Thymoquinone, a Novel Multi-Strike Inhibitor of Pro-Tumorigenic Breast Cancer (BC) Markers: CALR, NLRP3 Pathway and sPD-L1 in PBMCs of HR+ and TNBC Patients
Abstract
:1. Introduction
2. Results
2.1. TQ-Inhibited CALR in PBMCs of HR+ BC Patients
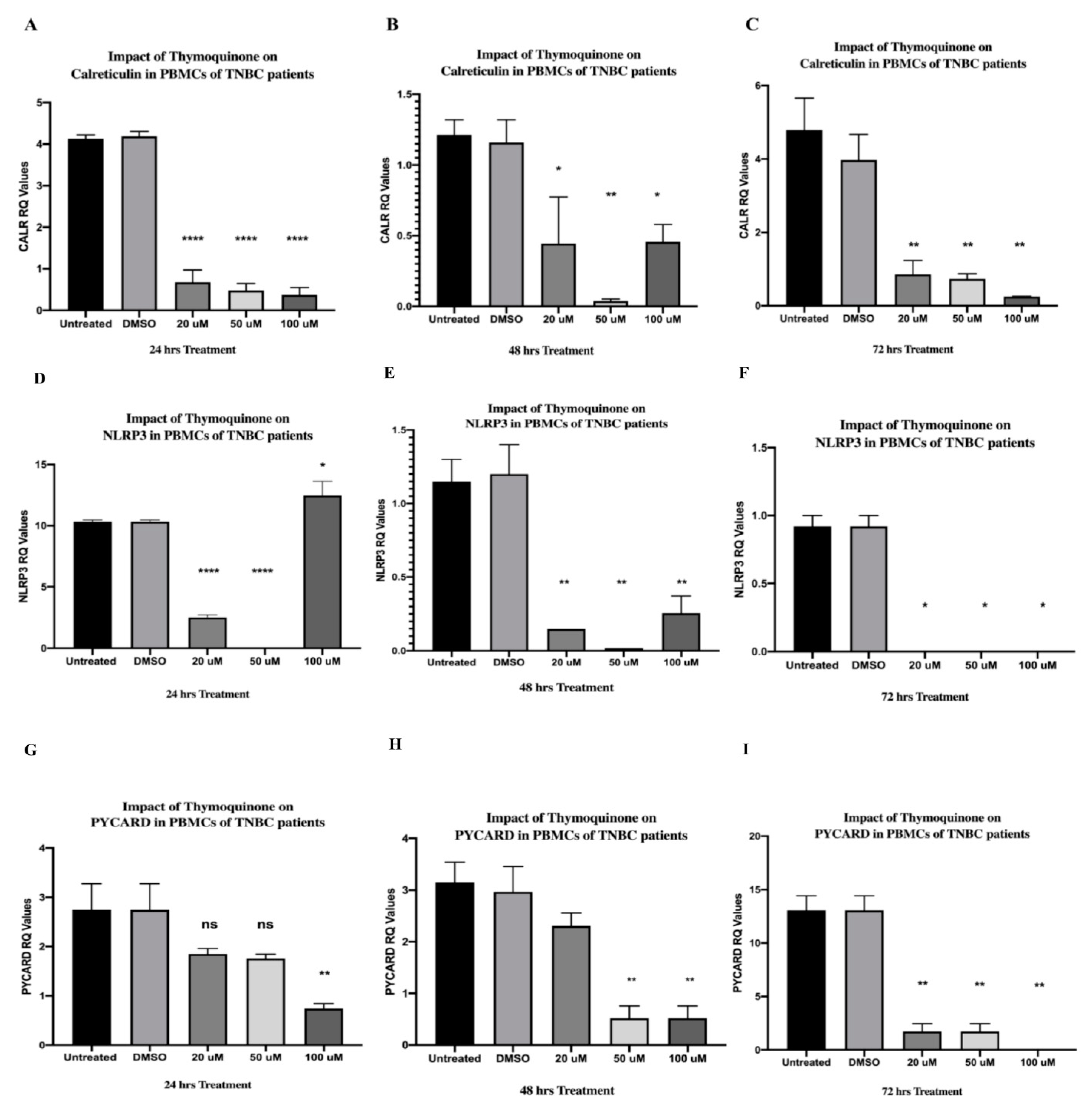
2.2. TQ Significantly Downregulated CALR Expression in PBMCs Isolated from TNBC Patients in a Dose-Dependent Manner
2.3. TQ Showed an Interesting Inhibitory Pattern in NLRP3 and PYCARD in PBMCs of HR+ BC Patients after 24 h of Treatment
2.4. TQ Inhibited NLRP3 and PYCARD Expressions in PBMCs of HR+ BC Patients after 48 and 72 h
2.5. TQ Strongly Inhibited NLRP3 Expression in PBMCs of TNBC Patients after 24 and 48 h of Treatment In Vitro and Completely Abolished Its Expression after 72 h
2.6. TQ Significantly Inhibited PYCARD Expression in PBMCs Isolated from TNBC Patients after 24 and 48 h of Treatment
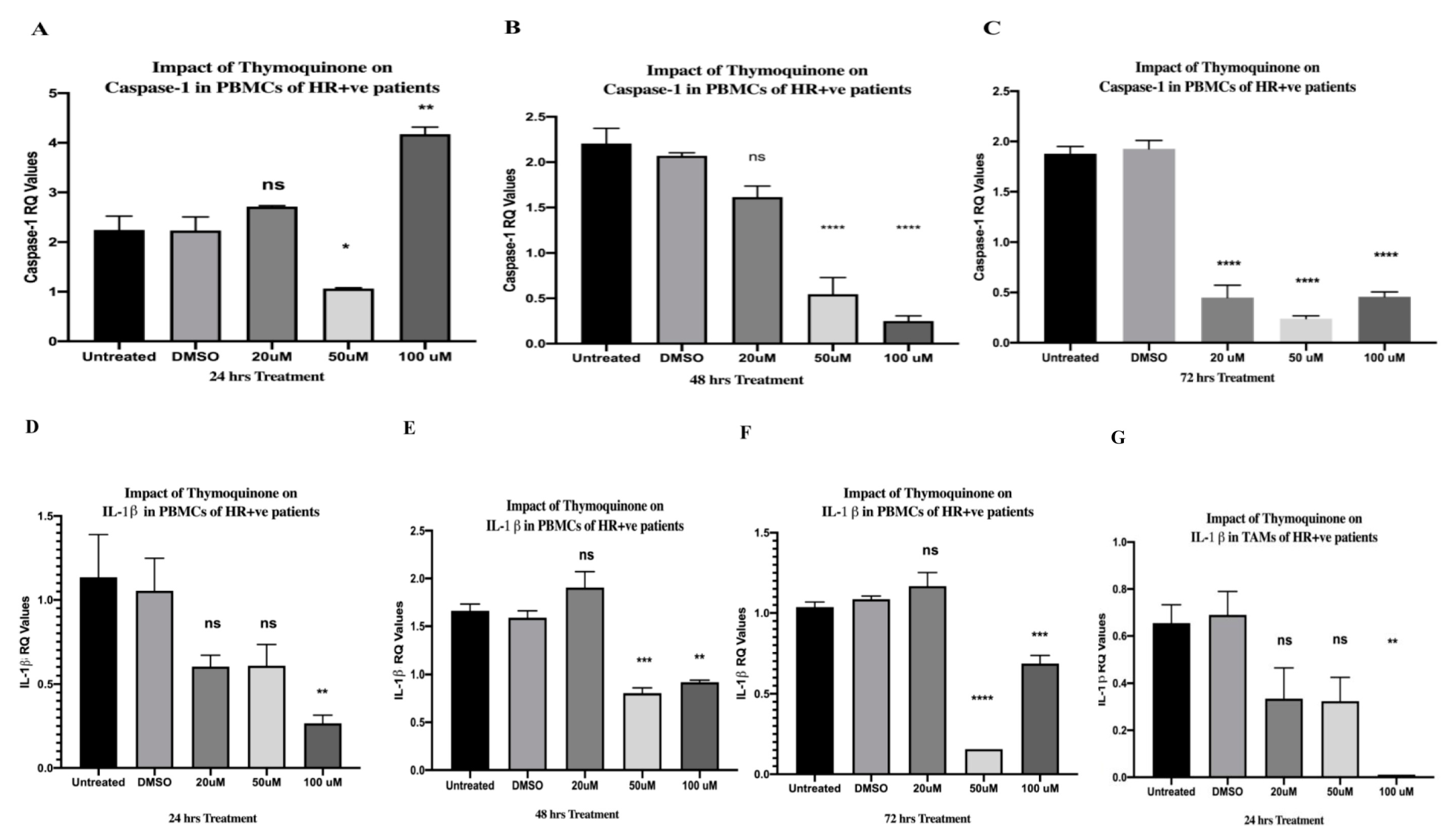
2.7. TQ Significantly Inhibited Caspase-1 after 24, 48, and 72 h of Treatment in PBMCs of HR+ BC Patients
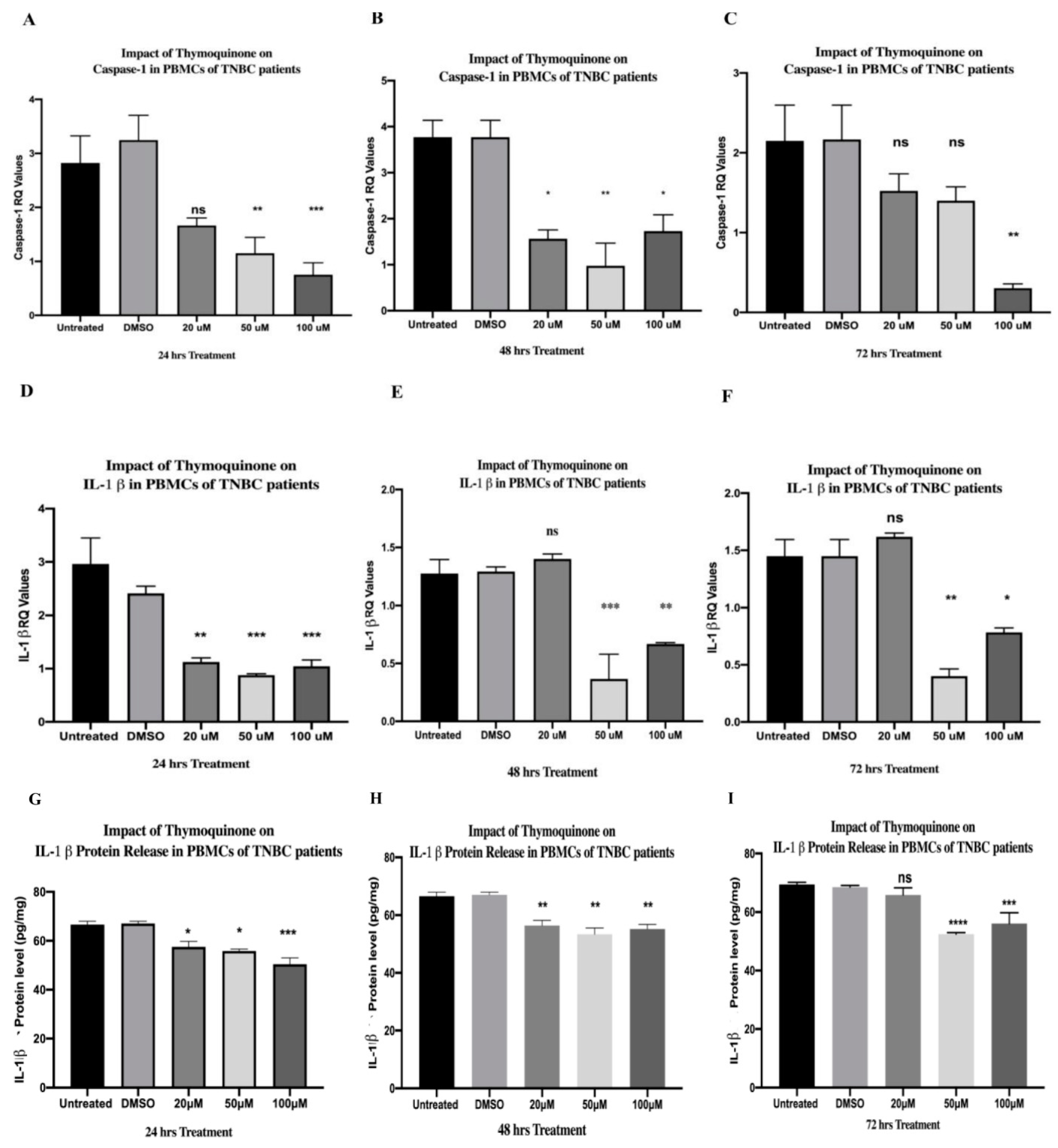
2.8. TQ Showed a Dose-Dependent Inhibitory Effect on Caspase-1 Expression in PBMCs Isolated from TNBC In Vitro
2.9. TQ Significantly Downregulated IL-1β Expression in a Dose-Dependent Manner in PBMCs of HR+ BC Patients
2.10. TQ Significantly Downregulated IL-1β Expression in PBMCs of TNBCs
2.11. Microscopic and Flow Cytometry Results of Efficient CD14+ Monocyte Differentiation to Tumor-Associated Macrophages (TAMs)
2.12. TQ Significantly Abolished the Expression of IL-1β in TAMs Isolated from HR+ BC Patients
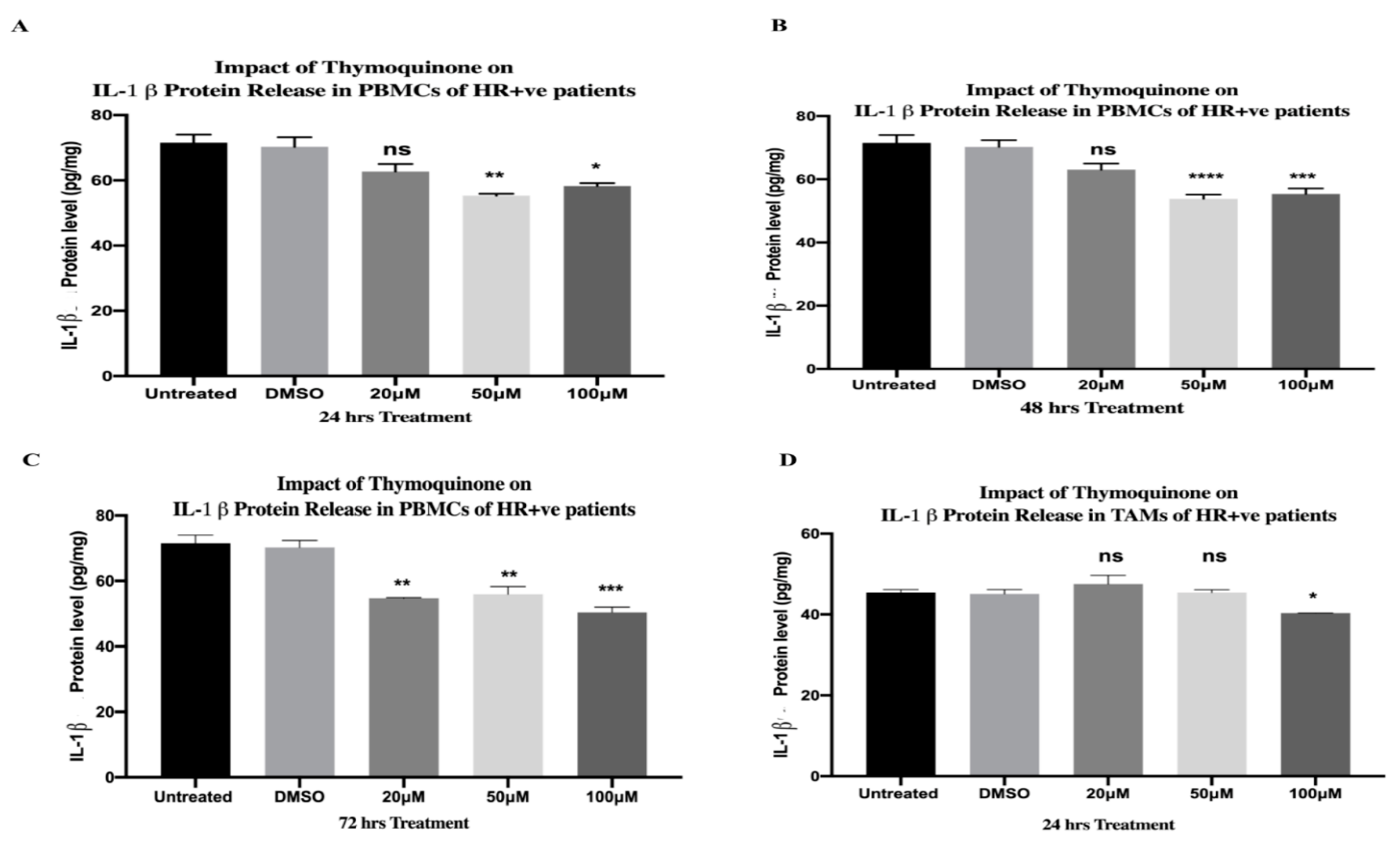
2.13. TQ Significantly Downregulated Protein Release of IL-1β in PBMCs and TAMs of HR+ BC Patients
2.14. TQ Significantly Downregulated IL-1β Protein Release from PBMCs of TNBC Patients
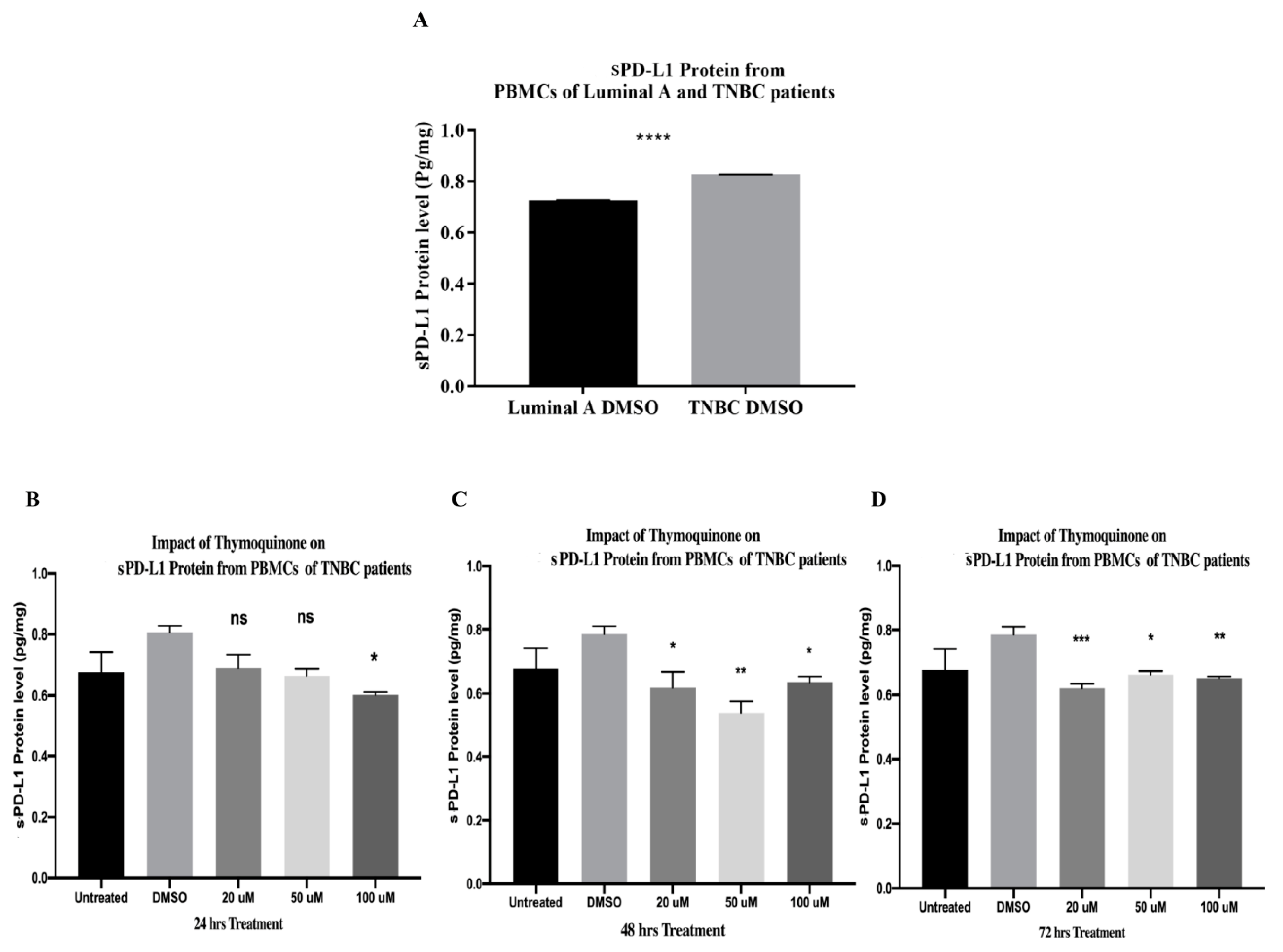
2.15. PBMCs of TNBC Patients Released Significantly Higher sPD-L1 Than That of HR+ BC Patients
2.16. TQ Significantly Downregulated sPD-L1 Release from PBMCs of TNBC Patients after 24, 48, and 72 h of Treatment In Vitro
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Peripheral Blood Mononuclear Cell Isolation
4.3. PBMCs Pooling
4.4. MDA-MB231 Culture and Tumor-Conditioned Media (TCM) Preparation
4.5. Monocyte Isolation from Hormonal BC Patients and Tumor-Associated Macrophage Differentiation In Vitro
4.6. Flow Cytometry
4.7. Thymoquinone (TQ) Preparations
4.8. PBMCs and TAMs Coculture with TQ
4.9. RNA Extraction, Complementary DNA (cDNA), and Quantified Real-Time Polymerase Chain Reaction (qRT-PCR)
4.10. Enzyme-Linked Immunosorbent Assay
4.11. Statistical Analysis
5. Conclusions and Future Insights
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Cho, N. Molecular subtypes and imaging phenotypes of breast cancer. Ultrasonography 2016, 35, 281–288. [Google Scholar] [CrossRef]
- Cichon, M.A.; Degnim, A.C.; Visscher, D.W.; Radisky, D.C. Microenvironmental influences that drive progression from benign breast disease to invasive breast cancer. J. Mammary Gland Biol. Neoplasia 2010, 15, 389–397. [Google Scholar] [CrossRef]
- Dumeaux, V.; Fjukstad, B.; Fjosne, H.E.; Frantzen, J.O.; Holmen, M.M.; Rodegerdts, E.; Schlichting, E.; Børresen-Dale, A.L.; Bongo, L.A.; Lund, E.; et al. Interactions between the tumor and the blood systemic response of breast cancer patients. PLoS Comput. Biol. 2017, 13, e1005680. [Google Scholar] [CrossRef]
- Danforth, D.N. The role of chronic inflammation in the development of breast cancer. Cancers 2021, 13, 3918. [Google Scholar] [CrossRef]
- Batalha, S.; Ferreira, S.; Brito, C. The peripheral immune landscape of breast cancer: Clinical findings and in vitro models for biomarker discovery. Cancers 2021, 13, 1305. [Google Scholar] [CrossRef]
- Moradpoor, R.; Gharebaghian, A.; Shahi, F.; Mousavi, A.; Salari, S.; Akbari, M.E.; Ajdari, S.; Salimi, M. Identification and Validation of Stage-Associated PBMC Biomarkers in Breast Cancer Using MS-Based Proteomics. Front. Oncol. 2020, 10, 1101. [Google Scholar] [CrossRef]
- Dumeaux, V.; Ursini-Siegel, J.; Flatberg, A.; Fjosne, H.E.; Frantzen, J.O.; Holmen, M.M.; Rodegerdts, E.; Schlichting, E.; Lund, E. Peripheral blood cells inform on the presence of breast cancer: A population-based case-control study. Int. J. Cancer 2015, 136, 656–667. [Google Scholar] [CrossRef]
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Marroquin-Muciño, M.; Perez-Medina, M.; Benito-Lopez, J.J.; Camarena, A.; Rumbo-Nava, U.; Lopez-Gonzalez, J.S. The systemic-level repercussions of cancer-associated inflammation mediators produced in the tumor microenvironment. Front. Endocrinol. 2022, 13, 929572. [Google Scholar] [CrossRef]
- Hernandez, C.; Huebener, P.; Schwabe, R.F. Damage-associated molecular patterns in cancer: A double-edged sword. Oncogene 2016, 35, 5931–5941. [Google Scholar] [CrossRef]
- Kim, R.; Kin, T. Current and future therapies for immunogenic cell death and related molecules to potentially cure primary breast cancer. Cancers 2021, 13, 4756. [Google Scholar] [CrossRef]
- Kwa, M.J.; Adams, S. Checkpoint inhibitors in triple-negative breast cancer (TNBC): Where to go from here. Cancer 2018, 124, 2086–2103. [Google Scholar] [CrossRef]
- Peng, Z.; Su, P.; Yang, Y.; Yao, X.; Zhang, Y.; Jin, F.; Yang, B. Identification of CTLA-4 associated with tumor microenvironment and competing interactions in triple negative breast cancer by co-expression network analysis. J. Cancer 2020, 11, 6365–6375. [Google Scholar] [CrossRef]
- Hammerl, D.; Smid, M.; Timmermans, A.M.; Sleijfer, S.; Martens, J.W.M.; Debets, R. Breast cancer genomics and immuno-oncological markers to guide immune therapies. Semin. Cancer Biol. 2018, 52, 178–188. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-associated molecular patterns in inflammatory diseases. Immune Netw. 2018, 18, e27. [Google Scholar] [CrossRef]
- Fucikova, J.; Spisek, R.; Kroemer, G.; Galluzzi, L. Calreticulin and cancer. Cell Res. 2021, 31, 5–16. [Google Scholar] [CrossRef]
- Ocadlikova, D.; Lecciso, M.; Isidori, A.; Loscocco, F.; Visani, G.; Amadori, S.; Cavo, M.; Curti, A. Chemotherapy-Induced Tumor Cell Death at the Crossroads Between Immunogenicity and Immunotolerance: Focus on Acute Myeloid Leukemia. Front. Oncol. 2019, 9, 1004. [Google Scholar] [CrossRef]
- Gameiro, S.R.; Jammeh, M.L.; Wattenberg, M.M.; Tsang, K.Y.; Ferrone, S.; Hodge, J.W. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killign. Oncotarget 2014, 5, 403–416. [Google Scholar] [CrossRef]
- Zhou, H.; Forveille, S.; Sauvat, A.; Yamazaki, T.; Senovilla, L.; Ma, Y.; Liu, P.; Yang, H.; Bezu, L.; Müller, K.; et al. The oncolytic peptide LTX-315 triggers immunogenic cell death. Cell Death Dis. 2016, 7, e2134. [Google Scholar] [CrossRef]
- Gardai, S.J.; McPhillips, K.A.; Frasch, S.C.; Janssen, W.J.; Starefeldt, A.; Murphy-Ullrich, J.E.; Bratton, D.L.; Oldenborg, P.A.; Michalak, M.; Henson, P.M. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 2005, 123, 321–334. [Google Scholar] [CrossRef]
