Abstract
Through whole-genome bisulfite sequencing and RNA-seq, we determined the potential impact of autophagy in regulating DNA methylation in Arabidopsis, providing a solid foundation for further understanding the molecular mechanism of autophagy and how plants cope with nitrogen deficiency. A total of 335 notable differentially expressed genes (DEGs) were discovered in wild-type Arabidopsis (Col-0-N) and an autophagic mutant cultivated under nitrogen starvation (atg5-1-N). Among these, 142 DEGs were associated with hypomethylated regions (hypo-DMRs) and were upregulated. This suggests a correlation between DNA demethylation and the ability of Arabidopsis to cope with nitrogen deficiency. Examination of the hypo-DMR-linked upregulated DEGs indicated that the expression of MYB101, an ABA pathway regulator, may be regulated by DNA demethylation and the recruitment of transcription factors (TFs; ERF57, ERF105, ERF48, and ERF111), which may contribute to the growth arrest induced by abscisic acid (ABA). Additionally, we found that DNA methylation might impact the biosynthesis of salicylic acid (SA). The promoter region of ATGH3.12 (PBS3), a key enzyme in SA synthesis, was hypomethylated, combined with overexpression of PBS3 and its potential TF AT3G46070, suggesting that autophagy defects may lead to SA-activated senescence, depending on DNA demethylation. These findings suggest that DNA hypomethylation may impact the mechanism by which Arabidopsis autophagy mutants (atg5-1) respond to nitrogen deficiency, specifically in relation to ABA and SA regulation. Our evaluation of hormone levels verified that these two hormones are significantly enriched under nitrogen deficiency in atg5-1-N compared to Col-0-N.
1. Introduction
As a universal mechanism in eukaryotes that promotes cell longevity and nutrient recycling, autophagy helps plants cope with various biotic/abiotic stresses [1]. Autophagy in plants primarily encompasses three types: microautophagy, macroautophagy, and mega-autophagy. In the process of microautophagy, the separation and extraction of components in vacuoles are realized by direct wrapping within vacuole/lysosome membranes. This is related to the accumulation of anthocyanidin in vacuoles and the elimination of damaged chloroplasts in plants [2]. Mega-autophagy is implicated in both programmed cell death (PCD) that occurs during developmental processes and pathogenic invasion [3]. During macroautophagy (hereafter referred to as autophagy), cargos are trapped in newly formed cytoplasmic vesicles, which are created when a cup-shaped phagophore (also known as an isolation membrane) expands and envelops the cytoplasm, eventually sealing off to form the autophagosome, a double-membrane-bound structure. Subsequently, the outer membrane of the autophagosome fuses with the tonoplast, leading to the release of the internal vesicle as an autophagic body [3]. The autophagy pathway is regulated by a sophisticated mechanism for which over 40 ATG genes have been identified in plants [4,5,6,7,8]. In recent years, various studies have indicated that autophagy plays a crucial role in diverse biological processes in plants, including growth, development, degradation of starch, senescence, and control of lipid metabolism [9]. In Arabidopsis, autophagy also affects the growth of pollen tubes. Through the specific mediation of ATG8-family interacting motif (AIM) recognition sites, ATG8e and mitochondria can bind and interact spatiotemporally, thereby inducing mitochondrial autophagy [10]. In addition, the relationship between autophagy and epigenetics in animals has also been validated. Under nutritional deficiency, fibroblast growth factor-21 (FGF21) signaling activates the global expression of autophagy-related genes through the histone H3K27-ME3 demethylase Jumonji-D3 (JMJD3/KDM6B), thus mediating lipid degradation [11]. The autophagy regulatory network may also be regulated by DNA methylation. It has been reported that MAP1LC3 is a key component of the core mechanism of autophagy. DNA methylation mediated by the de novo DNA methyltransferase DNMT3A at the MAP1LC3 locus leads to continuous downregulation of the transcription level of MAP1LC [12]. The relationship between autophagic function and epigenetic regulation has been explored in animals. However, it remains unknown whether plant autophagy and/or nitrogen starvation affect whole-genome DNA methylation.
Numerous studies have examined the function of DNA methylation in the abiotic stress response. DNA methylation resulting from abiotic stress is not limited to specific gene regions; it can occur throughout the genome. Moreover, the genes that are susceptible to modification in response to stress are ostensibly linked to methylation regulation [13]. In research conducted by Jiang, C. et al. on the methylation profile of Arabidopsis cultivated in soil with high salt concentration, in comparison to the control group, the stressed lineages amassed approximately 45% more differentially methylated cytosine positions (DMPs) at CG sites (CG DMPs), and the majority of these DMPs were observed to be inherited [14]. In Arabidopsis, exposure to low levels of Pi results in a significant alteration in the methylation level of the entire genome, which in turn is linked with the regulation of gene expression for responding to Pi starvation [15]. In a study of DNA methylation of the VRN-A1 gene of winter wheat, Abdul Rehman Khan et al. reported methylation at both CG and non-CG sites. Furthermore, they observed that site-specific hypermethylation induced by cold was transmitted through mitosis at non-CG sites [16]. To date, comprehensive research has been conducted on the response of various plants and crops to abiotic stress. This includes studies on the correlation between autophagy and abiotic stress and the regulatory mechanism of DNA methylation in plants or crops for resistance to abiotic stress [17,18,19,20,21].
Based on the above research background, the response of plants to abiotic stress seems to have a profound relationship with DNA methylation, so we hypothesized that DNA methylation may be involved in the response of autophagy-deficient mutants to nitrogen deficiency. However, up to now, no relevant research has been conducted on the relationship between autophagy and DNA methylation in plants and how to regulate gene expression by affecting the DNA methylation level. Thus, in this study, whole-genome bisulfite sequencing (WGBS) was performed on Arabidopsis autophagic mutants (atg5-1) developing under nitrogen starvation to address relevant concerns, in conjunction with transcriptome sequencing (RNA-seq).
2. Results
2.1. The Whole-Genome Methylation Level of Autophagic Mutants Was Strongly Affected under Nitrogen Deficiency Conditions
To investigate whether autophagy influences plant growth under nitrogen starvation stress through DNA methylation/demethylation, we performed WGBS and RNA-seq for Arabidopsis wild-type (Col-0) and autophagic mutant (atg5-1) lines under MS growth conditions (MS liquid culture medium) or nitrogen starvation conditions (MS-N liquid culture medium) (Figure 1A). Over 4G clean reads (paired-end reads) were produced for each sample in the WGBS experiment.
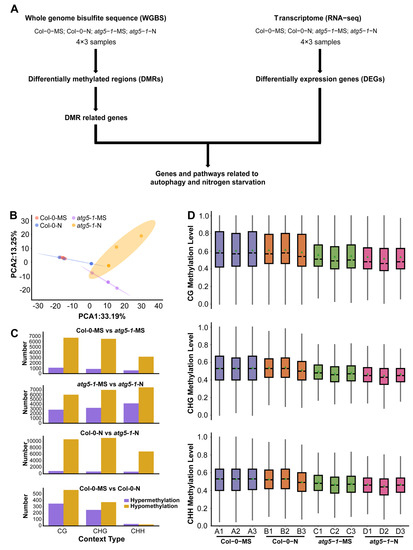
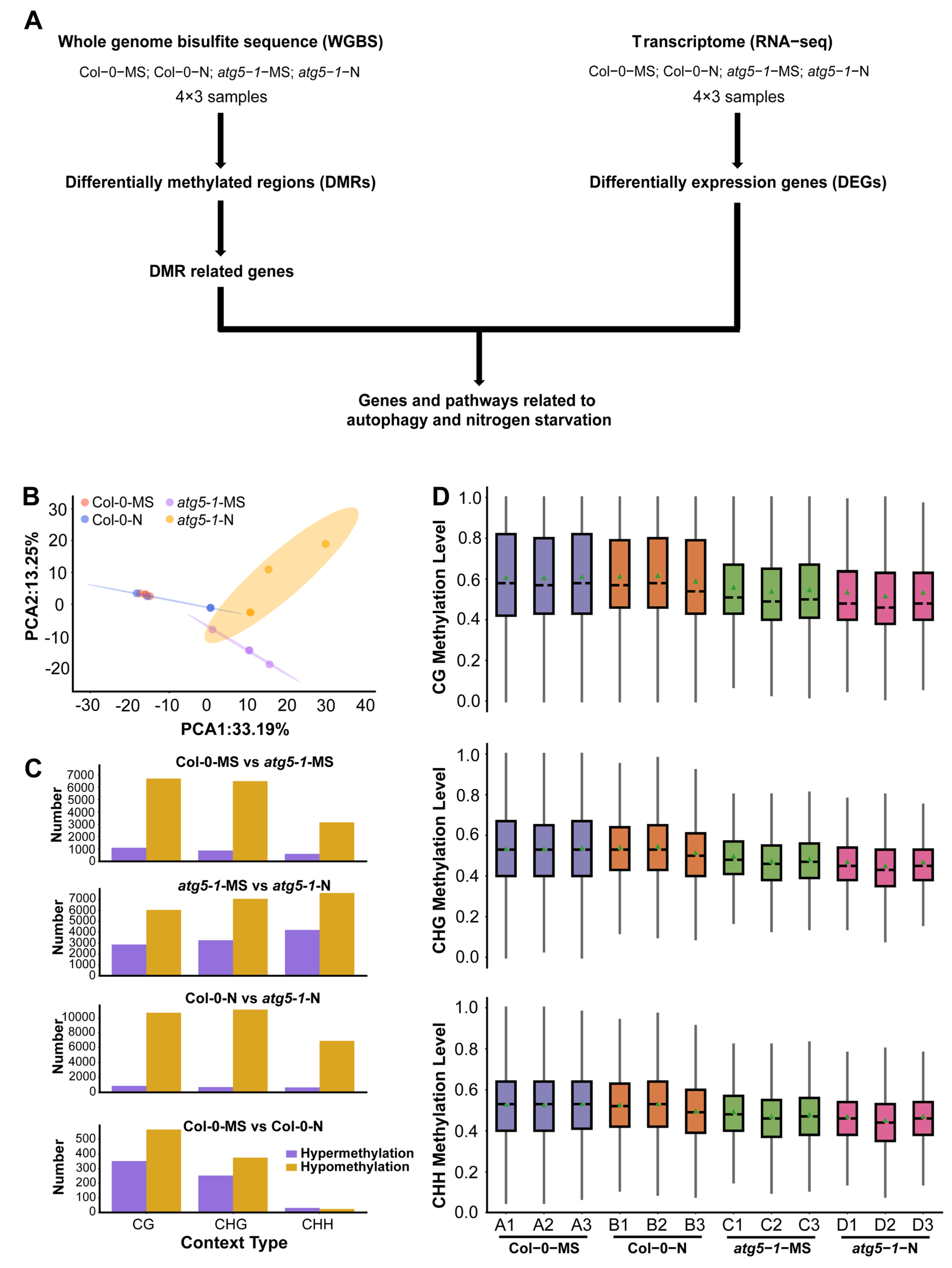
Figure 1.
