Rapid and Sensitive Detection of Toxigenic Fusarium asiaticum Integrating Recombinase Polymerase Amplification, CRISPR/Cas12a, and Lateral Flow Techniques
Abstract
:1. Introduction
2. Results
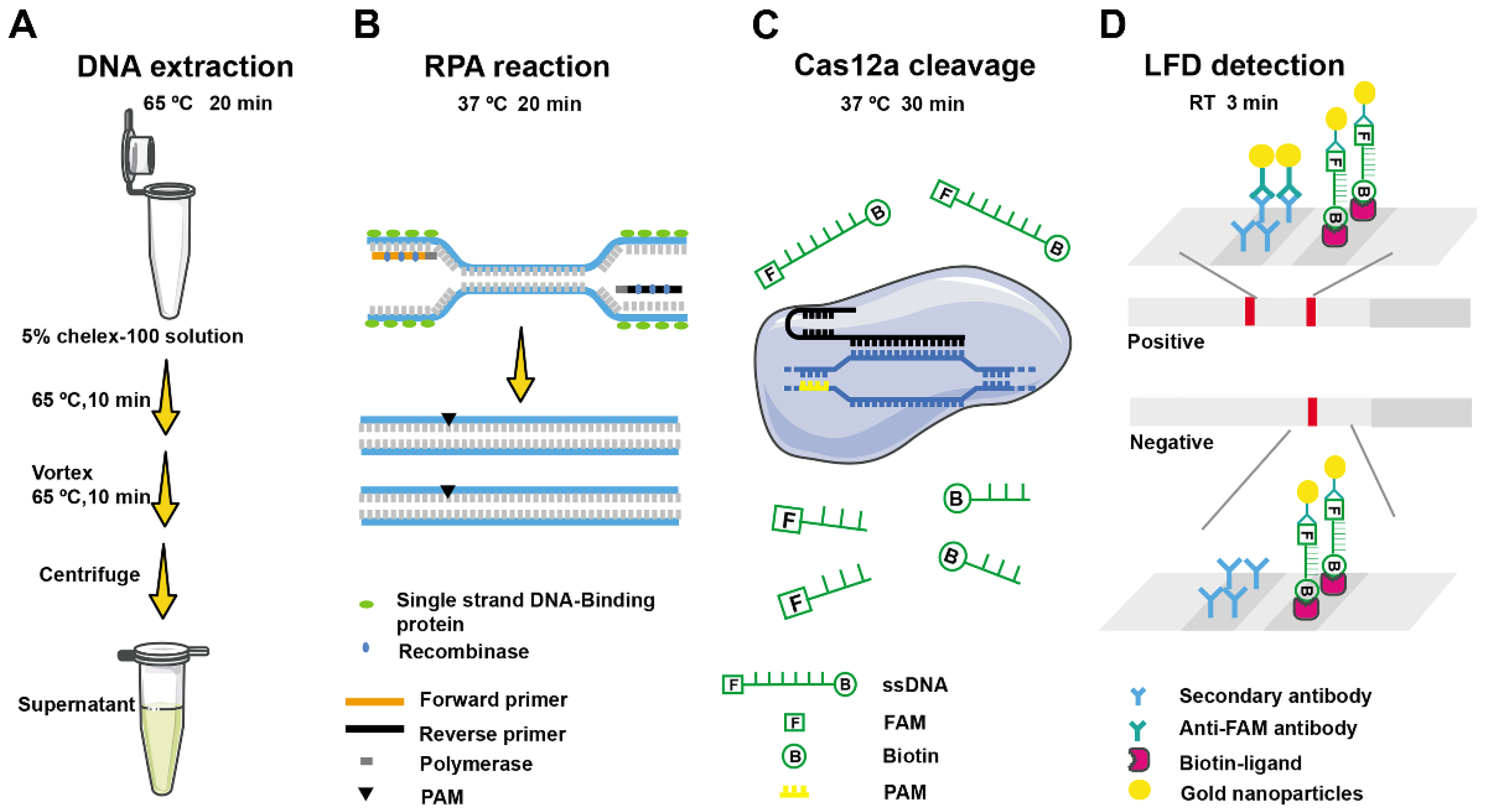
2.1. Schematic Diagram of F. asiaticum Detection Based on RPA-Cas12a-LFD Assay
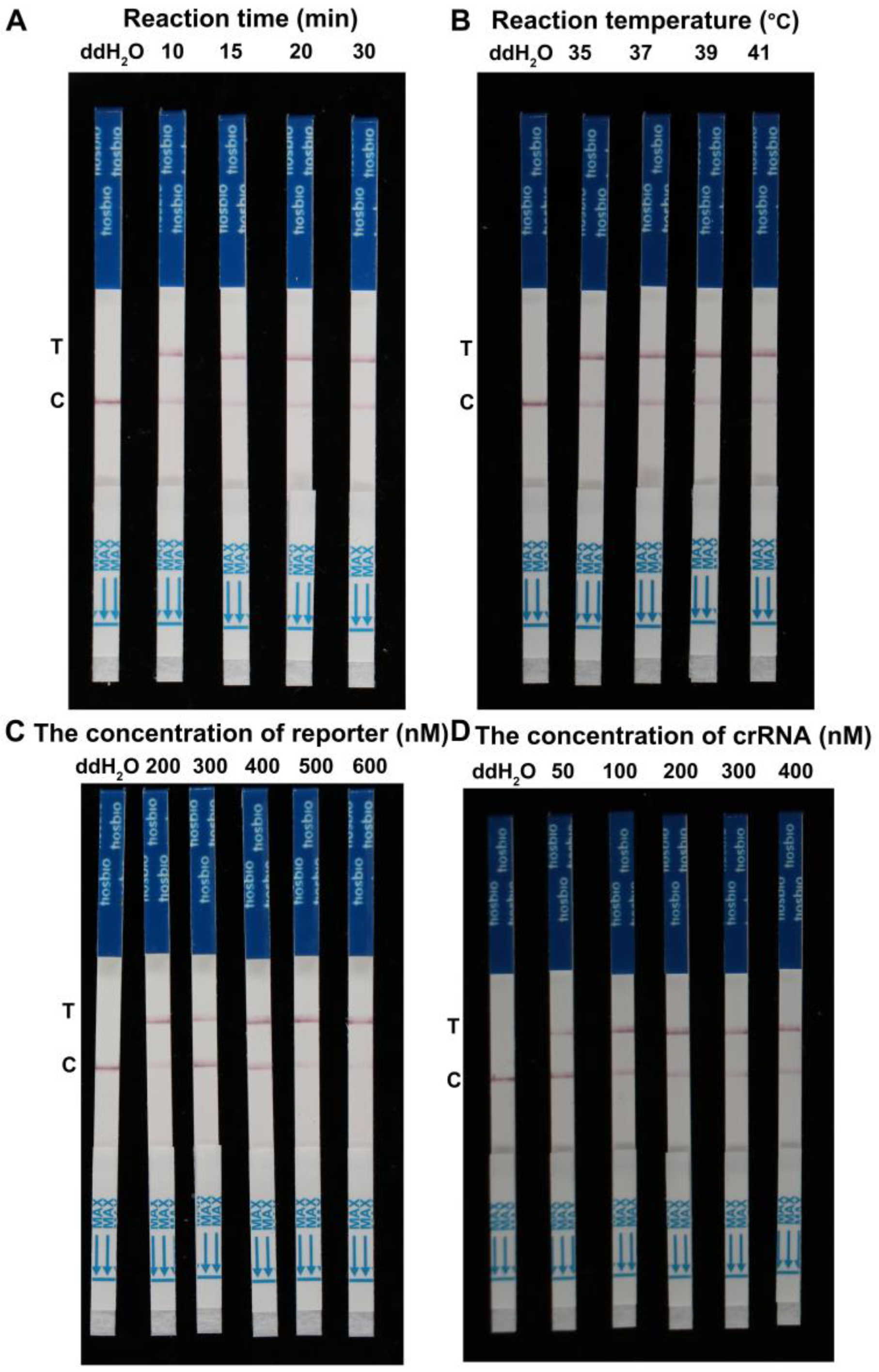
2.2. Optimal Reaction Conditions of RPA-Cas12a-LFD Assay for F. asiaticum Detection
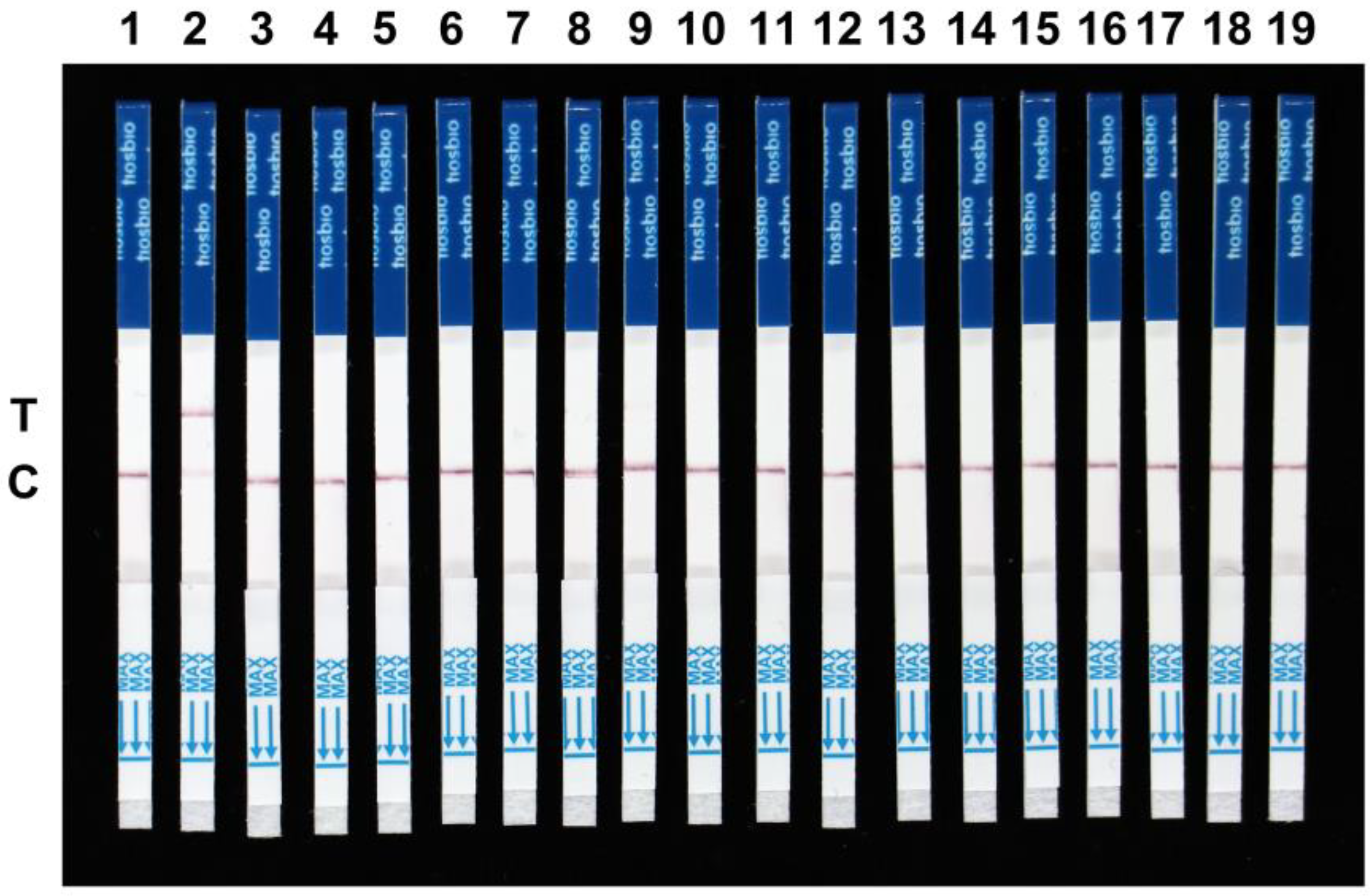
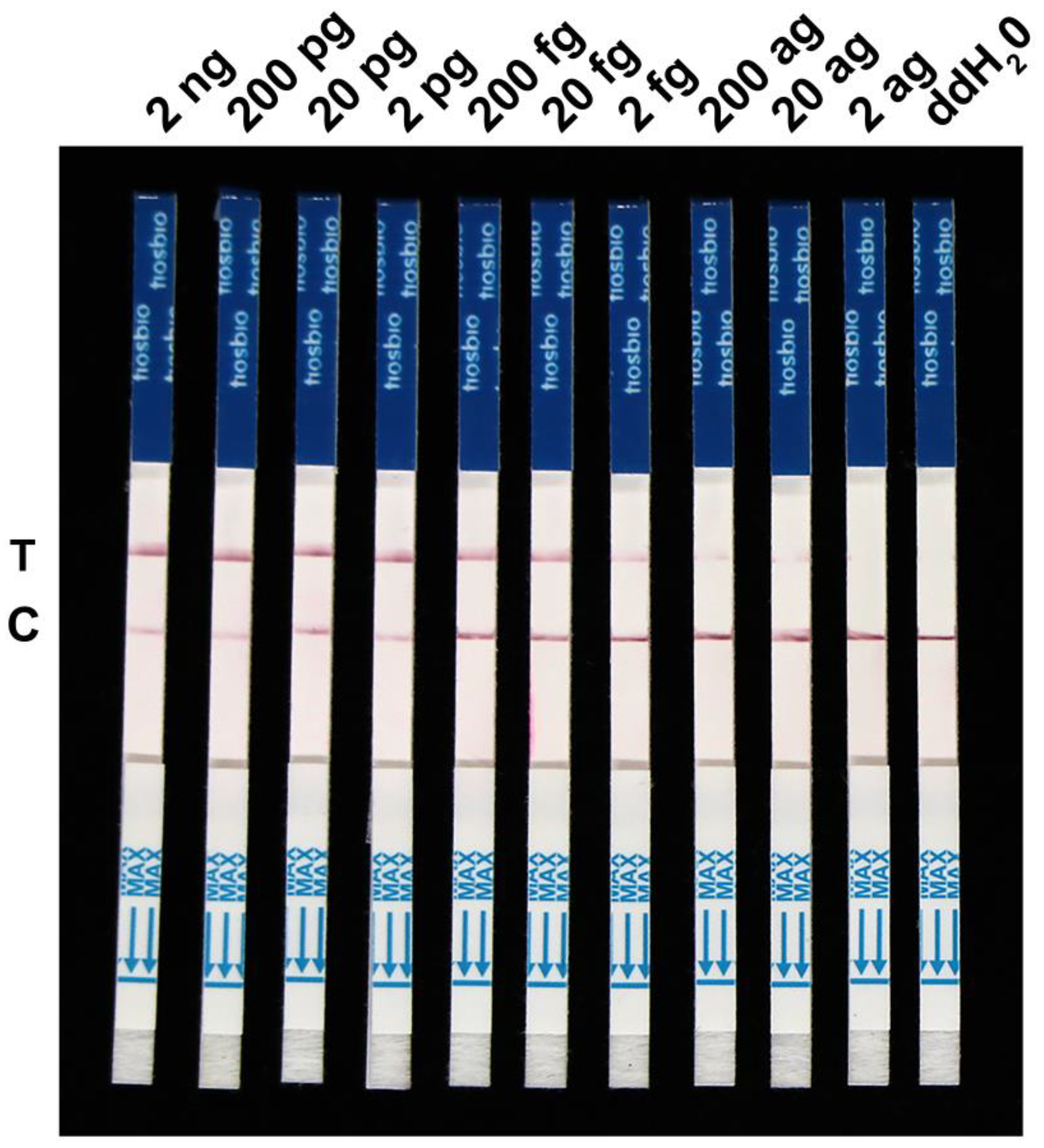
2.3. Specificity and Sensitivity Evaluation of the RPA-Cas12a-LFD Assay for F. asiaticum Detection
2.4. Early Diagnosis of F. asiaticum Infection in Wheat and Maize
3. Discussion
4. Materials and Methods
4.1. Source of Strain Preparation and DNA Extraction
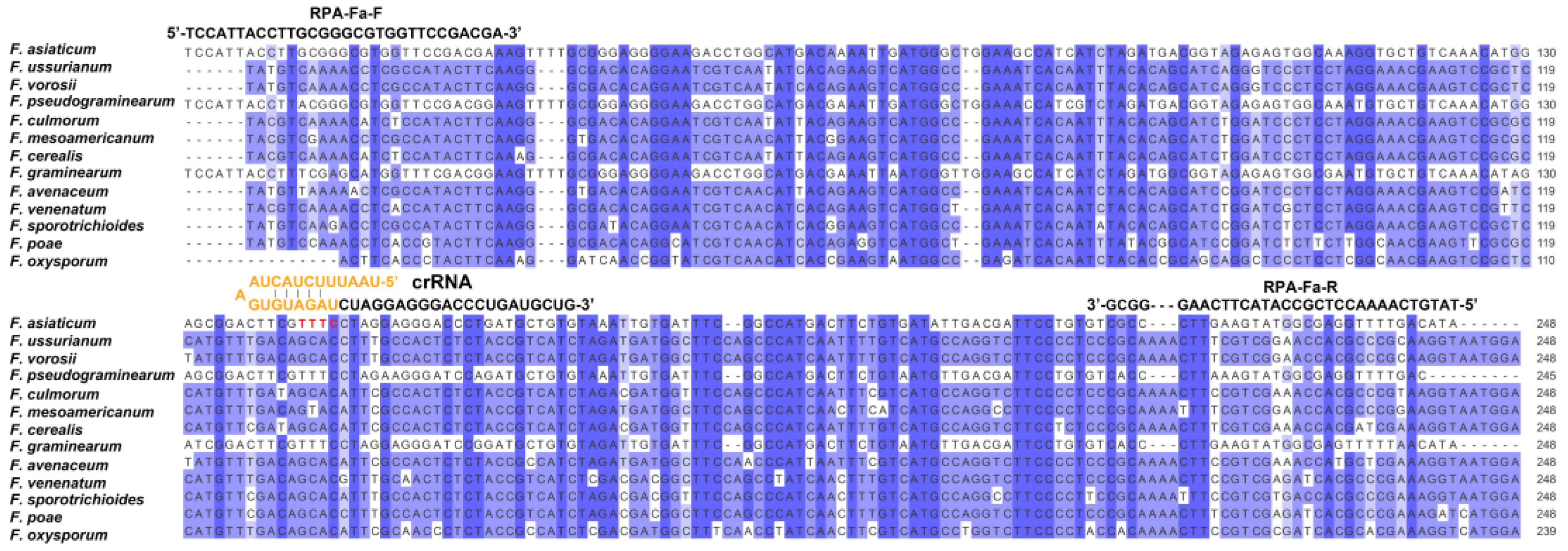
4.2. RPA Primers and CRISPR RNA Design for F. asiaticum Detection
4.3. Optimizing Reaction Conditions of RPA-Cas12a-LFD Assay for F. asiaticum Detection
4.4. CRISPR/Cas12a-Based Lateral Flow Strip Assay
4.5. Specificity and Sensitivity of RPA-Cas12a-LFD Assay for F. asiaticum Detection
4.6. Detection of F. asiaticum-Infected Wheat and Maize Samples by RPA-Cas12a-LFD Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goswami, R.S.; Kistler, H.C. Heading for disaster: Fusarium graminearum on cereal crops. Mol. Plant Pathol. 