Advanced Techniques Using In Vivo Electroporation to Study the Molecular Mechanisms of Cerebral Development Disorders
Abstract
1. Introduction
2. Basic Technique for IUE
2.1. Developmental Stage and Animals
2.2. Selection of Promoters for Exogenous Gene Expression
2.2.1. Ubiquitous Promoters
2.2.2. Specific Promoters
2.3. Pathological Mutant Analysis by IUE
2.4. Loss-of-Function Studies to Investigate the Pathology of Brain Development Disorders
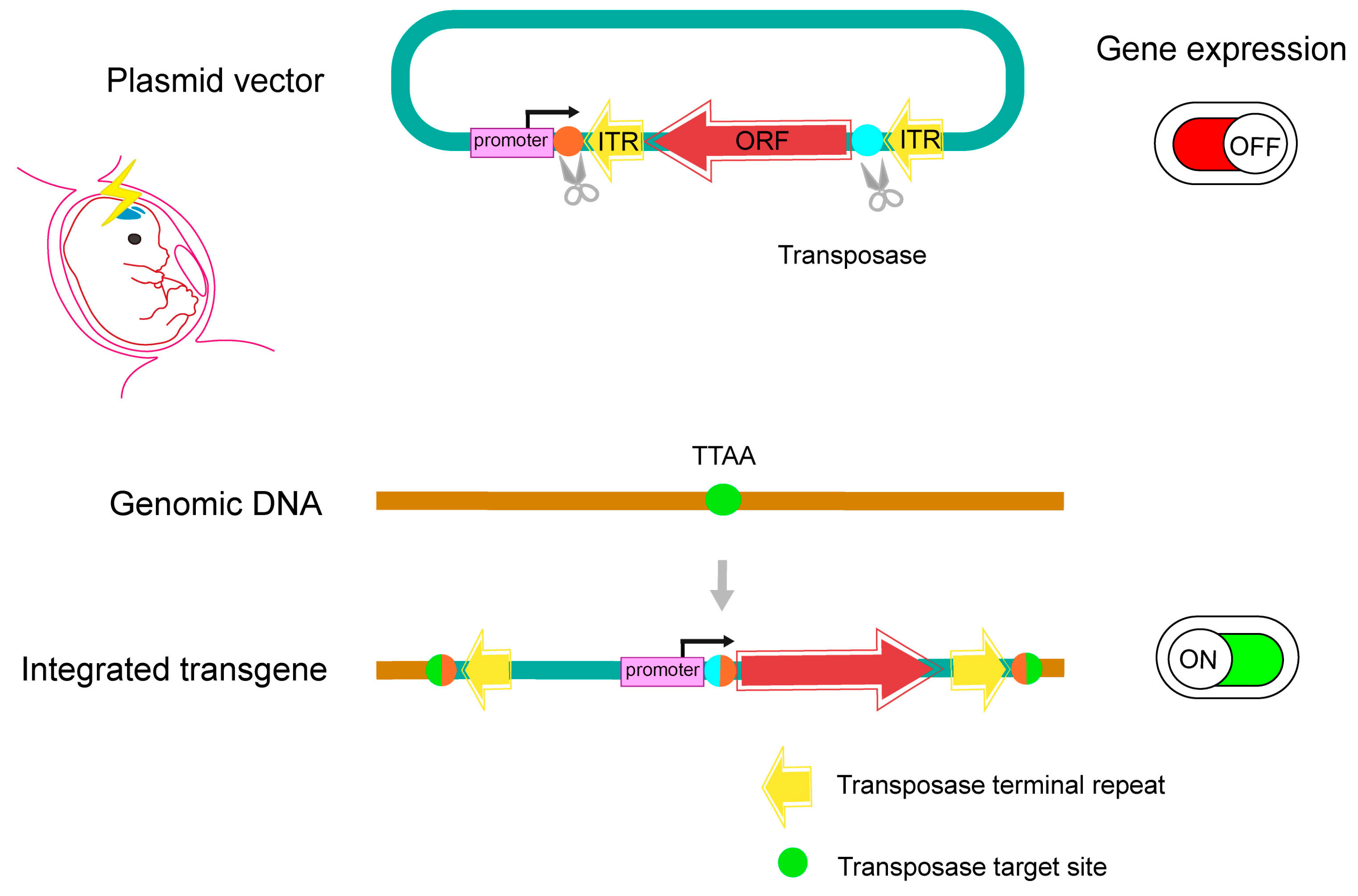
2.5. Application of Electroporation to Organoid Disease Model
2.6. Limitations of IUE
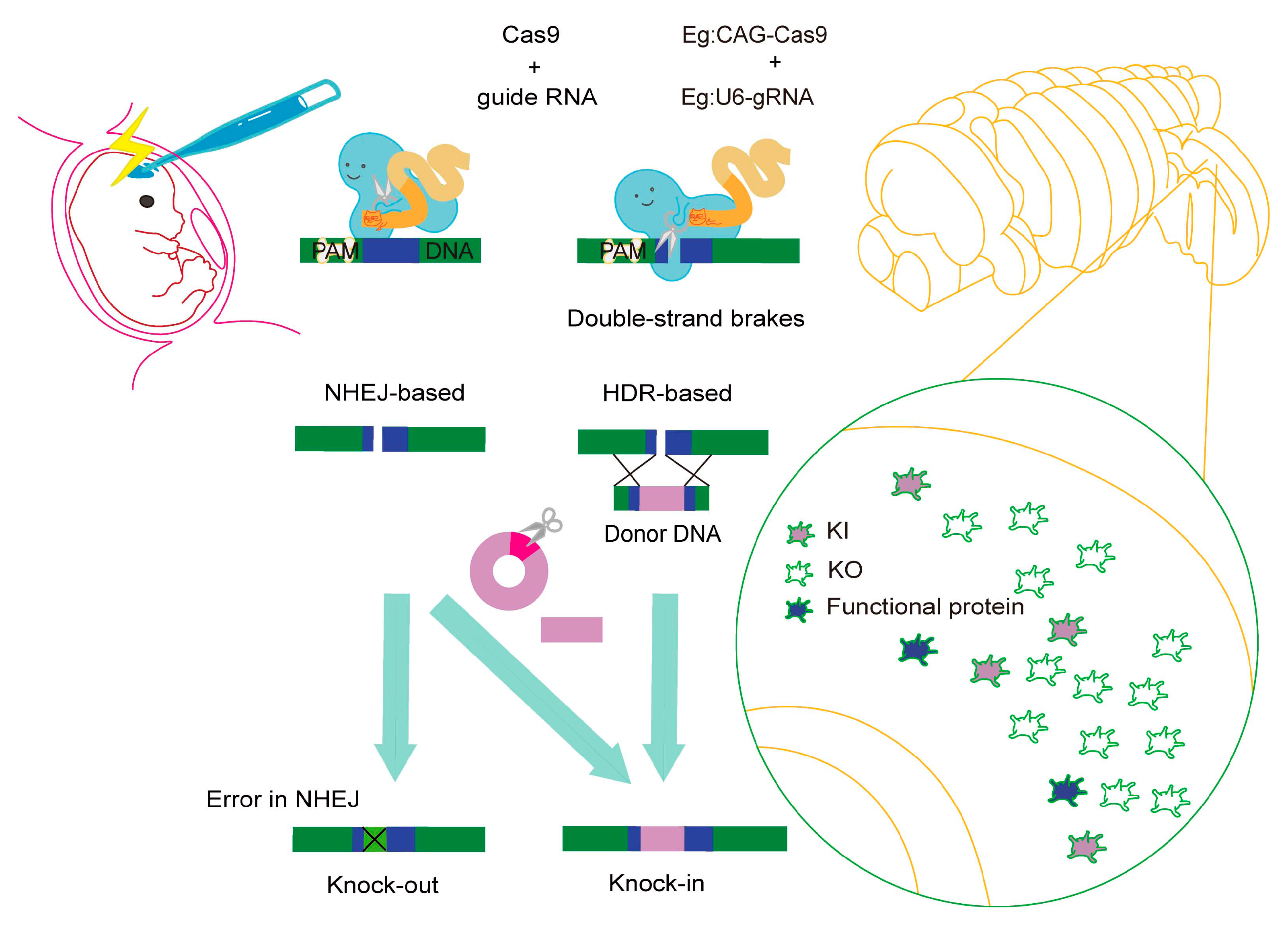
3. Genome Editing by IUE
3.1. Knockout (KO)
3.2. Knock-In (KI)
3.3. In Vivo Epigenetic Editing in a Brain Disease Model
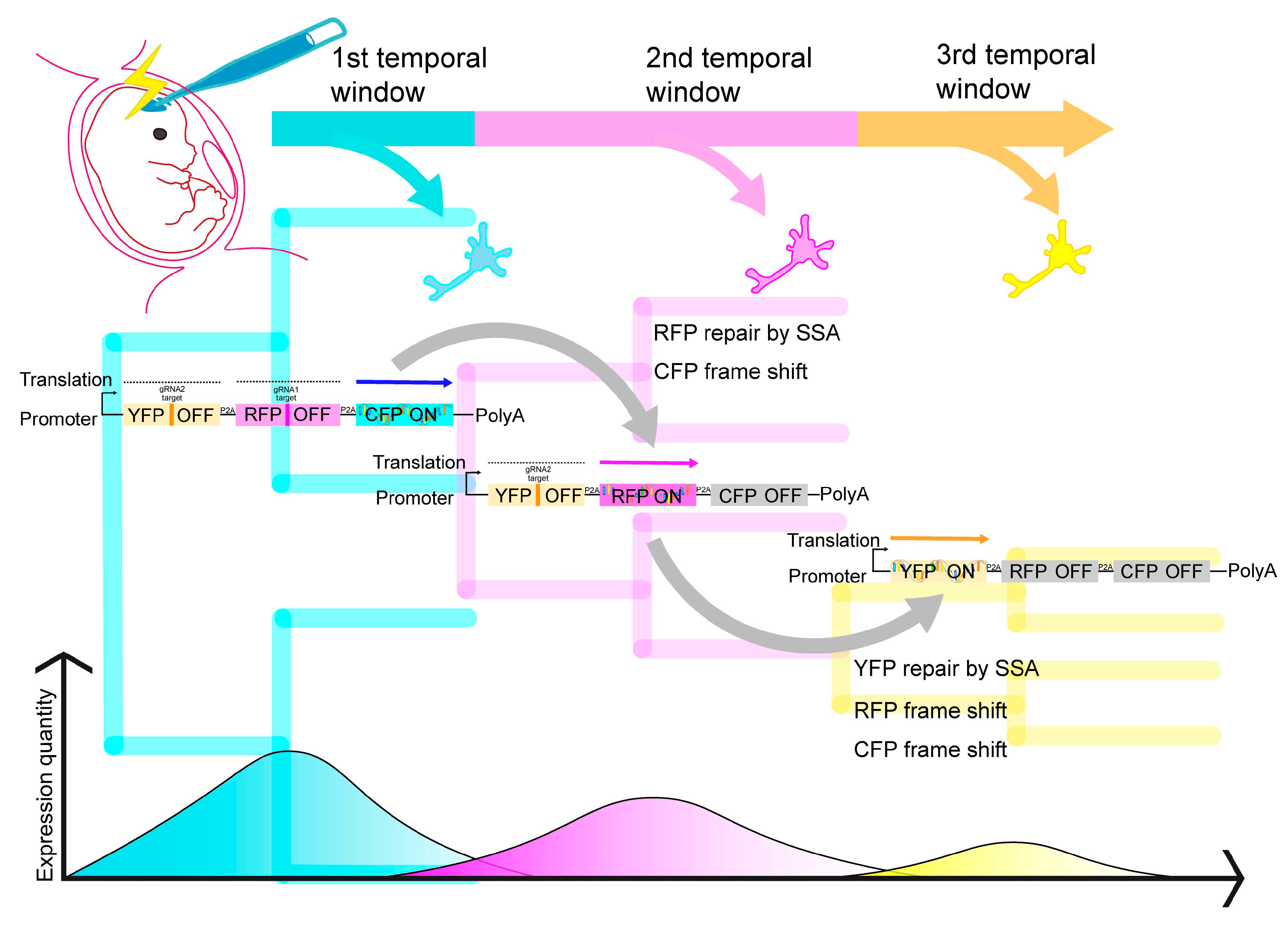
4. Spatiotemporal Expression Control and Lineage Tracing by IUE
4.1. Sparse Labeling and Live Imaging
4.2. iON Expression Switch
4.3. TEMPO
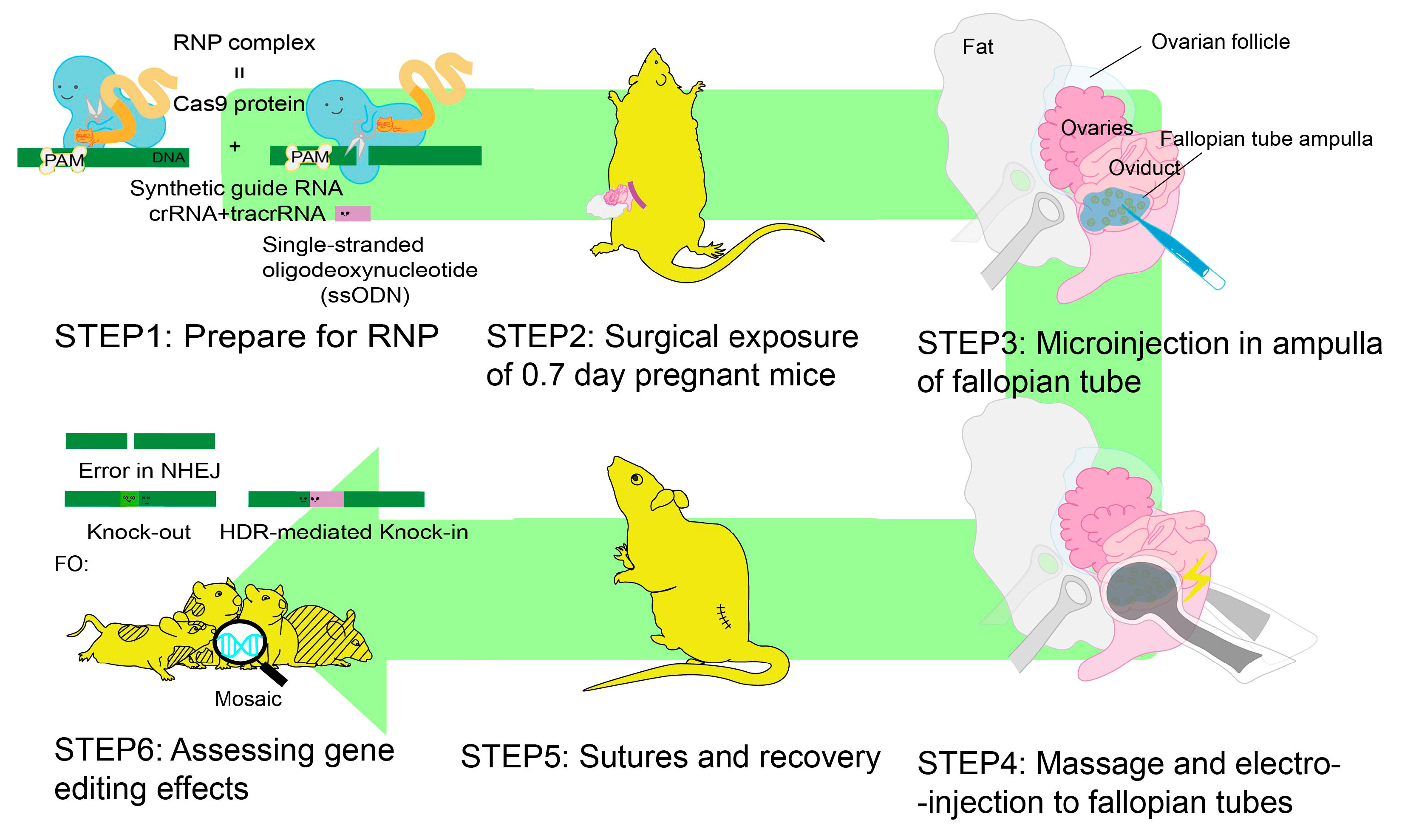
5. iGONAD
6. Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawaguchi, A. Temporal patterning of neocortical progenitor cells: How do they know the right time? Neurosci. Res. 2019, 138, 3–11. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, J.; Shen, Z.; Ma, J.; Simons, B.D.; Shi, S.H. Behavior and lineage progression of neural progenitors in the mammalian cortex. Curr. Opin. Neurobiol. 2021, 66, 144–157. [Google Scholar] [CrossRef]
- Hippenmeyer, S. Principles of neural stem cell lineage progression: Insights from developing cerebral cortex. Curr. Opin. Neurobiol. 2023, 79, 102695. [Google Scholar] [CrossRef]
- Jabaudon, D. Fate and freedom in developing neocortical circuits. Nat. Commun. 2017, 8, 16042. [Google Scholar] [CrossRef]
- Cadwell, C.R.; Bhaduri, A.; Mostajo-Radji, M.A.; Keefe, M.G.; Nowakowski, T.J. Development and Arealization of the Cerebral Cortex. Neuron 2019, 103, 980–1004. [Google Scholar] [CrossRef]
- Klingler, E. Temporal controls over cortical projection neuron fate diversity. Curr. Opin. Neurobiol. 2023, 79, 102677. [Google Scholar] [CrossRef]
- Bizzotto, S.; Walsh, C.A. Genetic mosaicism in the human brain: From lineage tracing to neuropsychiatric disorders. Nat. Rev. Neurosci. 2022, 23, 275–286. [Google Scholar] [CrossRef]
- Saito, T.; Nakatsuji, N. Efficient gene transfer into the embryonic mouse brain using in vivo electroporation. Dev. Biol. 2001, 240, 237–246. [Google Scholar] [CrossRef]
- Tabata, H.; Nakajima, K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: Visualization of neuronal migration in the developing cortex. Neuroscience 2001, 103, 865–872. [Google Scholar] [CrossRef]
- Kittock, C.M.; Pilaz, L.J. Advances in in utero electroporation. Dev. Neurobiol. 2023, 83, 73–90. [Google Scholar] [CrossRef]
- Espinosa-Medina, I.; Feliciano, D.; Belmonte-Mateos, C.; Linda Miyares, R.; Garcia-Marques, J.; Foster, B.; Lindo, S.; Pujades, C.; Koyama, M.; Lee, T. TEMPO enables sequential genetic labeling and manipulation of vertebrate cell lineages. Neuron 2023, 111, 345–361.e310. [Google Scholar] [CrossRef]
- McGrail, M.; Sakuma, T.; Bleris, L. Genome editing. Sci. Rep. 2022, 12, 20497. [Google Scholar] [CrossRef]
- Nishiyama, J. Genome editing in the mammalian brain using the CRISPR-Cas system. Neurosci. Res. 2019, 141, 4–12. [Google Scholar] [CrossRef]
- Sato, M.; Miyagasako, R.; Takabayashi, S.; Ohtsuka, M.; Hatada, I.; Horii, T. Sequential i-GONAD: An Improved In Vivo Technique for CRISPR/Cas9-Based Genetic Manipulations in Mice. Cells 2020, 9, 546. [Google Scholar] [CrossRef]
- Ohtsuka, M.; Sato, M.; Miura, H.; Takabayashi, S.; Matsuyama, M.; Koyano, T.; Arifin, N.; Nakamura, S.; Wada, K.; Gurumurthy, C.B. i-GONAD: A robust method for in situ germline genome engineering using CRISPR nucleases. Genome Biol. 2018, 19, 25. [Google Scholar] [CrossRef]
- Szczurkowska, J.; Cwetsch, A.W.; dal Maschio, M.; Ghezzi, D.; Ratto, G.M.; Cancedda, L. Targeted in vivo genetic manipulation of the mouse or rat brain by in utero electroporation with a triple-electrode probe. Nat. Protoc. 2016, 11, 399–412. [Google Scholar] [CrossRef]
