Identification of Novel Glycans in the Mucus Layer of Shark and Skate Skin
Abstract
:1. Introduction
2. Results
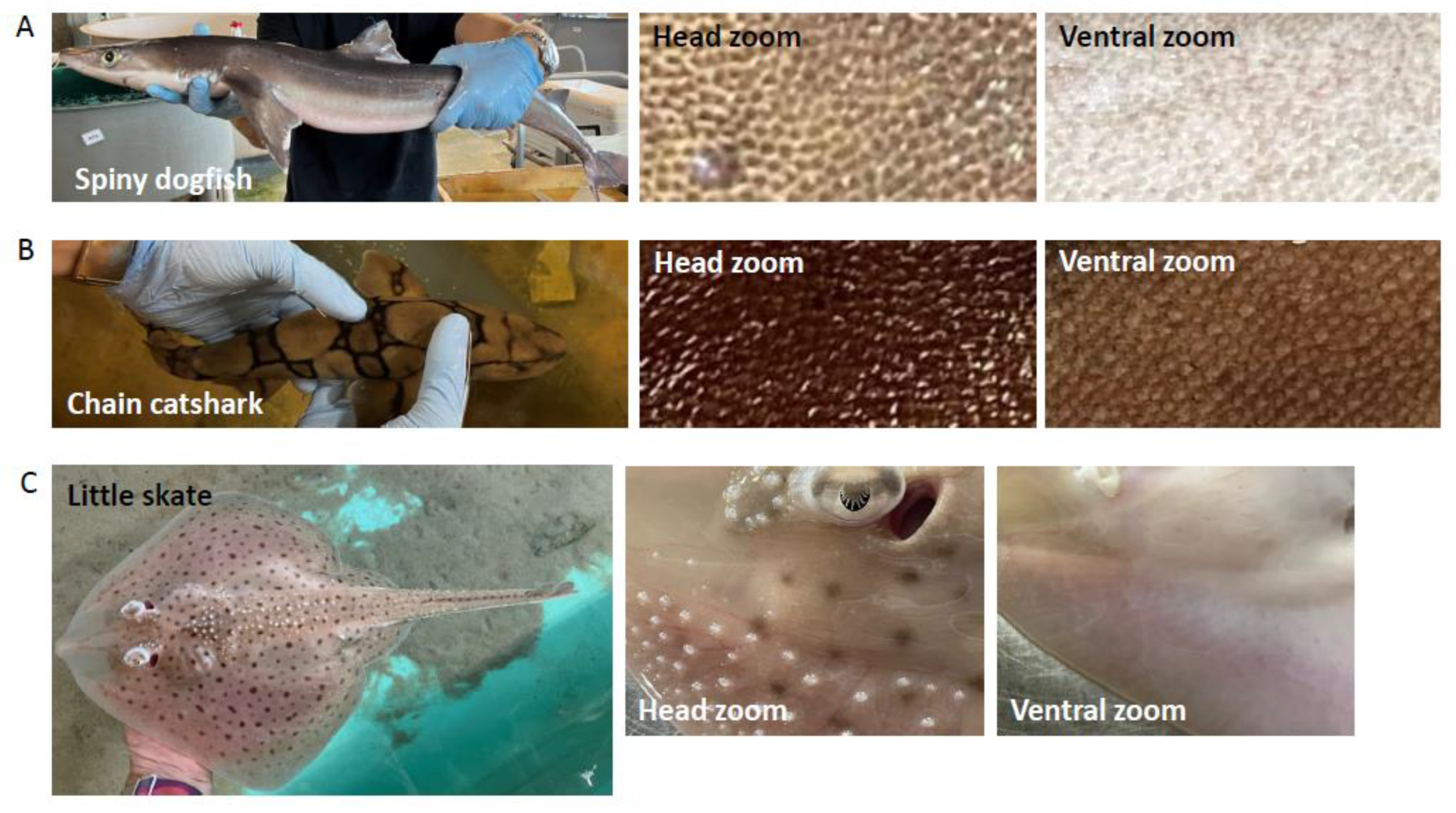
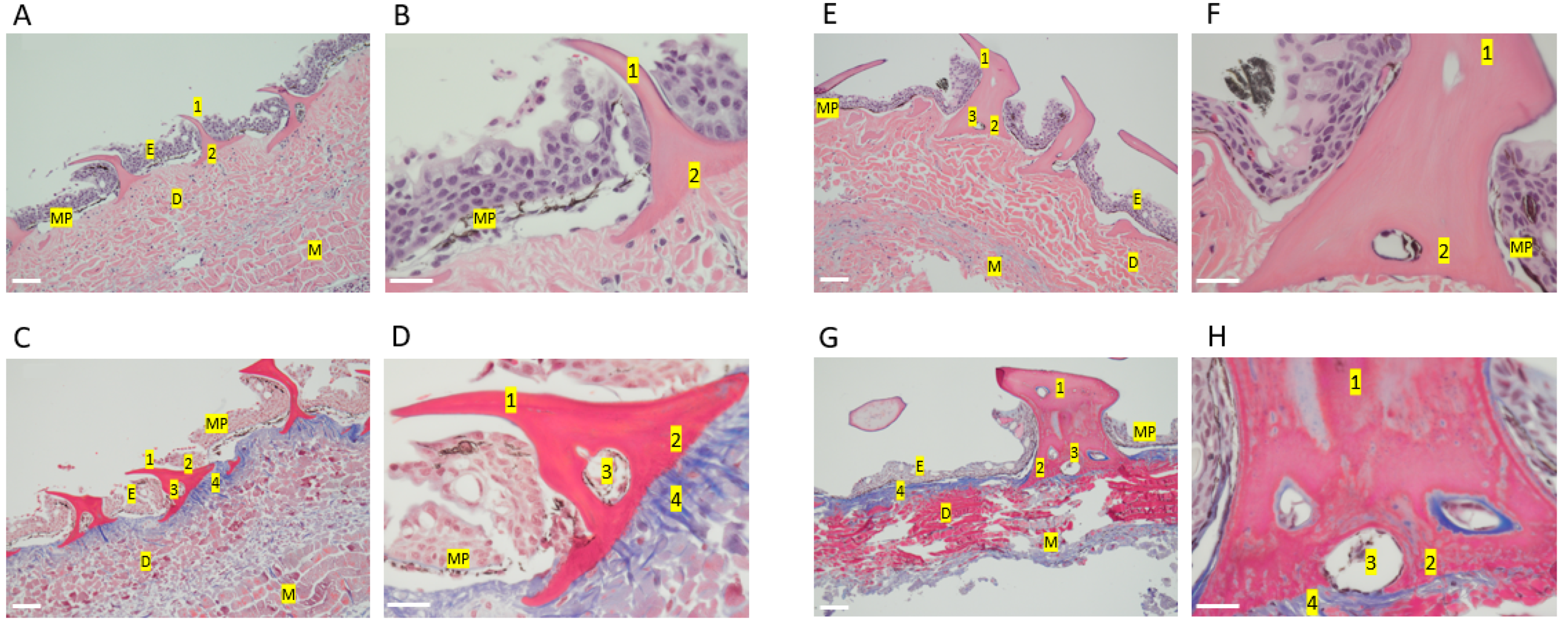
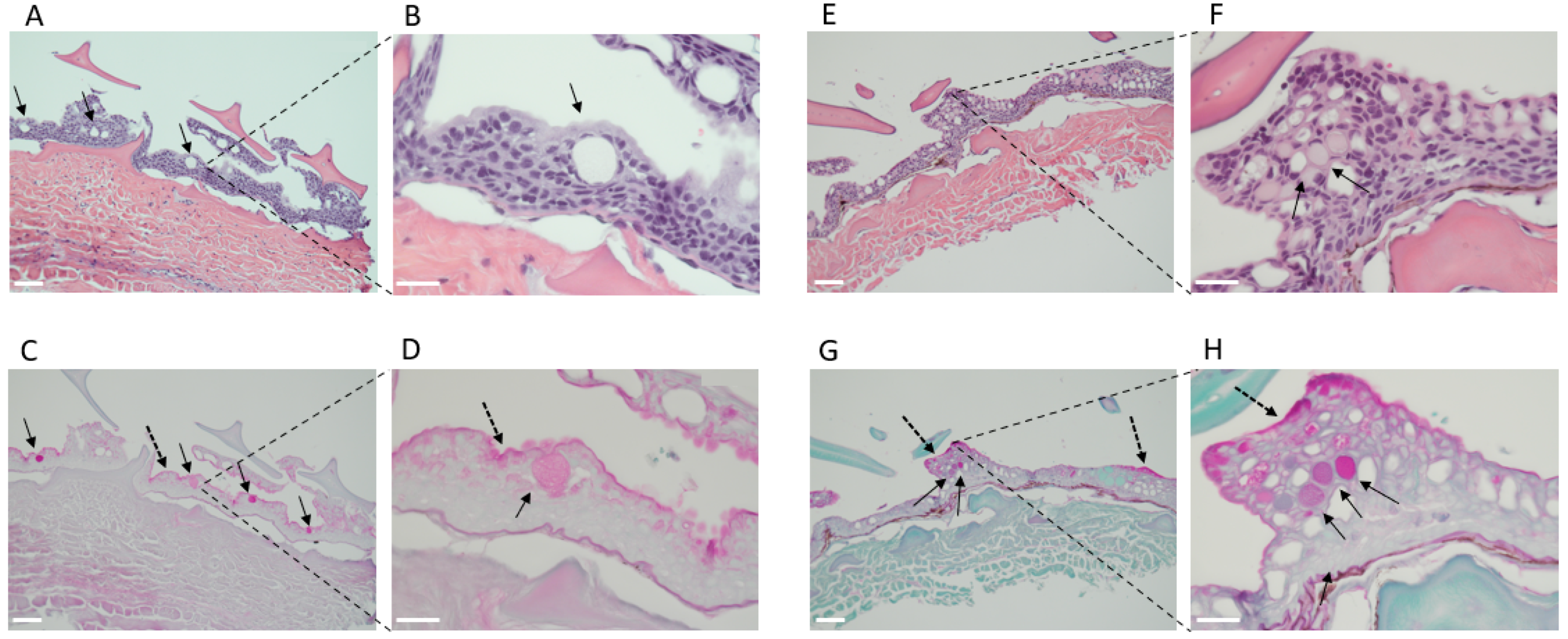
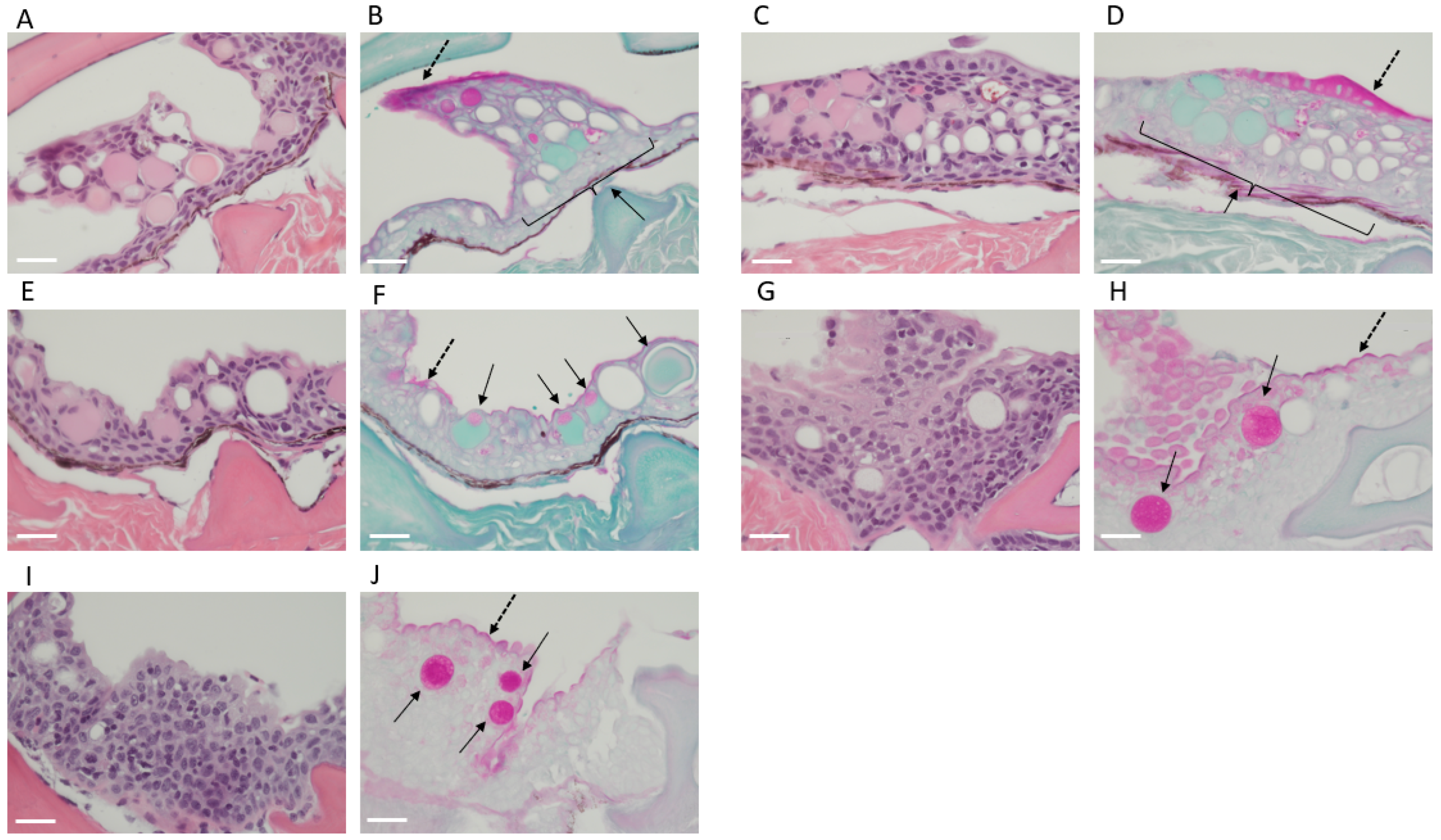
2.1. Shark Skin Histology
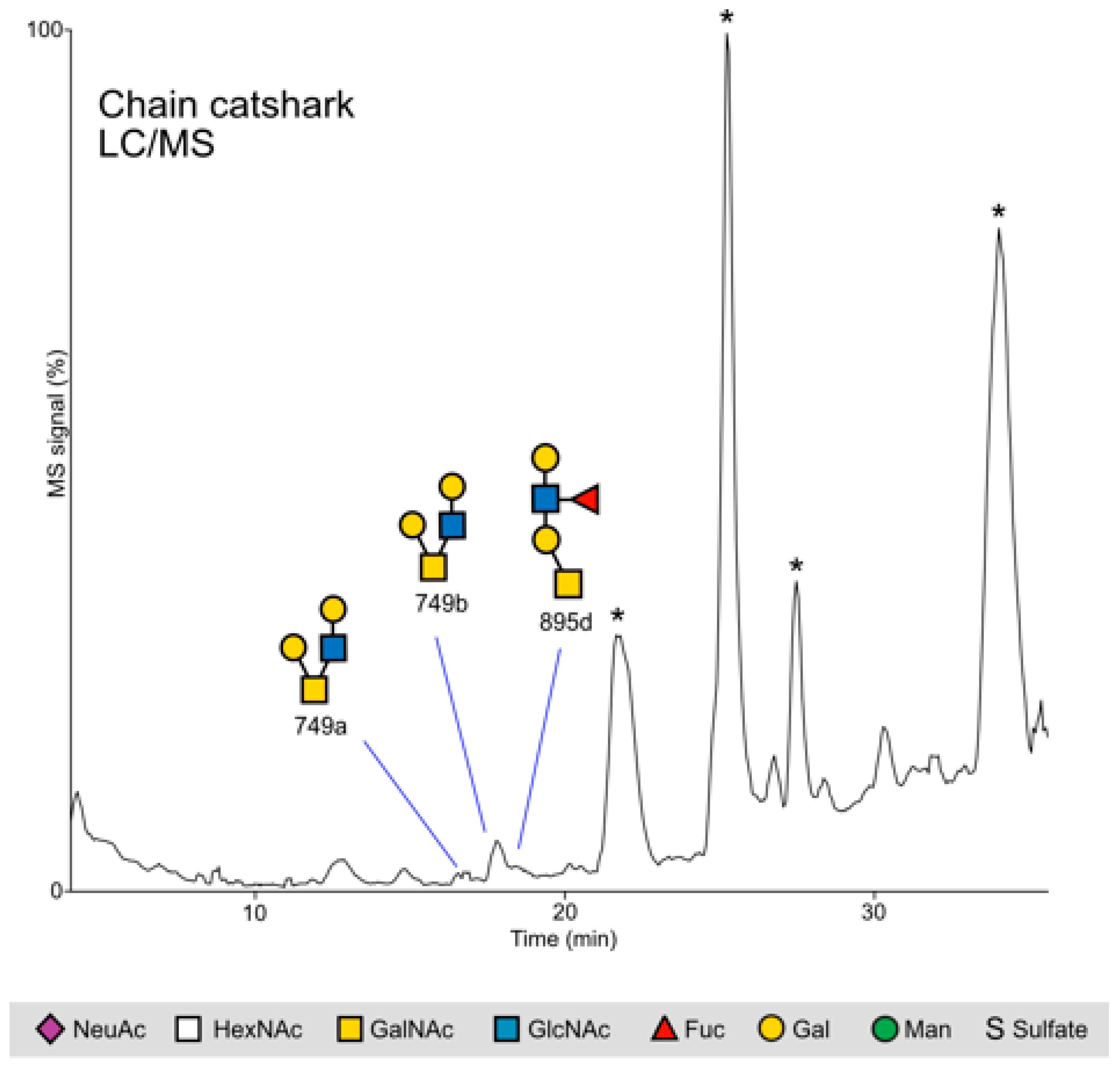
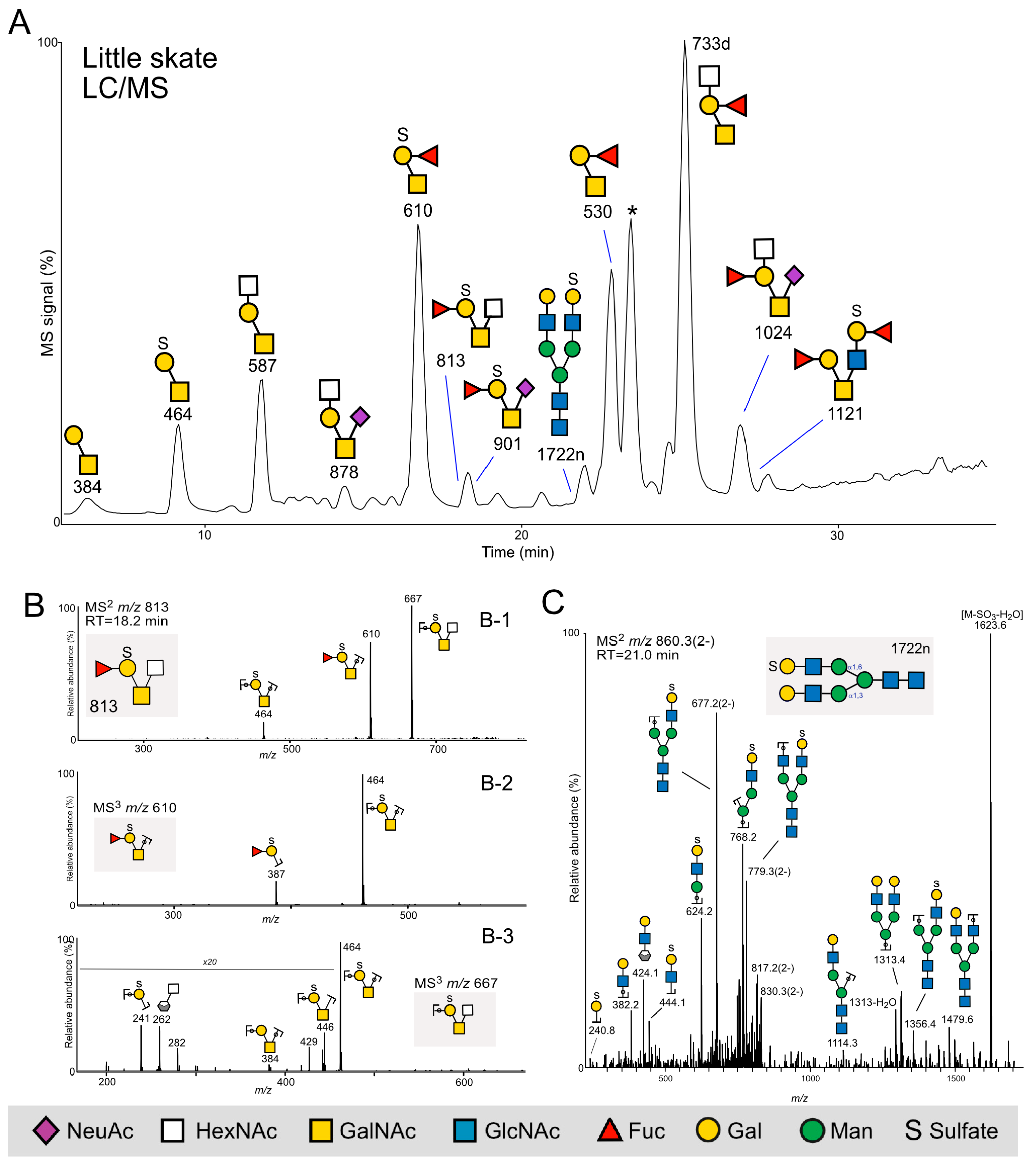
2.2. Glycan Mass Spectrometry
2.2.1. Spiny Dogfish
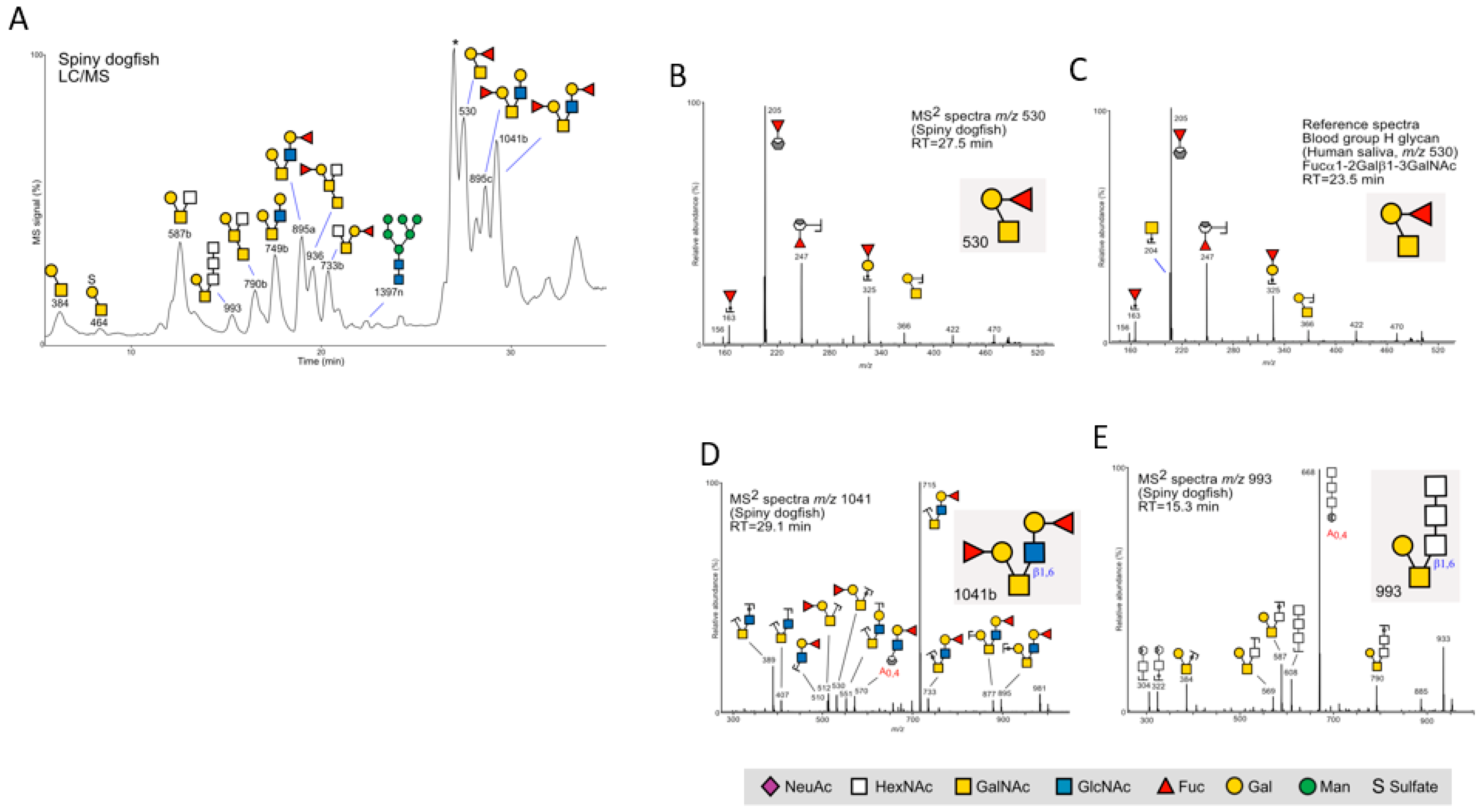
2.2.2. Chain Catshark
2.2.3. Little Skate
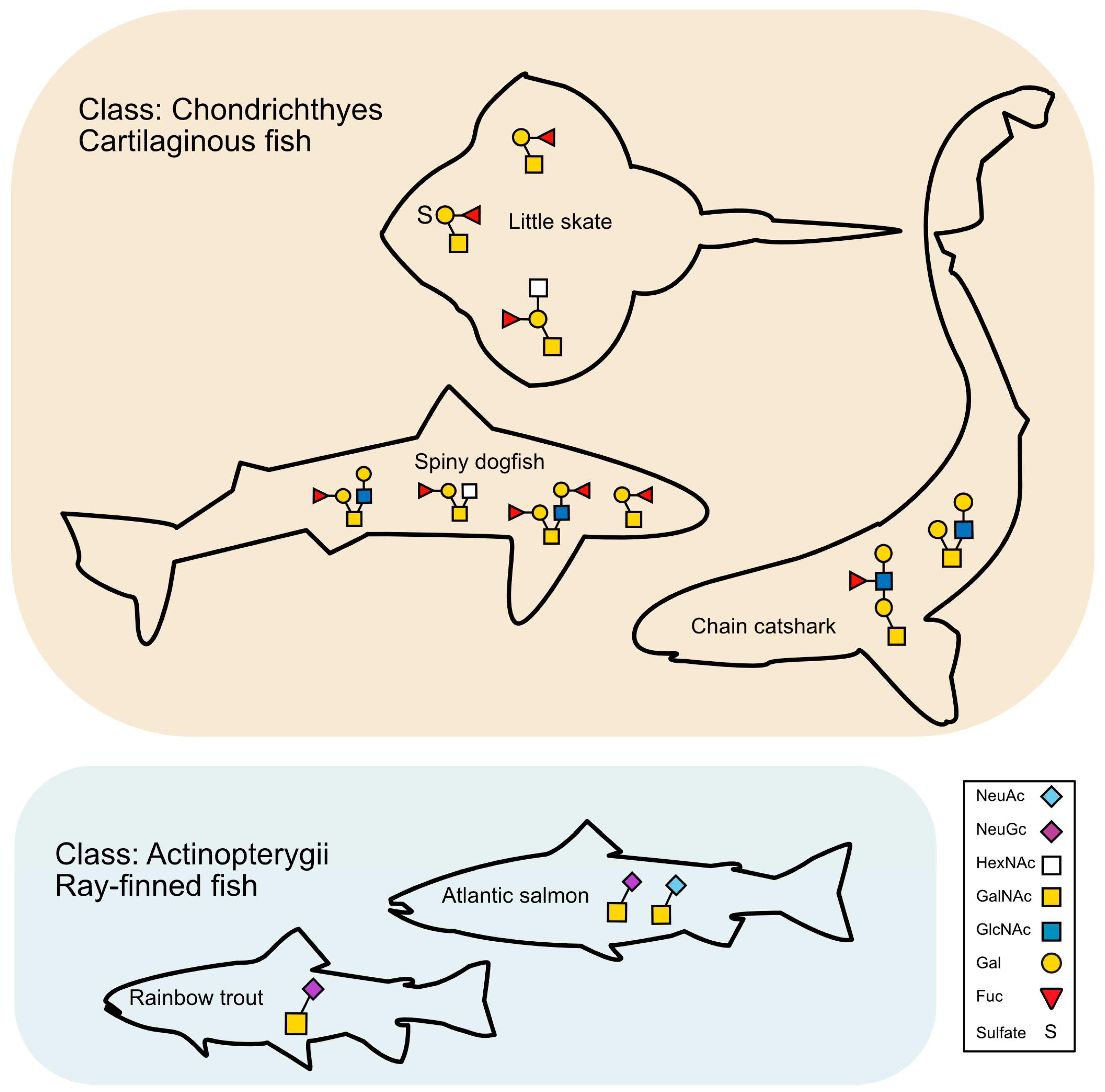
3. Discussion
3.1. The Mucus Layer in Bony Fish and Elasmobranchs
3.2. Shark Skin Histology
3.3. Glycans Unique to Elasmobranchs and Potential Roles
3.4. N-Glycans
3.5. Study Limitations
4. Materials and Methods
4.1. Animals
4.2. Skin Mucus Sampling
4.3. Skin Biopsy Sampling and Histology
4.4. Mass Spectrometry
4.4.1. Glycan Release
4.4.2. Glycan Analyses with LC/MS
4.4.3. MS2 Spectra Interpretation
4.4.4. Chemicals
4.4.5. Pilot Experiments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Hex | Gal (galactose); |
| HexNAc | N-acetylhexosamine; |
| GalNAc | N-acetylgalactosamine; |
| GlcNAc | N-acetylglucosamine; |
| Deoxyhexose | Fuc (fucose); |
| SO3 | Sulfate; |
| NeuAc | N-acetylneuraminic acid; |
| MS/MS | Tandem mass spectrometry; |
| LC–MS | Liquid chromatography–mass spectrometry. |
References
- Moore, K.S.; Wehrli, S.; Roder, H.; Rogers, M.; Forrest, J.N., Jr.; McCrimmon, D.; Zasloff, M. Squalamine: An aminosterol antibiotic from the shark. Proc. Natl. Acad. Sci. USA 1993, 90, 1354–1358. [Google Scholar] [CrossRef] [PubMed]
- Forrest, J.N., Jr. The Shark Rectal Gland Model: A Champion of Receptor Mediated Chloride Secretion through Cftr. Trans. Am. Clin. Climatol. Assoc. 2016, 127, 162–175. [Google Scholar] [PubMed]
- Henrikson, R.C.; Matoltsy, A.G. The fine structure of teleost epidermis. II. Mucous cells. J. Ultrastruct. Res. 1967, 21, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Henrikson, R.C.; Matoltsy, A.G. The fine structure of teleost epidermis. 1. Introduction and filament-containing cells. J. Ultrastruct. Res. 1967, 21, 194–212. [Google Scholar] [CrossRef] [PubMed]
