The Cytotoxic Effect of Septic Plasma on Healthy RBCs: Is Eryptosis a New Mechanism for Sepsis?
Abstract
:1. Introduction
2. Results
2.1. Subjects Baseline Characteristics
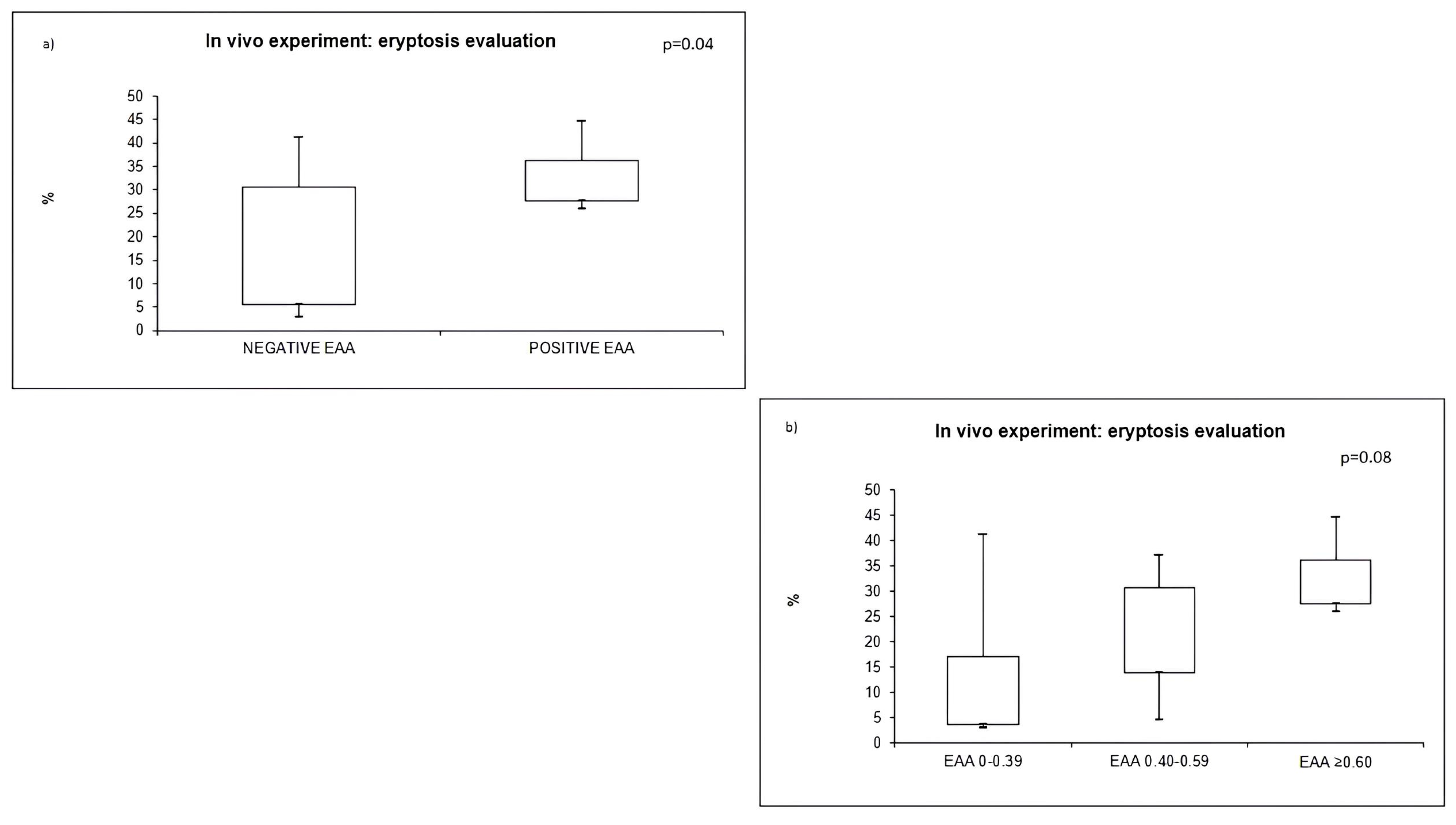
2.2. EAA Evaluation and Outcome
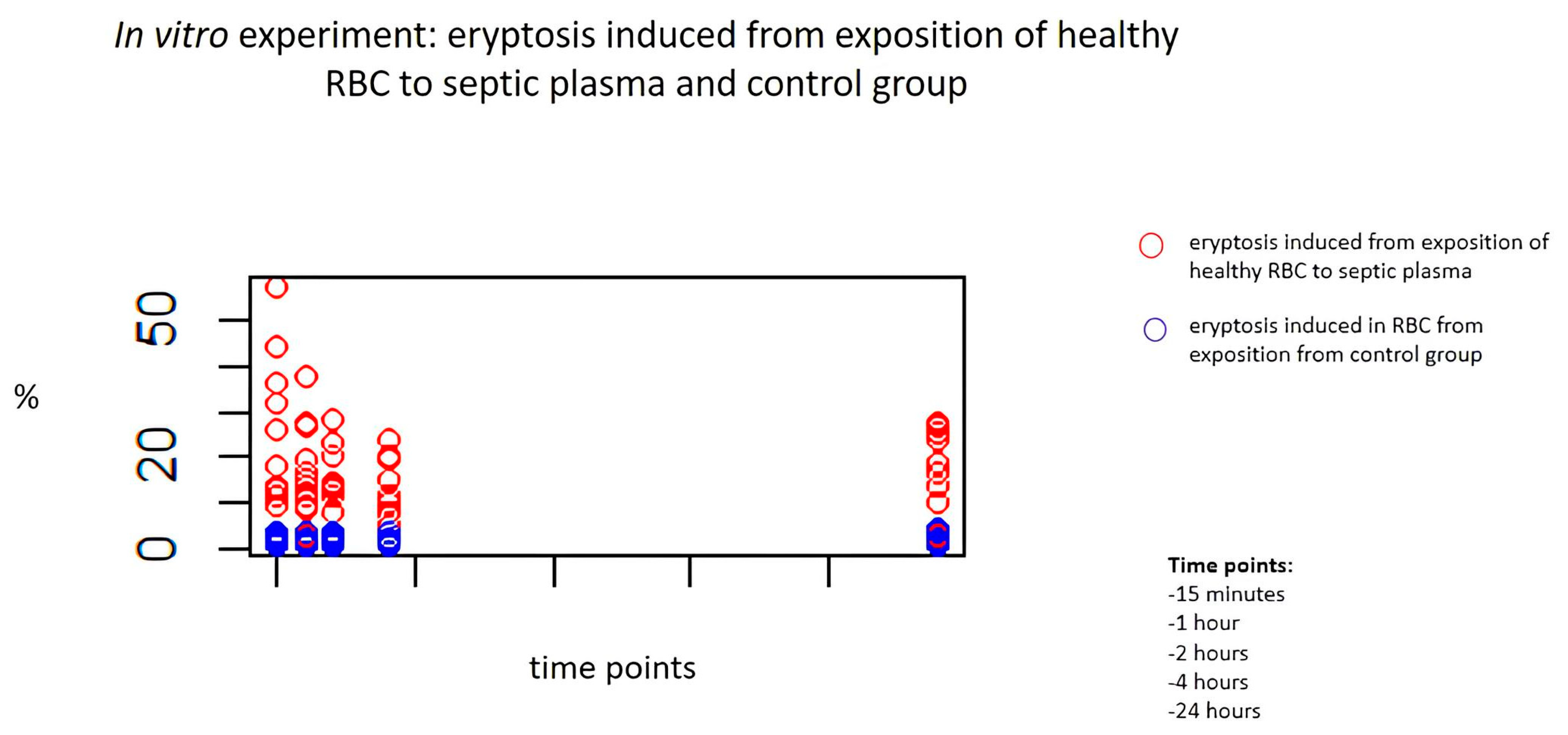
2.3. In Vitro Exposure to Septic Plasma and Induction of Eryptosis
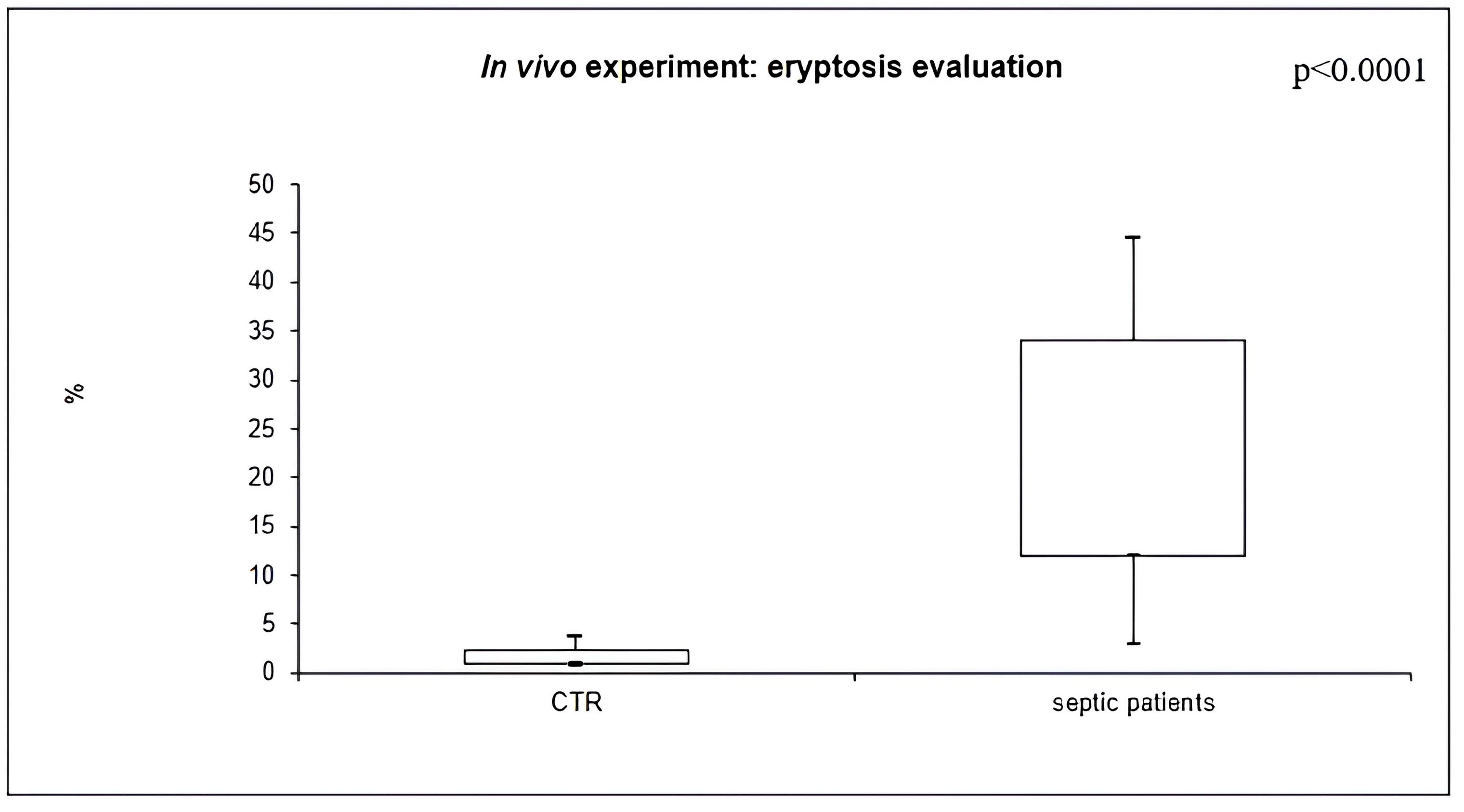
2.4. In Vivo Eryptosis Evaluation
2.5. Relation to Other Biomarkers
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Sample Collection
4.3. Endotoxin Activity Assay (EAA)
4.4. Enzyme-Linked Immunosorbent Assay (ELISA) for IL-6 and MPO
4.5. In Vitro Exposure to Septic Plasma and Induction of Eryptosis
4.6. In Vivo Eryptosis
4.7. Flow Cytometry Evaluation
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis-Pathophysiology and Therapeutic Concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Stearns-Kurosawa, D.J.; Osuchowski, M.F.; Valentine, C.; Kurosawa, S.; Remick, D.G. The pathogenesis of sepsis. Annu. Rev. Pathol. 2011, 6, 19–48. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C.; Foster, D.; Vincent, J.L.; Cook, D.J.; Cohen, J.; Dellinger, R.P.; Opal, S.; Abraham, E.; Brett, S.J.; Smith, T.; et al. Diagnostic and prognostic implications of endotoxemia in critical illness: Results of the MEDIC study. J. Infect. Dis. 2004, 190, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Romaschin, A.D.; Harris, D.M.; Ribeiro, M.B.; Paice, J.; Foster, D.M.; Walker, P.M.; Marshall, J.C. A rapid assay of endotoxin in whole blood using autologous neutrophil dependent chemiluminescence. J. Immunol. Methods 1998, 212, 169–185. [Google Scholar] [CrossRef]
- Molano Franco, D.; Arevalo-Rodriguez, I.; Roque, I.F.M.; Montero Oleas, N.G.; Nuvials, X.; Zamora, J. Plasma interleukin-6 concentration for the diagnosis of sepsis in critically ill adults. Cochrane Database Syst. Rev. 2019, 4, CD011811. [Google Scholar] [CrossRef]
