin, Z.-Y.; Chuang, Y.-H.; Chuang, W.-L. Cancer-Associated Fibroblasts up-Regulate CCL2, CCL26, IL6 and LOXL2 Genes Related to Promotion of Cancer Progression in Hepatocellular Carcinoma Cells. Biomed. Pharmacother. 2012, 66, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Cho, H.-J.; Park, K.-S.; Jung, H.-Y. ELK3 Controls Gastric Cancer Cell Migration and Invasion by Regulating ECM Remodeling-Related Genes. Int. J. Mol. Sci. 2022, 23, 3709. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.; Aoyagi, K.; Takahashi, H.; Kawamura, T.; Mabuchi, T.; Igaki, H.; Tachimori, Y.; Kato, H.; Ochiai, A.; Honda, H.; et al. Forkhead Box A1 Transcriptional Pathway in KRT7-Expressing Esophageal Squamous Cell Carcinomas with Extensive Lymph Node Metastasis. Int. J. Oncol. 2010, 36, 321–330. [Google Scholar]
- Wang, M.; Han, X.; Zha, W.; Wang, X.; Liu, L.; Li, Z.; Shi, Y.; Kan, X.; Wang, G.; Gao, D.; et al. GDNF Promotes Astrocyte Abnormal Proliferation and Migration Through the GFRα1/RET/MAPK/PCREB/LOXL2 Signaling Axis. Mol. Neurobiol. 2022, 59, 6321–6340. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Song, Y.-N.; He, S.-F.; Zhuang, J.-H.; Wang, G.-Y.; Xia, W. GINS2 Promotes Cell Proliferation and Inhibits Cell Apoptosis in Thyroid Cancer by Regulating CITED2 and LOXL2. Cancer Gene Ther. 2019, 26, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Dinca, S.C.; Greiner, D.; Weidenfeld, K.; Bond, L.; Barkan, D.; Jorcyk, C.L. Novel Mechanism for OSM-Promoted Extracellular Matrix Remodeling in Breast Cancer: LOXL2 Upregulation and Subsequent ECM Alignment. Breast Cancer Res. 2021, 23, 56. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, D.; Li, X.; Liu, Z.; Li, T.; Jiang, P.; He, Q.; Tian, F.; Gao, Y.; Wang, D.; et al. 67 Laminin Receptor Promotes the Malignant Potential of Tumour Cells Up-Regulating Lysyl Oxidase-like 2 Expression in Cholangiocarcinoma. Dig. Liver Dis. 2014, 46, 750–757. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, M.-X.; Zhang, X.-D.; Xu, X.-E.; Wu, Z.-Y.; Liao, L.-D.; Li, L.-Y.; Xie, Y.-M.; Wu, J.-Y.; Zou, H.-Y.; et al. SMYD3 Stimulates EZR and LOXL2 Transcription to Enhance Proliferation, Migration, and Invasion in Esophageal Squamous Cell Carcinoma. Hum. Pathol. 2016, 52, 153–163. [Google Scholar] [CrossRef]
- Loriot, C.; Burnichon, N.; Gadessaud, N.; Vescovo, L.; Amar, L.; Libé, R.; Bertherat, J.; Plouin, P.-F.; Jeunemaitre, X.; Gimenez-Roqueplo, A.-P.; et al. Epithelial to Mesenchymal Transition Is Activated in Metastatic Pheochromocytomas and Paragangliomas Caused by SDHB Gene Mutations. J. Clin. Endocrinol. Metab. 2012, 97, E954–E962. [Google Scholar] [CrossRef]
- Kim, I.; Lee, Y.S.; Kim, H.S.; Dong, S.M.; Park, J.S.; Yoon, D.S. Specific Protein 1(SP1) Regulates the Epithelial-Mesenchymal Transition via Lysyl Oxidase-like 2(LOXL2) in Pancreatic Ductal Adenocarcinoma. Sci. Rep. 2019, 9, 5933. [Google Scholar] [CrossRef]
- Liu, X.; Liu, T.; Hu, L.; Jiang, T.; Liu, H.; Wang, Y.; Lei, Y.; Zhu, J.; Bu, Y. Identification and Characterization of the Promoter of Cancer-Related Gene LOXL2. Exp. Cell Res. 2020, 387, 111786. [Google Scholar] [CrossRef] [PubMed]
- Ezzoukhry, Z.; Henriet, E.; Piquet, L.; Boyé, K.; Bioulac-Sage, P.; Balabaud, C.; Couchy, G.; Zucman-Rossi, J.; Moreau, V.; Saltel, F. TGF-Β1 Promotes Linear Invadosome Formation in Hepatocellular Carcinoma Cells, through DDR1 up-Regulation and Collagen I Cross-Linking. Eur. J. Cell Biol. 2016, 95, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Nagaoka, H.; Mochida, Y.; Atsawasuwan, P.; Kaku, M.; Kondoh, T.; Yamauchi, M. 1,25(OH) 2 D 3 Regulates Collagen Quality in an Osteoblastic Cell Culture System. Biochem. Biophys. Res. Commun. 2008, 377, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Tse, A.P.-W.; Sze, K.M.-F.; Shea, Q.T.-K.; Chiu, E.Y.-T.; Tsang, F.H.-C.; Chiu, D.K.-C.; Zhang, M.S.; Lee, D.; Xu, I.M.-J.; Chan, C.Y.-K.; et al. Hepatitis Transactivator Protein X Promotes Extracellular Matrix Modification through HIF/LOX Pathway in Liver Cancer. Oncogenesis 2018, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Li, D.; Cheng, L.; Wu, H.; Gao, Z.; Liu, Z.; Jiang, W.; Gao, Y.H.; Tian, F.; Zhao, L.; et al. Epithelial-Mesenchymal Transition Induced by Hepatitis C Virus Core Protein in Cholangiocarcinoma. Ann. Surg. Oncol. 2010, 17, 1937–1944. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Kurozumi, A.; Goto, Y.; Matsushita, R.; Okato, A.; Nishikawa, R.; Fukumoto, I.; Koshizuka, K.; Ichikawa, T.; Seki, N. Regulation of Metastasis-Promoting LOXL2 Gene Expression by Antitumor MicroRNAs in Prostate Cancer. J. Hum. Genet. 2017, 62, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, I.; Kikkawa, N.; Matsushita, R.; Kato, M.; Kurozumi, A.; Nishikawa, R.; Goto, Y.; Koshizuka, K.; Hanazawa, T.; Enokida, H.; et al. Tumor-Suppressive MicroRNAs (MiR-26a/b, MiR-29a/b/c and MiR-218) Concertedly Suppressed Metastasis-Promoting LOXL2 in Head and Neck Squamous Cell Carcinoma. J. Hum. Genet. 2016, 61, 109–118. [Google Scholar] [CrossRef]
