ASAP1 Expression in Invasive Breast Cancer and Its Prognostic Role
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics of the Patients
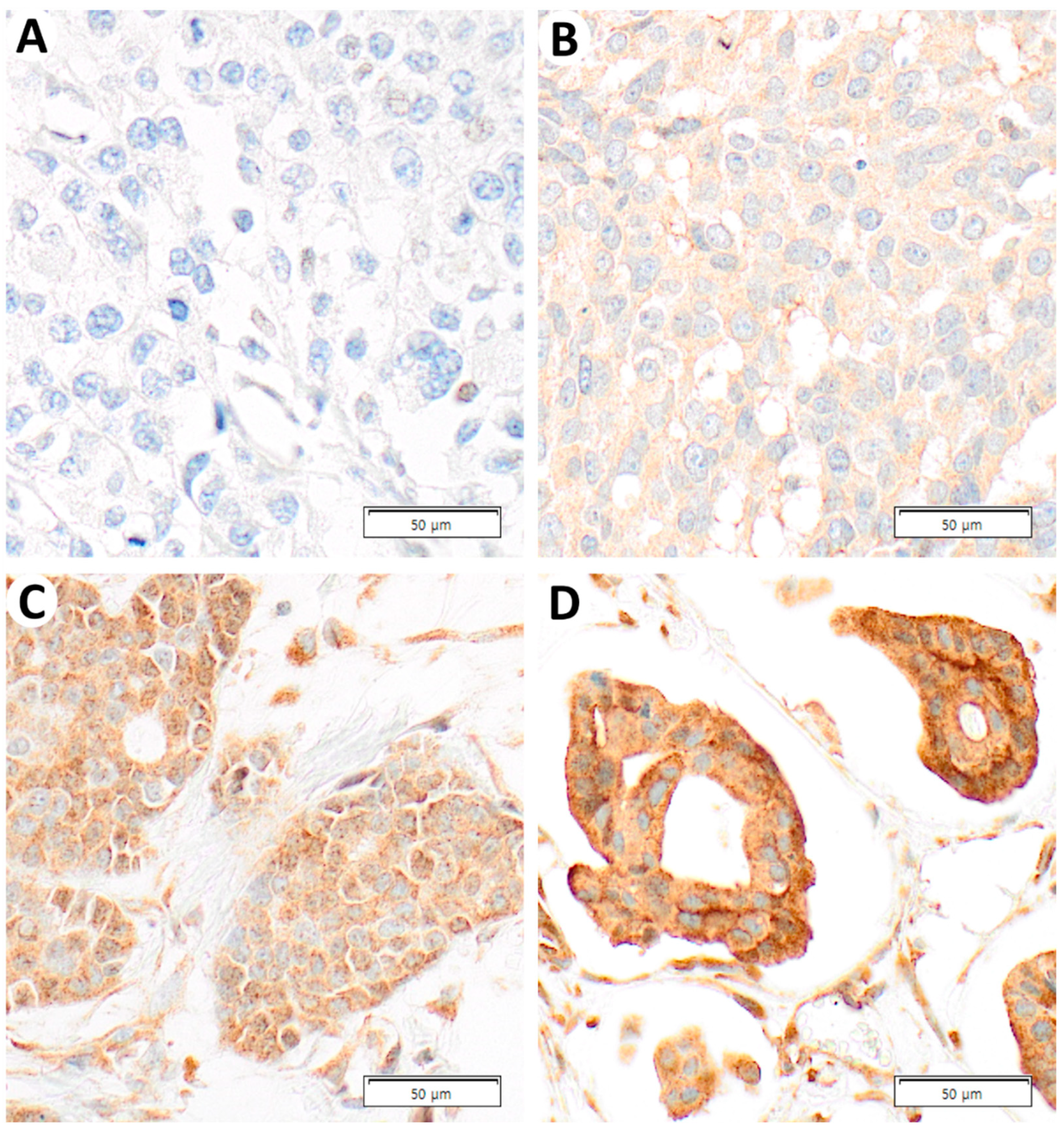
2.2. ASAP1 Expression and Its Correlations with Clinicopathological Characteristics in Total Cases
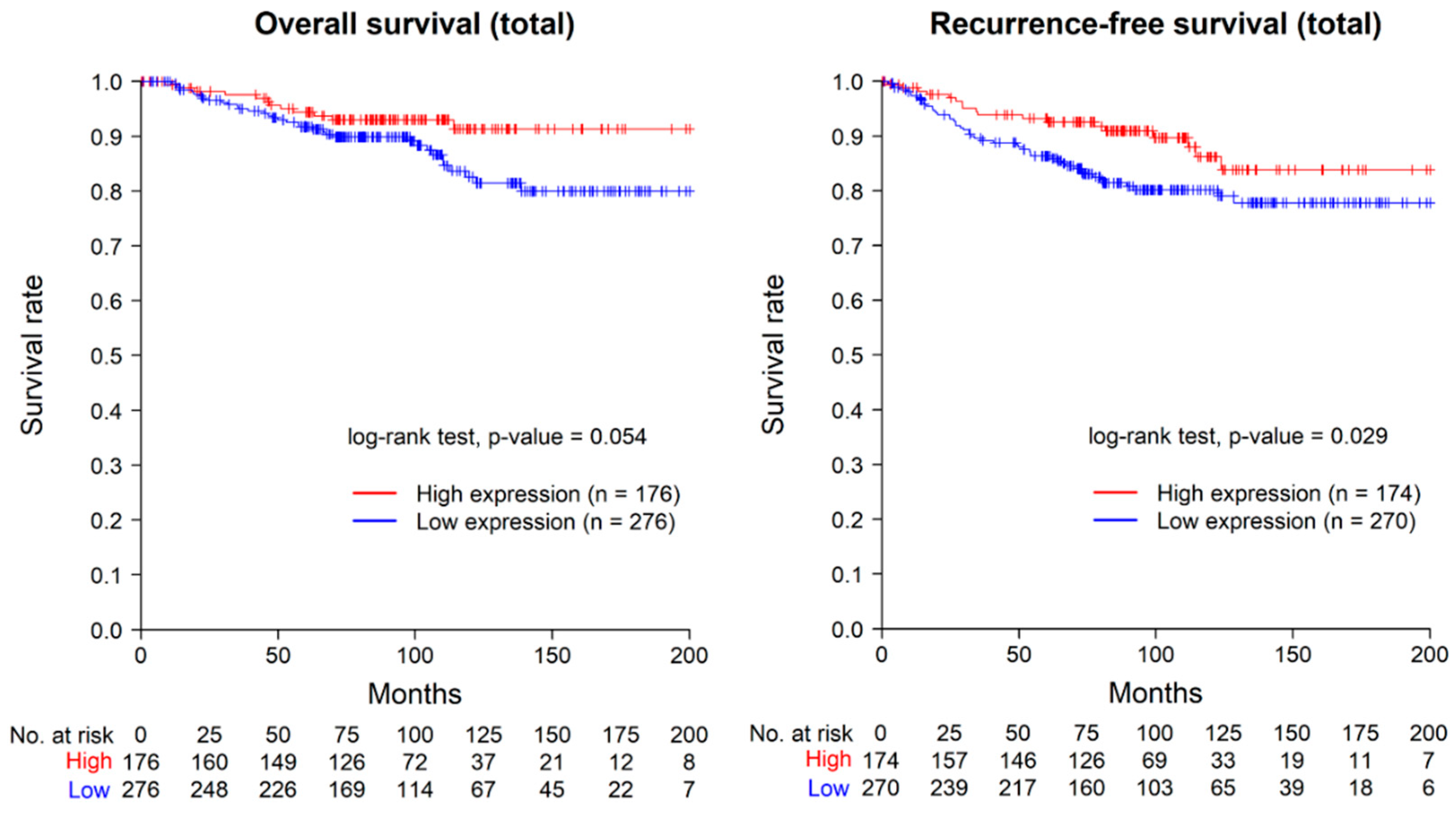
2.3. Prognostic Value of ASAP1 Expression in Total Cases
2.4. Correlations between ASAP1 Expression and Clinicopathological Characteristics in ER-Positive Cases
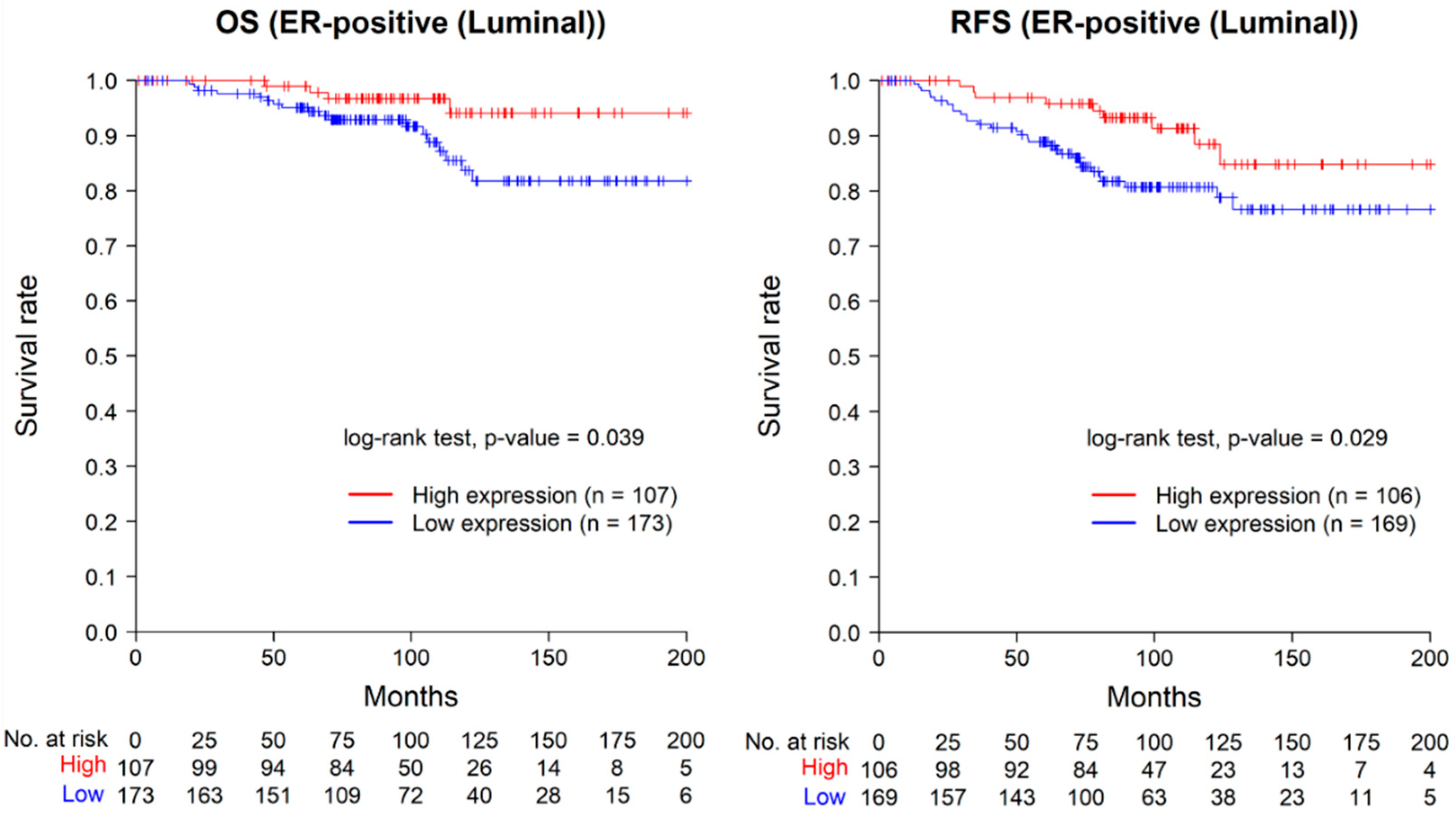
2.5. Prognostic Value of ASAP1 Expression in ER-Positive Cases
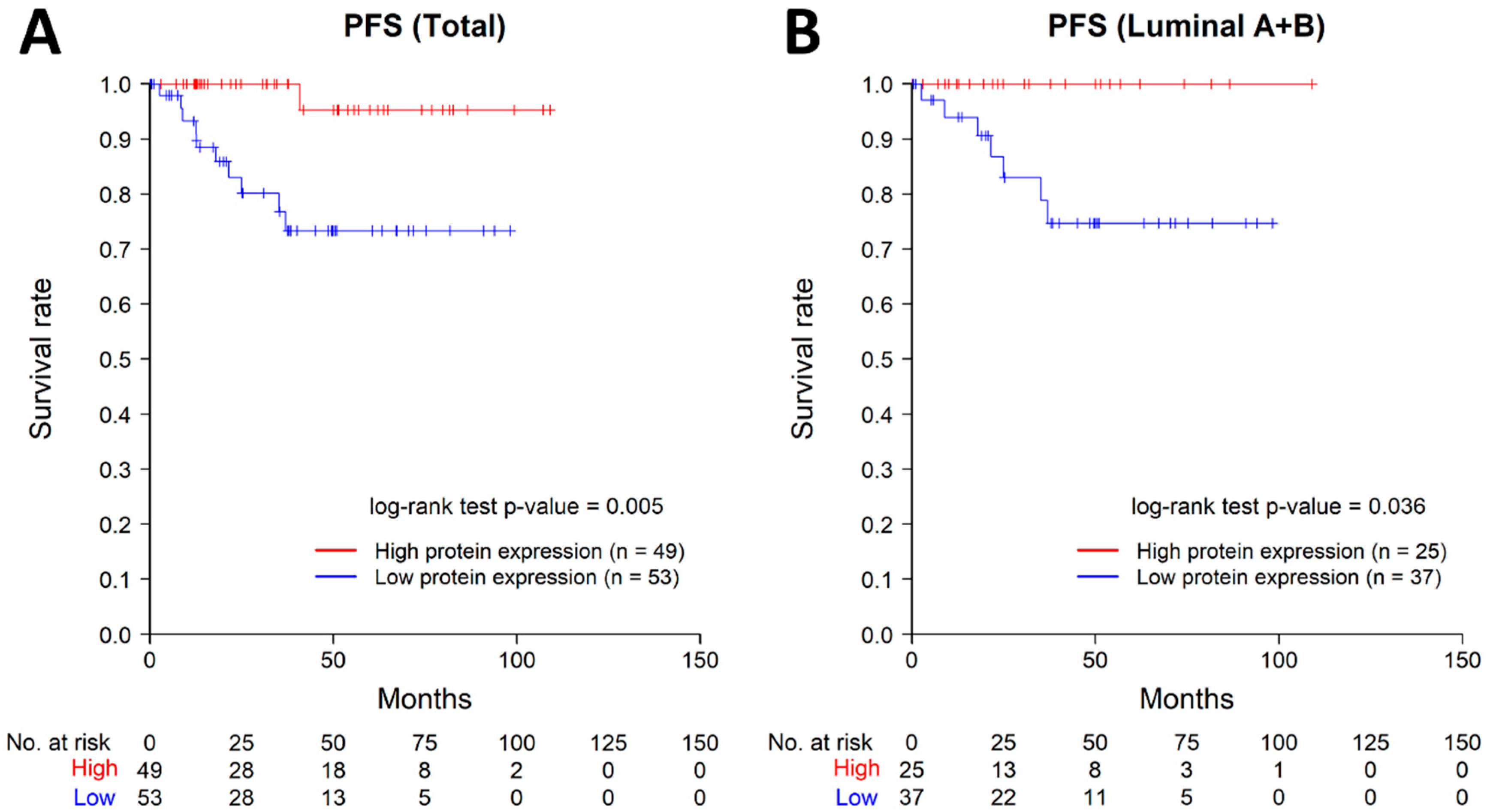
2.6. Results of Public Dataset Analysis
3. Discussion
4. Materials and Methods
4.1. Patients and Tissue Samples
4.2. Tissue Microarray (TMA) Construction
4.3. Immunohistochemical (IHC) Staining
4.4. Interpretation of IHC Staining
4.5. The Cancer Genome Atlas (TCGA) PanCancer Atlas Data
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.J.; Jung, K.-W.; Bang, S.H.; Choi, S.H.; Park, E.H.; Yun, E.H.; Kim, H.-J.; Kong, H.-J.; Im, J.-S.; Seo, H.G.; et al. Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2020. Cancer Res. Treat. 2023, 55, 385–399. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.-J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef]
