Potential Molecular Markers Related to Lymph Node Metastasis and Stalk Resection Margins in Pedunculated T1 Colorectal Cancers Using Digital Spatial Profiling: A Pilot Study with a Small Case Series
Abstract
1. Introduction
2. Results
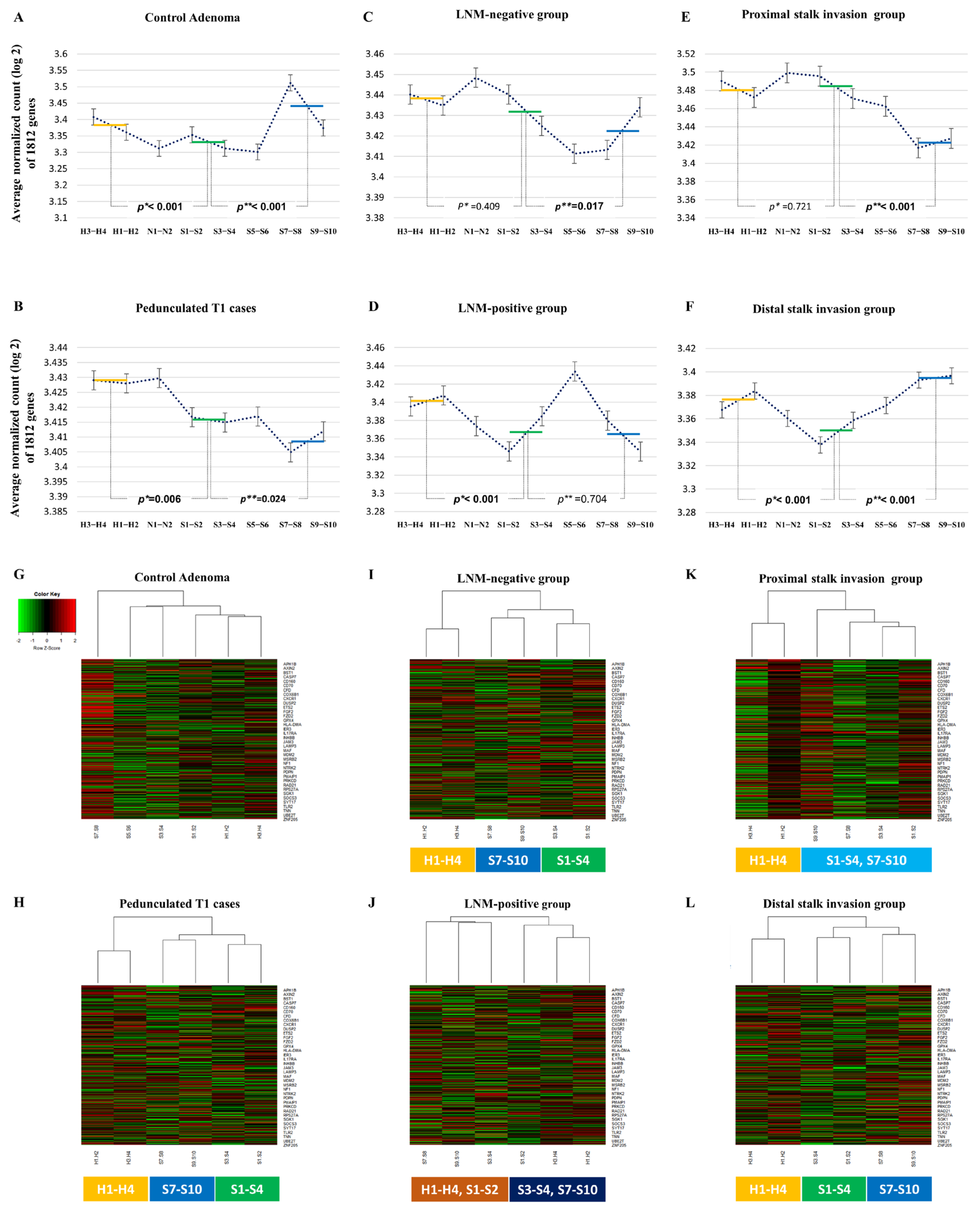
2.1. The Overall Gene Expression Pattern Related Distance from Haggitt Line
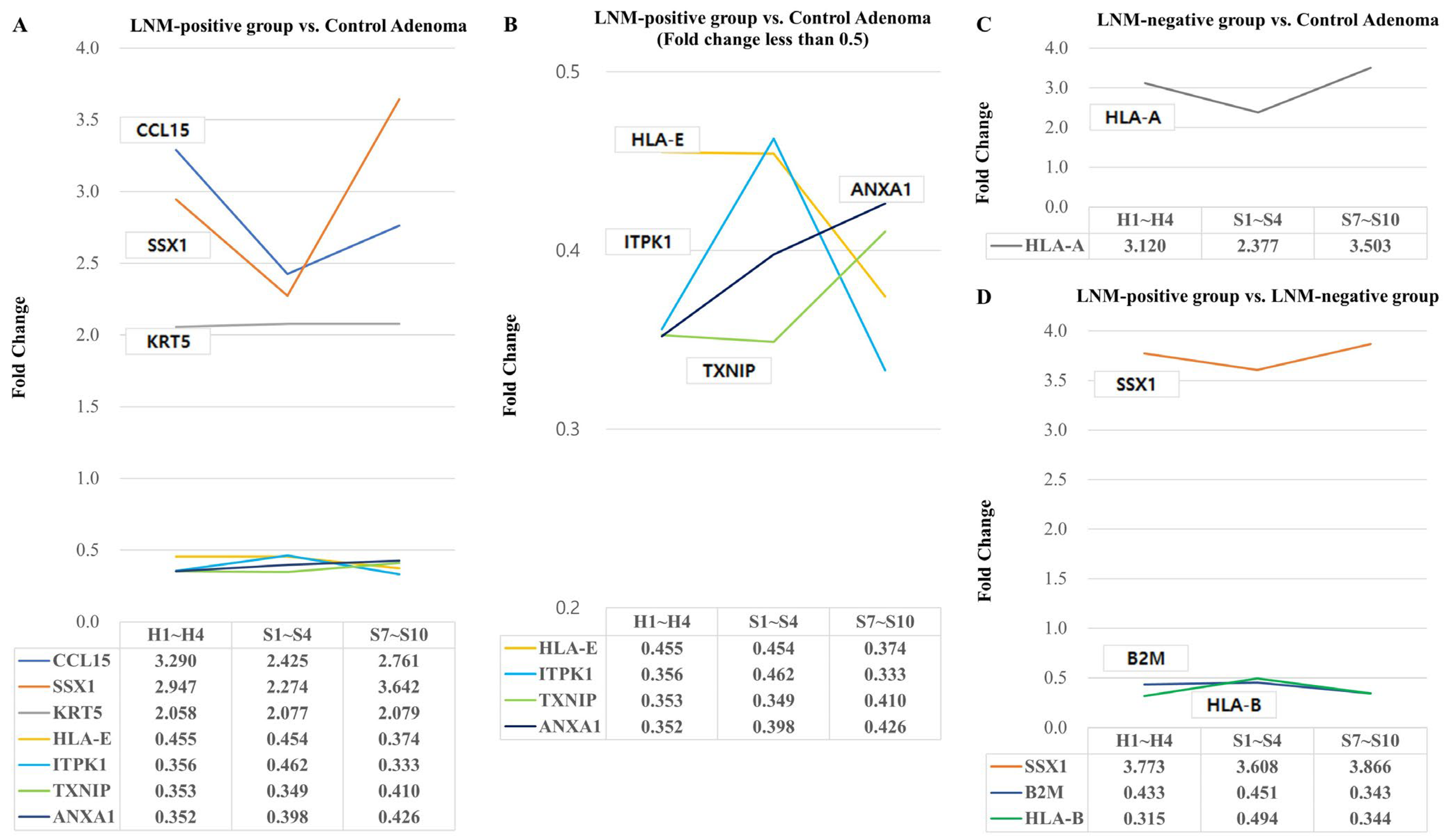
2.2. Differentially Expressed Genes Associated with Lymph Node Metastasis
2.3. Differentially Expressed Genes Associated with Proximal and Distal Stalk Invasion
2.4. KEGG Pathway Analysis of Differentially Expressed Genes
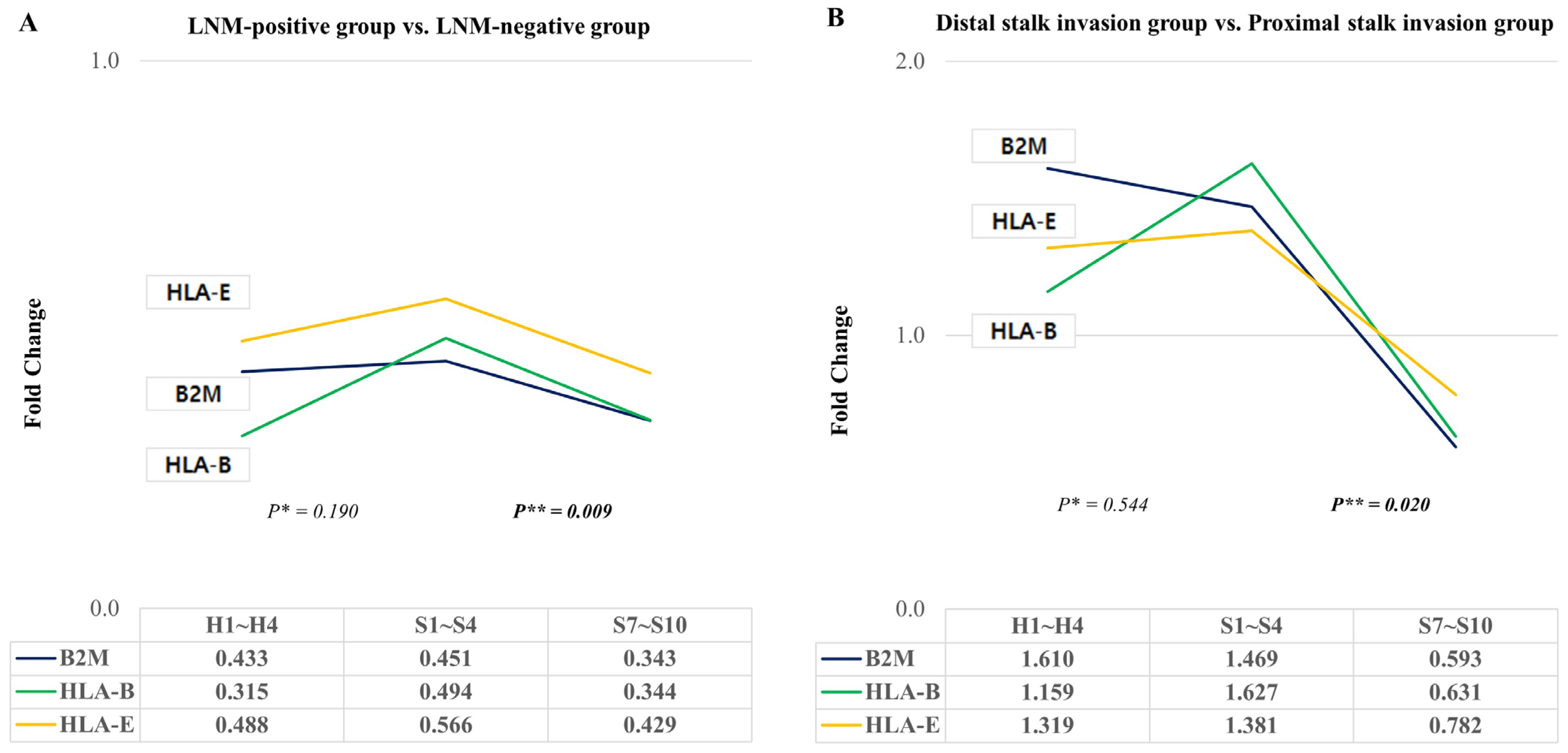
2.5. Changes in HLA-E, B2M, and HLA-B According to Lymph Node Metastasis or Stalk Invasion
3. Discussion
4. Materials and Methods
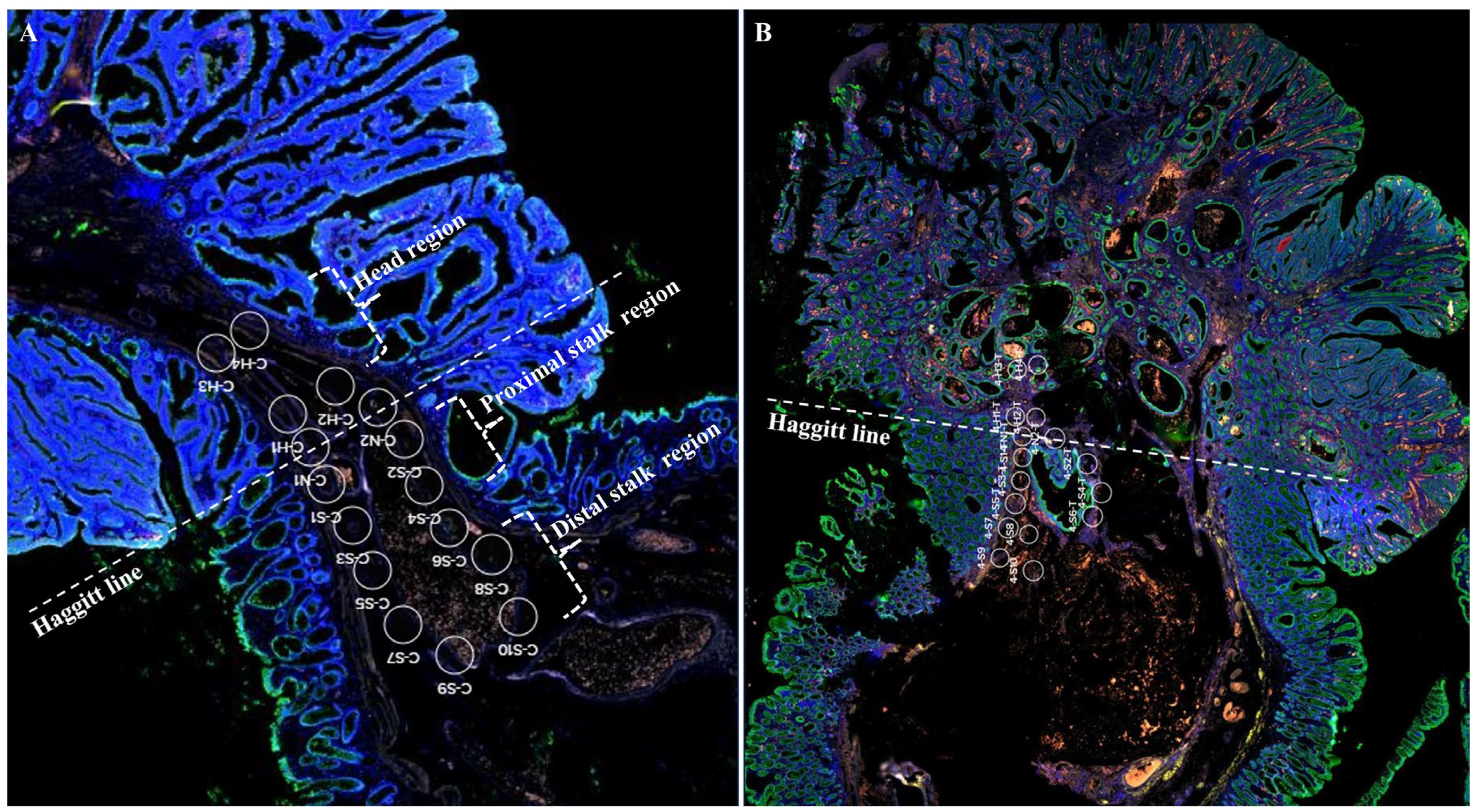
4.1. Patients and Histologic Evaluation
4.2. Digital Spatial Profiling and nCounter Data Analysis
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ichimasa, K.; Kudo, S.-E.; Miyachi, H.; Kouyama, Y.; Misawa, M.; Mori, Y. Risk Stratification of T1 Colorectal Cancer Metastasis to Lymph Nodes: Current Status and Perspective. Gut Liver 2021, 15, 818–826. [Google Scholar] [CrossRef]
- Bartel, M.J.; Brahmbhatt, B.S.; Wallace, M.B. Management of colorectal T1 carcinoma treated by endoscopic resection from the Western perspective. Dig. Endosc. 2016, 28, 330–341. [Google Scholar] [CrossRef] [PubMed]
- The Paris endoscopic classification of superficial neoplastic lesions: Esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest. Endosc. 2003, 58, S3–S43. [CrossRef]