Autophagy function defects lead to changes in genome-wide methylation levels. (A) Data analysis workflow for whole-genome bisulfite sequencing (WGBS) and transcriptome analysis (RNA-seq). (B) PCA of the DNA methylation level in each sample. PCA of Col-0 and atg5-1 genotypes, displaying obvious differences under MS and nitrogen starvation conditions. (C) The number of hypomethylated and hypermethylated DMRs in each comparison group. (D) The distribution of DNA methylation levels. The distribution plot of the methylation level of common sites showed that DNA methylation was affected by autophagy.
The average mapping ratios for alignment with the reference genome for the different treatment groups were 94.06% (Col-0-MS), 92.50% (Col-0-N), 87.64% (atg5-1-MS), and 85.06% (atg5-1-N), and the conversion ratios were >99.5% (Table S1). We then separated DNA methylation into three context types (CG, CHG, and CHH) and detected methylation sites based on the above context types [22,23]. According to the methylation level of common sites in all samples, a principal component analysis (PCA) was performed and showed obvious differentiation between wild-type Arabidopsis and the autophagic mutant (Figure 1B).
To characterize the methylation changes under nitrogen starvation conditions and in the autophagy mutant, differentially methylated cytosines (DMCs) and differentially methylated regions (DMRs) were identified in different comparison groups (Col-0-MS vs. Col-0-N, atg5-1-MS vs. atg5-1-N, Col-0-MS vs. atg5-1-MS, and Col-0-N vs. atg5-1-N).
In Col-0-MS vs. Col-0-N, there were 351, 252, and 31 upregulated DMRs and 565, 375, and 24 downregulated DMRs in the CG, CHG, and CHH contexts, respectively, and among the comparison groups, the fewest DMRs were identified in Col-0-MS vs. Col-0-N. Apparently, wild-type Arabidopsis was not significantly impacted by nitrogen starvation, and relatively obvious differences were observed in the comparison between the wild-type (Col-0-MS) and autophagic mutant (atg5-1-MS) under normal conditions (1088, 874, and 602 upregulated DMRs and 6690, 6483, and 3149 downregulated DMRs). Autophagy seems to have a greater influence on the whole-genome methylation level than nitrogen starvation, although autophagy is one of the most important pathways for responding to N stress in plants.
By comparing the DMR numbers of atg5-1 samples in MS and nitrogen starvation conditions (atg5-1-MS vs. atg5-1-N), 2868, 3265, and 4213 hypermethylated DMRs and 6052, 7070, and 7603 hypomethylated DMRs were identified in the CG, CHG, and CHH contexts, respectively, showing a dramatic difference in Col-0-MS vs. Col-0-N. Significant differences in DMR number were observed in atg5-1 genotype samples and wild-type samples growing in MS and nitrogen starvation conditions, which seemingly indicated that in wild-type samples, some compensation effects probably resisted the influence of nitrogen starvation on methylation levels; however, this kind of compensation effect might be inhibited due to autophagy defects. Under the same nitrogen starvation conditions, 863, 698, and 659 hypermethylated DMRs and 10691, 11110, and 6913 hypomethylated DMRs were identified in Col-0-N vs. atg5-1-N in the CG, CHG, and CHH contexts, respectively (Table S2) (Figure 1C). We examined the common methylation site for each treatment group, and the results showed that the overall methylation level of the atg5-1 sample was significantly lower than that of the wild-type sample, which further suggested that the decrease in the DNA methylation level could be largely attributed to the autophagy defect (Figure 1D).
In summary, nitrogen starvation may not have a significant impact on Arabidopsis in terms of methylation. However, when autophagy in Arabidopsis produces defects, the impact of nitrogen starvation may be amplified.
2.2. Autophagy Stimulates Global Expression of the Arabidopsis Genome under Nitrogen Starvation Conditions
To examine the gene expression changes involved in resistance to autophagy and nitrogen starvation in Arabidopsis, transcriptome profiles under different conditions were generated by RNA-seq (Table S3), and more than 6G clean reads (paired-end reads) were obtained for each RNA-seq sample.
The number of upregulated genes was obviously greater than the number of downregulated genes in the Col-0-MS vs. Col-0-N comparison group, suggesting that global gene expression was activated by nitrogen starvation to maintain growth. In atg5-1-MS vs. atg5-1-N, there were similar changes in differential gene expression, indicating that in the case of autophagy-related functional defects, nitrogen starvation may still activate the expression of many genes in response. However, a minimum number of DEGs appeared in Col-0-MS vs. atg5-1-MS, indicating that autophagy-related functional defects probably mildly impact plant growth under normal conditions (Figure 2A,B).
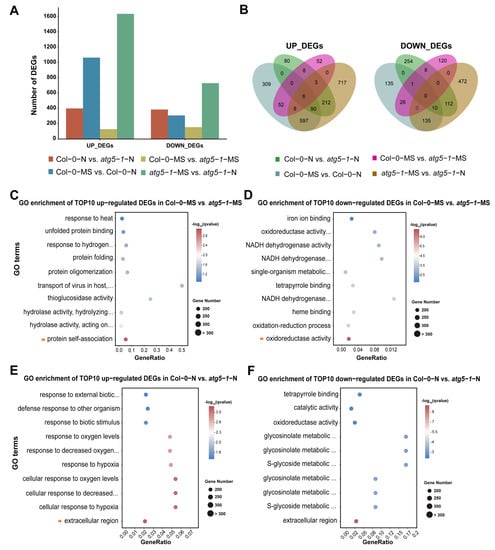
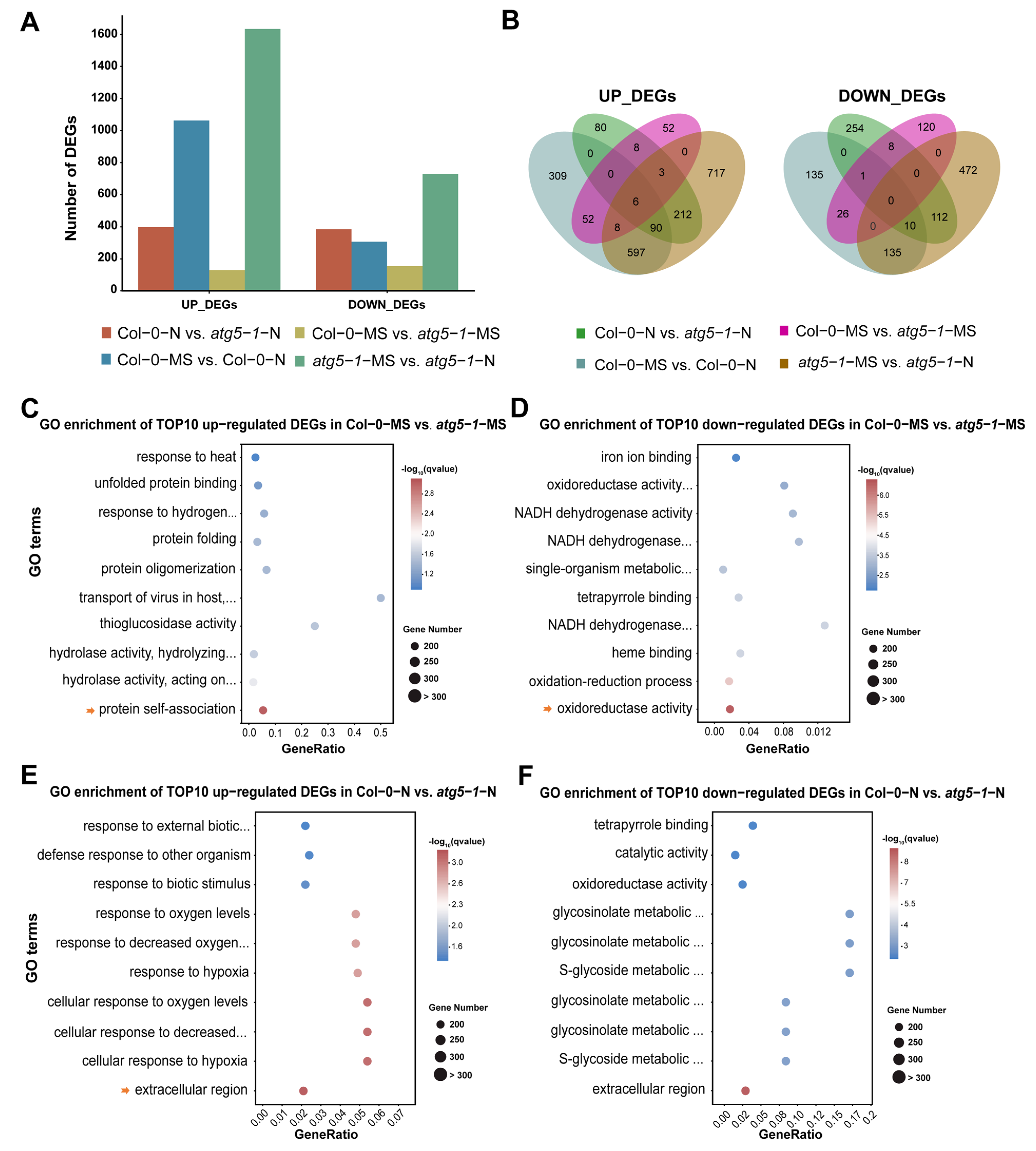
Figure 2.