- Regan, M.M.; Francis, P.A.; Pagani, O.; Fleming, G.F.; Walley, B.A.; Viale, G.; Colleoni, M.; Láng, I.; Gómez, H.L.; Tondini, C.; et al. Absolute Benefit of Adjuvant Endocrine Therapies for Premenopausal Women with Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Early Breast Cancer: TEXT and SOFT Trials. J. Clin. Oncol. 2016, 34, 2221–2231. [Google Scholar] [CrossRef]
- Dowling, R.J.O.; Sparano, J.A.; Goodwin, P.J.; Bidard, F.-C.; Cescon, D.W.; Chandarlapaty, S.; Deasy, J.O.; Dowsett, M.; Gray, R.J.; Henry, N.L.; et al. Toronto Workshop on Late Recurrence in Estrogen Receptor-Positive Breast Cancer: Part 2: Approaches to Predict and Identify Late Recurrence, Research Directions. JNCI Cancer Spectr. 2019, 3, pkz049. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, J.G.; Jackson, C.L. ARF family G proteins and their regulators: Roles in membrane transport, development and disease. Nat. Rev. Mol. Cell Biol. 2011, 12, 362–375. [Google Scholar] [CrossRef]
- Müller, T.; Stein, U.; Poletti, A.; Garzia, L.; Rothley, M.; Plaumann, D.; Thiele, W.; Bauer, M.; Galasso, A.; Schlag, P.; et al. ASAP1 promotes tumor cell motility and invasiveness, stimulates metastasis formation in vivo, and correlates with poor survival in colorectal cancer patients. Oncogene 2010, 29, 2393–2403. [Google Scholar] [CrossRef]
- Lin, D.; Watahiki, A.; Bayani, J.; Zhang, F.; Liu, L.; Ling, V.; Sadar, M.D.; English, J.; Fazli, L.; So, A.; et al. ASAP1, a Gene at 8q24, Is Associated with Prostate Cancer Metastasis. Cancer Res 2008, 68, 4352–4359. [Google Scholar] [CrossRef]
- Li, M.; Tian, L.; Yao, H.; Lu, J.; Ge, J.; Guo, Y.; Liu, M.; Xiao, H. ASAP1 mediates the invasive phenotype of human laryngeal squamous cell carcinoma to affect survival prognosis. Oncol. Rep. 2014, 31, 2676–2682. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sato, H.; Hatanaka, K.C.; Hatanaka, Y.; Hatakeyama, H.; Hashimoto, A.; Matsuno, Y.; Fukuda, S.; Sabe, H. High level expression of AMAP1 protein correlates with poor prognosis and survival after surgery of head and neck squamous cell carcinoma patients. Cell Commun. Signal. 2014, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Bang, S.; Jee, S.; Son, H.; Cha, H.; Sim, J.; Kim, Y.; Park, H.; Myung, J.; Kim, H.; Paik, S. Clinicopathological Implications of ASAP1 Expression in Hepatocellular Carcinoma. Pathol. Oncol. Res. 2022, 28, 1610635. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Zhang, S.; Zhang, D.; Yuan, F.; Chen, X.; Yang, S. Expression of ASAP1 and FAK in gastric cancer and its clinicopathological significance. Oncol. Lett. 2020, 20, 974–980. [Google Scholar] [CrossRef]
- Hou, T.; Yang, C.; Tong, C.; Zhang, H.; Xiao, J.; Li, J. Overexpression of ASAP1 is associated with poor prognosis in epithelial ovarian cancer. Int. J. Clin. Exp. Pathol. 2013, 7, 280–287. [Google Scholar]
- Hashimoto, S.; Mikami, S.; Sugino, H.; Yoshikawa, A.; Hashimoto, A.; Onodera, Y.; Furukawa, S.; Handa, H.; Oikawa, T.; Okada, Y.; et al. Lysophosphatidic acid activates Arf6 to promote the mesenchymal malignancy of renal cancer. Nat. Commun. 2016, 7, 10656. [Google Scholar] [CrossRef]
- Tsutaho, A.; Hashimoto, A.; Hashimoto, S.; Hata, S.; Kachi, S.; Hirano, S.; Sabe, H. High expression of AMAP1, an ARF6 effector, is associated with elevated levels of PD-L1 and fibrosis of pancreatic cancer. Cell Commun. Signal. 2020, 18, 1–8. [Google Scholar] [CrossRef]
- Ranganathan, S.; Ningappa, M.; Ashokkumar, C.; Higgs, B.W.; Min, J.; Sun, Q.; Schmitt, L.; Subramaniam, S.; Hakonarson, H.; Sindhi, R. Loss of EGFR-ASAP1 signaling in metastatic and unresectable hepatoblastoma. Sci. Rep. 2016, 6, 38347. [Google Scholar] [CrossRef]
