Red-Light Transmittance Changes in Variegated Pelargonium zonale—Diurnal Variation in Chloroplast Movement and Photosystem II Efficiency
Abstract
1. Introduction
2. Results
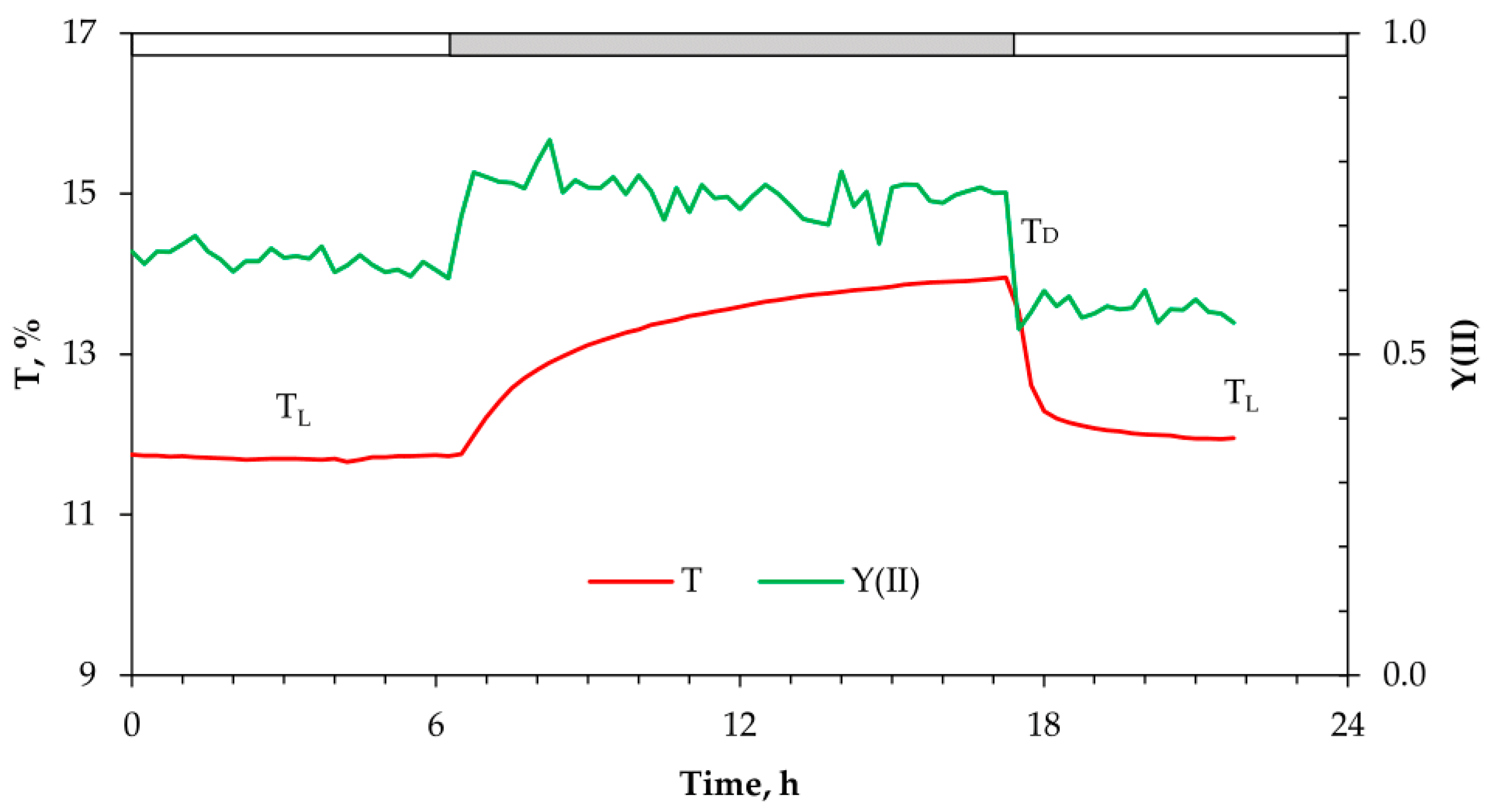
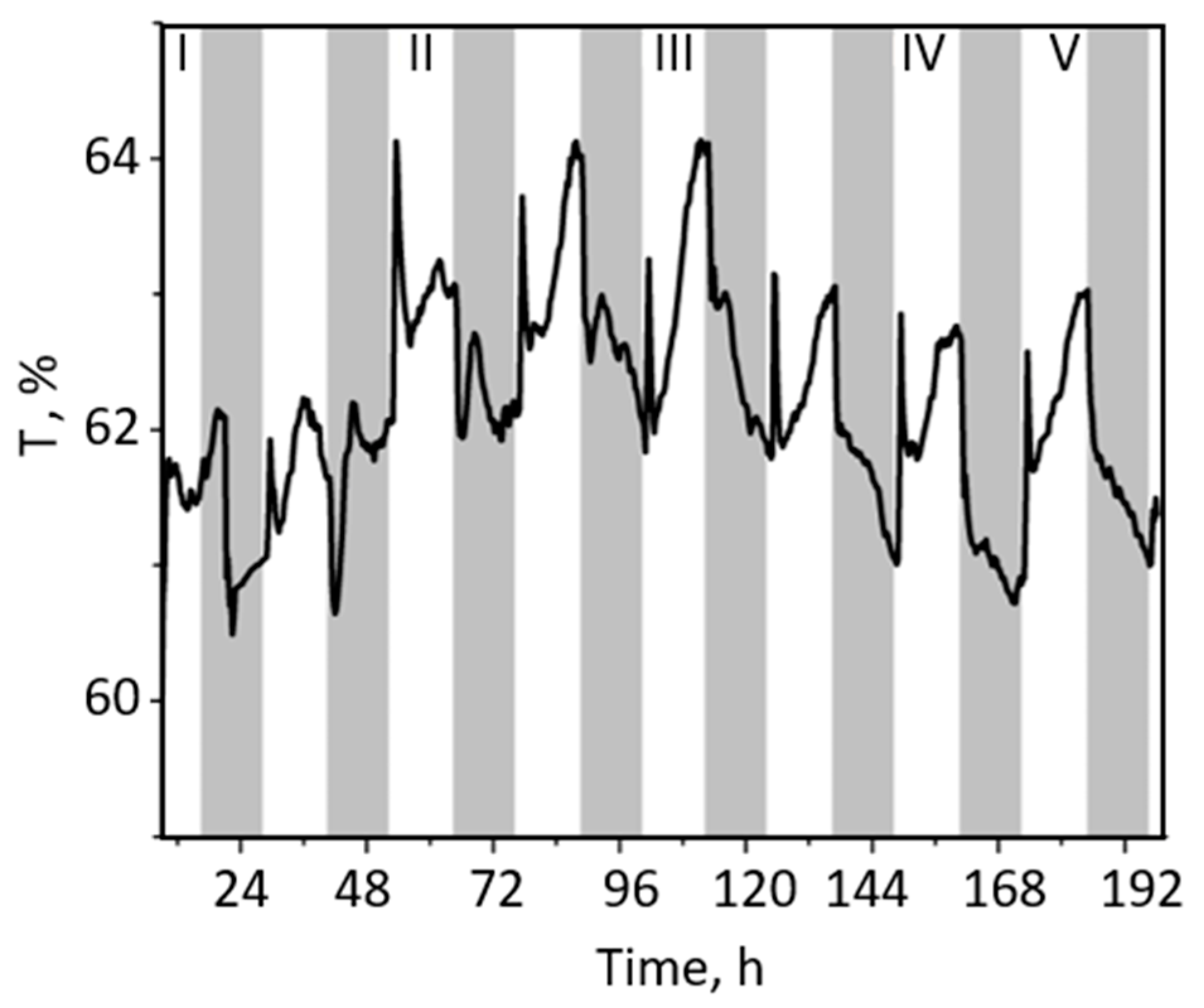
2.1. Changes in the Red-Light Transmittance and chl Fluorescence Changes at Dark–Light–Dark Transitions in Green Leaf Sectors
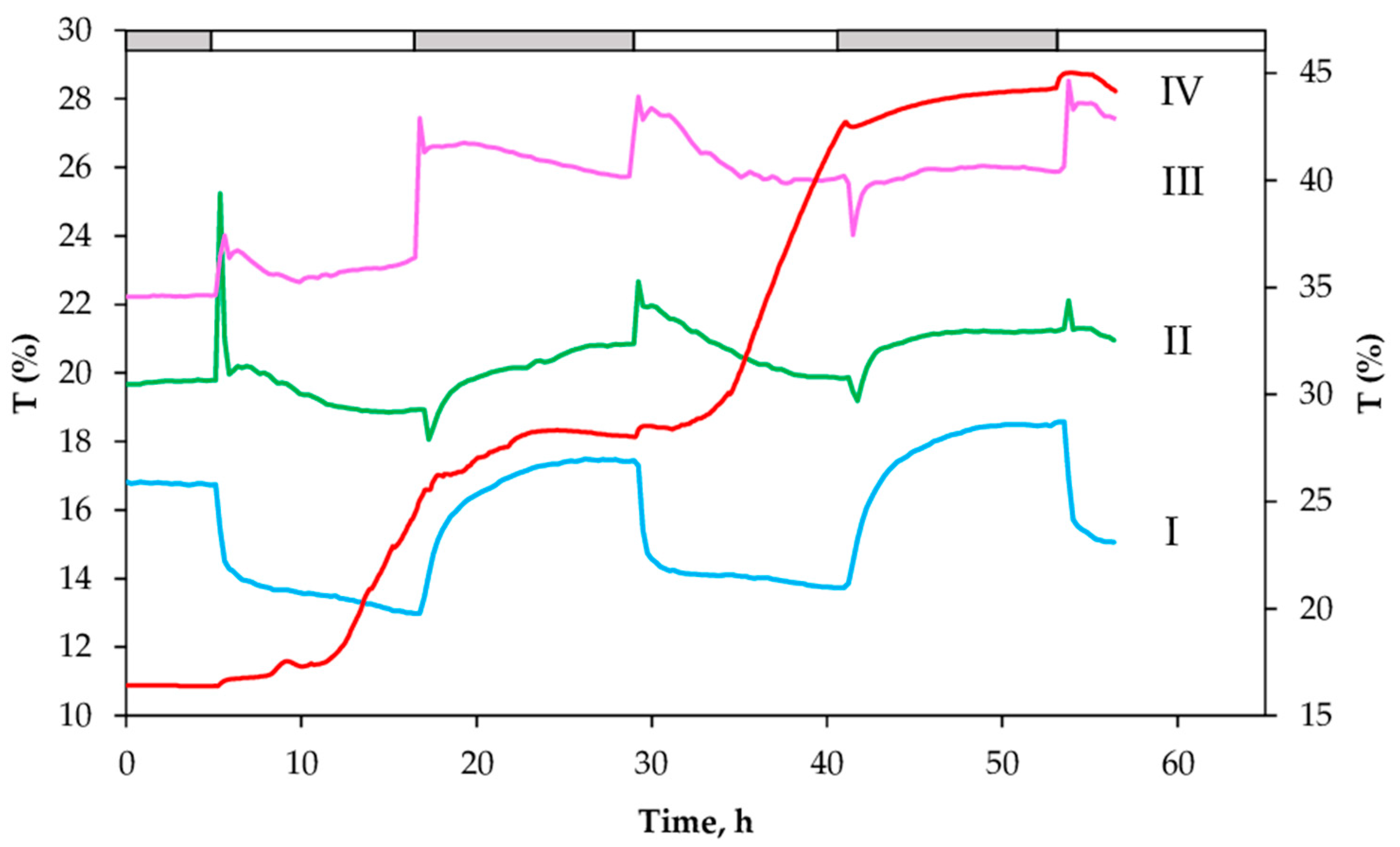
2.2. Changes in the Red-Light Transmittance and chl Fluorescence Changes at Dark–Light–Dark Transitions in White Leaf Sectors (WS)
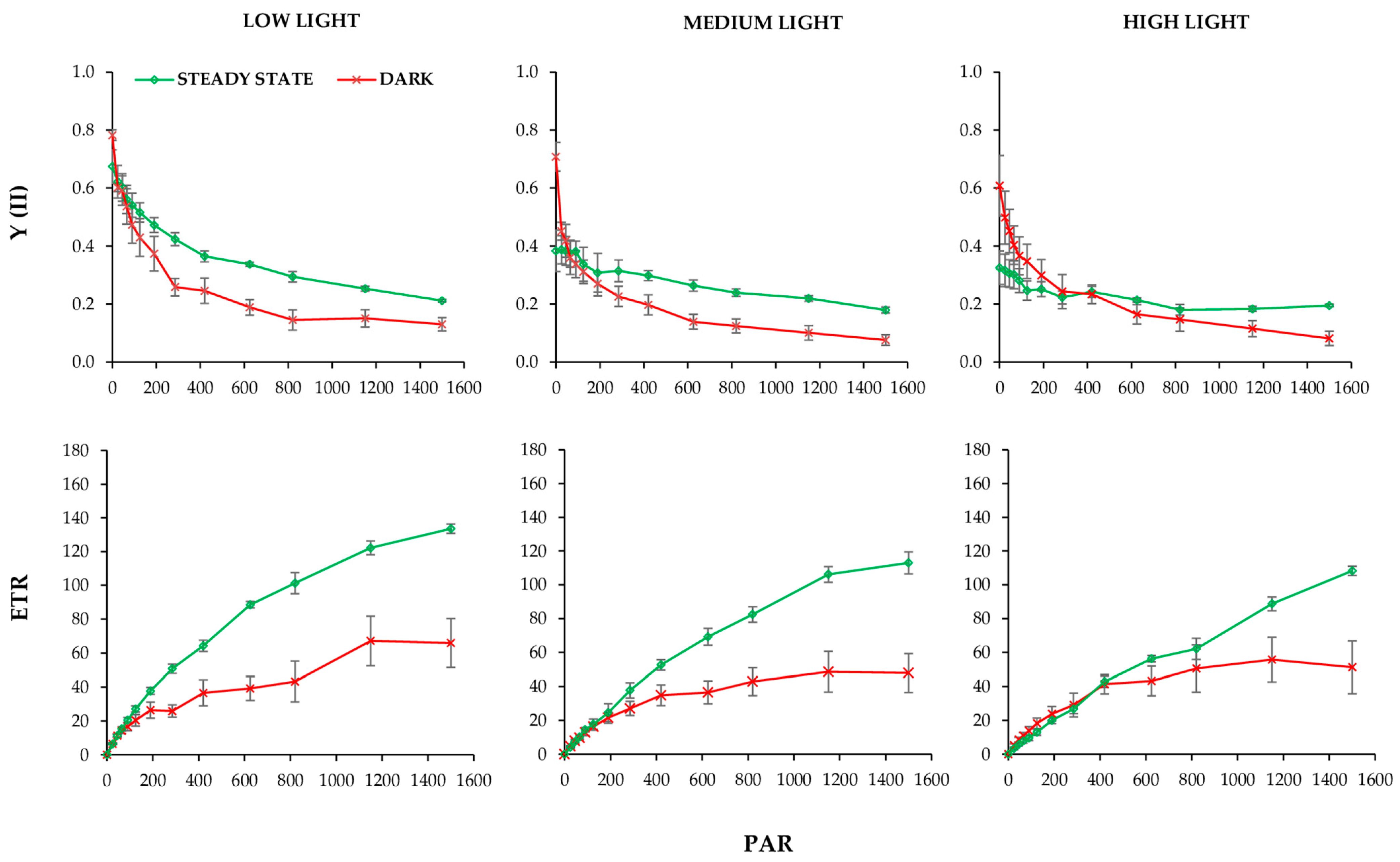
2.3. Chlorophyll Fluorescence Parameters
2.4. Non-Invasive Measurements of Chlorophyll (chl) and Epidermal Flavonoids (Flav)
2.5. Differentially Expressed Genes (DEGs) Involved in Blue-Light Sensing and Chloroplast Movement in GS and WS
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Transmittance Measurements
4.3. Chlorophyll Fluorescence Parameters
4.4. Dertermination of chl, Flav and NBI
4.5. Annotation of the Subcellular Localisation of Photoreceptors in P. zonale
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lichtenthaler, H.K.; Wenzel, O.; Buschmann, C.; Gitelson, A. Plant stress detection by reflectance and fluorescence. Ann. N. Y. Acad. Sci. 1998, 851, 271–285. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I. Visible and near-infrared reflectance techniques for diagnosing plant physiological status. Trends Plant Sci. 1998, 3, 151–156. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Ustin, S.L. Leaf optical properties: A state of the art. In Proceedings of the 8th International Symposium of Physical Measurements & Signatures in Remote Sensing, Aussois, France, 8–12 January 2001; pp. 223–332. [Google Scholar]
- Estrada, F.; Flexas, J.; Araus, J.L.; Mora-Poblete, F.; Gonzalez-Talice, J.; Castillo, D.; Matus, I.A.; Méndez-Espinoza, A.M.; Garriga, M.; Araya-Riquelme, C.; et al. Exploring plant responses to abiotic stress by contrasting spectral signature changes. Front. Plant Sci. 2023, 13, 5601. [Google Scholar] [CrossRef] [PubMed]
- Powles, S.B. Photoinhibition of photosynthesis induced by visible light. Annu. Rev. Plant Biol. 1984, 35, 15–44. [Google Scholar] [CrossRef]