2004, 5, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Sarver, B.A.J.; Ward, T.J.; Gale, L.R.; Broz, K.; Corby Kistler, H.; Aoki, T.; Nicholson, P.; Carter, J.; O’Donnell, K. Novel Fusarium head blight pathogens from Nepal and Louisiana revealed by multilocus genealogical concordance. Fungal Genet. Biol. 2011, 48, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Wegulo, S.N.; Baenziger, P.S.; Hernandez Nopsa, J.; Bockus, W.W.; Hallen-Adams, H. Management of Fusarium head blight of wheat and barley. Crop Prot. 2015, 73, 100–107. [Google Scholar] [CrossRef]
- van der Lee, T.; Zhang, H.; van Diepeningen, A.; Waalwijk, C. Biogeography of Fusarium graminearum species complex and chemotypes: A review. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2015, 32, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Van der Lee, T.; Waalwijk, C.; Chen, W.; Xu, J.; Xu, J.; Zhang, Y.; Feng, J. Population analysis of the Fusarium graminearum species complex from wheat in China show a shift to more aggressive isolates. PLoS ONE 2012, 7, e31722. [Google Scholar] [CrossRef]
- Xu, F.; Liu, W.; Song, Y.; Zhou, Y.; Xu, X.; Yang, G.; Wang, J.; Zhang, J.; Liu, L. The distribution of Fusarium graminearum and Fusarium asiaticum causing Fusarium Head Blight of wheat in relation to climate and cropping system. Plant Dis. 2021, 105, 2830–2835. [Google Scholar] [CrossRef]
