Choroideremia: The Endpoint Endgame
Abstract
:1. Introduction
1.1. Choroideremia and Genetics
1.2. Alternative Subretinal Gene Therapy Approaches for Choroideremia
1.2.1. Nonsense Suppression Therapy
1.2.2. Antisense Oligonucleotides
2. Lessons from Choroideremia Clinical Trials
3. Clinical Endpoints
3.1. What Makes a Good Outcome Measure
3.1.1. Target Population
3.1.2. Length of Clinical Trials
3.1.3. Choice of Controls
- (i)
- a demonstration that the inter-individual variability is greater than the intra-individual variability for the respective endpoint;
- (ii)
- evidence that unilateral treatment has no effect on the endpoint measured in the fellow eye.
3.2. Functional and Structural Outcome Measures Used in Previous Choroideremia Clinical Trials
3.3. Functional Outcome Measures
3.3.1. Best Corrected Visual Acuity
3.3.2. Low-Luminance Visual Acuity
3.3.3. Microperimetry
3.3.4. Scotopic Microperimetry
3.3.5. Full-Field Perimetry
3.4. Full-Field Stimulus Testing (FST)
3.5. Patient-Reported Outcome Measures
Multi-Luminance Mobility Testing (MLMT)
3.6. Structural Outcome Measures
3.6.1. Ocular Coherence Tomography (OCT)
3.6.2. Fundus Autofluorescence
3.6.3. Concluding Remarks about Endpoints
4. Future Directions for Choroideremia Clinical Trials
4.1. Earlier Intervention
4.2. Novel Structural and Functional Measure
4.3. Optical Coherence Tomography Angiography
4.4. Future Therapies
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cremers, F.P.; Sankila, E.M.; Brunsmann, F.; Jay, M.; Jay, B.; Wright, A.; Pinckers, A.J.; Schwartz, M.; Van De Pol, D.J.; Wieringa, B. Deletions in patients with classical choroideremia vary in size from 45 kb to several megabases. Am. J. Hum. Genet. 1990, 47, 622–628. [Google Scholar]
- Zeitz, C.; Nassisi, M.; Laurent-Coriat, C.; Andrieu, C.; Boyard, F.; Condroyer, C.; Démontant, V.; Antonio, A.; Lancelot, M.; Frederiksen, H.; et al. CHM mutation spectrum and disease: An update at the time of human therapeutic trials. Hum. Mutat. 2021, 42, 323–341. [Google Scholar] [CrossRef]
- Cremers, F.P.; Molloy, C.M.; van de Pol, D.J.; Hurk, J.A.; Bach, I.; Kessel, A.H.; Ropers, H.H. An autosomal homologue of the choroideremia gene colocalizes with the usher syndrome type II locus on the distal part of chromosome 1q. Hum. Mol. Genet. 1992, 1, 71–75. [Google Scholar] [CrossRef]
- Patrício, M.I.; Barnard, A.R.; Cox, C.I.; Blue, C.; MacLaren, R.E. The Biological Activity of AAV Vectors for Choroideremia Gene Therapy Can Be Measured by In Vitro Prenylation of RAB6A. Mol. Ther.—Methods Clin. Dev. 2018, 9, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Larijani, B.; Hume, A.N.; Tarafder, A.K.; Seabra, M.C. Multiple Factors Contribute to Inefficient Prenylation of Rab27a in Rab Prenylation Diseases. J. Biol. Chem. 2003, 278, 46798–46804. [Google Scholar] [CrossRef] [PubMed]
- Jolly, J.K.; Xue, K.; Edwards, T.L.; Groppe, M.; MacLaren, R.E. Characterizing the Natural History of Visual Function in Choroideremia Using Microperimetry and Multimodal Retinal Imaging. Investig. Opthalmol. Vis. Sci. 2017, 58, 5575–5583. [Google Scholar] [CrossRef]
- Hariri, A.H.; Ip, M.S.; Girach, A.; Lam, B.L.; Fischer, M.D.; Sankila, E.-M.; Pennesi, M.E.; Holz, F.G.; Maclaren, R.E.; Birch, D.G.; et al. Macular spatial distribution of preserved autofluorescence in patients with choroideremia. Br. J. Ophthalmol. 2018, 103, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Fry, L.E.; Patrício, M.I.; Jolly, J.K.; Xue, K.; MacLaren, R.E. Expression of Rab Prenylation Pathway Genes and Relation to Disease Progression in Choroideremia. Transl. Vis. Sci. Technol. 2021, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Cunha, D.L.; Richardson, R.; Tracey-White, D.; Abbouda, A.; Mitsios, A.; Horneffer-van der Sluis, V.; Takis, P.; Owen, N.; Skinner, J.; Welch, A.A.; et al. REP1 deficiency causes systemic dysfunction of lipid metabolism and oxidative stress in choroideremia. JCI Insight 2021, 6, e146934. [Google Scholar] [CrossRef] [PubMed]
- Futter, C.E.; Ramalho, J.S.; Jaissle, G.B.; Seeliger, M.W.; Seabra, M.C. The role of Rab27a in the regulation of melanosome distribution within retinal pigment epithelial cells. Mol. Biol. Cell 2004, 15, 2264–2275. [Google Scholar] [CrossRef]
- Kwok, M.C.M.; Holopainen, J.M.; Molday, L.L.; Foster, L.J.; Molday, R.S. Proteomics of photoreceptor outer segments identifies a subset of snare and rab proteins implicated in membrane vesicle trafficking and fusion. Mol. Cell. Proteom. 2008, 7, 1053–1066. [Google Scholar] [CrossRef] [PubMed]