- Whitear, M. The skin of fishes including cyclostomes. In Biology of the Integument. II, Vertebrates; Bereiter-Hahn, J., Matoltsy, A.G., Ricards, K.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 8–73. [Google Scholar]
- Meyer, W.; Seegers, U.; Stelzer, R. Sulphur, thiols, and disulphides in the fish epidermis, with remarks on keratinization. J. Fish Biol. 2007, 71, 1135–1144. [Google Scholar] [CrossRef]
- Webb, A.E.; Kimelman, D. Analysis of early epidermal development in zebrafish. Methods Mol. Biol. 2005, 289, 137–146. [Google Scholar] [CrossRef]
- Shephard, K.L. Functions for fish mucus. Rev. Fish. Biol. Fish. 1994, 4, 401–429. [Google Scholar] [CrossRef]
- Noga, E.J. Skin ulcers in fish: Pfiesteria and other etiologies. Toxicol. Pathol. 2000, 28, 807–823. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef]
- Brockhausen, I.; Schachter, H.; Stanley, P. O-GalNAc Glycans. In Essentials of Glycobiology, 2nd ed.; Varki, A., Cummings, R.D., Esko, J.D., Eds.; Cold Spring Harbour Press: Cold Spring Harbour, NY, USA, 2009. [Google Scholar]
- Watson, M.E.; Diepeveen, L.A.; Stubbs, K.A.; Yeoh, G.C. Glycosylation-related Diagnostic and Therapeutic Drug Target Markers in Hepatocellular Carcinoma. J. Gastrointestin Liver Dis. 2015, 24, 349–357. [Google Scholar] [CrossRef]
- Wasik, B.R.; Barnard, K.N.; Parrish, C.R. Effects of Sialic Acid Modifications on Virus Binding and Infection. Trends Microbiol. 2016, 24, 991–1001. [Google Scholar] [CrossRef]
- Jin, C.; Padra, J.T.; Sundell, K.; Sundh, H.; Karlsson, N.G.; Linden, S.K. Atlantic Salmon Carries a Range of Novel O-Glycan Structures Differentially Localized on Skin and Intestinal Mucins. J. Proteome Res. 2015, 14, 3239–3251. [Google Scholar] [CrossRef]