- Öquist, G.; Chow, W.S.; Anderson, J.M. Photoinhibition of photosynthesis represents a mechanism for the long-term regulation of photosystem II. Planta 1992, 186, 450–460. [Google Scholar] [CrossRef]
- Krause, G.H. The high-energy state of the thylakoid system as indicated by chlorophyll fluorescence and chloroplast shrinkage. Biochim. Biophys. Acta Bioenerg. 1973, 292, 715–728. [Google Scholar] [CrossRef]
- Briantais, J.M.; Vernotte, C.; Picaud, M.; Krause, G.H. Chlorophyll fluorescence as a probe for the determination of the photo-induced proton gradient in isolated chloroplasts. Biochim. Biophys. Acta Bioenerg. 1980, 591, 198–202. [Google Scholar] [CrossRef]
- Demmig-Adams, B. Carotenoids and photoprotection in plants: A role for the xanthophyll zeaxanthin. Biochim. Biophys. Acta Bioenerg. 1990, 1020, 1–24. [Google Scholar] [CrossRef]
- Krause, A.G.; Weis, E. Chlorophyll fluorescence and photosynthesis: The basics. Annu. Rev. Plant Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Allen, J.F.; Bennett, J.; Steinback, K.E.; Arntzen, C.J. Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature 1981, 291, 25–29. [Google Scholar] [CrossRef]
- Mekala, N.R.; Suorsa, M.; Rantala, M.; Aro, E.M.; Tikkanen, M. Plants actively avoid state transitions upon changes in light intensity: Role of light-harvesting complex II protein dephosphorylation in high light. Plant Physiol. 2015, 168, 721–734. [Google Scholar] [CrossRef]
- Edwards, G.; Walker, D. C Three C Four: Mechanisms, Cellular and Environmental Regulation of Photosynthesis; University of California Press: Berkeley, CA, USA, 1983. [Google Scholar]
- Jurić, S.; Hazler-Pilepić, K.; Tomašić, A.; Lepeduš, H.; Jeličić, B.; Puthiyaveetil, S.; Bionda, T.; Vojta, L.; Allen, J.F.; Schleiff, E.; et al. Tethering of ferredoxin: NADP+ oxidoreductase to thylakoid membranes is mediated by novel chloroplast protein TROL. Plant J. 2009, 60, 783–794. [Google Scholar]
- Wada, M.; Kagawa, T.; Sato, Y. Chloroplast movement. Annu. Rev. Plant Biol. 2003, 54, 455–468. [Google Scholar] [CrossRef]
