A Route for Investigating Psoriasis: From the Perspective of the Pathological Mechanisms and Therapeutic Strategies of Cancer
Abstract
:1. Introduction
2. Common Pathological Mechanisms between Psoriasis and Cancer
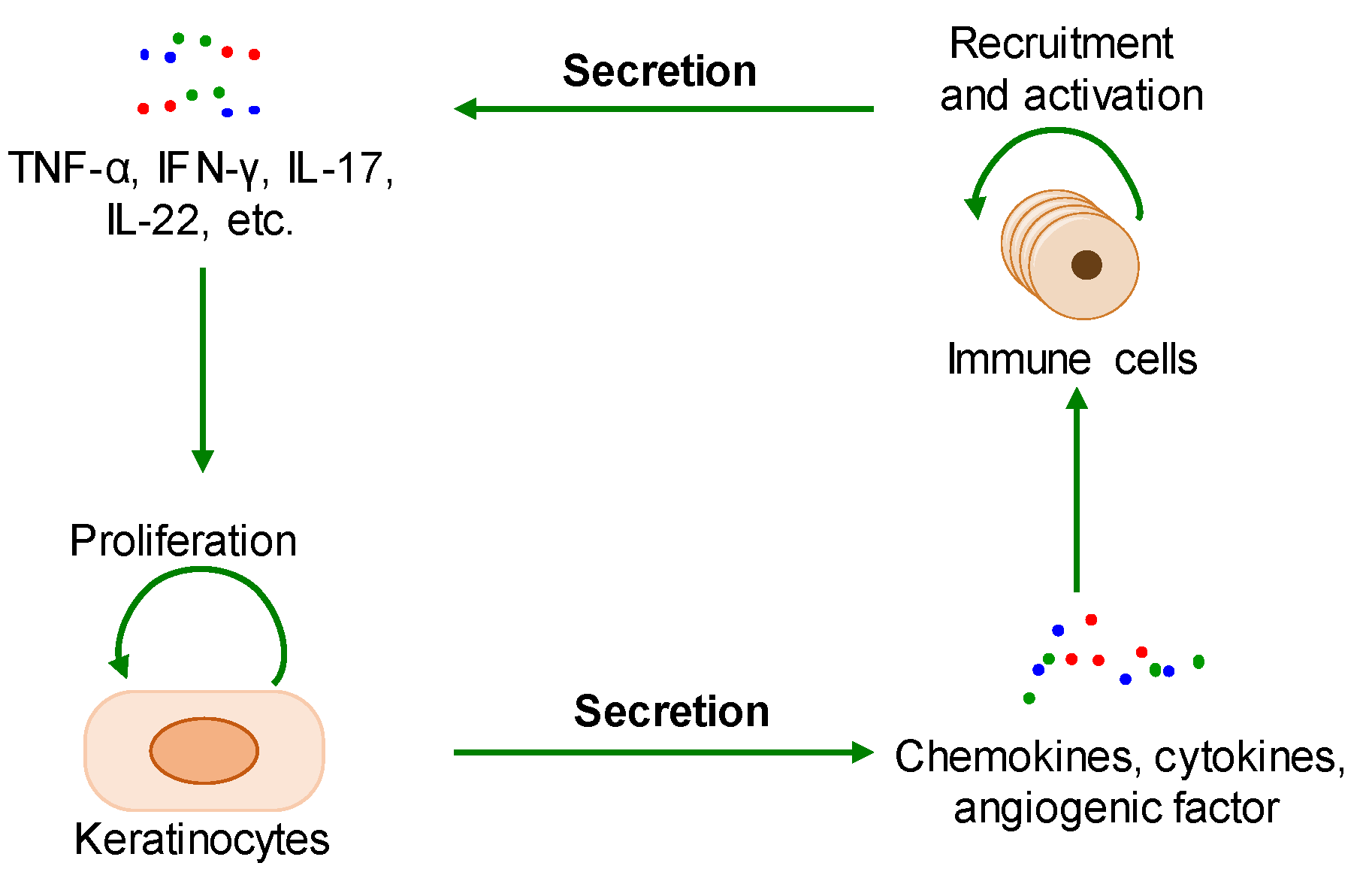
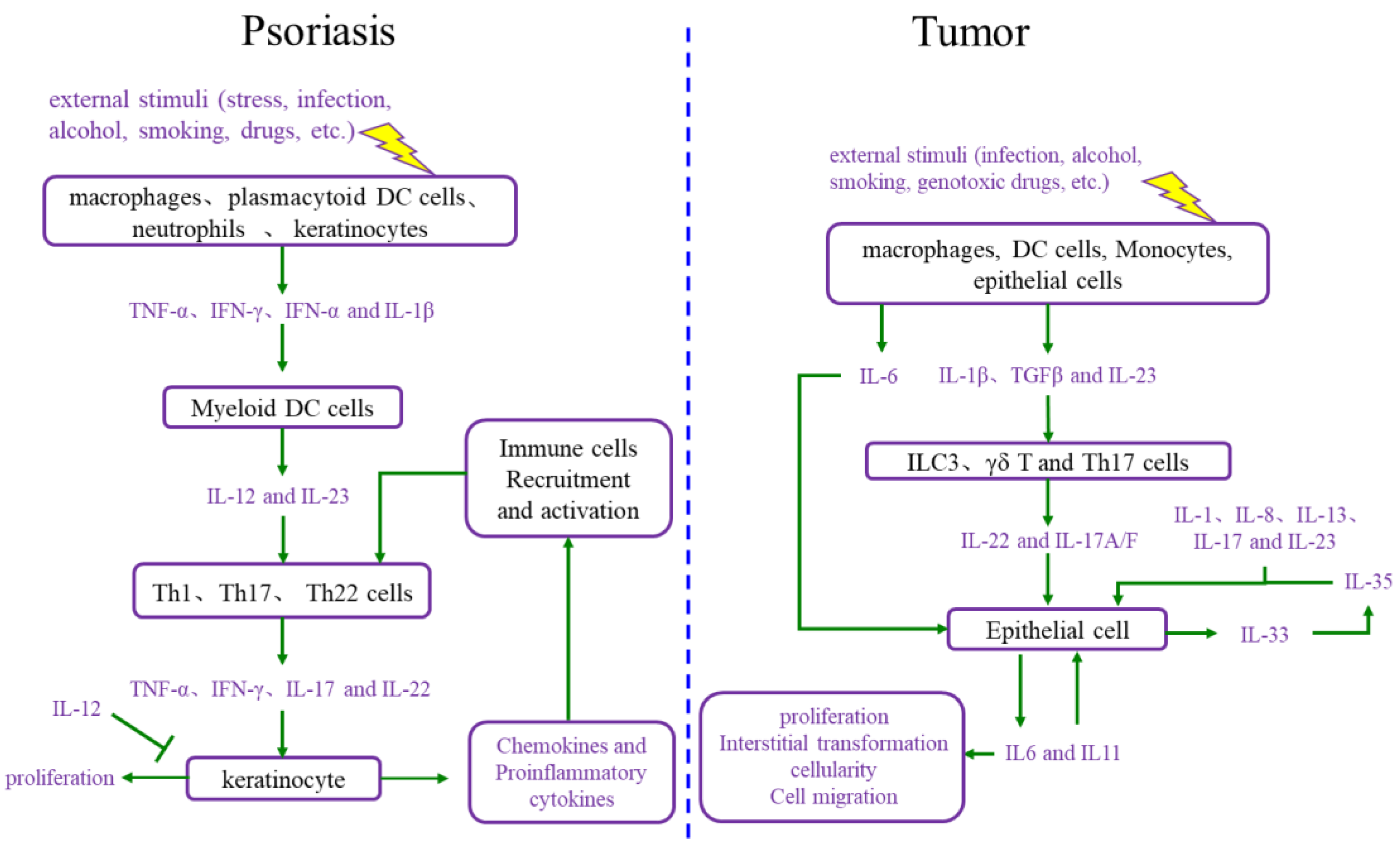
2.1. The Interaction between Cell Proliferation and Abnormal Immune Microenvironment
2.2. Metabolic Reprogramming
2.3. Epigenetic Reprogramming
2.4. Others
3. Common Therapeutic Agents and Therapeutic Targets in Psoriasis and Cancer
3.1. Current Therapeutic Agents and Their Targets
3.1.1. Cytotoxic Agents Regulating the Cell Cycle
3.1.2. Immune Modulators
3.1.3. Epigenetic Modulators
3.1.4. Metabolic Modulators
3.1.5. Others
3.2. Novel Potential Therapeutic Targets
| Drugs | Targets | Clinical Phase | Ref | ||
|---|---|---|---|---|---|
| Psoriasis | Cancer | ||||
| Cytotoxic agents | Camptothecin | Topoisomerase I | Clinical application (topical) | Clinical application for various cancers | [46,47] |
| Bimolane and ICRF-154 | Topoisomerase II | Clinical application (oral) | Clinical application for various cancers | [49,50,51,52] | |
| THZ1 | CDK7 | Preclinical study (topical) | Preclinical study | [53,54] | |
| Paclitaxel | Microtubule | Clinical application (topical) | Clinical application for various cancers | [55,56] | |
| Methotrexate | Folate-dependent enzyme | Clinical application | Clinical application for various cancers | [57,58] | |
| Immune modulators | Deucravacitinib | TYK2 | Clinical application (oral) | Preclinical study for MPNST | [62,63] |
| Tildrakizumab | IL-23 | Clinical application | Clinical phase I/II for prostatic cancer | [64,65] | |
| CJM112 | IL-17 | Clinical phase I | Clinical phase I for colorectal cancer | [66,67,68] | |
| Epigenetic modulators | JQ1 | BRD4 | Preclinical study | Clinical phase I for melanoma | [69,70,71,72] |
| A485 | p300/CBP | Preclinical study | Preclinical study for hematological malignancies and prostate cancer | [73,74] | |
| Vorinostat | HDACs | Preclinical study | Clinical application for hematologic cancer | [75,76] | |
| Decitabine | DNMTs | Preclinical study | Clinical application for MDS | [77,78] | |
| Metabolic modulators | WZB117 | GLUT1 | Preclinical study | Preclinical study for lung cancer | [22,79,80] |
| 2′-Hydroxycinnamaldehyde | PKM2 | Preclinical study | Preclinical study for various cancers | [83,84] | |
| Others | VEGF antagonist | VEGF | Proven effective based on clinical observation | Clinical application for various cancers | [85,86] |
| Calcipotriol | VDR | Clinical application (topical) | Preclinical study for gastric cancer | [87] | |
| RGRN-305 | Hsp90 | Clinical phase Ib | Clinical phase I for various cancers | [88,89,90] | |
| OTS514 | TOPK | Preclinical study (topical) | Preclinical study for various cancers | [91,92] | |
4. Discussion
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Z.; Lu, J.; Ou, J.; Yu, J.; Lu, C. Effect of Chinese herbal medicine injections for treatment of psoriasis vulgaris: A systematic review and meta-analysis. Front. Pharmacol. 2023, 14, 1148445. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Wang, Z.; Li, W.; Li, Y.; Liu, M. Biomarkers and biologics related with psoriasis and psoriatic arthritis. Int. Immunopharmacol. 2023, 122, 110646. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Jiang, L.; Lei, L.; Luo, L.; Guo, H.; Zhou, Y.; Huang, J.; Chen, J.; Zeng, Q. Establishment and validation of psoriasis evaluation models. Fundam. Res. 2022, 2, 166–176. [Google Scholar] [CrossRef]
- Committee on Psoriasis, Chinese Society of Dermatology; Committee on Children, Chinese Society of Dermatology. Consensus on the diagnosis and treatment of childhood psoriasis in China (2021). Chin. J. Dermatol. 2021, 54, 559–581. [Google Scholar]
- Committee on Psoriasis; Chinese Society of Dermatology. Guideline for the diagnosis and treatment of psoriasis in China (2018 complete edition). Chin. J. Dermatol. 2019, 52, 667–710. [Google Scholar]