- Backes, Y.; Elias, S.G.; Groen, J.N.; Schwartz, M.P.; Wolfhagen, F.H.J.; Geesing, J.M.J.; Ter Borg, F.; van Bergeijk, J.; Spanier, B.W.M.; de Vos Tot Nederveen Cappel, W.H.; et al. Histologic Factors Associated with Need for Surgery in Patients with Pedunculated T1 Colorectal Carcinomas. Gastroenterology 2018, 154, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- Backes, Y.; de Vos Tot Nederveen Cappel, W.H.; van Bergeijk, J.; Ter Borg, F.; Schwartz, M.P.; Spanier, B.W.M.; Geesing, J.M.J.; Kessels, K.; Kerkhof, M.; Groen, J.N.; et al. Risk for Incomplete Resection after Macroscopic Radical Endoscopic Resection of T1 Colorectal Cancer: A Multicenter Cohort Study. Am. J. Gastroenterol. 2017, 112, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Fukuzawa, M.; Uraoka, T.; Nishi, M.; Yamaguchi, Y.; Kobayashi, N.; Ikematsu, H.; Saito, Y.; Nakajima, T.; Fujii, T.; et al. Risk of lymph node metastasis in patients with pedunculated type early invasive colorectal cancer: A retrospective multicenter study. Cancer Sci. 2011, 102, 1693–1697. [Google Scholar] [CrossRef] [PubMed]
- Asayama, N.; Oka, S.; Tanaka, S.; Nagata, S.; Furudoi, A.; Kuwai, T.; Onogawa, S.; Tamura, T.; Kanao, H.; Hiraga, Y.; et al. Long-term outcomes after treatment for pedunculated-type T1 colorectal carcinoma: A multicenter retrospective cohort study. J. Gastroenterol. 2016, 51, 702–710. [Google Scholar] [CrossRef]
- Backes, Y.; Moons, L.M.; Novelli, M.R.; van Bergeijk, J.D.; Groen, J.N.; Seerden, T.C.; Schwartz, M.P.; de Vos Tot Nederveen Cappel, W.H.; Spanier, B.W.; Geesing, J.M.; et al. Diagnosis of T1 colorectal cancer in pedunculated polyps in daily clinical practice: A multicenter study. Mod. Pathol. 2017, 30, 104–112. [Google Scholar] [CrossRef]