- Kawai, H.; Kanegae, T.; Christensen, S.; Kiyosue, T.; Sato, Y.; Imaizumi, T.; Kadota, A.; Wada, M. Responses of ferns to red light are mediated by an unconventional photoreceptor. Nature 2003, 421, 287–290. [Google Scholar] [CrossRef] [PubMed]
- DeBlasio, S.L.; Mullen, J.L.; Luesse, D.R.; Hangarter, R.P. Phytochrome modulation of blue light-induced chloroplast movements in Arabidopsis. Plant Phys. 2003, 133, 1471–1479. [Google Scholar] [CrossRef][Green Version]
- Wada, M. Chloroplast movement. Plant Sci. 2013, 210, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, M.; Kagawa, T.; Oikawa, K.; Suetsugu, N.; Miyao, M.; Wada, M. Chloroplast avoidance movement reduces photodamage in plants. Nature 2002, 420, 829–832. [Google Scholar] [CrossRef]
- Sztatelman, O.; Waloszek, A.; Banaś, A.K.; Gabryś, H. Photoprotective function of chloroplast avoidance movement: In vivo chlorophyll fluorescence study. J. Plant Phys. 2010, 167, 709–716. [Google Scholar] [CrossRef]
- Davis, P.A.; Hangarter, R.P. Chloroplast movement provides photoprotection to plants by redistributing PSII damage within leaves. Photosynth. Res. 2012, 112, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Cazzaniga, S.; Dall’Osto, L.; Kong, S.G.; Wada, M.; Bassi, R. Interaction between avoidance of photon absorption, excess energy dissipation and zeaxanthin synthesis against photooxidative stress in Arabidopsis. Plant J. 2013, 76, 568–579. [Google Scholar] [CrossRef]
- Wilson, S.; Ruban, A.V. Rethinking the influence of chloroplast movements on non-photochemical quenching and photoprotection. Plant Phys. 2020, 183, 1213–1223. [Google Scholar] [CrossRef]
- Boehm, L. Der Nystagmus und Dessen Heilung: Eine Monographie; Hirschwald: Berlin, Germany, 1857. [Google Scholar]
- Frank, B. Über lichtwärts sich bewegende Chlorophyllkörner. Bot. Ztg. 1871, 29, 209. [Google Scholar]
- Senn, G. Die Gestalts-und Lageveränderung der Pflanzen-Chromatophoren: Mit Einer Beilage: Die Lichtbrechung der Lebenden Pflanzenzelle; W. Engelmann: Leipzig, Germany, 1908. [Google Scholar]