- Yu, H.; Liu, Y.; Wang, M.; Restrepo, R.J.; Wang, D.; Kalogeris, T.J.; Neumann, W.L.; Ford, D.A.; Korthuis, R.J. Myeloperoxidase instigates proinflammatory responses in a cecal ligation and puncture rat model of sepsis. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H705–H721. [Google Scholar] [CrossRef]
- Wong, G.G.; Clark, S.C. Multiple actions of interleukin 6 within a cytokine network. Immunol. Today 1988, 9, 137–139. [Google Scholar] [CrossRef]
- Van Snick, J. Interleukin-6: An overview. Annu. Rev. Immunol. 1990, 8, 253–278. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 77, 598–625. [Google Scholar] [CrossRef]
- Pishchany, G.; McCoy, A.L.; Torres, V.J.; Krause, J.C.; Crowe, J.E., Jr.; Fabry, M.E.; Skaar, E.P. Specificity for human hemoglobin enhances Staphylococcus aureus infection. Cell Host Microbe 2010, 8, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; du Plooy, J.N.; Bester, J. A Comprehensive Review on Eryptosis. Cell. Physiol. Biochem. 2016, 39, 1977–2000. [Google Scholar] [CrossRef] [PubMed]
- Lang, E.; Lang, F. Triggers, inhibitors, mechanisms, and significance of eryptosis: The suicidal erythrocyte death. BioMed Res. Int. 2015, 2015, 513518. [Google Scholar] [CrossRef]
- Lang, K.S.; Lang, P.A.; Bauer, C.; Duranton, C.; Wieder, T.; Huber, S.M.; Lang, F. Mechanisms of suicidal erythrocyte death. Cell. Physiol. Biochem. 2005, 15, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Virzi, G.M.; Mattiotti, M.; Clementi, A.; Milan Manani, S.; Battaglia, G.G.; Ronco, C.; Zanella, M. In Vitro Induction of Eryptosis by Uremic Toxins and Inflammation Mediators in Healthy Red Blood Cells. J. Clin. Med. 2022, 11, 5329. [Google Scholar] [CrossRef]