- Sohn, D.K.; Chang, H.J.; Park, J.W.; Choi, D.H.; Han, K.S.; Hong, C.W.; Jung, K.H.; Kim, D.Y.; Lim, S.B.; Choi, H.S.; et al. Histopathological risk factors for lymph node metastasis in submucosal invasive colorectal carcinoma of pedunculated or semipedunculated type. J. Clin. Pathol. 2007, 60, 912–915. [Google Scholar] [CrossRef]
- Haggitt, R.C.; Glotzbach, R.E.; Soffer, E.E.; Wruble, L.D. Prognostic factors in colorectal carcinomas arising in adenomas: Implications for lesions removed by endoscopic polypectomy. Gastroenterology 1985, 89, 328–336. [Google Scholar] [CrossRef]
- Kimura, Y.J.; Kudo, S.E.; Miyachi, H.; Ichimasa, K.; Kouyama, Y.; Misawa, M.; Sato, Y.; Matsudaira, S.; Oikawa, H.; Hisayuki, T.; et al. ‘Head Invasion’ Is Not a Metastasis-Free Condition in Pedunculated T1 Colorectal Carcinomas Based on the Precise Histopathological Assessment. Digestion 2016, 94, 166–175. [Google Scholar] [CrossRef]
- Jeon, M.H.; Jang, S.W.; Lee, C.M.; Kim, S.B. Early Colon Cancer Recurring as Liver Metastasis without Local Recurrence Three Years after Complete Endoscopic Mucosal Resection. Case Rep. Gastroenterol. 2019, 13, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Ciocalteu, A.; Gheonea, D.I.; Saftoiu, A.; Streba, L.; Dragoescu, N.A.; Tenea-Cojan, T.S. Current strategies for malignant pedunculated colorectal polyps. World J. Gastrointest. Oncol. 2018, 10, 465–475. [Google Scholar] [CrossRef]
- Shaukat, A.; Kaltenbach, T.; Dominitz, J.A.; Robertson, D.J.; Anderson, J.C.; Cruise, M.; Burke, C.A.; Gupta, S.; Lieberman, D.; Syngal, S.; et al. Endoscopic Recognition and Management Strategies for Malignant Colorectal Polyps: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2020, 159, 1916–1934.e1912. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.S.; Kwon, M.J.; Haynes, P.; Liang, Y.; Ren, Y.; Lim, H.; Soh, J.S.; Kim, N.Y.; Lee, H.K. Molecular risk markers related to local tumor recurrence at histological margin-free endoscopically resected early gastric cancers: A pilot study. Pathol. Res. Pract. 2021, 222, 153434. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.M.; Fropf, R.; Kulasekara, B.R.; Griswold, M.; Appelbe, O.; Bahrami, A.; Boykin, R.; Buhr, D.L.; Fuhrman, K.; Hoang, M.L.; et al. Spatially resolved whole transcriptome profiling in human and mouse tissue using Digital Spatial Profiling. Genome Res. 2022, 32, 1892–1905. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, M.; Monti, M.; Pezzola, D.C.; Lonardi, S.; Bugatti, M.; Missale, F.; Cioncada, R.; Melocchi, L.; Giustini, V.; Villanacci, V.; et al. Immuno-Contexture and Immune Checkpoint Molecule Expression in Mismatch Repair Proficient Colorectal Carcinoma. Cancers 2023, 15, 3097. [Google Scholar] [CrossRef]
- Mizuki, N.; Ando, H.; Kimura, M.; Ohno, S.; Miyata, S.; Yamazaki, M.; Tashiro, H.; Watanabe, K.; Ono, A.; Taguchi, S.; et al. Nucleotide sequence analysis of the HLA class I region spanning the 237-kb segment around the HLA-B and -C genes. Genomics 1997, 42, 55–66. [Google Scholar] [CrossRef]
- Anderson, P.; Aptsiauri, N.; Ruiz-Cabello, F.; Garrido, F. HLA class I loss in colorectal cancer: Implications for immune escape and immunotherapy. Cell Mol. Immunol. 2021, 18, 556–565. [Google Scholar] [CrossRef]
- Najafimehr, H.; Hajizadeh, N.; Nazemalhosseini-Mojarad, E.; Pourhoseingholi, M.A.; Abdollahpour-Alitappeh, M.; Ashtari, S.; Zali, M.R. The role of Human leukocyte antigen class I on patient survival in Gastrointestinal cancers: A systematic review and meta- analysis. Sci. Rep. 2020, 10, 728. [Google Scholar] [CrossRef]
