Slowed Intestinal Transit Induced by Less Mucus in Intestinal Goblet Cell Piezo1-Deficient Mice through Impaired Epithelial Homeostasis
Abstract
:1. Introduction
2. Results
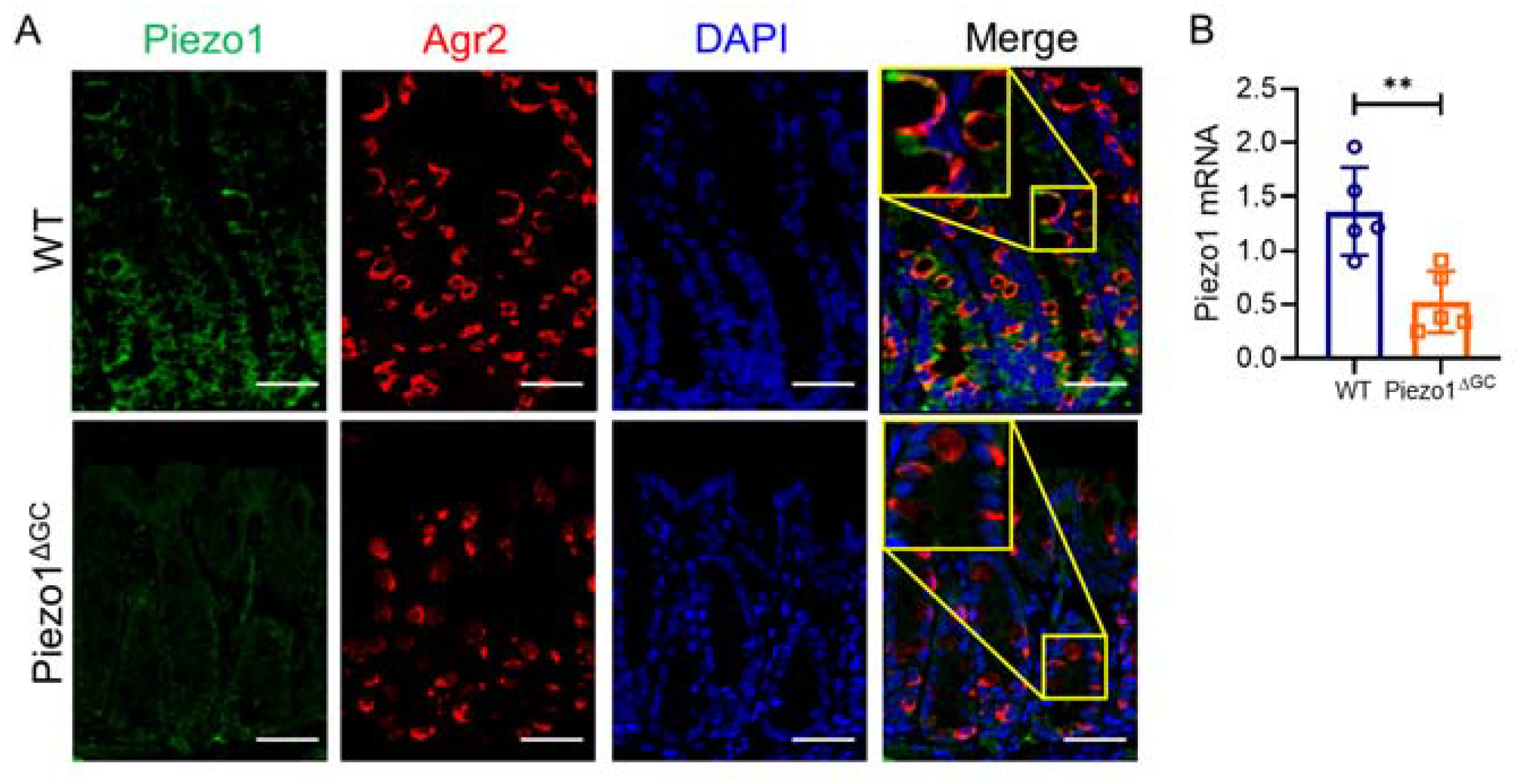
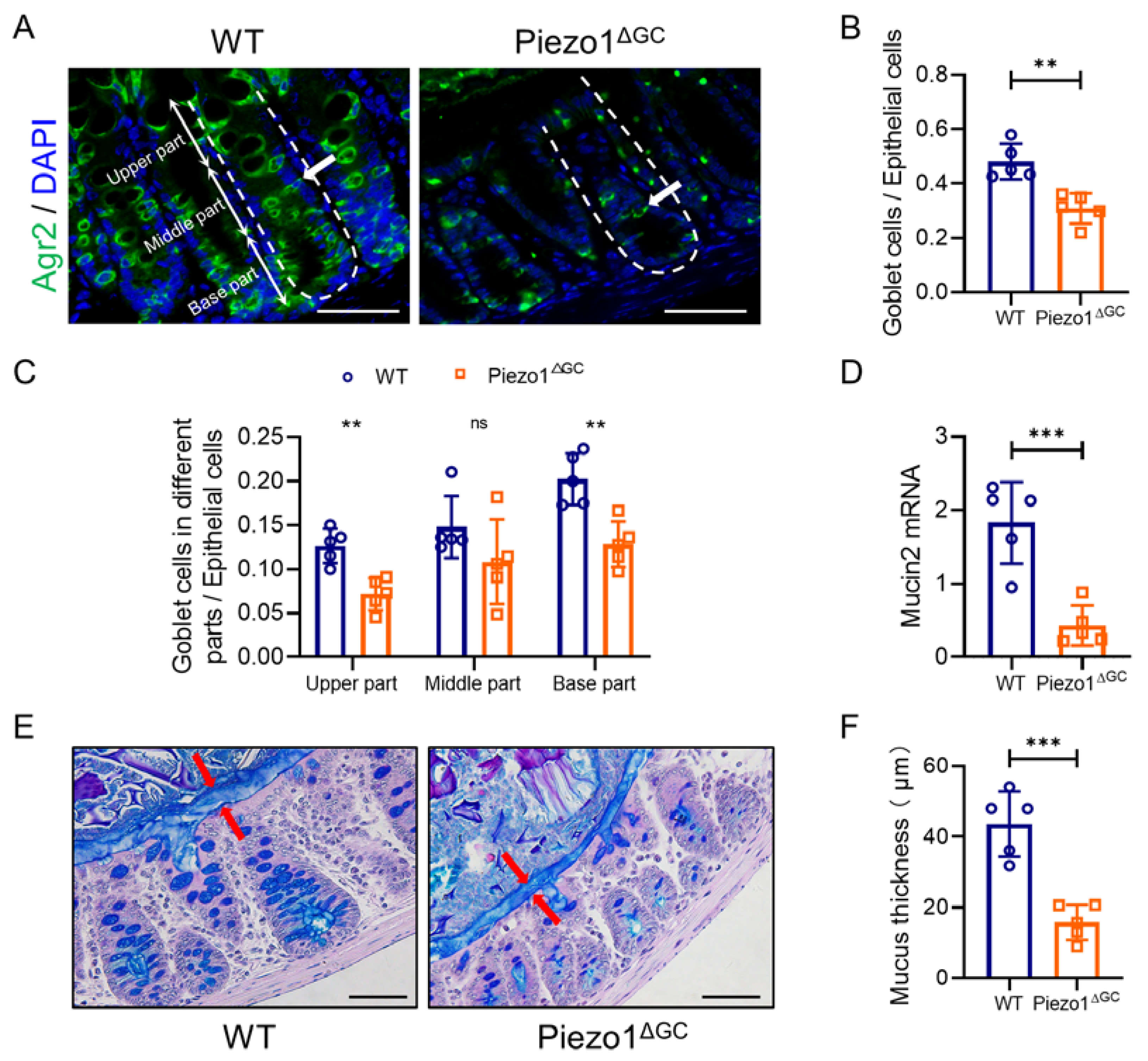
2.1. Decreased Goblet Cell Numbers and Thinner Mucus Layer in Piezo1ΔGC Mouse Colons
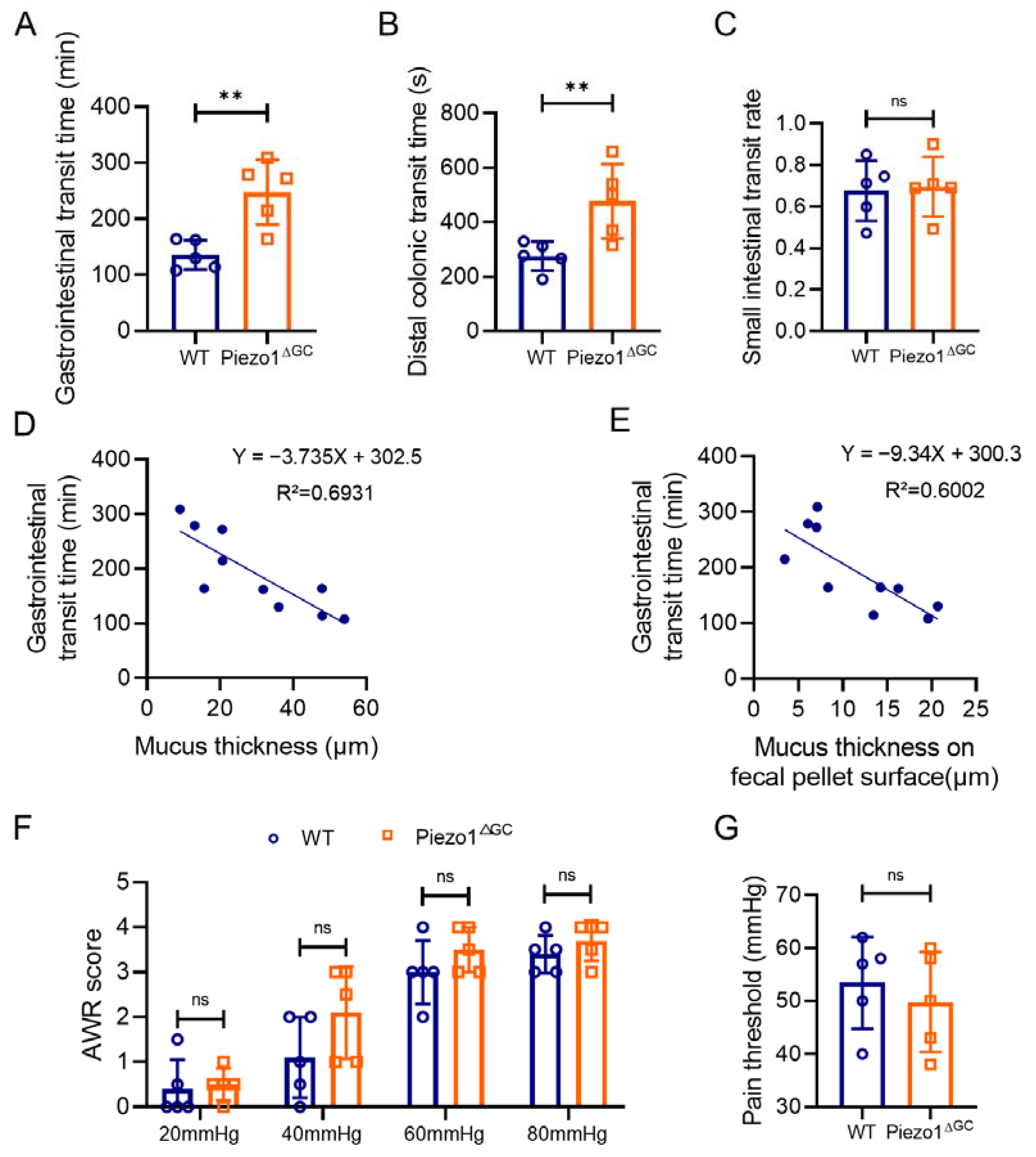
2.2. Decreased Fecal Excretion and Thinning Mucus Layer on the Fecal Pellet Surface in Piezo1ΔGC Mice
2.3. Prolonged Gastrointestinal Transit Time in Piezo1ΔGC Mice Correlates with Intestinal Mucus Thickness
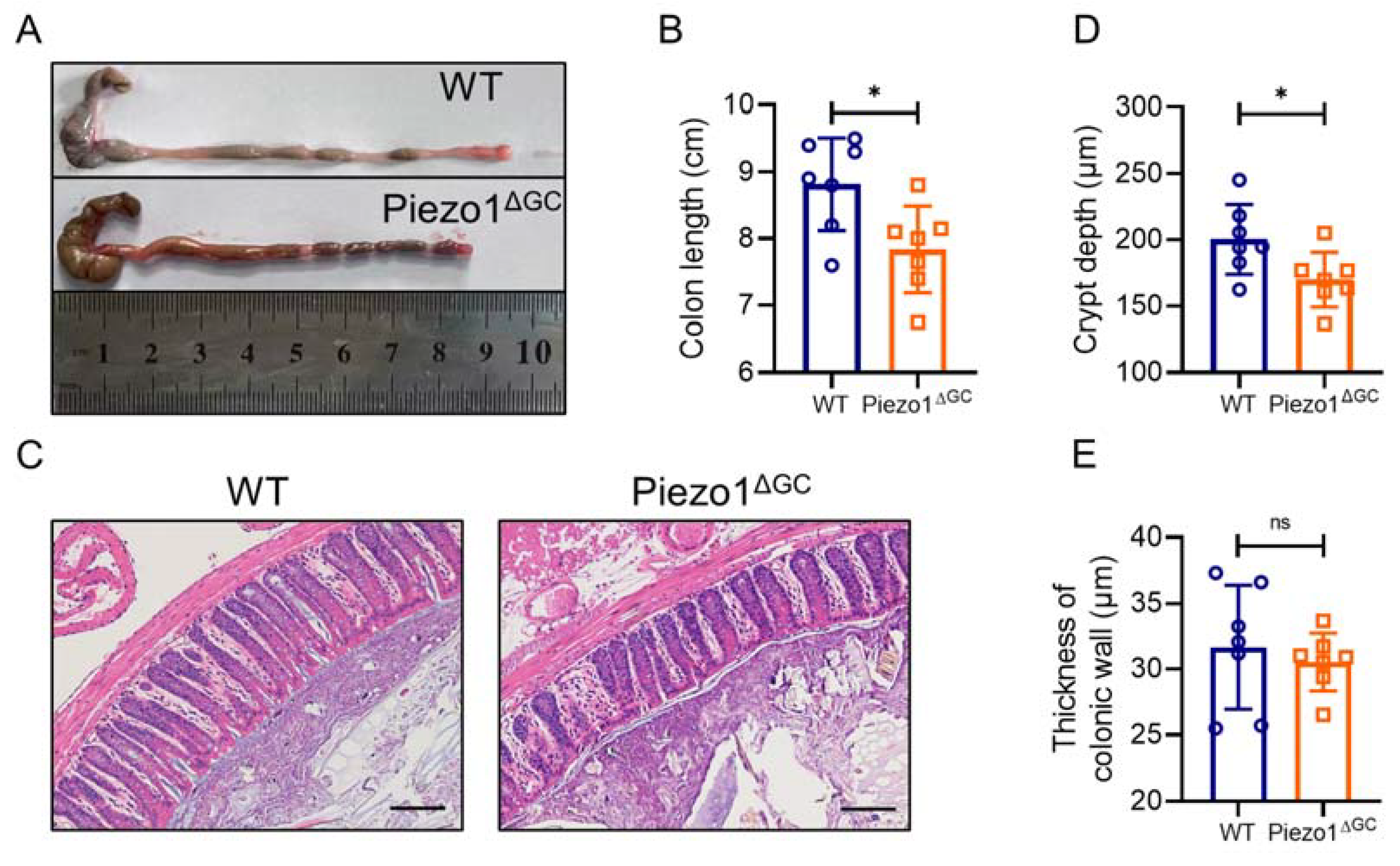
2.4. Abnormal Colonic Development in Piezo1ΔGC Mice
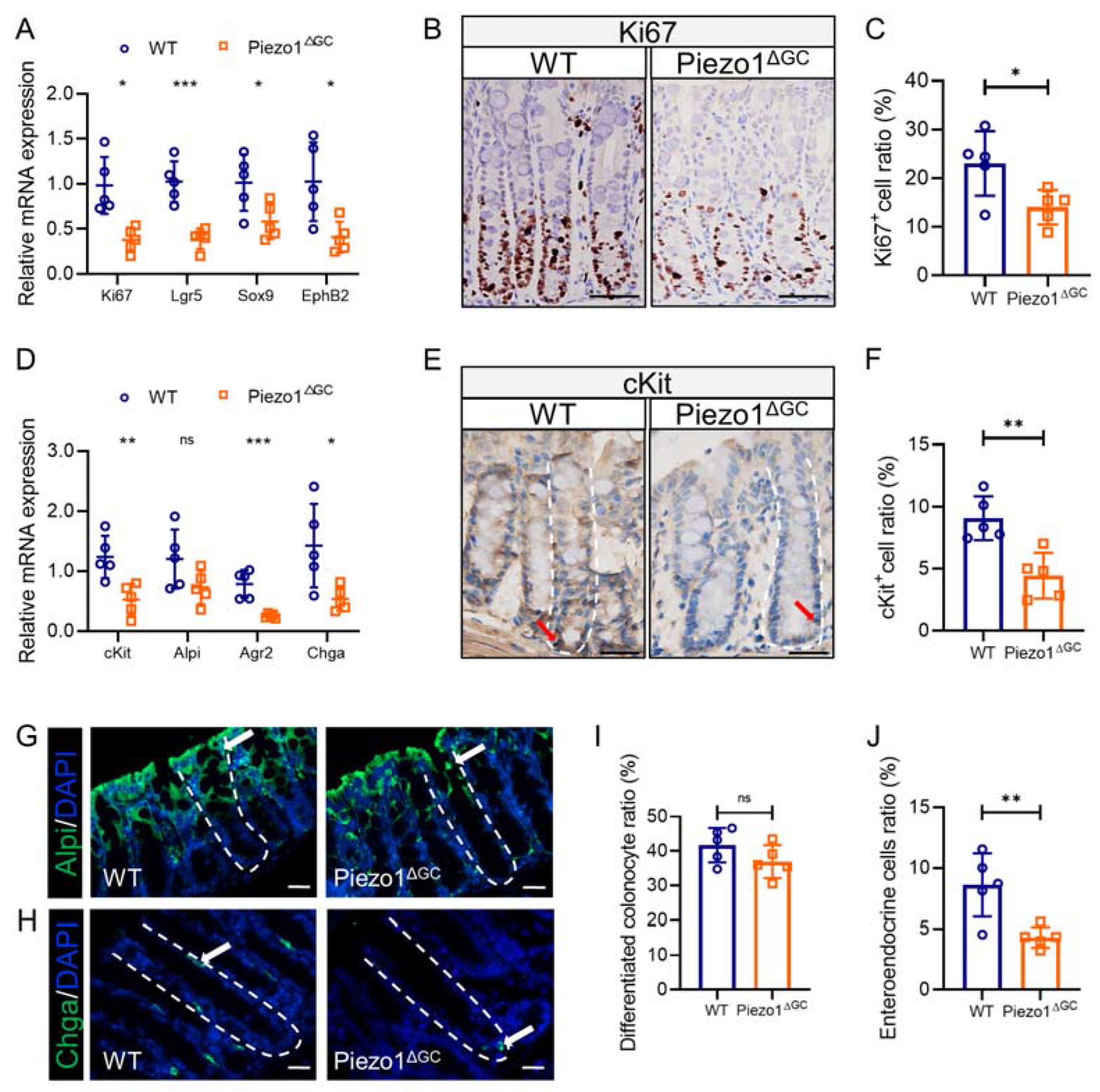
2.5. Abnormal Intestinal Epithelial Cell Composition and Impaired Colon Stem Cell Niche in Piezo1ΔGC Mice
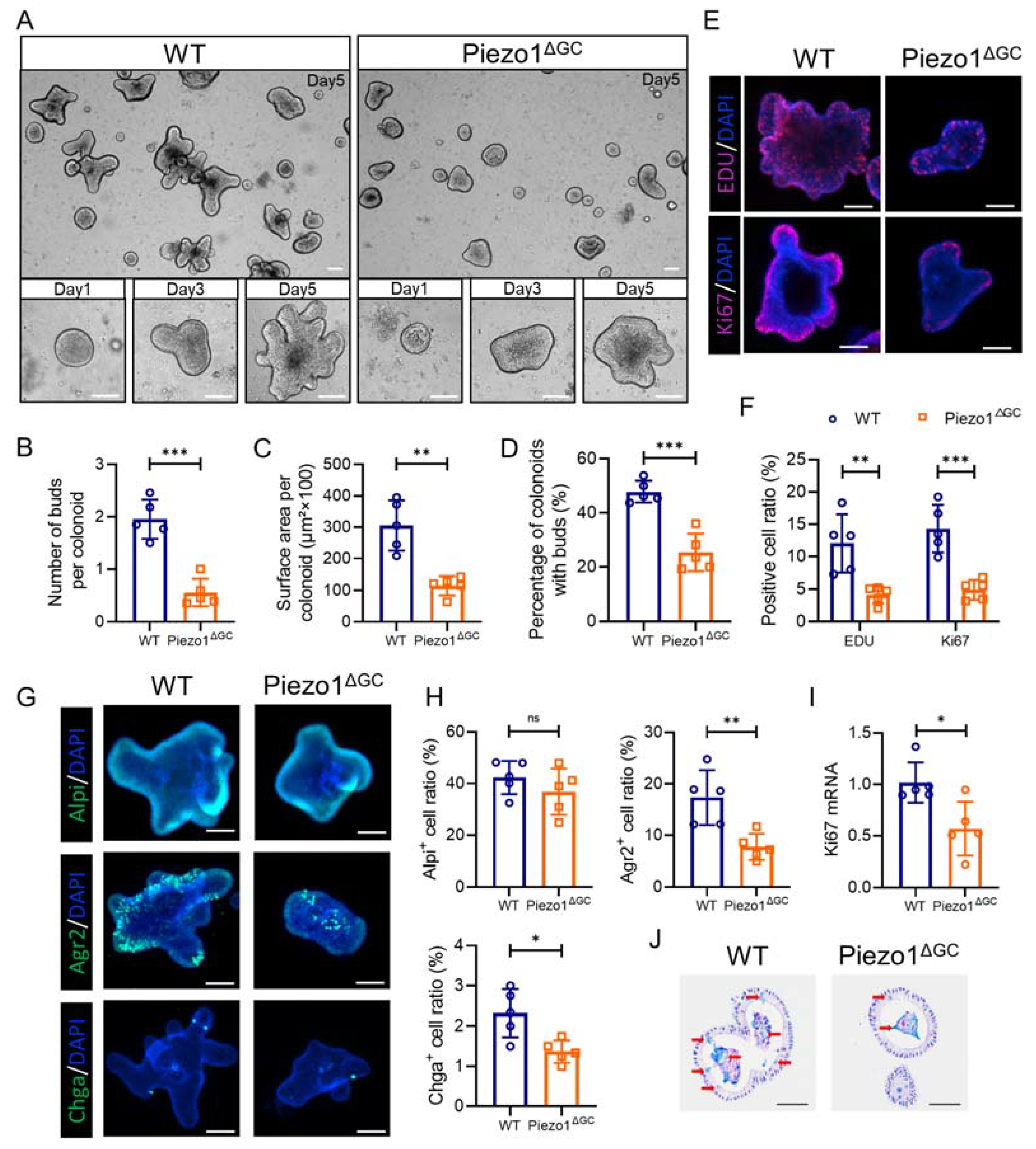
2.6. Decreased Self-Renewal Capacity of Colon Stem Cells from Piezo1ΔGC Mice
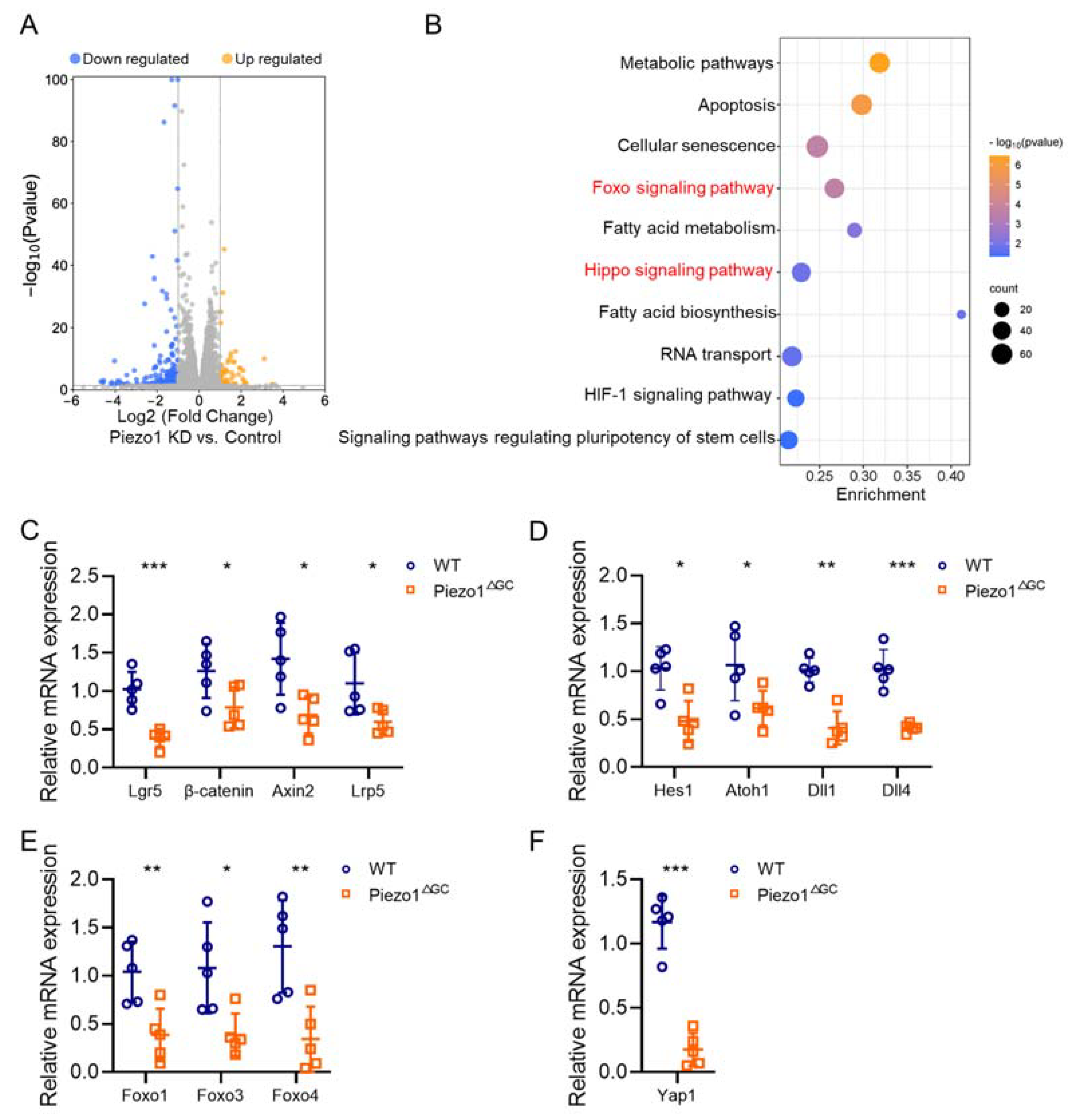
2.7. Reshaped Wnt and Notch Signaling Expression in Piezo1ΔGC Mouse Colon Crypts Leads to Reduced GCs
3. Discussion
4. Materials and Methods
4.1. Mouse Model
4.2. Tissue Immunostaining
4.3. Alcian Blue Staining (AB-PAS Staining)
4.4. Hematoxylin and Eosin (H&E) Staining
4.5. Measurement of Fecal Parameters
4.6. Measurement of Intestinal Transit
4.7. Abdominal Withdrawal Reflex (AWR) Score
4.8. Colonic Smooth Muscle Contraction Test
4.9. Crypt Isolation and Organoid Culture
4.10. Immunofluorescence and EDU Staining in Colonoids
4.11. Cell Culture
4.12. RT-PCR Analysis
4.13. RNA-Seq and Bioinformatics Analysis
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Camilleri, M.; Ford, A.C.; Mawe, G.M.; Dinning, P.G.; Rao, S.S.; Chey, W.D.; Simren, M.; Lembo, A.; Young-Fadok, T.M.; Chang, L. Chronic constipation. Nat. Rev. Dis. Primers 2017, 3, 17095. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xiong, Y.; Liu, Y.; Li, G.; Bai, T.; Zheng, G.; Hou, X.; Song, J. Activation of goblet cell Piezo1 alleviates mucus barrier damage in mice exposed to WAS by inhibiting H3K9me3 modification. Cell Biosci. 2023, 13, 7. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, G.; Chen, J.; Chen, X.; Chia, N.; O’Connor, H.M.; Wolf, P.G.; Gaskins, H.R.; Bharucha, A.E. Relationship Between Microbiota of the Colonic Mucosa vs Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients With Chronic Constipation. Gastroenterology 2016, 150, 367–379.e1. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, P.T.; Vollebregt, P.F.; Knowles, C.H.; Lunniss, P.J.; Dinning, P.G.; Scott, S.M. Understanding the physiology of human defaecation and disorders of continence and evacuation. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 751–769. [Google Scholar] [CrossRef]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Bergstrom, K.; Shan, X.; Casero, D.; Batushansky, A.; Lagishetty, V.; Jacobs, J.P.; Hoover, C.; Kondo, Y.; Shao, B.; Gao, L.; et al. Proximal colon-derived O-glycosylated mucus encapsulates and modulates the microbiota. Science 2020, 370, 467–472. [Google Scholar] [CrossRef]
- Yang, P.J.; LaMarca, M.; Kaminski, C.; Chu, D.I.; Hu, D.L. Hydrodynamics of defecation. Soft Matter 2017, 13, 4960–4970. [Google Scholar] [CrossRef]
- Gao, C.C.; Li, G.W.; Wang, T.T.; Gao, L.; Wang, F.F.; Shang, H.W.; Yang, Z.J.; Guo, Y.X.; Wang, B.Y.; Xu, J.D. Rhubarb extract relieves constipation by stimulating mucus production in the colon and altering the intestinal flora. Biomed. Pharm. 2021, 138, 111479. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, X.; Jiang, J.; Xiao, B. Structural Designs and Mechanogating Mechanisms of the Mechanosensitive Piezo Channels. Trends Biochem. Sci. 2021, 46, 472–488. [Google Scholar] [CrossRef]
- Mercado-Perez, A.; Beyder, A. Gut feelings: Mechanosensing in the gastrointestinal tract. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 283–296. [Google Scholar] [CrossRef]