Nitrogen starvation activates differential gene expression across the whole genome of Col-0 and autophagic mutants. (A) DEG statistics. Gene expression was activated in the atg5-1 and Columbia genotype groups under nitrogen starvation, showing the notable influence of nitrogen starvation. (B) Venn plot of DEGs in different comparison groups. (C–F) GO enrichment of the top 10 upregulated DEGs in Col-0-MS vs. atg5-1-MS, Col-0-MS vs. atg5-1-MS, Col-0-N vs. atg5-1-N, and Col-0-N vs. atg5-1-N. The arrow represents the GO terms with the most significant enrichment or that were meaningful for our research.
In Col-0-MS vs. Col-0-N, the genes associated with the integral component of the membrane, the intrinsic component of the membrane, and the defense response were the most significantly enriched among the upregulated DEGs. KEGG analysis showed that genes associated with ABC transporters were significantly enriched in membrane transport-related pathways, suggesting that ABC transporters probably play an important role when Arabidopsis suffers nitrogen starvation. The genes associated with the chloroplast thylakoid membrane, the plastid thylakoid membrane, and the photosynthetic membrane were the most significantly enriched among the downregulated DEGs. Interestingly, most of the top 10 GO enrichment annotations of the downregulated DEGs seemed to be related to thylakoids. We performed a KEGG analysis for DEGs annotated in thylakoid-related functional modules. The DEGs were mainly involved in pathways related to photosynthesis, ATPase, and NADH dehydrogenase (Table S4).
In Col-0-MS vs. atg5-1-MS, the upregulated DEGs were extremely enriched in protein self-association, and most of the functional modules in the top 10 enriched GO terms were associated with protein structure, unfolded protein processing, and hydrolase activity. Understandably, in autophagy, degradation, and recycling of unwanted content and misfolded proteins are the main functions (Figure 2C) [24,25]. A KEGG analysis was performed for these DEGs, which showed that the protein processing in the endoplasmic reticulum and tryptophan metabolism pathways were significantly enriched, and the DEGs enriched in these two pathways were associated with heat-shock-protein- and myrosinase-related genes. (Table S5). GO enrichment analysis was performed for the downregulated DEGs and showed that oxidoreductase activity and the oxidation–reduction process were the most significantly enriched, while other significantly enriched GO terms were mainly related to the binding of substances and NADH dehydrogenase activity (Figure 2D). KEGG results produced based on GO enrichment showed that most DEGs were associated with oxidative reactions and encoded glutathione transferase (Table S6).
To explore the functions that were influenced differentially between Col-0-N and atg5-1-N, we performed GO enrichment analysis of the upregulated DEGs and removed the DEGs that were present in Col-0-MS vs. atg5-1-MS. The results clearly showed that most of the top 10 GO terms were related to oxygen levels and the response to hypoxia, suggesting that plants with autophagy defects were more sensitive to oxygen under nitrogen starvation conditions (Figure 2E). Additionally, we annotated the pathways associated with these upregulated genes using KEGG enrichment. The most upregulated DEGs, which were enriched in oxygen-level-related functions, were associated with the amino acid metabolism pathway, while carotenoid biosynthesis, the MAPK signaling pathway, and the biosynthesis of other secondary metabolites were also enriched (Table S7). Subsequently, exploration of gene function was carried out through GO and KEGG enrichment for the downregulated DEGs, from which, for functional analysis, DEGs that genes were also present in Col-0-MS vs. atg5-1-MS were removed. The DEGs associated with the extracellular region, the glucosinolate metabolic process, and the glucosinolate catabolic process were significantly enriched in metabolic pathways and the biosynthesis of secondary metabolites, mainly including genes encoding various enzymes (Figure 2F and Table S8).
To explore functional changes between atg5-1-MS and atg5-1-N, the DEGs that were also present in Col-0-MS vs. Col-0-N were removed. The GO results for the upregulated genes were similar to those for Col-0-N vs. atg5-1-N. The top 10 GO terms were mainly related to the response to changes in oxygen levels, and the KEGG results for DEGs enriched in the GO terms mentioned above showed that the significantly enriched terms were mainly related to metabolic and biosynthetic pathways of various substances, which mainly involved genes associated with a cytidine/deoxycytidylate deaminase family protein and genes encoding a cytidine deaminase, a gamma-glutamyltransferase and glycosyl hydrolase family protein (Tables S9 and S10). For downregulated DEGs, GO enrichment showed that extracellular function and oxygenase activity-related function were significantly enriched. KEGG annotation indicated that the genes with the functions mentioned above were mainly enriched in biosynthesis- and metabolism-related pathways (Tables S11 and S12).
2.3. Autophagy Deficiency May Induce Downregulation of DNA Methylation Levels, Thus Activating Gene Expression Related to Cell Death and Catabolic Pathways
To investigate the potential influence of DNA hypomethylation induced by autophagy defects, we counted the number of hypo-DMRs associated with upregulated DEGs. The results showed that most of the DEGs were identified in Col-0-MS vs. Col-0-N, but only a few of these DEGs were regulated by DNA methylation, which indicates that DNA methylation is not closely involved in the mechanism underlying the response to nitrogen deficiency in the growth process of wild-type Arabidopsis under nitrogen deficiency conditions.
In Col-0-MS vs. atg5-1-MS, atg5-1-MS vs. atg5-1-N, and Col-0-N vs. atg5-1-N, there were 38, 73, 142 hypo-DMRs associated with upregulated DEGs, respectively, which was comparable to the number of upregulated DEGs identified in the comparison groups mentioned above. The number of upregulated DEGs regulated by methylation was also considerable (Figure 3A). To explain the relationship between DNA methylation and autophagy and the possible mechanism by which DNA methylation regulates autophagy mutants’ resistance to nitrogen deficiency, we mainly focused on Col-0-MS vs. atg5-1-MS and Col-0-N vs. atg5-1-N in the analysis process.
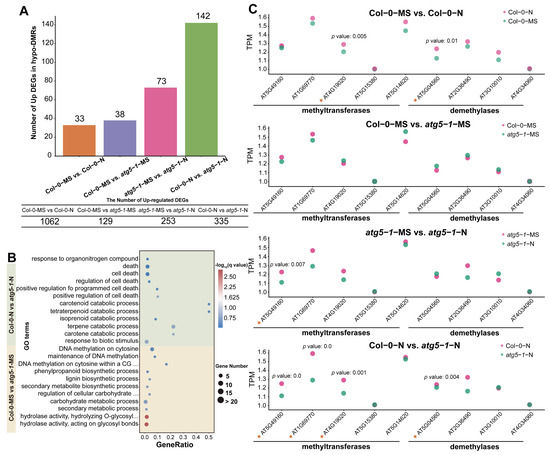
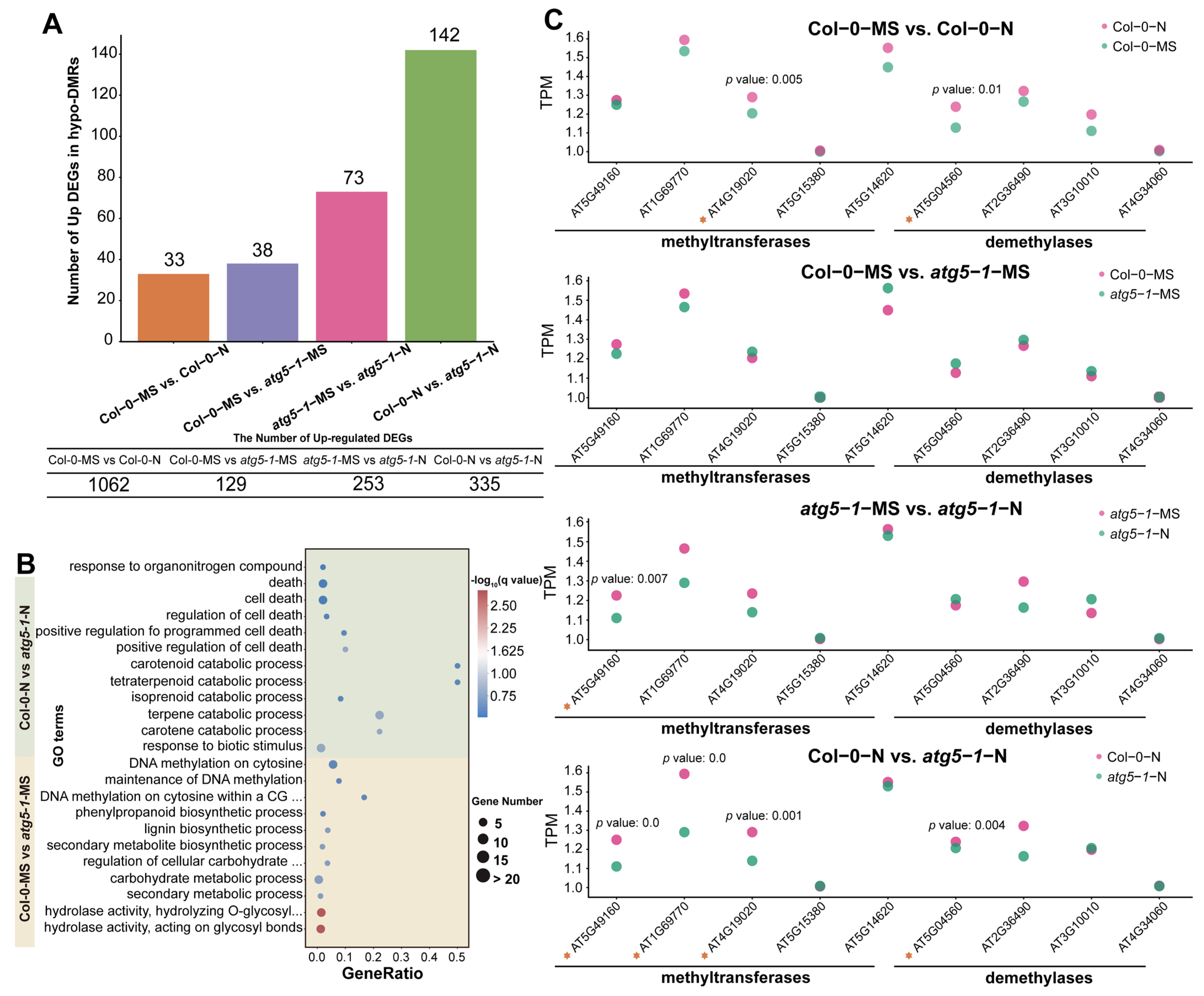
Figure 3.
