Anti-Amnesia-like Effect of Pinus densiflora Extract by Improving Apoptosis and Neuroinflammation on Trimethyltin-Induced ICR Mice
Abstract
1. Introduction
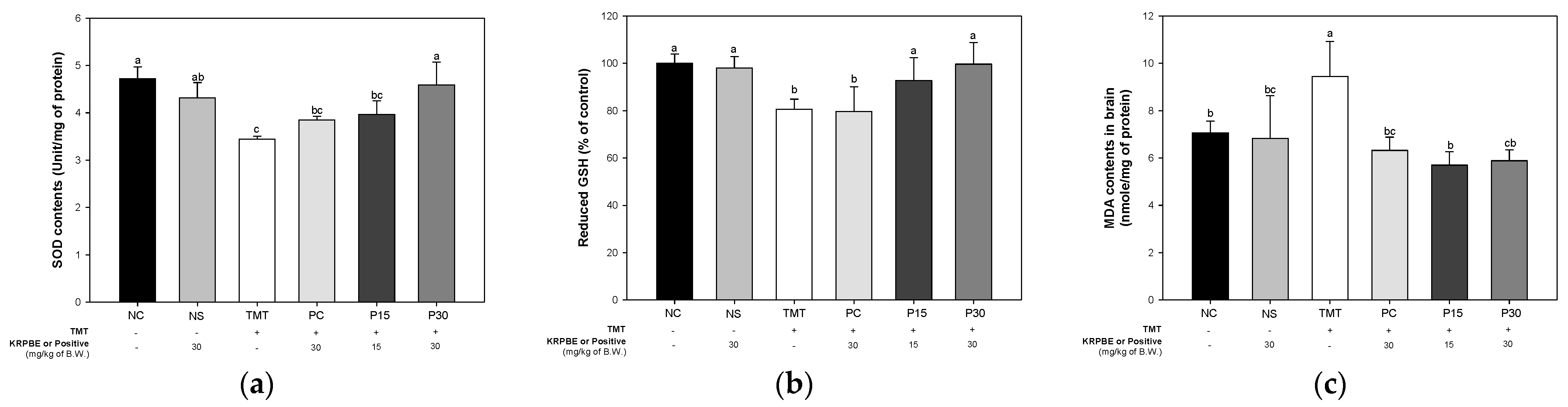
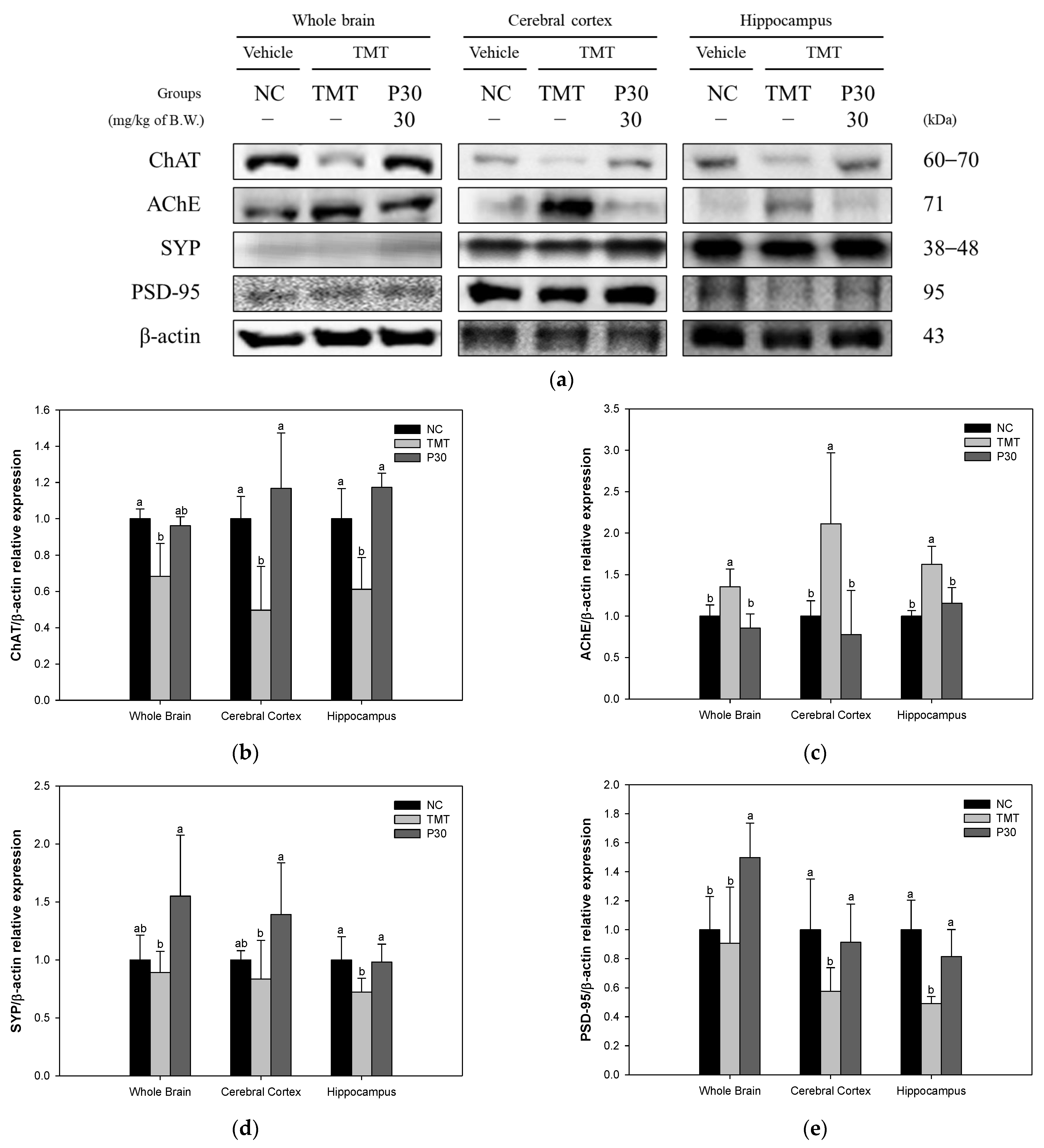
2. Results
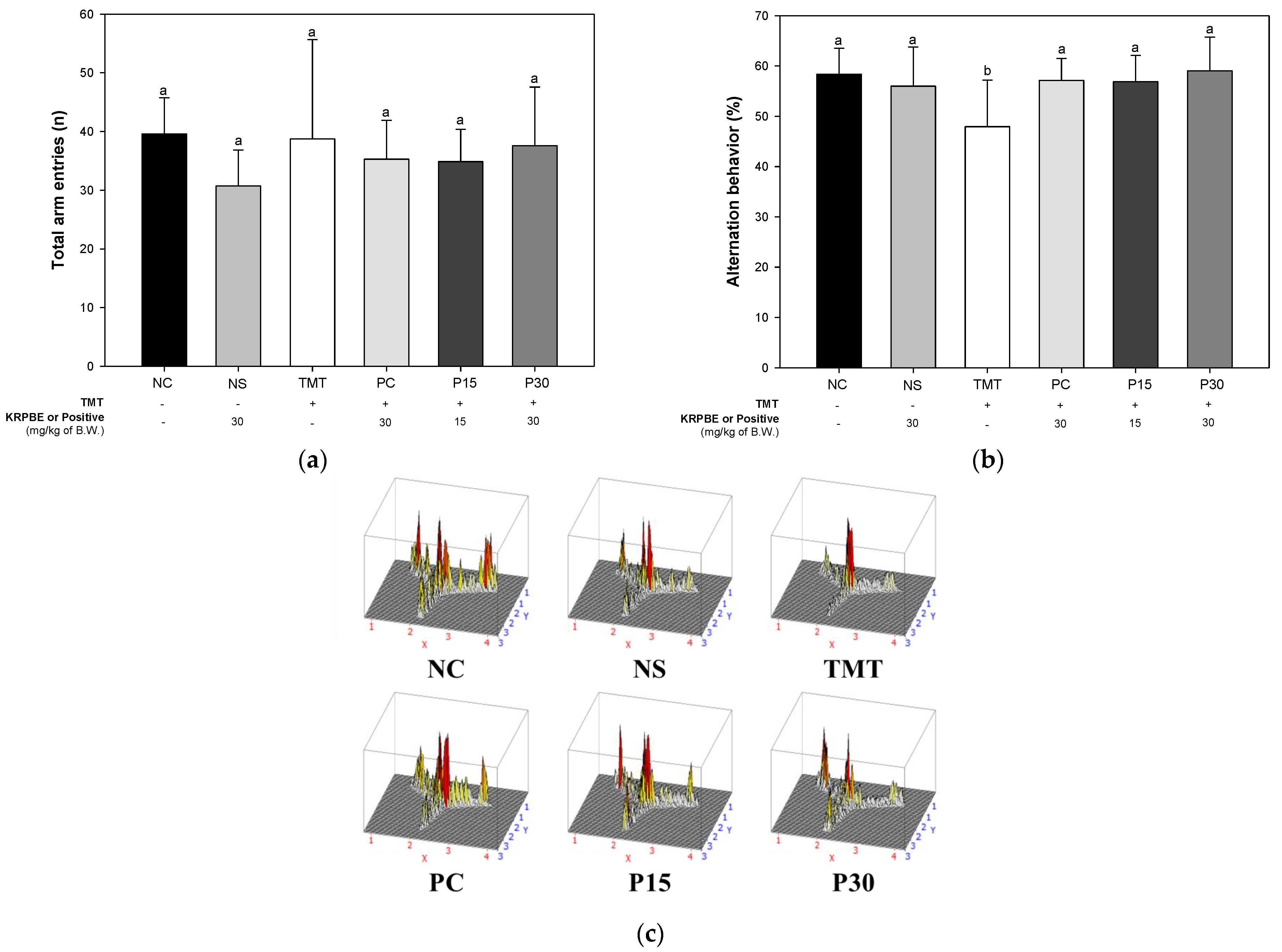
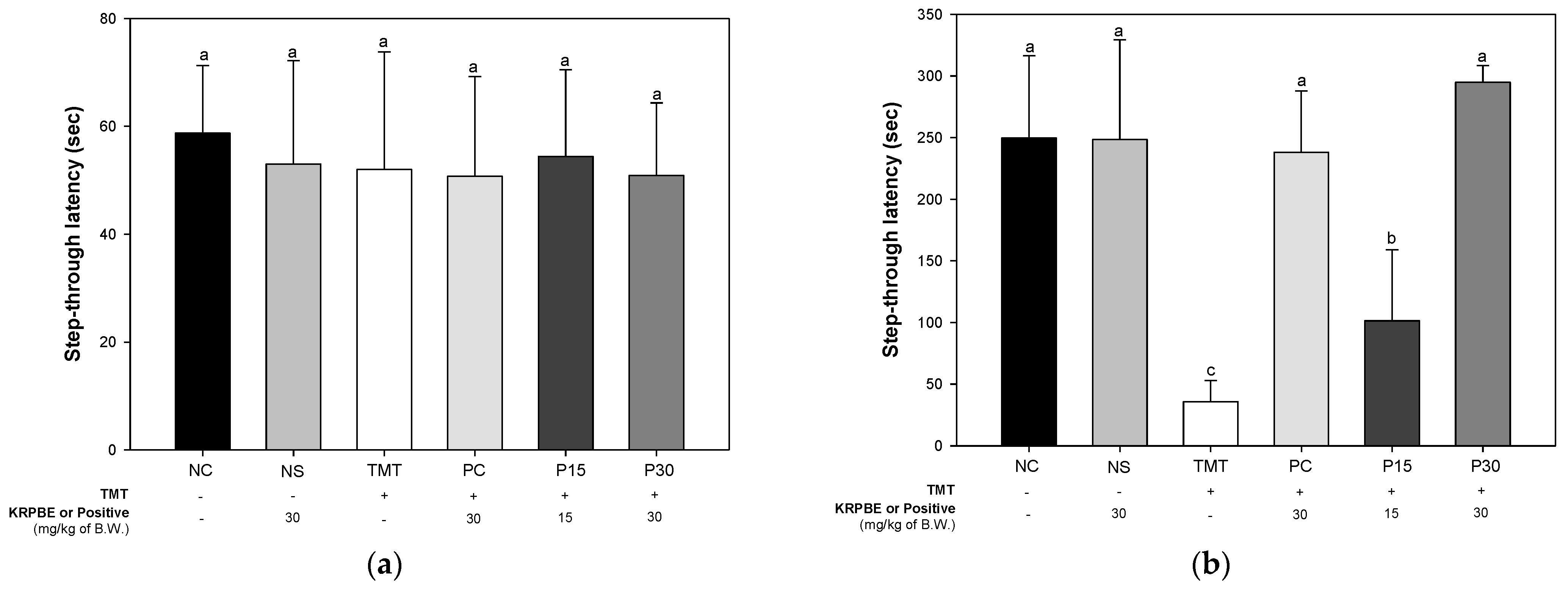
2.1. Behavioral Tests
2.1.1. Y-Maze Test
2.1.2. Passive Avoidance Test
2.1.3. Morris Water Maze Test
2.2. Antioxidant System
2.3. Cholinergic System and Synaptic Function
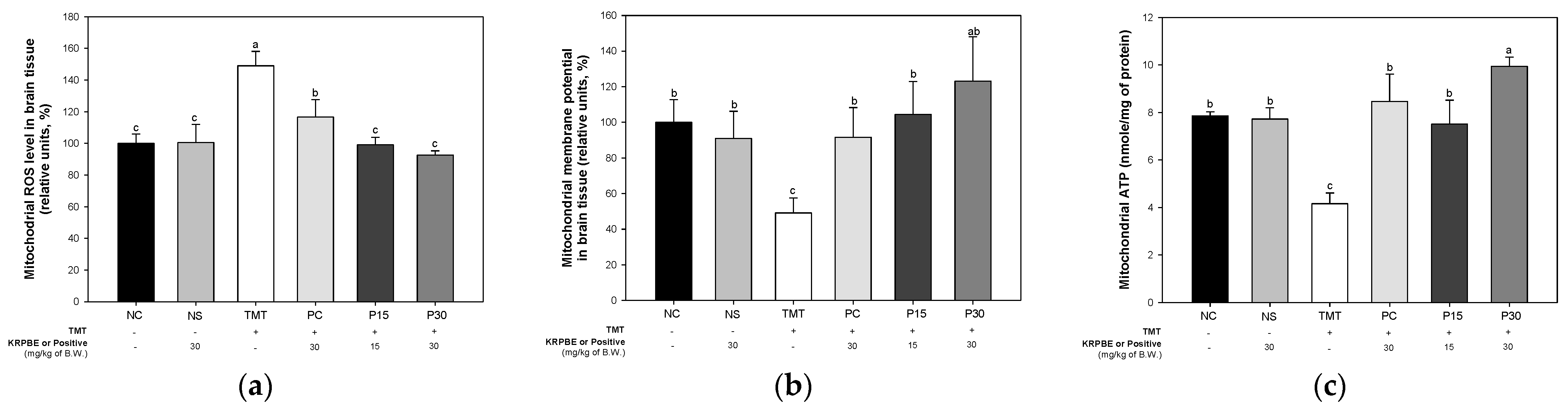
2.4. Mitochondrial Function
2.5. Aβ and Tau Contents
2.6. Apoptosis-Relative Factors
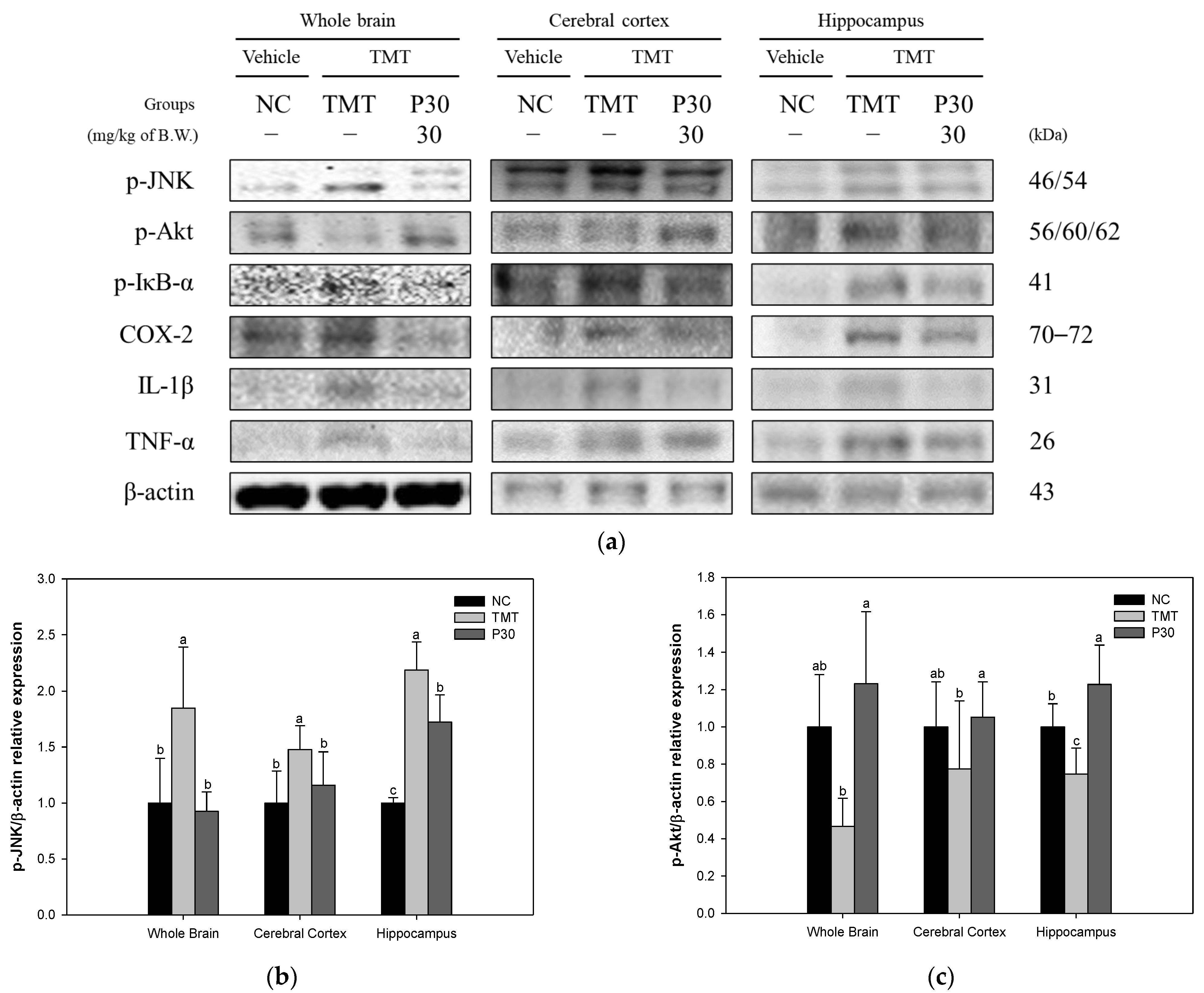
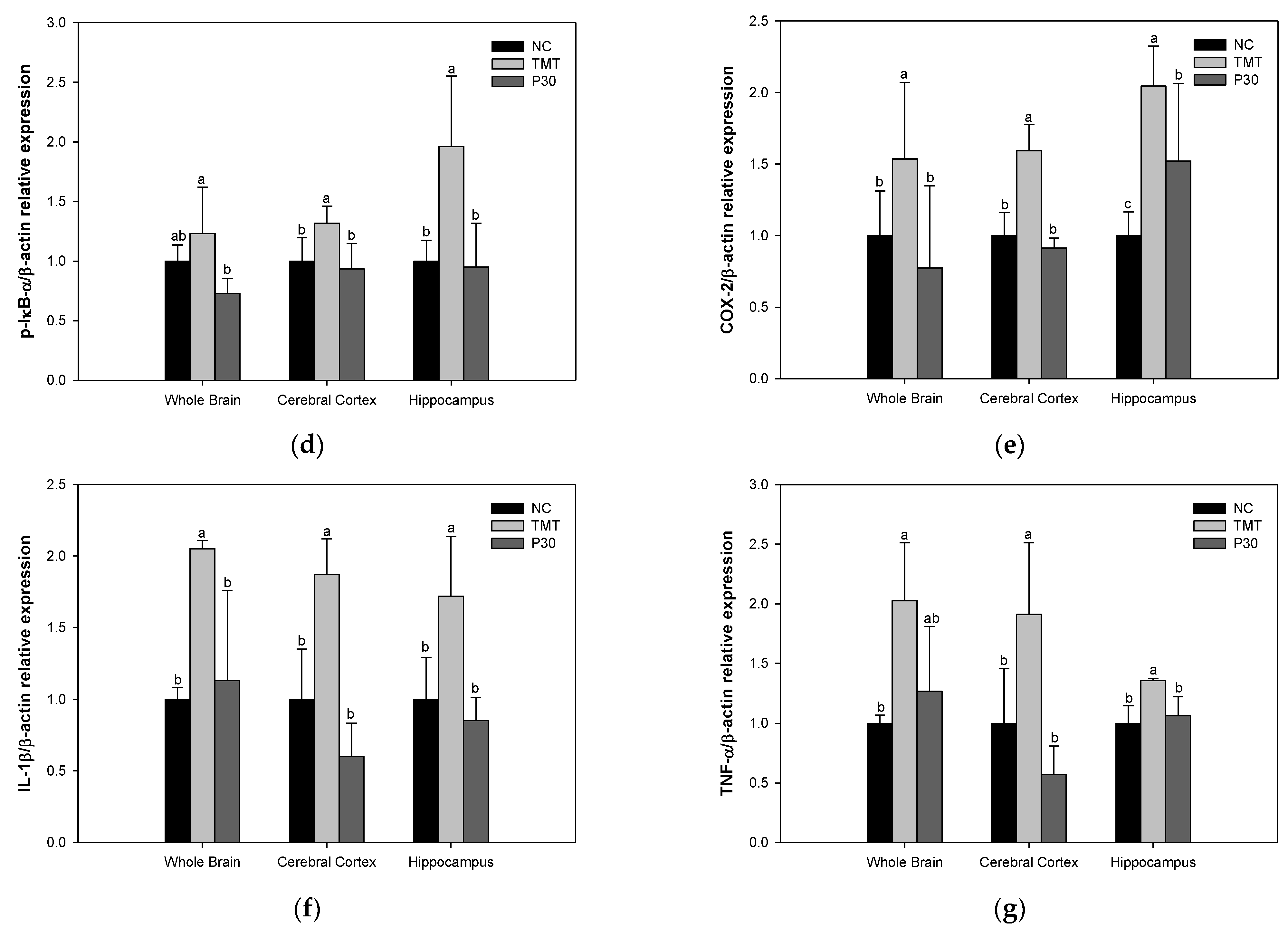
2.7. Neuroinflammation-Relative Factors
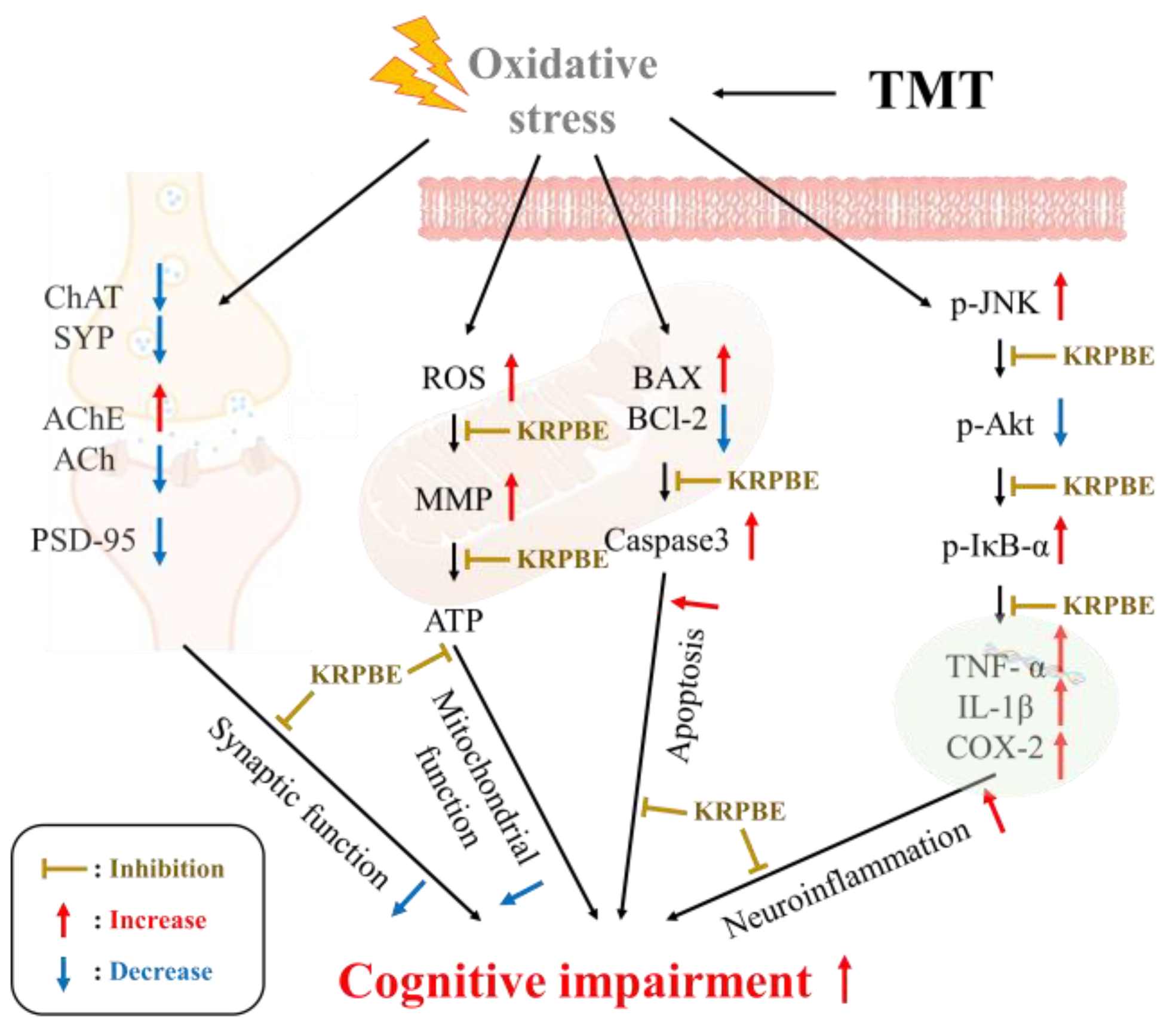
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Animal Experimental
Animal Experimental Design
4.3. Animal Experimental
4.3.1. Y-Maze Test
4.3.2. Passive Avoidance Test
4.3.3. Morris Water Maze Test
4.4. Preparation of Tissue
4.5. Measurement of Antioxidant System Biomarker
4.6. Measurement of ACh Content and AChE Inhibitory Activity
4.7. Extraction of Mitochondria from Brain Tissure
4.8. Evaluation of Mitochondrial Activity
4.9. Western Blot
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2013, 6, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Zvěřová, M. Clinical aspects of Alzheimer’s disease. Clin. Biochem. 2019, 72, 3–6. [Google Scholar] [CrossRef]
- Liu, P.-P.; Xie, Y.; Meng, X.-Y.; Kang, J.-S. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Signal Transduct. Target. Ther. 2019, 4, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxid. Med. Cell. Longev. 2013, 2013, 316523. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.; Chauhan, A. Oxidative stress in Alzheimer’s disease. Pathophysiology 2006, 13, 195–208. [Google Scholar] [CrossRef]
- Kim, J.M.; Park, S.K.; Kang, J.Y.; Park, S.B.; Yoo, S.K.; Han, H.J.; Kim, C.-W.; Lee, U.; Kim, S.-H.; Heo, H.J. Ethyl acetate fraction from persimmon (Diospyros kaki) ameliorates cerebral neuronal loss and cognitive deficit via the JNK/Akt pathway in TMT-induced mice. Int. J. Mol. Sci. 2018, 19, 1499. [Google Scholar] [CrossRef]
- Wang, S.; Su, G.; Zhang, X.; Song, G.; Zhang, L.; Zheng, L.; Zhao, M. Characterization and exploration of potential neuroprotective peptides in walnut (Juglans regia) protein hydrolysate against cholinergic system damage and oxidative stress in scopolamine-induced cognitive and memory impairment mice and zebrafish. J. Agric. Food Chem. 2021, 69, 2773–2783. [Google Scholar] [CrossRef]
