Murine Mast Cells That Are Deficient in IFNAR-Signaling Respond to Viral Infection by Producing a Large Amount of Inflammatory Cytokines, a Low Level of Reactive Oxygen Species, and a High Rate of Cell Death
Abstract
1. Introduction
2. Results
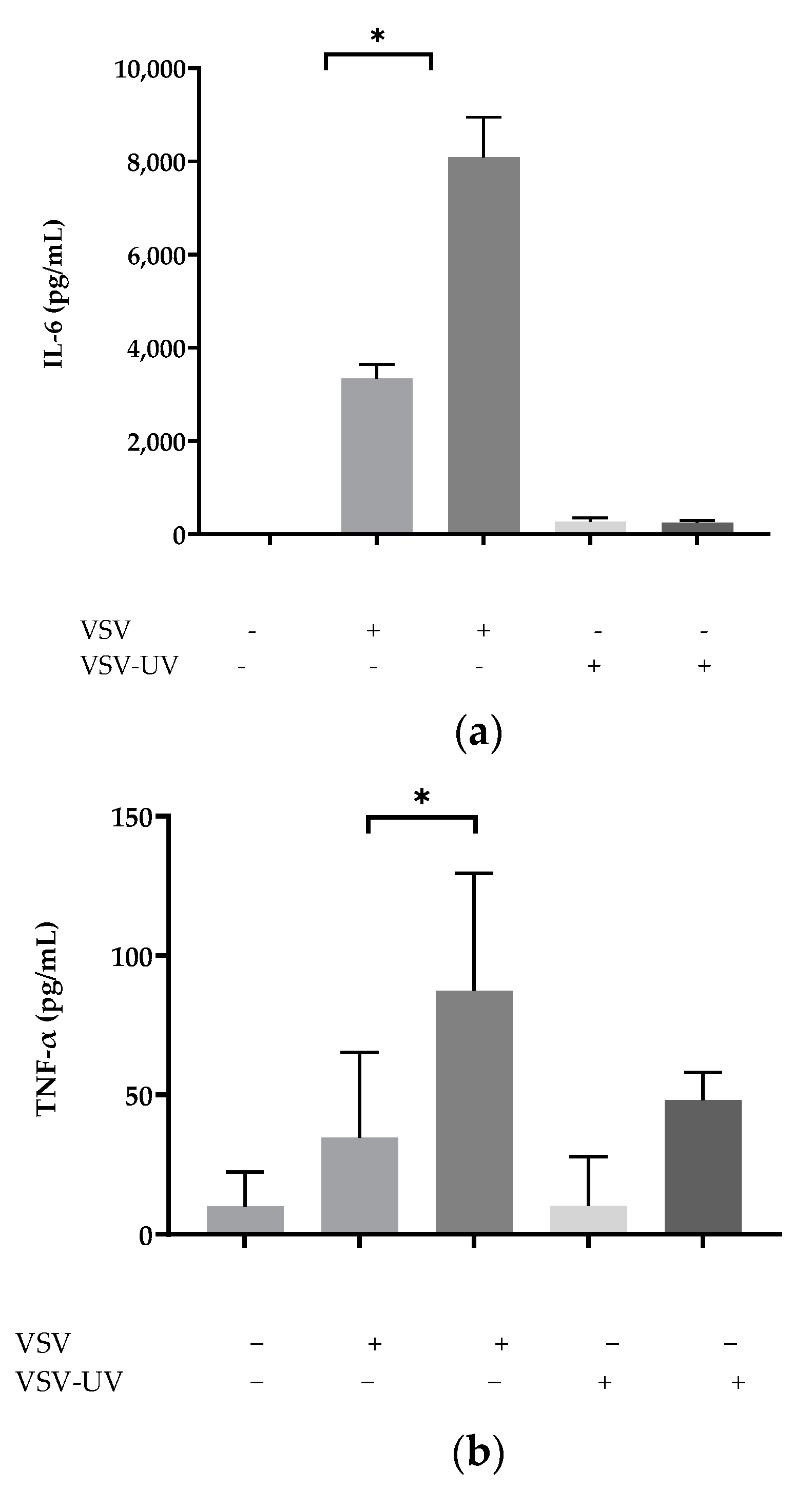
2.1. Ultraviolet Irradiation of rVSV∆M51 Failed to Induce Cytokines in BMMCs
2.2. IFNAR Signaling Protected BMMCs from rVSV∆M51-Induced Cell Death
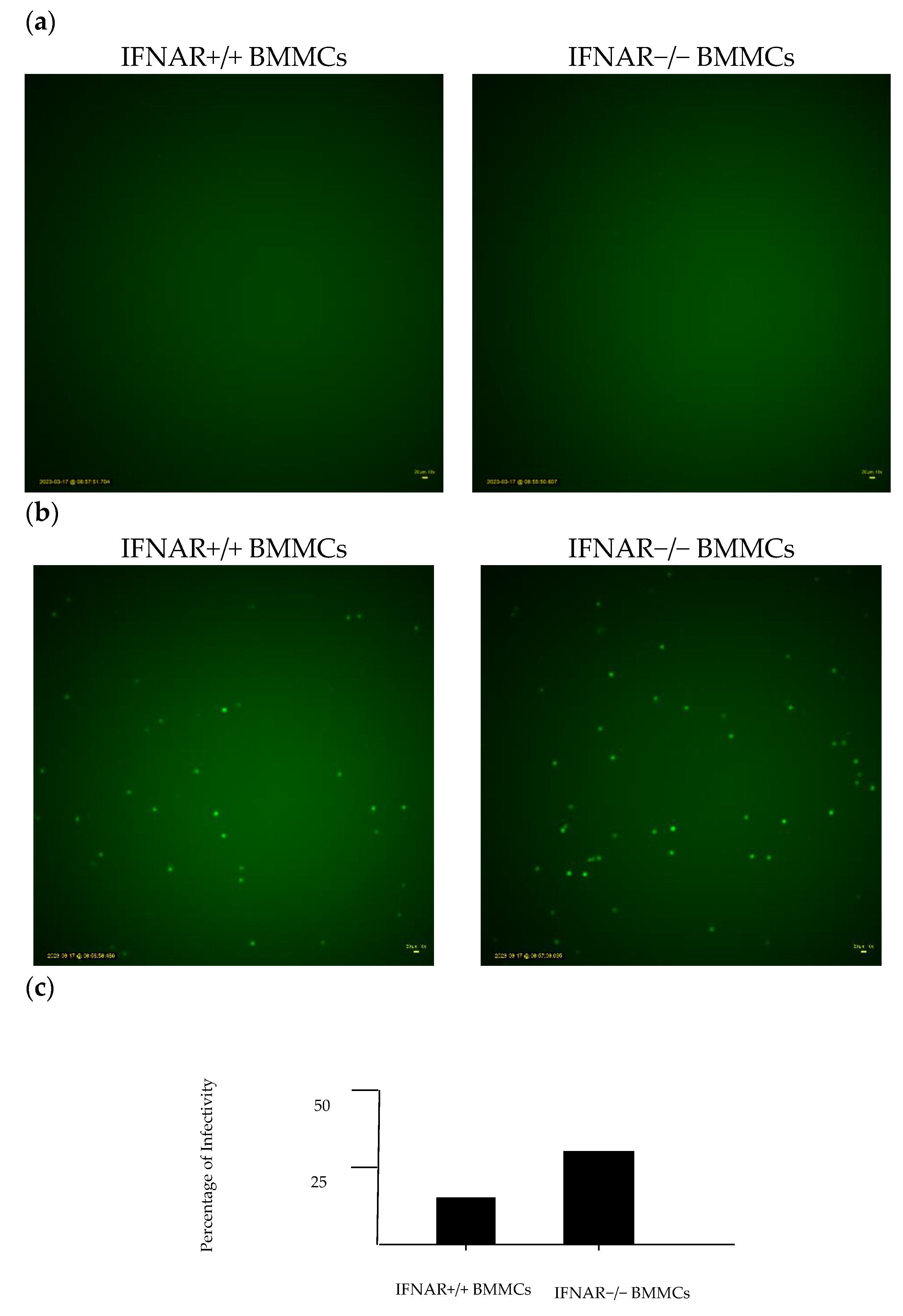
2.3. Lack of IFNAR Signaling in BMMCs Affects rVSV∆M51 Infectivity
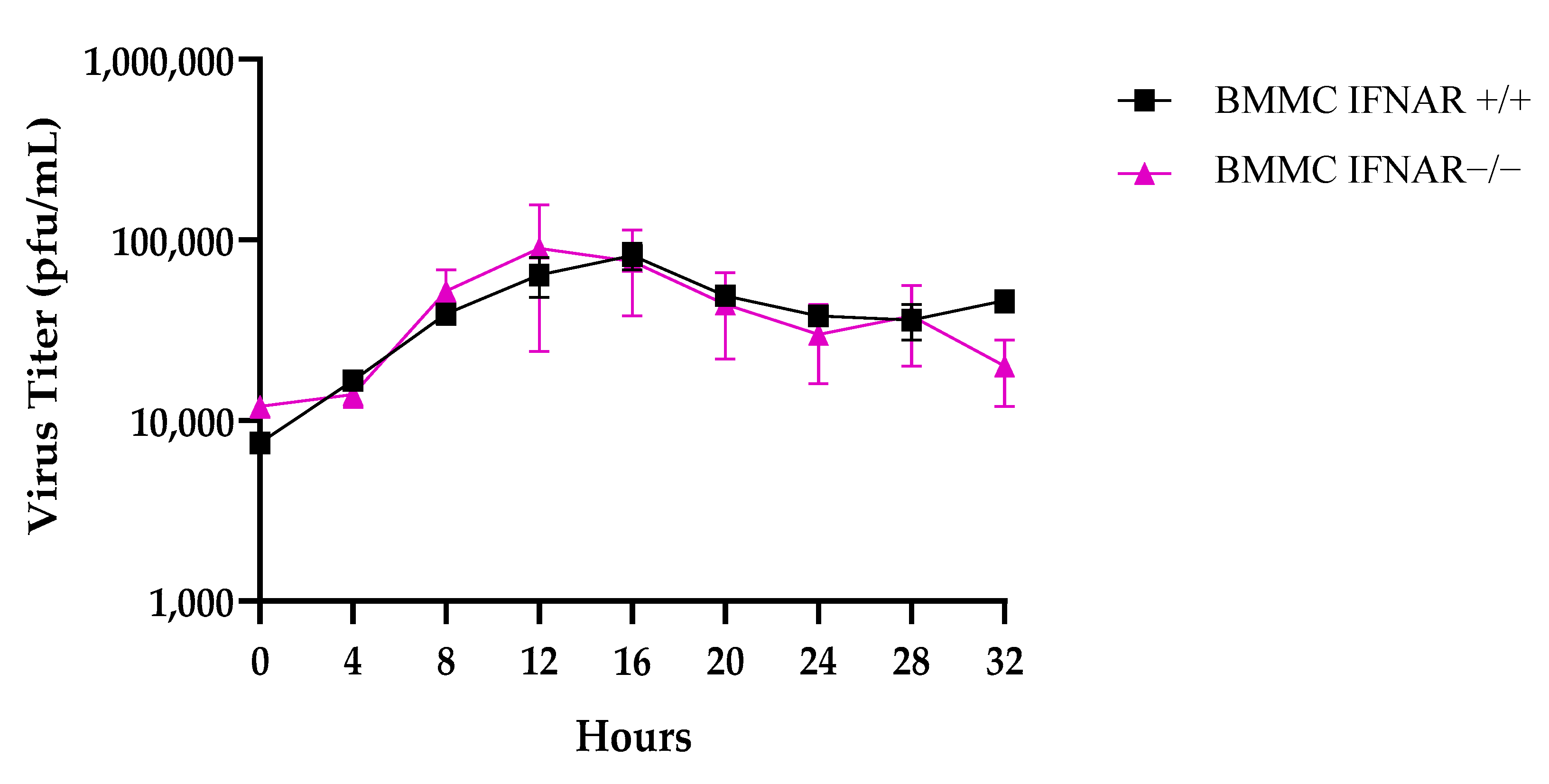
2.4. Comparable Viral Titration in IFNAR+/+ and IFNAR−/− BMMCs
2.5. ROS Assay Revealed Significant ROS Production in IFNAR+/+ BMMCs
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Virus
4.3. Generation of BMMCs
4.4. UV-Inactivated VSV-Δm51-hDCT
4.5. Cell Viability Assay
4.6. Time-Lapse Images of VSV-Infected BMMCs
4.7. Plaque Assay for Quantifying Viral Titers
4.8. Reactive Oxygen Species Assay
4.9. Statistics
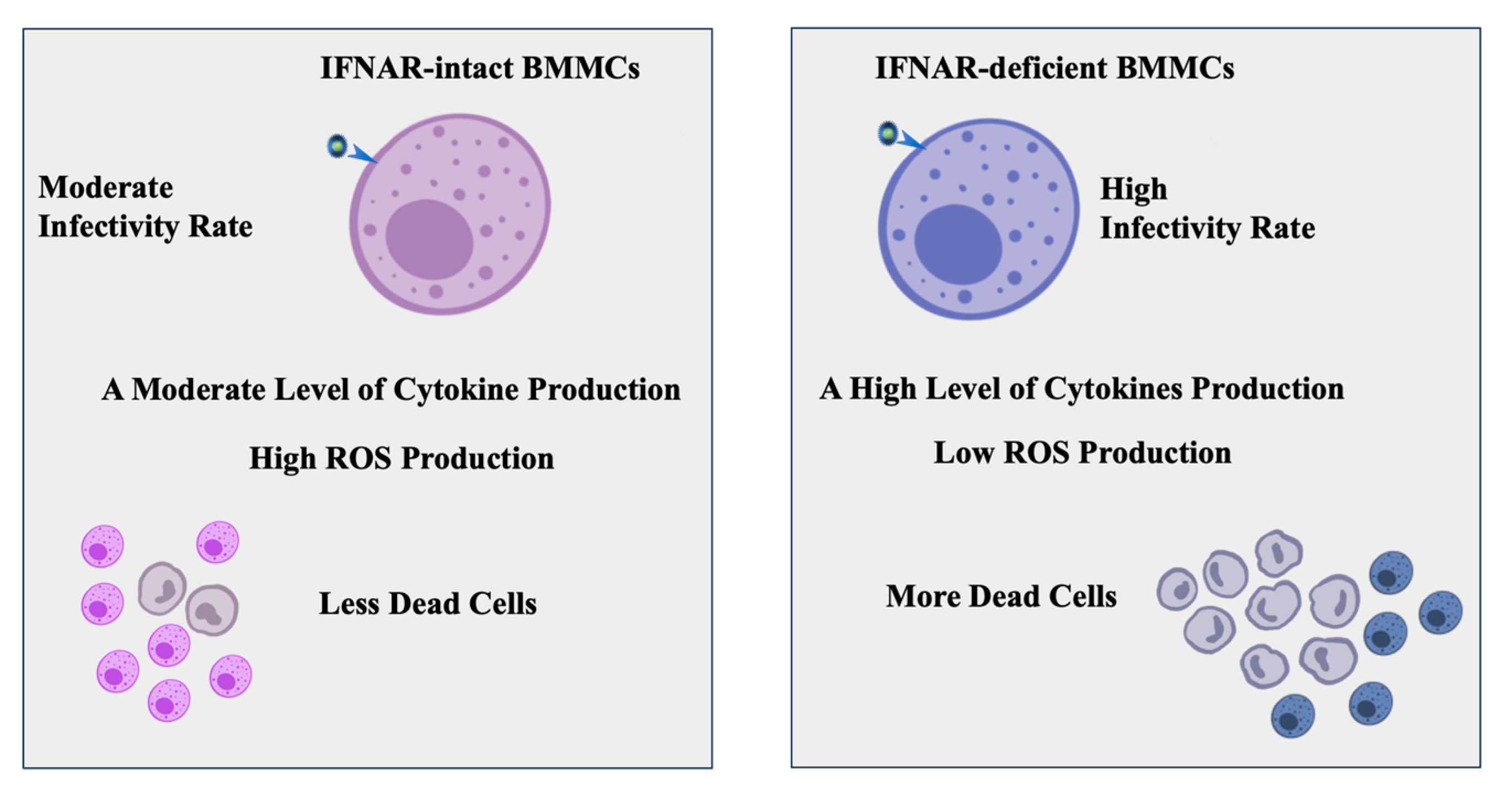
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nirmal, S.; Vaykole, A.; Khadse, G.; Pal, S.; Mandal, S. Mast cell degranulation: A target for bioactive natural products. Phytopharmacology 2013, 4, 575–597. [Google Scholar]
- Darzianiazizi, M.; Mehrani, Y.; Chan, L.; Mould, R.C.; Kulkarni, R.R.; Sharif, S.; Bridle, B.W.; Karimi, K. Type I Interferon α/β Receptor-Mediated Signaling Negatively Regulates Antiviral Cytokine Responses in Murine Bone-Marrow-Derived Mast Cells and Protects the Cells from Virus-Induced Cell Death. Int. J. Mol. Sci. 2020, 21, 9041. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Kawakita, K.; Kiso, Y.; Nakano, T.; Kitamura, Y. Substance P induces granulocyte infiltration through degranulation of mast cells. J. Immunol. 1989, 142, 927–931. [Google Scholar] [CrossRef]