- Ramic, L.; Sator, P. Topical treatment of psoriasis vulgaris. J. Dtsch. Dermatol. Ges. 2023, 21, 631–642. [Google Scholar] [CrossRef]
- Balak, D.M.W.; Gerdes, S.; Parodi, A.; Salgado-Boquete, L. Long-term safety of oral systemic therapies for psoriasis: A comprehensive review of the literature. Dermatol. Ther. 2020, 10, 589–613. [Google Scholar] [CrossRef]
- Phan, D.B.; Elyoussfi, S.; Stevenson, M.; Lunt, M.; Warren, R.B.; Yiu, Z.Z.N. Biosimilars for the treatment of psoriasis: A systematic review of clinical trials and observational studies. JAMA Dermatol. 2023, 159, 763–771. [Google Scholar] [CrossRef]
- Abduelmula, A.; Rankin, B.D.; Sood, S.; Georgakopoulos, J.R.; Mufti, A.; Vender, R.; Yeung, J.; Prajapati, V.H. Management of adult generalized pustular psoriasis using biologics: A systematic review. J. Am. Acad. Dermatol. 2023, 89, 417–419. [Google Scholar] [CrossRef]
- Wang, W.M.; Jin, H.Z. Biologics in the treatment of pustular psoriasis. Expert. Opin. Drug Saf. 2020, 19, 969–980. [Google Scholar] [CrossRef]
- Sreya, R.; Nene, S.; Pathade, V.; Singh, S.B.; Srivastava, S. Emerging trends in combination strategies with phototherapy in advanced psoriasis management. Inflammopharmacology 2023, 31, 1761–1778. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z.; Wu, S.; Guo, L.; Jiang, X. Oral small-molecule tyrosine kinase 2 and phosphodiesterase 4 inhibitors in plaque psoriasis: A network meta-analysis. Front. Immunol. 2023, 14, 1180170. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, Y.; Cui, L.; Shi, Y.; Guo, C. Advances in the pathogenesis of psoriasis: From keratinocyte perspective. Cell Death Dis. 2022, 13, 81. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Read, C. Pathophysiology, clinical presentation, and treatment of psoriasis: A review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Dainichi, T.; Kitoh, A.; Otsuka, A.; Nakajima, S.; Nomura, T.; Kaplan, D.H.; Kabashima, K. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat. Immunol. 2018, 19, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of cancer: New dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Briukhovetska, D.; Dorr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nat. Rev. Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef]
- Elia, I.; Haigis, M.C. Metabolites and the tumour microenvironment: From cellular mechanisms to systemic metabolism. Nat. Metab. 2021, 3, 21–32. [Google Scholar] [CrossRef]
- Martinez-Reyes, I.; Chandel, N.S. Cancer metabolism: Looking forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [CrossRef]
- Zheng, X.; Li, W.; Ren, L.; Liu, J.; Pang, X.; Chen, X.; Kang, D.; Wang, J.; Du, G. The sphingosine kinase-1/sphingosine-1-phosphate axis in cancer: Potential target for anticancer therapy. Pharmacol. Ther. 2019, 195, 85–99. [Google Scholar] [CrossRef]
- Zhang, Z.; Zi, Z.; Lee, E.E.; Zhao, J.; Contreras, D.C.; South, A.P.; Abel, E.D.; Chong, B.F.; Vandergriff, T.; Hosler, G.A.; et al. Differential glucose requirement in skin homeostasis and injury identifies a therapeutic target for psoriasis. Nat. Med. 2018, 24, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Xu, M.Y.; Dai, X.Y.; Yan, L.; Li, L.; Zhu, R.Z.; Ren, L.J.; Zhang, J.Q.; Zhang, X.F.; Li, J.F.; et al. Pyruvate kinase M2 mediates glycolysis contributes to psoriasis by promoting keratinocyte proliferation. Front. Pharmacol. 2021, 12, 765790. [Google Scholar] [CrossRef] [PubMed]
- Lou, F.; Sun, Y.; Xu, Z.; Niu, L.; Wang, Z.; Deng, S.; Liu, Z.; Zhou, H.; Bai, J.; Yin, Q.; et al. Excessive polyamine generation in keratinocytes promotes self-RNA sensing by dendritic cells in psoriasis. Immunity 2020, 53, 204–216.e10. [Google Scholar] [CrossRef]
- Xia, X.; Cao, G.; Sun, G.; Zhu, L.; Tian, Y.; Song, Y.; Guo, C.; Wang, X.; Zhong, J.; Zhou, W.; et al. GLS1-mediated glutaminolysis unbridled by MALT1 protease promotes psoriasis pathogenesis. J. Clin. Investig. 2020, 130, 5180–5196. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Hou, G.; Zeng, C.; Ren, Y.; Chen, X.; Peng, C. Metabolomic profiling reveals amino acid and carnitine alterations as metabolic signatures in psoriasis. Theranostics 2021, 11, 754–767. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Molloy, P.L. Early Insights into cancer epigenetics: Gene promoter hypermethylation emerges as a potential biomarker for cancer detection. Cancer Res. 2022, 82, 1461–1463. [Google Scholar] [CrossRef] [PubMed]