- Kurozumi, A.; Kato, M.; Goto, Y.; Matsushita, R.; Nishikawa, R.; Okato, A.; Fukumoto, I.; Ichikawa, T.; Seki, N. Regulation of the Collagen Cross-Linking Enzymes LOXL2 and PLOD2 by Tumor-Suppressive MicroRNA-26a/b in Renal Cell Carcinoma. Int. J. Oncol. 2016, 48, 1837–1846. [Google Scholar] [CrossRef]
- Mizuno, K.; Seki, N.; Mataki, H.; Matsushita, R.; KamikawajI, K.; Kumamoto, T.; Takagi, K.; Goto, Y.; Nishikawa, R.; Kato, M.; et al. Tumor-Suppressive MicroRNA-29 Family Inhibits Cancer Cell Migration and Invasion Directly Targeting LOXL2 in Lung Squamous Cell Carcinoma. Int. J. Oncol. 2016, 48, 450–460. [Google Scholar] [CrossRef]
- Dey, S.; Kwon, J.J.; Liu, S.; Hodge, G.A.; Taleb, S.; Zimmers, T.A.; Wan, J.; Kota, J. MiR-29a Is Repressed by MYC in Pancreatic Cancer and Its Restoration Drives Tumor-Suppressive Effects via Downregulation of LOXL2. Mol. Cancer Res. 2020, 18, 311–323. [Google Scholar] [CrossRef]
- Nishikawa, R.; Chiyomaru, T.; Enokida, H.; Inoguchi, S.; Ishihara, T.; Matsushita, R.; Goto, Y.; Fukumoto, I.; Nakagawa, M.; Seki, N. Tumour-Suppressive MicroRNA-29s Directly Regulate LOXL2 Expression and Inhibit Cancer Cell Migration and Invasion in Renal Cell Carcinoma. FEBS Lett. 2015, 589, 2136–2145. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-L.; Tsai, M.-C.; Chang, Y.-H.; Wang, C.-C.; Chu, P.-Y.; Lin, H.-Y.; Huang, Y.-H. MIR29A Impedes Metastatic Behaviors in Hepatocellular Carcinoma via Targeting LOX, LOXL2, and VEGFA. Int. J. Mol. Sci. 2021, 22, 6001. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Xiao, H.; Xiao, W.; Xiong, Z.; Hu, W.; Gao, Y.; Ru, Z.; Wang, C.; Bao, L.; Wang, K.; et al. Upregulation of MIAT Regulates LOXL2 Expression by Competitively Binding MiR-29c in Clear Cell Renal Cell Carcinoma. Cell Physiol. Biochem. 2018, 48, 1075–1087. [Google Scholar] [CrossRef]
- Tan, J.; Zhou, M.; Yang, S.; Lin, J. Long Noncoding RNA HCP5 Promotes Osteosarcoma Cell Proliferation, Invasion, and Migration via the miR-29b-3p-LOXL2 Axis. Kaohsiung J. Med. Sci. 2022, 38, 960–970. [Google Scholar] [CrossRef]
- Ye, M.; Zhang, J.; Guo, T.; Pan, X. MiR-504 Inhibits Cell Proliferation and Invasion by Targeting LOXL2 in Non Small Cell Lung Cancer. Biomed. Pharmacother. 2018, 97, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, B.; Li, Y.; Song, H. Circular RNA Circ_0000228 Promotes the Malignancy of Cervical Cancer via MicroRNA-195-5p/ Lysyl Oxidase-like Protein 2 Axis. Bioengineered 2021, 12, 4397–4406. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Jin, H.; Gong, S.; Han, K.; Li, Z.; Zhang, W.; Tian, J. LncRNA KCNQ1OT1 mediated Cervical Cancer Progression by Sponging miR-1270 as a ceRNA of LOXL2 through PI3k/Akt Pathway. J. Obstet. Gynaecol. Res. 2022, 48, 1001–1010. [Google Scholar] [CrossRef]
- Zheng, G.-L.; Liu, Y.-L.; Yan, Z.-X.; Xie, X.-Y.; Xiang, Z.; Yin, L.; Wang, Q.-Q.; Chong, D.-C.; Xue, G.-L.; Xu, L.-L.; et al. Elevated LOXL2 Expression by LINC01347/MiR-328-5p Axis Contributes to 5-FU Chemotherapy Resistance of Colorectal Cancer. Am. J. Cancer Res. 2021, 11, 1572–1585. [Google Scholar]
- Wang, X.; Wu, S.; Yang, Y.; Zhao, J. LncRNA CARMN Affects Hepatocellular Carcinoma Prognosis by Regulating the MiR-192-5p/LOXL2 Axis. Oxid. Med. Cell Longev. 2022, 2022, 9277360. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, X.; Zeng, L.; Yang, X.; Cheng, X.; Tian, H.; Chen, C.; Sun, X.; Zhao, C.; Ma, H.; et al. Circular RNA PDK1Targets miR-4731-5p to Enhance TNXB Expression in Ligamentum Flavum Hypertrophy. FASEB J. 2023, 37, e22877. [Google Scholar] [CrossRef]
- Okada, K.; Moon, H.-J.; Finney, J.; Meier, A.; Mure, M. Extracellular Processing of Lysyl Oxidase-like 2 and Its Effect on Amine Oxidase Activity. Biochemistry 2018, 57, 6973–6983. [Google Scholar] [CrossRef] [PubMed]
- López-Jiménez, A.J.; Basak, T.; Vanacore, R.M. Proteolytic Processing of Lysyl Oxidase–like-2 in the Extracellular Matrix Is Required for Crosslinking of Basement Membrane Collagen IV. J. Biol. Chem. 2017, 292, 16970–16982. [Google Scholar] [CrossRef]
- Wang, H.; Poe, A.; Martinez Yus, M.; Pak, L.; Nandakumar, K.; Santhanam, L. Lysyl Oxidase-like 2 Processing by Factor Xa Modulates Its Activity and Substrate Preference. Commun. Biol. 2023, 6, 375. [Google Scholar] [CrossRef] [PubMed]
- McCready, J.; Wong, D.; Burlison, J.; Ying, W.; Jay, D. An Impermeant Ganetespib Analog Inhibits Extracellular Hsp90-Mediated Cancer Cell Migration That Involves Lysyl Oxidase 2-like Protein. Cancers 2014, 6, 1031–1046. [Google Scholar] [CrossRef] [PubMed]