- Schreiber, C.; Gruber, A.; Roßwag, S.; Saraswati, S.; Harkins, S.; Thiele, W.; Foroushani, Z.H.; Munding, N.; Schmaus, A.; Rothley, M.; et al. Loss of ASAP1 in the MMTV-PyMT model of luminal breast cancer activates AKT, accelerates tumorigenesis, and promotes metastasis. Cancer Lett. 2022, 533, 215600. [Google Scholar] [CrossRef]
- Onodera, Y.; Hashimoto, S.; Hashimoto, A.; Morishige, M.; Mazaki, Y.; Yamada, A.; Ogawa, E.; Adachi, M.; Sakurai, T.; Manabe, T.; et al. Expression of AMAP1, an ArfGAP, provides novel targets to inhibit breast cancer invasive activities. EMBO J. 2005, 24, 963–973. [Google Scholar] [CrossRef]
- Kinoshita, R.; Nam, J.-M.; Ito, Y.M.; Hatanaka, K.C.; Hashimoto, A.; Handa, H.; Otsuka, Y.; Hashimoto, S.; Onodera, Y.; Hosoda, M.; et al. Co-Overexpression of GEP100 and AMAP1 Proteins Correlates with Rapid Local Recurrence after Breast Conservative Therapy. PLoS ONE 2013, 8, e76791. [Google Scholar] [CrossRef] [PubMed]