- Casado-Pelaez, M.; Bueno-Costa, A.; Esteller, M. Single cell cancer epigenetics. Trends Cancer 2022, 8, 820–838. [Google Scholar] [CrossRef]
- Mobus, L.; Weidinger, S.; Emmert, H. Epigenetic factors involved in the pathophysiology of inflammatory skin diseases. J. Allergy Clin. Immunol. 2020, 145, 1049–1060. [Google Scholar] [CrossRef]
- Pollock, R.A.; Abji, F.; Gladman, D.D. Epigenetics of psoriatic disease: A systematic review and critical appraisal. J. Autoimmun. 2017, 78, 29–38. [Google Scholar] [CrossRef]
- Roberson, E.D.; Liu, Y.; Ryan, C.; Joyce, C.E.; Duan, S.; Cao, L.; Martin, A.; Liao, W.; Menter, A.; Bowcock, A.M. A subset of methylated CpG sites differentiate psoriatic from normal skin. J. Investig. Dermatol. 2012, 132 Pt 1, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Chen, Z.Q.; Cui, P.G.; Yao, X.; Li, Y.M.; Li, A.S.; Gong, J.Q.; Cao, Y.H. The methylation pattern of p16INK4a gene promoter in psoriatic epidermis and its clinical significance. Br. J. Dermatol. 2008, 158, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, W.; Li, B.; Wang, G. Keratin 17 in psoriasis: Current understanding and future perspectives. Semin. Cell Dev. Biol. 2021, 128, 112–119. [Google Scholar] [CrossRef]
- Depianto, D.; Kerns, M.L.; Dlugosz, A.A.; Coulombe, P.A. Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nat. Genet. 2010, 42, 910–914. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, S.; Wang, G. Keratin 17 in disease pathogenesis: From cancer to dermatoses. J. Pathol. 2019, 247, 158–165. [Google Scholar] [CrossRef]
- Baraks, G.; Tseng, R.; Pan, C.H.; Kasliwal, S.; Leiton, C.V.; Shroyer, K.R.; Escobar-Hoyos, L.F. Dissecting the oncogenic roles of keratin 17 in the hallmarks of cancer. Cancer Res. 2021, 82, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.C.; Pan, H.F.; Leng, R.X.; Wang, D.G.; Li, X.P.; Li, X.M.; Ye, D.Q. Emerging role of long noncoding RNAs in autoimmune diseases. Autoimmun. Rev. 2015, 14, 798–805. [Google Scholar] [CrossRef]
- Elamir, A.M.; Shaker, O.G.; El-Komy, M.H.; Mahmoud Sharabi, M.; Aboraia, N.M. The role of lncRNA MALAT-1 and miRNA-9 in psoriasis. Biochem. Biophys. Rep. 2021, 26, 101030. [Google Scholar] [CrossRef]
- Fang, Y.; Fullwood, M.J. Roles, functions, and mechanisms of long non-coding RNAs in cancer. Genom. Proteom. Bioinform. 2016, 14, 42–54. [Google Scholar] [CrossRef]
- Zhou, Q.; Mrowietz, U.; Rostami-Yazdi, M. Oxidative stress in the pathogenesis of psoriasis. Free Radic. Biol. Med. 2009, 47, 891–905. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, A.W.; Voyles, S.V.; Armstrong, E.J.; Fuller, E.N.; Rutledge, J.C. Angiogenesis and oxidative stress: Common mechanisms linking psoriasis with atherosclerosis. J. Dermatol. Sci. 2011, 63, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Rosada, C.; Stenderup, K.; de Darko, E.; Dagnaes-Hansen, F.; Kamp, S.; Dam, T.N. Valrubicin in a topical formulation treats psoriasis in a xenograft transplantation model. J. Investig. Dermatol. 2010, 130, 455–463. [Google Scholar] [CrossRef]
- Zhang, W.J.; Li, P.H.; Zhao, M.C.; Gu, Y.H.; Dong, C.Z.; Chen, H.X.; Du, Z.Y. Synthesis and identification of quinoline derivatives as topoisomerase I inhibitors with potent antipsoriasis activity in an animal model. Bioorg. Chem. 2019, 88, 102899. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, X.; Bao, Y.; Hou, S.; An, L.; Lin, X. Effects of isocamptothecin, a novel camptothecin analogue, on proliferation, apoptosis and telomerase activity in HaCaT cells. Exp. Dermatol. 2008, 17, 530–536. [Google Scholar] [CrossRef]
- Wang, X.; Zhuang, Y.; Wang, Y.; Jiang, M.; Yao, L. The recent developments of camptothecin and its derivatives as potential anti-tumor agents. Eur. J. Med. Chem. 2023, 260, 115710. [Google Scholar] [CrossRef]