- Lelièvre, E.; Hinek, A.; Lupu, F.; Buquet, C.; Soncin, F.; Mattot, V. VE-Statin/Egfl7 Regulates Vascular Elastogenesis by Interacting with Lysyl Oxidases. EMBO J. 2008, 27, 1658–1670. [Google Scholar] [CrossRef] [PubMed]
- Neumann, P.; Jaé, N.; Knau, A.; Glaser, S.F.; Fouani, Y.; Rossbach, O.; Krüger, M.; John, D.; Bindereif, A.; Grote, P.; et al. The LncRNA GATA6-AS Epigenetically Regulates Endothelial Gene Expression via Interaction with LOXL2. Nat. Commun. 2018, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, X.; Sun, Y.; Chu, Y.; Liu, F.; Chen, C. TRIM44 Regulates Tumor Immunity in Gastric Cancer through LOXL2-Dependent Extracellular Matrix Remodeling. Cell Oncol. 2023, 46, 423–435. [Google Scholar] [CrossRef]
- Cai, J.-H.; Sun, Y.-T.; Bao, S. HucMSCs-Exosomes Containing MiR-21 Promoted Estrogen Production in Ovarian Granulosa Cells via LATS1-Mediated Phosphorylation of LOXL2 and YAP. Gen. Comp. Endocrinol. 2022, 321–322, 114015. [Google Scholar] [CrossRef]
- Peng, L.; Ran, Y.-L.; Hu, H.; Yu, L.; Liu, Q.; Zhou, Z.; Sun, Y.-M.; Sun, L.-C.; Pan, J.; Sun, L.-X.; et al. Secreted LOXL2 Is a Novel Therapeutic Target That Promotes Gastric Cancer Metastasis via the Src/FAK Pathway. Carcinogenesis 2009, 30, 1660–1669. [Google Scholar] [CrossRef]
- Barker, H.E.; Bird, D.; Lang, G.; Erler, J.T. Tumor-Secreted LOXL2 Activates Fibroblasts through FAK Signaling. Mol. Cancer Res. 2013, 11, 1425–1436. [Google Scholar] [CrossRef]
- Hong, X.; Yu, J. Silencing of Lysyl Oxidase like 2 Inhibits the Migration, Invasion and Epithelial to mesenchymal Transition of Renal Cell Carcinoma Cells through the Src/FAK Signaling Pathway. Int. J. Oncol. 2019, 54, 1676–1690. [Google Scholar] [CrossRef]
- Mahjour, F.; Dambal, V.; Shrestha, N.; Singh, V.; Noonan, V.; Kantarci, A.; Trackman, P.C. Mechanism for Oral Tumor Cell Lysyl Oxidase Like-2 in Cancer Development: Synergy with PDGF-AB. Oncogenesis 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.-L.; Tse, A.P.-W.; Huang, Y.-P.; Zhu, Y.-T.; Chiu, D.K.-C.; Lai, R.K.-H.; Au, S.L.-K.; Kai, A.K.-L.; Lee, J.M.-F.; Wei, L.L.; et al. Lysyl Oxidase-like 2 Is Critical to Tumor Microenvironment and Metastatic Niche Formation in Hepatocellular Carcinoma. Hepatology 2014, 60, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, L.; Meng, W.; Lu, S.; Cao, B.; Liang, X.; He, C.; Hao, Y.; Du, X.; Wang, X.; et al. LOXL2-Enriched Small Extracellular Vesicles Mediate Hypoxia-Induced Premetastatic Niche and Indicates Poor Outcome of Head and Neck Cancer. Theranostics 2021, 11, 9198–9216. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liang, A.; Zhou, Y.; Huang, Y.; Liao, C.; Zhang, X.; Gong, Q. Hypoxia Preconditioned DPSC-Derived Exosomes Regulate Angiogenesis via Transferring LOXL2. Exp. Cell Res. 2023, 425, 113543. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.C.-L.; Gilkes, D.M.; Zhang, H.; Chen, J.; Wei, H.; Chaturvedi, P.; Fraley, S.I.; Wong, C.-M.; Khoo, U.-S.; Ng, I.O.-L.; et al. Hypoxia-Inducible Factor 1 Is a Master Regulator of Breast Cancer Metastatic Niche Formation. Proc. Natl. Acad. Sci. USA 2011, 108, 16369–16374. [Google Scholar] [CrossRef]
- Canesin, G.; Cuevas, E.P.; Santos, V.; López-Menéndez, C.; Moreno-Bueno, G.; Huang, Y.; Csiszar, K.; Portillo, F.; Peinado, H.; Lyden, D.; et al. Lysyl Oxidase-like 2 (LOXL2) and E47 EMT Factor: Novel Partners in E-Cadherin Repression and Early Metastasis Colonization. Oncogene 2015, 34, 951–964. [Google Scholar] [CrossRef]
- Chang, J.; Nicolau, M.M.; Cox, T.R.; Wetterskog, D.; Martens, J.W.; E Barker, H.; Erler, J.T. LOXL2 Induces Aberrant Acinar Morphogenesis via ErbB2 Signaling. Breast Cancer Res. 2013, 15, R67. [Google Scholar] [CrossRef]
- Jong, O.G.; Waals, L.M.; Kools, F.R.W.; Verhaar, M.C.; Balkom, B.W.M. Lysyl Oxidase-like 2 Is a Regulator of Angiogenesis through Modulation of Endothelial-to-mesenchymal Transition. J. Cell Physiol. 2019, 234, 10260–10269. [Google Scholar] [CrossRef]
- Moreno-Bueno, G.; Salvador, F.; Martín, A.; Floristán, A.; Cuevas, E.P.; Santos, V.; Montes, A.; Morales, S.; Castilla, M.A.; Rojo-Sebastián, A.; et al. Lysyl Oxidase-like 2 (LOXL2), a New Regulator of Cell Polarity Required for Metastatic Dissemination of Basal-like Breast Carcinomas. EMBO Mol. Med. 2011, 3, 528–544. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, Y.; Liu, X.; Nian, W.; Huang, X.; Yang, Q.; Hou, S.; Chen, F. Phosphorylation of AKT by Lysyl Oxidase-like 2 Activates the PI3K/AKT Signaling Pathway to Promote Proliferation, Invasion and Metastasis in Esophageal Squamous Carcinoma. Clin. Transl. Oncol. 2023, 25, 2487–2498. [Google Scholar] [CrossRef] [PubMed]