- Thomsson, K.A.; Benktander, J.; Quintana-Hayashi, M.P.; Sharba, S.; Linden, S.K. Mucin O-glycosylation and pathogen binding ability differ between rainbow trout epithelial sites. Fish. Shellfish Immunol. 2022, 131, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Benktander, J.; Sundh, H.; Sundell, K.; Murugan, A.V.M.; Venkatakrishnan, V.; Padra, J.T.; Kolarevic, J.; Terjesen, B.F.; Gorissen, M.; Linden, S.K. Stress Impairs Skin Barrier Function and Induces alpha2-3 Linked N-Acetylneuraminic Acid and Core 1 O-Glycans on Skin Mucins in Atlantic Salmon, Salmo salar. Int. J. Mol. Sci. 2021, 22, 1488. [Google Scholar] [CrossRef]
- Ankhelyi, M.V.; Wainwright, D.K.; Lauder, G.V. Diversity of dermal denticle structure in sharks: Skin surface roughness and three-dimensional morphology. J. Morphol. 2018, 279, 1132–1154. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.L.; Nicklin, E.F.; Rasch, L.J.; Fraser, G.J. Teeth outside the mouth: The evolution and development of shark denticles. Evol. Dev. 2023, 25, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Gabler-Smith, M.K.; Wainwright, D.K.; Wong, G.A.; Lauder, G.V. Dermal Denticle Diversity in Sharks: Novel Patterns on the Interbranchial Skin. Integr. Org. Biol. 2021, 3, obab034. [Google Scholar] [CrossRef]
- Ritter, E.K.; Amin, R.W. Mating scars among sharks: Evidence of coercive mating? Acta Ethol. 2019, 22, 9–16. [Google Scholar] [CrossRef]
- Genten, F.; Terwinghe, E.; Danguy, A. Atlas of Fish Histology; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Benktander, J.; Venkatakrishnan, V.; Padra, J.T.; Sundh, H.; Sundell, K.; Murugan, A.V.M.; Maynard, B.; Linden, S.K. Effects of Size and Geographical Origin on Atlantic salmon, Salmo salar, Mucin O-Glycan Repertoire. Mol. Cell Proteom. 2019, 18, 1183–1196. [Google Scholar] [CrossRef]
- Padra, J.T.; Linden, S.K. Optimization of Alcian blue pH 1.0 histo-staining protocols to match mass spectrometric quantification of sulfomucins and circumvent false positive results due to sialomucins. Glycobiology 2022, 32, 6–10. [Google Scholar] [CrossRef]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol Nomenclature for Graphical Representations of Glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef] [PubMed]