- Bailly, C. Contemporary challenges in the design of topoisomerase II inhibitors for cancer chemotherapy. Chem. Rev. 2012, 112, 3611–3640. [Google Scholar] [CrossRef]
- Frantz, C.E.; Smith, H.; Eades, D.M.; Grosovsky, A.J.; Eastmond, D.A. Bimolane: In vitro inhibitor of human topoisomerase II. Cancer Lett. 1997, 120, 135–140. [Google Scholar] [CrossRef]
- Xu, B.; Noah, P.W.; Skinner, R.B., Jr.; Bale, G.; Chesney, T.M.; Rosenberg, E.W. Efficacy of bimolane in the Malassezia ovalis model of psoriasis. J. Dermatol. 1991, 18, 707–713. [Google Scholar] [CrossRef]
- Vuong, M.C.; Hasegawa, L.S.; Eastmond, D.A. A comparative study of the cytotoxic and genotoxic effects of ICRF-154 and bimolane, two catalytic inhibitors of topoisomerase II. Mutat. Res. 2013, 750, 63–71. [Google Scholar] [CrossRef]
- Azarova, A.M.; Lyu, Y.L.; Lin, C.P.; Tsai, Y.C.; Lau, J.Y.; Wang, J.C.; Liu, L.F. Roles of DNA topoisomerase II isozymes in chemotherapy and secondary malignancies. Proc. Natl. Acad. Sci. USA 2007, 104, 11014–11019. [Google Scholar] [CrossRef] [PubMed]
- Mughal, M.J.; Bhadresha, K.; Kwok, H.F. CDK inhibitors from past to present: A new wave of cancer therapy. Semin. Cancer Biol. 2023, 88, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Xue, K.; Li, Q.; Liu, Z.; Zhu, Z.; Chen, J.; Dang, E.; Wang, L.; Zhang, W.; Wang, G.; et al. Cyclin-dependent kinase 7 promotes Th17/Th1 cell differentiation in psoriasis by modulating glycolytic metabolism. J. Investig. Dermatol. 2021, 141, 2656–2667.e11. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, A.; Booher, S.; Becerra, Y.; Borris, D.L.; Figg, W.D.; Turner, M.L.; Blauvelt, A. Micellar paclitaxel improves severe psoriasis in a prospective phase II pilot study. J. Am. Acad. Dermatol. 2004, 50, 533–540. [Google Scholar] [CrossRef]
- Christensen, S.B. Drugs that changed society: Microtubule-targeting agents belonging to taxanoids, macrolides and non-ribosomal peptides. Molecules 2022, 27, 5648. [Google Scholar] [CrossRef]
- Rana, R.M.; Rampogu, S.; Bin Abid, N.; Zeb, A.; Parate, S.; Lee, G.H.; Yoon, S.; Kim, Y.; Kim, D.; Lee, K.W. In silico study identified methotrexate analog as potential inhibitor of drug resistant human dihydrofolate reductase for cancer therapeutics. Molecules 2020, 25, 3510. [Google Scholar] [CrossRef] [PubMed]
- Bharadwaj, R.; Lusi, C.F.; Mashayekh, S.; Nagar, A.; Subbarao, M.; Kane, G.I.; Wodzanowski, K.A.; Brown, A.R.; Okuda, K.; Monahan, A.; et al. Methotrexate suppresses psoriatic skin inflammation by inhibiting muropeptide transporter SLC46A2 activity. Immunity 2023, 56, 998–1012. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Nogueira, M.; Puig, L.; Torres, T. JAK inhibitors for treatment of psoriasis: Focus on selective TYK2 inhibitors. Drugs 2020, 80, 341–352. [Google Scholar] [CrossRef]
- Krueger, J.G.; McInnes, I.B.; Blauvelt, A. Tyrosine kinase 2 and Janus kinase–signal transducer and activator of transcription signaling and inhibition in plaque psoriasis. J. Am. Acad. Dermatol. 2022, 86, 148–157. [Google Scholar] [CrossRef]
- Hoy, S.M. Deucravacitinib: First approval. Drugs 2022, 82, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Borcherding, D.C.; Amin, N.V.; He, K.; Zhang, X.; Lyu, Y.; Dehner, C.; Bhatia, H.; Gothra, A.; Daud, L.; Ruminski, P.; et al. MEK inhibition synergizes with TYK2 inhibitors in NF1-associated malignant peripheral nerve sheath tumors. Clin. Cancer Res. 2023, 29, 1592–1604. [Google Scholar] [CrossRef] [PubMed]
- Reich, K.; Papp, K.A.; Blauvelt, A.; Tyring, S.K.; Sinclair, R.; Thaci, D.; Nograles, K.; Mehta, A.; Cichanowitz, N.; Li, Q.; et al. Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): Results from two randomised controlled, phase 3 trials. Lancet 2017, 390, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Abiraterone Acetate in Combination with Tildrakizumab (ACTIon). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04458311?term=Tildrakizumab&cond=prostatic+cancer&draw=2&rank=1 (accessed on 6 September 2023).