- Retailleau, K.; Duprat, F.; Arhatte, M.; Ranade, S.S.; Peyronnet, R.; Martins, J.R.; Jodar, M.; Moro, C.; Offermanns, S.; Feng, Y.; et al. Piezo1 in Smooth Muscle Cells Is Involved in Hypertension-Dependent Arterial Remodeling. Cell Rep. 2015, 13, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; You, X.; Lotinun, S.; Zhang, L.; Wu, N.; Zou, W. Mechanical sensing protein PIEZO1 regulates bone homeostasis via osteoblast-osteoclast crosstalk. Nat. Commun. 2020, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Solis, A.G.; Bielecki, P.; Steach, H.R.; Sharma, L.; Harman, C.C.D.; Yun, S.; de Zoete, M.R.; Warnock, J.N.; To, S.D.F.; York, A.G.; et al. Author Correction: Mechanosensation of cyclical force by PIEZO1 is essential for innate immunity. Nature 2019, 575, E7. [Google Scholar] [CrossRef]
- Geng, J.; Shi, Y.; Zhang, J.; Yang, B.; Wang, P.; Yuan, W.; Zhao, H.; Li, J.; Qin, F.; Hong, L.; et al. TLR4 signalling via Piezo1 engages and enhances the macrophage mediated host response during bacterial infection. Nat. Commun. 2021, 12, 3519. [Google Scholar] [CrossRef]
- Koser, D.E.; Thompson, A.J.; Foster, S.K.; Dwivedy, A.; Pillai, E.K.; Sheridan, G.K.; Svoboda, H.; Viana, M.; Costa, L.D.; Guck, J.; et al. Mechanosensing is critical for axon growth in the developing brain. Nat. Neurosci. 2016, 19, 1592–1598. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Hou, F.; Fang, J.; Bahrani Fard, M.R.; Liu, Y.; Ren, S.; Wu, S.; Qi, Y.; Sui, S.; Read, A.T.; et al. The role of Piezo1 in conventional aqueous humor outflow dynamics. iScience 2021, 24, 102042. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Chi, S.; Li, Y.; Ling, S.; Tan, Y.; Xu, Y.; Jiang, F.; Li, J.; Liu, C.; Zhong, G.; et al. The mechanosensitive Piezo1 channel is required for bone formation. eLife 2019, 8, e47454. [Google Scholar] [CrossRef]
- Romac, J.M.; Shahid, R.A.; Swain, S.M.; Vigna, S.R.; Liddle, R.A. Piezo1 is a mechanically activated ion channel and mediates pressure induced pancreatitis. Nat. Commun. 2018, 9, 1715. [Google Scholar] [CrossRef]
- Yu, J.L.; Liao, H.Y. Piezo-type mechanosensitive ion channel component 1 (Piezo1) in human cancer. Biomed. Pharm. 2021, 140, 111692. [Google Scholar] [CrossRef]