- Akoto, C.; Davies, D.E.; Swindle, E.J. Mast cells are permissive for rhinovirus replication: Potential implications for asthma exacerbations. Clin. Exp. Allergy 2017, 47, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Fensterl, V.; Sen, G.C. Interferon-induced Ifit proteins: Their role in viral pathogenesis. J. Virol. 2015, 89, 2462–2468. [Google Scholar] [CrossRef]
- Snell, L.M.; McGaha, T.L.; Brooks, D.G. Type I interferon in chronic virus infection and cancer. Trends Immunol. 2017, 38, 542–557. [Google Scholar] [CrossRef]
- Zhou, Z.; Hamming, O.J.; Ank, N.; Paludan, S.R.; Nielsen, A.L.; Hartmann, R. Type III interferon (IFN) induces a type I IFN-like response in a restricted subset of cells through signaling pathways involving both the Jak-STAT pathway and the mitogen-activated protein kinases. J. Virol. 2007, 81, 7749–7758. [Google Scholar] [CrossRef]
- Ivashkiv, L.B.; Donlin, L.T. Regulation of type I interferon responses. Nat. Rev. Immunol. 2014, 14, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Katze, M.G.; He, Y.; Gale, M. Viruses and interferon: A fight for supremacy. Nat. Rev. Immunol. 2002, 2, 675–687. [Google Scholar] [CrossRef] [PubMed]
- Stanifer, M.L.; Guo, C.; Doldan, P.; Boulant, S. Importance of Type I and III interferons at respiratory and intestinal barrier surfaces. Front. Immunol. 2020, 11, 608645. [Google Scholar] [CrossRef]