- Arosa, F.A.; De Jesus, O.; Porto, G.; Carmo, A.M.; De Sousa, M. Calreticulin is expressed on the cell surface of activated human peripheral blood T lymphocytes in association with major histocompatibility complex class I molecules. J. Biol. Chem. 1999, 274, 16917–16922. [Google Scholar] [CrossRef]
- Feng, M.; Chen, J.Y.; Weissman-Tsukamoto, R.; Volkmer, J.P.; Ho, P.Y.; McKenna, K.M.; Cheshier, S.; Zhang, M.; Guo, N.; Gip, P.; et al. Macrophages eat cancer cells using their own calreticulin as a guide: Roles of TLR and Btk. Proc. Natl. Acad. Sci. USA 2015, 112, 2145–2150. [Google Scholar] [CrossRef]
- Zeng, G.; Aldridge, M.E.; Tian, X.; Seiler, D.; Zhang, X.; Jin, Y.; Rao, J.; Li, W.; Chen, D.; Langford, M.P.; et al. Dendritic Cell Surface Calreticulin Is a Receptor for NY-ESO-1: Direct Interactions between Tumor-Associated Antigen and the Innate Immune System. J. Immunol. 2006, 177, 3582–3589. [Google Scholar] [CrossRef]
- Zhou, P.; Teruya-Feldstein, J.; Lu, P.; Fleisher, M.; Olshen, A.; Comenzo, R.L. Calreticulin expression in the clonal plasma cells of patients with systemic light-chain (AL-) amyloidosis is associated with response to high-dose melphalan. Blood 2008, 111, 549–557. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, C.; Xin, P.; Peng, Q.; Zhang, W.; Liu, S.; Zhu, X. Calreticulin increases growth and progression of natural killer/T-cell lymphoma. Aging 2020, 12, 23822–23835. [Google Scholar] [CrossRef]
- Osman, R.; Tacnet-Delorme, P.; Kleman, J.P.; Millet, A.; Frachet, P. Calreticulin release at an early stage of death modulates the clearance by macrophages of apoptotic cells. Front. Immunol. 2017, 8, 1034. [Google Scholar] [CrossRef]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. A Simple and Low-Cost Electrochemical Immunosensor for Ultrasensitive Determination of Calreticulin Biomarker in Human Serum. Macromol. Biosci. 2023, 23, e2200390. [Google Scholar] [CrossRef]
- SUN, M.; YANG, G.; ZHAO, Y.; QU, F. Screening of Aptamer for Breast Cancer Biomarker Calreticulin and Its Application to Detection of Serum and Recognition of Breast Cancer Cell. Chin. J. Anal. Chem. 2020, 48, 642–649. [Google Scholar] [CrossRef]
- Cruz-Ramos, E.; Sandoval-Hernández, A.; Tecalco-Cruz, A.C. Differential expression and molecular interactions of chromosome region maintenance 1 and calreticulin exportins in breast cancer cells. J. Steroid Biochem. Mol. Biol. 2019, 185, 7–16. [Google Scholar] [CrossRef]
- Erić, A.; Juranić, Z.; Milovanović, Z.; Marković, I.; Inić, M.; Stanojević-Bakić, N.; Vojinović-Golubović, V. Effects of humoral immunity and calreticulin overexpression on postoperative course in breast cancer. Pathol. Oncol. Res. 2009, 15, 89–90. [Google Scholar] [CrossRef]
- Gromov, P.; Gromova, I.; Bunkenborg, J.; Cabezon, T.; Moreira, J.M.A.; Timmermans-Wielenga, V.; Roepstorff, P.; Rank, F.; Celis, J.E. Up-regulated Proteins in the Fluid Bathing the Tumour Cell Microenvironment as Potential Serological Markers for Early Detection of Cancer of the Breast. Mol. Oncol. 2010, 4, 65–89. [Google Scholar] [CrossRef]
- Lwin, Z.M.; Guo, C.; Salim, A.; Yip, G.W.C.; Chew, F.T.; Nan, J.; Thike, A.A.; Tan, P.H.; Bay, B.H. Clinicopathological significance of calreticulin in breast invasive ductal carcinoma. Mod. Pathol. 2010, 23, 1559–1566. [Google Scholar] [CrossRef]
- Zamanian, M.; Qader Hamadneh, L.A.; Veerakumarasivam, A.; Abdul Rahman, S.; Shohaimi, S.; Rosli, R. Calreticulin mediates an invasive breast cancer phenotype through the transcriptional dysregulation of p53 and MAPK pathways. Cancer Cell Int. 2016, 16, 56. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Kouadir, M.; Song, H.; Shi, F. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 2019, 10, 128. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, W.; Wang, Y.; Wan, C.; Bai, Y.; Sun, X.; Ma, J.; Zheng, F. TNF-α/calreticulin dual signaling induced NLRP3 inflammasome activation associated with HuR nucleocytoplasmic shuttling in rheumatoid arthritis. Inflamm. Res. 2019, 68, 597–611. [Google Scholar] [CrossRef]
- Jiang, Z.; Chen, Z.; Hu, L.; Qiu, L.; Zhu, L. Calreticulin Blockade Attenuates Murine Acute Lung Injury by Inducing Polarization of M2 Subtype Macrophages. Front. Immunol. 2020, 11, 11. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, D.; She, L.; Wang, Z.; Yang, N.; Sun, R.; Zhang, Y.; Yan, C.; Wei, Q.; Aa, J.; et al. Silybin inhibits NLRP3 inflammasome assembly through the NAD+/SIRT2 pathway in mice with nonalcoholic fatty liver disease. FASEB J. 2018, 32, 757–767. [Google Scholar] [CrossRef]
- Mónaco, A.; Chilibroste, S.; Yim, L.; Chabalgoity, J.A.; Moreno, M. Inflammasome activation, NLRP3 engagement and macrophage recruitment to tumor microenvironment are all required for Salmonella antitumor effect. Cancer Immunol. Immunother. 2022, 71, 2141–2150. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, X.; Liao, N.; Mi, L.; Peng, Y.; Liu, B.; Zhang, S.; Wen, F. Enhanced expression of NLRP3 inflammasome-related inflammation in diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2018, 59, 978–985. [Google Scholar] [CrossRef]
- Silva, M.D.S.; Lopes, J.A.; Paloschi, M.V.; Boeno, C.N.; Rego, C.M.A.; de Oliveira Sousa, O.; Santana, H.M.; dos Reis, V.P.; Serrath, S.N.; Setúbal, S.d.S.; et al. NLRP3 inflammasome activation in human peripheral blood mononuclear cells induced by venoms secreted PLA2s. Int. J. Biol. Macromol. 2022, 202, 597–607. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, S.; Hu, S.; Li, H.; Li, M.; Geng, X.; Wang, H. NLRP3 inflammasome expression in peripheral blood monocytes of coronary heart disease patients and its modulation by rosuvastatin. Mol. Med. Rep. 2019, 20, 1826–1836. [Google Scholar] [CrossRef]
- Kummer, J.A.; Broekhuizen, R.; Everett, H.; Agostini, L.; Kuijk, L.; Martinon, F.; Van Bruggen, R.; Tschopp, J. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J. Histochem. Cytochem. 2007, 55, 443–452. [Google Scholar] [CrossRef]
- Sand, J.; Haertel, E.; Biedermann, T.; Contassot, E.; Reichmann, E.; French, L.E.; Werner, S.; Beer, H.D. Expression of inflammasome proteins and inflammasome activation occurs in human, but not in murine keratinocytes article. Cell Death Dis. 2018, 9, 24. [Google Scholar] [CrossRef]
- Hsieh, C.W.; Chen, Y.M.; Lin, C.C.; Tang, K.T.; Chen, H.H.; Hung, W.T.; Lai, K.L.; Chen, D.Y. Elevated expression of the NLRP3 inflammasome and its correlation with disease activity in adult-onset still disease. J. Rheumatol. 2017, 44, 1142–1150. [Google Scholar] [CrossRef]
- Peelen, E.; Damoiseaux, J.; Muris, A.H.; Knippenberg, S.; Smolders, J.; Hupperts, R.; Thewissen, M. Increased inflammasome related gene expression profile in PBMC may facilitate T helper 17 cell induction in multiple sclerosis. Mol. Immunol. 2015, 63, 521–529. [Google Scholar] [CrossRef]
- Qiu, D.; Zhang, D.; Yu, Z.; Jiang, Y.; Zhu, D. Bioinformatics approach reveals the critical role of the NOD-like receptor signaling pathway in COVID-19-associated multiple sclerosis syndrome. J. Neural Transm. 2022, 129, 1031–1038. [Google Scholar] [CrossRef]