- Gustafsson, J.K.; Johansson, M.E.V. The role of goblet cells and mucus in intestinal homeostasis. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 785–803. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, F.; Xiong, Y.; Wu, J.; Li, X.; Li, G.; Bai, T.; Hou, X.; Song, J. Reprogrammed fecal and mucosa-associated intestinal microbiota and weakened mucus layer in intestinal goblet cell—Specific Piezo1-deficient mice. Front. Cell Infect. Microbiol. 2022, 12, 1035386. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, M.E.; Nusse, Y.; Kalisky, T.; Lee, J.J.; Dalerba, P.; Scheeren, F.; Lobo, N.; Kulkarni, S.; Sim, S.; Qian, D.; et al. Identification of a cKit(+) colonic crypt base secretory cell that supports Lgr5(+) stem cells in mice. Gastroenterology 2012, 142, 1195–1205.e6. [Google Scholar] [CrossRef] [PubMed]
- Houtekamer, R.M.; van der Net, M.C.; Maurice, M.M.; Gloerich, M. Mechanical forces directing intestinal form and function. Curr. Biol. 2022, 32, R791–R805. [Google Scholar] [CrossRef] [PubMed]
- Gudipaty, S.A.; Lindblom, J.; Loftus, P.D.; Redd, M.J.; Edes, K.; Davey, C.F.; Krishnegowda, V.; Rosenblatt, J. Mechanical stretch triggers rapid epithelial cell division through Piezo1. Nature 2017, 543, 118–121. [Google Scholar] [CrossRef]
- Ludikhuize, M.C.; Meerlo, M.; Gallego, M.P.; Xanthakis, D.; Burgaya Julia, M.; Nguyen, N.T.B.; Brombacher, E.C.; Liv, N.; Maurice, M.M.; Paik, J.H.; et al. Mitochondria Define Intestinal Stem Cell Differentiation Downstream of a FOXO/Notch Axis. Cell Metab. 2020, 32, 889–900.e7. [Google Scholar] [CrossRef]
- Beumer, J.; Clevers, H. Cell fate specification and differentiation in the adult mammalian intestine. Nat. Rev. Mol. Cell Biol. 2021, 22, 39–53. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, T.; Xiong, Y.; Liu, C.; Liu, Y.; Hou, X.; Song, J. Mechanical stimulation activates Piezo1 to promote mucin2 expression in goblet cells. J. Gastroenterol. Hepatol. 2021, 36, 3127–3139. [Google Scholar] [CrossRef]
- Tao, Y.; Qiao, S.M.; Lv, C.J.; Yun, X.M.; Yue, M.F.; Fang, Y.L.; Wei, Z.F.; Dai, Y.; Xia, Y.F. Phytoestrogen arctigenin preserves the mucus barrier in inflammatory bowel diseases by inhibiting goblet cell apoptosis via the ERbeta/TRIM21/PHB1 pathway. Phytother Res. 2022, 36, 3248–3264. [Google Scholar] [CrossRef]