- Murira, A.; Lamarre, A. Type-I interferon responses: From friend to foe in the battle against chronic viral infection. Front. Immunol. 2016, 7, 609. [Google Scholar] [CrossRef] [PubMed]
- Darzianiazizi, M.; Allison, K.E.; Kulkarni, R.R.; Sharif, S.; Karimi, K.; Bridle, B.W. Disruption of type I interferon signaling causes sexually dimorphic dysregulation of anti-viral cytokines. Cytokine X 2021, 3, 100053. [Google Scholar] [CrossRef] [PubMed]
- Chelombitko, M.; Fedorov, A.; Ilyinskaya, O.; Zinovkin, R.; Chernyak, B. Role of reactive oxygen species in mast cell degranulation. Biochemistry 2016, 81, 1564–1577. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.-H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Kang, K.S. N-acetylcysteine modulates lipopolysaccharide-induced intestinal dysfunction. Sci. Rep. 2019, 9, 1004. [Google Scholar] [CrossRef]
- Yang, Y.; Bazhin, A.V.; Werner, J.; Karakhanova, S. Reactive oxygen species in the immune system. Int. Rev. Immunol. 2013, 32, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Sander, W.J.; Fourie, C.; Sabiu, S.; O’Neill, F.H.; Pohl, C.H.; O’Neill, H.G. Reactive oxygen species as potential antiviral targets. Rev. Med. Virol. 2022, 32, e2240. [Google Scholar] [CrossRef]
- Canton, M.; Sánchez-Rodríguez, R.; Spera, I.; Venegas, F.C.; Favia, M.; Viola, A.; Castegna, A. Reactive oxygen species in macrophages: Sources and targets. Front. Immunol. 2021, 12, 734229. [Google Scholar] [CrossRef]
- Reshi, M.L.; Su, Y.-C.; Hong, J.-R. RNA viruses: ROS-mediated cell death. Int. J. Cell Biol. 2014, 2014, 467452. [Google Scholar] [CrossRef]