- He, J.; McLaughlin, R.P.; van der Beek, L.; Canisius, S.; Wessels, L.; Smid, M.; Martens, J.W.M.; Foekens, J.A.; Zhang, Y.; van de Water, B. Integrative analysis of genomic amplification-dependent expression and loss-of-function screen identifies ASAP1 as a driver gene in triple-negative breast cancer progression. Oncogene 2020, 39, 4118–4131. [Google Scholar] [CrossRef]
- Elston, C.; Ellis, I. pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Meyerholz, D.K.; Beck, A.P. Principles and approaches for reproducible scoring of tissue stains in research. Lab. Investig. 2018, 98, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Fluss, R.; Faraggi, D.; Reiser, B. Estimation of the Youden Index and its Associated Cutoff Point. Biom. J. 2005, 47, 458–472. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef]
- Wolff, A.C.; Hammond, M.E.H.; Allison, K.H.; Harvey, B.E.; Mangu, P.B.; Bartlett, J.M.S.; Bilous, M.; Ellis, I.O.; Fitzgibbons, P.; Hanna, W.; et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2018, 36, 2105–2122. [Google Scholar] [CrossRef]
- Dowsett, M.; Nielsen, T.O.; A’hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in breast cancer working group. JNCI J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef]
- cBioPortal for Cancer Genomics. Available online: https://www.cbioportal.org/study/summary?id=brca_tcga_pan_can_atlas_2018 (accessed on 14 May 2023).