- Chen, L.; Jiao, T.; Liu, W.; Luo, Y.; Wang, J.; Guo, X.; Tong, X.; Lin, Z.; Sun, C.; Wang, K.; et al. Hepatic cytochrome P450 8B1 and cholic acid potentiate intestinal epithelial injury in colitis by suppressing intestinal stem cell renewal. Cell Stem. Cell 2022, 29, 1366–1381.e9. [Google Scholar] [CrossRef]
- Eisenhoffer, G.T.; Loftus, P.D.; Yoshigi, M.; Otsuna, H.; Chien, C.B.; Morcos, P.A.; Rosenblatt, J. Crowding induces live cell extrusion to maintain homeostatic cell numbers in epithelia. Nature 2012, 484, 546–549. [Google Scholar] [CrossRef]
- Duckworth, C.A. Identifying key regulators of the intestinal stem cell niche. Biochem. Soc. Trans. 2021, 49, 2163–2176. [Google Scholar] [CrossRef] [PubMed]
- Huelsz-Prince, G.; Kok, R.N.U.; Goos, Y.; Bruens, L.; Zheng, X.; Ellenbroek, S.; Van Rheenen, J.; Tans, S.; van Zon, J.S. Mother cells control daughter cell proliferation in intestinal organoids to minimize proliferation fluctuations. eLife 2022, 11, e80682. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, H.; Zheng, J.; Jiang, N.; Sun, G.; Bao, X.; Lin, A.; Liu, H. Chitosan oligosaccharides attenuate loperamide-induced constipation through regulation of gut microbiota in mice. Carbohydr. Polym. 2021, 253, 117218. [Google Scholar] [CrossRef] [PubMed]
- Ermund, A.; Schutte, A.; Johansson, M.E.; Gustafsson, J.K.; Hansson, G.C. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 305, G341–G347. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.K.; Wei, W.; Zhao, D.Y.; Wang, H.F.; Zhang, Y.L.; Lei, J.P.; Yao, S.K. Intestinal mucosal barrier in functional constipation: Dose it change? World J. Clin. Cases 2022, 10, 6385–6398. [Google Scholar] [CrossRef]
- Pellegrinet, L.; Rodilla, V.; Liu, Z.; Chen, S.; Koch, U.; Espinosa, L.; Kaestner, K.H.; Kopan, R.; Lewis, J.; Radtke, F. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology 2011, 140, 1230–1240.e7. [Google Scholar] [CrossRef]
- Hong, A.W.; Meng, Z.; Guan, K.L. The Hippo pathway in intestinal regeneration and disease. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 324–337. [Google Scholar] [CrossRef]