- Leszek, J.; Barreto, G.E.; Gasiorowski, K.; Koutsouraki, E.; Aliev, G. Inflammatory mechanisms and oxidative stress as key factors responsible for progression of neurodegeneration: Role of brain innate immune system. CNS Neurol. Disord. Drug Targets 2016, 15, 329–336. [Google Scholar] [CrossRef]
- Chong, Z.Z.; Li, F.; Maiese, K. Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog. Neurobiol. 2005, 75, 207–246. [Google Scholar] [CrossRef]
- Zilka, N.; Kazmerova, Z.; Jadhav, S.; Neradil, P.; Madari, A.; Obetkova, D.; Bugos, O.; Novak, M. Who fans the flames of Alzheimer’s disease brains? Misfolded tau on the crossroad of neurodegenerative and inflammatory pathways. J. Neuroinflamm. 2012, 9, 47. [Google Scholar] [CrossRef]
- Chen, X.; Drew, J.; Berney, W.; Lei, W. Neuroprotective natural products for Alzheimer’s disease. Cells 2021, 10, 1309. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.-J.; Nah, S.-Y.; Chae, J.S.; Bing, G.; Shin, S.W.; Yen, T.P.H.; Baek, I.-H.; Kim, W.-K.; Maurice, T.; Nabeshima, T. Dextromethorphan attenuates trimethyltin-induced neurotoxicity via σ1 receptor activation in rats. Neurochem. Int. 2007, 50, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Yoo, S.K.; Han, H.J.; Chung, D.H.; Kim, D.-O.; Kim, G.-H.; Heo, H.J. Fucoidan-rich substances from Ecklonia cava improve trimethyltin-induced cognitive dysfunction via down-regulation of amyloid β production/tau hyperphosphorylation. Mar. Drugs 2019, 17, 591. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choung, S.-Y. Pinus densiflora bark extract prevents selenite-induced cataract formation in the lens of Sprague Dawley rat pups. Mol. Vision 2017, 23, 638. [Google Scholar]
- Kim, K.J.; Hwang, E.-S.; Kim, M.-J.; Park, J.-H.; Kim, D.-O. Antihypertensive effects of polyphenolic extract from Korean red pine (Pinus densiflora Sieb. et Zucc.) bark in spontaneously hypertensive rats. Antioxidants 2020, 9, 333. [Google Scholar] [CrossRef] [PubMed]
- Her, Y.; Lee, T.-K.; Sim, H.; Lee, J.-C.; Kim, D.W.; Choi, S.Y.; Hong, J.K.; Lee, J.-W.; Kim, J.-D.; Won, M.-H. Pinus thunbergii bark extract rich in flavonoids promotes hair growth in dorsal skin by regulating inflammatory cytokines and increasing growth factors in mice. Mol. Med. Rep. 2022, 25, 100. [Google Scholar] [CrossRef]
- Ahn, H.; Go, G.-w. Pinus densiflora bark extract (PineXol) decreases adiposity in mice by down-regulation of hepatic de novo lipogenesis and adipogenesis in white adipose tissue. J. Microbiol. Biotechnol. 2017, 27, 660–667. [Google Scholar] [CrossRef]
- Lane, C.A.; Hardy, J.; Schott, J.M. Alzheimer’s disease. Eur. J. Neurol. 2018, 25, 59–70. [Google Scholar] [CrossRef]