- Garrido, F.; Cabrera, T.; Concha, A.; Glew, S.; Ruiz-Cabello, F.; Stern, P.L. Natural history of HLA expression during tumour development. Immunol. Today 1993, 14, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Bodmer, W.F.; Browning, M.J.; Krausa, P.; Rowan, A.; Bicknell, D.C.; Bodmer, J.G. Tumor escape from immune response by variation in HLA expression and other mechanisms. Ann. N. Y. Acad. Sci. 1993, 690, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Marincola, F.M.; Jaffee, E.M.; Hicklin, D.J.; Ferrone, S. Escape of human solid tumors from T-cell recognition: Molecular mechanisms and functional significance. Adv. Immunol. 2000, 74, 181–273. [Google Scholar] [CrossRef] [PubMed]
- Seliger, B.; Cabrera, T.; Garrido, F.; Ferrone, S. HLA class I antigen abnormalities and immune escape by malignant cells. Semin. Cancer Biol. 2002, 12, 3–13. [Google Scholar] [CrossRef]
- Aptsiauri, N.; Ruiz-Cabello, F.; Garrido, F. The transition from HLA-I positive to HLA-I negative primary tumors: The road to escape from T-cell responses. Curr. Opin. Immunol. 2018, 51, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Beaton, C.; Twine, C.P.; Williams, G.L.; Radcliffe, A.G. Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Color. Dis. 2013, 15, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Bosch, S.L.; Teerenstra, S.; de Wilt, J.H.; Cunningham, C.; Nagtegaal, I.D. Predicting lymph node metastasis in pT1 colorectal cancer: A systematic review of risk factors providing rationale for therapy decisions. Endoscopy 2013, 45, 827–834. [Google Scholar] [CrossRef]