- Hase, H.; Jingushi, K.; Ueda, Y.; Kitae, K.; Egawa, H.; Ohshio, I.; Kawakami, R.; Kashiwagi, Y.; Tsukada, Y.; Kobayashi, T.; et al. LOXL2 Status Correlates with Tumor Stage and Regulates Integrin Levels to Promote Tumor Progression in CcRCC. Mol. Cancer Res. 2014, 12, 1807–1817. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Antin, P.; Berx, G.; Blanpain, C.; Brabletz, T.; Bronner, M.; Campbell, K.; Cano, A.; Casanova, J.; Christofori, G.; et al. Guidelines and Definitions for Research on Epithelial–Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2020, 21, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Peinado, H.; Olmeda, D.; Cano, A. Snail, Zeb and BHLH Factors in Tumour Progression: An Alliance against the Epithelial Phenotype? Nat. Rev. Cancer 2007, 7, 415–428. [Google Scholar] [CrossRef] [PubMed]
- De Craene, B.; Berx, G. Regulatory Networks Defining EMT during Cancer Initiation and Progression. Nat. Rev. Cancer 2013, 13, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Goossens, S.; Vandamme, N.; Van Vlierberghe, P.; Berx, G. EMT Transcription Factors in Cancer Development Re-Evaluated: Beyond EMT and MET. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 584–591. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, L.; Guan, G.; Cheng, P.; Cheng, W.; Wu, A. LOXL2 Upregulation in Gliomas Drives Tumorigenicity by Activating Autophagy to Promote TMZ Resistance and Trigger EMT. Front. Oncol. 2020, 10, 569584. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-R.; Dong, S.M.; Seo, S.H.; Lee, J.-H.; Lee, J.M.; Lee, S.-H.; Rho, S.B. Lysyl Oxidase-like 2 (LOXL2) Controls Tumor-Associated Cell Proliferation through the Interaction with MARCKSL1. Cell. Signal. 2014, 26, 1765–1773. [Google Scholar] [CrossRef]
- Peinado, H.; Portillo, F.; Cano, A. Switching On-Off Snail: LOXL2 Versus GSK3? Cell Cycle 2005, 4, 1749–1752. [Google Scholar] [CrossRef]
- Peinado, H.; del Carmen Iglesias-de la Cruz, M.; Olmeda, D.; Csiszar, K.; Fong, K.S.K.; Vega, S.; Nieto, M.A.; Cano, A.; Portillo, F. A Molecular Role for Lysyl Oxidase-like 2 Enzyme in Snail Regulation and Tumor Progression. EMBO J. 2005, 24, 3446–3458. [Google Scholar] [CrossRef]
- Millanes-Romero, A.; Herranz, N.; Perrera, V.; Iturbide, A.; Loubat-Casanovas, J.; Gil, J.; Jenuwein, T.; García de Herreros, A.; Peiró, S. Regulation of Heterochromatin Transcription by Snail1/LOXL2 during Epithelial-to-Mesenchymal Transition. Mol. Cell. 2013, 52, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Salvador, F.; Moreno-Bueno, G.; Floristán, A.; Ruiz-Herguido, C.; Cuevas, E.P.; Morales, S.; Santos, V.; Csiszar, K.; Dubus, P.; et al. Lysyl Oxidase-like 2 Represses Notch1 Expression in the Skin to Promote Squamous Cell Carcinoma Progression. EMBO J. 2015, 34, 1090–1109. [Google Scholar] [CrossRef] [PubMed]
- Brekhman, V.; Lugassie, J.; Zaffryar-Eilot, S.; Sabo, E.; Kessler, O.; Smith, V.; Golding, H.; Neufeld, G. Receptor Activity Modifying Protein-3 Mediates the Pro-tumorigenic Activity of Lysyl Oxidase-like Protein-2. FASEB J. 2011, 25, 55–65. [Google Scholar] [CrossRef]
- Fan, Z.; Zheng, W.; Li, H.; Wu, W.; Liu, X.; Sun, Z.; Hu, H.; Du, L.; Jia, Q.; Liu, Q. LOXL2 Upregulates Hypoxia inducible Factor 1α Signaling through Snail FBP1 Axis in Hepatocellular Carcinoma Cells. Oncol. Rep. 2020, 43, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Lu, F.-T.; Cao, J.-X.; Ma, W.-J.; Xia, Z.-F.; Zhan, J.-H.; Zeng, K.-M.; Huang, Y.; Zhao, H.-Y.; Zhang, L. HIF-1α Inhibition Promotes the Efficacy of Immune Checkpoint Blockade in the Treatment of Non-Small Cell Lung Cancer. Cancer Lett. 2022, 531, 39–56. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Deng, X.; Tian, F.; Li, Z.; Jiang, P.; Zhao, X.; Chen, G.; Chen, Y.; Zheng, P.; Li, D.; et al. The Interaction of LOXL2 with GATA6 Induces VEGFA Expression and Angiogenesis in Cholangiocarcinoma. Int. J. Oncol. 2019, 55, 657–670. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Qiu, S.; Mao, W.; Lin, W.; Peng, Q.; Chang, H. LOXL2 Reduces 5-FU Sensitivity through the Hedgehog/BCL2 Signaling Pathway in Colorectal Cancer. Exp. Biol. Med. 2023, 248, 457–468. [Google Scholar] [CrossRef]
- Li, N.; Gu, H.; Liu, L.; Zhang, X.-L.; Cheng, Q.-L.; Zhu, Y. Inhibitory Effects of LOXL2 Knockdown on Cellular Functions of Liver Cancer Stem Cells. Transl. Cancer Res. 2022, 11, 2013–2025. [Google Scholar] [CrossRef]
- Mäki, J.M.; Räsänen, J.; Tikkanen, H.; Sormunen, R.; Mäkikallio, K.; Kivirikko, K.I.; Soininen, R. Inactivation of the Lysyl Oxidase Gene Lox Leads to Aortic Aneurysms, Cardiovascular Dysfunction, and Perinatal Death in Mice. Circulation 2002, 106, 2503–2509. [Google Scholar] [CrossRef]
- Hornstra, I.K.; Birge, S.; Starcher, B.; Bailey, A.J.; Mecham, R.P.; Shapiro, S.D. Lysyl Oxidase Is Required for Vascular and Diaphragmatic Development in Mice. J. Biol. Chem. 2003, 278, 14387–14393. [Google Scholar] [CrossRef]