- Shamsabadi, R.M.; Basafa, S.; Yarahmadi, R.; Goorani, S.; Khani, M.; Kamarehei, M.; Hossein Kiani, A. Elevated Expression of NLRP1 and IPAF Are Related to Oral Pemphigus Vulgaris Pathogenesis. Inflammation 2015, 38, 205–208. [Google Scholar] [CrossRef]
- Ugurel, E.; Erdag, E.; Kucukali, C.I.; Olcay, A.; Sanli, E.; Akbayir, E.; Kurtuncu, M.; Gunduz, T.; Yilmaz, V.; Tuzun, E.; et al. Enhanced NLRP3 and DEFA1B expression during the active stage of parenchymal neuro-behçet’s disease. In Vivo 2019, 33, 1493–1497. [Google Scholar] [CrossRef]
- Scianaro, R.; Insalaco, A.; Bracci Laudiero, L.; De Vito, R.; Pezzullo, M.; Teti, A.; De Benedetti, F.; Prencipe, G. Deregulation of the IL-1β axis in chronic recurrent multifocal osteomyelitis. Pediatr. Rheumatol. 2014, 12, 30. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Karrabi, M.; Atabti, H.; Raoofi, A.; Mousavi Khaneghah, A. Investigation of the role of IL18, IL-1β and NLRP3 inflammasome in reducing expression of FLG-2 protein in Psoriasis vulgaris skin lesions. Biotech. Histochem. 2022, 97, 277–283. [Google Scholar] [CrossRef]
- Wu, X.; Cakmak, S.; Wortmann, M.; Hakimi, M.; Zhang, J.; Böckler, D.; Dihlmann, S. Sex-and disease-specific inflammasome signatures in circulating blood leukocytes of patients with abdominal aortic aneurysm. Mol. Med. 2016, 22, 508–518. [Google Scholar] [CrossRef]
- Wu, X.; Hakimi, M.; Wortmann, M.; Zhang, J.; Böckler, D.; Dihlmann, S. Gene expression of inflammasome components in peripheral blood mononuclear cells (PBMC) of vascular patients increases with age. Immun. Ageing 2015, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Ferlazzo, N.; Currò, M.; Isola, G.; Maggio, S.; Bertuccio, M.P.; Trovato-Salinaro, A.; Matarese, G.; Alibrandi, A.; Caccamo, D.; Ientile, R. Changes in the biomarkers of oxidative/nitrosative stress and endothelial dysfunction are associated with cardiovascular risk in periodontitis patients. Curr. Issues Mol. Biol. 2021, 43, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.d.S.; Queiroga, T.B.D.; Nunes, D.F.; Andrade, C.d.M.; Nascimento, M.S.L.; Do-Valle-Matta, M.A.; da Câmara, A.C.J.; Galvão, L.M.d.C.; Guedes, P.M.M.; Chiari, E. Innate immune receptors over expression correlate with chronic chagasic cardiomyopathy and digestive damage in patients. PLoS Negl. Trop. Dis. 2018, 12, e0006589. [Google Scholar] [CrossRef]
- Rodrigues, T.S.; de Sá, K.S.G.; Ishimoto, A.Y.; Becerra, A.; Oliveira, S.; Almeida, L.; Gonçalves, A.V.; Perucello, D.B.; Andrade, W.A.; Castro, R.; et al. Inflammasomes are activated in response to SARS-cov-2 infection and are associated with COVID-19 severity in patients. J. Exp. Med. 2020, 218, e20201707. [Google Scholar] [CrossRef] [PubMed]
- Vakrakou, A.G.; Boiu, S.; Ziakas, P.D.; Xingi, E.; Boleti, H.; Manoussakis, M.N. Systemic activation of NLRP3 inflammasome in patients with severe primary Sjögren’s syndrome fueled by inflammagenic DNA accumulations. J. Autoimmun. 2018, 91, 23–33. [Google Scholar] [CrossRef]
- Pakvisal, N.; Kongkavitoon, P.; Sathitruangsak, C.; Pornpattanarak, N.; Boonsirikamchai, P.; Ouwongprayoon, P.; Aporntewan, C.; Chantranuwatana, P.; Mutirangura, A.; Vinayanuwattikun, C. Differential expression of immune-regulatory proteins C5AR1, CLEC4A and NLRP3 on peripheral blood mononuclear cells in early-stage non-small cell lung cancer patients. Sci. Rep. 2022, 12, 18439. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, Y.; Dong, Y.; Xu, J.; Su, W.; Shi, W.; Zou, Q.; Yang, X. NLRP3 promotes immune escape by regulating immune checkpoints: A pan-cancer analysis. Int. Immunopharmacol. 2022, 104, 108512. [Google Scholar] [CrossRef] [PubMed]
- Felicetti, F.; Aimaretti, E.; Dal Bello, F.; Gatti, F.; Godono, A.; Saba, F.; Einaudi, G.; Collino, M.; Fagioli, F.; Aragno, M.; et al. Advanced glycation end products and their related signaling cascades in adult survivors of childhood Hodgkin lymphoma: A possible role in the onset of late complications. Free Radic. Biol. Med. 2022, 178, 76–82. [Google Scholar] [CrossRef]
- Saponaro, C.; Scarpi, E.; Sonnessa, M.; Cioffi, A.; Buccino, F.; Giotta, F.; Pastena, M.I.; Zito, F.A.; Mangia, A. Prognostic Value of NLRP3 Inflammasome and TLR4 Expression in Breast Cancer Patients. Front. Oncol. 2021, 11, 705331. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, H.; Xu, Y.; Peng, T.; Meng, X.; Zou, F. NLRP3 induces the autocrine secretion of IL-1β to promote epithelial–mesenchymal transition and metastasis in breast cancer. Biochem. Biophys. Res. Commun. 2021, 560, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, H.; Zang, Y.; Wang, F. NLRP3 inflammasome inactivation driven by miR-223-3p reduces tumor growth and increases anticancer immunity in breast cancer. Mol. Med. Rep. 2019, 19, 2180–2188. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhao, F.; Guo, F.; Wang, C.; Fu, Z. Polymeric Nanoparticles Induce NLRP3 Inflammasome Activation and Promote Breast Cancer Metastasis. Macromol. Biosci. 2017, 17, 1700273. [Google Scholar] [CrossRef]
- Yentis, S.M.; Rowbottom, A.W.; Riches, P.G. Detection of cytoplasmic IL-1β in peripheral blood mononuclear cells from intensive care unit (ICU) patients. Clin. Exp. Immunol. 1995, 100, 330–335. [Google Scholar] [CrossRef]
- Perez, R.L.; Roman, J. Fibrin enhances the expression of IL-1 beta by human peripheral blood mononuclear cells. Implications in pulmonary inflammation. J. Immunol. 1995, 154, 1879–1887. [Google Scholar] [CrossRef]
- Ferentinos, P.; Maratou, E.; Antoniou, A.; Serretti, A.; Smyrnis, N.; Moutsatsou, P. Interleukin-1 Beta in Peripheral Blood Mononuclear Cell Lysates as a Longitudinal Biomarker of Response to Antidepressants: A Pilot Study. Front. Psychiatry 2021, 12, 801738. [Google Scholar] [CrossRef]
- Adachi, M.; Inoue, H.; Arinaga, S.; Li, J.; Ueo, H.; Mori, M.; Akiyoshi, T. Quantitative analysis of cytokine mRNA expression in peripheral blood mononuclear cells following treatment with interleukin-2. Cancer Immunol. Immunother. 1997, 44, 329–334. [Google Scholar] [CrossRef]
- Autenshlyus, A.I.; Davletova, K.I.; Mikhaylova, E.S.; Proskura, A.V.; Varaksin, N.A.; Bogachuk, A.P.; Sidorov, S.V.; Lyakhovich, V.V.; Lipkin, V.M. Influence of Internal and External Factors on the Production of Cytokines by Peripheral Blood Cells in Breast Cancer. Dokl. Biochem. Biophys. 2020, 493, 178–180. [Google Scholar] [CrossRef]
- Cai, S.; Zheng, J.; Song, H.; Wu, H.; Cai, W. Relationship between serum TGF- β 1, MMP-9 and IL-1β and pathological features and prognosis in breast cancer. Front. Genet. 2023, 13, 1095338. [Google Scholar] [CrossRef]
- Alipour, S.; Khalighfard, S.; Khori, V.; Amiriani, T.; Tajaldini, M.; Dehghan, M.; Sadani, S.; Omranipour, R.; Vahabzadeh, G.; Eslami, B.; et al. Innovative targets of the lncRNA-miR-mRNA network in response to low-dose aspirin in breast cancer patients. Sci. Rep. 2022, 12, 12054. [Google Scholar] [CrossRef]