- Kumamoto, T.; Ohtaka-Maruyama, C. Visualizing Cortical Development and Evolution: A Toolkit Update. Front. Neurosci. 2022, 16, 876406. [Google Scholar] [CrossRef]
- Shimogori, T.; Banuchi, V.; Ng, H.Y.; Strauss, J.B.; Grove, E.A. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development 2004, 131, 5639–5647. [Google Scholar] [CrossRef]
- Kawaue, T.; Shitamukai, A.; Nagasaka, A.; Tsunekawa, Y.; Shinoda, T.; Saito, K.; Terada, R.; Bilgic, M.; Miyata, T.; Matsuzaki, F.; et al. Lzts1 controls both neuronal delamination and outer radial glial-like cell generation during mammalian cerebral development. Nat. Commun. 2019, 10, 2780. [Google Scholar] [CrossRef]
- Okamoto, M.; Miyata, T.; Konno, D.; Ueda, H.R.; Kasukawa, T.; Hashimoto, M.; Matsuzaki, F.; Kawaguchi, A. Cell-cycle-independent transitions in temporal identity of mammalian neural progenitor cells. Nat. Commun. 2016, 7, 11349. [Google Scholar] [CrossRef]
- Mizutani, K.; Saito, T. Progenitors resume generating neurons after temporary inhibition of neurogenesis by Notch activation in the mammalian cerebral cortex. Development 2005, 132, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Watanabe, Y.; Funahashi, J. Misexpression of genes in brain vesicles by in ovo electroporation. Dev. Growth Differ. 2000, 42, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, A.; Borrell, V. A protocol for in ovo electroporation of chicken and snake embryos to study forebrain development. STAR Protoc. 2021, 2, 100692. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Gotoh, H.; Ono, K. Changes in the regulation of cortical neurogenesis contribute to encephalization during amniote brain evolution. Nat. Commun. 2013, 4, 2206. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, A.; Villalba, A.; de Juan Romero, C.; Pico, E.; Kyrousi, C.; Tzika, A.C.; Tessier-Lavigne, M.; Ma, L.; Drukker, M.; Cappello, S.; et al. Evolution of Cortical Neurogenesis in Amniotes Controlled by Robo Signaling Levels. Cell 2018, 174, 590–606.e521. [Google Scholar] [CrossRef]
- Paolino, A.; Fenlon, L.R.; Kozulin, P.; Richards, L.J.; Suarez, R. Multiple events of gene manipulation via in pouch electroporation in a marsupial model of mammalian forebrain development. J. Neurosci. Methods 2018, 293, 45–52. [Google Scholar] [CrossRef]
- Hatakeyama, J.; Sato, H.; Shimamura, K. Developing guinea pig brain as a model for cortical folding. Dev. Growth Differ. 2017, 59, 286–301. [Google Scholar] [CrossRef]
- Matsui, A.; Tran, M.; Yoshida, A.C.; Kikuchi, S.S.; Mami, U.; Ogawa, M.; Shimogori, T. BTBD3 controls dendrite orientation toward active axons in mammalian neocortex. Science 2013, 342, 1114–1118. [Google Scholar] [CrossRef]
- Kawasaki, H.; Iwai, L.; Tanno, K. Rapid and efficient genetic manipulation of gyrencephalic carnivores using in utero electroporation. Mol. Brain 2012, 5, 24. [Google Scholar] [CrossRef]
- Kawaguchi, A.; Miyata, T.; Sawamoto, K.; Takashita, N.; Murayama, A.; Akamatsu, W.; Ogawa, M.; Okabe, M.; Tano, Y.; Goldman, S.A.; et al. Nestin-EGFP transgenic mice: Visualization of the self-renewal and multipotency of CNS stem cells. Mol. Cell Neurosci. 2001, 17, 259–273. [Google Scholar] [CrossRef]
- Hamabe-Horiike, T.; Kawasaki, K.; Sakashita, M.; Ishizu, C.; Yoshizaki, T.; Harada, S.I.; Ogawa-Ochiai, K.; Shinmyo, Y.; Kawasaki, H. Glial cell type-specific gene expression in the mouse cerebrum using the piggyBac system and in utero electroporation. Sci. Rep. 2021, 11, 4864. [Google Scholar] [CrossRef] [PubMed]
- Schmid, R.S.; Yokota, Y.; Anton, E.S. Generation and characterization of brain lipid-binding protein promoter-based transgenic mouse models for the study of radial glia. Glia 2006, 53, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Yamaguchi, Y.; Nonomura, K.; Kawakami, K.; Takahashi, Y.; Miura, M. Simultaneous expression of different transgenes in neurons and glia by combining in utero electroporation with the Tol2 transposon-mediated gene transfer system. Genes. Cells 2010, 15, 501–512. [Google Scholar] [CrossRef]
- Kawaue, T.; Sagou, K.; Kiyonari, H.; Ota, K.; Okamoto, M.; Shinoda, T.; Kawaguchi, A.; Miyata, T. Neurogenin2-d4Venus and Gadd45g-d4Venus transgenic mice: Visualizing mitotic and migratory behaviors of cells committed to the neuronal lineage in the developing mammalian brain. Dev. Growth Differ. 2014, 56, 293–304. [Google Scholar] [CrossRef]
