Early Alzheimer’s Disease Screening Approach Using Plasma Biomarkers
Abstract
:1. Introduction
2. Results and Discussion
2.1. Demographic and Clinical Description of Participants
2.2. Plasma Biomarkers
2.3. Clinical Variables Association
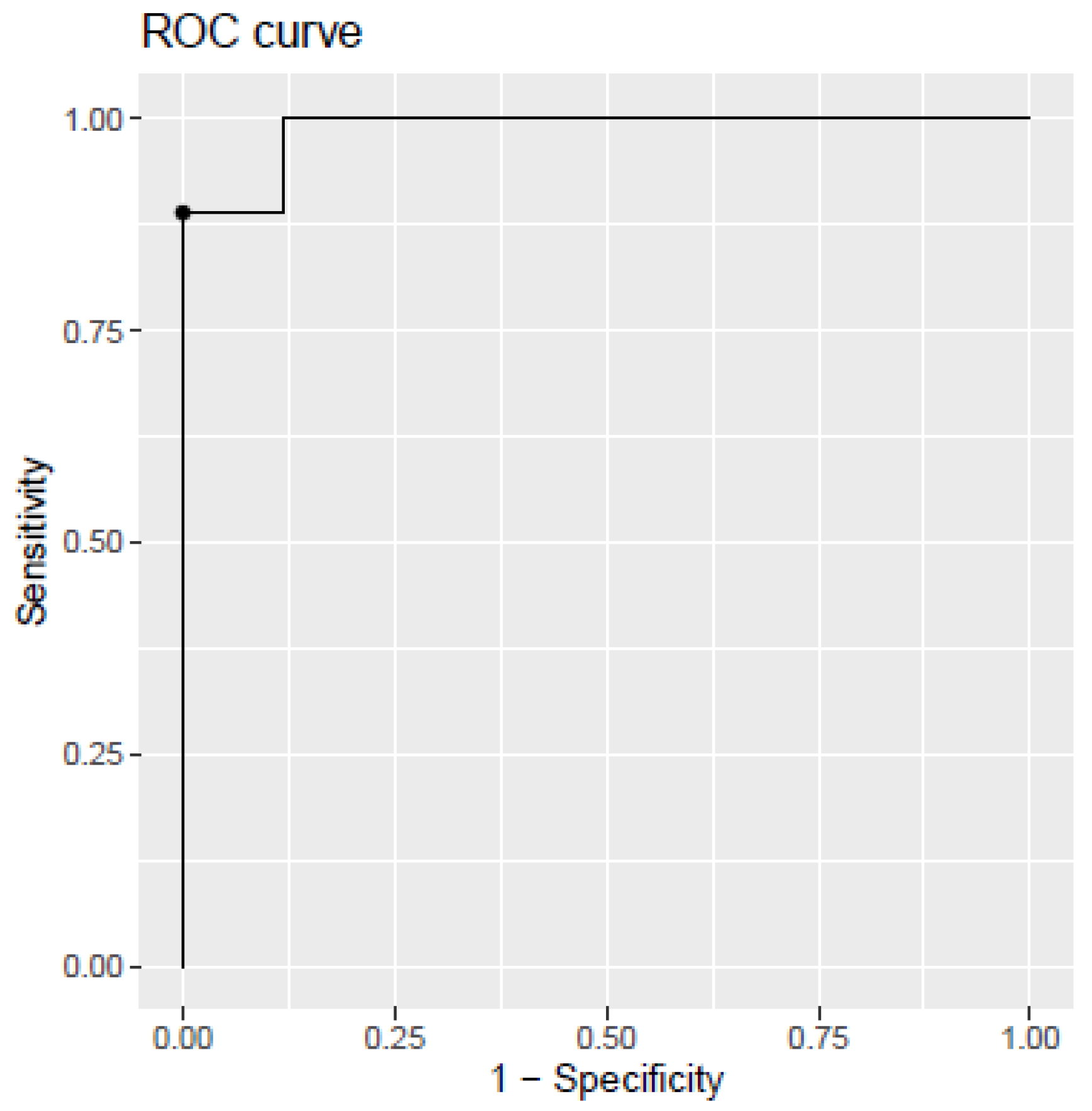
2.4. Development of Diagnosis Model
2.5. Discussion
3. Materials and Methods
3.1. Participants and Samples Collection
3.2. Equipment and Reagents
3.3. Plasma Sample Treatment and Biomarkers Determination
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scheltens, P.; Blennow, K.; Breteler, M.M.B.; de Strooper, B.; Frisoni, G.B.; Salloway, S.; Van der Flier, W.M. Alzheimer’s Disease. Lancet 2016, 388, 505–517. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Disease International. World Alzheimer Report 2018—The State of the Art of Dementia Research: New Frontiers; Alzheimer’s Disease International: London, UK, 2018. [Google Scholar]
- Jankovic, J.; Mazziot, C.J.; Pmeroy, S. Bradley and Daroff’s Neurology in Clinical Practice. In Bradley and Daroff’s Neurology in Clinical Practice; Newman, N.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 1465–1471. [Google Scholar]
- Zhang, H.; Wei, W.; Zhao, M.; Ma, L.; Jiang, X.; Pei, H.; Cao, Y.; Li, H. Interaction between Aβ and Tau in the Pathogenesis of Alzheimer’s Disease. Int. J. Biol. Sci. 2021, 17, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Hanon, O.; Vidal, J.; Lehmann, S.; Bombois, S.; Allinquant, B.; Tréluyer, J.; Gelé, P.; Delmaire, C.; Blanc, F.; Mangin, J.; et al. Plasma Amyloid Levels within the Alzheimer’s Process and Correlations with Central Biomarkers. Alzheimer’s Dement. 2018, 14, 858–868. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-C.; Han, S.-H.; Yi, D.; Byun, M.S.; Lee, J.H.; Jang, S.; Ko, K.; Jeon, S.Y.; Lee, Y.-S.; Kim, Y.K.; et al. Plasma Tau/Amyloid-Β1–42 Ratio Predicts Brain Tau Deposition and Neurodegeneration in Alzheimer’s Disease. Brain 2019, 142, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Shepardson, N.; Yang, T.; Chen, G.; Walsh, D.; Selkoe, D.J. Soluble Amyloid β-Protein Dimers Isolated from Alzheimer Cortex Directly Induce Tau Hyperphosphorylation and Neuritic Degeneration. Proc. Natl. Acad. Sci. USA 2011, 108, 5819–5824. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a Biological Definition of Alzheimer’s Disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Hansson, O.; Zetterberg, H.; Vanmechelen, E.; Vanderstichele, H.; Andreasson, U.; Londos, E.; Wallin, A.; Minthon, L.; Blennow, K. Evaluation of Plasma Aβ40 and Aβ42 as Predictors of Conversion to Alzheimer’s Disease in Patients with Mild Cognitive Impairment. Neurobiol. Aging 2010, 31, 357–367. [Google Scholar] [CrossRef]
- Chatterjee, P.; Elmi, M.; Goozee, K.; Shah, T.; Sohrabi, H.R.; Dias, C.B.; Pedrini, S.; Shen, K.; Asih, P.R.; Dave, P.; et al. Ultrasensitive Detection of Plasma Amyloid-β as a Biomarker for Cognitively Normal Elderly Individuals at Risk of Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 71, 775–783. [Google Scholar] [CrossRef]
- Bjerke, M.; Engelborghs, S. Cerebrospinal Fluid Biomarkers for Early and Differential Alzheimer’s Disease Diagnosis. J. Alzheimer’s Dis. 2018, 62, 1199–1209. [Google Scholar] [CrossRef]
- Chouliaras, L.; Thomas, A.; Malpetti, M.; Donaghy, P.; Kane, J.; Mak, E.; Savulich, G.; Prats-Sedano, M.A.; Heslegrave, A.J.; Zetterberg, H.; et al. Differential Levels of Plasma Biomarkers of Neurodegeneration in Lewy Body Dementia, Alzheimer’s Disease, Frontotemporal Dementia and Progressive Supranuclear Palsy. J. Neurol. Neurosurg. Psychiatry 2022, 93, 651–658. [Google Scholar] [CrossRef]