- Yin, Y.; Liu, X.; Li, B.; Ma, Z. Characterization of sterol demethylation inhibitor-resistant isolates of Fusarium asiaticum and F. graminearum collected from wheat in China. Phytopathology 2009, 99, 487–497. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, H.; van der Lee, T.A.J.; Waalwijk, C.; van Diepeningen, A.D.; Feng, J.; Brankovics, B.; Chen, W. Population genomic analysis reveals a highly conserved mitochondrial genome in Fusarium asiaticum. Front Microbiol. 2020, 11, 839. [Google Scholar] [CrossRef]
- Polak-Śliwińska, M.; Paszczyk, B. Trichothecenes in food and feed, relevance to human and animal health and methods of detection: A systematic review. Molecules 2021, 26, 454. [Google Scholar] [CrossRef]
- Wang, S.; Cui, H.; Chen, M.; Wu, Y.; Wang, S. Quantitative PCR assays for the species-specific detection of Fusarium graminearum sensu stricto and Fusarium asiaticum in winter wheat growing regions in China. Int. J. Food Microbiol. 2023, 387, 110061. [Google Scholar] [CrossRef]
- Xu, M.; Ye, W.; Zeng, D.; Wang, Y.; Zheng, X. Rapid diagnosis of wheat head blight caused by Fusarium asiaticum using a loop-mediated isothermal amplification assay. Australas Plant Path. 2017, 46, 261–266. [Google Scholar] [CrossRef]
- Soroka, M.; Wasowicz, B.; Rymaszewska, A. Loop-mediated isothermal amplification (LAMP): The better sibling of PCR? Cells 2021, 10, 1931. [Google Scholar] [CrossRef]
- Lobato, I.M.; O’Sullivan, C.K. Recombinase polymerase amplification: Basics, applications and recent advances. Trends Analyt. Chem. 2018, 98, 19–35. [Google Scholar] [CrossRef]