DNA methylation affects the response of autophagic mutants to nitrogen deficiency. (A) The number of hypo-DMRs associated with upregulated DEGs. For Col-0-N vs. atg5-1-N, half of the upregulated DEGs were associated with DMRs. (B) GO enrichment of hypo-DMRs associated with upregulated DEGs. (C) The expression of methyltransferases and demethylases calculated by RNA-seq indicated that DNA methylation was involved in the regulation of gene expression. The asterisks represent the transcription factors.
GO enrichment analysis of hypo-DMRs associated with the upregulation of DEGs in different comparison groups showed that DNA hypomethylation may have a regulatory effect on nitrogen starvation and autophagy defects. This effect may be controlled by specific genes in different biological pathways. The GO enrichment of hypo-DMRs associated with upregulated DEGs in Col-0-MS vs. atg5-1-MS showed that the upregulated DEGs were most significantly enriched in GO terms associated with hydrolase activity and meta-hydroxylase activity, suggesting that DNA hypomethylation significantly regulated hydrolase activity under autophagy defects (Figure 3B). The genes encoding cellulase (glycosyl hydrolase family 5) protein (AT1G13130), glycosyl hydrolase 28 (GH28) family polygalacturonase (PG) protein, cell wall invertase 5, disease resistance protein (TIR–NBS–LRR class) family and tricoumaroylspermidine meta-hydroxylase had the highest fold change. Surprisingly, several hypo-DMR-associated DEGs were enriched in GO terms related to various substance metabolic processes and biosynthetic processes, such as the secondary metabolite biosynthetic process, the lignin biosynthetic process, the lignin metabolic process, and the negative regulation of starch metabolic process, suggesting that metabolic and biosynthetic activity is induced by autophagy defects. Interestingly, the QQS (AT3G30720), ATMYB29 (AT5G07690), XTH6 (AT5G65730), AT4G08160, and BGLU34 (AT1G47600) genes were enriched in multiple metabolism-related GO terms, and in biosynthesis-related GO terms, ATCAD8 (AT4G37990), LAC11 (AT5G03260), and ATMYB29 (AT5G07690) were also enriched. In addition, the DNA methylation processes were probably regulated. For example, DNA methylation on cytosine within a CG sequence, maintenance of DNA methylation, DNA methylation on cytosine, and all GO terms related to methylation were enriched, including only ORTH3 (AT1G57800), which indicates that ORTH3 probably plays an important role in regulating the methylation process induced by autophagy defects (Table S13).
To explore the functions regulated by the autophagy mutant compared with the wild type under nitrogen starvation, GO enrichment was performed for Col-0-N vs. atg5-1-N. The results indicated that hypomethylation greatly affected the response to biotic stimuli (Figure 3B). The DEGs that exhibited the highest fold change were RLP3 (AT1G17250), AT1G32763, AIG1 (AT1G33960), LECRK-III.2 (AT2G29250), AT5G10530 (LECRK-IX.1), AT5G42223, and SAG12 (AT5G45890). In addition, the catabolic processes for many substances were significantly enriched, and five hypo-DMR-related upregulated DEGs, AT4G18350, AT4G32810, AT4G39650, AT5G24540, and AT4G29640, were mainly involved in these processes. AT4G29640 and AT4G39650 exhibited the highest fold change, suggesting that these two genes probably play an important role in resistance when autophagic mutants are subjected to nitrogen starvation stress. This response mechanism may be due to the deficiency of autophagy function, which leads to Arabidopsis being unable to maintain the original level of DNA methylation under nitrogen deficiency, thus activating the decomposition of some substances. Meanwhile, the regulatory pathways related to cell death were also affected, and AT2G32460, AT5G10530, AT5G13320, and AT5G45890 were significantly enriched in these GO terms, suggesting that they may be key for establishing the relationship between DNA methylation and premature aging caused by autophagy deficiency. Similar to the catabolic process for many substances, the downregulation of DNA methylation levels may activate gene expression regulating senescence or death in Arabidopsis. This regulatory process may be activated by nitrogen deficiency, as there was no enrichment observed for many cell-death-related functions in the Col-0-MS vs. Col-0-N comparison group. Therefore, we speculate that defects in autophagy may affect the level of DNA methylation, and this effect reduces the tolerance of Arabidopsis to nitrogen deficiency.
Additionally, compared with the result for Col-0-MS vs. atg5-1-MS, the response to organonitrogen compounds was exclusively enriched when Arabidopsis was under nitrogen starvation, and it was probably influenced by AT2G14610, AT2G46400, AT3G44350, and AT5G59820, which likely indicates that under nitrogen deficiency, changes in the level of DNA methylation may be involved in mediating the utilization of nitrogen by Arabidopsis when the autophagy function has broken down.
We performed Student’s t-tests for the TPM (transcripts per million) value of several methyltransferases and demethylases of different comparison groups. A maximum number of methyltransferases and demethylases were differentially expressed in Col-0-N vs. atg5-1-N, which indicates that DNA methylation might be regulated by autophagy under nitrogen starvation conditions (Figure 3C).
2.4. Nitrogen Deficiency May Induce Transcription Factors of the ERF and C2H2 Type Zinc Finger Families to Regulate ABA-Induced Growth Arrest and SA Biosynthesis in atg5-1 Autophagic Mutants
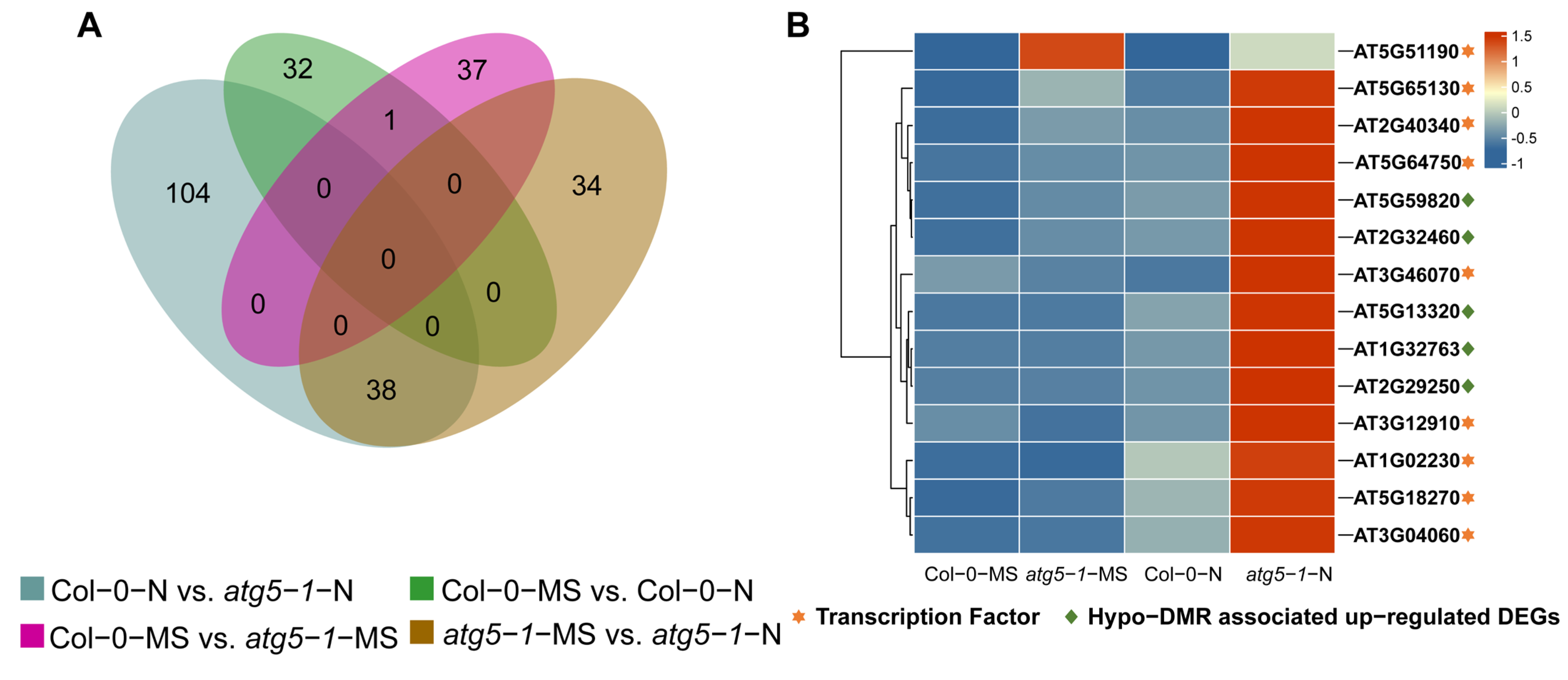
A Venn diagram was plotted for different comparison groups, and most of the upregulated DEGs were methylated exclusively in Col-0-N vs. atg5-1-N (Figure 4A). Hypo-DMR is usually associated with upregulated expression of genes through the recruitment of transcription factors (TFs). It has been reported that low-methylation regions (LMRs) can be used to identify transcriptional enhancers, and TF binding sites can usually be detected in LMRs, which tend to be occupied by cell-type-specific TFs [26,27]. Thus, we predicted TFs that can bind to hypo-DMRs for Col-0-MS vs. atg5-1-MS using PlantTFDB, and a total of 53 TFs were identified, which included 17 types of TF families, the most abundant among which were the bZIP, Dof, and GATA families [28,29]. However, the TFs associated with hypo-DMRs did not exhibit significant differences in the expression profiles determined by RNA-seq. In Col-0-N vs. atg5-1-N, a total of 26 types of TF families were predicted, the most abundant of which were the ERF, NAC, MYB, C2H2, LBD, GATA, and Trihelix families. By analyzing TF expression, AT1G02230 (ANAC004), AT2G40340 (ATERF48), AT3G04060 (ANAC046), AT3G12910, AT3G46070 (C2H2-type zinc finger family protein), AT5G18270 (ANAC087), AT5G51190 (ERF105), AT5G64750 (ERF111), and AT5G65130 (ERF57) were shown to be significantly differentially expressed (p-value < 0.05; FC ≥ 1.5), and most of them belong to the NAC and ERF families (Table S14 and Figure 4B). Among the predicted genes, TFs showed the possibility of binding to the DMRs in the AT2G32460 (MYB101), AT2G29250, AT1G32763, AT5G13320 (PBS3) and AT5G59820 (RHL41) promoter regions; GO enrichment analysis showed that these genes are involved in biotic stimulus, cell death and response to organonitrogen compounds, indicating that these functions were probably regulated by hypo-DMR-recruiting TFs.