- Moosajee, M.; Ramsden, S.C.; Black, G.C.; Seabra, M.C.; Webster, A.R. Clinical utility gene card for: Choroideremia. Eur. J. Hum. Genet. 2014, 22, 572. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.; Smart, M.; Tracey-White, D.; Webster, A.R.; Moosajee, M. Mechanism and evidence of nonsense suppression therapy for genetic eye disorders. Exp. Eye Res. 2017, 155, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Moosajee, M.; Tracey-White, D.; Smart, M.; Weetall, M.; Torriano, S.; Kalatzis, V.; da Cruz, L.; Coffey, P.; Webster, A.R.; Welch, E. Functional rescue of REP1 following treatment with PTC124 and novel derivative PTC-414 in human choroideremia fibroblasts and the nonsense-mediated zebrafish model. Hum. Mol. Genet. 2016, 25, 3416–3431. [Google Scholar] [CrossRef]
- Duijkers, L.; Born, L.I.V.D.; Neidhardt, J.; Bax, N.M.; Pierrache, L.H.M.; Klevering, B.J.; Collin, R.W.J.; Garanto, A. Antisense Oligonucleotide-Based Splicing Correction in Individuals with Leber Congenital Amaurosis due to Compound Heterozygosity for the c.2991+1655A>G Mutation in CEP290. Int. J. Mol. Sci. 2018, 19, 753. [Google Scholar] [CrossRef]
- Garanto, A.; van der Velde-Visser, S.D.; Cremers, F.P.M.; Collin, R.W.J. Antisense Oligonucleotide-Based Splice Correction of a Deep-Intronic Mutation in CHM Underlying Choroideremia. In Retinal Degenerative Diseases: Mechanisms and Experimental Therapy; Springer: Berlin/Heidelberg, Germany, 2018; Volume 1074, pp. 83–89. [Google Scholar] [CrossRef]
- Zhai, Y.; Xu, M.; Radziwon, A.; Dimopoulos, I.S.; Crichton, P.; Mah, R.; MacLaren, R.E.; Somani, R.; Tennant, M.T.; MacDonald, I.M. AAV2-mediated gene therapy for choroideremia: 5-year results and alternate anti-sense oligonucleotide therapy. Am. J. Ophthalmol. 2022, 248, 145–156. [Google Scholar] [CrossRef]
- Russell, S.R.; Drack, A.V.; Cideciyan, A.V.; Jacobson, S.G.; Leroy, B.P.; Van Cauwenbergh, C.; Ho, A.C.; Dumitrescu, A.V.; Han, I.C.; Martin, M.; et al. Intravitreal antisense oligonucleotide sepofarsen in Leber congenital amaurosis type 10: A phase 1b/2 trial. Nat. Med. 2022, 28, 1014–1021. [Google Scholar] [CrossRef]
- Simunovic, M.P.; Jolly, J.K.; Xue, K.; Edwards, T.L.; Groppe, M.; Downes, S.M.; MacLaren, R.E. The Spectrum of CHM Gene Mutations in Choroideremia and Their Relationship to Clinical Phenotype. Investig. Opthalmol. Vis. Sci. 2016, 57, 6033–6039. [Google Scholar] [CrossRef]
- Edwards, T.L.; Jolly, J.K.; Groppe, M.; Barnard, A.R.; Cottriall, C.L.; Tolmachova, T.; Black, G.C.; Webster, A.R.; Lotery, A.J.; Holder, G.E.; et al. Visual Acuity after Retinal Gene Therapy for Choroideremia. N. Engl. J. Med. 2016, 374, 1996–1998. [Google Scholar] [CrossRef]
- Xue, K.; Groppe, M.; Salvetti, A.P.; MacLaren, R.E. Technique of retinal gene therapy: Delivery of viral vector into the subretinal space. Eye 2017, 31, 1308–1316. [Google Scholar] [CrossRef]
- MacLaren, R.E.; Groppe, M.; Barnard, A.R.; Cottriall, C.L.; Tolmachova, T.; Seymour, L.; Clark, K.R.; During, M.J.; Cremers, F.P.; Black, G.C.; et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet 2014, 383, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, I.S.; Hoang, S.C.; Radziwon, A.; Binczyk, N.M.; Seabra, M.C.; MacLaren, R.E.; Somani, R.; Tennant, M.T.; MacDonald, I.M. Two-Year Results After AAV2-Mediated Gene Therapy for Choroideremia: The Alberta Experience. Am. J. Ophthalmol. 2018, 193, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.L.; Davis, J.L.; Gregori, N.Z.; MacLaren, R.E.; Girach, A.; Verriotto, J.D.; Rodriguez, B.; Rosa, P.R.; Zhang, X.; Feuer, W.J. Choroideremia Gene Therapy Phase 2 Clinical Trial: 24-Month Results. Am. J. Ophthalmol. 2018, 197, 65–73. [Google Scholar] [CrossRef]
- Fischer, M.D.; Ochakovski, G.A.; Beier, B.; Seitz, I.P.; Vaheb, Y.; Kortuem, C.; Reichel, F.F.L.; Kuehlewein, L.; Kahle, N.A.; Peters, T.; et al. Efficacy and Safety of Retinal Gene Therapy Using Adeno-Associated Virus Vector for Patients with Choroideremia. JAMA Ophthalmol. 2019, 137, 1247–1254. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.D.; Ochakovski, G.A.; Beier, B.; Seitz, I.P.; Vaheb, Y.; Kortuem, C.; Reichel, F.F.L.; Kuehlewein, L.; Kahle, N.A.; Peters, T.; et al. Changes in retinal sensitivity after gene therapy in choroideremia. Retina 2020, 40, 160–168. [Google Scholar] [CrossRef]