- Panis, C.; Victorino, V.J.; Herrera, A.C.S.A.; Freitas, L.F.; De Rossi, T.; Campos, F.C.; Colado Simão, A.N.; Barbosa, D.S.; Pinge-Filho, P.; Cecchini, R.; et al. Differential oxidative status and immune characterization of the early and advanced stages of human breast cancer. Breast Cancer Res. Treat. 2012, 133, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Woo, Y.; Kim, H.; Kim, K.C.; Han, J.A.; Jung, Y.J. Tumor-secreted factors induce IL-1β maturation via the glucose-mediated synergistic axis of mTOR and NF-κB pathways in mouse macrophages. PLoS ONE 2018, 13, e0209653. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Ren, P.; Luo, S.; Ji, Q.; Xu, M.; Lu, N.; Wang, Y. Detection and analysis of phenotypes of tumor-associated macrophages in mouse model of spontaneous breast cancer. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2017, 33, 721–725. [Google Scholar] [PubMed]
- Qin, Q.; Ji, H.; Li, D.; Zhang, H.; Zhang, Z.; Zhang, Q. Tumor-associated macrophages increase COX-2 expression promoting endocrine resistance in breast cancer via the PI3K/Akt/mTOR pathway. Neoplasma 2021, 68, 938–946. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Garduño, A.M.; Mendoza-Rodríguez, M.G.; Urrutia-Cabrera, D.; Domínguez-Robles, M.C.; Pérez-Yépez, E.A.; Ayala-Sumuano, J.T.; Meza, I. IL-1β induced methylation of the estrogen receptor ERα gene correlates with EMT and chemoresistance in breast cancer cells. Biochem. Biophys. Res. Commun. 2017, 490, 780–785. [Google Scholar] [CrossRef]
- Rani, A.; Stebbing, J.; Giamas, G.; Murphy, J. Endocrine resistance in hormone receptor positive breast cancer–from mechanism to therapy. Front. Endocrinol. 2019, 10, 245. [Google Scholar] [CrossRef]
- Wein, L.; Loi, S. Mechanisms of resistance of chemotherapy in early-stage triple negative breast cancer (TNBC). Breast 2017, 34, S27–S30. [Google Scholar] [CrossRef]
- Schütz, F.; Stefanovic, S.; Mayer, L.; Von Au, A.; Domschke, C.; Sohn, C. PD-1/PD-L1 Pathway in Breast Cancer. Oncol. Res. Treat. 2017, 40, 294–297. [Google Scholar] [CrossRef]
- Dong, Y.; Sun, Q.; Zhang, X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget 2017, 8, 2171–2186. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Katz, H.; Alsharedi, M. Immunotherapy in triple-negative breast cancer. Med. Oncol. 2018, 35, 13. [Google Scholar] [CrossRef] [PubMed]
- Frigola, X.; Inman, B.A.; Lohse, C.M.; Krco, C.J.; Cheville, J.C.; Thompson, R.H.; Leibovich, B.; Blute, M.L.; Dong, H.; Kwon, E.D. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin. Cancer Res. 2011, 17, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Q.; Shi, B.; Xu, P.; Hu, Z.; Bai, L.; Zhang, X. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1 + cell lines. Cytokine 2011, 56, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Asanuma, K.; Nakamura, T.; Hayashi, A.; Okamoto, T.; Iino, T.; Asanuma, Y.; Hagi, T.; Kita, K.; Nakamura, K.; Sudo, A. Soluble programmed death-ligand 1 rather than PD-L1 on tumor cells effectively predicts metastasis and prognosis in soft tissue sarcomas. Sci. Rep. 2020, 10, 9077. [Google Scholar] [CrossRef]
- Baggio, C.; Ramaschi, G.E.; Oliviero, F.; Ramonda, R.; Sfriso, P.; Trevisi, L.; Cignarella, A.; Bolego, C. Sex-dependent PD-L1/sPD-L1 trafficking in human endothelial cells in response to inflammatory cytokines and VEGF. Biomed. Pharmacother. 2023, 162, 114670. [Google Scholar] [CrossRef]
- Han, B.; Dong, L.; Zhou, J.; Yang, Y.; Guo, J.; Xuan, Q.; Gao, K.; Xu, Z.; Lei, W.; Wang, J.; et al. The clinical implication of soluble PD-L1 (sPD-L1) in patients with breast cancer and its biological function in regulating the function of T lymphocyte. Cancer Immunol. Immunother. 2021, 70, 2893–2909. [Google Scholar] [CrossRef]
- Li, Y.; Cui, X.; Yang, Y.J.; Chen, Q.Q.; Zhong, L.; Zhang, T.; Cai, R.L.; Miao, J.Y.; Yu, S.C.; Zhang, F. Serum sPD-1 and sPD-L1 as Biomarkers for Evaluating the Efficacy of Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer Patients. Clin. Breast Cancer 2019, 19, 326–332.e1. [Google Scholar] [CrossRef]
- Botticelli, A.; Pomati, G.; Cirillo, A.; Scagnoli, S.; Pisegna, S.; Chiavassa, A.; Rossi, E.; Schinzari, G.; Tortora, G.; Di Pietro, F.R.; et al. The role of immune profile in predicting outcomes in cancer patients treated with immunotherapy. Front. Immunol. 2022, 13, 974087. [Google Scholar] [CrossRef]
- Hossen, M.J.; Yang, W.S.; Kim, D.; Aravinthan, A.; Kim, J.H.; Cho, J.Y. Thymoquinone: An IRAK1 inhibitor with in vivo and in vitro anti-inflammatory activities. Sci. Rep. 2017, 7, 42995. [Google Scholar] [CrossRef]
- Elgohary, S.; Elkhodiry, A.A.; Amin, N.S.; Stein, U.; El Tayebi, H.M. Thymoquinone: A tie-breaker in SARS-CoV-2-infected cancer patients? Cells 2021, 10, 302. [Google Scholar] [CrossRef] [PubMed]
- Bashmail, H.A.; Alamoudi, A.A.; Noorwali, A.; Hegazy, G.A.; AJabnoor, G.; Choudhry, H.; Al-Abd, A.M. Thymoquinone synergizes gemcitabine anti-breast cancer activity via modulating its apoptotic and autophagic activities. Sci. Rep. 2018, 8, 11674. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.; Kumar, B.N.P.; Dey, K.K.; Pal, I.; Parekh, A.; Mandal, M. Molecular targeting of Akt by thymoquinone promotes G1 arrest through translation inhibition of cyclin D1 and induces apoptosis in breast cancer cells. Life Sci. 2013, 93, 783–790. [Google Scholar] [CrossRef]
- Bashmail, H.A.; Alamoudi, A.A.; Noorwali, A.; Hegazy, G.A.; Ajabnoor, G.M.; Al-Abd, A.M. Thymoquinone enhances paclitaxel anti-breast cancer activity via inhibiting tumor-associated stem cells despite apparent mathematical antagonism. Molecules 2020, 25, 426. [Google Scholar] [CrossRef] [PubMed]
- Alobaedi, O.H.; Talib, W.H.; Basheti, I.A. Antitumor effect of thymoquinone combined with resveratrol on mice transplanted with breast cancer. Asian Pac. J. Trop. Med. 2017, 10, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Kabil, N.; Bayraktar, R.; Kahraman, N.; Mokhlis, H.A.; Calin, G.A.; Lopez-Berestein, G.; Ozpolat, B. Thymoquinone inhibits cell proliferation, migration, and invasion by regulating the elongation factor 2 kinase (eEF-2K) signaling axis in triple-negative breast cancer. Breast Cancer Res. Treat. 2018, 171, 593–605. [Google Scholar] [CrossRef]
- Moubarak, M.M.; Chanouha, N.; Abou Ibrahim, N.; Khalife, H.; Gali-Muhtasib, H. Thymoquinone anticancer activity is enhanced when combined with royal jelly in human breast cancer. World J. Clin. Oncol. 2021, 12, 342–354. [Google Scholar] [CrossRef]
- Parbin, S.; Shilpi, A.; Kar, S.; Pradhan, N.; Sengupta, D.; Deb, M.; Rath, S.K.; Patra, S.K. Insights into the molecular interactions of thymoquinone with histone deacetylase: Evaluation of the therapeutic intervention potential against breast cancer. Mol. Biosyst. 2016, 12, 48–58. [Google Scholar] [CrossRef]
- Alandağ, C.; Kancaği, D.D.; Karakuş, G.S.; Çakirsoy, D.; Ovali, E.; Karaman, E.; Yüce, E.; Özdemir, F. The effects of thymoquinone on pancreatic cancer and immune cells. Rev. Assoc. Med. Bras. 2022, 68, 1023–1026. [Google Scholar] [CrossRef]
- Alkhattabi, N.A.; Hussein, S.A.; Tarbiah, N.I.; Alzahri, R.Y.; Khalifa, R. Thymoquinone Effect on Monocyte-Derived Macrophages, Cell-Surface Molecule Expression, and Phagocytosis. Nutrients 2022, 14, 5240. [Google Scholar] [CrossRef]