| Clinicopathological Characteristics | Value |
|---|---|
| Age (years, mean ± SD) | 52.8 ± 10.8 (27–83) |
| Size (cm, mean ± SD) | 2.6 ± 1.8 (0.12–16) |
| Histological grade * | |
| G1 | 99 (21.90%) |
| G2 | 200 (44.25%) |
| G3 | 153 (33.85%) |
| T stage † | |
| T1 | 202 (44.69%) |
| T2 | 214 (47.34%) |
| T3 | 23 (5.09%) |
| T4 | 13 (2.88%) |
| N stage † | |
| N0 | 285 (63.05%) |
| N1 | 98 (21.68%) |
| N2 | 38 (8.41%) |
| N3 | 31 (6.86%) |
| Distant metastasis | |
| Negative | 444 (98.23%) |
| Positive | 8 (1.77%) |
| ER status | |
| Negative | 172 (38.05%) |
| Positive | 280 (61.95%) |
| HER2 status | |
| Negative | 309 (68.36%) |
| Positive | 143 (31.64%) |
| Ki-67 index | |
| Low (≤10%) | 279 (61.725%) |
| Intermediate (>10%, ≤20%) | 53 (11.725%) |
| High (>20%) | 120 (26.55%) |
| Molecular subtype ‡ | |
| Luminal A-like | 187 (41.37%) |
| Luminal B-like | 93 (20.58%) |
| HER2 (non-luminal) | 76 (16.81%) |
| Triple-negative | 96 (21.24%) |
| ASAP1 expression | |
| High | 176 (38.94%) |
| Low | 276 (61.06%) |
| Recurrence | |
| Negative | 377 (83.41%) |
| Positive | 75 (16.59%) |
| Survival | |
| Survive | 405 (89.60%) |
| Dead | 47 (10.40%) |
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| Age | (per year) | 0.992 | 0.969–1.016 | 0.503 | 1.007 | 0.984–1.031 | 0.551 |
| Size | (per cm) | 1.24 | 1.144–1.344 | <0.001 | 1.103 | 0.981–1.24 | 0.102 |
| T stage | T4 (vs. T1,2,3) | 7.792 | 3.357–18.089 | <0.001 | 4.579 | 1.493–14.044 | 0.008 |
| Histological grade | G2 (vs. G1) | 3.015 | 1.17–7.773 | 0.022 | 2.393 | 0.91–6.292 | 0.077 |
| G3 (vs. G1) | 4.547 | 1.771–11.67 | 0.002 | 3.106 | 1.102–8.753 | 0.032 | |
| N stage | N1 (vs. N0) | 3.337 | 1.86–5.988 | <0.001 | 2.935 | 1.593–5.407 | 0.001 |
| N2 (vs. N0) | 4.013 | 1.945–8.278 | <0.001 | 4.155 | 1.958–8.82 | <0.001 | |
| N3 (vs. N0) | 5.189 | 2.515–10.708 | <0.001 | 4.454 | 2.05–9.677 | <0.001 | |
| ER status | Positive (vs. Negative) | 0.786 | 0.484–1.278 | 0.332 | 0.95 | 0.54–1.671 | 0.858 |
| HER2 status | Positive (vs. Negative) | 1.305 | 0.795–2.142 | 0.293 | 1.114 | 0.669–1.856 | 0.678 |
| Ki-67 index | Intermediate (vs. Low) | 0.719 | 0.305–1.697 | 0.452 | 0.843 | 0.352–2.018 | 0.702 |
| High (vs. Low) | 1.206 | 0.711–2.046 | 0.487 | 0.868 | 0.486–1.551 | 0.632 | |
| ASAP1 expression | Low (vs. High) | 1.813 | 1.056–3.113 | 0.031 | 2.566 | 1.429–4.607 | 0.002 |