- Kaul, M.; Jarvis, P.; Rozenberg, I.; Kolbinger, F.; Di Padova, F.; Calonder, C.; Espie, P.; Rondeau, J.M.; Cebe, R.; Huber, T.; et al. First-in-human study demonstrating the safety and clinical efficacy of novel anti-IL-17A monoclonal antibody CJM112 in moderate to severe plaque psoriasis. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Study of Single Agent CJM112, and PDR001 in Combination with LCL161 or CJM112 in Patients with Multiple Myeloma. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT03111992?term=CJM112&cond=cancer&draw=2&rank=2 (accessed on 6 September 2023).
- A Study of PDR001 in Combination with CJM112, EGF816, Ilaris® (Canakinumab) or Mekinist® (Trametinib). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT02900664?term=CJM112&cond=cancer&draw=2&rank=1 (accessed on 6 September 2023).
- Sun, X.; Yang, P. Inhibition of BRD4 inhibits proliferation and promotes apoptosis of psoriatic keratinocytes. Biomed. Eng. Online 2021, 20, 107. [Google Scholar] [CrossRef]
- Nadeem, A.; Al-Harbi, N.O.; Al-Harbi, M.M.; El-Sherbeeny, A.M.; Ahmad, S.F.; Siddiqui, N.; Ansari, M.A.; Zoheir, K.M.; Attia, S.M.; Al-Hosaini, K.A.; et al. Imiquimod-induced psoriasis-like skin inflammation is suppressed by BET bromodomain inhibitor in mice through RORC/IL-17A pathway modulation. Pharmacol. Res. 2015, 99, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Qi, J. Bromodomain and extraterminal domain inhibitors (BETi) for cancer therapy: Chemical modulation of chromatin structure. Cold Spring Harb. Perspect. Biol. 2014, 6, a018663. [Google Scholar] [CrossRef]
- Delmore, J.E.; Issa, G.C.; Lemieux, M.E.; Rahl, P.B.; Shi, J.; Jacobs, H.M.; Kastritis, E.; Gilpatrick, T.; Paranal, R.M.; Qi, J.; et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell 2011, 146, 904–917. [Google Scholar] [CrossRef]
- Lasko, L.M.; Jakob, C.G.; Edalji, R.P.; Qiu, W.; Montgomery, D.; Digiammarino, E.L.; Hansen, T.M.; Risi, R.M.; Frey, R.; Manaves, V.; et al. Discovery of a selective catalytic p300/CBP inhibitor that targets lineage-specific tumours. Nature 2017, 550, 128–132. [Google Scholar] [CrossRef]
- Kim, J.; He, Y.; Tormen, S.; Kleindienst, P.; Ducoli, L.; Restivo, G.; Drach, M.; Levesque, M.P.; Navarini, A.A.; Tacconi, C.; et al. The p300/CBP inhibitor A485 normalizes psoriatic fibroblast gene expression in vitro and reduces psoriasis-like skin inflammation in vivo. J. Investig. Dermatol. 2023, 143, 431–443.e19. [Google Scholar] [CrossRef] [PubMed]
- Samuelov, L.; Bochner, R.; Magal, L.; Malovitski, K.; Sagiv, N.; Nousbeck, J.; Keren, A.; Fuchs-Telem, D.; Sarig, O.; Gilhar, A.; et al. Vorinostat, a histone deacetylase inhibitor, as a potential novel treatment for psoriasis. Exp. Dermatol. 2021, 31, 567–576. [Google Scholar] [CrossRef]
- Zagni, C.; Floresta, G.; Monciino, G.; Rescifina, A. The search for potent, small-molecule HDACIs in cancer treatment: A decade after vorinostat. Med. Res. Rev. 2017, 37, 1373–1428. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhou, Y.; Luo, D.; Sun, X.; Li, H.; Lu, Y.; Wang, J.; Zhang, M.; Lin, N.; Yin, C.; et al. Aberrant promoter methylation of Wnt inhibitory factor-1 gene is a potential target for treating psoriasis. Clin. Immunol. 2023, 250, 109294. [Google Scholar] [CrossRef]
- Itzykson, R.; Santini, V.; Thepot, S.; Ades, L.; Chaffaut, C.; Giagounidis, A.; Morabito, M.; Droin, N.; Lubbert, M.; Sapena, R.; et al. Decitabine versus hydroxyurea for advanced proliferative chronic myelomonocytic leukemia: Results of a randomized phase III trial within the EMSCO network. J. Clin. Oncol. 2023, 41, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Xu, X.; Luan, H.; Li, L.; Dai, W.; Li, Z.; Bian, J. The progress and development of GLUT1 inhibitors targeting cancer energy metabolism. Future Med. Chem. 2019, 11, 2333–2352. [Google Scholar] [CrossRef]
- Goodwin, J.; Neugent, M.L.; Lee, S.Y.; Choe, J.H.; Choi, H.; Jenkins, D.M.R.; Ruthenborg, R.J.; Robinson, M.W.; Jeong, J.Y.; Wake, M.; et al. The distinct metabolic phenotype of lung squamous cell carcinoma defines selective vulnerability to glycolytic inhibition. Nat. Commun. 2017, 8, 15503. [Google Scholar] [CrossRef]
- Veras, F.P.; Publio, G.A.; Melo, B.M.; Prado, D.S.; Norbiato, T.; Cecilio, N.T.; Hiroki, C.; Damasceno, L.E.A.; Jung, R.; Toller-Kawahisa, J.E.; et al. Pyruvate kinase M2 mediates IL-17 signaling in keratinocytes driving psoriatic skin inflammation. Cell Rep. 2022, 41, 111897. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, Y.; Zhang, X.; Liu, H.; Yin, M.; Chen, X.; Peng, C. Pyruvate kinase M2 (PKM2) in cancer and cancer therapeutics. Cancer Lett. 2021, 503, 240–248. [Google Scholar] [CrossRef]