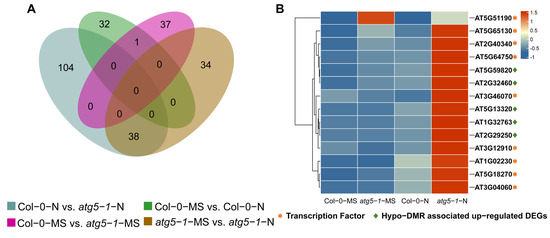
Figure 4.
Senescence-related genes were strongly influenced by DNA methylation under nitrogen deficiency. (A) Venn plot for hypo-DMRs in different comparison groups. (B) Transcription factors (TFs) predicted to bind to the promoters of hypo-DMRs associated with upregulated DEGs. Most TFs belonged to the ERF family and NAC family, and most of them were highly expressed exclusively in atg5-1-N, which shows that these TFs and hypo-DMRs associated with upregulated DEGs probably play a key role in resisting nitrogen starvation in the atg5-1 genotype. The asterisks and diamond represent the transcription factors and key genes related to plant death.
MYB101 has been confirmed to be associated with ABA-induced plant growth arrest, and transcription factors of the ERF family also play an important role in the mechanism of plant death. Our results predicted that the transcription factors binding to MYB101 belong to the ERF family, and these transcription factors were also differentially upregulated in the RNA-seq-based differential expression analysis. This seems to indicate that under nitrogen deficiency, the methylation level of the promoter region of MYB101 is downregulated, and the introduction of ERF family transcription factors binding to this region activates the ABA-induced plant growth arrest process. In addition, the transcription factor prediction results indicated that AT3G46070 (C2H2 type zinc finger family protein) could bind to ATGH3.12 (PBS3). PBS3 is the rate-limiting enzyme in the last two steps of SA biosynthesis, which controls plant aging. Therefore, we speculate that autophagic deficiency can cause significant downregulation of the DNA methylation level in the PBS3 promoter region, thus recruiting AT3G46070 to combine with this low methylation region to promote SA biosynthesis and control the premature senescence of Arabidopsis.
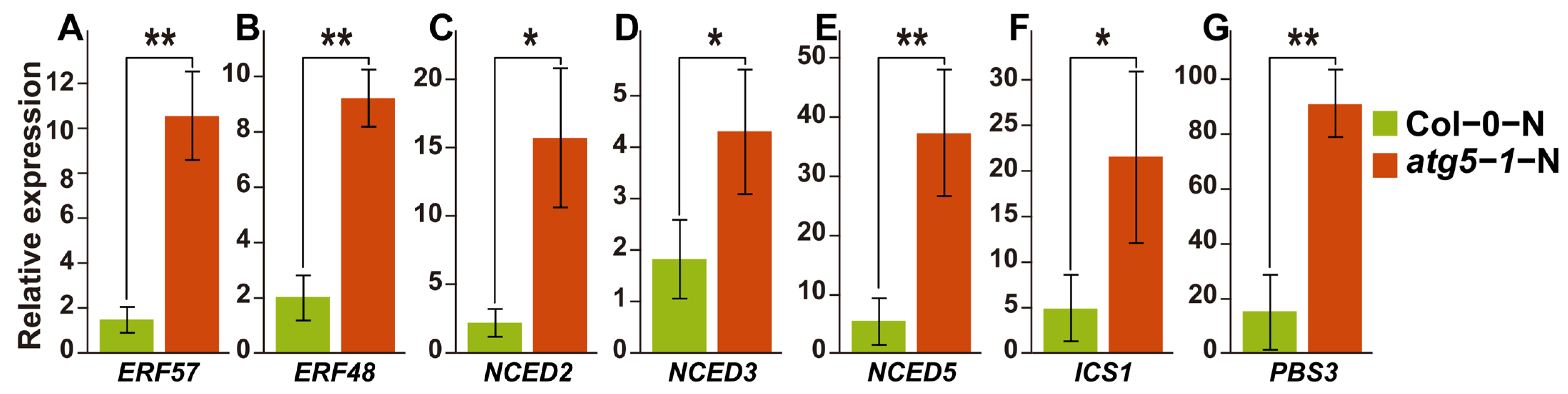
2.5. The qRT–PCR Experiments on Key Genes Involved in the SA and ABA Synthesis Pathways Confirmed the WGBS and RNA-seq Analysis Results
Based on our hypothesis, DNA methylation is involved in the process of ABA-induced growth arrest of Arabidopsis in Col-0-N vs. atg5-1-N. We thus performed qRT–PCR for the DEGs mentioned above (Figure 5A). We speculate that the level of ABA would increase if it was involved in the premature senescence of the autophagy mutant under nitrogen deficiency, and this is also fundamental evidence that ABA-induced senescence is impacted by DNA methylation. Therefore, we also verified the expression levels of key genes related to ABA biosynthesis, and the results also corresponded to the RNA-seq profile and our hypothesis (Figure 5B).
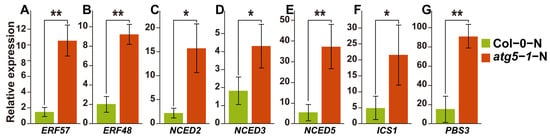
Figure 5.
qRT–PCR results of key genes and predicted transcription factors that regulate the SA and ABA hormone pathways. ** Significant difference at p < 0.01 and * significant difference at p < 0.05. Vertical bars represent the standard deviation. (A–E) qRT–PCR of key genes in ABA biosynthesis pathway and predicted transcription factors that may be associated with ABA-induced growth arrest. (F,G) The expression levels of key genes regulated by DNA methylation in the SA biosynthesis pathway.
SA biosynthesis was probably also associated with DNA methylation in our WGBS and RNA-seq analysis results, and DNA methylation might affect the expression of ATGH3.12 (PBS3), which participates in the last two steps of SA biosynthesis. We also performed qRT–PCR for the genes involved in the SA biosynthesis pathway, and the results showed that the expression of ATGH3.12 (PBS3) increased dramatically and that of key genes of the ABA biosynthesis pathway, such as ICS1, also changed significantly (Figure 5C).
The qPCR results of key genes suggested that the levels of the hormones SA and ABA were significantly upregulated, consistent with our hypothesis. This confirms the accuracy of the RNA-seq results and indicates that differential DNA methylation is highly likely related to the mechanism by which ABA and SA regulate plant growth arrest or premature senescence (Table S15).
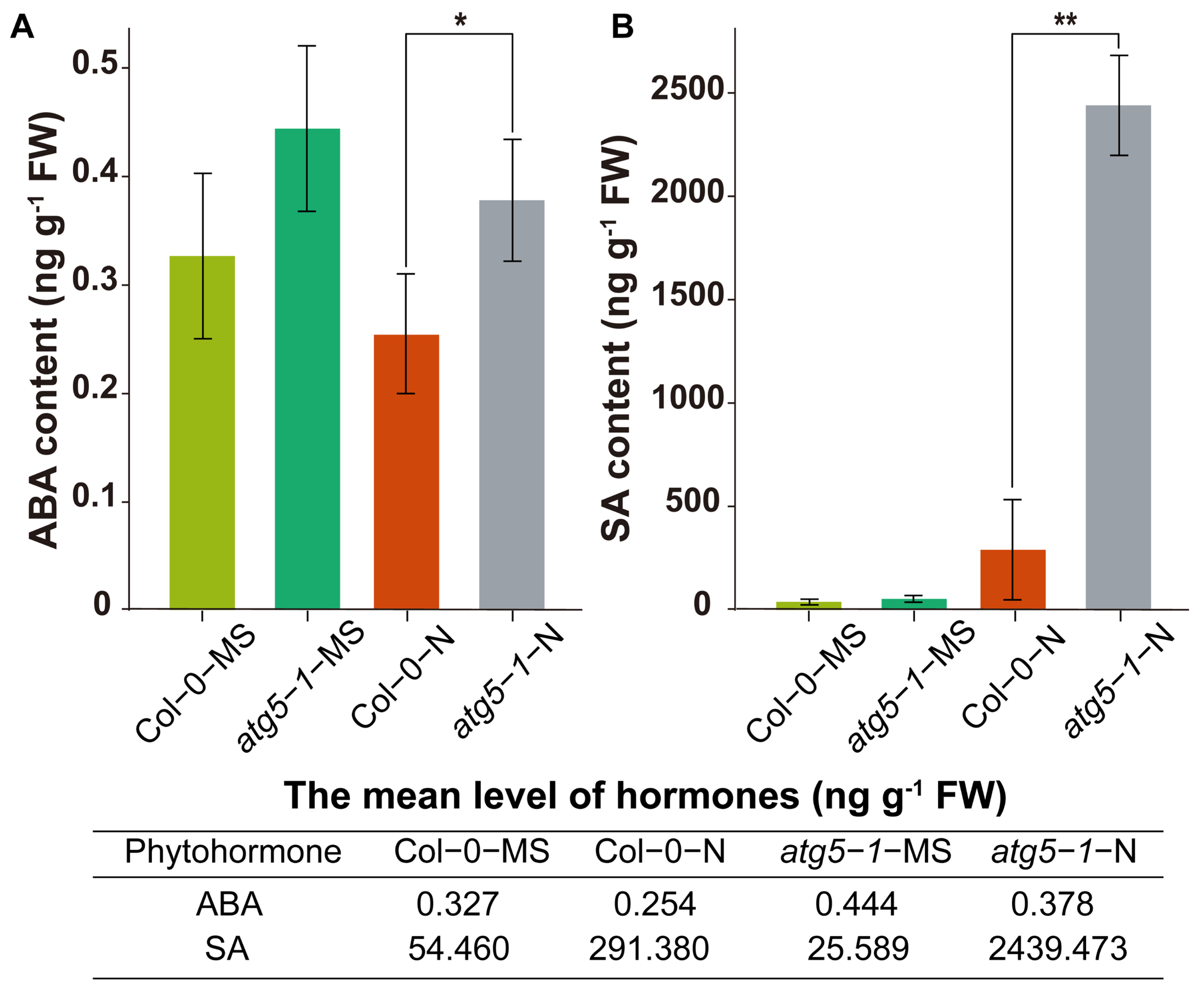
2.6. The Levels of the Hormones SA and ABA in the atg5-1 Mutant Significantly Increased under Nitrogen Deficiency
We hypothesize that the ABA and SA pathways may be regulated by DNA methylation to affect premature plant senescence. Therefore, we measured the levels of SA and ABA. The results of ABA determination showed that the hormone levels were significantly higher in the atg5-1-N group than in the Col-0-N group (Figure 6A). The level of SA showed a significant increase in atg5-1-N, exhibiting a more than eight-fold change compared with Col0-N (Figure 6B).