- Markesbery, W.R. Oxidative stress hypothesis in Alzheimer’s disease. Free Radic. Biol. Med. 1997, 23, 134–147. [Google Scholar] [CrossRef]
- Smith, M.A.; Rottkamp, C.A.; Nunomura, A.; Raina, A.K.; Perry, G. Oxidative stress in Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2000, 1502, 139–144. [Google Scholar] [CrossRef]
- Haga, S.; Haga, C.; Aizawa, T.; Ikeda, K. Neuronal degeneration and glial cell-responses following trimethyltin intoxication in the rat. Acta Neuropathol. 2002, 103, 575–582. [Google Scholar] [PubMed]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Takuma, K.; Yan, S.S.; Stern, D.M.; Yamada, K. Mitochondrial dysfunction, endoplasmic reticulum stress, and apoptosis in Alzheimer’s disease. J. Pharmacol. Sci. 2005, 97, 312–316. [Google Scholar] [CrossRef]
- Rojas-Gutierrez, E.; Muñoz-Arenas, G.; Treviño, S.; Espinosa, B.; Chavez, R.; Rojas, K.; Flores, G.; Díaz, A.; Guevara, J. Alzheimer’s disease and metabolic syndrome: A link from oxidative stress and inflammation to neurodegeneration. Synapse 2017, 71, e21990. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Hwang, E.-S.; Kim, M.-J.; Rha, C.-S.; Song, M.C.; Maeng, S.; Park, J.-H.; Kim, D.-O. Effects of phenolic-rich Pinus densiflora extract on learning, memory, and hippocampal long-term potentiation in scopolamine-induced amnesic rats. Antioxidants 2022, 11, 2497. [Google Scholar] [CrossRef]
- Wang, C.; He, L.; Yan, M.; Zheng, G.-Y.; Liu, X.-Y. Effects of polyprenols from pine needles of Pinus massoniana on ameliorating cognitive impairment in a D-galactose-induced mouse model. Age 2014, 36, 9676. [Google Scholar] [CrossRef][Green Version]
- Lee, J.-S.; Kim, H.-G.; Lee, H.-W.; Han, J.-M.; Lee, S.-K.; Kim, D.-W.; Saravanakumar, A.; Son, C.-G. Hippocampal memory enhancing activity of pine needle extract against scopolamine-induced amnesia in a mouse model. Sci. Rep. 2015, 5, 9651. [Google Scholar] [CrossRef]
- Lee, T.-K.; Park, J.H.; Shin, M.C.; Cho, J.H.; Ahn, J.H.; Kim, D.W.; Lee, J.-C.; Lee, C.-H.; Hong, S.; Won, M.-H. Therapeutic treatment with Pycnogenol® attenuates ischemic brain injury in gerbils focusing on cognitive impairment, neuronal death, BBB leakage and neuroinflammation in the hippocampus. J. Integr. Neurosci. 2023, 22, 26. [Google Scholar] [CrossRef]