- Hao, L.; Mao, Y.; Park, J.; Kwon, B.M.; Bae, E.J.; Park, B.H. 2′-Hydroxycinnamaldehyde ameliorates imiquimod-induced psoriasiform inflammation by targeting PKM2-STAT3 signaling in mice. Exp. Mol. Med. 2021, 53, 875–884. [Google Scholar] [CrossRef]
- Yoon, Y.J.; Kim, Y.H.; Jin, Y.; Chi, S.W.; Moon, J.H.; Han, D.C.; Kwon, B.M. 2′-hydroxycinnamaldehyde inhibits cancer cell proliferation and tumor growth by targeting the pyruvate kinase M2. Cancer Lett. 2018, 434, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Luengas-Martinez, A.; Paus, R.; Young, H.S. Antivascular endothelial growth factor—A therapy: A novel personalized treatment approach for psoriasis. Br. J. Dermatol. 2022, 186, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Lacal, P.M.; Graziani, G. Therapeutic implication of vascular endothelial growth factor receptor-1 (VEGFR-1) targeting in cancer cells and tumor microenvironment by competitive and non-competitive inhibitors. Pharmacol. Res. 2018, 136, 97–107. [Google Scholar] [CrossRef]
- Kong, W.; Liu, Z.; Sun, M.; Liu, H.; Kong, C.; Ma, J.; Wang, R.; Qian, F. Synergistic autophagy blockade and VDR signaling activation enhance stellate cell reprogramming in pancreatic ductal adenocarcinoma. Cancer Lett. 2022, 539, 215718. [Google Scholar] [CrossRef]
- Bregnhoj, A.; Thuesen, K.K.H.; Emmanuel, T.; Litman, T.; Grek, C.L.; Ghatnekar, G.S.; Johansen, C.; Iversen, L. HSP90 inhibitor RGRN-305 for oral treatment of plaque-type psoriasis: Efficacy, safety and biomarker results in an open-label proof-of-concept study. Br. J. Dermatol. 2022, 186, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Kakeda, M.; Arock, M.; Schlapbach, C.; Yawalkar, N. Increased expression of heat shock protein 90 in keratinocytes and mast cells in patients with psoriasis. J. Am. Acad. Dermatol. 2014, 70, 683–690.e1. [Google Scholar] [CrossRef]
- Bao, R.; Lai, C.J.; Qu, H.; Wang, D.; Yin, L.; Zifcak, B.; Atoyan, R.; Wang, J.; Samson, M.; Forrester, J.; et al. CUDC-305, a novel synthetic HSP90 inhibitor with unique pharmacologic properties for cancer therapy. Clin. Cancer Res. 2009, 15, 4046–4057. [Google Scholar] [CrossRef]
- Matsuo, Y.; Park, J.H.; Miyamoto, T.; Yamamoto, S.; Hisada, S.; Alachkar, H.; Nakamura, Y. TOPK inhibitor induces complete tumor regression in xenograft models of human cancer through inhibition of cytokinesis. Sci. Transl. Med. 2014, 6, 259ra145. [Google Scholar] [CrossRef]
- Zeng, F.; Lu, H.; Wu, M.; Dai, C.; Li, J.; Wang, J.; Hu, G. Topical application of TOPK inhibitor OTS514 suppresses psoriatic progression by inducing keratinocytes cell cycle arrest and apoptosis. Exp. Dermatol. 2023. [Google Scholar] [CrossRef]
- Xiao, C.Y.; Zhu, Z.L.; Zhang, C.; Fu, M.; Qiao, H.J.; Wang, G.; Dang, E.L. Small interfering RNA targeting of keratin 17 reduces inflammation in imiquimod-induced psoriasis-like dermatitis. Chin. Med. J. 2020, 133, 2910–2918. [Google Scholar] [CrossRef]
- Chivu-Economescu, M.; Dragu, D.L.; Necula, L.G.; Matei, L.; Enciu, A.M.; Bleotu, C.; Diaconu, C.C. Knockdown of KRT17 by siRNA induces antitumoral effects on gastric cancer cells. Gastric Cancer 2017, 20, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Luistro, L.; Zhong, H.; Smith, M.; Nevins, T.; Schostack, K.; Hilton, H.; Lin, T.A.; Truitt, T.; Biondi, D.; et al. RG7212 anti-TWEAK mAb inhibits tumor growth through inhibition of tumor cell proliferation and survival signaling and by enhancing the host antitumor immune response. Clin. Cancer Res. 2013, 19, 5686–5698. [Google Scholar] [CrossRef]
- Cherry, E.M.; Lee, D.W.; Jung, J.U.; Sitcheran, R. Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) promotes glioma cell invasion through induction of NF-kappaB-inducing kinase (NIK) and noncanonical NF-kappaB signaling. Mol. Cancer 2015, 14, 9. [Google Scholar] [CrossRef]
- Dwyer, B.J.; Jarman, E.J.; Gogoi-Tiwari, J.; Ferreira-Gonzalez, S.; Boulter, L.; Guest, R.V.; Kendall, T.J.; Kurian, D.; Kilpatrick, A.M.; Robson, A.J.; et al. TWEAK/Fn14 signalling promotes cholangiocarcinoma niche formation and progression. J. Hepatol. 2021, 74, 860–872. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Gracias, D.T.; Figueroa, D.S.; Miki, H.; Miller, J.; Fung, K.; Ay, F.; Burkly, L.; Croft, M. TWEAK functions with TNF and IL-17 on keratinocytes and is a potential target for psoriasis therapy. Sci. Immunol. 2021, 6, eabi8823. [Google Scholar] [CrossRef] [PubMed]