- Simunovic, M.P.; Xue, K.; Jolly, J.K.; MacLaren, R.E. Structural and Functional Recovery Following Limited Iatrogenic Macular Detachment for Retinal Gene Therapy. JAMA Ophthalmol. 2017, 135, 234–241. [Google Scholar] [CrossRef]
- Xue, K.; Jolly, J.K.; Barnard, A.R.; Rudenko, A.; Salvetti, A.P.; Patrício, M.I.; Edwards, T.L.; Groppe, M.; Orlans, H.O.; Tolmachova, T.; et al. Beneficial effects on vision in patients undergoing retinal gene therapy for choroideremia. Nat. Med. 2018, 24, 1507–1512. [Google Scholar] [CrossRef]
- Edwards, T.L.; Xue, K.; Meenink, H.C.M.; Beelen, M.J.; Naus, G.J.L.; Simunovic, M.P.; Latasiewicz, M.; Farmery, A.D.; de Smet, M.D.; MacLaren, R.E. First-in-human study of the safety and viability of intraocular robotic surgery. Nat. Biomed. Eng. 2018, 2, 649–656. [Google Scholar] [CrossRef]
- Maguire, A.M.; Simonelli, F.; Pierce, E.A.; Pugh, E.N.; Mingozzi, F.; Bennicelli, J.; Banfi, S.; Marshall, K.A.; Testa, F.; Surace, E.M.; et al. Safety and Efficacy of Gene Transfer for Leber’s Congenital Amaurosis. N. Engl. J. Med. 2008, 358, 2240–2248. [Google Scholar] [CrossRef]
- Patrício, M.I.; Barnard, A.R.; Orlans, H.O.; McClements, M.E.; MacLaren, R.E. Inclusion of the Woodchuck Hepatitis Virus Posttranscriptional Regulatory Element Enhances AAV2-Driven Transduction of Mouse and Human Retina. Mol. Ther.—Nucleic Acids 2017, 6, 198–208. [Google Scholar] [CrossRef]
- Li, W.; Kong, F.; Li, X.; Dai, X.; Liu, X.; Zheng, Q.; Wu, R.; Zhou, X.; Lü, F.; Chang, B.; et al. Gene therapy following subretinal AAV5 vector delivery is not affected by a previous intravitreal AAV5 vector administration in the partner eye. Mol. Vis. 2009, 15, 267–275. [Google Scholar] [PubMed]
- Barker, S.E.; Broderick, C.A.; Robbie, S.J.; Duran, Y.; Natkunarajah, M.; Buch, P.; Balaggan, K.S.; MacLaren, R.E.; Bainbridge, J.W.B.; Smith, A.J.; et al. Subretinal delivery of adeno-associated virus serotype 2 results in minimal immune responses that allow repeat vector administration in immunocompetent mice. J. Gene Med. 2009, 11, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.I.W.; Jiang, Y.Y.; Vergilio, G.K.; Serrano, L.W.; Pearson, D.J.; Bennett, J.; Maguire, A.M.; Aleman, T.S. Short-term Assessment of Subfoveal Injection of Adeno-Associated Virus-Mediated hCHM Gene Augmentation in Choroideremia Using Adaptive Optics Ophthalmoscopy. JAMA Ophthalmol. 2022, 140, 411–420. [Google Scholar] [CrossRef]
- Hariri, A.H.; Velaga, S.B.; Girach, A.; Ip, M.S.; Le, P.V.; Lam, B.L.; Fischer, M.D.; Sankila, E.-M.; Pennesi, M.E.; Holz, F.G.; et al. Measurement and Reproducibility of Preserved Ellipsoid Zone Area and Preserved Retinal Pigment Epithelium Area in Eyes With Choroideremia. Am. J. Ophthalmol. 2017, 179, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, J.R.; Bowden, C.L.; Sachs, G.S.; Ascher, J.A.; Monaghan, E.; Rudd, G.D. A Double-Blind Placebo-Controlled Study of Lamotrigine Monotherapy in Outpatients With Bipolar I Depression. J. Clin. Psychiatry 1999, 60, 79–88. [Google Scholar] [CrossRef]
- Geddes, J.R.; Calabrese, J.R.; Goodwin, G.M. Lamotrigine for treatment of bipolar depression: Independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br. J. Psychiatry 2009, 194, 4–9. [Google Scholar] [CrossRef]
- Shen, L.L.; Ahluwalia, A.; Sun, M.; Young, B.K.; Nardini, H.K.G.; Del Priore, L.V. Long-term Natural History of Atrophy in Eyes with Choroideremia—A Systematic Review and Meta-analysis of Individual-Level Data. Ophthalmol. Retin. 2020, 4, 840–852. [Google Scholar] [CrossRef]
- Shen, L.L.; Ahluwalia, A.; Sun, M.; Young, B.K.; Nardini, H.K.G.; Del Priore, L.V. Long-term natural history of visual acuity in eyes with choroideremia: A systematic review and meta-analysis of data from 1004 individual eyes. Br. J. Ophthalmol. 2020, 105, 271–278. [Google Scholar] [CrossRef]
- Seitz, I.P.; Zhour, A.; Kohl, S.; Llavona, P.; Peter, T.; Wilhelm, B.; Zrenner, E.; Ueffing, M.; Bartz-Schmidt, K.U.; Fischer, M.D. Multimodal assessment of choroideremia patients defines pre-treatment characteristics. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 2143–2150. [Google Scholar] [CrossRef]
- Gelman, A. Data Analysis Using Regression and Multilevel/Hierarchical Models; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Ferris, F.L.; Kassoff, A.; Bresnick, G.H.; Bailey, I. New visual acuity charts for clinical research. Am. J. Ophthalmol. 1982, 94, 91–96. [Google Scholar] [CrossRef]