- Tan, C.-C.; Yu, J.-T.; Tan, L. Biomarkers for Preclinical Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 42, 1051–1069. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Stomrud, E.; Palmqvist, S.; Zetterberg, H.; van Westen, D.; Jeromin, A.; Song, L.; Hanlon, D.; Tan Hehir, C.A.; Baker, D.; et al. Plasma β-Amyloid in Alzheimer’s Disease and Vascular Disease. Sci. Rep. 2016, 6, 26801. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, S.; Schaeverbeke, J.M.; Verberk, I.M.W.; Gille, B.; De Schaepdryver, M.; Luckett, E.S.; Gabel, S.; Bruffaerts, R.; Mauroo, K.; Thijssen, E.H.; et al. Comparison of ELISA- and SIMOA-Based Quantification of Plasma Aβ Ratios for Early Detection of Cerebral Amyloidosis. Alzheimer’s Res. Ther. 2020, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Rembach, A.; Faux, N.G.; Watt, A.D.; Pertile, K.K.; Rumble, R.L.; Trounson, B.O.; Fowler, C.J.; Roberts, B.R.; Perez, K.A.; Li, Q.; et al. Changes in Plasma Amyloid Beta in a Longitudinal Study of Aging and Alzheimer’s Disease. Alzheimer’s Dement. 2014, 10, 53–61. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Palmqvist, S.; Smith, R.; Beach, T.G.; Serrano, G.E.; Chai, X.; Proctor, N.K.; Eichenlaub, U.; Zetterberg, H.; et al. Plasma P-Tau181 in Alzheimer’s Disease: Relationship to Other Biomarkers, Differential Diagnosis, Neuropathology and Longitudinal Progression to Alzheimer’s Dementia. Nat. Med. 2020, 26, 379–386. [Google Scholar] [CrossRef]
- Nabizadeh, F.; Balabandian, M.; Rostami, M.R.; Ward, R.T.; Ahmadi, N.; Pourhamzeh, M. Plasma P-Tau181 Associated with Structural Changes in Mild Cognitive Impairment. Aging Clin. Exp. Res. 2022, 34, 2139–2147. [Google Scholar] [CrossRef]
- Milà-Alomà, M.; Ashton, N.J.; Shekari, M.; Salvadó, G.; Ortiz-Romero, P.; Montoliu-Gaya, L.; Benedet, A.L.; Karikari, T.K.; Lantero-Rodriguez, J.; Vanmechelen, E.; et al. Plasma P-Tau231 and p-Tau217 as State Markers of Amyloid-β Pathology in Preclinical Alzheimer’s Disease. Nat. Med. 2022, 28, 1797–1801. [Google Scholar] [CrossRef]
- Brickman, A.M.; Manly, J.J.; Honig, L.S.; Sanchez, D.; Reyes-Dumeyer, D.; Lantigua, R.A.; Lao, P.J.; Stern, Y.; Vonsattel, J.P.; Teich, A.F.; et al. Plasma P-tau181, P-tau217, and Other Blood-based Alzheimer’s Disease Biomarkers in a Multi-ethnic, Community Study. Alzheimer’s Dement. 2021, 17, 1353–1364. [Google Scholar] [CrossRef]
- Moscoso, A.; Grothe, M.J.; Ashton, N.J.; Karikari, T.K.; Lantero Rodríguez, J.; Snellman, A.; Suárez-Calvet, M.; Blennow, K.; Zetterberg, H.; Schöll, M.; et al. Longitudinal Associations of Blood Phosphorylated Tau181 and Neurofilament Light Chain With Neurodegeneration in Alzheimer Disease. JAMA Neurol. 2021, 78, 396. [Google Scholar] [CrossRef]
- Álvarez-Sánchez, L.; Peña-Bautista, C.; Baquero, M.; Cháfer-Pericás, C. Novel Ultrasensitive Detection Technologies for the Identification of Early and Minimally Invasive Alzheimer’s Disease Blood Biomarkers. J. Alzheimer’s Dis. 2022, 86, 1337–1369. [Google Scholar] [CrossRef]
- Pereira, J.B.; Janelidze, S.; Smith, R.; Mattsson-Carlgren, N.; Palmqvist, S.; Teunissen, C.E.; Zetterberg, H.; Stomrud, E.; Ashton, N.J.; Blennow, K.; et al. Plasma GFAP Is an Early Marker of Amyloid-β but Not Tau Pathology in Alzheimer’s Disease. Brain 2021, 144, 3505–3516. [Google Scholar] [CrossRef] [PubMed]