- Chen, J.S.; Ma, E.; Harrington, L.B.; Da Costa, M.; Tian, X.; Palefsky, J.M.; Doudna, J.A. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science 2018, 360, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Gootenberg, J.S.; Abudayyeh, O.O.; Kellner, M.J.; Joung, J.; Collins, J.J.; Zhang, F. Multiplexed and portable nucleic acid detection platform with Cas13, Cas12a, and Csm6. Science 2018, 360, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-Y.; Cheng, Q.-X.; Wang, J.-M.; Li, X.-Y.; Zhang, Z.-L.; Gao, S.; Cao, R.-B.; Zhao, G.-P.; Wang, J. CRISPR-Cas12a-assisted nucleic acid detection. Cell Discov. 2018, 4, 20. [Google Scholar] [CrossRef]
- Li, L.; Duan, C.; Weng, J.; Qi, X.; Liu, C.; Li, X.; Zhu, J.; Xie, C. A field-deployable method for single and multiplex detection of DNA or RNA from pathogens using Cas12 and Cas13. Sci. China Life Sci. 2022, 65, 1456–1465. [Google Scholar] [CrossRef]
- Haile, J.K.; N’Diaye, A.; Walkowiak, S.; Nilsen, K.T.; Clarke, J.M.; Kutcher, H.R.; Steiner, B.; Buerstmayr, H.; Pozniak, C.J. Fusarium head blight in durum wheat: Recent status, breeding directions, and future research prospects. Phytopathology 2019, 109, 1664–1675. [Google Scholar] [CrossRef]
- Wang, H.; Sun, S.; Ge, W.; Zhao, L.; Hou, B.; Wang, K.; Lyu, Z.; Chen, L.; Xu, S.; Guo, J.; et al. Horizontal gene transfer of Fhb7 from fungus underlies Fusarium head blight resistance in wheat. Science 2020, 368, eaba5435. [Google Scholar] [CrossRef] [PubMed]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; Armes, N.A. DNA Detection Using Recombination Proteins. PLoS Biol. 2006, 4, e204. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.; Hu, T.; Yang, X.; Shen, D.; Jiao, B.; Tian, W.; Xu, Y. A recombinase polymerase amplification-lateral flow dipstick assay for rapid detection of the quarantine citrus pathogen in China, Phytophthora hibernalis. PeerJ 2019, 7, e8083. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, D.K.; Kokane, S.B.; Kokane, A.D.; Warghane, A.J.; Motghare, M.R.; Bhose, S.; Sharma, A.K.; Reddy, M.K. Development of a recombinase polymerase based isothermal amplification combined with lateral flow assay (HLB-RPA-LFA) for rapid detection of “Candidatus Liberibacter asiaticus”. PLoS ONE 2018, 13, e0208530. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiong, E.; Tian, T.; Cheng, M.; Lin, W.; Wang, H.; Zhang, G.; Sun, J.; Zhou, X. Clustered regularly interspaced short palindromic repeats/Cas9-mediated lateral flow nucleic acid assay. ACS Nano 2020, 14, 2497–2508. [Google Scholar] [CrossRef]
- Danks, C.; Barker, I. On-site detection of plant pathogens using lateral-flow devices. EPPO Bull. 2000, 30, 421–426. [Google Scholar] [CrossRef]
- Kaminski, M.M.; Abudayyeh, O.O.; Gootenberg, J.S.; Zhang, F.; Collins, J.J. CRISPR-based diagnostics. Nat. Biomed. Eng. 2021, 5, 643–656. [Google Scholar] [CrossRef]