- Ward, D.; Montes Olivas, S.; Fletcher, A.; Homer, M.; Marucci, L. Cross-talk between Hippo and Wnt signalling pathways in intestinal crypts: Insights from an agent-based model. Comput. Struct. Biotechnol. J. 2020, 18, 230–240. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, Y.; Wu, H.; Barry, E.; Yin, Y.; Lawrence, E.; Dawson, D.; Willis, J.E.; Markowitz, S.D.; Camargo, F.D.; et al. Mst1 and Mst2 protein kinases restrain intestinal stem cell proliferation and colonic tumorigenesis by inhibition of Yes-associated protein (Yap) overabundance. Proc. Natl. Acad. Sci. USA 2011, 108, E1312–E1320. [Google Scholar] [CrossRef]
- Gopinath, S.D.; Webb, A.E.; Brunet, A.; Rando, T.A. FOXO3 promotes quiescence in adult muscle stem cells during the process of self-renewal. Stem. Cell Rep. 2014, 2, 414–426. [Google Scholar] [CrossRef]
- Kim, D.Y.; Hwang, I.; Muller, F.L.; Paik, J.H. Functional regulation of FoxO1 in neural stem cell differentiation. Cell Death Differ. 2015, 22, 2034–2045. [Google Scholar] [CrossRef] [PubMed]
- Ludikhuize, M.C.; Rodriguez Colman, M.J. Metabolic Regulation of Stem Cells and Differentiation: A Forkhead Box O Transcription Factor Perspective. Antioxid Redox Signal 2021, 34, 1004–1024. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Su, S.; Li, J.; Zhao, H.; Hu, D.; Chen, J.; Li, S.; Lin, Y.; Wen, L.; Lin, X.; et al. Activation of Piezo1 promotes osteogenic differentiation of aortic valve interstitial cell through YAP-dependent glutaminolysis. Sci. Adv. 2023, 9, eadg0478. [Google Scholar] [CrossRef]
- Daoud, F.; Holmberg, J.; Alajbegovic, A.; Grossi, M.; Rippe, C.; Sward, K.; Albinsson, S. Inducible Deletion of YAP and TAZ in Adult Mouse Smooth Muscle Causes Rapid and Lethal Colonic Pseudo-Obstruction. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 623–637. [Google Scholar] [CrossRef]