- Elahi, F.M.; Casaletto, K.B.; La Joie, R.; Walters, S.M.; Harvey, D.; Wolf, A.; Edwards, L.; Rivera-Contreras, W.; Karydas, A.; Cobigo, Y.; et al. Plasma Biomarkers of Astrocytic and Neuronal Dysfunction in Early- and Late-onset Alzheimer’s Disease. Alzheimer’s Dement. 2020, 16, 681–695. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, P.; Pedrini, S.; Ashton, N.J.; Tegg, M.; Goozee, K.; Singh, A.K.; Karikari, T.K.; Simrén, J.; Vanmechelen, E.; Armstrong, N.J.; et al. Diagnostic and Prognostic Plasma Biomarkers for Preclinical Alzheimer’s Disease. Alzheimer’s Dement. 2022, 18, 1141–1154. [Google Scholar] [CrossRef] [PubMed]
- Benedet, A.L.; Milà-Alomà, M.; Vrillon, A.; Ashton, N.J.; Pascoal, T.A.; Lussier, F.; Karikari, T.K.; Hourregue, C.; Cognat, E.; Dumurgier, J.; et al. Differences Between Plasma and Cerebrospinal Fluid Glial Fibrillary Acidic Protein Levels Across the Alzheimer Disease Continuum. JAMA Neurol. 2021, 78, 1471. [Google Scholar] [CrossRef]
- Escartin, C.; Galea, E.; Lakatos, A.; O’Callaghan, J.P.; Petzold, G.C.; Serrano-Pozo, A.; Steinhäuser, C.; Volterra, A.; Carmignoto, G.; Agarwal, A.; et al. Reactive Astrocyte Nomenclature, Definitions, and Future Directions. Nat. Neurosci. 2021, 24, 312–325. [Google Scholar] [CrossRef]
- Frost, G.R.; Li, Y.-M. The Role of Astrocytes in Amyloid Production and Alzheimer’s Disease. Open Biol. 2017, 7, 170228. [Google Scholar] [CrossRef]
- Kamphuis, W.; Middeldorp, J.; Kooijman, L.; Sluijs, J.A.; Kooi, E.-J.; Moeton, M.; Freriks, M.; Mizee, M.R.; Hol, E.M. Glial Fibrillary Acidic Protein Isoform Expression in Plaque Related Astrogliosis in Alzheimer’s Disease. Neurobiol. Aging 2014, 35, 492–510. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/news-events/press-announcements/fda-converts-novel-alzheimers-disease-treatment-traditional-approval (accessed on 6 April 2023).
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.P.; Onyike, C.U.; et al. Sensitivity of Revised Diagnostic Criteria for the Behavioural Variant of Frontotemporal Dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef]
- Gorno-Tempini, M.L.; Hillis, A.E.; Weintraub, S.; Kertesz, A.; Mendez, M.; Cappa, S.F.; Ogar, J.M.; Rohrer, J.D.; Black, S.; Boeve, B.F.; et al. Classification of Primary Progressive Aphasia and Its Variants. Neurology 2011, 76, 1006–1014. [Google Scholar] [CrossRef]
- Hogan, D.B.; Jetté, N.; Fiest, K.M.; Roberts, J.I.; Pearson, D.; Smith, E.E.; Roach, P.; Kirk, A.; Pringsheim, T.; Maxwell, C.J. The Prevalence and Incidence of Frontotemporal Dementia: A Systematic Review. Can. J. Neurol. Sci. 2016, 43 (Suppl. S1), S96–S109. [Google Scholar] [CrossRef]
- Ikeda, M.; Ishikawa, T.; Tanabe, H. Epidemiology of Frontotemporal Lobar Degeneration. Dement. Geriatr. Cogn. Disord. 2004, 17, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Snowden, J.; Neary, D.; Mann, D. Frontotemporal Lobar Degeneration: Clinical and Pathological Relationships. Acta Neuropathol. 2007, 114, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Perry, D.C.; Brown, J.A.; Possin, K.L.; Datta, S.; Trujillo, A.; Radke, A.; Karydas, A.; Kornak, J.; Sias, A.C.; Rabinovici, G.D.; et al. Clinicopathological Correlations in Behavioural Variant Frontotemporal Dementia. Brain 2017, 140, 3329–3345. [Google Scholar] [CrossRef]
- Robinson, J.L.; Richardson, H.; Xie, S.X.; Suh, E.; Van Deerlin, V.M.; Alfaro, B.; Loh, N.; Porras-Paniagua, M.; Nirschl, J.J.; Wolk, D.; et al. The Development and Convergence of Co-Pathologies in Alzheimer’s Disease. Brain 2021, 144, 953–962. [Google Scholar] [CrossRef]