- Fang, F.C. Antimicrobial actions of reactive oxygen species. mBio 2011, 2, e00141-11. [Google Scholar] [CrossRef]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973, 52, 741–744. [Google Scholar] [CrossRef] [PubMed]
- Hadaschik, B.A.; Zhang, K.; So, A.I.; Fazli, L.; Jia, W.; Bell, J.C.; Gleave, M.E.; Rennie, P.S. Oncolytic vesicular stomatitis viruses are potent agents for intravesical treatment of high-risk bladder cancer. Cancer Res. 2008, 68, 4506–4510. [Google Scholar] [CrossRef] [PubMed]
- Stojdl, D.F.; Lichty, B.; Knowles, S.; Marius, R.; Atkins, H.; Sonenberg, N.; Bell, J.C. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000, 6, 821–825. [Google Scholar] [CrossRef]
- Graham, A.C.; Hilmer, K.M.; Zickovich, J.M.; Obar, J.J. Inflammatory response of mast cells during influenza A virus infection is mediated by active infection and RIG-I signaling. J. Immunol. 2013, 190, 4676–4684. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.G.; McAlpine, S.M.; Huang, Y.Y.; Haidl, I.D.; Al-Afif, A.; Marshall, J.S.; Anderson, R. RNA sensors enable human mast cell anti-viral chemokine production and IFN-mediated protection in response to antibody-enhanced dengue virus infection. PLoS ONE 2012, 7, e34055. [Google Scholar] [CrossRef]
- Marcet, C.W.; Laurent, C.D.S.; Moon, T.C.; Singh, N.; Befus, A.D. Limited replication of influenza A virus in human mast cells. Immunol. Res. 2013, 56, 32–43. [Google Scholar] [CrossRef]
- Troupin, A.; Shirley, D.; Londono-Renteria, B.; Watson, A.M.; McHale, C.; Hall, A.; Hartstone-Rose, A.; Klimstra, W.B.; Gomez, G.; Colpitts, T.M. A role for human skin mast cells in dengue virus infection and systemic spread. J. Immunol. 2016, 197, 382–391. [Google Scholar] [CrossRef]