- Lang, F.; Lang, E.; Foller, M. Physiology and pathophysiology of eryptosis. Transfus. Med. Hemother. 2012, 39, 308–314. [Google Scholar] [CrossRef]
- Bonan, N.B.; Steiner, T.M.; Kuntsevich, V.; Virzi, G.M.; Azevedo, M.; Nakao, L.S.; Barreto, F.C.; Ronco, C.; Thijssen, S.; Kotanko, P.; et al. Uremic Toxicity-Induced Eryptosis and Monocyte Modulation: The Erythrophagocytosis as a Novel Pathway to Renal Anemia. Blood Purif. 2016, 41, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.B.; Wagner-Britz, L.; Maia, S.; Steffen, P.; Wagner, C.; Kaestner, L.; Bernhardt, I. Regulation of phosphatidylserine exposure in red blood cells. Cell. Physiol. Biochem. 2011, 28, 847–856. [Google Scholar] [CrossRef]
- Lang, E.; Qadri, S.M.; Lang, F. Killing me softly-suicidal erythrocyte death. Int. J. Biochem. Cell Biol. 2012, 44, 1236–1243. [Google Scholar] [CrossRef]
- Chan, C.Y.; Cheng, C.F.; Shui, H.A.; Ku, H.C.; Su, W.L. Erythrocyte degradation, metabolism, secretion, and communication with immune cells in the blood during sepsis: A review. Tzu Chi Med. J. 2022, 34, 125–133. [Google Scholar] [CrossRef]
- Lang, F.; Abed, M.; Lang, E.; Foller, M. Oxidative stress and suicidal erythrocyte death. Antioxid. Redox Signal. 2014, 21, 138–153. [Google Scholar] [CrossRef]
- Lang, F.; Lang, K.S.; Lang, P.A.; Huber, S.M.; Wieder, T. Osmotic shock-induced suicidal death of erythrocytes. Acta Physiol. 2006, 187, 191–198. [Google Scholar] [CrossRef]
- Lang, F.; Gulbins, E.; Lang, P.A.; Zappulla, D.; Foller, M. Ceramide in suicidal death of erythrocytes. Cell. Physiol. Biochem. 2010, 26, 21–28. [Google Scholar] [CrossRef]