- Geloso, M.C.; Corvino, V.; Michetti, F. Trimethyltin-induced hippocampal degeneration as a tool to investigate neurodegenerative processes. Neurochem. Int. 2011, 58, 729–738. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Choi, W.S.; Kwon, O.S.; Lee, J.S.; Son, S.Y.; Lee, C.H.; Lee, S.; Song, J.Y.; Lee, Y.J.; Lee, J.-Y. Extract of Pinus densiflora needles suppresses acute inflammation by regulating inflammatory mediators in RAW264. 7 macrophages and mice. Pharm. Biol. 2022, 60, 1148–1159. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, H.-G.; Lee, H.-W.; Kim, W.-Y.; Ahn, Y.-C.; Son, C.-G. Pine needle extract prevents hippocampal memory impairment in acute restraint stress mouse model. J. Ethnopharmacol. 2017, 207, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Y.; Zheng, Y.; Zhao, J.; Yu, H.; Zhu, J. Relationship between neuroprotective effects and structure of procyanidins. Molecules 2022, 27, 2308. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Zhang, N.; Hu, N.; Jiang, R.; Lu, H.; Xuan, A.; Long, D.; Chen, Y. Neural stem cell transplantation improves learning and memory by protecting cholinergic neurons and restoring synaptic impairment in an amyloid precursor protein/presenilin 1 transgenic mouse model of Alzheimer’s disease. Mol. Med. Rep. 2020, 21, 1172–1180. [Google Scholar] [CrossRef] [PubMed]
- Yuki, D.; Sugiura, Y.; Zaima, N.; Akatsu, H.; Takei, S.; Yao, I.; Maesako, M.; Kinoshita, A.; Yamamoto, T.; Kon, R. DHA-PC and PSD-95 decrease after loss of synaptophysin and before neuronal loss in patients with Alzheimer’s disease. Sci. Rep. 2014, 4, 7130. [Google Scholar] [CrossRef] [PubMed]
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Statti, G.A.; Menichini, F. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of Pinus species essential oils and their constituents. J. Enzym. Inhib. Med. Chem. 2010, 25, 622–628. [Google Scholar] [CrossRef]
- Adedara, I.A.; Fasina, O.B.; Ayeni, M.F.; Ajayi, O.M.; Farombi, E.O. Protocatechuic acid ameliorates neurobehavioral deficits via suppression of oxidative damage, inflammation, caspase-3 and acetylcholinesterase activities in diabetic rats. Food Chem. Toxicol. 2019, 125, 170–181. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, X.; Lv, C.; Li, C.; Yu, Y.; Wang, X.; Han, F. Protocatechuic acid ameliorates neurocognitive functions impairment induced by chronic intermittent hypoxia. Sci. Rep. 2015, 5, 14507. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Q.; Bao, X.; Ding, Y.; Shentu, J.; Cui, W.; Chen, X.; Wei, X.; Xu, S. Taxifolin prevents β-amyloid-induced impairments of synaptic formation and deficits of memory via the inhibition of cytosolic phospholipase A2/prostaglandin E2 content. Metab. Brain Dis. 2018, 33, 1069–1079. [Google Scholar] [CrossRef]