- Sawamoto, K.; Yamamoto, A.; Kawaguchi, A.; Yamaguchi, M.; Mori, K.; Goldman, S.; Okano, H. Direct isolation of committed neuronal progenitor cells from transgenic mice coexpressing spectrally distinct fluorescent proteins regulated by stage-specific neural promoters. J. Neurosci. Res. 2001, 65, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Kawaue, T.; Miyata, T. Differentiating cells mechanically limit the interkinetic nuclear migration of progenitor cells to secure apical cytogenesis. Development 2018, 145, dev162883. [Google Scholar] [CrossRef]
- Hevner, R.F.; Hodge, R.D.; Daza, R.A.; Englund, C. Transcription factors in glutamatergic neurogenesis: Conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci. Res. 2006, 55, 223–233. [Google Scholar] [CrossRef]
- Huang, L.; Yuan, Z.; Liu, P.; Zhou, T. Effects of promoter leakage on dynamics of gene expression. BMC Syst. Biol. 2015, 9, 16. [Google Scholar] [CrossRef]
- Broix, L.; Jagline, H.; Ivanova, E.; Schmucker, S.; Drouot, N.; Clayton-Smith, J.; Pagnamenta, A.T.; Metcalfe, K.A.; Isidor, B.; Louvier, U.W.; et al. Mutations in the HECT domain of NEDD4L lead to AKT-mTOR pathway deregulation and cause periventricular nodular heterotopia. Nat. Genet. 2016, 48, 1349–1358. [Google Scholar] [CrossRef]
- Baek, S.T.; Copeland, B.; Yun, E.J.; Kwon, S.K.; Guemez-Gamboa, A.; Schaffer, A.E.; Kim, S.; Kang, H.C.; Song, S.; Mathern, G.W.; et al. An AKT3-FOXG1-reelin network underlies defective migration in human focal malformations of cortical development. Nat. Med. 2015, 21, 1445–1454. [Google Scholar] [CrossRef]
- Khadka, P.; Reitman, Z.J.; Lu, S.; Buchan, G.; Gionet, G.; Dubois, F.; Carvalho, D.M.; Shih, J.; Zhang, S.; Greenwald, N.F.; et al. PPM1D mutations are oncogenic drivers of de novo diffuse midline glioma formation. Nat. Commun. 2022, 13, 604. [Google Scholar] [CrossRef]
- Weng, Y.T.; Chien, T.; Kuan, I.I.; Chern, Y. The TRAX, DISC1, and GSK3 complex in mental disorders and therapeutic interventions. J. Biomed. Sci. 2018, 25, 71. [Google Scholar] [CrossRef] [PubMed]
- Hikida, T.; Gamo, N.J.; Sawa, A. DISC1 as a therapeutic target for mental illnesses. Expert Opin. Ther. Targets 2012, 1612, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Ge, X.; Frank, C.L.; Madison, J.M.; Koehler, A.N.; Doud, M.K.; Tassa, C.; Berry, E.M.; Soda, T.; Singh, K.K.; et al. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell 2009, 136, 1017–1031. [Google Scholar] [CrossRef] [PubMed]
- Steinecke, A.; Gampe, C.; Nitzsche, F.; Bolz, J. DISC1 knockdown impairs the tangential migration of cortical interneurons by affecting the actin cytoskeleton. Front. Cell Neurosci. 2014, 8, 190. [Google Scholar] [CrossRef]
- Vomund, S.; de Souza Silva, M.A.; Huston, J.P.; Korth, C. Behavioral Resilience and Sensitivity to Locally Restricted Cortical Migration Deficits Induced by In Utero Knockdown of Disabled-1 in the Adult Rat. Cereb. Cortex 2017, 27, 2052–2063. [Google Scholar] [CrossRef][Green Version]
- Rao, M.K.; Wilkinson, M.F. Tissue-specific and cell type-specific RNA interference in vivo. Nat. Protoc. 2006, 1, 1494–1501. [Google Scholar] [CrossRef]
- Di Lullo, E.; Kriegstein, A.R. The use of brain organoids to investigate neural development and disease. Nat. Rev. Neurosci. 2017, 18, 573–584. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Denoth-Lippuner, A.; Royall, L.N.; Gonzalez-Bohorquez, D.; Machado, D.; Jessberger, S. Injection and electroporation of plasmid DNA into human cortical organoids. STAR Protoc. 2022, 3, 101129. [Google Scholar] [CrossRef]
- Tynianskaia, L.; Esiyok, N.; Huttner, W.B.; Heide, M. Targeted Microinjection and Electroporation of Primate Cerebral Organoids for Genetic Modification. J. Vis. Exp. 2023, 193, e65176. [Google Scholar] [CrossRef]
- Ogawa, J.; Pao, G.M.; Shokhirev, M.N.; Verma, I.M. Glioblastoma Model Using Human Cerebral Organoids. Cell Rep. 2018, 23, 1220–1229. [Google Scholar] [CrossRef]