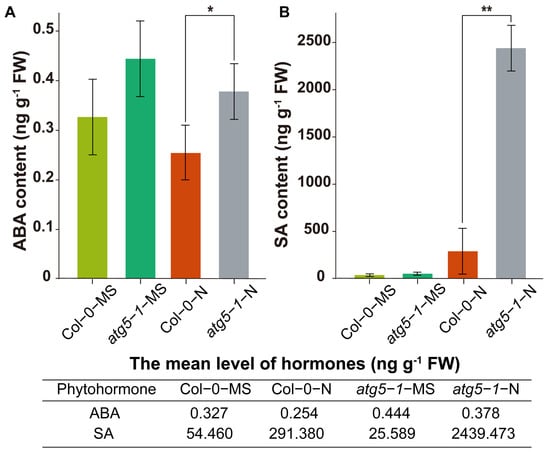
Figure 6.
Quantification of the phytohormones ABA and SA. ** Significant difference at p < 0.01 and * significant difference at p < 0.05. Vertical bars represent the standard deviation. The levels of the hormones ABA (A) and SA (B) in the autophagic mutant were significantly higher than those in the wild type under nitrogen starvation.
Hormonal measurements indicated that deficiencies in autophagy function may reduce the tolerance of Arabidopsis to nitrogen starvation, thus directly or indirectly changing the DNA methylation level, thereby triggering an increase in SA and ABA levels and leading to premature aging.
3. Discussion
DNA methylation is an epigenetic modification that commonly occurs during plant growth and development and plays an important role in regulating various biological processes [30], including leaf senescence and plant cell death [31,32,33]. In research on autophagy in plants, it has been proven that the response to nutrition deficiency, senescence, intracellular degradation and recycling of amino acids, and other stresses from various environments can be regulated by autophagy [34,35]. Nitrogen is a crucial nutrient element required for plant growth, and autophagy, which is associated with plant aging, is activated as a response to nitrogen deprivation [36,37,38]. Nevertheless, it remains ambiguous whether DNA methylation plays a role in autophagy triggered by nitrogen deprivation. In previous research, Arabidopsis Col-0 and atg5-1 mutant lines exhibited distinct phenotypic characteristics while growing under MS and nitrogen starvation conditions. Notably, the atg5-1 genotype exhibited faster senescence and cell death under nitrogen starvation conditions, implying that autophagy potentially expedites senescence and cell death in comparison to Col-0-N. The dynamic changes in DNA methylation in Col-0-N and atg5-1-N corresponded to phenotype, wherein the number of hypo-DMRs was highest in the CG, CHG, and CHH contexts (Figure 1C), and among all the groups, 142 upregulated DEGs associated with hypo-DMRs were the most abundant in this comparison group (Figure 3A). The findings suggest that under conditions of nitrogen starvation, the atg5-1 genotype may reduce the expression of methylated regions and potentially attract transcription factors to regulate gene expression and react to nitrogen starvation [39,40]. Based on the above results, the prediction of TFs that bind to hypo-DMRs was performed on Col-0-N vs. atg5-1-N, identifying nine TFs (ERF105, ERF57, ATERF48, ERF111, AT3G46070, AT3G12910, ANAC004, ANAC087, ANAC046) and five upregulated DEGs (ATZAT12, ATMYB101, ATGH3.12, AT1G32763, LECRK-III.2) binding with the above nine TFs from the GO terms response to biotic stimulus, cell death, catabolic process, and response to organonitrogen compound.
3.1. DNA Methylation Participates in Arabidopsis Senescence through the Regulation of ABA Biosynthesis
The process of PCD, known as senescence, has been found to be regulated by endogenous ethylene production under the control of specific transcription factors [41,42]. In our study, hypo-DMR-associated upregulated DEGs that might be regulated by TFs were identified, including MYB101 (AT2G32460) and ATGH3.12 (AT5G13320). Previous research indicates that MYB101 functions as a constructive regulatory element in ABA-stimulated growth cessation, and an ineffective MYB101 mutant reduces the sensitivity of plants to ABA [43,44]. It has been observed that ABA has the potential to induce senescence in leaves through a pathway that is independent of ethylene. Various scientific reports have indicated that the genes that encode rate-limiting enzymes involved in the biosynthesis of ABA include NCED2, NCED3, NCED5, NCED6, and NCED9 [45,46]. These five genes were differentially expressed (p-value < 0.1) in our analysis of gene expression differences between Col-0-N and atg5-1-N. Notably, NCED3 was identified as the most crucial rate-limiting enzyme (Figure 5B). It can be inferred that atg5-1 induces high expression of MYB101 under nitrogen starvation conditions, which consequentially exerts a positive regulatory effect on ABA synthesis and consequentially regulates Arabidopsis senescence. This regulatory relationship may be due to the downregulation of the methylation of the MYB101 promoter region, which is achieved by recruiting TFs. Our investigation revealed that there are five TFs that have the capacity to bind to hypo-DMRs within the promoter region of the MYB101 gene. Notably, these genes belong predominantly to the ERF family. Several research studies have demonstrated the participation of transcription factors from the ERF family in the deterioration and mortality mechanisms of plants. Based on research conducted by Chen, Y. et al., ERF.F5 plays a pivotal role in the senescence of tomato leaves. Specifically, the study indicated that ERF.F5 can directly regulate the promoter activity of ACS6 and interact with MYC2, which ultimately leads to the regulation of tomato leaf senescence [47]. The transcription factor EPI1, also belonging to the ERF family, plays a negative regulatory role in dark-induced and JA-stimulated aging [48]. Furthermore, Juanxu Liu and colleagues discovered a robust correlation between numerous ERF genes and an increase in ethylene levels in petals and pistils during their examination of petunia blooms. This correlation is intimately linked with the process of petunia flower aging [49]. Therefore, we speculate that ERF57, ERF105, ERF48, and ERF111 may be potential transcription factors that affect the aging of autophagic mutants under nitrogen starvation conditions. They are highly likely to bind to the hypo-DMR of the MYB101 promoter, which affects the premature aging of the atg5-1 mutant by upregulating the expression of MYB101.
3.2. SA Biosynthesis Was Probably Regulated by DNA Methylation
The ATGH3.12/PBS3 enzyme belongs to the GH3 acyl adenosylase enzyme family and plays a vital role in the accumulation of SA. Its main function is facilitating the conversion of isochorismate to unstable intermediates. Together with EPS1, it forms a two-step metabolic pathway in Arabidopsis to form SA, and this pathway is involved in the last two steps of SA formation [50]. SA plays an important role in regulating innate immunity and various developmental processes in plants. Research conducted by Niu, F. et al. showed that the transcription factor WRKY42 can regulate the aging process in Arabidopsis leaves by regulating the synthesis of SA [51,52]. According to reports, ICS1 is the key enzyme controlling SA synthesis, controlling 90% of this process [53,54]. Our study has demonstrated that there are significant changes in gene expression for two crucial enzymes, namely, ATGH3.12(PBS3) and ICS1 (p-value < 0.05; FC > 1.5) (Figure 5), which means that SA biosynthesis is likely to be regulated, and DNA methylation may be involved in regulating the expression level of ATGH3.12/PBS3, thereby affecting SA biosynthesis. We also predicted a transcription factor (AT3G46070) that can probably bind to the hypo-DMR of the promoter of ATGH3.12 (PBS3) and is differentially expressed; this transcription factor belongs to the C2H2-type zinc finger family of proteins.
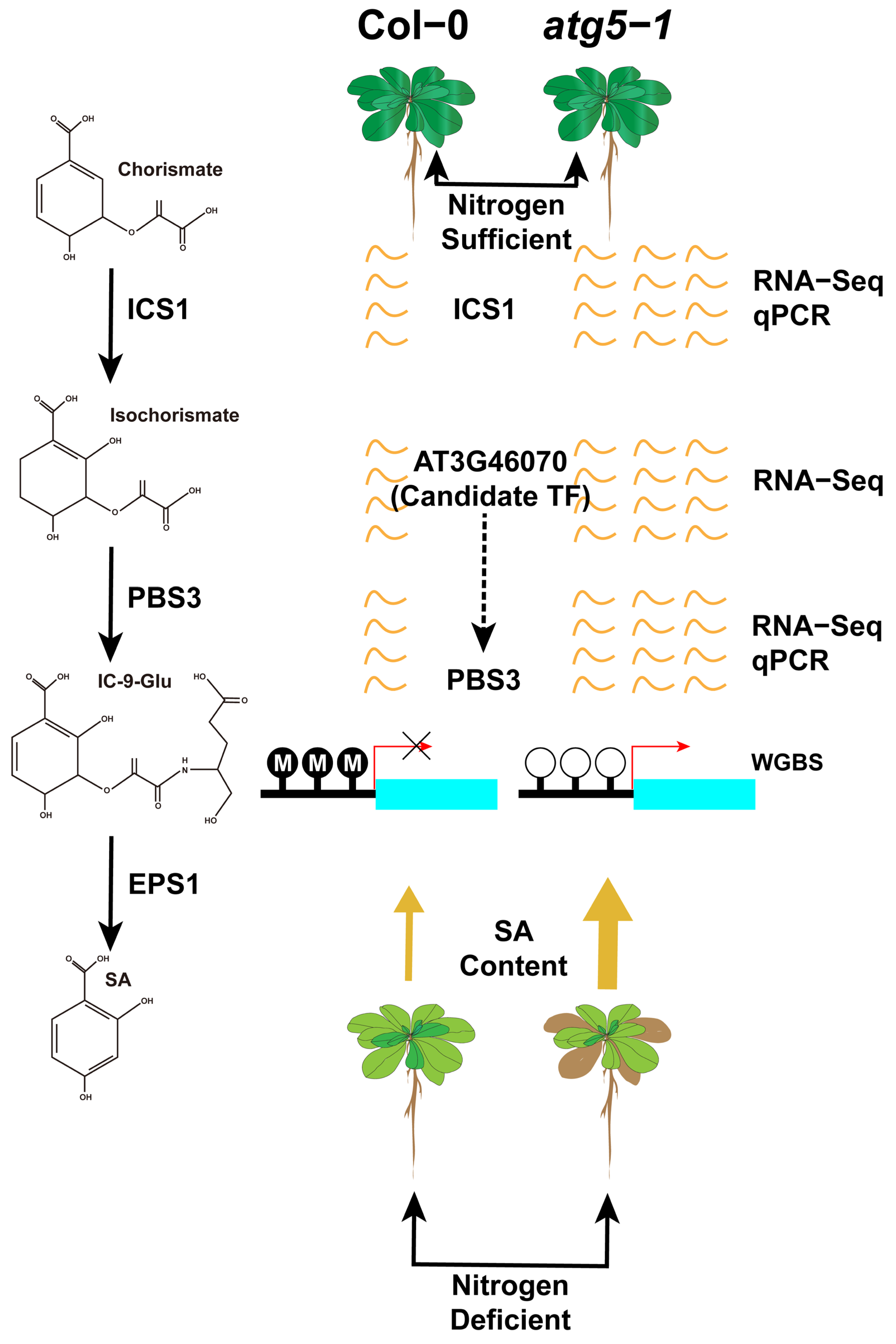
Based on hormone level determination, our findings indicate that DNA methylation has a greater impact on SA biosynthesis, which probably contributes greatly to the premature senescence of Arabidopsis (Figure 7).