- Jolly, J.K.; Bridge, H.; MacLaren, R.E. Outcome Measures Used in Ocular Gene Therapy Trials: A Scoping Review of Current Practice. Front. Pharmacol. 2019, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Hagag, A.M.; Mitsios, A.; Narayan, A.; Abbouda, A.; Webster, A.R.; Dubis, A.M.; Moosajee, M. Prospective deep phenotyping of choroideremia patients using multimodal structure-function approaches. Eye 2020, 35, 838–852. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, I.S.; Freund, P.R.; Knowles, J.A.; MacDonald, I.M. The natural history of full-field stimulus threshold decline in choroideremia. Retina 2018, 38, 1731–1742. [Google Scholar] [CrossRef] [PubMed]
- Finger, R.P.; Schmitz-Valckenberg, S.; Schmid, M.; Rubin, G.S.; Dunbar, H.; Tufail, A.; Crabb, D.P.; Binns, A.; Sánchez, C.I.; Margaron, P.; et al. MACUSTAR: Development and Clinical Validation of Functional, Structural, and Patient-Reported Endpoints in Intermediate Age-Related Macular Degeneration. Ophthalmologica 2018, 241, 61–72. [Google Scholar] [CrossRef]
- Ayton, L.N.; Rizzo, J.F.; Bailey, I.L.; Colenbrander, A.; Dagnelie, G.; Geruschat, D.R.; Hessburg, P.C.; McCarthy, C.D.; Petoe, M.A.; Rubin, G.S.; et al. Harmonization of Outcomes and Vision Endpoints in Vision Restoration Trials: Recommendations from the International HOVER Taskforce. Transl. Vis. Sci. Technol. 2020, 9, 25. [Google Scholar] [CrossRef]
- Baffour-Awuah, K.; Taylor, L.J.; Josan, A.S.; Jolly, J.K.; Ahmed, R.; MacLaren, R.E. Investigating central visual field loss and its effects on how patients read the ETDRS chart. Investig. Ophthalmol. Vis. Sci. 2022, 63, 4061-F0025. [Google Scholar]
- Wood, L.J.; Jolly, J.K.; Buckley, T.M.; Josan, A.S.; MacLaren, R.E. Low luminance visual acuity as a clinical measure and clinical trial outcome measure: A scoping review. Ophthalmic Physiol. Opt. 2021, 41, 213–223. [Google Scholar] [CrossRef]
- Wood, L.J.; Jolly, J.K.; Andrews, C.D.; Wilson, I.R.; Hickey, D.; Cehajic-Kapetanovic, J.; MacLaren, R.E. Low-contrast visual acuity versus low-luminance visual acuity in choroideremia. Clin. Exp. Optom. 2021, 104, 90–94. [Google Scholar] [CrossRef]
- Wood, L.J.; Jolly, J.K.; Josan, A.S.; Buckley, T.M.W.; MacLaren, R.E. Low Luminance Visual Acuity and Low Luminance Deficit in Choroideremia and RPGR-Associated Retinitis Pigmentosa. Transl. Vis. Sci. Technol. 2021, 10, 28. [Google Scholar] [CrossRef]
- Pfau, M.; Jolly, J.K.; Wu, Z.; Denniss, J.; Lad, E.M.; Guymer, R.H.; Fleckenstein, M.; Holz, F.G.; Schmitz-Valckenberg, S. Fundus-controlled perimetry (microperimetry): Application as outcome measure in clinical trials. Prog. Retin. Eye Res. 2021, 82, 100907. [Google Scholar] [CrossRef]
- Han, R.C.; Jolly, J.K.; Xue, K.; MacLaren, R.E. Effects of pupil dilation on MAIA microperimetry. Clin. Exp. Ophthalmol. 2017, 45, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Han, R.C.; Gray, J.M.; Han, J.; Maclaren, R.E.; Jolly, J.K. Optimisation of dark adaptation time required for mesopic microperimetry. Br. J. Ophthalmol. 2018, 103, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Josan, A.S.; Buckley, T.M.W.; Wood, L.J.; Jolly, J.K.; Cehajic-Kapetanovic, J.; MacLaren, R.E. Microperimetry Hill of Vision and Volumetric Measures of Retinal Sensitivity. Transl. Vis. Sci. Technol. 2021, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.J.; Josan, A.S.; Pfau, M.; Simunovic, M.P.; Jolly, J.K. Scotopic microperimetry: Evolution, applications and future directions. Clin. Exp. Optom. 2022, 105, 793–800. [Google Scholar] [CrossRef]
- Parmann, R.; Greenstein, V.C.; Tsang, S.H.; Sparrow, J.R. Choroideremia Carriers: Dark-Adapted Perimetry and Retinal Structures. Investig. Opthalmol. Vis. Sci. 2022, 63, 4. [Google Scholar] [CrossRef]
- Marques, A.P.; Ramke, J.; Cairns, J.; Butt, T.; Zhang, J.H.; Faal, H.B.; Taylor, H.; Jones, I.; Congdon, N.; Bastawrous, A.; et al. Estimating the global cost of vision impairment and its major causes: Protocol for a systematic review. BMJ Open 2020, 10, e036689. [Google Scholar] [CrossRef]
- Roman, A.J.; Cideciyan, A.V.; Wu, V.; Garafalo, A.V.; Jacobson, S.G. Full-field stimulus testing: Role in the clinic and as an outcome measure in clinical trials of severe childhood retinal disease. Prog. Retin. Eye Res. 2021, 87, 101000. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims; FDA: Rockville, MD, USA, 2009. Available online: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM193282.pdf (accessed on 28 June 2023).