- Klaus, J.; Kanton, S.; Kyrousi, C.; Ayo-Martin, A.C.; Di Giaimo, R.; Riesenberg, S.; O’Neill, A.C.; Camp, J.G.; Tocco, C.; Santel, M.; et al. Altered neuronal migratory trajectories in human cerebral organoids derived from individuals with neuronal heterotopia. Nat. Med. 2019, 25, 561–568. [Google Scholar] [CrossRef]
- Giandomenico, S.L.; Mierau, S.B.; Gibbons, G.M.; Wenger, L.M.D.; Masullo, L.; Sit, T.; Sutcliffe, M.; Boulanger, J.; Tripodi, M.; Derivery, E.; et al. Cerebral organoids at the air-liquid interface generate diverse nerve tracts with functional output. Nat. Neurosci. 2019, 22, 669–679. [Google Scholar] [CrossRef]
- Rocha-Martins, M.; Nerli, E.; Kretzschmar, J.; Weigert, M.; Icha, J.; Myers, E.W.; Norden, C. Neuronal migration prevents spatial competition in retinal morphogenesis. Nature 2023, 620, 615–624. [Google Scholar] [CrossRef]
- Yamashiro, K.; Ikegaya, Y.; Matsumoto, N. In Utero Electroporation for Manipulation of Specific Neuronal Populations. Membranes 2022, 12, 513. [Google Scholar] [CrossRef]
- Tian, L.; Hires, S.A.; Mao, T.; Huber, D.; Chiappe, M.E.; Chalasani, S.H.; Petreanu, L.; Akerboom, J.; McKinney, S.A.; Schreiter, E.R.; et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 2009, 6, 875–881. [Google Scholar] [CrossRef]
- Gee, J.M.; Gibbons, M.B.; Taheri, M.; Palumbos, S.; Morris, S.C.; Smeal, R.M.; Flynn, K.F.; Economo, M.N.; Cizek, C.G.; Capecchi, M.R.; et al. Imaging activity in astrocytes and neurons with genetically encoded calcium indicators following in utero electroporation. Front. Mol. Neurosci. 2015, 8, 10. [Google Scholar] [CrossRef]
- Fujita, I.; Shitamukai, A.; Kusumoto, F.; Mase, S.; Suetsugu, T.; Omori, A.; Kato, K.; Abe, T.; Shioi, G.; Konno, D.; et al. Endfoot regeneration restricts radial glial state and prevents translocation into the outer subventricular zone in early mammalian brain development. Nat. Cell Biol. 2020, 22, 26–37. [Google Scholar] [CrossRef]
- Kumamoto, T.; Maurinot, F.; Barry-Martinet, R.; Vaslin, C.; Vandormael-Pournin, S.; Le, M.; Lerat, M.; Niculescu, D.; Cohen-Tannoudji, M.; Rebsam, A.; et al. Direct Readout of Neural Stem Cell Transgenesis with an Integration-Coupled Gene Expression Switch. Neuron 2020, 107, 617–630.e616. [Google Scholar] [CrossRef]
- Hattori, Y.; Miyata, T. Embryonic Neocortical Microglia Express Toll-Like Receptor 9 and Respond to Plasmid DNA Injected into the Ventricle: Technical Considerations Regarding Microglial Distribution in Electroporated Brain Walls. eNeuro 2018, 5, ENEURO.0312-18.2018. [Google Scholar] [CrossRef]
- Kalebic, N.; Taverna, E.; Tavano, S.; Wong, F.K.; Suchold, D.; Winkler, S.; Huttner, W.B.; Sarov, M. CRISPR/Cas9-induced disruption of gene expression in mouse embryonic brain and single neural stem cells in vivo. EMBO Rep. 2016, 17, 338–348. [Google Scholar] [CrossRef]
- Straub, C.; Granger, A.J.; Saulnier, J.L.; Sabatini, B.L. CRISPR/Cas9-mediated gene knock-down in post-mitotic neurons. PLoS ONE 2014, 9, e105584. [Google Scholar] [CrossRef]
- Chen, F.; Rosiene, J.; Che, A.; Becker, A.; LoTurco, J. Tracking and transforming neocortical progenitors by CRISPR/Cas9 gene targeting and piggyBac transposase lineage labeling. Development 2015, 142, 3601–3611. [Google Scholar] [CrossRef]
- Vierl, F.; Kaur, M.; Gotz, M. Non-codon Optimized PiggyBac Transposase Induces Developmental Brain Aberrations: A Call for in vivo Analysis. Front. Cell Dev. Biol. 2021, 9, 698002. [Google Scholar] [CrossRef]
- Swiech, L.; Heidenreich, M.; Banerjee, A.; Habib, N.; Li, Y.; Trombetta, J.; Sur, M.; Zhang, F. In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9. Nat. Biotechnol. 2015, 33, 102–106. [Google Scholar] [CrossRef]
- Bukhari, H.; Muller, T. Endogenous Fluorescence Tagging by CRISPR. Trends Cell Biol. 2019, 29, 912–928. [Google Scholar] [CrossRef]
- Nishizono, H.; Hayano, Y.; Nakahata, Y.; Ishigaki, Y.; Yasuda, R. Rapid generation of conditional knockout mice using the CRISPR-Cas9 system and electroporation for neuroscience research. Mol. Brain 2021, 14, 148. [Google Scholar] [CrossRef]
- Shinmyo, Y.; Hamabe-Horiike, T.; Saito, K.; Kawasaki, H. Investigation of the Mechanisms Underlying the Development and Evolution of the Cerebral Cortex Using Gyrencephalic Ferrets. Front. Cell Dev. Biol. 2022, 10, 847159. [Google Scholar] [CrossRef]