- Orriols, M.; Guadall, A.; Galán, M.; Martí-Pàmies, I.; Varona, S.; Rodríguez-Calvo, R.; Briones, A.M.; Navarro, M.; de Diego, A.; Osada, J.; et al. Lysyl Oxidase (LOX) in Vascular Remodelling. Thromb. Haemost. 2014, 112, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, Y.; Gao, J.; Pawlyk, B.; Starcher, B.; Spencer, J.A.; Yanagisawa, H.; Zuo, J.; Li, T. Elastic Fiber Homeostasis Requires Lysyl Oxidase–like 1 Protein. Nat. Genet. 2004, 36, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Alsofi, L.; Daley, E.; Hornstra, I.; Morgan, E.F.; Mason, Z.D.; Acevedo, J.F.; Word, R.A.; Gerstenfeld, L.C.; Trackman, P.C. Sex-Linked Skeletal Phenotype of Lysyl Oxidase Like-1 Mutant Mice. Calcif. Tissue Int. 2016, 98, 172–185. [Google Scholar] [CrossRef]
- Chen, Y.; He, L.-X.; Chen, J.-L.; Xu, X.; Wang, J.-J.; Zhan, X.-H.; Jiao, J.-W.; Dong, G.; Li, E.-M.; Xu, L.-Y. L2Δ13, a Splicing Isoform of Lysyl Oxidase-like 2, Causes Adipose Tissue Loss via the Gut Microbiota and Lipid Metabolism. iScience 2022, 25, 104894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, R.; Liu, Z.; Hou, C.; Zong, W.; Zhang, A.; Sun, X.; Gao, J. Loss of Lysyl Oxidase-like 3 Causes Cleft Palate and Spinal Deformity in Mice. Hum. Mol. Genet. 2015, 24, 6174–6185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, Z.; Zhang, T.; Lin, Z.; Li, Z.; Zhang, A.; Sun, X.; Gao, J. Loss of Lysyl Oxidase-like 3 Attenuates Embryonic Lung Development in Mice. Sci. Rep. 2016, 6, 33856. [Google Scholar] [CrossRef] [PubMed]
- Kraft-Sheleg, O.; Zaffryar-Eilot, S.; Genin, O.; Yaseen, W.; Soueid-Baumgarten, S.; Kessler, O.; Smolkin, T.; Akiri, G.; Neufeld, G.; Cinnamon, Y.; et al. Localized LoxL3-Dependent Fibronectin Oxidation Regulates Myofiber Stretch and Integrin-Mediated Adhesion. Dev. Cell 2016, 36, 550–561. [Google Scholar] [CrossRef]
- Santamaría, P.G.; Dubus, P.; Bustos-Tauler, J.; Floristán, A.; Vázquez-Naharro, A.; Morales, S.; Cano, A.; Portillo, F. Loxl2 and Loxl3 Paralogues Play Redundant Roles during Mouse Development. Int. J. Mol. Sci. 2022, 23, 5730. [Google Scholar] [CrossRef]
- Liu, Z.; Bai, X.; Wan, P.; Mo, F.; Chen, G.; Zhang, J.; Gao, J. Targeted Deletion of Loxl3 by Col2a1-Cre Leads to Progressive Hearing Loss. Front. Cell Dev. Biol. 2021, 9, 683495. [Google Scholar] [CrossRef]
- Li, H.; Guo, J.; Jia, Y.; Kong, W.; Li, W. LOXL4 Abrogation Does Not Exaggerate Angiotensin II-Induced Thoracic or Abdominal Aortic Aneurysm in Mice. Genes 2021, 12, 513. [Google Scholar] [CrossRef]
- Yang, J.; Savvatis, K.; Kang, J.S.; Fan, P.; Zhong, H.; Schwartz, K.; Barry, V.; Mikels-Vigdal, A.; Karpinski, S.; Kornyeyev, D.; et al. Targeting LOXL2 for Cardiac Interstitial Fibrosis and Heart Failure Treatment. Nat. Commun. 2016, 7, 13710. [Google Scholar] [CrossRef] [PubMed]
- Salvador, F.; Martin, A.; López-Menéndez, C.; Moreno-Bueno, G.; Santos, V.; Vázquez-Naharro, A.; Santamaría, P.G.; Morales, S.; Dubus, P.R.; Muinelo-Romay, L.; et al. Lysyl Oxidase–like Protein LOXL2 Promotes Lung Metastasis of Breast Cancer. Cancer Res. 2017, 77, 5846–5859. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Naharro, A.; Bustos-Tauler, J.; Floristán, A.; Yuste, L.; Oltra, S.S.; Vinyals, A.; Moreno-Bueno, G.; Fabra, À.; Portillo, F.; Cano, A.; et al. Loxl3 Promotes Melanoma Progression and Dissemination Influencing Cell Plasticity and Survival. Cancers 2022, 14, 1200. [Google Scholar] [CrossRef] [PubMed]
- Yuspa, S.H. The Pathogenesis of Squamous Cell Cancer: Lessons Learned from Studies of Skin Carcinogenesis—Thirty-Third GHA Clowes Memorial Award Lecture. Cancer Res. 1994, 54, 1178–1189. [Google Scholar] [PubMed]
- Peinado, H.; Moreno-Bueno, G.; Hardisson, D.; Pérez-Gómez, E.; Santos, V.; Mendiola, M.; de Diego, J.I.; Nistal, M.; Quintanilla, M.; Portillo, F.; et al. Lysyl Oxidase–Like 2 as a New Poor Prognosis Marker of Squamous Cell Carcinomas. Cancer Res. 2008, 68, 4541–4550. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, P.G.; Floristán, A.; Fontanals-Cirera, B.; Vázquez-Naharro, A.; Santos, V.; Morales, S.; Yuste, L.; Peinado, H.; García-Gómez, A.; Portillo, F.; et al. Lysyl Oxidase-like 3 Is Required for Melanoma Cell Survival by Maintaining Genomic Stability. Cell Death Differ. 2018, 25, 935–950. [Google Scholar] [CrossRef]
- Smithen, D.A.; Leung, L.M.H.; Challinor, M.; Lawrence, R.; Tang, H.; Niculescu-Duvaz, D.; Pearce, S.P.; Mcleary, R.; Lopes, F.; Aljarah, M.; et al. 2-Aminomethylene-5-Sulfonylthiazole Inhibitors of Lysyl Oxidase (LOX) and LOXL2 Show Significant Efficacy in Delaying Tumor Growth. J. Med. Chem. 2020, 63, 2308–2324. [Google Scholar] [CrossRef]