- Ebran, N.; Julien, S.; Orange, N.; Auperin, B.; Molle, G. Isolation and characterization of novel glycoproteins from fish epidermal mucus: Correlation between their pore-forming properties and their antibacterial activities. Biochim. Biophys. Acta 2000, 1467, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Abdayem, R.; Formanek, F.; Minondo, A.M.; Potter, A.; Haftek, M. Cell surface glycans in the human stratum corneum: Distribution and depth-related changes. Exp. Dermatol. 2016, 25, 865–871. [Google Scholar] [CrossRef]
- Venkatakrishnan, V.; Padra, J.T.; Sundh, H.; Sundell, K.; Jin, C.; Langeland, M.; Carlberg, H.; Vidakovic, A.; Lundh, T.; Karlsson, N.G.; et al. Exploring the Arctic Charr Intestinal Glycome: Evidence of Increased N-Glycolylneuraminic Acid Levels and Changed Host-Pathogen Interactions in Response to Inflammation. J. Proteome Res. 2019, 18, 1760–1773. [Google Scholar] [CrossRef]
- Reverter, M.; Tapissier-Bontemps, N.; Lecchini, D.; Banaigs, B.; Sasal, P. Biological and Ecological Roles of External Fish Mucus: A Review. Fishes 2018, 3, 41. [Google Scholar] [CrossRef]
- Gomez, D.; Sunyer, J.O.; Salinas, I. The mucosal immune system of fish: The evolution of tolerating commensals while fighting pathogens. Fish. Shellfish Immunol. 2013, 35, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Bechert, D.W.; Bartenwerfer, M. The viscous flow on surfaces with longitudinal ribs. J. Fluid. Mech. 1989, 206, 105–129. [Google Scholar] [CrossRef]
- Meyer, W.; Seegers, U. Basics of skin structure and function in elasmobranchs: A review. J. Fish. Biol. 2012, 80, 1940–1967. [Google Scholar] [CrossRef]
- Tsutsui, S.; Dotsuta, Y.; Ono, A.; Suzuki, M.; Tateno, H.; Hirabayashi, J.; Nakamura, O. A C-type lectin isolated from the skin of Japanese bullhead shark (Heterodontus japonicus) binds a remarkably broad range of sugars and induces blood coagulation. J. Biochem. 2015, 157, 345–356. [Google Scholar] [CrossRef]