- Goritzka, M.; Durant, L.R.; Pereira, C.; Salek-Ardakani, S.; Openshaw, P.J.; Johansson, C. Alpha/beta interferon receptor signaling amplifies early proinflammatory cytokine production in the lung during respiratory syncytial virus infection. J. Virol. 2014, 88, 6128–6136. [Google Scholar] [CrossRef]
- Dietrich, N.; Rohde, M.; Geffers, R.; Kröger, A.; Hauser, H.; Weiss, S.; Gekara, N.O. Mast cells elicit proinflammatory but not type I interferon responses upon activation of TLRs by bacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 8748–8753. [Google Scholar] [CrossRef]
- McNab, F.; Mayer-Barber, K.; Sher, A.; Wack, A.; O’garra, A. Type I interferons in infectious disease. Nat. Rev. Immunol. 2015, 15, 87–103. [Google Scholar] [CrossRef]
- Shahangian, A.; Chow, E.K.; Tian, X.; Kang, J.R.; Ghaffari, A.; Liu, S.Y.; Belperio, J.A.; Cheng, G.; Deng, J.C. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J. Clin. Investig. 2009, 119, 1910–1920. [Google Scholar] [CrossRef] [PubMed]
- Al-Afif, A.; Alyazidi, R.; Oldford, S.A.; Huang, Y.Y.; King, C.A.; Marr, N.; Haidl, I.D.; Anderson, R.; Marshall, J.S. Respiratory syncytial virus infection of primary human mast cells induces the selective production of type I interferons, CXCL10, and CCL4. J. Allergy Clin. Immunol. 2015, 136, 1346–1354.e1. [Google Scholar] [CrossRef] [PubMed]
- Paiva, C.N.; Bozza, M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid. Redox Signal. 2014, 20, 1000–1037. [Google Scholar] [CrossRef] [PubMed]