- McDade, E.; Cummings, J.L.; Dhadda, S.; Swanson, C.J.; Reyderman, L.; Kanekiyo, M.; Koyama, A.; Irizarry, M.; Kramer, L.D.; Bateman, R.J. Lecanemab in Patients with Early Alzheimer’s Disease: Detailed Results on Biomarker, Cognitive, and Clinical Effects from the Randomized and Open-Label Extension of the Phase 2 Proof-of-Concept Study. Alzheimer’s Res. Ther. 2022, 14, 191. [Google Scholar] [CrossRef] [PubMed]
- Shir, D.; Graff-Radford, J.; Hofrenning, E.I.; Lesnick, T.G.; Przybelski, S.A.; Lowe, V.J.; Knopman, D.S.; Petersen, R.C.; Jack, C.R.; Vemuri, P.; et al. Association of Plasma Glial Fibrillary Acidic Protein (GFAP) with Neuroimaging of Alzheimer’s Disease and Vascular Pathology. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2022, 14, e12291. [Google Scholar] [CrossRef]
- Álvarez-Sánchez, L.; Peña-Bautista, C.; Ferré-González, L.; Balaguer, A.; Baquero, M.; Casanova-Estruch, B.; Cháfer-Pericás, C. Assessment of Plasma and Cerebrospinal Fluid Biomarkers in Different Stages of Alzheimer’s Disease and Frontotemporal Dementia. Int. J. Mol. Sci. 2023, 24, 1226. [Google Scholar] [CrossRef]
- Wang, Y.-L.; Chen, J.; Du, Z.-L.; Weng, H.; Zhang, Y.; Li, R.; Jia, Z.; Sun, M.; Jiang, J.; Wang, F.-Z.; et al. Plasma P-Tau181 Level Predicts Neurodegeneration and Progression to Alzheimer’s Dementia: A Longitudinal Study. Front. Neurol. 2021, 12, 695696. [Google Scholar] [CrossRef]
- Braak, H.; Thal, D.R.; Ghebremedhin, E.; Del Tredici, K. Stages of the Pathologic Process in Alzheimer Disease: Age Categories from 1 to 100 Years. J. Neuropathol. Exp. Neurol. 2011, 70, 960–969. [Google Scholar] [CrossRef]
- Grober, E.; Dickson, D.; Sliwinski, M.J.; Buschke, H.; Katz, M.; Crystal, H.; Lipton, R.B. Memory and Mental Status Correlates of Modified Braak Staging. Neurobiol. Aging 1999, 20, 573–579. [Google Scholar] [CrossRef]
- Rojas, J.C.; Wang, P.; Staffaroni, A.M.; Heller, C.; Cobigo, Y.; Wolf, A.; Goh, S.-Y.M.; Ljubenkov, P.A.; Heuer, H.W.; Fong, J.C.; et al. Plasma Neurofilament Light for Prediction of Disease Progression in Familial Frontotemporal Lobar Degeneration. Neurology 2021, 96, e2296–e2312. [Google Scholar] [CrossRef] [PubMed]
- Illán-Gala, I.; Lleo, A.; Karydas, A.; Staffaroni, A.M.; Zetterberg, H.; Sivasankaran, R.; Grinberg, L.T.; Spina, S.; Kramer, J.H.; Ramos, E.M.; et al. Plasma Tau and Neurofilament Light in Frontotemporal Lobar Degeneration and Alzheimer Disease. Neurology 2021, 96, e671–e683. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, N.; Cullen, N.C.; Andreasson, U.; Zetterberg, H.; Blennow, K. Association Between Longitudinal Plasma Neurofilament Light and Neurodegeneration in Patients with Alzheimer Disease. JAMA Neurol. 2019, 76, 791. [Google Scholar] [CrossRef]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as Biomarkers in Neurological Disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Verde, F.; Steinacker, P.; Weishaupt, J.H.; Kassubek, J.; Oeckl, P.; Halbgebauer, S.; Tumani, H.; von Arnim, C.A.F.; Dorst, J.; Feneberg, E.; et al. Neurofilament Light Chain in Serum for the Diagnosis of Amyotrophic Lateral Sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Marutani, N.; Akamine, S.; Kanayama, D.; Gotoh, S.; Yanagida, K.; Maruyama, R.; Mori, K.; Miyamoto, T.; Adachi, H.; Sakagami, Y.; et al. Plasma NfL Is Associated with Mild Cognitive Decline in Patients with Diabetes. Psychogeriatrics 2022, 22, 353–359. [Google Scholar] [CrossRef]