- Choi, J.Y.; Jung, S.A.; Shim, K.N.; Cho, W.Y.; Keum, B.; Byeon, J.S.; Huh, K.C.; Jang, B.I.; Chang, D.K.; Jung, H.Y.; et al. Meta-analysis of predictive clinicopathologic factors for lymph node metastasis in patients with early colorectal carcinoma. J. Korean Med. Sci. 2015, 30, 398–406. [Google Scholar] [CrossRef]
- Mou, S.; Soetikno, R.; Shimoda, T.; Rouse, R.; Kaltenbach, T. Pathologic predictive factors for lymph node metastasis in submucosal invasive (T1) colorectal cancer: A systematic review and meta-analysis. Surg. Endosc. 2013, 27, 2692–2703. [Google Scholar] [CrossRef]
- Zwager, L.W.; Bastiaansen, B.A.J.; Montazeri, N.S.M.; Hompes, R.; Barresi, V.; Ichimasa, K.; Kawachi, H.; Machado, I.; Masaki, T.; Sheng, W.; et al. Deep Submucosal Invasion Is Not an Independent Risk Factor for Lymph Node Metastasis in T1 Colorectal Cancer: A Meta-Analysis. Gastroenterology 2022, 163, 174–189. [Google Scholar] [CrossRef]
- Vogel, J.D.; Felder, S.I.; Bhama, A.R.; Hawkins, A.T.; Langenfeld, S.J.; Shaffer, V.O.; Thorsen, A.J.; Weiser, M.R.; Chang, G.J.; Lightner, A.L.; et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Colon Cancer. Dis. Colon Rectum 2022, 65, 148–177. [Google Scholar] [CrossRef]
- Roelands, J.; Ploeg, M.v.d.; Ijsselsteijn, M.E.; Dang, H.; Boonstra, J.J.; Hardwick, J.C.H.; Hawinkels, L.J.A.C.; Morreau, H.; Miranda, N.F. Transcriptomic and immunophenotypic profiling reveals molecular and immunological hallmarks of colorectal cancer tumourigenesis. Gut 2023, 72, 1326–1339. [Google Scholar] [CrossRef]
- Van Hijfte, L.; Geurts, M.; Vallentgoed, W.R.; Eilers, P.H.C.; Sillevis Smitt, P.A.E.; Debets, R.; French, P.J. Alternative normalization and analysis pipeline to address systematic bias in NanoString GeoMx Digital Spatial Profiling data. iScience 2023, 26, 105760. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]