- Perry, C.T.; Pratte, Z.A.; Clavere-Graciette, A.; Ritchie, K.B.; Hueter, R.E.; Newton, A.L.; Fischer, G.C.; Dinsdale, E.A.; Doane, M.P.; Wilkinson, K.A.; et al. Elasmobranch microbiomes: Emerging patterns and implications for host health and ecology. Anim. Microbiome 2021, 3, 61. [Google Scholar] [CrossRef]
- Ritchie, K.B.; Schwarz, M.; Mueller, J.; Lapacek, V.A.; Merselis, D.; Walsh, C.J.; Luer, C.A. Survey of Antibiotic-producing Bacteria Associated with the Epidermal Mucus Layers of Rays and Skates. Front. Microbiol. 2017, 8, 1050. [Google Scholar] [CrossRef] [PubMed]
- Quintero-Hunter, I.; Grier, H.; Muscato, M. Enhancement of histological detail using metanil yellow as counterstain in periodic acid Schiff’s hematoxylin staining of glycol methacrylate tissue sections. Biotech. Histochem. 1991, 66, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Yantiss, R.K. Diagnostic challenges in the pathologic evaluation of Barrett esophagus. Arch. Pathol. Lab. Med. 2010, 134, 1589–1600. [Google Scholar] [CrossRef] [PubMed]
- Linden, S.K.; Sutton, P.; Karlsson, N.G.; Korolik, V.; McGuckin, M.A. Mucins in the mucosal barrier to infection. Mucosal Immunol. 2008, 1, 183–197. [Google Scholar] [CrossRef]
- Giron, L.B.; Tanes, C.E.; Schleimann, M.H.; Engen, P.A.; Mattei, L.M.; Anzurez, A.; Damra, M.; Zhang, H.; Bittinger, K.; Bushman, F.; et al. Sialylation and fucosylation modulate inflammasome-activating eIF2 Signaling and microbial translocation during HIV infection. Mucosal Immunol. 2020, 13, 753–766. [Google Scholar] [CrossRef]
- Linden, S.K.; Sheng, Y.H.; Every, A.L.; Miles, K.M.; Skoog, E.C.; Florin, T.H.; Sutton, P.; McGuckin, M.A. MUC1 limits Helicobacter pylori infection both by steric hindrance and by acting as a releasable decoy. PLoS Pathog. 2009, 5, e1000617. [Google Scholar] [CrossRef]