- Kataoka, H. Gustav Senn (1875–1945): The pioneer of chloroplast movement research. J. Integr. Plant Biol. 2015, 57, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Sakai, T.; Kagawa, T.; Kasahara, M.; Swartz, T.E.; Christie, J.M.; Briggs, W.R.; Wada, M.; Okada, K. Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl. Acad. Sci. USA 2001, 98, 6969–6974. [Google Scholar] [CrossRef]
- Jarillo, J.A.; Gabrys, H.; Capel, J.; Alonso, J.M.; Ecker, J.R.; Cashmore, A.R. Phototropin-related NPL1 controls chloroplast relocation induced by blue light. Nature 2001, 410, 952–954. [Google Scholar] [CrossRef]
- Huala, E.; Oeller, P.W.; Liscum, E.; Han, I.S.; Larsen, E.; Briggs, W.R. Arabidopsis NPH1: A protein kinase with a putative redox-sensing domain. Science 1997, 278, 2120–2123. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Doi, M.; Suetsugu, N.; Kagawa, T.; Wada, M.; Shimazaki, K.I. Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 2001, 414, 656–660. [Google Scholar] [CrossRef]
- Briggs, W.R.; Christie, J.M. Phototropins 1 and 2: Versatile plant blue-light receptors. Trends Plant Sci. 2002, 7, 204–210. [Google Scholar] [CrossRef]
- Briggs, W.R.; Olney, M.A. Photoreceptors in plant photomorphogenesis to date. Five phytochromes, two cryptochromes, one phototropin, and one superchrome. Plant Physiol. 2001, 125, 85–88. [Google Scholar] [CrossRef]
- Briggs, W.R.; Beck, C.F.; Cashmore, A.R.; Christie, J.M.; Hughes, J.; Jarillo, J.A.; Kagawa, T.; Kanegae, H.; Liscum, E.; Nagatani, A.; et al. The phototropin family of photoreceptors. Plant Cell 2001, 13, 993–997. [Google Scholar] [CrossRef]
- Kagawa, T.; Sakai, T.; Suetsugu, N.; Oikawa, K.; Ishiguro, S.; Kato, T.; Tabata, S.; Okada, K.; Wada, M. Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science 2001, 291, 2138–2141. [Google Scholar] [CrossRef] [PubMed]
- Pfündel, E.E.; Latouche, G.; Meister, A.; Cerovic, Z.G. Linking chloroplast relocation to different responses of photosynthesis to blue and red radiation in low and high light-acclimated leaves of Arabidopsis thaliana (L.). Photosynth. Res. 2018, 137, 105–128. [Google Scholar] [CrossRef]
- Wada, M.; Kong, S.G. Analysis of chloroplast movement and relocation in Arabidopsis. In Chloroplast Research in Arabidopsis: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2011; Volume I, pp. 87–102. [Google Scholar]