- Verri, M.; Pastoris, O.; Dossena, M.; Aquilani, R.; Guerriero, F.; Cuzzoni, G.; Venturini, L.; Ricevuti, G.; Bongiorno, A. Mitochondrial alterations, oxidative stress and neuroinflammation in Alzheimer’s disease. Int. J. Immunopathol. Pharmacol. 2012, 25, 345–353. [Google Scholar] [CrossRef]
- Eckert, A.; Keil, U.; Marques, C.A.; Bonert, A.; Frey, C.; Schüssel, K.; Müller, W.E. Mitochondrial dysfunction, apoptotic cell death, and Alzheimer’s disease. Biochem. Pharmacol. 2003, 66, 1627–1634. [Google Scholar] [CrossRef]
- Dhanalakshmi, C.; Manivasagam, T.; Nataraj, J.; Justin Thenmozhi, A.; Essa, M.M. Neurosupportive role of vanillin, a natural phenolic compound, on rotenone induced neurotoxicity in SH-SY5Y neuroblastoma cells. Evid. Based Complement. Altern. Med. 2015, 2015, 626028. [Google Scholar] [CrossRef] [PubMed]
- Josiah, S.S.; Saliu, I.; Umar, H.; Famusiwa, C.; Akinmoladun, A. Taxifolin alleviates metabolic and neurochemical alterations in hippocampus and cortex of brain of rotenone-toxified rats in vivo and effectively interacts with enzymes involve in neurotransmission in silico. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Jiang, B.; Bao, Y.-M.; An, L.-J. Protocatechuic acid inhibits apoptosis by mitochondrial dysfunction in rotenone-induced PC12 cells. Toxicol. In Vitro 2008, 22, 430–437. [Google Scholar] [CrossRef]
- Sajjad, R.; Arif, R.; Shah, A.; Manzoor, I.; Mustafa, G. Pathogenesis of Alzheimer’s disease: Role of amyloid-beta and hyperphosphorylated tau protein. Indian J. Pharm. Sci. 2018, 80, 581–591. [Google Scholar] [CrossRef]
- Tanaka, M.; Saito, S.; Inoue, T.; Satoh-Asahara, N.; Ihara, M. Novel therapeutic potentials of taxifolin for amyloid-β-associated neurodegenerative diseases and other diseases: Recent advances and future perspectives. Int. J. Mol. Sci. 2019, 20, 2139. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Alvarez-Fernandez, M.A.; Cerezo, A.B.; Richard, T.; Troncoso, A.M.; Garcia-Parrilla, M.C. Protocatechuic acid: Inhibition of fibril formation, destabilization of preformed fibrils of amyloid-β and α-synuclein, and neuroprotection. J. Agric. Food Chem. 2016, 64, 7722–7732. [Google Scholar] [CrossRef]
- Chang, W.; Huang, D.; Lo, Y.M.; Tee, Q.; Kuo, P.; Wu, J.S.; Huang, W.; Shen, S. Protective effect of caffeic acid against Alzheimer’s disease pathogenesis via modulating cerebral insulin signaling, β-amyloid accumulation, and synaptic plasticity in hyperinsulinemic rats. J. Agric. Food Chem. 2019, 67, 7684–7693. [Google Scholar] [CrossRef]
- Ruan, W.; Shen, S.; Xu, Y.; Ran, N.; Zhang, H. Mechanistic insights into procyanidins as therapies for Alzheimer’s disease: A review. J. Funct. Foods 2021, 86, 104683. [Google Scholar] [CrossRef]