- Pilotto, A.; Imarisio, A.; Conforti, F.; Scalvini, A.; Masciocchi, S.; Nocivelli, S.; Turrone, R.; Gipponi, S.; Cottini, E.; Borroni, B.; et al. Plasma NfL, Clinical Subtypes and Motor Progression in Parkinson’s Disease. Park. Relat. Disord. 2021, 87, 41–47. [Google Scholar] [CrossRef]
- Aamodt, W.W.; Waligorska, T.; Shen, J.; Tropea, T.F.; Siderowf, A.; Weintraub, D.; Grossman, M.; Irwin, D.; Wolk, D.A.; Xie, S.X.; et al. Neurofilament Light Chain as a Biomarker for Cognitive Decline in Parkinson Disease. Mov. Disord. 2021, 36, 2945–2950. [Google Scholar] [CrossRef]
- Ntymenou, S.; Tsantzali, I.; Kalamatianos, T.; Voumvourakis, K.I.; Kapaki, E.; Tsivgoulis, G.; Stranjalis, G.; Paraskevas, G.P. Blood Biomarkers in Frontotemporal Dementia: Review and Meta-Analysis. Brain Sci. 2021, 11, 244. [Google Scholar] [CrossRef]
- Beyer, L.; Stocker, H.; Rujescu, D.; Holleczek, B.; Stockmann, J.; Nabers, A.; Brenner, H.; Gerwert, K. Amyloid-beta Misfolding and GFAP Predict Risk of Clinical Alzheimer’s Disease Diagnosis within 17 Years. Alzheimer’s Dement. 2023, 19, 1020–1028. [Google Scholar] [CrossRef]
- Cicognola, C.; Janelidze, S.; Hertze, J.; Zetterberg, H.; Blennow, K.; Mattsson-Carlgren, N.; Hansson, O. Plasma Glial Fibrillary Acidic Protein Detects Alzheimer Pathology and Predicts Future Conversion to Alzheimer Dementia in Patients with Mild Cognitive Impairment. Alzheimer’s Res. Ther. 2021, 13, 68. [Google Scholar] [CrossRef] [PubMed]
- Oeckl, P.; Anderl-Straub, S.; Von Arnim, C.A.F.; Baldeiras, I.; Diehl-Schmid, J.; Grimmer, T.; Halbgebauer, S.; Kort, A.M.; Lima, M.; Marques, T.M.; et al. Serum GFAP Differentiates Alzheimer’s Disease from Frontotemporal Dementia and Predicts MCI-to-Dementia Conversion. J. Neurol. Neurosurg. Psychiatry 2022, 93, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Cousins, K.A.Q.; Irwin, D.J.; Chen-Plotkin, A.; Shaw, L.M.; Arezoumandan, S.; Lee, E.B.; Wolk, D.A.; Weintraub, D.; Spindler, M.; Deik, A.; et al. Plasma GFAP Associates with Secondary Alzheimer’s Pathology in Lewy Body Disease. Ann. Clin. Transl. Neurol. 2023, 10, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Abdelhak, A.; Foschi, M.; Abu-Rumeileh, S.; Yue, J.K.; D’Anna, L.; Huss, A.; Oeckl, P.; Ludolph, A.C.; Kuhle, J.; Petzold, A.; et al. Blood GFAP as an Emerging Biomarker in Brain and Spinal Cord Disorders. Nat. Rev. Neurol. 2022, 18, 158–172. [Google Scholar] [CrossRef]
- Hol, E.M.; Pekny, M. Glial Fibrillary Acidic Protein (GFAP) and the Astrocyte Intermediate Filament System in Diseases of the Central Nervous System. Curr. Opin. Cell Biol. 2015, 32, 121–130. [Google Scholar] [CrossRef]
- Dvorak, F.; Haberer, I.; Sitzer, M.; Foerch, C. Characterisation of the Diagnostic Window of Serum Glial Fibrillary Acidic Protein for the Differentiation of Intracerebral Haemorrhage and Ischaemic Stroke. Cerebrovasc. Dis. 2009, 27, 37–41. [Google Scholar] [CrossRef]
- Lange, R.P.; Everett, A.; Dulloor, P.; Korley, F.K.; Bettegowda, C.; Blair, C.; Grossman, S.A.; Holdhoff, M. Evaluation of Eight Plasma Proteins as Candidate Blood-Based Biomarkers for Malignant Gliomas. Cancer Investig. 2014, 32, 423–429. [Google Scholar] [CrossRef]
- Thijssen, E.H.; Verberk, I.M.W.; Stoops, E.; Boxer, A.L.; Teunissen, C.E. Amyloid, PTau, NfL, and GFAP as Biomarkers for Alzheimer’s Disease. Alzheimer’s Dement. 2020, 16, e038179. [Google Scholar] [CrossRef]