| Patient | Sex | Age (y) | EMR Histology | cTNM | Op | LN Status | pTNM | Recurrence | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Location | Size (mm) | Grade | EMR Margin | Invasion Depth | LVI | (F/U Days) | |||||||
| 1 | F | 71 | Distal sigmoid | 10 | Well | Clear | 1000 µm from HL | + | cT1N0M0 | LAR | 0/5 | pT1N0M0 | No (3997) |
| 2 | M | 76 | Distal sigmoid | 13 | Moderately | Clear | 500 µm from HL | + | cT1N0M0 | LAR | 0/3 | pT1N0M0 | No (3935) |
| 3 * | M | 75 | Transverse colon | 17 | Moderately | Clear | 300 µm from HL | + | cT1N0M0 | LH | 0/17 | pT1N0M0 | No (2114) |
| 4 | M | 68 | Mid sigmoid | 15 | Well | Clear | 1000 µm from HL | + | cT1N0M0 | LAR | 1/12 | pT1N1aM0 | No (1169) |
| Signaling Pathway | p-Value | Related Genes | KEGG ID |
|---|---|---|---|
| Antigen processing and presentation | 0.000137 | HLA-E, B2M, HLA-B | KEGG:04612 |
| Human T-cell leukemia virus 1 infection | 0.00068 | HLA-E, B2M, HLA-B | KEGG:05166 |
| Allograft rejection | 0.00068 | HLA-E, HLA-B | KEGG:05330 |
| Human immunodeficiency virus 1 infection | 0.00068 | HLA-E, B2M, HLA-B | KEGG:05170 |
| Epstein–Barr virus infection | 0.00068 | HLA-E, B2M, HLA-B | KEGG:05169 |
| Human cytomegalovirus infection | 0.00068 | HLA-E, B2M, HLA-B | KEGG:05163 |
| Graft-versus-host disease | 0.00068 | HLA-E, HLA-B | KEGG:05332 |
| Type I diabetes mellitus | 0.000696 | HLA-E, HLA-B | KEGG:04940 |
| Autoimmune thyroid disease | 0.000931 | HLA-E, HLA-B | KEGG:05320 |