- Tauffenberger, A.; Magistretti, P.J. Reactive oxygen species: Beyond their reactive behavior. Neurochem. Res. 2021, 46, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Martinez, S.M.; Prieto-Garcia, L.; Prieto, M.; Fuentes-Calvo, I.; Lopez-Novoa, J.M.; Morales, A.I.; Martinez-Salgado, C.; Lopez-Hernandez, F.J. N-acetylcysteine transforms necrosis into apoptosis and affords tailored protection from cisplatin cytotoxicity. Toxicol. Appl. Pharmacol. 2018, 349, 83–93. [Google Scholar] [CrossRef]
- Erkkilä, K.; Hirvonen, V.; Wuokko, E.; Parvinen, M.; Dunkel, L. N-acetyl-L-cysteine inhibits apoptosis in human male germ cells in vitro. J. Clin. Endocrinol. Metab. 1998, 83, 2523–2531. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Gu, L.; Wang, S.; Yuan, J.; Yang, H.; Zhu, J.; Zhang, H. N-acetylcysteine protects against apoptosis through modulation of group I metabotropic glutamate receptor activity. PLoS ONE 2012, 7, e32503. [Google Scholar] [CrossRef]
- Mlejnek, P.; Dolezel, P.; Kriegova, E.; Pastvova, N. N-acetylcysteine can induce massive oxidative stress, resulting in cell death with apoptotic features in human leukemia cells. Int. J. Mol. Sci. 2021, 22, 12635. [Google Scholar] [CrossRef]
- Qanungo, S.; Wang, M.; Nieminen, A.-L. N-acetyl-L-cysteine enhances apoptosis through inhibition of nuclear factor-κB in hypoxic murine embryonic fibroblasts. J. Biol. Chem. 2004, 279, 50455–50464. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehrani, Y.; Knapp, J.P.; Kakish, J.E.; Tieu, S.; Javadi, H.; Chan, L.; Stegelmeier, A.A.; Napoleoni, C.; Bridle, B.W.; Karimi, K. Murine Mast Cells That Are Deficient in IFNAR-Signaling Respond to Viral Infection by Producing a Large Amount of Inflammatory Cytokines, a Low Level of Reactive Oxygen Species, and a High Rate of Cell Death. Int. J. Mol. Sci. 2023, 24, 14141. https://doi.org/10.3390/ijms241814141