- Amano, K.; Hazama, S.; Araki, Y.; Ito, E. Isolation and characterization of structural components of Bacillus cereus AHU 1356 cell walls. Eur. J. Biochem. 1977, 75, 513–522. [Google Scholar] [CrossRef]
- Martini, F.; Eckmair, B.; Stefanic, S.; Jin, C.; Garg, M.; Yan, S.; Jimenez-Castells, C.; Hykollari, A.; Neupert, C.; Venco, L.; et al. Highly modified and immunoactive N-glycans of the canine heartworm. Nat. Commun. 2019, 10, 75. [Google Scholar] [CrossRef]
- Chen, S.; Siu, K.C.; Wang, W.Q.; Liu, X.X.; Wu, J.Y. Structure and antioxidant activity of a novel poly-N-acetylhexosamine produced by a medicinal fungus. Carbohydr. Polym. 2013, 94, 332–338. [Google Scholar] [CrossRef]
- Rossez, Y.; Maes, E.; Lefebvre Darroman, T.; Gosset, P.; Ecobichon, C.; Joncquel Chevalier Curt, M.; Boneca, I.G.; Michalski, J.C.; Robbe-Masselot, C. Almost all human gastric mucin O-glycans harbor blood group A, B or H antigens and are potential binding sites for Helicobacter pylori. Glycobiology 2012, 22, 1193–1206. [Google Scholar] [CrossRef]
- Padra, J.T.; Murugan, A.V.M.; Sundell, K.; Sundh, H.; Benktander, J.; Linden, S.K. Fish pathogen binding to mucins from Atlantic salmon and Arctic char differs in avidity and specificity and is modulated by fluid velocity. PLoS ONE 2019, 14, e0215583. [Google Scholar] [CrossRef] [PubMed]
- Padra, J.T.; Pagneux, Q.; Bouckaert, J.; Jijie, R.; Sundh, H.; Boukherroub, R.; Szunerits, S.; Linden, S.K. Mucin modified SPR interfaces for studying the effect of flow on pathogen binding to Atlantic salmon mucins. Biosens. Bioelectron. 2019, 146, 111736. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Harduin-Lepers, A.; Kitajima, K.; Sato, C.; Huang, C.J.; Khoo, K.H.; Guerardel, Y. Developmental regulation of oligosialylation in zebrafish. Glycoconj. J. 2009, 26, 247–261. [Google Scholar] [CrossRef] [PubMed]
- Thomes, L.; Bojar, D. The Role of Fucose-Containing Glycan Motifs Across Taxonomic Kingdoms. Front. Mol. Biosci. 2021, 8, 755577. [Google Scholar] [CrossRef]