- Lacy, G.D.; Abalem, M.F.; Musch, D.C.; Jayasundera, K.T. Patient-reported outcome measures in inherited retinal degeneration gene therapy trials. Ophthalmic Genet. 2020, 41, 1–6. [Google Scholar] [CrossRef]
- Mangione, C.M.; Lee, P.P.; Gutierrez, P.R.; Spritzer, K.; Berry, S.; Hays, R.D. Development of the 25-list-item National Eye Institute Visual Function Questionnaire. Arch. Ophthalmol. 2001, 119, 1050–1058. [Google Scholar] [CrossRef]
- Klein, R.; Moss, S.E.; Klein, B.E.K.; Gutierrez, P.; Mangione, C.M. The NEI-VFQ-25 in People With Long-term Type 1 Diabetes Mellitus. Arch. Ophthalmol. 2001, 119, 733–740. [Google Scholar] [CrossRef]
- Cahill, M.; Banks, A.; Stinnett, S.; Toth, C. Vision-related quality of life in patients with bilateral severe age-related macular degeneration. Ophthalmology 2005, 112, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Nordmann, J.-P.; Viala, M.; Sullivan, K.; Arnould, B.; Berdeaux, G. Psychometric Validation of the National Eye Institute Visual Function Questionnaire—25 (NEI VFQ-25) French Version. Pharmacoeconomics 2004, 22, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Suzukamo, Y.; Oshika, T.; Yuzawa, M.; Tokuda, Y.; Tomidokoro, A.; Oki, K.; Mangione, C.M.; Green, J.; Fukuhara, S. Psychometric properties of the 25-item National Eye Institute Visual Function Questionnaire (NEI VFQ-25), Japanese version. Health Qual. Life Outcomes 2005, 3, 65. [Google Scholar] [CrossRef] [PubMed]
- Costela, F.M.; Pesudovs, K.; Sandberg, M.A.; Weigel-DiFranco, C.; Woods, R.L. Validation of a vision-related activity scale for patients with retinitis pigmentosa. Health Qual. Life Outcomes 2020, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, T.; Hagiwara, A.; Hiramatsu, A.; Ogata, K.; Mitamura, Y.; Yamamoto, S. Relationship between peripheral visual field loss and vision-related quality of life in patients with retinitis pigmentosa. Eye 2009, 24, 535–539. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Cochrane, A.L. Design of the low vision quality-of-life questionnaire (LVQOL) and measuring the outcome of low-vision rehabilitation. Am. J. Ophthalmol. 2000, 130, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Schofield, D.; Kraindler, J.; Tan, O.; Shrestha, R.; Jelovic, D.; West, S.; Ma, A.; Grigg, J.; Jamieson, R.V. Patient-Reported Health-Related Quality of Life in Individuals with Inherited Retinal Diseases. Ophthalmol. Sci. 2022, 2, 100106. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services FDA Center for Drug Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Biologics Evaluation and Research; U.S. Department of Health and Human Services FDA Center for Devices and Radiological Health. Guidance for industry: Patient-reported outcome measures: Use in medical product development to support labeling claims: Draft guidance. Health Qual. Life Outcomes 2006, 4, 79. [Google Scholar] [CrossRef]
- Scanlon, P.H.; Loftus, J.; Starita, C.; Stratton, I.M. The use of weighted health-related Quality of Life scores in people with diabetic macular oedema at baseline in a randomized clinical trial. Diabet. Med. 2014, 32, 97–101. [Google Scholar] [CrossRef]