- Inoue, Y.; Shibata, K. Light-induced chloroplast rearrangements and their action spectra as measured by absorption spectrophotometry. Planta 1973, 114, 341–358. [Google Scholar] [CrossRef]
- Kasalica, B.V.; Miletic, K.M.; Sabovljevic, A.D.; Vujicic, M.M.; Jeremic, D.A.; Belca, I.D.; Petkovic-Benazzouz, M.M. Nondestructive optical method for plant overall health evaluation. Acta Agric. Scand. B Soil Plant Sci. 2021, 71, 1017–1023. [Google Scholar] [CrossRef]
- Suetsugu, N.; Wada, M. Chloroplast photorelocation movement mediated by phototropin family proteins in green plants. Biol. Chem. 2007, 927–935. [Google Scholar] [CrossRef]
- Allorent, G.; Petroutsos, D. Photoreceptor-dependent regulation of photoprotection. Curr. Opin. Plant Biol. 2017, 37, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.E.; Gorton, H.L.; Witiak, S.M. Chloroplast movements in the field. Plant Cell Environ. 2003, 26, 2005–2014. [Google Scholar] [CrossRef]
- Banaś, A.K.; Aggarwal, C.; Łabuz, J.; Sztatelman, O.; Gabryś, H. Blue light signalling in chloroplast movements. J. Exp. Bot. 2012, 63, 1559–1574. [Google Scholar] [CrossRef]
- Gotoh, E.; Suetsugu, N.; Yamori, W.; Ishishita, K.; Kiyabu, R.; Fukuda, M.; Higa, T.; Shirouchi, B.; Wada, M. Chloroplast accumulation response enhances leaf photosynthesis and plant biomass production. Plant Phys. 2018, 178, 1358–1369. [Google Scholar] [CrossRef]
- Aihara, Y.; Tabata, R.; Suzuki, T.; Shimazaki, K.I.; Nagatani, A. Molecular basis of the functional specificities of phototropin 1 and 2. Plant J. 2008, 56, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.; Pedmale, U.V.; Morrow, J.; Sachdev, S.; Lechner, E.; Tang, X.; Zheng, N.; Hannink, M.; Genschik, P.; Liscum, E. Modulation of phototropic responsiveness in Arabidopsis through ubiquitination of phototropin 1 by the CUL3-Ring E3 ubiquitin ligase CRL3NPH3. Plant Cell 2011, 23, 3627–3640. [Google Scholar] [CrossRef] [PubMed]
- Vidović, M.; Morina, F.; Milić, S.; Albert, A.; Zechmann, B.; Tosti, T.; Winkler, J.B.; Veljović Jovanović, S. Carbon allocation from source to sink leaf tissue in relation to flavonoid biosynthesis in variegated Pelargonium zonale under UV-B radiation and high PAR intensity. Plant Phys. Biochem. 2015, 93, 44–55. [Google Scholar] [CrossRef]