- Mikuni, T.; Nishiyama, J.; Sun, Y.; Kamasawa, N.; Yasuda, R. High-Throughput, High-Resolution Mapping of Protein Localization in Mammalian Brain by In Vivo Genome Editing. Cell 2016, 165, 1803–1817. [Google Scholar] [CrossRef]
- Tsunekawa, Y.; Terhune, R.K.; Fujita, I.; Shitamukai, A.; Suetsugu, T.; Matsuzaki, F. Developing a de novo targeted knock-in method based on in utero electroporation into the mammalian brain. Development 2016, 143, 3216–3222. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Mori, T.; Kurihara, T.; Kawase, S.; Koike, R.; Satoga, M.; Cao, X.; Li, X.; Yanagawa, T.; Sakurai, T.; et al. Fluorescent protein tagging of endogenous protein in brain neurons using CRISPR/Cas9-mediated knock-in and in utero electroporation techniques. Sci. Rep. 2016, 6, 35861. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Wang, X.; Hu, X.; Liu, Z.; Liu, J.; Zhou, H.; Shen, X.; Wei, Y.; Huang, Z.; Ying, W.; et al. Homology-mediated end joining-based targeted integration using CRISPR/Cas9. Cell Res. 2017, 27, 801–814. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Tsunekawa, Y.; Hernandez-Benitez, R.; Wu, J.; Zhu, J.; Kim, E.J.; Hatanaka, F.; Yamamoto, M.; Araoka, T.; Li, Z.; et al. In vivo genome editing via CRISPR/Cas9 mediated homology-independent targeted integration. Nature 2016, 540, 144–149. [Google Scholar] [CrossRef]
- Miura, H.; Quadros, R.M.; Gurumurthy, C.B.; Ohtsuka, M. Easi-CRISPR for creating knock-in and conditional knockout mouse models using long ssDNA donors. Nat. Protoc. 2018, 13, 195–215. [Google Scholar] [CrossRef]
- Meyerink, B.L.; Kc, P.; Tiwari, N.K.; Kittock, C.M.; Klein, A.; Evans, C.M.; Pilaz, L.J. Breasi-CRISPR: An efficient genome-editing method to interrogate protein localization and protein-protein interactions in the embryonic mouse cortex. Development 2022, 149, dev200616. [Google Scholar] [CrossRef]
- Nishiyama, J.; Mikuni, T.; Yasuda, R. Virus-Mediated Genome Editing via Homology-Directed Repair in Mitotic and Postmitotic Cells in Mammalian Brain. Neuron 2017, 96, 755–768. [Google Scholar] [CrossRef]
- Gao, Y.; Hisey, E.; Bradshaw, T.W.A.; Erata, E.; Brown, W.E.; Courtland, J.L.; Uezu, A.; Xiang, Y.; Diao, Y.; Soderling, S.H. Plug-and-Play Protein Modification Using Homology-Independent Universal Genome Engineering. Neuron 2019, 103, 583–597.e588. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, M.; Wang, X.; Ying, W.; Hu, X.; Dai, P.; Meng, F.; Shi, L.; Sun, Y.; Yao, N.; et al. Tild-CRISPR Allows for Efficient and Precise Gene Knockin in Mouse and Human Cells. Dev. Cell 2018, 45, 526–536.e525. [Google Scholar] [CrossRef]
- Tabata, H.; Sasaki, M.; Agetsuma, M.; Sano, H.; Hirota, Y.; Miyajima, M.; Hayashi, K.; Honda, T.; Nishikawa, M.; Inaguma, Y.; et al. Erratic and blood vessel-guided migration of astrocyte progenitors in the cerebral cortex. Nat. Commun. 2022, 13, 6571. [Google Scholar] [CrossRef]
- Wang, J.; He, Z.; Wang, G.; Zhang, R.; Duan, J.; Gao, P.; Lei, X.; Qiu, H.; Zhang, C.; Zhang, Y.; et al. Efficient targeted insertion of large DNA fragments without DNA donors. Nat. Methods 2022, 19, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Yarnall, M.T.N.; Ioannidi, E.I.; Schmitt-Ulms, C.; Krajeski, R.N.; Lim, J.; Villiger, L.; Zhou, W.; Jiang, K.; Garushyants, S.K.; Roberts, N.; et al. Drag-and-drop genome insertion of large sequences without double-strand DNA cleavage using CRISPR-directed integrases. Nat. Biotechnol. 2023, 41, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Lampe, G.D.; King, R.T.; Halpin-Healy, T.S.; Klompe, S.E.; Hogan, M.I.; Vo, P.L.H.; Tang, S.; Chavez, A.; Sternberg, S.H. Targeted DNA integration in human cells without double-strand breaks using CRISPR-associated transposases. Nat. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Tou, C.J.; Orr, B.; Kleinstiver, B.P. Precise cut-and-paste DNA insertion using engineered type V-K CRISPR-associated transposases. Nat. Biotechnol. 2023, 41, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.; Kalebic, N.; Florio, M.; Lakshmanaperumal, N.; Haffner, C.; Brandl, H.; Henry, I.; Huttner, W.B. Epigenome profiling and editing of neocortical progenitor cells during development. EMBO J. 2017, 36, 2642–2658. [Google Scholar] [CrossRef]