- Lei, R.; Li, Y.; Li, L.; Wang, J.; Cui, Z.; Ju, R.; Jiang, L.; Liao, X.; Wu, P.; Wang, X. A CRISPR/Cas12a-based portable platform for rapid detection of Leptosphaeria maculans in Brassica crops. Front. Plant Sci. 2022, 13, 976510. [Google Scholar] [CrossRef]
- Bai, J.; Lin, H.; Li, H.; Zhou, Y.; Liu, J.; Zhong, G.; Wu, L.; Jiang, W.; Du, H.; Yang, J.; et al. Cas12a-based on-site and rapid nucleic acid detection of African Swine Fever. Front. Microbiol. 2019, 10, 2830. [Google Scholar] [CrossRef]
- Fernández-Ortuño, D.; Loza-Reyes, E.; Atkins, S.L.; Fraaije, B.A. The CYP51C gene, a reliable marker to resolve interspecific phylogenetic relationships within the Fusarium species complex and a novel target for species-specific PCR. Int. J. Food Microbiol. 2010, 144, 301–309. [Google Scholar] [CrossRef]
- Germini, A.; Masola, A.; Carnevali, P.; Marchelli, R. Simultaneous detection of Escherichia coli O175:H7, Salmonella spp., and Listeria monocytogenes by multiplex PCR. Food Control 2009, 20, 733–738. [Google Scholar] [CrossRef]
- Sopko, B.; Zitek, J.; Nesvorna, M.; Markovic, M.; Kamler, M.; Titera, D.; Erban, T.; Hubert, J. Detection and quantification of Melissococcus plutonius in honey bee workers exposed to European foulbrood in Czechia through conventional PCR, qPCR, and barcode sequencing. J. Apicult. Res. 2020, 59, 503–514. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.B.; Song, B.; Li, H.P.; Xu, H.Q.; Qu, B.; Dang, F.J.; Liao, Y.C. Resistance to Fusarium head blight and seedling blight in wheat is associated with activation of a cytochrome P450 gene. Phytopathology 2010, 100, 183–191. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Liang, X.; Zhang, H.; Ishfaq, S.; Xi, K.; Zhou, X.; Yang, X.; Guo, W. Rapid and Sensitive Detection of Toxigenic Fusarium asiaticum Integrating Recombinase Polymerase Amplification, CRISPR/Cas12a, and Lateral Flow Techniques. Int. J. Mol. Sci. 2023, 24, 14134. https://doi.org/10.3390/ijms241814134
Zhang J, Liang X, Zhang H, Ishfaq S, Xi K, Zhou X, Yang X, Guo W. Rapid and Sensitive Detection of Toxigenic Fusarium asiaticum Integrating Recombinase Polymerase Amplification, CRISPR/Cas12a, and Lateral Flow Techniques. International Journal of Molecular Sciences. 2023; 24(18):14134. https://doi.org/10.3390/ijms241814134
Chicago/Turabian StyleZhang, Jun, Xiaoyan Liang, Hao Zhang, Shumila Ishfaq, Kaifei Xi, Xueping Zhou, Xiuling Yang, and Wei Guo. 2023. "Rapid and Sensitive Detection of Toxigenic Fusarium asiaticum Integrating Recombinase Polymerase Amplification, CRISPR/Cas12a, and Lateral Flow Techniques" International Journal of Molecular Sciences 24, no. 18: 14134. https://doi.org/10.3390/ijms241814134
APA StyleZhang, J., Liang, X., Zhang, H., Ishfaq, S., Xi, K., Zhou, X., Yang, X., & Guo, W. (2023). Rapid and Sensitive Detection of Toxigenic Fusarium asiaticum Integrating Recombinase Polymerase Amplification, CRISPR/Cas12a, and Lateral Flow Techniques. International Journal of Molecular Sciences, 24(18), 14134. https://doi.org/10.3390/ijms241814134