- Veljović-Jovanović, S.; Vidović, M.; Morina, F.; Prokić, L.; Todorović, D.M. Comparison of photoacoustic signals in photosynthetic and nonphotosynthetic leaf tissues of variegated Pelargonium zonale. Int. J. Thermophys. 2016, 37, 91–101. [Google Scholar] [CrossRef]
- Walczak, T.; Gabryś, H. New type of photometer for measurements of transmission changes corresponding to chloroplast movements in leaves. Photosynthetica 1980, 14, 65–72. [Google Scholar]
- Berg, R.; Königer, M.; Schjeide, B.M.; Dikmak, G.; Kohler, S.; Harris, G.C. A simple low-cost microcontroller-based photometric instrument for monitoring chloroplast movement. Photosynth. Res. 2006, 87, 303–311. [Google Scholar] [CrossRef]
- Luesse, D.R.; DeBlasio, S.L.; Hangarter, R.P. Integration of phot1, phot2, and PhyB signalling in light-induced chloroplast movements. J. Exp. Bot. 2010, 61, 4387–4397. [Google Scholar] [CrossRef]
- Higa, T.; Wada, M. Clues to the signals for chloroplast photo-relocation from the lifetimes of accumulation and avoidance responses. J. Integr. Plant Biol. 2015, 57, 120–126. [Google Scholar] [CrossRef]
- Vidović, M.; Morina, F.; Prokić, L.; Milić-Komić, S.; Živanović, B.; Veljovic Jovanović, S. Antioxidative response in variegated Pelargonium zonale leaves and generation of extracellular H2O2 in (peri) vascular tissue induced by sunlight and paraquat. J. Plant Phys. 2016, 206, 25–39. [Google Scholar] [CrossRef]
- Milić, D.; Pantelić, A.; Banović-Djeri, B.; Samardžić, J.; Vidović, M. Contrasting metabolisms in green and white leaf sectors of variegated Pelargonium zonale—An integrated transcriptomic and metabolomic study. Int. J. Mol. Sci. 2022, 24, 5288. [Google Scholar] [CrossRef]
- Vogelmann, T.C.; Martin, G. The functional significance of palisade tissue: Penetration of directional versus diffuse light. Plant Cell Environ. 1993, 16, 65–72. [Google Scholar] [CrossRef]
- Hikosaka, K.; Terashima, I. A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ. 1995, 18, 605–618. [Google Scholar] [CrossRef]