- Lang, F.; Gulbins, E.; Lerche, H.; Huber, S.M.; Kempe, D.S.; Foller, M. Eryptosis, a window to systemic disease. Cell. Physiol. Biochem. 2008, 22, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Virzi, G.M.; Milan Manani, S.; Marturano, D.; Clementi, A.; Lerco, S.; Tantillo, I.; Giuliani, A.; Battaglia, G.G.; Ronco, C.; Zanella, M. Eryptosis in Peritoneal Dialysis-Related Peritonitis: The Potential Role of Inflammation in Mediating the Increase in Eryptosis in PD. J. Clin. Med. 2022, 11, 6918. [Google Scholar] [CrossRef] [PubMed]
- Virzi, G.M.; Milan Manani, S.; Clementi, A.; Castegnaro, S.; Brocca, A.; Riello, C.; de Cal, M.; Giuliani, A.; Battaglia, G.G.; Crepaldi, C.; et al. Eryptosis Is Altered in Peritoneal Dialysis Patients. Blood Purif. 2019, 48, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Gok, M.G.; Paydas, S.; Boral, B.; Onan, E.; Kaya, B. Evaluation of eryptosis in patients with chronic kidney disease. Int. Urol. Nephrol. 2022, 54, 2919–2928. [Google Scholar] [CrossRef]
- Adamzik, M.; Hamburger, T.; Petrat, F.; Peters, J.; de Groot, H.; Hartmann, M. Free hemoglobin concentration in severe sepsis: Methods of measurement and prediction of outcome. Crit. Care 2012, 16, R125. [Google Scholar] [CrossRef] [PubMed]
- Kempe, D.S.; Akel, A.; Lang, P.A.; Hermle, T.; Biswas, R.; Muresanu, J.; Friedrich, B.; Dreischer, P.; Wolz, C.; Schumacher, U.; et al. Suicidal erythrocyte death in sepsis. J. Mol. Med. 2007, 85, 273–281. [Google Scholar] [CrossRef]
- Piagnerelli, M.; Boudjeltia, K.Z.; Rapotec, A.; Richard, T.; Brohee, D.; Babar, S.; Bouckaert, V.; Simon, A.C.; Toko, J.P.; Walravens, T.; et al. Neuraminidase alters red blood cells in sepsis. Crit. Care Med. 2009, 37, 1244–1250. [Google Scholar] [CrossRef]
- Reggiori, G.; Occhipinti, G.; De Gasperi, A.; Vincent, J.L.; Piagnerelli, M. Early alterations of red blood cell rheology in critically ill patients. Crit. Care Med. 2009, 37, 3041–3046. [Google Scholar] [CrossRef]