- Chatterjee, P.; Pedrini, S.; Doecke, J.D.; Thota, R.; Villemagne, V.L.; Doré, V.; Singh, A.K.; Wang, P.; Rainey-Smith, S.; Fowler, C.; et al. Plasma Aβ42/40 Ratio, P-tau181, GFAP, and NfL across the Alzheimer’s Disease Continuum: A Cross-sectional and Longitudinal Study in the AIBL Cohort. Alzheimer’s Dement. 2023, 19, 1117–1134. [Google Scholar] [CrossRef]
- Stocker, H.; Beyer, L.; Perna, L.; Rujescu, D.; Holleczek, B.; Beyreuther, K.; Stockmann, J.; Schöttker, B.; Gerwert, K.; Brenner, H. Association of Plasma Biomarkers, P-tau181, Glial Fibrillary Acidic Protein, and Neurofilament Light, with Intermediate and Long-term Clinical Alzheimer’s Disease Risk: Results from a Prospective Cohort Followed over 17 Years. Alzheimer’s Dement. 2023, 19, 25–35. [Google Scholar] [CrossRef]
- Baiardi, S.; Quadalti, C.; Mammana, A.; Dellavalle, S.; Zenesini, C.; Sambati, L.; Pantieri, R.; Polischi, B.; Romano, L.; Suffritti, M.; et al. Diagnostic Value of Plasma P-Tau181, NfL, and GFAP in a Clinical Setting Cohort of Prevalent Neurodegenerative Dementias. Alzheimer’s Res. Ther. 2022, 14, 153. [Google Scholar] [CrossRef]
- Truffi, M.; Garofalo, M.; Ricciardi, A.; Cotta Ramusino, M.; Perini, G.; Scaranzin, S.; Gastaldi, M.; Albasini, S.; Costa, A.; Chiavetta, V.; et al. Neurofilament-Light Chain Quantification by SIMOA and Ella in Plasma from Patients with Dementia: A Comparative Study. Sci. Rep. 2023, 13, 4041. [Google Scholar] [CrossRef] [PubMed]
- Blennow, K.; Zetterberg, H. Biomarkers for Alzheimer’s Disease: Current Status and Prospects for the Future. J. Intern. Med. 2018, 284, 643–663. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Tseng, Y.; Chen, Y.; Chen, P.; Chiu, H. Diagnostic Accuracy of the Clinical Dementia Rating Scale for Detecting Mild Cognitive Impairment and Dementia: A Bivariate Meta-analysis. Int. J. Geriatr. Psychiatry 2021, 36, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiong, C.; Aschenbrenner, A.J.; Chang, C.; Weiner, M.W.; Nosheny, R.L.; Mungas, D.; Bateman, R.J.; Hassenstab, J.; Moulder, K.L.; et al. Item Response Theory Analysis of the Clinical Dementia Rating. Alzheimer’s Dement. 2021, 17, 534–542. [Google Scholar] [CrossRef]
- Tsoi, K.K.F.; Chan, J.Y.C.; Hirai, H.W.; Wong, S.Y.S.; Kwok, T.C.Y. Cognitive Tests to Detect Dementia. JAMA Intern. Med. 2015, 175, 1450. [Google Scholar] [CrossRef]
- Mitchell, A.J. A Meta-Analysis of the Accuracy of the Mini-Mental State Examination in the Detection of Dementia and Mild Cognitive Impairment. J. Psychiatr. Res. 2009, 43, 411–431. [Google Scholar] [CrossRef]
- Muntal Encinas, S.; Gramunt-Fombuena, N.; Badenes Guia, D.; Casas Hernanz, L.; Aguilar Barbera, M. Traducción y Adaptación Española de La Batería Neuropsicológica Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) Forma A En Una Muestra Piloto. Neurología 2012, 27, 531–546. [Google Scholar] [CrossRef]
- Freilich, B.M.; Hyer, L.A. Relation of the Repeatable Battery for Assessment of Neuropsychological Status to Measures of Daily Functioning in Dementia. Psychol. Rep. 2007, 101, 119–129. [Google Scholar] [CrossRef]
- Duff, K.; Humphreysclark, J.; Obryant, S.; Mold, J.; Schiffer, R.; Sutker, P. Utility of the RBANS in Detecting Cognitive Impairment Associated with Alzheimer’s Disease: Sensitivity, Specificity, and Positive and Negative Predictive Powers. Arch. Clin. Neuropsychol. 2008, 23, 603–612. [Google Scholar] [CrossRef]