- Wang, T.T. IgG Fc Glycosylation in Human Immunity. Curr. Top. Microbiol. Immunol. 2019, 423, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Nakao, H.; Matsumoto, S.; Nagai, Y.; Kojima, A.; Toyoda, H.; Hashii, N.; Takakura, D.; Kawasaki, N.; Yamaguchi, T.; Kawabata, K.; et al. Characterization of glycoproteins expressing the blood group H type 1 epitope on human induced pluripotent stem (hiPS) cells. Glycoconj. J. 2017, 34, 779–787. [Google Scholar] [CrossRef]
- Larsen, R.D.; Ernst, L.K.; Nair, R.P.; Lowe, J.B. Molecular cloning, sequence, and expression of a human GDP-L-fucose:beta-D-galactoside 2-alpha-L-fucosyltransferase cDNA that can form the H blood group antigen. Proc. Natl. Acad. Sci. USA 1990, 87, 6674–6678. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Ryu, J.S.; Ko, J.H.; Kim, J.Y.; Kim, H.J.; Lee, H.J.; Oh, J.H.; Chung, J.H.; Oh, J.Y. FUT1 deficiency elicits immune dysregulation and corneal opacity in steady state and under stress. Cell Death Dis. 2020, 11, 285. [Google Scholar] [CrossRef]
- Li, J.; Hsu, H.C.; Mountz, J.D.; Allen, J.G. Unmasking Fucosylation: From Cell Adhesion to Immune System Regulation and Diseases. Cell Chem. Biol. 2018, 25, 499–512. [Google Scholar] [CrossRef]
- Pickard, J.M.; Chervonsky, A.V. Intestinal fucose as a mediator of host-microbe symbiosis. J. Immunol. 2015, 194, 5588–5593. [Google Scholar] [CrossRef]
- Stanley, P.; Moreman, K.W.; Lewis, N.E.; Taniguchi, N.; Aebi, M. Essentials of Glycobiology [Internet], 4th ed.; Cold Spring Harbour Press: Cold Spring Harbour, NY, USA, 2022. [Google Scholar]
- Nagao-Kitamoto, H.; Leslie, J.L.; Kitamoto, S.; Jin, C.; Thomsson, K.A.; Gillilland, M.G., 3rd; Kuffa, P.; Goto, Y.; Jenq, R.R.; Ishii, C.; et al. Interleukin-22-mediated host glycosylation prevents Clostridioides difficile infection by modulating the metabolic activity of the gut microbiota. Nat. Med. 2020, 26, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Haugen, J.B.; Curtis, T.H.; Fernandes, P.G.; Sosebee, K.A.; Rago, P.J. Sexual segregation of spiny dogfish (Squalus acanthias) off the northeastern United States: Implications for a male-directed fishery. Fish. Res. 2017, 193, 121–128. [Google Scholar] [CrossRef]