- Chung, D.C.; McCague, S.; Yu, Z.-F.; Thill, S.; DiStefano-Pappas, J.; Bennett, J.; Cross, D.; Marshall, K.; Wellman, J.; High, K.A. Novel mobility test to assess functional vision in patients with inherited retinal dystrophies. Clin. Exp. Ophthalmol. 2017, 46, 247–259. [Google Scholar] [CrossRef]
- Spaide, R.F.; Curcio, C.A. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography. Retina 2011, 31, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Zhou, L.; Locke, K.G.; Birch, D.G.; Hood, D.C. A Comparison of Methods for Tracking Progression in X-Linked Retinitis Pigmentosa Using Frequency Domain OCT. Transl. Vis. Sci. Technol. 2013, 2, 5. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Oldani, M.; Jolly, J.; Edwards, T.; Groppe, M.; Downes, S.M.; MacLaren, R. Correlation of Optical Coherence Tomography and Autofluorescence in the Outer Retina and Choroid of Patients With Choroideremia. Investig. Opthalmol. Vis. Sci. 2016, 57, 3674–3684. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Oke, S.; MacDonald, I.M. Validating Ellipsoid Zone Area Measurement With Multimodal Imaging in Choroideremia. Transl. Vis. Sci. Technol. 2021, 10, 17. [Google Scholar] [CrossRef]
- Birtel, J.; Salvetti, A.P.; Jolly, J.K.; Xue, K.; Gliem, M.; Müller, P.L.; Holz, F.G.; MacLaren, R.E.; Issa, P.C. Near-Infrared Autofluorescence in Choroideremia: Anatomic and Functional Correlations. Am. J. Ophthalmol. 2018, 199, 19–27. [Google Scholar] [CrossRef]
- Dubis, A.; Lim, W.; Jolly, J.; Toms, M.; MacLaren, R.; Webster, A.; Moosajee, M. Longitudinal Study to Assess the Quantitative Use of Fundus Autofluorescence for Monitoring Disease Progression in Choroideremia. J. Clin. Med. 2021, 10, 232. [Google Scholar] [CrossRef]
- Poli, F.E.; Yusuf, I.H.; Jolly, J.K.; Taylor, L.J.; Adejoyu, D.; Josan, A.S.; Kapetanovic, J.C.; da Cruz, L.; MacLaren, R.E. Correlation between fundus autofluorescence pattern and retinal sensitivity measured by microperimetry in patients with choroideremia. Investig. Ophthalmol. Vis. Sci. 2022, 63, 3788-F0209. [Google Scholar]
- Burns, S.A.; Elsner, A.E.; Sapoznik, K.A.; Warner, R.L.; Gast, T.J. Adaptive optics imaging of the human retina. Prog. Retin. Eye Res. 2018, 68, 1–30. [Google Scholar] [CrossRef]
- Foote, K.G.; Wong, J.J.; Boehm, A.E.; Bensinger, E.; Porco, T.C.; Roorda, A.; Duncan, J.L. Comparing Cone Structure and Function in RHO- and RPGR-Associated Retinitis Pigmentosa. Investig. Opthalmol. Vis. Sci. 2020, 61, 42. [Google Scholar] [CrossRef]
- Cunefare, D.; Fang, L.; Cooper, R.F.; Dubra, A.; Carroll, J.; Farsiu, S. Open source software for automatic detection of cone photoreceptors in adaptive optics ophthalmoscopy using convolutional neural networks. Sci. Rep. 2017, 7, 6620. [Google Scholar] [CrossRef]
- Aleman, T.S.; Miller, A.J.; Maguire, K.H.; Aleman, E.M.; Serrano, L.W.; O’Connor, K.B.; Bedoukian, E.C.; Leroy, B.P.; Maguire, A.M.; Bennett, J. A Virtual Reality Orientation and Mobility Test for Inherited Retinal Degenerations: Testing a Proof-of-Concept After Gene Therapy. Clin. Ophthalmol. 2021, 15, 939–952. [Google Scholar] [CrossRef] [PubMed]