- Chien, J.W.; Richards, T.J.; Gibson, K.F.; Zhang, Y.; Lindell, K.O.; Shao, L.; Lyman, S.K.; Adamkewicz, J.I.; Smith, V.; Kaminski, N.; et al. Serum Lysyl Oxidase-like 2 Levels and Idiopathic Pulmonary Fibrosis Disease Progression. Eur. Respir. J. 2014, 43, 1430–1438. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, K.; Tianbao, X.; Wang, J.; Yang, J.; Li, D. Increased Serum Lysyl Oxidase-like 2 Levels Correlate with the Degree of Left Atrial Fibrosis in Patients with Atrial Fibrillation. Biosci. Rep. 2017, 37, BSR20171332. [Google Scholar] [CrossRef]
- Fu, Q.; Bai, Y.; Liu, Y.; Zhou, J.; Zheng, Y. The Serum Level and Significance of Lysyl Oxidase-like 2 in Patients with Rheumatoid Arthritis-Associated Interstitial Lung Disease. Clin. Rheumatol. 2018, 37, 193–198. [Google Scholar] [CrossRef]
- Turhan, U.; Şahin, B.; Dağ, İ. Lysyl Oxidase like Protein-2 (LOXL-2); a Novel Marker for Prediction of Intrahepatic Cholestasis of Pregnancy. J. Matern. Fetal Neonatal Med. 2021, 34, 2363–2368. [Google Scholar] [CrossRef] [PubMed]
- Sanada, T.; Islam, A.; Kaminota, T.; Kirino, Y.; Tanimoto, R.; Yoshimitsu, H.; Yano, H.; Mizuno, Y.; Okada, M.; Mitani, S.; et al. Elevated Exosomal Lysyl Oxidase like 2 Is a Potential Biomarker for Head and Neck Squamous Cell Carcinoma. Laryngoscope 2020, 130, E327–E334. [Google Scholar] [CrossRef] [PubMed]
- Leeming, D.J.; Willumsen, N.; Sand, J.M.B.; Holm Nielsen, S.; Dasgupta, B.; Brodmerkel, C.; Curran, M.; Bager, C.L.; Karsdal, M.A. A Serological Marker of the N-Terminal Neoepitope Generated during LOXL2 Maturation Is Elevated in Patients with Cancer or Idiopathic Pulmonary Fibrosis. Biochem. Biophys. Rep. 2019, 17, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Setargew, Y.F.I.; Wyllie, K.; Grant, R.D.; Chitty, J.L.; Cox, T.R. Targeting Lysyl Oxidase Family Meditated Matrix Cross-Linking as an Anti-Stromal Therapy in Solid Tumours. Cancers 2021, 13, 491. [Google Scholar] [CrossRef] [PubMed]
- Chopra, V.; Sangarappillai, R.M.; Romero-Canelón, I.; Jones, A.M. Lysyl Oxidase Like-2 (LOXL2): An Emerging Oncology Target. Adv. Ther. 2020, 3, 1900119. [Google Scholar] [CrossRef]
- Ferreira, S.; Saraiva, N.; Rijo, P.; Fernandes, A.S. LOXL2 Inhibitors and Breast Cancer Progression. Antioxidants 2021, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, H.M.; Vaysberg, M.; Mikels, A.; McCauley, S.; Velayo, A.C.; Garcia, C.; Smith, V. Modulation of Lysyl Oxidase-like 2 Enzymatic Activity by an Allosteric Antibody Inhibitor. J. Biol. Chem. 2010, 285, 20964–20974. [Google Scholar] [CrossRef] [PubMed]
- Zaffryar-Eilot, S.; Marshall, D.; Voloshin, T.; Bar-Zion, A.; Spangler, R.; Kessler, O.; Ghermazien, H.; Brekhman, V.; Suss-Toby, E.; Adam, D.; et al. Lysyl Oxidase-like-2 Promotes Tumour Angiogenesis and Is a Potential Therapeutic Target in Angiogenic Tumours. Carcinogenesis 2013, 34, 2370–2379. [Google Scholar] [CrossRef][Green Version]
- Barry-Hamilton, V.; Spangler, R.; Marshall, D.; McCauley, S.; Rodriguez, H.M.; Oyasu, M.; Mikels, A.; Vaysberg, M.; Ghermazien, H.; Wai, C.; et al. Allosteric Inhibition of Lysyl Oxidase–like-2 Impedes the Development of a Pathologic Microenvironment. Nat. Med. 2010, 16, 1009–1017. [Google Scholar] [CrossRef]
- Muir, A.J.; Levy, C.; Janssen, H.L.A.; Montano-Loza, A.J.; Shiffman, M.L.; Caldwell, S.; Luketic, V.; Ding, D.; Jia, C.; McColgan, B.J.; et al. Simtuzumab for Primary Sclerosing Cholangitis: Phase 2 Study Results with Insights on the Natural History of the Disease. Hepatology 2019, 69, 684–698. [Google Scholar] [CrossRef]
- Meissner, E.G.; McLaughlin, M.; Matthews, L.; Gharib, A.M.; Wood, B.J.; Levy, E.; Sinkus, R.; Virtaneva, K.; Sturdevant, D.; Martens, C.; et al. Simtuzumab Treatment of Advanced Liver Fibrosis in HIV and HCV-Infected Adults: Results of a 6-Month Open-Label Safety Trial. Liver Int. 2016, 36, 1783–1792. [Google Scholar] [CrossRef] [PubMed]
- Verstovsek, S.; Savona, M.R.; Mesa, R.A.; Dong, H.; Maltzman, J.D.; Sharma, S.; Silverman, J.; Oh, S.T.; Gotlib, J. A Phase 2 Study of Simtuzumab in Patients with Primary, Post-Polycythaemia Vera or Post-Essential Thrombocythaemia Myelofibrosis. Br. J. Haematol. 2017, 176, 939–949. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Brown, K.K.; Collard, H.R.; Cottin, V.; Gibson, K.F.; Kaner, R.J.; Lederer, D.J.; Martinez, F.J.; Noble, P.W.; Song, J.W.; et al. Efficacy of Simtuzumab versus Placebo in Patients with Idiopathic Pulmonary Fibrosis: A Randomised, Double-Blind, Controlled, Phase 2 Trial. Lancet Respir. Med. 2017, 5, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Abdelmalek, M.F.; Caldwell, S.; Shiffman, M.L.; Diehl, A.M.; Ghalib, R.; Lawitz, E.J.; Rockey, D.C.; Schall, R.A.; Jia, C.; et al. Simtuzumab Is Ineffective for Patients with Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology 2018, 155, 1140–1153. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Benson, A.B.; Vyushkov, D.; Yang, Y.; Bendell, J.; Verma, U. A Phase II, Randomized, Double-Blind, Placebo-Controlled Study of Simtuzumab in Combination with FOLFIRI for the Second-Line Treatment of Metastatic KRAS Mutant Colorectal Adenocarcinoma. Oncologist 2017, 22, 243-e23. [Google Scholar] [CrossRef] [PubMed]