Mehrani Y, Knapp JP, Kakish JE, Tieu S, Javadi H, Chan L, Stegelmeier AA, Napoleoni C, Bridle BW, Karimi K. Murine Mast Cells That Are Deficient in IFNAR-Signaling Respond to Viral Infection by Producing a Large Amount of Inflammatory Cytokines, a Low Level of Reactive Oxygen Species, and a High Rate of Cell Death. International Journal of Molecular Sciences. 2023; 24(18):14141. https://doi.org/10.3390/ijms241814141
Chicago/Turabian StyleMehrani, Yeganeh, Jason P. Knapp, Julia E. Kakish, Sophie Tieu, Helia Javadi, Lily Chan, Ashley A. Stegelmeier, Christina Napoleoni, Byram W. Bridle, and Khalil Karimi. 2023. "Murine Mast Cells That Are Deficient in IFNAR-Signaling Respond to Viral Infection by Producing a Large Amount of Inflammatory Cytokines, a Low Level of Reactive Oxygen Species, and a High Rate of Cell Death" International Journal of Molecular Sciences 24, no. 18: 14141. https://doi.org/10.3390/ijms241814141
APA StyleMehrani, Y., Knapp, J. P., Kakish, J. E., Tieu, S., Javadi, H., Chan, L., Stegelmeier, A. A., Napoleoni, C., Bridle, B. W., & Karimi, K. (2023). Murine Mast Cells That Are Deficient in IFNAR-Signaling Respond to Viral Infection by Producing a Large Amount of Inflammatory Cytokines, a Low Level of Reactive Oxygen Species, and a High Rate of Cell Death. International Journal of Molecular Sciences, 24(18), 14141. https://doi.org/10.3390/ijms241814141