- Kannan, K.; Jain, S.K. Oxidative stress and apoptosis. Pathophysiology 2000, 7, 153–163. [Google Scholar] [CrossRef]
- Paradis, E.; Douillard, H.; Koutroumanis, M.; Goodyer, C.; LeBlanc, A. Amyloid β peptide of Alzheimer’s disease downregulates Bcl-2 and upregulates Bax expression in human neurons. J. Neurosci. 1996, 16, 7533–7539. [Google Scholar] [CrossRef]
- Luo, Y.; Smith, J.V.; Paramasivam, V.; Burdick, A.; Curry, K.J.; Buford, J.P.; Khan, I.; Netzer, W.J.; Xu, H.; Butko, P. Inhibition of amyloid-β aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc. Natl. Acad. Sci. USA 2002, 99, 12197–12202. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Yang, M.; Kim, J.; Kang, S.; Kim, J.; Kim, J.-C.; Jung, C.; Shin, T.; Kim, S.-H.; Moon, C. Trimethyltin-induced hippocampal neurodegeneration: A mechanism-based review. Brain Res. Bull. 2016, 125, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Song, D.-Q.; Liu, J.; Wang, F.; Li, X.-F.; Liu, M.-H.; Zhang, Z.; Cao, S.-S.; Jiang, X. Procyanidin B2 inhibits lipopolysaccharide-induced apoptosis by suppressing the Bcl-2/Bax and NF-κB signalling pathways in human umbilical vein endothelial cells. Mol. Med. Rep. 2021, 23, 267. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, C.; Liccardo, M.; Sirangelo, I. Overview of the role of vanillin in neurodegenerative diseases and neuropathophysiological conditions. Int. J. Mol. Sci. 2023, 24, 1817. [Google Scholar] [CrossRef]
- Xia, R.; Ji, C.; Zhang, L. Neuroprotective effects of pycnogenol against oxygen-glucose deprivation/reoxygenation-induced injury in primary rat astrocytes via NF-κB and ERK1/2 MAPK pathways. Cell. Physiol. Biochem. 2017, 42, 987–998. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Laurent, C.; Buée, L.; Blum, D. Tau and neuroinflammation: What impact for Alzheimer’s Disease and Tauopathies? Biomed. J. 2018, 41, 21–33. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Gong, T.; Chen, W.; Mao, S.; Kong, Y.; Yu, J.; Sun, J. Anti-neuroinflammatory effect of short-chain fatty acid acetate against Alzheimer’s disease via upregulating GPR41 and inhibiting ERK/JNK/NF-κB. J. Agric. Food Chem. 2020, 68, 7152–7161. [Google Scholar] [CrossRef]
- Kim, M.J.; Rehman, S.U.; Amin, F.U.; Kim, M.O. Enhanced neuroprotection of anthocyanin-loaded PEG-gold nanoparticles against Aβ1-42-induced neuroinflammation and neurodegeneration via the NF-κB/JNK/GSK3β signaling pathway. Nanomedicine 2017, 13, 2533–2544. [Google Scholar] [CrossRef]