- Findlay, A.; Turner, C.; Schilter, H.; Deodhar, M.; Zhou, W.; Perryman, L.; Foot, J.; Zahoor, A.; Yao, Y.; Hamilton, R.; et al. An activity-based bioprobe differentiates a novel small molecule inhibitor from a LOXL2 antibody and provides renewed promise for anti-fibrotic therapeutic strategies. Clin. Transl. Med. 2021, 11, e572. [Google Scholar] [CrossRef] [PubMed]
- Grossman, M.; Ben-Chetrit, N.; Zhuravlev, A.; Afik, R.; Bassat, E.; Solomonov, I.; Yarden, Y.; Sagi, I. Tumor Cell Invasion Can Be Blocked by Modulators of Collagen Fibril Alignment That Control Assembly of the Extracellular Matrix. Cancer Res. 2016, 76, 4249–4258. [Google Scholar] [CrossRef]
- Brewer, G.J. Copper Control as an Antiangiogenic Anticancer Therapy: Lessons from Treating Wilson’s Disease. Exp. Biol. Med. 2001, 226, 665–673. [Google Scholar] [CrossRef]
- Cox, T.R.; Gartland, A.; Erler, J.T. Lysyl Oxidase, a Targetable Secreted Molecule Involved in Cancer Metastasis. Cancer Res. 2016, 76, 188–192. [Google Scholar] [CrossRef]
- Chan, N.; Willis, A.; Kornhauser, N.; Ward, M.M.; Lee, S.B.; Nackos, E.; Seo, B.R.; Chuang, E.; Cigler, T.; Moore, A.; et al. Influencing the Tumor Microenvironment: A Phase II Study of Copper Depletion Using Tetrathiomolybdate in Patients with Breast Cancer at High Risk for Recurrence and in Preclinical Models of Lung Metastases. Clin. Cancer Res. 2017, 23, 666–676. [Google Scholar] [CrossRef]
- Barker, H.E.; Chang, J.; Cox, T.R.; Lang, G.; Bird, D.; Nicolau, M.; Evans, H.R.; Gartland, A.; Erler, J.T. LOXL2-Mediated Matrix Remodeling in Metastasis and Mammary Gland Involution. Cancer Res. 2011, 71, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Ramchandani, D.; Berisa, M.; Tavarez, D.A.; Li, Z.; Miele, M.; Bai, Y.; Lee, S.B.; Ban, Y.; Dephoure, N.; Hendrickson, R.C.; et al. Copper Depletion Modulates Mitochondrial Oxidative Phosphorylation to Impair Triple Negative Breast Cancer Metastasis. Nat. Commun. 2021, 12, 7311. [Google Scholar] [CrossRef] [PubMed]
- Mohankumar, A.; Renganathan, B.; Karunakaran, C.; Chidambaram, S.; Konerirajapuram Natarajan, S. Peptides Derived from the Copper-Binding Region of Lysyl Oxidase Exhibit Antiangiogeneic Properties by Inhibiting Enzyme Activity: An In Vitro Study. J. Pept. Sci. 2014, 20, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Pinnell, S.R.; Martin, G.R. The Cross-Linking of Collagen and Elastin: Enzymatic Conversion of Lysine in Peptide Linkage to Alpha-Aminoadipic-Delta-Semialdehyde (Allysine) by an Extract from Bone. Proc. Natl. Acad. Sci. USA 1968, 61, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.S.; Trackman, P.C.; Kagan, H.M. Reaction of Aortic Lysyl Oxidase with Beta-Aminopropionitrile. J. Biol. Chem. 1983, 258, 4331–4338. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.H.; Rowbottom, M.W.; Lonergan, D.; Darlington, J.; Prodanovich, P.; King, C.D.; Evans, J.F.; Bain, G. Small Molecule Lysyl Oxidase-like 2 (LOXL2) Inhibitors: The Identification of an Inhibitor Selective for LOXL2 over LOX. ACS Med. Chem. Lett. 2017, 8, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Lucas, M.C.; Leonte, L.E.; Garcia-Montolio, M.; Singh, L.B.; Findlay, A.D.; Deodhar, M.; Foot, J.S.; Jarolimek, W.; Timpson, P.; et al. Pre-Clinical Evaluation of Small Molecule LOXL2 Inhibitors in Breast Cancer. Oncotarget 2017, 8, 26066–26078. [Google Scholar] [CrossRef]
- Schilter, H.; Findlay, A.D.; Perryman, L.; Yow, T.T.; Moses, J.; Zahoor, A.; Turner, C.I.; Deodhar, M.; Foot, J.S.; Zhou, W.; et al. The lysyl oxidase like 2/3 enzymatic inhibitor, PXS-5153A, reduces crosslinks and ameliorates fibrosis. J. Cell Mol. Med. 2019, 23, 1759–1770. [Google Scholar] [CrossRef]
- Rowbottom, M.W.; Bain, G.; Calderon, I.; Lasof, T.; Lonergan, D.; Lai, A.; Huang, F.; Darlington, J.; Prodanovich, P.; Santini, A.M.; et al. Identification of 4-(Aminomethyl)-6-(trifluoromethyl)-2-(phenoxy)pyridine Derivatives as Potent, Selective, and Orally Efficacious Inhibitors of the Copper-Dependent Amine Oxidase, Lysyl Oxidase-Like 2 (LOXL2). J. Med. Chem. 2017, 25, 4403–4423. [Google Scholar] [CrossRef]
- Wei, Y.; Kim, T.J.; Peng, D.H.; Duan, D.; Gibbons, D.L.; Yamauchi, M.; Jackson, J.R.; Le Saux, C.J.; Calhoun, C.; Peters, J.; et al. Fibroblast-Specific Inhibition of TGF-Β1 Signaling Attenuates Lung and Tumor Fibrosis. J. Clin. Investig. 2017, 127, 3675–3688. [Google Scholar] [CrossRef]