- Jemaa, M.; Fezai, M.; Bissinger, R.; Lang, F. Methods Employed in Cytofluorometric Assessment of Eryptosis, the Suicidal Erythrocyte Death. Cell. Physiol. Biochem. 2017, 43, 431–444. [Google Scholar] [CrossRef]
- Veillette, A.; Thibaudeau, E.; Latour, S. High expression of inhibitory receptor SHPS-1 and its association with protein-tyrosine phosphatase SHP-1 in macrophages. J. Biol. Chem. 1998, 273, 22719–22728. [Google Scholar] [CrossRef] [PubMed]
- Effenberger-Neidnicht, K.; Hartmann, M. Mechanisms of Hemolysis During Sepsis. Inflammation 2018, 41, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Hortova-Kohoutkova, M.; Tidu, F.; De Zuani, M.; Sramek, V.; Helan, M.; Fric, J. Phagocytosis-Inflammation Crosstalk in Sepsis: New Avenues for Therapeutic Intervention. Shock 2020, 54, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Larsen, R.; Gozzelino, R.; Jeney, V.; Tokaji, L.; Bozza, F.A.; Japiassu, A.M.; Bonaparte, D.; Cavalcante, M.M.; Chora, A.; Ferreira, A.; et al. A central role for free heme in the pathogenesis of severe sepsis. Sci. Transl. Med. 2010, 2, 51ra71. [Google Scholar] [CrossRef] [PubMed]
- Danner, R.L.; Elin, R.J.; Hosseini, J.M.; Wesley, R.A.; Reilly, J.M.; Parillo, J.E. Endotoxemia in human septic shock. Chest 1991, 99, 169–175. [Google Scholar] [CrossRef]
- Klein, D.J.; Foster, D.; Schorr, C.A.; Kazempour, K.; Walker, P.M.; Dellinger, R.P. The EUPHRATES trial (Evaluating the Use of Polymyxin B Hemoperfusion in a Randomized controlled trial of Adults Treated for Endotoxemia and Septic shock): Study protocol for a randomized controlled trial. Trials 2014, 15, 218. [Google Scholar] [CrossRef]
- Hoareau, L.; Bencharif, K.; Rondeau, P.; Murumalla, R.; Ravanan, P.; Tallet, F.; Delarue, P.; Cesari, M.; Roche, R.; Festy, F. Signaling pathways involved in LPS induced TNFalpha production in human adipocytes. J. Inflamm 2010, 7, 1. [Google Scholar] [CrossRef]