- Peña-Bautista, C.; Álvarez-Sánchez, L.; Pascual, R.; Moreno, M.J.; Baquero, M.; Cháfer-Pericás, C. Clinical usefulness of cerebrospinal fluid biomarkers in Alzheimer’s disease. Eur. J. Clin. Investig. 2023, 53, e13910. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.G.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.-P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C.G.; et al. Diagnosis and Management of Dementia with Lewy Bodies. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Available online: www.quanterix.com/simoa-technology/ (accessed on 5 January 2023).
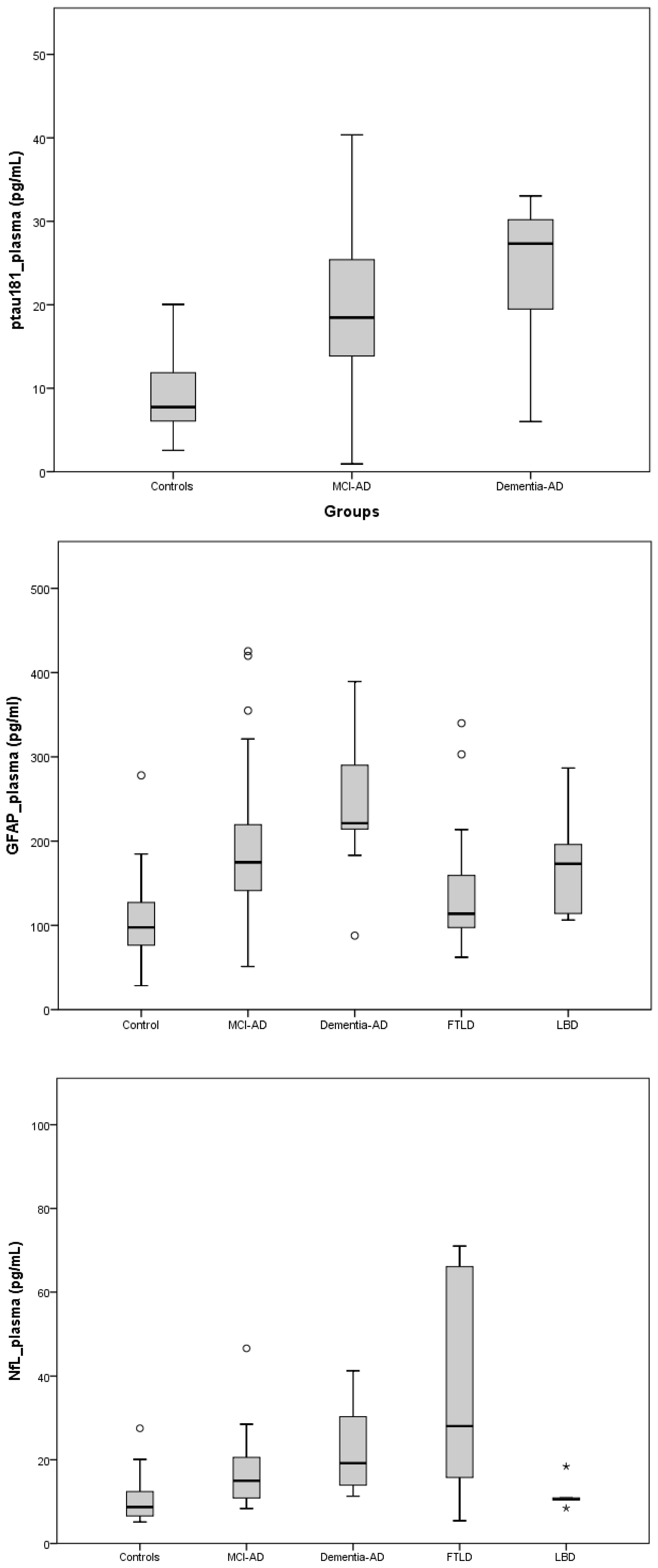


| SCI (n = 21) | MCI-AD (n = 50) | Mild Dementia-AD (n = 10) | FTD (n = 20) | LBD (n = 5) | p-Value (Kruskal–Wallis) | ||
|---|---|---|---|---|---|---|---|
| Age (years, median (IQR)) | 62 (59–70) | 71 (68.75–74) | 75 (71.25–77) | 66.50 (59.75–70.75) | 70 (70–73) | <0.01 | |
| Sex (% women) | 57.1% | 56% | 80% | 65% | 40% | >0.05 | |
| Educational level (n%) | Primary | 28.6% | 54% | 70% | 55% | - | <0.05 |
| Secondary | 23.8% | 26% | 10% | 40% | 40% | ||
| University | 47.6% | 20% | 20% | 5% | 60% | ||
| ApoE genotype (% ε4 carrier) | 9% | 38% | 30% | 0% | 20% | <0.05 | |
| CSF Aβ42 (pg mL−1) (median (IQR)) | 1043.64 (938.5–1431.5) | 509 (450.1–760) | 483 (372–674) | 1192 (867.75–1669) | 819.24 (657–1204.62) | <0.01 | |
| CSF p-Tau181 (pg mL−1) (median (IQR)) | 32 (24.5–42.5) | 80.5 (58.75–113.5) | 98 (71.5–159) | 39.5 (24.25–57) | 57.5 (34.25–92.75) | <0.01 | |
| CSF t-Tau (pg mL−1) (median (IQR)) | 233 (152.5–302.5) | 477.5 (328.25–769.5) | 768 (432.5–997) | 316 (227–549.5) | 379.5 (203.75–516.25) | <0.01 | |
| CSF Aβ40 (pg mL−1) (median (IQR)) | 10,279 (8882–13,519) | 13,217.5 (8545.5–15,064.25) | 13,807 (8457–15,054) | 12,133 (8770.5–16,159.25) | 12,307 (11,964–/) | >0.05 | |
| CSF NfL (pg mL−1) (median (IQR)) | 533.71 (443.89–775.17) | 1160.52 (825.26–1308.24) | 1261.21 (788.26–1717.72) | 3019.16 (965.55–4880.87) | 948.67 (813.14–/) | <0.01 | |
| CSF Aβ42/Aβ40 (median (IQR)) | 0.107 (0.099–0.114) | 0.052 (0.043–0.059) | 0.047 (0.043–0.056) | 0.107 (0.104–0.115) | 0.066 (0.05–/) | <0.01 | |
| CSF t-Tau/Aβ 42 (median (IQR)) | 0.19 (0.16–0.24) | 0.72 (0.5–1.7) | 98 (71.5–159) | 0.27 (0.21–037) | 0.46 (0.11–0.46) | <0.01 | |
| CDR (median (IQR)) | 0 (0–0) | 0.5 (0.5–0.5) | 1 (1–2) | 0.5 (0.5–1) | 0.5 (0.5–0.75) | <0.01 | |
| MMSE (median (IQR)) | 29 (27.75–30) | 26 (23.75–28.25) | 17.50 (12.5–27) | 23 (20–27) | 24 (20.50–27.50) | <0.01 | |
| RBANS-IM (median (IQR)) | 92 (82–100) | 69 (60–78.75) | 61 (44–69) | 59 (47.75–73.75) | 65 (52.50–84) | <0.01 | |
| RBANS-V/C (median (IQR)) | 101 (91.25–114.25) | 84 (71.25–96) | 66 (56–72) | 78 (61.5–89) | 69 (58.5–82) | <0.01 | |
| RBANS-L (median (IQR)) | 89 (84.25–94.5) | 85 (64–96) | 57 (51–85) | 55.5 (44–69) | 87 (69.5–92) | <0.01 | |
| RBANS-A (median (IQR)) | 91 (89.50–100) | 73.5 (56–88) | 49 (49–56) | 67.5 (53 –94.75) | 75 (60.5–84.5) | <0.01 | |
| RBANS-DM (median (IQR)) | 100.5 (96.25–109) | 69.5 (52–88.5) | 44 (40–44) | 52 (47–70.5) | 78 (57.5–83) | <0.01 | |
| Total RBANS (median (IQR)) | 90.5 (85.75–105.25) | 71.5 (56.75–80.25) | 50 (45–53) | 54 (48–69) | 55 (49–74.50) | <0.01 | |
| Plasma Biomarker (pg mL−1, Median (IQR)) | SCI (n = 21) | MCI-AD (n = 50) | Mild Dementia-AD (n = 10) | FTD (n = 20) | LBD (n = 5) | p-Value (Kruskal–Wallis) | p-Value (Mann–Whitney) |
|---|---|---|---|---|---|---|---|
| p-Tau181 | 7.745 (5.97–11.99) | 18.47 (13.48–25.43) | 27.33 (18.69–30.46) | – | – | <0.01 | 0 <0.01 1 <0.01 |
| NfL | 8.72 (6.51–12.59) | 14.98 (10.82–20.84) | 19.18 (12.75–33.34) | 28.04 (15.37–67.23) | 10.52 (9.42–14.66) | <0.01 | 0 <0.01 1 <0.01 2 <0.01 3 >0.05 4 >0.05 5 >0.05 6 <0.01 |