- Parodi, M.B.; Arrigo, A.; MacLaren, R.E.; Aragona, E.; Toto, L.; Mastropasqua, R.; Manitto, M.P.; Bandello, F. Vascular alterations revealed with optical coherence tomography angiography in patients with choroideremia. Retina 2019, 39, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Arrigo, A.; Romano, F.; Parodi, M.B.; Issa, P.C.; Birtel, J.; Bandello, F.; MacLaren, R.E. Reduced vessel density in deep capillary plexus correlates with retinal layer thickness in choroideremia. Br. J. Ophthalmol. 2020, 105, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Tolmachova, T.; Tolmachov, O.E.; Barnard, A.R.; de Silva, S.R.; Lipinski, D.M.; Walker, N.J.; MacLaren, R.E.; Seabra, M.C. Functional expression of Rab escort protein 1 following AAV2-mediated gene delivery in the retina of choroideremia mice and human cells ex vivo. J. Mol. Med. 2013, 91, 825–837. [Google Scholar] [CrossRef]
- Fry, L.E.; Patrício, M.I.; Williams, J.; Aylward, J.W.; Hewitt, H.; Clouston, P.; Xue, K.; Barnard, A.R.; MacLaren, R.E. Association of Messenger RNA Level with Phenotype in Patients with Choroideremia. JAMA Ophthalmol. 2020, 138, 128–135. [Google Scholar] [CrossRef]
- Cideciyan, A.V.; Jacobson, S.G.; Drack, A.V.; Ho, A.C.; Charng, J.; Garafalo, A.V.; Roman, A.J.; Sumaroka, A.; Han, I.C.; Hochstedler, M.D.; et al. Effect of an intravitreal antisense oligonucleotide on vision in Leber congenital amaurosis due to a photoreceptor cilium defect. Nat. Med. 2018, 25, 225–228. [Google Scholar] [CrossRef]
- Chung, D.C.; Bertelsen, M.; Lorenz, B.; Pennesi, M.E.; Leroy, B.P.; Hamel, C.P.; Pierce, E.; Sallum, J.; Larsen, M.; Stieger, K.; et al. The Natural History of Inherited Retinal Dystrophy Due to Biallelic Mutations in the RPE65 Gene. Am. J. Ophthalmol. 2018, 199, 58–70. [Google Scholar] [CrossRef]
- Maclaren, R.E. Assessment of safety and efficacy of subretinal timrepigene emparvovec in adult men with choroideremia in a randomized parallel-controlled phase 3 trial: The STAR Study. In Proceedings of the Seventh Annual Retinal Cell and Gene Therapy Innovation Summit, Denver, CO, USA, 29 April 2022. [Google Scholar]
- Russell, S.; Bennett, J.; Wellman, J.A.; Chung, D.C.; Yu, Z.-F.; Tillman, A.; Wittes, J.; Pappas, J.; Elci, O.; McCague, S.; et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet 2017, 390, 849–860. [Google Scholar] [CrossRef]
- Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: The Scandinavian Simvastatin Survival Study (4S). Lancet 1994, 344, 1383–1389. [Google Scholar]
| Clinicaltrials.gov Identifier and Study Start Date | Phase | Drug Design | Location | Vector | Study Status | Outcome | Reference |
|---|---|---|---|---|---|---|---|
| 01461213 (October 2011) | I/II | r.AAV-REP1 | University of Oxford, UK | Subretinal | Completed | 3.5 years—Two patients with poor baseline BCVA gained 11 and 21 letters. Three patients with good baseline BCVA maintained BCVA. One patient had a surgical complication leading to a lower dose of the vector and had a decline in BCVA from 6 months to 3.5 years, likely due to degeneration in the fovea. | [20,21,22] |
| 02341807 (January 2015) | I/II | AAV2-hCHM | University of Philadelphia, USA | Subretinal | Active, not recruiting | 2 years—unchanged BCVA in 13/15. Acute foveal thinning in one patient. Macular hole in one patient. | |
| 02077361 (April 2015) | I/II | r.AAV-REP1 | University of Alberta, Edmonton, Alberta, Canada | Subretinal | Completed | 2 years—BCVA change of −8 to >15 letters. One serious adverse event: a localized intraretinal immune response. | [23] |