- Regis, G.; Icardi, L.; Conti, L.; Chiarle, R.; Piva, R.; Giovarelli, M.; Poli, V.; Novelli, F. IL-6, but not IFN-gamma, triggers apoptosis and inhibits in vivo growth of human malignant T cells on STAT3 silencing. Leukemia 2009, 23, 2102–2108. [Google Scholar] [CrossRef]
- Schrijver, I.T.; Kemperman, H.; Roest, M.; Kesecioglu, J.; de Lange, D.W. Myeloperoxidase can differentiate between sepsis and non-infectious SIRS and predicts mortality in intensive care patients with SIRS. Intensive Care Med. Exp. 2017, 5, 43. [Google Scholar] [CrossRef]
- Bester, J.; Pretorius, E. Effects of IL-1beta, IL-6 and IL-8 on erythrocytes, platelets and clot viscoelasticity. Sci. Rep. 2016, 6, 32188. [Google Scholar] [CrossRef]
- Sadaka, F.; O’Brien, J.; Prakash, S. Red cell distribution width and outcome in patients with septic shock. J. Intensive Care Med. 2013, 28, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Virzi, G.M.; Mattiotti, M.; de Cal, M.; Ronco, C.; Zanella, M.; De Rosa, S. Endotoxin in Sepsis: Methods for LPS Detection and the Use of Omics Techniques. Diagnostics 2022, 13, 79. [Google Scholar] [CrossRef]
- Cruz, D.N.; Antonelli, M.; Fumagalli, R.; Foltran, F.; Brienza, N.; Donati, A.; Malcangi, V.; Petrini, F.; Volta, G.; Bobbio Pallavicini, F.M.; et al. Early use of polymyxin B hemoperfusion in abdominal septic shock: The EUPHAS randomized controlled trial. JAMA 2009, 301, 2445–2452. [Google Scholar] [CrossRef] [PubMed]

| Septic Patients, n = 16 | |
|---|---|
| Age, years | 58.25 ± 14.4 |
| Sex | 11 male |
| Diabetes | 7 |
| Hypertension | 5 |
| CVD | 5 |
| CKD | 4 |
| EAA level | 0.55, IQR 0.42–0.77 |
| In Vitro Induced Eryptosis | |||
|---|---|---|---|
| Septic Plasma | Healthy Plasma | p-Value | |