- Sugisawa, E.; Takayama, Y.; Takemura, N.; Kondo, T.; Hatakeyama, S.; Kumagai, Y.; Sunagawa, M.; Tominaga, M.; Maruyama, K. RNA Sensing by Gut Piezo1 Is Essential for Systemic Serotonin Synthesis. Cell 2020, 182, 609–624.e21. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, S.; Taguchi, M.; Sumi, S.; Oka, K.; Okamura, K. Establishment of a novel protocol for formalin-fixed paraffin-embedded organoids and spheroids. Biol. Open 2023, 12, bio059882. [Google Scholar] [CrossRef] [PubMed]
- Israelyan, N.; Del Colle, A.; Li, Z.; Park, Y.; Xing, A.; Jacobsen, J.P.R.; Luna, R.A.; Jensen, D.D.; Madra, M.; Saurman, V.; et al. Effects of Serotonin and Slow-Release 5-Hydroxytryptophan on Gastrointestinal Motility in a Mouse Model of Depression. Gastroenterology 2019, 157, 507–521.e4. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, D.; Lei, Z.; Ge, P.; Lu, Z.; Chai, Q.; Zhang, Y.; Qiang, L.; Yu, Y.; Zhang, X.; et al. TRIM27 maintains gut homeostasis by promoting intestinal stem cell self-renewal. Cell. Mol. Immunol. 2023, 20, 158–174. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, R.; Bai, T.; Xiang, X.; Qian, W.; Song, J.; Hou, X. EphrinB2/ephB2-mediated myenteric synaptic plasticity: Mechanisms underlying the persistent muscle hypercontractility and pain in postinfectious IBS. FASEB J. 2019, 33, 13644–13659. [Google Scholar] [CrossRef]
- Mahe, M.M.; Sundaram, N.; Watson, C.L.; Shroyer, N.F.; Helmrath, M.A. Establishment of human epithelial enteroids and colonoids from whole tissue and biopsy. J. Vis. Exp. (JoVE) 2015, 97, e52483. [Google Scholar] [CrossRef]
- Lindemans, C.A.; Calafiore, M.; Mertelsmann, A.M.; O’Connor, M.H.; Dudakov, J.A.; Jenq, R.R.; Velardi, E.; Young, L.F.; Smith, O.M.; Lawrence, G.; et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature 2015, 528, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]

| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| GAPDH | AACAGCAACTCCCACTCTTC | CCTGTTGCTGTAGCCGTATT |
| Piezo1 | TAACACCCTCTGTGTGTCATGGT | GGGAGGTCTACGAAGTTCTTGAG |
| Mucin2 | ATGCCCACCTCCTCAAAGAC | GTAGTTTCCGTTGGAACAGTGAA |
| Alpi | GGCCATCTAGGACCGGAGA | TGTCCACGTTGTATGTCTTGG |
| Chga | CCAAGGTGATGAAGTGCGTC | GGTGTCGCAGGATAGAGAGGA |
| cKit | GACCCGACGCAACTTCCTTA | GAGCATCTTCACGGCAACTGT |
| Ki67 | ATCATTGACCGCTCCTTTAGGT | GCTCGCCTTGATGGTTCCT |