- Tanenbaum, M.E.; Gilbert, L.A.; Qi, L.S.; Weissman, J.S.; Vale, R.D. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell 2014, 159, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Peter, C.J.; Saito, A.; Hasegawa, Y.; Tanaka, Y.; Nagpal, M.; Perez, G.; Alway, E.; Espeso-Gil, S.; Fayyad, T.; Ratner, C.; et al. In vivo epigenetic editing of Sema6a promoter reverses transcallosal dysconnectivity caused by C11orf46/Arl14ep risk gene. Nat. Commun. 2019, 10, 4112. [Google Scholar] [CrossRef]
- Fraser, M.J.; Smith, G.E.; Summers, M.D. Acquisition of Host Cell DNA Sequences by Baculoviruses: Relationship Between Host DNA Insertions and FP Mutants of Autographa californica and Galleria mellonella Nuclear Polyhedrosis Viruses. J. Virol. 1983, 47, 287–300. [Google Scholar] [CrossRef]
- Yusa, K.; Zhou, L.; Li, M.A.; Bradley, A.; Craig, N.L. A hyperactive piggyBac transposase for mammalian applications. Proc. Natl. Acad. Sci. US 2011, 108, 1531–1536. [Google Scholar] [CrossRef]
- Mates, L.; Chuah, M.K.; Belay, E.; Jerchow, B.; Manoj, N.; Acosta-Sanchez, A.; Grzela, D.P.; Schmitt, A.; Becker, K.; Matrai, J.; et al. Molecular evolution of a novel hyperactive Sleeping Beauty transposase enables robust stable gene transfer in vertebrates. Nat. Genet. 2009, 41, 753–761. [Google Scholar] [CrossRef]
- Ivics, Z.; Hackett, P.B.; Plasterk, R.H.; Izsvak, Z. Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 1997, 91, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K.; Shima, A.; Kawakami, N. Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proc. Natl. Acad. Sci. USA 2000, 97, 11403–11408. [Google Scholar] [CrossRef] [PubMed]
- Loulier, K.; Barry, R.; Mahou, P.; Le Franc, Y.; Supatto, W.; Matho, K.S.; Ieng, S.; Fouquet, S.; Dupin, E.; Benosman, R.; et al. Multiplex cell and lineage tracking with combinatorial labels. Neuron 2014, 81, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Dumas, L.; Clavreul, S.; Durand, J.; Hernandez-Garzon, E.; Abdeladim, L.; Barry-Martinet, R.; Caballero-Megido, A.; Beaurepaire, E.; Bonvento, G.; Livet, J.; et al. In Utero Electroporation of Multiaddressable Genome-Integrating Color (MAGIC) Markers to Individualize Cortical Mouse Astrocytes. J. Vis. Exp. 2020, 159, e61110. [Google Scholar] [CrossRef]
- Sumiyoshi, K.; Koso, H.; Watanabe, S. Spontaneous development of intratumoral heterogeneity in a transposon-induced mouse model of glioma. Cancer Sci. 2018, 109, 1513–1523. [Google Scholar] [CrossRef]
- Morin, X.; Jaouen, F.; Durbec, P. Control of planar divisions by the G-protein regulator LGN maintains progenitors in the chick neuroepithelium. Nat. Neurosci. 2007, 10, 1440–1448. [Google Scholar] [CrossRef]
- Humpel, C. Organotypic brain slice cultures: A review. Neuroscience 2015, 305, 86–98. [Google Scholar] [CrossRef]
- Alfadil, E.; Bradke, F.; Dupraz, S. In Situ Visualization of Axon Growth and Growth Cone Dynamics in Acute Ex Vivo Embryonic Brain Slice Cultures. J. Vis. Exp. 2021, 176, e63068. [Google Scholar] [CrossRef]
- Yang, T.; Hergenreder, T.; Ye, B. Analysis of Mouse Brain Sections by Live-cell Time-lapse Confocal Microscopy. Bio Protoc. 2023, 13, e4648. [Google Scholar] [CrossRef]
- Shitamukai, A.; Konno, D.; Matsuzaki, F. Oblique radial glial divisions in the developing mouse neocortex induce self-renewing progenitors outside the germinal zone that resemble primate outer subventricular zone progenitors. J. Neurosci. 2011, 31, 3683–3695. [Google Scholar] [CrossRef]
- Pilz, G.A.; Shitamukai, A.; Reillo, I.; Pacary, E.; Schwausch, J.; Stahl, R.; Ninkovic, J.; Snippert, H.J.; Clevers, H.; Godinho, L.; et al. Amplification of progenitors in the mammalian telencephalon includes a new radial glial cell type. Nat. Commun. 2013, 4, 2125. [Google Scholar] [CrossRef] [PubMed]
- Lepiemme, F.; Silva, C.G.; Nguyen, L. Time lapse recording of cortical interneuron migration in mouse organotypic brain slices and explants. STAR Protoc. 2021, 2, 100467. [Google Scholar] [CrossRef] [PubMed]