- Fæste, C.K.; Tartor, H.; Moen, A.; Kristoffersen, A.B.; Dhanasiri, A.K.S.; Anonsen, J.H.; Furmanek, T.; Grove, S. Proteomic profiling of salmon skin mucus for the comparison of sampling methods. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2020, 1138, 121965. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.H.; Karlsson, N.G.; Kolarich, D.; Packer, N.H. Structural analysis of N- and O-glycans released from glycoproteins. Nat. Protoc. 2012, 7, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Ceroni, A.; Maass, K.; Geyer, H.; Geyer, R.; Dell, A.; Haslam, S.M. GlycoWorkbench: A tool for the computer-assisted annotation of mass spectra of glycans. J. Proteome. Res. 2008, 7, 1650–1659. [Google Scholar] [CrossRef]
- Karlsson, N.G.; Schulz, B.L.; Packer, N.H. Structural determination of neutral O-linked oligosaccharide alditols by negative ion LC-electrospray-MSn. J. Am. Soc. Mass Spectrom. 2004, 15, 659–672. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bachar-Wikstrom, E.; Thomsson, K.A.; Sihlbom, C.; Abbo, L.; Tartor, H.; Lindén, S.K.; Wikstrom, J.D. Identification of Novel Glycans in the Mucus Layer of Shark and Skate Skin. Int. J. Mol. Sci. 2023, 24, 14331. https://doi.org/10.3390/ijms241814331
Bachar-Wikstrom E, Thomsson KA, Sihlbom C, Abbo L, Tartor H, Lindén SK, Wikstrom JD. Identification of Novel Glycans in the Mucus Layer of Shark and Skate Skin. International Journal of Molecular Sciences. 2023; 24(18):14331. https://doi.org/10.3390/ijms241814331
Chicago/Turabian StyleBachar-Wikstrom, Etty, Kristina A. Thomsson, Carina Sihlbom, Lisa Abbo, Haitham Tartor, Sara K. Lindén, and Jakob D. Wikstrom. 2023. "Identification of Novel Glycans in the Mucus Layer of Shark and Skate Skin" International Journal of Molecular Sciences 24, no. 18: 14331. https://doi.org/10.3390/ijms241814331