- Deshpande, H. Levoleucovorin Inhibits LOXL2 (Lysyl Oxidase like-2) to Control Breast Cancer Proliferation: A Repurposing Approach. J. Biomol. Struct. Dyn. 2023, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Zhao, P.; Tong, B.; Wei, Z.; Dai, Y. Escin Ia Suppresses the Metastasis of Triple-Negative Breast Cancer by Inhibiting Epithelial-Mesenchymal Transition via down-Regulating LOXL2 Expression. Oncotarget 2016, 7, 23684–23699. [Google Scholar] [CrossRef]
- Chen, X.; Kou, Y.; Lu, Y.; Pu, Y. Salidroside Ameliorated Hypoxia-induced Tumorigenesis of BxPC-3 Cells via Downregulating Hypoxia-inducible Factor (HIF)-1α and LOXL2. J. Cell. Biochem. 2020, 121, 165–173. [Google Scholar] [CrossRef]
- Ding, X.; Zhang, Y.; Liang, J.; Li, Q.; Hu, H.; Zhou, Y.; Zhang, B. Dihydroartemisinin Potentiates VEGFR-TKIs Antitumorigenic Effect on Osteosarcoma by Regulating Loxl2/VEGFA Expression and Lipid Metabolism Pathway. J. Cancer 2023, 14, 809–820. [Google Scholar] [CrossRef]


| Factors | Ref. |
|---|---|
| Cancer-associated fibroblasts | [61] |
| ETS transcription factor ELK3 (ELK3) | [62] |
| Transcription factor forkhead box A1 (FOXA1) | [63] |
| Glial cell-line derived neurotrophic factor (GDNF) | [64] |
| DNA replication GINS complex, subunit 2 (GINS2) | [65] |
| Oncostatin M | [39,66] |
| 37/67 kDa laminin-1 receptor ribosomal protein SA (RPSA) | [67] |
| Histone methyltransferase, SET, and MYND domain containing 3 (SMYD3) | [68] |
| Succinate dehydrogenase complex iron sulphur subunit B (SDHB) | [69] |
| Transcription factor SP1 | [70,71] |
| Transforming growth factor beta (TGFβ) | [72] |
| Vitamin D | [73] |
| Hepatitis transactivator protein X (HBx) | [74] |
| Hepatitis C virus core protein | [75] |
| Gene | Genetic Model | Phenotype | Ref. |
|---|---|---|---|
| Lox | Lox KO | Alteration in cardiovascular and respiratory systems. Perinatal lethality | [129,130] |
| Lox KI | Alteration in vascular remodelling | [131] | |
| Loxl1 | Loxl1 KO | Pelvic prolapse | [132,133] |
| Sex-linked skeletal alterations | |||
| Loxl2 | Loxl2 KO | Congenital heart defects. Hepatic vessel distention. Incomplete perinatal lethality | [122] |
| Loxl2 KO | Female uterine hyperplasia | [17] | |
| Conditional LOX2 KO (adult) | Stress induced cardiac fibrosis and increased cardiac injury | [133] | |
| Loxl2 KI | Male sterility | [122] | |
| L2ΔE13 KI (splice variant) | Loss of adipose tissue | [16,134] | |
| Loxl3 | Loxl3 KO | Defects in muscle–skeletal and lung system development. Incomplete perinatal lethality | [135,136,137,138] |
| Conditional Loxl3 KO (adult) | Progressive loss of hearing via Loxl3 ablation in inner ear | [139] | |
| Loxl4 | Loxl4 KO | No phenotype | [140] |
| Gene | Genetic Model | Cancer Model | Phenotype | Ref. |
|---|---|---|---|---|
| Loxl2 constitutive | Loxl2 KO | DMBA/TPA mouse skin carcinogenesis | Decreased tumour burden and malignant progression | [122] |
| Loxl2 KI | DMBA/TPA mouse skin carcinogenesis | Decreased latency, increased tumour burden, and malignant progression | [122] | |
| Loxl2 KO | Spontaneous uterine cancer | Uterine hyperplasia and uterine carcinomas | [17] | |
| L2ΔE3 KI | Oesophageal cancer | Metabolic reprograming | [16] | |
| Loxl2 conditional | Loxl2 KO (mammary glands) | MMTV-PyMT-breast cancer | Decreased lung metastasis | [142] |
| Loxl2 KI (mammary glands) | MMTV-PyMT-breast cancer | Increased lung metastasis | [142] | |
| Loxl2 KO (pancreatic tumours) | KPC (Kras/Tp53/Pdx1-Cre)-L2-KO/ KC (Kras/Pdx1-Cre)-L2-KO | Decreased metastasis, increased overall survival. Alteration in collagen crosslinking | [39] | |
| Loxl2 KI (pancreatic tumours) | KPC (Kras/Tp53/Pdx1-Cre)-L2-KI/ KC (Kras/Pdx1-Cre)-L2-KI | Increased metastasis and tumour growth, decreased overall survival. Induction of EMT and stemness. | [39] | |
| Loxl3 conditional/ inducible | Loxl3 (melanoma) | Tyr-CreER/Braf/Pten/L3-KO | Decreased tumour burden and reduced lymphatic dissemination | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano, A.; Eraso, P.; Mazón, M.J.; Portillo, F. LOXL2 in Cancer: A Two-Decade Perspective. Int. J. Mol. Sci. 2023, 24, 14405. https://doi.org/10.3390/ijms241814405
Cano A, Eraso P, Mazón MJ, Portillo F. LOXL2 in Cancer: A Two-Decade Perspective. International Journal of Molecular Sciences. 2023; 24(18):14405. https://doi.org/10.3390/ijms241814405
Chicago/Turabian StyleCano, Amparo, Pilar Eraso, María J. Mazón, and Francisco Portillo. 2023. "LOXL2 in Cancer: A Two-Decade Perspective" International Journal of Molecular Sciences 24, no. 18: 14405. https://doi.org/10.3390/ijms241814405
APA StyleCano, A., Eraso, P., Mazón, M. J., & Portillo, F. (2023). LOXL2 in Cancer: A Two-Decade Perspective. International Journal of Molecular Sciences, 24(18), 14405. https://doi.org/10.3390/ijms241814405