| Viral myocarditis | 0.001095 | HLA-E, HLA-B | KEGG:05416 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, M.J.; Park, H.Y.; Lim, H.; Son, I.T.; Kim, M.-J.; Kim, N.Y.; Kim, M.J.; Nam, E.S.; Cho, S.J.; Bang, W.J.; et al. Potential Molecular Markers Related to Lymph Node Metastasis and Stalk Resection Margins in Pedunculated T1 Colorectal Cancers Using Digital Spatial Profiling: A Pilot Study with a Small Case Series. Int. J. Mol. Sci. 2024, 25, 1103. https://doi.org/10.3390/ijms25021103
Kwon MJ, Park HY, Lim H, Son IT, Kim M-J, Kim NY, Kim MJ, Nam ES, Cho SJ, Bang WJ, et al. Potential Molecular Markers Related to Lymph Node Metastasis and Stalk Resection Margins in Pedunculated T1 Colorectal Cancers Using Digital Spatial Profiling: A Pilot Study with a Small Case Series. International Journal of Molecular Sciences. 2024; 25(2):1103. https://doi.org/10.3390/ijms25021103
Chicago/Turabian StyleKwon, Mi Jung, Ha Young Park, Hyun Lim, Il Tae Son, Min-Jeong Kim, Nan Young Kim, Min Jeong Kim, Eun Sook Nam, Seong Jin Cho, Woo Jin Bang, and et al. 2024. "Potential Molecular Markers Related to Lymph Node Metastasis and Stalk Resection Margins in Pedunculated T1 Colorectal Cancers Using Digital Spatial Profiling: A Pilot Study with a Small Case Series" International Journal of Molecular Sciences 25, no. 2: 1103. https://doi.org/10.3390/ijms25021103
APA StyleKwon, M. J., Park, H. Y., Lim, H., Son, I. T., Kim, M.-J., Kim, N. Y., Kim, M. J., Nam, E. S., Cho, S. J., Bang, W. J., & Kang, H. S. (2024). Potential Molecular Markers Related to Lymph Node Metastasis and Stalk Resection Margins in Pedunculated T1 Colorectal Cancers Using Digital Spatial Profiling: A Pilot Study with a Small Case Series. International Journal of Molecular Sciences, 25(2), 1103. https://doi.org/10.3390/ijms25021103