- Go, M.J.; Kim, J.M.; Kang, J.Y.; Park, S.K.; Lee, C.J.; Kim, M.J.; Lee, H.R.; Kim, T.Y.; Joo, S.G.; Kim, D.-O. Korean red pine (Pinus densiflora) bark extract attenuates Aβ-induced cognitive impairment by regulating cholinergic dysfunction and neuroinflammation. J. Microbiol. Biotechnol. 2022, 32, 1154–1167. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, A.; Xu, L.; Zhang, X. The role of TLR4-mediated PTEN/PI3K/AKT/NF-κB signaling pathway in neuroinflammation in hippocampal neurons. Neuroscience 2014, 269, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, M.; Scali, C.; Prosperi, C.; Bellucci, A.; Pepeu, G.; Casamenti, F. Experimental brain inflammation and neurodegeneration as model of Alzheimer’s disease: Protective effects of selective COX-2 inhibitors. Int. J. Immunopathol. Pharmacol. 2003, 16, 31–40. [Google Scholar]
- Yen, G.-C.; Duh, P.-D.; Huang, D.-W.; Hsu, C.-L.; Fu, T.Y.-C. Protective effect of pine (Pinus morrisonicola Hay.) needle on LDL oxidation and its anti-inflammatory action by modulation of iNOS and COX-2 expression in LPS-stimulated RAW 264.7 macrophages. Food Chem. Toxicol. 2008, 46, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, T.; Choi, Y.-W.; Lee, J.; Kim, Y.-K. Pinus densiflora needle supercritical fluid extract suppresses the expression of pro-inflammatory mediators iNOS, IL-6 and IL-1β, and activation of inflammatory STAT1 and STAT3 signaling proteins in bacterial lipopolysaccharide-challenged murine macrophages. Daru J. Pharm. Sci. 2017, 25, 18. [Google Scholar]
- Kang, J.Y.; Park, S.K.; Guo, T.J.; Ha, J.S.; Lee, D.S.; Kim, J.M.; Lee, U.; Kim, D.O.; Heo, H.J. Reversal of trimethyltin-induced learning and memory deficits by 3, 5-dicaffeoylquinic acid. Oxid. Med. Cell. Longev. 2016, 2016, 6981595. [Google Scholar] [CrossRef]
- Nitta, A.; Murai, R.; Suzuki, N.; Ito, H.; Nomoto, H.; Katoh, G.; Furukawa, Y.; Furukawa, S. Diabetic neuropathies in brain are induced by deficiency of BDNF. Neurotoxicol. Teratol. 2002, 24, 695–701. [Google Scholar] [CrossRef]
- DeNoble, V.J. Vinpocetine enhances retrieval of a step-through passive avoidance response in rats. Pharmacol. Biochem. Behav. 1987, 26, 183–186. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Vincent, D.; Segonzac, G.; Vincent, M.C. Colorimetric determination of acetylcholine by the Hestrin hydroxylamine reaction and its application in pharmacy. Ann. Pharm. Françaises 1958, 16, 179–185. [Google Scholar]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]




| Antibody | Catalog No. | Concentration | Manufacturer |
|---|---|---|---|
| β-actin | sc-69879 | 1:1000 | Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