- Ahmad, I.; Muneer, K.M.; Tamimi, I.A.; Chang, M.E.; Ata, M.O.; Yusuf, N. Thymoquinone suppresses metastasis of melanoma cells by inhibition of NLRP3 inflammasome. Toxicol. Appl. Pharmacol. 2013, 270, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Periyanayagam, S.; Arumugam, G.; Ravikumar, A.; Ganesan, V.S. Thymoquinone ameliorates NLRP3-mediated inflammation in the pancreas of albino Wistar rats fed ethanol and high-fat diet. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.P.; Liu, S.X.; Yang, Q.; Liu, H.Y.; Xu, L.L.; Hao, Y.H.; Zhang, X.Q. Effect of Thymoquinone on Acute Kidney Injury Induced by Sepsis in BALB/c Mice. Biomed Res. Int. 2020, 2020, 1594726. [Google Scholar] [CrossRef]
- Liu, H.; Sun, Y.; Zhang, Y.; Yang, G.; Guo, L.; Zhao, Y.; Pei, Z. Role of Thymoquinone in Cardiac Damage Caused by Sepsis from BALB/c Mice. Inflammation 2019, 42, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.W.; Guo, Y.; Zhu, H.L.; Dong, M.; Zhang, Q.; Wang, F. Thymoquinone Protects against Hyperlipidemia-Induced Cardiac Damage in Low-Density Lipoprotein Receptor-Deficient (LDL-R−/−) Mice via Its Anti-inflammatory and Antipyroptotic Effects. Biomed Res. Int. 2020, 2020, 4878704. [Google Scholar] [CrossRef]
- Hamdan, A.M.E.; Alharthi, F.H.J.; Alanazi, A.H.; El-Emam, S.Z.; Zaghlool, S.S.; Metwally, K.; Albalawi, S.A.; Abdu, Y.S.; Mansour, R.E.S.; Salem, H.A.; et al. Neuroprotective Effects of Phytochemicals against Aluminum Chloride-Induced Alzheimer’s Disease through ApoE4/LRP1, Wnt3/β-Catenin/GSK3β, and TLR4/NLRP3 Pathways with Physical and Mental Activities in a Rat Model. Pharmaceuticals 2022, 15, 1008. [Google Scholar] [CrossRef]
- Adinew, G.M.; Messeha, S.S.; Taka, E.; Badisa, R.B.; Soliman, K.F.A. Anticancer Effects of Thymoquinone through the Antioxidant Activity, Upregulation of Nrf2, and Downregulation of PD-L1 in Triple-Negative Breast Cancer Cells. Nutrients 2022, 14, 4787. [Google Scholar] [CrossRef]
- Pelekanou, V.; Villarroel-Espindola, F.; Schalper, K.A.; Pusztai, L.; Rimm, D.L. CD68, CD163, and matrix metalloproteinase 9 (MMP-9) co-localization in breast tumor microenvironment predicts survival differently in ER-positive and -negative cancers. Breast Cancer Res. 2018, 20, 154. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef]
- Rivera Vargas, T.; Apetoh, L. Danger signals: Chemotherapy enhancers? Immunol. Rev. 2017, 280, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Fan, X.; Yuan, B.; Takagi, N.; Liu, S.; Han, X.; Ren, J.; Liu, J. Berberine inhibits NLRP3 Inflammasome pathway in human triple-negative breast cancer MDA-MB-231 cell. BMC Complement. Altern. Med. 2019, 19, 216. [Google Scholar] [CrossRef] [PubMed]
- Faria, S.S.; Costantini, S.; de Lima, V.C.C.; de Andrade, V.P.; Rialland, M.; Cedric, R.; Budillon, A.; Magalhães, K.G. NLRP3 inflammasome-mediated cytokine production and pyroptosis cell death in breast cancer. J. Biomed. Sci. 2021, 28, 26. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Gaston, C.L.; Reddy, K.; Iwata, S.; Nishio, J. Inflammatory Biomarkers in Cancer. Mediat. Inflamm. 2016, 2016, 7282797. [Google Scholar] [CrossRef]
- Lebreton, F.; Berishvili, E.; Parnaud, G.; Rouget, C.; Bosco, D.; Berney, T.; Lavallard, V. NLRP3 inflammasome is expressed and regulated in human islets article. Cell Death Dis. 2018, 9, 726. [Google Scholar] [CrossRef]
- Moossavi, M.; Parsamanesh, N.; Bahrami, A.; Atkin, S.L.; Sahebkar, A. Role of the NLRP3 inflammasome in cancer. Mol. Cancer 2018, 17, 158. [Google Scholar] [CrossRef]
- Hamarsheh, S.; Zeiser, R. NLRP3 Inflammasome Activation in Cancer: A Double-Edged Sword. Front. Immunol. 2020, 11, 1444. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Ahn, K.S.; Hsu, A.; Woo, C.C.; Yuan, Y.; Tan, K.H.B.; Chinnathambi, A.; Alahmadi, T.A.; Alharbi, S.A.; Koh, A.P.F.; et al. Thymoquinone Inhibits Bone Metastasis of Breast Cancer Cells Through Abrogation of the CXCR4 Signaling Axis. Front. Pharmacol. 2018, 9, 1294. [Google Scholar] [CrossRef]
- Obeid, M.; Tesniere, A.; Ghiringhelli, F.; Fimia, G.M.; Apetoh, L.; Perfettini, J.L.; Castedo, M.; Mignot, G.; Panaretakis, T.; Casares, N.; et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 2007, 13, 54–61. [Google Scholar] [CrossRef]
- Elangovan, M.; Chong, H.K.; Park, J.H.; Yeo, E.J.; Yoo, Y.J. The role of ubiquitin-conjugating enzyme Ube2j1 phosphorylation and its degradation by proteasome during endoplasmic stress recovery. J. Cell Commun. Signal. 2017, 11, 265–273. [Google Scholar] [CrossRef]
- Lenin, R.; Nagy, P.G.; Jha, K.A.; Gangaraju, R. GRP78 translocation to the cell surface and O-GlcNAcylation of VE-Cadherin contribute to ER stress-mediated endothelial permeability. Sci. Rep. 2019, 9, 10783. [Google Scholar] [CrossRef]
- Cook, K.L.; Clarke, P.A.G.; Clarke, R. Targeting GRP78 and antiestrogen resistance in breast cancer. Future Med. Chem. 2013, 5, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-W.; Yang, R.-Y.; Chan, L.-C.; Li, C.-F.; Sun, L.; Lee, H.-H.; Lee, P.-C.; Sher, Y.-P.; Ying, H.; Hung, M.-C. The stabilization of PD-L1 by the endoplasmic reticulum stress protein GRP78 in triple-negative breast cancer. Am. J. Cancer Res. 2020, 10, 2621–2634. [Google Scholar] [PubMed]
- Bouhlel, A.; Mosbah, B.; Abdallah, H.; Ribault, C.; Viel, R.; Mannaï, S.; Corlu, A.; Abdennebi, B. Thymoquinone prevents endoplasmic reticulum stress and mitochondria-induced apoptosis in a rat model of partial hepatic warm ischemia reperfusion. Biomed. Pharmacother. 2017, 94, 964–973. [Google Scholar] [CrossRef] [PubMed]
- Serrano Del Valle, A.; Beltrán-Visiedo, M.; de Poo-Rodríguez, V.; Jiménez-Alduán, N.; Azaceta, G.; Díez, R.; Martínez-Lázaro, B.; Izquierdo, I.; Palomera, L.; Naval, J.; et al. Ecto-calreticulin expression in multiple myeloma correlates with a failed anti-tumoral immune response and bad prognosis. Oncoimmunology 2022, 11, 2141973. [Google Scholar] [CrossRef]
- Najibi, A.J.; Larkin, K.; Feng, Z.; Jeffreys, N.; Dacus, M.T.; Rustagi, Y.; Hodi, F.S.; Mooney, D.J. Chemotherapy Dose Shapes the Expression of Immune-Interacting Markers on Cancer Cells. Cell. Mol. Bioeng. 2022, 15, 535–551. [Google Scholar] [CrossRef]
- Kepp, O.; Liu, P.; Zhao, L.; Plo, I.; Kroemer, G. Surface-exposed and soluble calreticulin: Conflicting biomarkers for cancer prognosis. Oncoimmunology 2020, 9, 1792037. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Zhao, L.; Loos, F.; Marty, C.; Xie, W.; Martins, I.; Lachkar, S.; Qu, B.; Waeckel-Énée, E.; Plo, I.; et al. Immunosuppression by Mutated Calreticulin Released from Malignant Cells. Mol. Cell 2020, 77, 748–760.e9. [Google Scholar] [CrossRef]
- Kroemer, G.; Zitvogel, L. Subversion of calreticulin exposure as a strategy of immune escape. Cancer Cell 2021, 39, 449–451. [Google Scholar] [CrossRef]
- Dastjerdi, M.N.; Mehdiabady, E.M.; Iranpour, F.G.; Bahramian, H. Effect of thymoquinone on P53 gene expression and consequence apoptosis in breast cancer cell line. Int. J. Prev. Med. 2016, 7, 66. [Google Scholar] [CrossRef]