- Coquand, L.; Victoria, G.S.; Tata, A.; Carpentieri, J.A.; Brault, J.B.; Guimiot, F.; Fraisier, V.; Baffet, A.D. CAMSAPs organize an acentrosomal microtubule network from basal varicosities in radial glial cells. J. Cell Biol. 2021, 220, e202003151. [Google Scholar] [CrossRef] [PubMed]
- Brault, J.B.; Bardin, S.; Lampic, M.; Carpentieri, J.A.; Coquand, L.; Penisson, M.; Lachuer, H.; Victoria, G.S.; Baloul, S.; El Marjou, F.; et al. RAB6 and dynein drive post-Golgi apical transport to prevent neuronal progenitor delamination. EMBO Rep. 2022, 23, e54605. [Google Scholar] [CrossRef]
- Livet, J.; Weissman, T.A.; Kang, H.; Draft, R.W.; Lu, J.; Bennis, R.A.; Sanes, J.R.; Lichtman, J.W. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 2007, 450, 56–62. [Google Scholar] [CrossRef]
- Dumas, L.; Clavreul, S.; Michon, F.; Loulier, K. Multicolor strategies for investigating clonal expansion and tissue plasticity. Cell Mol. Life Sci. 2022, 79, 141. [Google Scholar] [CrossRef]
- Liu, Z.; Lu, Z.; Yang, G.; Huang, S.; Li, G.; Feng, S.; Liu, Y.; Li, J.; Yu, W.; Zhang, Y.; et al. Efficient generation of mouse models of human diseases via ABE- and BE-mediated base editing. Nat. Commun. 2018, 9, 2338. [Google Scholar] [CrossRef]
- Takahashi, G.; Gurumurthy, C.B.; Wada, K.; Miura, H.; Sato, M.; Ohtsuka, M. GONAD: Genome-editing via Oviductal Nucleic Acids Delivery system: A novel microinjection independent genome engineering method in mice. Sci. Rep. 2015, 5, 11406. [Google Scholar] [CrossRef]
- Ohtsuka, M.; Sato, M. i-GONAD: A method for generating genome-edited animals without ex vivo handling of embryos. Dev. Growth Differ. 2019, 61, 306–315. [Google Scholar] [CrossRef]
- Aoto, K.; Takabayashi, S.; Mutoh, H.; Saitsu, H. Generation of Flag/DYKDDDDK Epitope Tag Knock-In Mice Using i-GONAD Enables Detection of Endogenous CaMKIIalpha and beta Proteins. Int. J. Mol. Sci. 2022, 23, 11915. [Google Scholar] [CrossRef]
- Yoshimi, K.; Kunihiro, Y.; Kaneko, T.; Nagahora, H.; Voigt, B.; Mashimo, T. ssODN-mediated knock-in with CRISPR-Cas for large genomic regions in zygotes. Nat. Commun. 2016, 7, 10431. [Google Scholar] [CrossRef] [PubMed]
- Nishizono, H.; Yasuda, R.; Laviv, T. Methodologies and Challenges for CRISPR/Cas9 Mediated Genome Editing of the Mammalian Brain. Front. Genome Ed. 2020, 2, 602970. [Google Scholar] [CrossRef] [PubMed]
- Cochard, L.M.; Levros, L.C.; Joppé, S.E.; Pratesi, F.; Aumont, A.; Fernandes, K.J.L. Manipulation of EGFR-Induced Signaling for the Recruitment of Quiescent Neural Stem Cells in the Adult Mouse Forebrain. Front. Neurosci. 2021, 15, 621076. [Google Scholar] [CrossRef] [PubMed]
- Nomura, T.; Nishimura, Y.; Gotoh, H.; Ono, K. Rapid and efficient gene delivery into the adult mouse brain via focal electroporation. Sci. Rep. 2016, 6, 29817. [Google Scholar] [CrossRef]
- De Vry, J.; Martínez-Martínez, P.; Losen, M.; Bode, G.H.; Temel, Y.; Steckler, T.; Steinbusch, H.W.; De Baets, M.; Prickaerts, J. Low Current-driven Micro-electroporation Allows Efficient In Vivo Delivery of Nonviral DNA into the Adult Mouse Brain. Mol. Ther. 2010, 18, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, S.C.M.; Masullo, L.A.; Baudrexel, I.; Steen, P.R.; Kowalewski, R.; Eklund, A.S.; Strauss, S.; Unterauer, E.M.; Schlichthaerle, T.; Strauss, M.T.; et al. Angstrom-resolution fluorescence microscopy. Nature 2023, 617, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Damstra, H.G.J.; Mohar, B.; Eddison, M.; Akhmanova, A.; Kapitein, L.C.; Tillberg, P.W. Visualizing cellular and tissue ultrastructure using Ten-fold Robust Expansion Microscopy (TREx). Elife 2022, 11, e73775. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.E.; Fan, X.; Shi, Y.; Zhang, H.; Huang, Z.; Cheng, B.; Tang, Q.; Li, W.; Zhu, Y.; Bai, J.; et al. Click-ExM enables expansion microscopy for all biomolecules. Nat. Methods 2021, 18, 107–113. [Google Scholar] [CrossRef]
- Chen, F.; Tillberg, P.W.; Boyden, E.S. Optical imaging. Expansion microscopy. Science 2015, 347, 543–548. [Google Scholar] [CrossRef]