| Univariate Analysis | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | ||
| Age | (per year) | 0.993 | 0.963–1.024 | 0.643 | 1.015 | 0.984–1.048 | 0.345 |
| Size | (per cm) | 1.319 | 1.159–1.502 | <0.001 | 1.238 | 1.03–1.488 | 0.023 |
| T stage | T4 (vs. T1,2,3) | 3.888 | 0.531–28.477 | 0.181 | 2.458 | 0.236–25.626 | 0.452 |
| Histological grade | G2 (vs. G1) | 3.195 | 1.101–9.275 | 0.033 | 2.35 | 0.783–7.054 | 0.128 |
| G3 (vs. G1) | 6.48 | 2.113–19.878 | 0.001 | 6.53 | 1.839–23.182 | 0.004 | |
| N stage | N1 (vs. N0) | 4.657 | 2.132–10.174 | <0.001 | 3.416 | 1.469–7.945 | 0.004 |
| N2 (vs. N0) | 4.015 | 1.372–11.749 | 0.011 | 2.889 | 0.873–9.565 | 0.082 | |
| N3 (vs. N0) | 6.581 | 2.504–17.298 | <0.001 | 4.133 | 1.428–11.966 | 0.009 | |
| HER2 status | Positive (vs. Negative) | 0.981 | 0.477–2.016 | 0.957 | 0.596 | 0.259–1.371 | 0.224 |
| Ki-67 index | Intermediate (vs. Low) | 0.977 | 0.404–2.362 | 0.959 | 1.139 | 0.453–2.862 | 0.782 |
| High (vs. Low) | 0.806 | 0.311–2.089 | 0.658 | 0.257 | 0.08–0.831 | 0.023 | |
| ASAP1 expression | Low (vs. High) | 2.246 | 1.065–4.734 | 0.033 | 4.046 | 1.644–9.957 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, H.; Son, H.; Cha, H.; Song, K.; Bang, S.; Jee, S.; Kim, H.; Myung, J.; Shin, S.-J.; Cha, C.; et al. ASAP1 Expression in Invasive Breast Cancer and Its Prognostic Role. Int. J. Mol. Sci. 2023, 24, 14355. https://doi.org/10.3390/ijms241814355
Park H, Son H, Cha H, Song K, Bang S, Jee S, Kim H, Myung J, Shin S-J, Cha C, et al. ASAP1 Expression in Invasive Breast Cancer and Its Prognostic Role. International Journal of Molecular Sciences. 2023; 24(18):14355. https://doi.org/10.3390/ijms241814355
Chicago/Turabian StylePark, Hosub, Hwangkyu Son, Hyebin Cha, Kihyuk Song, Seongsik Bang, Seungyun Jee, Hyunsung Kim, Jaekyung Myung, Su-Jin Shin, Chihwan Cha, and et al. 2023. "ASAP1 Expression in Invasive Breast Cancer and Its Prognostic Role" International Journal of Molecular Sciences 24, no. 18: 14355. https://doi.org/10.3390/ijms241814355
APA StylePark, H., Son, H., Cha, H., Song, K., Bang, S., Jee, S., Kim, H., Myung, J., Shin, S.-J., Cha, C., Chung, M. S., & Paik, S. (2023). ASAP1 Expression in Invasive Breast Cancer and Its Prognostic Role. International Journal of Molecular Sciences, 24(18), 14355. https://doi.org/10.3390/ijms241814355