- Kume, A. Importance of the green color, absorption gradient, and spectral absorption of chloroplasts for the radiative energy balance of leaves. J. Plant Res. 2017, 130, 501–514. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R.; Kaldenhoff, R.; Genty, B.; Terashima, I. Resistances along the CO2 diffusion pathway inside leaves. J. Exp. Bot. 2009, 60, 2235–2248. [Google Scholar] [CrossRef]
- Zurzycki, J. Chloroplast arrangement as a factor in photosynthesis. Acta Soc. Bot. Pol. 1955, 24, 27–63. [Google Scholar] [CrossRef]
- Takemiya, A.; Inoue, S.I.; Doi, M.; Kinoshita, T.; Shimazaki, K.I. Phototropins promote plant growth in response to blue light in low light environments. Plant Cell 2005, 17, 1120–1127. [Google Scholar] [CrossRef]
- Van Kooten, O.; Snel, J.F. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynth. Res. 1990, 25, 147–150. [Google Scholar] [CrossRef]
- Schreiber, U. Pulse-Amplitude-Modulation (PAM) fluorometry and saturation pulse method: An overview. In Chlorophyll a Fluorescence; Papageorgiou, G.C., Govindjee, Eds.; Advances in Photosynthesis and Respiration; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 279–319. [Google Scholar]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Garab, G.; Magyar, M.; Sipka, G.; Lambrev, P.H. Chlorophyll-a fluorescence induction on new grounds: Quantum efficiency versus the light-adapted state of photosystem II. J. Exp. Bot. 2023, erad252. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35 (Suppl. S2), W585–W587. [Google Scholar] [CrossRef] [PubMed]

| White Light Regime | Chl (µg cm−2) | Flav, (µg cm−2) | NBI |
|---|---|---|---|
| Low light—LL | 20.62 ± 0.74 a | 0.16 ± 0.01 a | 127.72 ± 0.88 c |
| Medium light—ML | 21.54 ± 2.68 a | 0.54 ± 0.01 b | 47.36 ± 3.52 b |
| High light—HL | 14.94 ± 1.02 a | 0.92 ± 0.09 c | 17.11 ± 3.11 a |
| Annotated Transcript | GS Value | WS Value | log2FC | p Value | p Adjust | Compartment |
|---|---|---|---|---|---|---|
| phototropin 1 (Phot1) | 0.554948 | 24.48923 | −5.46 | 2.23 × 10−5 | 0.000759 | nucleus |
| phototropin 2 (Phot2) | 5.424345 | 36.23852 | −2.74 | 1.58 × 10−9 | 2.62 × 10−7 | plastid |