- Bandler, R.C.; Vitali, I.; Delgado, R.N.; Ho, M.C.; Dvoretskova, E.; Ibarra Molinas, J.S.; Frazel, P.W.; Mohammadkhani, M.; Machold, R.; Maedler, S.; et al. Single-cell delineation of lineage and genetic identity in the mouse brain. Nature 2022, 601, 404–409. [Google Scholar] [CrossRef]
- Karlikow, M.; Amalfitano, E.; Yang, X.; Doucet, J.; Chapman, A.; Mousavi, P.S.; Homme, P.; Sutyrina, P.; Chan, W.; Lemak, S.; et al. CRISPR-induced DNA reorganization for multiplexed nucleic acid detection. Nat. Commun. 2023, 14, 1505. [Google Scholar] [CrossRef]
- McKenna, A.; Findlay, G.M.; Gagnon, J.A.; Horwitz, M.S.; Schier, A.F.; Shendure, J. Whole-organism lineage tracing by combinatorial and cumulative genome editing. Science 2016, 353, aaf7907. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Maynard, A.; Jain, A.; Gerber, T.; Petri, R.; Lin, H.C.; Santel, M.; Ly, K.; Dupre, J.S.; Sidow, L.; et al. Lineage recording in human cerebral organoids. Nat. Methods 2022, 19, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Masuyama, N.; Konno, N.; Yachie, N. Molecular recorders to track cellular events. Science 2022, 377, 469–470. [Google Scholar] [CrossRef] [PubMed]
- Lear, S.K.; Shipman, S.L. Molecular recording: Transcriptional data collection into the genome. Curr. Opin. Biotechnol. 2023, 79, 102855. [Google Scholar] [CrossRef]
- Xie, L.; Liu, H.; You, Z.; Wang, L.; Li, Y.; Zhang, X.; Ji, X.; He, H.; Yuan, T.; Zheng, W.; et al. Comprehensive spatiotemporal mapping of single-cell lineages in developing mouse brain by CRISPR-based barcoding. Nat. Methods 2023, 20, 1244–1255. [Google Scholar] [CrossRef]

| Animal Species | Stage, Sample | Voltage (V) | On Time (msec) | Off Time (msec) | Pulse Number | Electrode Size (mm) | References |
|---|---|---|---|---|---|---|---|
| Mouse | E(embryonic day) 9–E17 | 25–50 | 50 | 450–950 | 4–5 | 1–5 | [8,9,18,19,20,21] |
| Rat | E13–14 | 65 | 1 | – | – | 10 x 5 | [58] |
| Chick | HH10 (1.5 dpo [day post-ovoposition]) | 25 | 50 | 950 | 5 | 0.5 x 1.0 | [22] |
| Chick | 4 dpo | 30 | 5 | 500 | 5 | 3 | [23] |
| Snake | 4 dpo | 30 | 5 | 500 | 5 | 3 | [25] |
| Turtle | 14 dpo | 32 | 50 | 950 | 2 | needle type (CUY200S, NEPAGENE, Japan) | [24] |
| Gecko | 14 dpo | 32 | 50 | 950 | 2 | needle type (CUY200S, NEPAGENE, Japan) | [24] |
| Dunnart | Stage20 (Postnatal day 8–11) | 30–35 | 100 | 900 | 5 | 1 | [26] |
| Guinea pig | E28–37 | 40–54 | 50 | 950 | 4 | 5 | [27] |
| Ferret | E32 | 45 | 100 | 900 | 5 | 10 | [19] |
| Ferret | E35–E38 | 50–100 | 50 | 950 | 5 | 5 | [28,29] |
| Human brain organoid | 20–40 days in culture | 80 | 50 | 500–950 | 5 | chamber | [51,53,54] |
| Human brain organoid | 20–40 days in culture | – | – | – | – | cuvette (Nucleofector, A-23 program, LONZA, USA) | [50] |
| Human brain organoid | 4 months in culture | 45 | 50 | 950 | 5 | 3 | [52] |
| Human retinal organoid | 27 days in culture | 25 | 50 | 950 | 5 | chamber | [55] |
| Mouse (GONAD/iGONAD) step1 | E0.7–1.5 | 50, 10% decay | 5 | 50 | 3 | 3 | [14,15,108,109] |
| Mouse (GONAD/iGONAD) step2 | E0.7–1.5 | 10, 40% decay | 50 | 50 | 3 | 3 | [14,15,108,109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Shitamukai, A.; Yang, S.; Kawaguchi, A. Advanced Techniques Using In Vivo Electroporation to Study the Molecular Mechanisms of Cerebral Development Disorders. Int. J. Mol. Sci. 2023, 24, 14128. https://doi.org/10.3390/ijms241814128
Yang C, Shitamukai A, Yang S, Kawaguchi A. Advanced Techniques Using In Vivo Electroporation to Study the Molecular Mechanisms of Cerebral Development Disorders. International Journal of Molecular Sciences. 2023; 24(18):14128. https://doi.org/10.3390/ijms241814128
Chicago/Turabian StyleYang, Chen, Atsunori Shitamukai, Shucai Yang, and Ayano Kawaguchi. 2023. "Advanced Techniques Using In Vivo Electroporation to Study the Molecular Mechanisms of Cerebral Development Disorders" International Journal of Molecular Sciences 24, no. 18: 14128. https://doi.org/10.3390/ijms241814128
APA StyleYang, C., Shitamukai, A., Yang, S., & Kawaguchi, A. (2023). Advanced Techniques Using In Vivo Electroporation to Study the Molecular Mechanisms of Cerebral Development Disorders. International Journal of Molecular Sciences, 24(18), 14128. https://doi.org/10.3390/ijms241814128