- Garshick, M.S.; Baumer, Y.; Dey, A.K.; Grattan, R.; Ng, Q.; Teague, H.L.; Yu, Z.X.; Chen, M.Y.; Tawil, M.; Barrett, T.J.; et al. Characterization of PCSK9 in the blood and skin of psoriasis. J. Investig. Dermatol. 2021, 141, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Luan, C.; Chen, X.; Zhu, Y.; Osland, J.M.; Gerber, S.D.; Dodds, M.; Hu, Y.; Chen, M.; Yuan, R. Potentiation of psoriasis-like inflammation by PCSK9. J. Investig. Dermatol. 2019, 139, 859–867. [Google Scholar] [CrossRef]
- Merleev, A.; Ji-Xu, A.; Toussi, A.; Tsoi, L.C.; Le, S.T.; Luxardi, G.; Xing, X.; Wasikowski, R.; Liakos, W.; Bruggen, M.C.; et al. Proprotein convertase subtilisin/kexin type 9 is a psoriasis-susceptibility locus that is negatively related to IL36G. JCI Insight 2022, 7, e141193. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Chowdhury, A.; Chaudhury, K.; Shukla, P.C. Proprotein convertase subtilisin/kexin type 9 (PCSK9): A potential multifaceted player in cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188581. [Google Scholar] [CrossRef]
- Zuo, Y.; Dai, L.; Li, L.; Huang, Y.; Liu, X.; Liu, X.; Duan, X.; Jiang, S.; Deng, G.-M.; Chen, H. ANGPTL4 regulates psoriasis via modulating hyperproliferation and inflammation of keratinocytes. Front. Pharmacol. 2022, 13, 850967. [Google Scholar] [CrossRef]
- Yan, H.H.; Jung, K.H.; Lee, J.E.; Son, M.K.; Fang, Z.; Park, J.H.; Kim, S.J.; Kim, J.Y.; Lim, J.H.; Hong, S.S. ANGPTL4 accelerates KRAS(G12D)-Induced acinar to ductal metaplasia and pancreatic carcinogenesis. Cancer Lett. 2021, 519, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Liao, M.; Qin, R.; Zhu, S.; Peng, C.; Fu, L.; Chen, Y.; Han, B. Regulated cell death (RCD) in cancer: Key pathways and targeted therapies. Signal Transduct. Target. Ther. 2022, 7, 286. [Google Scholar] [CrossRef]
- Simonart, T.; Heenen, M.; Lejeune, O. Epidermal kinetic alterations required to generate the psoriatic phenotype: A reappraisal. Cell Prolif. 2010, 43, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Luo, X.; Zhang, S.; Su, Y.; Deng, M.; Zhu, Y.; Zhang, P.; Wu, R.; Zhao, M. Ferroptosis activation contributes to the formation of skin lesions in psoriasis vulgaris. Antioxidants 2023, 12, 310. [Google Scholar] [CrossRef] [PubMed]
- Shou, Y.; Yang, L.; Yang, Y.; Xu, J. Inhibition of keratinocyte ferroptosis suppresses psoriatic inflammation. Cell Death Dis. 2021, 12, 1009. [Google Scholar] [CrossRef]
- Zou, Y.; Schreiber, S.L. Progress in understanding ferroptosis and challenges in its targeting for therapeutic benefit. Cell Chem. Biol. 2020, 27, 463–471. [Google Scholar] [CrossRef]
- Lian, N.; Chen, Y.; Chen, S.; Zhang, Y.; Chen, H.; Yang, Y.; Gu, H.; Chen, Q.; Li, M.; Chen, X. Gasdermin D-mediated keratinocyte pyroptosis as a key step in psoriasis pathogenesis. Cell Death Dis. 2023, 14, 595. [Google Scholar] [CrossRef]
- Yang, F.; Bettadapura, S.N.; Smeltzer, M.S.; Zhu, H.; Wang, S.Z. Pyroptosis and pyroptosis-inducing cancer drugs. Acta Pharmacol. Sin. 2022, 43, 2462–2473. [Google Scholar] [CrossRef]
- Chen, H.L.; Lo, C.H.; Huang, C.C.; Lu, M.P.; Hu, P.Y.; Chen, C.S.; Chueh, D.Y.; Chen, P.; Lin, T.N.; Lo, Y.H.; et al. Galectin-7 downregulation in lesional keratinocytes contributes to enhanced IL-17A signaling and skin pathology in psoriasis. J. Clin. Investig. 2021, 131, e130740. [Google Scholar] [CrossRef]
- Demers, M.; Magnaldo, T.; St-Pierre, Y. A novel function for galectin-7: Promoting tumorigenesis by up-regulating MMP-9 gene expression. Cancer Res. 2005, 65, 5205–5210. [Google Scholar] [CrossRef]
- Matsui, Y.; Ueda, S.; Watanabe, J.; Kuwabara, I.; Ogawa, O.; Nishiyama, H. Sensitizing effect of galectin-7 in urothelial cancer to cisplatin through the accumulation of intracellular reactive oxygen species. Cancer Res. 2007, 67, 1212–1220. [Google Scholar] [CrossRef]
- Kanno, T.; Nakajima, T.; Miyako, K.; Endo, Y. Lipid metabolism in Th17 cell function. Pharmacol. Ther. 2023, 245, 108411. [Google Scholar] [CrossRef]
- Wang, M.; Li, T.; Ouyang, Z.; Tang, K.; Zhu, Y.; Song, C.; Sun, H.; Yu, B.; Ji, X.; Sun, Y. SHP2 allosteric inhibitor TK-453 alleviates psoriasis-like skin inflammation in mice via inhibition of IL-23/Th17 axis. iScience 2022, 25, 104009. [Google Scholar] [CrossRef] [PubMed]
- Martin-Orozco, N.; Muranski, P.; Chung, Y.; Yang, X.X.O.; Yamazaki, T.; Lu, S.J.; Hwu, P.; Restifo, N.P.; Overwijk, W.W.; Dong, C. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 2009, 31, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, G.A.; Miller, G. Targeting the interleukin-17 immune axis for cancer immunotherapy. J. Exp. Med. 2020, 217, e20190456. [Google Scholar] [CrossRef] [PubMed]