| GFAP | 97.53 (75.6–132.01) | 174.92 (141.1–221.93) | 221.32 (198.73–291.42) | 113.76 (96.93–162.89) | 173.16 (110.26–241.37) | <0.01 | 0 <0.01 1 <0.01 2 <0.05 3 <0.03 4 <0.01 5 <0.01 6 <0.02 |
| Plasma p-Tau181 (pg mL−1) Pearson (r (p-Value)) | Plasma NfL (pg mL−1) Pearson (r (p-Value)) | Plasma GFAP (pg mL−1) Pearson (r (p-Value)) | |
|---|---|---|---|
| Age | 0.322 (<0.01) * | 0.008 (>0.05) | 0.389 (<0.01) * |
| MMSE | −0.459 (<0.01) * | −0.198 (<0.05) * | −0.2 (<0.05) * |
| CDR | 0.367 (<0.01) * | −0.214 (<0.04) * | 0.238 (<0.02) * |
| RBANS-IM | −0.518 (<0.01) * | −1.76 (>0.05) | −0.264 (=0.01) * |
| RBANS-L | −0.179 (>0.05) | −0.183 (>0.05) | −0.211 (=0.04) * |
| RBANS-DM | −0.51 (<0.01) * | −0.231 (<0.03) * | −0.323 (<0.01) * |
| RBANS-V/C | −0.405 (<0.01) * | −0.214 (=0.04) * | −0.185 (>0.05) |
| RBANS-A | −0.322 (<0.01) * | −0.179 (>0.05) | −0.133 (>0.05) |
| TOTAL RBANS | −0.520 (<0.01) * | −0.248 (<0.02) * | −0.324 (<0.01) * |
| CSF Aβ40 (pg mL−1) | 0.046 (>0.05) | −0.054 (>0.05) | 0.048 (>0.05) |
| CSF Aβ42 (pg mL−1) | −0.587 (p < 0.01) * | 0.092 (>0.05) | −0.347 (<0.01) * |
| CSF t-Tau (pg mL−1) | 0.352 (<0.02) * | 0.19 (>0.05) | 0.351 (<0.01) * |
| CSF p-Tau181 (pg mL−1) | 0.414 (<0.01) * | −0.097 (>0.05) | 0.337 (<0.01) * |
| CSF NfL (pg mL−1) | 0.357 (>0.05) | 0.622 (<0.01) * | 0.26 (>0.05) |
| CSF Aβ42/Aβ40 | −0.543 (<0.01) * | 0.213 (>0.05) | −0.396 (<0.01) * |
| CSF t-Tau/Aβ42 | 0.471 (<0.01) * | 0.006 (>0.05) | 0.470 (<0.01) * |
| Coefficients | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Intercept | 2.530 | 2.644 | 2.150 |
| Sex | −0.051 | −0.196 | −0.066 |
| Age | +0.029 | +0.022 | +0.026 |
| p-Tau181 | +0.023 | +0.027 | +0.020 |
| GFAP | +0.002 | +0.003 | +0.002 |
| NfL | +0.023 | +0.023 | +0.018 |
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| AUC (CI (95%)) | 0.9256 (0.85–0.98) | 98.7 (94–100) | 93.5 (87–98) |
| Sensitivity (%, CI (95%)) | 89.8 (64–98) | 88.9 (83–100) | 86.2 (66–98) |
| Specificity (%, CI (95%)) | 82.3 (72–100) | 100 (81–100) | 88.2 (75–100) |
| Accuracy (%, CI (95%)) | 87.9 (73–95) | 96.1 (88–100) | 86.7 (73–96) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez-Sánchez, L.; Peña-Bautista, C.; Ferré-González, L.; Cubas, L.; Balaguer, A.; Casanova-Estruch, B.; Baquero, M.; Cháfer-Pericás, C. Early Alzheimer’s Disease Screening Approach Using Plasma Biomarkers. Int. J. Mol. Sci. 2023, 24, 14151. https://doi.org/10.3390/ijms241814151
Álvarez-Sánchez L, Peña-Bautista C, Ferré-González L, Cubas L, Balaguer A, Casanova-Estruch B, Baquero M, Cháfer-Pericás C. Early Alzheimer’s Disease Screening Approach Using Plasma Biomarkers. International Journal of Molecular Sciences. 2023; 24(18):14151. https://doi.org/10.3390/ijms241814151
Chicago/Turabian StyleÁlvarez-Sánchez, Lourdes, Carmen Peña-Bautista, Laura Ferré-González, Laura Cubas, Angel Balaguer, Bonaventura Casanova-Estruch, Miguel Baquero, and Consuelo Cháfer-Pericás. 2023. "Early Alzheimer’s Disease Screening Approach Using Plasma Biomarkers" International Journal of Molecular Sciences 24, no. 18: 14151. https://doi.org/10.3390/ijms241814151
APA StyleÁlvarez-Sánchez, L., Peña-Bautista, C., Ferré-González, L., Cubas, L., Balaguer, A., Casanova-Estruch, B., Baquero, M., & Cháfer-Pericás, C. (2023). Early Alzheimer’s Disease Screening Approach Using Plasma Biomarkers. International Journal of Molecular Sciences, 24(18), 14151. https://doi.org/10.3390/ijms241814151