| Lgr5 | GACGCTGGGTTATTTCAAGTTCAA | CAGCCAGCTACCAAATAGGTGCTC |
| Sox9 | GAGCCGGATCTGAAGAGGGA | GCTTGACGTGTGGCTTGTTC |
| EphB2 | CCATTGAACAGGACTACAGACTACC | CACCGTGTTAAAGCTGGTGTAG |
| β-catenin | ATGGAGCCGGACAGAAAAGC | TGGGAGGTGTCAACATCTTCT |
| Axin2 | GGACTGGGGAGCCTAAAGGT | AAGGAGGGACTCCATCTACGC |
| Lrp5 | AAGGGTGCTGTGTACTGGAC | AGAAGAGAACCTTACGGGACG |
| Hes1 | TCAACACGACACCGGACAAAC | ATGCCGGGAGCTATCTTTCTT |
| Atoh1 | CAGGGTGAGCTGGTAAGGAG | GCCAAGCTCGTCCACTACA |
| Dll1 | GCGACTGAGGTGTAAGATGGAA | TCTCAGCAGCATTCATCGGG |
| Dll4 | TTCCAGGCAACCTTCTCCGA | ACTGCCGCTATTCTTGTCCC |
| Yap1 | CGCTCTTCAATGCCGTCATG | AGTCATGGCTTGCTCCCATC |
| Foxo1 | AGGATAAGGGCGACAGCAAC | CTTGCCTCCCTCTGGATTGA |
| Foxo3 | TTGGTCAATCAGAACTTGCT | CCCATGTTGCTGACAGAAT |
| Foxo4 | GGTGCCCTACTTCAAGGACA | AGCTTGCTGCTGCTATCCAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, F.; Liu, Y.; Xiong, Y.; Li, X.; Li, G.; Jiang, Y.; Hou, X.; Song, J. Slowed Intestinal Transit Induced by Less Mucus in Intestinal Goblet Cell Piezo1-Deficient Mice through Impaired Epithelial Homeostasis. Int. J. Mol. Sci. 2023, 24, 14377. https://doi.org/10.3390/ijms241814377
Fang F, Liu Y, Xiong Y, Li X, Li G, Jiang Y, Hou X, Song J. Slowed Intestinal Transit Induced by Less Mucus in Intestinal Goblet Cell Piezo1-Deficient Mice through Impaired Epithelial Homeostasis. International Journal of Molecular Sciences. 2023; 24(18):14377. https://doi.org/10.3390/ijms241814377
Chicago/Turabian StyleFang, Feifei, Ying Liu, Yilin Xiong, Xueyan Li, Gangping Li, Yudong Jiang, Xiaohua Hou, and Jun Song. 2023. "Slowed Intestinal Transit Induced by Less Mucus in Intestinal Goblet Cell Piezo1-Deficient Mice through Impaired Epithelial Homeostasis" International Journal of Molecular Sciences 24, no. 18: 14377. https://doi.org/10.3390/ijms241814377
APA StyleFang, F., Liu, Y., Xiong, Y., Li, X., Li, G., Jiang, Y., Hou, X., & Song, J. (2023). Slowed Intestinal Transit Induced by Less Mucus in Intestinal Goblet Cell Piezo1-Deficient Mice through Impaired Epithelial Homeostasis. International Journal of Molecular Sciences, 24(18), 14377. https://doi.org/10.3390/ijms241814377