- Aslan, M.; Afşar, E.; Kırımlıoglu, E.; Çeker, T.; Yılmaz, Ç. Antiproliferative Effects of Thymoquinone in MCF-7 Breast and HepG2 Liver Cancer Cells: Possible Role of Ceramide and ER Stress. Nutr. Cancer 2021, 73, 460–472. [Google Scholar] [CrossRef]
- Arafa, E.S.A.; Zhu, Q.; Shah, Z.I.; Wani, G.; Barakat, B.M.; Racoma, I.; El-Mahdy, M.A.; Wani, A.A. Thymoquinone up-regulates PTEN expression and induces apoptosis in doxorubicin-resistant human breast cancer cells. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2011, 706, 28–35. [Google Scholar] [CrossRef]
- Al-Mutairi, A.; Rahman, A.; Rao, M.S. Low Doses of Thymoquinone and Ferulic Acid in Combination Effectively Inhibit Proliferation of Cultured MDA-MB 231 Breast Adenocarcinoma Cells. Nutr. Cancer 2021, 73, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Aldebasi, Y.H.; Alsuhaibani, S.A.; Khan, M.A. Thymoquinone augments cyclophosphamide-mediated inhibition of cell proliferation in breast cancer cells. Asian Pacific J. Cancer Prev. 2019, 20, 1153–1160. [Google Scholar] [CrossRef] [PubMed]
- Dehghani, H.; Hashemi, M.; Entezari, M.; Mohsenifar, A. The comparison of anticancer activity of thymoquinone and nanothymoquinone on human breast adenocarcinoma. Iran. J. Pharm. Res. 2015, 14, 539–546. [Google Scholar] [CrossRef]
- Sutton, K.M.; Greenshields, A.L.; Hoskin, D.W. Thymoquinone, a bioactive component of black caraway seeds, causes G1 phase cell cycle arrest and apoptosis in triple-negative breast cancer cells with mutant p53. Nutr. Cancer 2014, 66, 408–418. [Google Scholar] [CrossRef]
- Sutton, K.M.; Doucette, C.D.; Hoskin, D.W. NADPH quinone oxidoreductase 1 mediates breast cancer cell resistance to thymoquinone-induced apoptosis. Biochem. Biophys. Res. Commun. 2012, 426, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Perou, C.M.; Sørile, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Ress, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Prat, A.; Perou, C.M. Deconstructing the molecular portraits of breast cancer. Mol. Oncol. 2011, 5, 5–23. [Google Scholar] [CrossRef]
- Prat, A.; Parker, J.S.; Fan, C.; Perou, C.M. PAM50 assay and the three-gene model for identifying the major and clinically relevant molecular subtypes of breast cancer. Breast Cancer Res. Treat. 2012, 135, 301–306. [Google Scholar] [CrossRef]
- Prat, A.; Parker, J.S.; Karginova, O.; Fan, C.; Livasy, C.; Herschkowitz, J.I.; He, X.; Perou, C.M. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 2010, 12, R68. [Google Scholar] [CrossRef] [PubMed]
- Koboldt, D.C.; Fulton, R.S.; McLellan, M.D.; Schmidt, H.; Kalicki-Veizer, J.; McMichael, J.F.; Fulton, L.L.; Dooling, D.J.; Ding, L.; Mardis, E.R.; et al. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Sun, Q.; Scott, M.J. Caspase-1 as a multifunctional inflammatory mediator: Noncytokine maturation roles. J. Leukoc. Biol. 2016, 100, 961–967. [Google Scholar] [CrossRef] [PubMed]
- Honma, S.; Shimodaira, K.; Shimizu, Y.; Tsuchiya, N.; Saito, H.; Yanaihara, T.; Okai, T. The influence of inflammatory cytokines on estrogen production and cell proliferation in human breast cancer cells. Endocr. J. 2002, 49, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Todorović-Raković, N.; Radulovic, M.; Vujasinović, T.; Milovanović, J.; Nikolić-Vukosavljević, D. The time-dependent prognostic value of intratumoral cytokine expression profiles in a natural course of primary breast cancer with a long-term follow-up. Cytokine 2018, 102, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Paquette, B.; Therriault, H.; Wagner, J.R. Role of interleukin-1β in radiation-enhancement of MDA-MB-231 breast cancer cell invasion. Radiat. Res. 2013, 180, 292–298. [Google Scholar] [CrossRef]
- Ferrari, D.; Wesselborg, S.; Bauer, M.K.A.; Schulze-Osthoff, K. Extracellular ATP activates transcription factor NF-κB through the P2Z purinoreceptor by selectively targeting NF-κB p65 (RelA). J. Cell Biol. 1997, 139, 1635–1643. [Google Scholar] [CrossRef]
- Korcok, J.; Raimundo, L.N.; Ke, H.Z.; Sims, S.M.; Dixon, S.J. Extracellular nucleotides act through P2X7 receptors to activate NF-κB in osteoclasts. J. Bone Miner. Res. 2004, 19, 642–651. [Google Scholar] [CrossRef]
- Pantschenko, A.G.; Pushkar, I.; Anderson, K.H.; Wang, Y.; Miller, L.J.; Kurtzman, S.H.; Barrows, G.; Kreutzer, D.L. The interleukin-1 family of cytokines and receptors in human breast cancer: Implications for tumor progression. Int. J. Oncol. 2003, 23, 269–284. [Google Scholar] [CrossRef]
- Qiu, S.Q.; Waaijer, S.J.H.; Zwager, M.C.; de Vries, E.G.E.; van der Vegt, B.; Schröder, C.P. Tumor-associated macrophages in breast cancer: Innocent bystander or important player? Cancer Treat. Rev. 2018, 70, 178–189. [Google Scholar] [CrossRef]
- Szostakowska, M.; Trębińska-Stryjewska, A.; Grzybowska, E.A.; Fabisiewicz, A. Resistance to endocrine therapy in breast cancer: Molecular mechanisms and future goals. Breast Cancer Res. Treat. 2019, 173, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.E.; Shen, Q.; Cescon, D.W.; Reedijk, M. Exploring immune interactions in triple negative breast cancer: IL-1β inhibition and its therapeutic potential. Front. Genet. 2023, 14, 1086163. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, L.M.; Harhay, M.O.; Zhang, P.; Ugras, S. Impact of Neoadjuvant Chemotherapy on Breast Cancer Subtype: Does Subtype Change and, if so, How?: IHC Profile and Neoadjuvant Chemotherapy. Ann. Surg. Oncol. 2018, 25, 3535–3540. [Google Scholar] [CrossRef]
- Wang, D.; Qiao, J.; Zhao, X.; Chen, T.; Guan, D. Thymoquinone Inhibits IL-1β-Induced Inflammation in Human Osteoarthritis Chondrocytes by Suppressing NF-κB and MAPKs Signaling Pathway. Inflammation 2015, 38, 2235–2241. [Google Scholar] [CrossRef]
- Cui, B.W.; Bai, T.; Yang, Y.; Zhang, Y.; Jiang, M.; Yang, H.X.; Wu, M.; Liu, J.; Qiao, C.Y.; Zhan, Z.Y.; et al. Thymoquinone attenuates acetaminophen overdose-induced acute liver injury and inflammation via regulation of JNK and AMPK signaling pathway. Am. J. Chin. Med. 2019, 47, 577–594. [Google Scholar] [CrossRef]
- Adinolfi, E.; Giuliani, A.L.; De Marchi, E.; Pegoraro, A.; Orioli, E.; Di Virgilio, F. The P2X7 receptor: A main player in inflammation. Biochem. Pharmacol. 2018, 151, 234–244. [Google Scholar] [CrossRef]
- Lamkanfi, M.; Mueller, J.L.; Vitari, A.C.; Misaghi, S.; Fedorova, A.; Deshayes, K.; Lee, W.P.; Hoffman, H.M.; Dixit, V.M. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J. Cell Biol. 2009, 187, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Ashcroft, F.M. ATP-sensitive potassium channelopathies: Focus on insulin secretion. J. Clin. Investig. 2005, 115, 2047–2058. [Google Scholar] [CrossRef]
- Suddek, G.M. Thymoquinone-induced relaxation of isolated rat pulmonary artery. J. Ethnopharmacol. 2010, 127, 210–214. [Google Scholar] [CrossRef]
- Parvardeh, S.; Sabetkasaei, M.; Moghimi, M.; Masoudi, A.; Ghafghazi, S.; Mahboobifard, F. Role of L-Arginine/NO/cGMP/KATP channel signaling pathway in the central and peripheral antinociceptive effect of Thymoquinone in rats. Iran. J. Basic Med. Sci. 2018, 21, 625–633. [Google Scholar] [CrossRef]