| 15 min | 13.5, IQR 2.9–27.5 | 1.1, IQR 1.0–2.3 | 0.001 |
| 1 h | 12.9, IQR 2.7–17.6 | 1.6, IQR 1.1–1.9 | ≤0.001 |
| 2 h | 12.5, IQR 2.3–15.8 | 1.3, IQR 1.2–1.9 | ≤0.001 |
| 4 h | 8.8, IQR 1.8–16.5 | 1.2, IQR 1.3–2.2 | 0.001 |
| 24 h | 10.4, IQR 2.9–20 | 0.9, IQR 1.3–2.3 | 0.001 |
| Septic Patients | Controls | p-Value | |
|---|---|---|---|
| IL-6, pg/mL | 750.8, IQR 98.7–1342.8 | 5.4, IQR 4.4–7.8 | 0.0049 |
| MPO, pg/mL | 261.2, IQR 175.2–329.2 | 12, IQR 9.8–16.3 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcello, M.; Virzì, G.M.; Marturano, D.; de Cal, M.; Marchionna, N.; Sgarabotto, L.; De Rosa, S.; Ronco, C.; Zanella, M. The Cytotoxic Effect of Septic Plasma on Healthy RBCs: Is Eryptosis a New Mechanism for Sepsis? Int. J. Mol. Sci. 2023, 24, 14176. https://doi.org/10.3390/ijms241814176
Marcello M, Virzì GM, Marturano D, de Cal M, Marchionna N, Sgarabotto L, De Rosa S, Ronco C, Zanella M. The Cytotoxic Effect of Septic Plasma on Healthy RBCs: Is Eryptosis a New Mechanism for Sepsis? International Journal of Molecular Sciences. 2023; 24(18):14176. https://doi.org/10.3390/ijms241814176
Chicago/Turabian StyleMarcello, Matteo, Grazia Maria Virzì, Davide Marturano, Massimo de Cal, Nicola Marchionna, Luca Sgarabotto, Silvia De Rosa, Claudio Ronco, and Monica Zanella. 2023. "The Cytotoxic Effect of Septic Plasma on Healthy RBCs: Is Eryptosis a New Mechanism for Sepsis?" International Journal of Molecular Sciences 24, no. 18: 14176. https://doi.org/10.3390/ijms241814176
APA StyleMarcello, M., Virzì, G. M., Marturano, D., de Cal, M., Marchionna, N., Sgarabotto, L., De Rosa, S., Ronco, C., & Zanella, M. (2023). The Cytotoxic Effect of Septic Plasma on Healthy RBCs: Is Eryptosis a New Mechanism for Sepsis? International Journal of Molecular Sciences, 24(18), 14176. https://doi.org/10.3390/ijms241814176