| cryptochrome DASH | 0.927768 | 6.097461 | −2.72 | 9.55 × 10−9 | 1.28 × 10−6 | plastid |
| cryptochrome DASH | 0.016507 | 0.18674 | −3.49 | 9.69 × 10−4 | 1.37 × 10−2 | plastid |
| cryptochrome-1 isoform X1 | 15.17865 | 5.070029 | 1.58 | 8.40 × 10−5 | 2.13 × 10−3 | cytosol |
| cryptochrome-1 isoform X2 | 0.036141 | 0.285204 | −2.97 | 2.29 × 10−3 | 0.025591 | nucleus |
| CHUP1 | 0.014838 | 0.378728 | −4.68 | 2.74 × 10−6 | 0.000143 | plastid |
| 0.676587 | 6.034586 | −3.16 | 1.52 × 10−6 | 8.91 × 10−5 | plastid | |
| 7.423503 | 30.91456 | −2.06 | 0.000109 | 0.002619 | plastid | |
| 0.327659 | 0.01742 | 4.23 | 0.002075 | 0.023855 | plastid | |
| 0.982411 | 0.023832 | 5.37 | 1.92 × 10−5 | 0.000675 | plastid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veljović Jovanović, S.; Kasalica, B.; Miletić, K.; Vidović, M.; Šušić, N.; Jeremić, D.; Belča, I. Red-Light Transmittance Changes in Variegated Pelargonium zonale—Diurnal Variation in Chloroplast Movement and Photosystem II Efficiency. Int. J. Mol. Sci. 2023, 24, 14265. https://doi.org/10.3390/ijms241814265
Veljović Jovanović S, Kasalica B, Miletić K, Vidović M, Šušić N, Jeremić D, Belča I. Red-Light Transmittance Changes in Variegated Pelargonium zonale—Diurnal Variation in Chloroplast Movement and Photosystem II Efficiency. International Journal of Molecular Sciences. 2023; 24(18):14265. https://doi.org/10.3390/ijms241814265
Chicago/Turabian StyleVeljović Jovanović, Sonja, Bećko Kasalica, Katarina Miletić, Marija Vidović, Nikola Šušić, Dejan Jeremić, and Ivan Belča. 2023. "Red-Light Transmittance Changes in Variegated Pelargonium zonale—Diurnal Variation in Chloroplast Movement and Photosystem II Efficiency" International Journal of Molecular Sciences 24, no. 18: 14265. https://doi.org/10.3390/ijms241814265
APA StyleVeljović Jovanović, S., Kasalica, B., Miletić, K., Vidović, M., Šušić, N., Jeremić, D., & Belča, I. (2023). Red-Light Transmittance Changes in Variegated Pelargonium zonale—Diurnal Variation in Chloroplast Movement and Photosystem II Efficiency. International Journal of Molecular Sciences, 24(18), 14265. https://doi.org/10.3390/ijms241814265