- Shigaeva, M.I.; Talanov, E.Y.; Venediktova, N.I.; Murzaeva, S.V.; Mironova, G.D. A role for calreticulin in functioning of mitochondrial ATP-dependent potassium channel. Biophysics 2014, 59, 721–726. [Google Scholar] [CrossRef]
- Adinew, G.; Messeha, S.S.; Badisa, R.; Taka, E.; Soliman, K.F.A. Thymoquinone Anticancer Effects Through the Upregulation of NRF2 and the Downregulation of PD-L1 in MDA-MB-231 Triple-Negative Breast Cancer Cells. FASEB J. 2022, 36, 4787. [Google Scholar] [CrossRef] [PubMed]
- Heeke, A.L.; Tan, A.R. Checkpoint inhibitor therapy for metastatic triple-negative breast cancer. Cancer Metastasis Rev. 2021, 40, 537–547. [Google Scholar] [CrossRef]
- Slater, H. FDA Approves Pembrolizumab + Chemotherapy Combination for Locally Recurrent Unresectable or Metastatic TNBC. Oncology 2020, 34, 547. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.K.; Collier, A.L.; Lee, D.; Hoefer, R.A.; Zheleva, V.; van Reesema, L.L.S.; Tang-Tan, A.M.; Guye, M.L.; Chang, D.Z.; Winston, J.S.; et al. Perspectives on triple-negative breast cancer: Current treatment strategies, unmet needs, and potential targets for future therapies. Cancers 2020, 12, 2392. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Y.; Cheng, X.; Yuan, H.; Zhu, S.; Liu, J.; Wen, Q.; Xie, Y.; Liu, J.; Kroemer, G.; et al. PINK1 and PARK2 Suppress Pancreatic Tumorigenesis through Control of Mitochondrial Iron-Mediated Immunometabolism. Dev. Cell 2018, 46, 441–455.e8. [Google Scholar] [CrossRef] [PubMed]
- Elgohary, S.; El Tayebi, H.M. Inflammasomes in breast cancer: The ignition spark of progression and resistance? Expert Rev. Mol. Med. 2023, 25, e22. [Google Scholar] [CrossRef]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef]
- Guerrero-Zotano, A.; Mayer, I.A.; Arteaga, C.L. PI3K/AKT/mTOR: Role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev. 2016, 35, 515–524. [Google Scholar] [CrossRef]
- Papa, A.; Pandolfi, P.P. The pten–pi3k axis in cancer. Biomolecules 2019, 9, 153. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, C.C.; Jin, L.; Zhang, X.D. Regulation of PD-L1: A novel role of pro-survival signalling in cancer. Ann. Oncol. 2016, 27, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Zhang, T.; Deng, S.C.; Wei, J.C.; Yang, P.; Wang, Q.; Chen, Z.P.; Li, W.L.; Chen, H.C.; Hu, H.; et al. PD-L1 promotes colorectal cancer stem cell expansion by activating HMGA1-dependent signaling pathways. Cancer Lett. 2019, 450, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Shin, J.M.; Yang, H.W.; Kim, T.H.; Lee, S.H.; Lee, H.M.; Cho, J.G.; Park, I.H. Cigarette smoke extract stimulates mmp-2 production in nasal fibroblasts via ros/pi3k, akt, and nf-κb signaling pathways. Antioxidants 2020, 9, 739. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Wang, Q.; Fu, X.H.; Huang, X.H.; Chen, X.L.; Cao, L.Q.; Chen, L.Z.; Tan, H.X.; Li, W.; Bi, J.; et al. Involvement of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: Association with MMP-9. Hepatol. Res. 2009, 39, 177–186. [Google Scholar] [CrossRef]
- Yokoo, T.; Kitamura, M. Dual regulation of IL-1β-mediated matrix metalloproteinase-9 expression in mesangial cells by NF-KB and AP-1. Am. J. Physiol. 1996, 270, F123–F130. [Google Scholar] [CrossRef]
- Ruhul Amin, A.R.M.; Senga, T.; Oo, M.L.; Thant, A.A.; Hamaguchi, M. Secretion of matrix metalloproteinase-9 by the proinflammatory cytokine, IL-1β: A role for the dual signalling pathways, Akt and Erk. Genes Cells 2003, 8, 515–523. [Google Scholar] [CrossRef]
- Ren, P.; Wu, D.; Appel, R.; Zhang, L.; Zhang, C.; Luo, W.; Robertson, A.A.B.; Cooper, M.A.; Coselli, J.S.; Milewicz, D.M.; et al. Targeting the nlrp3 inflammasome with inhibitor mcc950 prevents aortic aneurysms and dissections in mice. J. Am. Heart Assoc. 2020, 9, e014044. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Lin, S.; Wu, Y.; He, D.; Qu, P. PI3K regulates the activation of NLRP3 inflammasome in atherosclerosis through part-dependent AKT signaling pathway. Exp. Anim. 2021, 70, 488–497. [Google Scholar] [CrossRef]
- Xu, D.; Ma, Y.; Zhao, B.; Li, S.; Zhang, Y.; Pan, S.; Wu, Y.; Wang, J.; Wang, D.; Pan, H.; et al. Thymoquinone induces G2/M arrest, inactivates PI3K/Akt and nuclear factor-κB pathways in human cholangiocarcinomas both in vitro and in vivo. Oncol. Rep. 2014, 31, 2063–2070. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Y.; Deng, H.; Liu, Y.; Lei, X.; He, P.; Dong, W. Thymoquinone inhibits the proliferation and invasion of esophageal cancer cells by disrupting the AKT/GSK-3β/Wnt signaling pathway via PTEN upregulation. Phyther. Res. 2020, 34, 3388–3399. [Google Scholar] [CrossRef]
- Hsu, H.H.; Chen, M.C.; Day, C.H.; Lin, Y.M.; Li, S.Y.; Tu, C.C.; Padma, V.V.; Shih, H.N.; Kuo, W.W.; Huang, C.Y. Thymoquinone suppresses migration of LoVo human colon cancer cells by reducing prostaglandin E2 induced COX-2 activation. World J. Gastroenterol. 2017, 23, 1171–1179. [Google Scholar] [CrossRef]
- Haiaty, S.; Rashidi, M.R.; Akbarzadeh, M.; Bazmani, A.; Mostafazadeh, M.; Nikanfar, S.; Zibaei, Z.; Rahbarghazi, R.; Nouri, M. Correction to: Thymoquinone inhibited vasculogenic capacity and promoted mesenchymal-epithelial transition of human breast cancer stem cells. BMC Complement. Med. Ther. 2021, 21, 266. [Google Scholar] [CrossRef]
- Tadros, S.A.; Attia, Y.M.; Maurice, N.W.; Fahim, S.A.; Abdelwahed, F.M.; Ibrahim, S.; Badary, O.A. Thymoquinone Suppresses Angiogenesis in DEN-Induced Hepatocellular Carcinoma by Targeting miR-1-3p. Int. J. Mol. Sci. 2022, 23, 15904. [Google Scholar] [CrossRef]
- Alshyarba, M.; Otifi, H.; Al Fayi, M.; A Dera, A.; Rajagopalan, P. Thymoquinone inhibits IL-7-induced tumor progression and metastatic invasion in prostate cancer cells by attenuating matrix metalloproteinase activity and Akt/NF-κB signaling. Biotechnol. Appl. Biochem. 2021, 68, 1403–1411. [Google Scholar] [CrossRef]
- Liou, Y.F.; Hsieh, Y.S.; Hung, T.W.; Chen, P.N.; Chang, Y.Z.; Kao, S.H.; Lin, S.W.; Chang, H.R. Thymoquinone inhibits metastasis of renal cell carcinoma cell 786-o-si3 associating with downregulation of MMP-2 and u-pa and suppression of PI3K/src signaling. Int. J. Med. Sci. 2019, 16, 686–695. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.; Subramanian, R.; Priyadharsini, J.V.; Gopalswamy, J. Thymoquinone inhibits the migration of mouse neuroblastoma (Neuro-2a) cells by down-regulating MMP-2 and MMP-9. Chin. J. Nat. Med. 2016, 14, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kuang, X.R.; Lv, P.T.; Yan, X.X. Thymoquinone inhibits proliferation and invasion of human nonsmall-cell lung cancer cells via ERK pathway. Tumor Biol. 2015, 36, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Kolli-Bouhafs, K.; Boukhari, A.; Abusnina, A.; Velot, E.; Gies, J.P.; Lugnier, C.; Rondé, P. Thymoquinone reduces migration and invasion of human glioblastoma cells associated with FAK, MMP-2 and MMP-9 down-regulation. Investig. New Drugs 2012, 30, 2121–2131. [Google Scholar] [CrossRef]