| AChE | sc-373901 | 1:1000 | Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
| PSD-95 | sc-32290 | 1:1000 | Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
| SYP | sc-17750 | 1:1000 | Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
| Aβ | sc-374527 | 1:1000 | Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
| p-Akt 1/2/3 | sc-393887 | 1:1000 | Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
| p-JNK | sc-6254 | 1:1000 | Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
| p-IκB-α | sc-8404 | 1:1000 | Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
| COX-2 | sc-376861 | 1:1000 | Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
| IL-1β | sc-373800 | 1:1000 | Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
| BAX | sc-7480 | 1:1000 | Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
| BCl-2 | sc-509 | 1:1000 | Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
| caspase 3 | sc-56053 | 1:1000 | Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
| ChAT | 20747-1AP | 1:1000 | Proteintech Group (Rosemont, IL, USA) |
| Anti-rabbit | 7074S | 1:5000 | Cell Signaling Technology (Beverly, MA, USA) |
| Anti-mouse | #1706516 | 1:5000 | Bio-Rad (Richmond, CA, USA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Go, M.J.; Kim, J.M.; Lee, H.L.; Kim, T.Y.; Joo, S.G.; Kim, J.H.; Lee, H.S.; Kim, D.-O.; Heo, H.J. Anti-Amnesia-like Effect of Pinus densiflora Extract by Improving Apoptosis and Neuroinflammation on Trimethyltin-Induced ICR Mice. Int. J. Mol. Sci. 2023, 24, 14084. https://doi.org/10.3390/ijms241814084
Go MJ, Kim JM, Lee HL, Kim TY, Joo SG, Kim JH, Lee HS, Kim D-O, Heo HJ. Anti-Amnesia-like Effect of Pinus densiflora Extract by Improving Apoptosis and Neuroinflammation on Trimethyltin-Induced ICR Mice. International Journal of Molecular Sciences. 2023; 24(18):14084. https://doi.org/10.3390/ijms241814084
Chicago/Turabian StyleGo, Min Ji, Jong Min Kim, Hyo Lim Lee, Tae Yoon Kim, Seung Gyum Joo, Ju Hui Kim, Han Su Lee, Dae-Ok Kim, and Ho Jin Heo. 2023. "Anti-Amnesia-like Effect of Pinus densiflora Extract by Improving Apoptosis and Neuroinflammation on Trimethyltin-Induced ICR Mice" International Journal of Molecular Sciences 24, no. 18: 14084. https://doi.org/10.3390/ijms241814084
APA StyleGo, M. J., Kim, J. M., Lee, H. L., Kim, T. Y., Joo, S. G., Kim, J. H., Lee, H. S., Kim, D.-O., & Heo, H. J. (2023). Anti-Amnesia-like Effect of Pinus densiflora Extract by Improving Apoptosis and Neuroinflammation on Trimethyltin-Induced ICR Mice. International Journal of Molecular Sciences, 24(18), 14084. https://doi.org/10.3390/ijms241814084