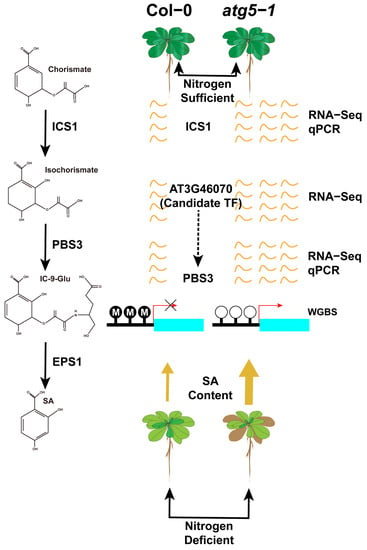
Figure 7.
DNA methylation may regulate premature aging of atg5-1 mutants under nitrogen starvation conditions by mediating the expression level of PBS3. By predicting transcription factors in the regions where hypomethylation occurs in the PBS3 promoter, it is speculated that AT3G46070 expressing transcription factors may be recruited to the hypomethylation regions, leading to an upregulation of PBS3 expression levels.
4. Materials and Methods
4.1. Plant Materials
The Arabidopsis thaliana Col-0 ecotype was used to elucidate methylation and transcription changes under nitrogen starvation and autophagy defects. The seeds were first cultivated in MS liquid culture medium for 7 days (16 h light/8 h darkness; constant temperature 22 °C; LED lighting; 120 µmol m−2 s−1) and then transferred into MS nitrogen starvation culture medium (MS-N) (MSP21-50LT) and cultivated for another 60 h (16 h light/8 h darkness; constant temperature 22 °C; LED lighting; 120 µmol m−2 s−1). The samples were dehydrated and snap-frozen in liquid nitrogen for subsequent WGBS, RNA-seq, and qRT–PCR analyses.
4.2. WGBS and Data Analysis
The DNA of the samples was extracted, and then an ultrasonic DNA fragmentation instrument was used to cleave the DNA sample (adding a certain proportion of Lambda DNA). A QSeq400 was used to verify cleavage (200–400 bp), and magnetic beads were used for purification. The purified product was subjected to bisulfite treatment and purification using the Method Gold Kit (ZYMO; Irvine, CA, USA). Finally, the product was amplified by PCR and sequenced using the Illumina platform at igenebook (www.ignenbook.com). The sequence of the sample was compared with that of lambda DNA to calculate the bisulfite conversion rate.
We used fastqc to evaluate raw data, and cutadapt [55] was used to remove low-quality bases from the 3′ ends, keeping the N base at no more than 5% of the total length and the length of reads at not less than 30 bp. In the mapping process, clean data were mapped to the Arabidopsis thaliana [56] reference genome by BSMAP [57]. The bisulfite conversion rate was calculated according to the result of mapping with lambda DNA. The coverage of cytosine bases across the genome and methylation level of three types of contexts (CG, CHG, CHH) were determined by CGmaptools. A PCA for each treatment group was performed according to the methylation level of each cytosine in the whole genome to evaluate the methylation level of each sample. The DMCs and DMRs were calculated using the CGmaptools dms and CGmaptools dmr tools (p-value < 0.001, ΔmC ≥ 0.2) [58]. For DMR-related genes, the differentially expressed genes (DEGs) identified from RNA-seq data (|log2FC| ≥ 2 and FDR < 0.01) and located within 2000 bp upstream or downstream of the DMR were defined as DMR-related genes. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analyses were established by the Omicshare cloud platform with a Q-value cutoff of 0.05 (https://www.omicshare.com/tools/ accessed on 9 September 2023).
4.3. Transcriptome Sequencing (RNA-seq) and Data Analysis
Total RNA was extracted from each sample to prepare RNA libraries, and then RNA sequencing libraries were sequenced by IGENEBOOK Biotechnology Co., Ltd. (Wuhan, China) Genes with |log2FC| ≥ 2 and p-value < 0.05 were defined as differentially expressed genes (DEGs) [59]. The Q-values obtained by Fisher’s exact test were adjusted for multiple comparisons with the false discovery rate [60].
4.4. qRT–PCR and Determination of Hormone Content
Col-0 and atg5-1 plants were grown in complete Murashige and Skoog (MS) medium for 1 week, which was then changed to −N or +N liquid medium. Sixty hours later, plants were harvested from different groups (three biological replicates). Total RNA was extracted by a FastPure Plant Total RNA Isolation Kit (Vazyme, Nanjing, China) and reverse transcribed to cDNA by HiScript III RT SuperMix (Vazyme) according to the manufacturer’s protocol. qPCR was performed using a CFX Connect PCR system (Bio-Rad, Hercules, CA, USA) according to the SYBR protocol (ChamQ Universal SYBR qPCR Master Mix, Vazyme), and the expression level was determined by the 2 − ΔΔCT method.
An amount of 50 mg of plant sample was weighed into a 2 mL plastic microtube, frozen in liquid nitrogen, and dissolved in 1 mL of methanol/water/formic acid (15:4:1, v/v/v). Ten microliters of internal standard mixed solution (100 ng/mL) were added to the extract as an internal standard (IS) for quantification. The mixture was vortexed for 10 min and then centrifuged for 5 min (12,000 r/min, 4 °C). The supernatant was transferred to a clean plastic microtube, evaporated to dryness, dissolved in 100 μL of 80% methanol (v/v), and passed through a 0.22 μM membrane filter for further LC–MS/MS analysis [61,62,63,64].
5. Conclusions
In this study, DNA methylation sequencing and RNA-seq sequencing of the whole genome of Arabidopsis thaliana showed that the expression of many genes was widely activated under nitrogen deficiency, especially when the autophagy function was defective, and the level of DNA methylation changed significantly. Under nitrogen deficiency, according to the analysis of DNA hypomethylation positions in autophagic mutants, many hypo-DMRs were found in the promoter regions of genes, which were largely related to the growth and death mechanisms of Arabidopsis. In our study, we found significant hypomethylation in the promoter regions of MYB101 and ATGH3.12 (PBS3). Based on the hypo-DMR sequence, we predicted transcription factors that may bind to the genes mentioned above (ERF57, ERF48, ERF111, ERF105, and AT3G46070) and verified differential upregulation of MYB101, ATGH3.12 (PBS3), and related transcription factors through RNA-seq and qRT–PCR. MYB101 and ATGH3.12 (PBS3) have been confirmed to be associated with the biosynthesis of ABA and SA and are involved in regulating plant aging. Therefore, we applied LC–MS/MS technology to determine the hormone content of SA and ABA, and the results showed that under nitrogen-deficient growth conditions, the levels of SA and ABA in autophagic mutants were significantly upregulated compared to those in the wild type. By combining the results of WBGS, RNA-seq, and qRT–PCR, we speculated that under nitrogen deficiency, the premature senescence of the autophagy mutant of Arabidopsis was probably due to the change in DNA methylation level in the promoter region of MYB101 and ATGH3.12 (PBS3), leading to the recruitment of ERF family and C2H2-type zinc-finger family transcription factors (AT3G46070) to activate their own expression and then regulate ABA and SA to promote premature senescence.
In addition, in subsequent studies, we should focus on the molecular mechanism of autophagy affecting DNA methylation and regulating key genes in SA- and ABA-related pathways under nitrogen deficiency conditions and evaluate the impact of DNA methylation during plant growth.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms241814047/s1.
Author Contributions
The authors confirm their contributions to this work: W.H. and F.L. in conceptualization; Y.S. in original draft preparation, data analysis, and writing; B.Y. and S.C. in sample preparation and qRT–PCR; and W.H. and F.L. in revising and review. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (32100297 to F.L.), the Jiangxi Provincial Natural Science Foundation of China (20224BAB215002 to F.L.), the Jiangxi Provincial Introduced Intelligence Program (20212BCJ25024 to F.L.), and the Jiangxi Provincial International Science and Technology Cooperation Program (S2023KJHZH0040 to F.L.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The raw sequence data reported in this paper have been deposited in the Genome Sequence Archive in National Genomics Data Center, China National Center for Bioinformation/Beijing Institute of Genomics, Chinese Academy of Sciences (GSA: CRA012212 for WGBS; GSA: CRA012214 for RNA-seq) that are publicly accessible at https://ngdc.cncb.ac.cn/gsa/s/InLHU0Ef and https://ngdc.cncb.ac.cn/gsa/s/W6sx307x, accessed on 9 September 2023.