- Markham, A. Alpelisib: First Global Approval. Drugs 2019, 79, 1249–1253. [Google Scholar] [CrossRef]
- Ghallab, A.M.; Eissa, R.A.; El Tayebi, H.M. CXCR2 Small-Molecule Antagonist Combats Chemoresistance and Enhances Immunotherapy in Triple-Negative Breast Cancer. Front. Pharmacol. 2022, 13, 862125. [Google Scholar] [CrossRef]
- Boro, M.; Balaji, K.N. CXCL1 and CXCL2 Regulate NLRP3 Inflammasome Activation via G-Protein–Coupled Receptor CXCR2. J. Immunol. 2017, 199, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Ge, K.; Zhou, W.; Lu, J.; Jia, C.; Zhu, H. C-X-C chemokine receptor 2 (Cxcr2) promotes hepatocellular carcinoma immune evasion via regulating programmed death-ligand 1 (PD-L1). Biol. Chem. 2021, 402, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Ashour, A.E.; Abd-Allah, A.R.; Korashy, H.M.; Attia, S.M.; Alzahrani, A.Z.; Saquib, Q.; Bakheet, S.A.; Abdel-Hamied, H.E.; Jamal, S.; Rishi, A.K. Thymoquinone suppression of the human hepatocellular carcinoma cell growth involves inhibition of IL-8 expression, elevated levels of TRAIL receptors, oxidative stress and apoptosis. Mol. Cell. Biochem. 2014, 389, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Dagar, G.; Chauhan, R.; Sadida, H.Q.; Almarzooqi, S.K.; Hashem, S.; Uddin, S.; Macha, M.A.; Akil, A.S.A.-S.; Pandita, T.K.; et al. Cyclin-dependent kinases in cancer: Role, regulation, and therapeutic targeting. In Control of Cell Cycle & Cell Proliferation; Donev, R., Ed.; Advances in Protein Chemistry and Structural Biology; Elsevier: Amsterdam, The Netherlands, 2023; Volume 135, pp. 21–55. Available online: https://www.sciencedirect.com/science/article/pii/S1876162323000135 (accessed on 16 March 2023).
- Rezaeian, A.H.; Inuzuka, H.; Wei, W. Insights into the aberrant CDK4/6 signaling pathway as a therapeutic target in tumorigenesis. Adv. Protein Chem. Struct. Biol. 2023, 135, 179–201. [Google Scholar]
- Pham, D.V.; Raut, P.K.; Pandit, M.; Chang, J.H.; Katila, N.; Choi, D.Y.; Jeong, J.H.; Park, P.H. Globular adiponectin inhibits breast cancer cell growth through modulation of inflammasome activation: Critical role of sestrin2 and AMPK signaling. Cancers 2020, 12, 613. [Google Scholar] [CrossRef] [PubMed]




| Patient | Age | Molecular Subtype | Size of Mass, cm | Type | Ki67 | Axillary Lymph Node | Treatment |
|---|---|---|---|---|---|---|---|
| Patient 1 | 78 | Luminal A | 3.5 × 3.5 cm | ILC | 12% | Negative | Neoadjuvant hormonal therapy Aromatase inhibitor for three months |
| Patient 2 | 69 | Luminal A | 2.5 × 1.8 cm | IDC | 3% | Positive | N/A |
| Patient 3 | 65 | Luminal A | 2.5 × 1.6 cm | IDC | 15% | Negative | N/A |
| Patient 4 | 75 | Luminal A | 1.5 cm | IDC | 7% | Negative | N/A |
| Patient 5 | 45 | Luminal A | 0.8 × 0.4 cm 0.7 × 0.5 cm | IDC | 8% | Negative | N/A |
| Patient 6 | 53 | Luminal A | 1.2 × 1 cm | IDC | 8% | Positive | N/A |
| Patient 7 | 77 | Luminal A | 1 cm | IDC | 5% | Negative | N/A |
| Patient 8 | 72 | Luminal A | 1.2 cm | ILC | 12% | Positive | N/A |
| Patient 9 | 55 | Luminal A | 1 × 1.5 cm | IDC | 3% | Positive | N/A |
| Patient 10 | 30 | Luminal A | 1.5 cm | IDC | 12% | Positive | N/A |
| Patient 11 | 60 | Luminal A | 1 cm | IDC | 18% | Positive | Chemotherapy one month before surgery |
| Patient 12 | 55 | Luminal A | 2.8 × 1.6 cm | IDC | 12% | Positive | N/A |
| Patient 13 | 60 | Luminal A | 1 cm | IDC | 15% | Positive | N/A |
| Patient 14 | 60 | Luminal A | 1.2 cm | IDC | 10% | Positive | N/A |
| Patient 15 | 65 | Luminal A | 1 cm | IDC | 10% | Positive | N/A |
| Patient 16 | 34 | Luminal A | 1.1 × 1 cm | IDC | 12% | Positive | N/A |
| Patient 17 | 60 | Luminal A | 1.5 cm | IDC | 7% | Negative | N/A |
| Patient 18 | 62 | Luminal A | 1.3 × 1.5 cm | ILC | 15% | Positive | N/A |
| Patient 19 | 44 | Luminal A | 1.2 cm | IDC | 14% | Positive | N/A |
| Patient 20 | 60 | Luminal A | 1 cm | IDC | 10% | Positive | N/A |
| Patient 21 | 64 | Luminal A | 1.5 cm | ILC | 12% | Positive | N/A |
| Patient 22 | 44 | Luminal A | 2.5 × 1.2 cm | IDC | 14% | Positive | N/A |
| Patient 23 | 62 | Luminal A | 1.3 cm | IDC | 10% | Positive | N/A |
| Patient 24 | 47 | Luminal A | 2 cm | IDC | 14% | Positive | N/A |
| Patient 25 | 53 | Luminal A | 1.3 × 1 cm | IDC | 12% | Negative | N/A |
| Patient 26 | 38 | Luminal A | 1.5 cm | IDC | 7% | Negative | N/A |
| Patient 27 | 79 | Luminal A | 1.5 × 1.2 cm | IDC | 8% | Positive | N/A |
| Patient 28 | 57 | Luminal A | 1 cm | IDC | 10% | Positive | N/A |
| Patient 29 | 59 | Luminal A | 1.3 cm | IDC | 12% | Positive | N/A |
| Patient 30 | 66 | Luminal A | 2 × 1.2 cm | IDC | 12% | Positive | N/A |
| Patient 31 | 46 | TNBC | 2 × 1.5 cm 1.1 × 1 cm | IDC | 25% | Positive | N/A |
| Patient 32 | 51 | TNBC | 2.5 cm | IDC | 60% | Negative | N/A |
| Patient 33 | 36 | TNBC | 1 cm | IDC | 50% | Negative | Finished six cycles of chemotherapy |
| Patient 34 | 68 | TNBC | 2.5 × 3.4 cm | IDC | 40% | Negative | N/A |
| Patient 35 | 73 | TNBC | 3.5 cm | IDC | 60% | Negative | Finished neoadjuvant chemotherapy |
| Patient 36 | 60 | TNBC | 2.5 × 1.5 cm | IDC | 50% | Negative | N/A |
| Patient 37 | 36 | TNBC | 4 × 2 × 2 cm | IDC | 70% | Negative | N/A |
| Patient 38 | 46 | TNBC | 3.5 cm | IDC | 50% | Negative | N/A |
| Patient 39 | 60 | TNBC | 2 × 1.5 cm | IDC | 40% | Negative | N/A |
| Patient 40 | 72 | TNBC | 1.2 cm | IDC | 50% | Negative | N/A |
| Patient 41 | 39 | TNBC | 1.5 cm | IDC | 70% | Positive | N/A |
| Patient 42 | 54 | TNBC | 1 cm | IDC | 50% | Negative | N/A |
| Patient 43 | 38 | TNBC | 2 × 1.5 cm | IDC | 40% | Negative | N/A |
| Patient 44 | 58 | TNBC | 1.5 cm | IDC | 70% | Negative | N/A |
| Patient 45 | 72 | TNBC | 1.2 × 1 cm | IDC | 60% | Negative | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elgohary, S.; Eissa, R.A.; El Tayebi, H.M. Thymoquinone, a Novel Multi-Strike Inhibitor of Pro-Tumorigenic Breast Cancer (BC) Markers: CALR, NLRP3 Pathway and sPD-L1 in PBMCs of HR+ and TNBC Patients. Int. J. Mol. Sci. 2023, 24, 14254. https://doi.org/10.3390/ijms241814254
Elgohary S, Eissa RA, El Tayebi HM. Thymoquinone, a Novel Multi-Strike Inhibitor of Pro-Tumorigenic Breast Cancer (BC) Markers: CALR, NLRP3 Pathway and sPD-L1 in PBMCs of HR+ and TNBC Patients. International Journal of Molecular Sciences. 2023; 24(18):14254. https://doi.org/10.3390/ijms241814254
Chicago/Turabian StyleElgohary, Sawsan, Reda A. Eissa, and Hend M. El Tayebi. 2023. "Thymoquinone, a Novel Multi-Strike Inhibitor of Pro-Tumorigenic Breast Cancer (BC) Markers: CALR, NLRP3 Pathway and sPD-L1 in PBMCs of HR+ and TNBC Patients" International Journal of Molecular Sciences 24, no. 18: 14254. https://doi.org/10.3390/ijms241814254
APA StyleElgohary, S., Eissa, R. A., & El Tayebi, H. M. (2023). Thymoquinone, a Novel Multi-Strike Inhibitor of Pro-Tumorigenic Breast Cancer (BC) Markers: CALR, NLRP3 Pathway and sPD-L1 in PBMCs of HR+ and TNBC Patients. International Journal of Molecular Sciences, 24(18), 14254. https://doi.org/10.3390/ijms241814254