- Costache, D.O.; Feroiu, O.; Ghilencea, A.; Georgescu, M.; Caruntu, A.; Caruntu, C.; Tiplica, S.G.; Jinga, M.; Costache, R.S. Skin inflammation modulation via TNF-alpha, IL-17, and IL-12 family inhibitors therapy and cancer control in patients with psoriasis. Int. J. Mol. Sci. 2022, 23, 5198. [Google Scholar] [CrossRef]
- Cirella, A.; Luri-Rey, C.; Di Trani, C.A.; Teijeira, A.; Olivera, I.; Bolanos, E.; Castanon, E.; Palencia, B.; Brocco, D.; Fernandez-Sendin, M.; et al. Novel strategies exploiting interleukin-12 in cancer immunotherapy. Pharmacol. Ther. 2022, 239, 108189. [Google Scholar] [CrossRef]
- Kulig, P.; Musiol, S.; Freiberger, S.N.; Schreiner, B.; Gyulveszi, G.; Russo, G.; Pantelyushin, S.; Kishihara, K.; Alessandrini, F.; Kundig, T.; et al. IL-12 protects from psoriasiform skin inflammation. Nat. Commun. 2016, 7, 13466. [Google Scholar] [CrossRef] [PubMed]
- Zwicky, P.; Ingelfinger, F.; de Melo, B.M.S.; Ruchti, F.; Scharli, S.; Puertas, N.; Lutz, M.; San Phan, T.; Kundig, T.M.; Levesque, M.P.; et al. IL-12 regulates type 3 immunity through interfollicular keratinocytes in psoriasiform inflammation. Sci. Immunol. 2021, 6, eabg9012. [Google Scholar] [CrossRef]
- Ergen, E.N.; Yusuf, N. Inhibition of interleukin-12 and/or interleukin-23 for the treatment of psoriasis: What is the evidence for an effect on malignancy? Exp. Dermatol. 2018, 27, 737–747. [Google Scholar] [CrossRef]
- Hu, P.; Wang, M.; Gao, H.; Zheng, A.; Li, J.; Mu, D.; Tong, J. The role of helper T cells in psoriasis. Front. Immunol. 2021, 12, 788940. [Google Scholar] [CrossRef] [PubMed]
- Boarder, E.; Rumberger, B.; Howell, M.D. Modeling skin inflammation using human in vitro models. Curr. Protoc. 2021, 1, e72. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Zhang, G.; Xia, Z.; Chen, N.; Yang, S.; Li, L. Identification of triazolopyridine derivatives as a new class of AhR agonists and evaluation of anti-psoriasis effect in a mouse model. Eur. J. Med. Chem. 2022, 231, 114122. [Google Scholar] [CrossRef]
- Rothhammer, V.; Quintana, F.J. The aryl hydrocarbon receptor: An environmental sensor integrating immune responses in health and disease. Nat. Rev. Immunol. 2019, 19, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Halle, B.R.; Betof Warner, A.; Zaman, F.Y.; Haydon, A.; Bhave, P.; Dewan, A.K.; Ye, F.; Irlmeier, R.; Mehta, P.; Kurtansky, N.R.; et al. Immune checkpoint inhibitors in patients with pre-existing psoriasis: Safety and efficacy. J. Immunother. Cancer 2021, 9, e003066. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Lai, Y. Keratinocyte: A trigger or an executor of psoriasis? J. Leukoc. Biol. 2020, 108, 485–491. [Google Scholar] [CrossRef]
- Luo, Y.X.; Pang, B.Y.; Hao, J.F.; Li, Q.Y.; Qiao, P.; Zhang, C.; Bai, Y.X.; Xiao, C.Y.; Chen, J.L.; Zhi, D.L.; et al. Keratin 17 covalently binds to alpha-enolase and exacerbates proliferation of keratinocytes in psoriasis. Int. J. Biol. Sci. 2023, 19, 3395–3411. [Google Scholar] [CrossRef]
- Prestonmartin, S.; Pike, M.C.; Ross, R.K.; Jones, P.A.; Henderson, B.E. Increased cell-division as a cause of human cancer. Cancer Res. 1990, 50, 7415–7421. [Google Scholar]
- Cohen, S.M.; Purtilo, D.T.; Ellwein, L.B. Pivotal role of increased cell-proliferation in human carcinogenesis. Modern Pathol. 1991, 4, 371–382. [Google Scholar]
- Galon, J.; Bruni, D. Tumor immunology and tumor evolution: Intertwined histories. Immunity 2020, 52, 55–81. [Google Scholar] [CrossRef]
- Ghoreschi, K. Targeting immune checkpoints and cytokines to protect from psoriasis relapse. JAMA Dermatol. 2021, 157, 1269–1271. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, X.; Ma, Y.; Wang, L.; Qin, X. A Route for Investigating Psoriasis: From the Perspective of the Pathological Mechanisms and Therapeutic Strategies of Cancer. Int. J. Mol. Sci. 2023, 24, 14390. https://doi.org/10.3390/ijms241814390
Wu X, Ma Y, Wang L, Qin X. A Route for Investigating Psoriasis: From the Perspective of the Pathological Mechanisms and Therapeutic Strategies of Cancer. International Journal of Molecular Sciences. 2023; 24(18):14390. https://doi.org/10.3390/ijms241814390
Chicago/Turabian StyleWu, Xingkang, Yushuang Ma, Lu Wang, and Xuemei Qin. 2023. "A Route for Investigating Psoriasis: From the Perspective of the Pathological Mechanisms and Therapeutic Strategies of Cancer" International Journal of Molecular Sciences 24, no. 18: 14390. https://doi.org/10.3390/ijms241814390
APA StyleWu, X., Ma, Y., Wang, L., & Qin, X. (2023). A Route for Investigating Psoriasis: From the Perspective of the Pathological Mechanisms and Therapeutic Strategies of Cancer. International Journal of Molecular Sciences, 24(18), 14390. https://doi.org/10.3390/ijms241814390