| 02553135 (September 2015) | II | r.AAV-REP1 | University of Miami, Miami, USA | Subretinal | Completed | 2 years—BCVA change of −1 to +10 letters. | [24] |
| 02671539 (January 2016) | II | r.AAV-REP1 | Tuebingen, Germany | Subretinal | Completed | 2 years—mean change in BCVA of +3.7 letters. | [25,26] |
| 02407678 (August 2016) | II | r.AAV-REP1 | University College London & University of Oxford, UK | Subretinal | Completed | Awaited | |
| 03507686 (November 2017) | II | r.AAV-REP1 | Gemini, Biogen | Subretinal | Completed | Awaited | |
| 03496012 (December 2017) | III | Low-dose and high-dose r.AAV-REP1 | STAR, Biogen | Subretinal | Completed | Failed to meet primary and secondary endpoints. | |
| 04483440 (June 2020) | 1 | AAV capsid variant (4D-100) carrying a transgene encoding a codon-optimized human CHM gene | 4D Molecular Therapeutics | Intravitreal | Ongoing | Initial clinical safety data at both of the two dose levels in the 4D molecular therapeutics trial indicate that it is well tolerated and did not result in any dose-limiting toxicity (n = 6; all patients followed up for between one and nine months). |
| Stage | Defining Characteristics | Potentially Appropriate Outcome Measures |
|---|---|---|
| Early stage | Rod degeneration in the mid-peripheral region with some secondary cone involvement and reduced para-central retinal sensitivity. | - Full field perimetry (static) - Mesopic/scotopic microperimetry - Patient-reported outcomes - OCT - FAF - Infrared AF |
| Moderate stage | Radial advancement of rod and secondary cone degeneration with a scotomatous mid-peripheral region and reduced retinal sensitivity encroaching the macula. | - Full field perimetry (static or kinetic) - Mesopic microperimetry - Patient-reported outcomes - OCT - FAF - BCVA/LLVA |
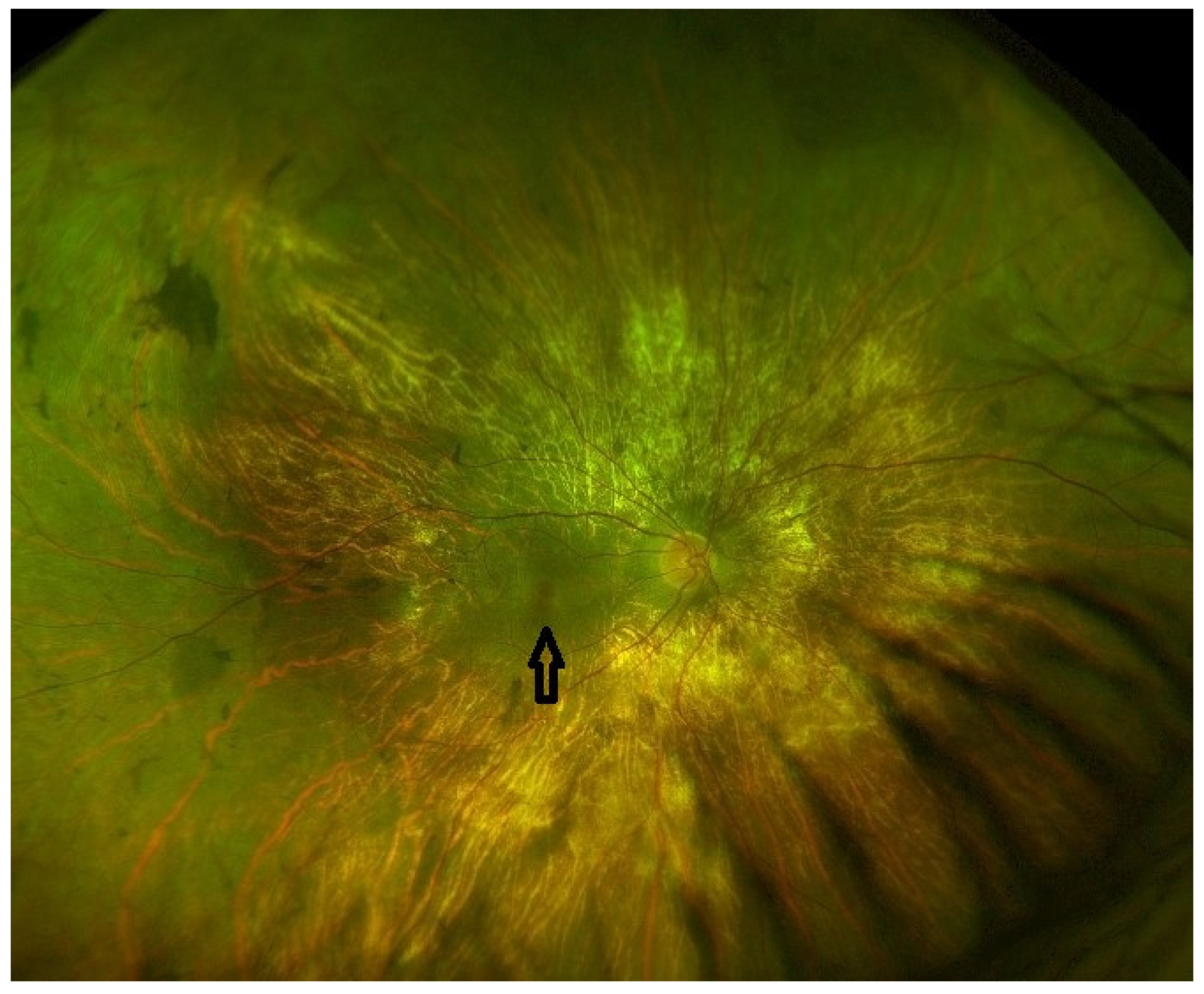
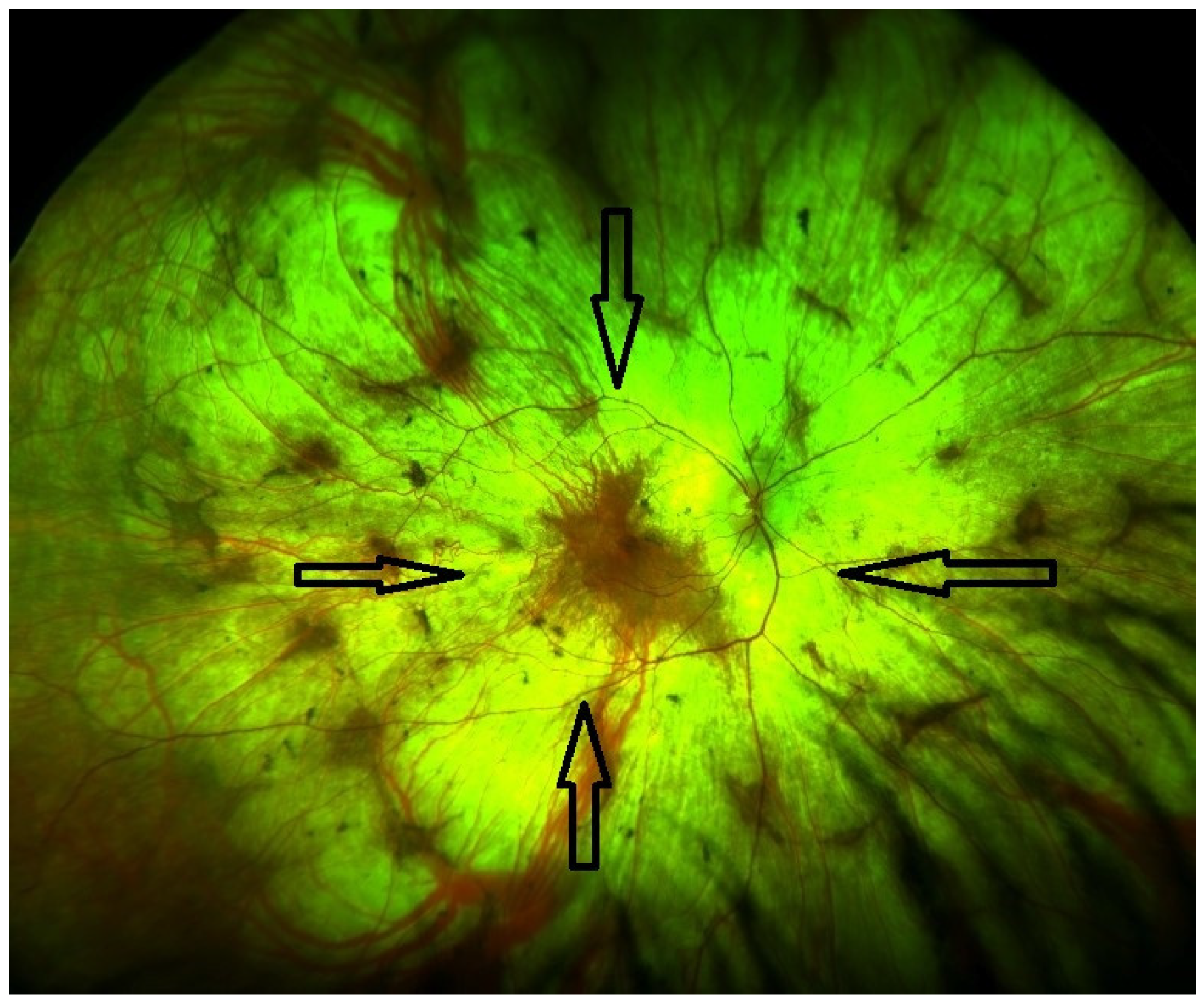
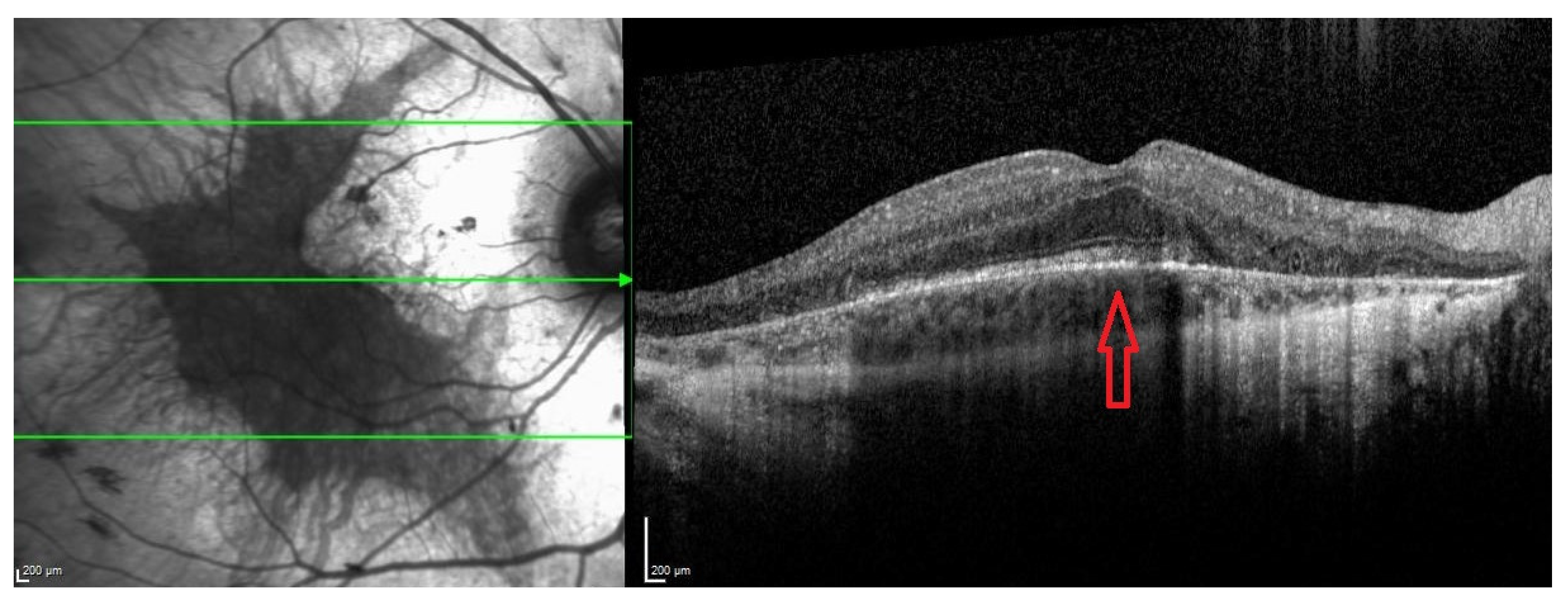
| Late stage | Defined by the encroachment of scotomas and/or reduced sensitivity well within the vascular arcades and encroaching the central region (Figure 1 and Figure 2). | - Full field perimetry (static or kinetic) - Mesopic microperimetry - Patient-reported outcomes - OCT (Figure 3) - FAF - FST - BCVA/LLVA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdalla Elsayed, M.E.A.; Taylor, L.J.; Josan, A.S.; Fischer, M.D.; MacLaren, R.E. Choroideremia: The Endpoint Endgame. Int. J. Mol. Sci. 2023, 24, 14354. https://doi.org/10.3390/ijms241814354
Abdalla Elsayed MEA, Taylor LJ, Josan AS, Fischer MD, MacLaren RE. Choroideremia: The Endpoint Endgame. International Journal of Molecular Sciences. 2023; 24(18):14354. https://doi.org/10.3390/ijms241814354
Chicago/Turabian StyleAbdalla Elsayed, Maram E. A., Laura J. Taylor, Amandeep S. Josan, M. Dominik Fischer, and Robert E. MacLaren. 2023. "Choroideremia: The Endpoint Endgame" International Journal of Molecular Sciences 24, no. 18: 14354. https://doi.org/10.3390/ijms241814354
APA StyleAbdalla Elsayed, M. E. A., Taylor, L. J., Josan, A. S., Fischer, M. D., & MacLaren, R. E. (2023). Choroideremia: The Endpoint Endgame. International Journal of Molecular Sciences, 24(18), 14354. https://doi.org/10.3390/ijms241814354