Acknowledgments
We apologize to the authors whose works are not cited because of space limitations.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhou, Y.; Manghwar, H.; Hu, W.; Liu, F. Degradation Mechanism of Autophagy-Related Proteins and Research Progress. Int. J. Mol. Sci. 2022, 23, 7301. [Google Scholar] [CrossRef] [PubMed]
- Sienko, K.; Poormassalehgoo, A.; Yamada, K.; Goto-Yamada, S. Microautophagy in Plants: Consideration of Its Molecular Mechanism. Cells 2020, 9, 887. [Google Scholar] [CrossRef] [PubMed]
- Marshall, R.S.; Vierstra, R.D. Autophagy: The Master of Bulk and Selective Recycling. Annu. Rev. Plant Biol. 2018, 69, 173–208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ding, X.; Marshall, R.S.; Paez-Valencia, J.; Lacey, P.; Vierstra, R.D.; Otegui, M.S. Reticulon proteins modulate autophagy of the endoplasmic reticulum in maize endosperm. Elife 2020, 9, e51918. [Google Scholar] [CrossRef]
- Suttangkakul, A.; Li, F.; Chung, T.; Vierstra, R.D. The ATG1/ATG13 protein kinase complex is both a regulator and a target of autophagic recycling in Arabidopsis. Plant Cell 2011, 23, 3761–3779. [Google Scholar] [CrossRef] [PubMed]
- Thirumalaikumar, V.P.; Gorka, M.; Schulz, K.; Masclaux-Daubresse, C.; Sampathkumar, A.; Skirycz, A.; Vierstra, R.D.; Balazadeh, S. Selective autophagy regulates heat stress memory in Arabidopsis by NBR1-mediated targeting of HSP90.1 and ROF1. Autophagy 2021, 17, 2184–2199. [Google Scholar] [CrossRef]
- Shangguan, L.; Fang, X.; Chen, L.; Cui, L.; Fang, J. Genome-wide analysis of autophagy-related genes (ARGs) in grapevine and plant tolerance to copper stress. Planta 2018, 247, 1449–1463. [Google Scholar] [CrossRef]
- Eshkiki, E.M.; Hajiahmadi, Z.; Abedi, A.; Kordrostami, M.; Jacquard, C. In Silico Analyses of Autophagy-Related Genes in Rapeseed (Brassica napus L.) under Different Abiotic Stresses and in Various Tissues. Plants 2020, 9, 1393. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wang, Q.; Manghwar, H.; Liu, F. Autophagy-Mediated Regulation of Different Meristems in Plants. Int. J. Mol. Sci. 2022, 23, 6236. [Google Scholar] [CrossRef]
- Yan, H.; Zhuang, M.; Xu, X.; Li, S.; Yang, M.; Li, N.; Du, X.; Hu, K.; Peng, X.; Huang, W.; et al. Autophagy and its mediated mitochondrial quality control maintain pollen tube growth and male fertility in Arabidopsis. Autophagy 2023, 19, 768–783. [Google Scholar] [CrossRef]
- Byun, S.; Seok, S.; Kim, Y.C.; Zhang, Y.; Yau, P.; Iwamori, N.; Xu, H.E.; Ma, J.; Kemper, B.; Kemper, J.K. Fasting-induced FGF21 signaling activates hepatic autophagy and lipid degradation via JMJD3 histone demethylase. Nat. Commun. 2020, 11, 807. [Google Scholar] [CrossRef]
- Gonzalez-Rodriguez, P.; Cheray, M.; Fullgrabe, J.; Salli, M.; Engskog-Vlachos, P.; Keane, L.; Cunha, V.; Lupa, A.; Li, W.; Ma, Q.; et al. The DNA methyltransferase DNMT3A contributes to autophagy long-term memory. Autophagy 2021, 17, 1259–1277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lang, Z.; Zhu, J.K. Dynamics and function of DNA methylation in plants. Nat. Rev. Mol. Cell Biol. 2018, 19, 489–506. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Mithani, A.; Belfield, E.J.; Mott, R.; Hurst, L.D.; Harberd, N.P. Environmentally responsive genome-wide accumulation of de novo Arabidopsis thaliana mutations and epimutations. Genome Res. 2014, 24, 1821–1829. [Google Scholar] [CrossRef]
- Yong-Villalobos, L.; Gonzalez-Morales, S.I.; Wrobel, K.; Gutierrez-Alanis, D.; Cervantes-Perez, S.A.; Hayano-Kanashiro, C.; Oropeza-Aburto, A.; Cruz-Ramirez, A.; Martinez, O.; Herrera-Estrella, L. Methylome analysis reveals an important role for epigenetic changes in the regulation of the Arabidopsis response to phosphate starvation. Proc. Natl. Acad. Sci. USA 2015, 112, E7293–E7302. [Google Scholar] [CrossRef]
- Khan, A.R.; Enjalbert, J.; Marsollier, A.C.; Rousselet, A.; Goldringer, I.; Vitte, C. Vernalization treatment induces site-specific DNA hypermethylation at the VERNALIZATION-A1 (VRN-A1) locus in hexaploid winter wheat. BMC Plant Biol. 2013, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, Q.; Sun, M.; Zhang, T.; Li, H.; Chen, B.; Xu, K.; Gao, G.; Li, F.; Yan, G.; et al. Global DNA methylation variations after short-term heat shock treatment in cultured microspores of Brassica napus cv. Topas. Sci. Rep. 2016, 6, 38401. [Google Scholar] [CrossRef]
- Li, S.; Xia, Q.; Wang, F.; Yu, X.; Ma, J.; Kou, H.; Lin, X.; Gao, X.; Liu, B. Laser Irradiation-Induced DNA Methylation Changes Are Heritable and Accompanied with Transpositional Activation of mPing in Rice. Front. Plant Sci. 2017, 8, 363. [Google Scholar] [CrossRef]
- Narsai, R.; Secco, D.; Schultz, M.D.; Ecker, J.R.; Lister, R.; Whelan, J. Dynamic and rapid changes in the transcriptome and epigenome during germination and in developing rice (Oryza sativa) coleoptiles under anoxia and re-oxygenation. Plant J. 2017, 89, 805–824. [Google Scholar] [CrossRef]
- Sanchez, D.H.; Paszkowski, J. Heat-induced release of epigenetic silencing reveals the concealed role of an imprinted plant gene. PLoS Genet. 2014, 10, e1004806. [Google Scholar] [CrossRef]
- Bocchini, M.; Bartucca, M.L.; Ciancaleoni, S.; Mimmo, T.; Cesco, S.; Pii, Y.; Albertini, E.; Del Buono, D. Iron deficiency in barley plants: Phytosiderophore release, iron translocation, and DNA methylation. Front. Plant Sci. 2015, 6, 514. [Google Scholar] [CrossRef] [PubMed]
- Henderson, I.R.; Jacobsen, S.E. Epigenetic inheritance in plants. Nature 2007, 447, 418–424. [Google Scholar] [CrossRef]
- Li, S.; Tollefsbol, T.O. DNA methylation methods: Global DNA methylation and methylomic analyses. Methods 2021, 187, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Nolan, T.M.; Yin, Y.; Bassham, D.C. Identification of transcription factors that regulate ATG8 expression and autophagy in Arabidopsis. Autophagy 2020, 16, 123–139. [Google Scholar] [CrossRef]
- Yang, X.; Srivastava, R.; Howell, S.H.; Bassham, D.C. Activation of autophagy by unfolded proteins during endoplasmic reticulum stress. Plant J. 2016, 85, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Stadler, M.B.; Murr, R.; Burger, L.; Ivanek, R.; Lienert, F.; Scholer, A.; van Nimwegen, E.; Wirbelauer, C.; Oakeley, E.J.; Gaidatzis, D.; et al. DNA-binding factors shape the mouse methylome at distal regulatory regions. Nature 2011, 480, 490–495. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, N.; Sklias, A.; Ecsedi, S.; Cahais, V.; Degli-Esposti, D.; Jay, A.; Ancey, P.B.; Woo, H.D.; Hernandez-Vargas, H.; Herceg, Z. Lowly methylated region analysis identifies EBF1 as a potential epigenetic modifier in breast cancer. Epigenetics 2017, 12, 964–972. [Google Scholar] [CrossRef]
- Zou, J.-J.; Cai, X.; Yang, J.; Zeng, X.; Liu, D.-X.; Huang, S.; Chen, X.; Yang, Q.-Y.; Wang, C.; Chen, H. DNA hypomethylation mediates flower opening and senescence in sweet osmanthus through auxin and ethylene responsive pathways. Postharvest Biol. Technol. 2023, 198, 112250. [Google Scholar] [CrossRef]
- Jin, J.; Tian, F.; Yang, D.C.; Meng, Y.Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2017, 45, D1040–D1045. [Google Scholar] [CrossRef]
- He, L.; Huang, H.; Bradai, M.; Zhao, C.; You, Y.; Ma, J.; Zhao, L.; Lozano-Duran, R.; Zhu, J.K. DNA methylation-free Arabidopsis reveals crucial roles of DNA methylation in regulating gene expression and development. Nat. Commun. 2022, 13, 1335. [Google Scholar] [CrossRef]
- Vatov, E.; Zentgraf, U.; Ludewig, U. Moderate DNA methylation changes associated with nitrogen remobilization and leaf senescence in Arabidopsis. J. Exp. Bot. 2022, 73, 4733–4752. [Google Scholar] [CrossRef]
- Manna, I.; Sahoo, S.; Bandyopadhyay, M. Dynamic changes in global methylation and plant cell death mechanism in response to NiO nanoparticles. Planta 2023, 257, 93. [Google Scholar] [CrossRef] [PubMed]
- Nie, W.F. DNA methylation: From model plants to vegetable crops. Biochem. Soc. Trans. 2021, 49, 1479–1487. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, M.; Wang, E.; Hu, L.; Hawkesford, M.J.; Zhong, L.; Chen, Z.; Xu, Z.; Li, L.; Zhou, Y.; et al. Genome-wide analysis of autophagy-associated genes in foxtail millet (Setaria italica L.) and characterization of the function of SiATG8a in conferring tolerance to nitrogen starvation in rice. BMC Genom. 2016, 17, 797. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, W.; Liu, F. Autophagy in the Lifetime of Plants: From Seed to Seed. Int. J. Mol. Sci. 2022, 23, 11410. [Google Scholar] [CrossRef]
- Guiboileau, A.; Yoshimoto, K.; Soulay, F.; Bataille, M.P.; Avice, J.C.; Masclaux-Daubresse, C. Autophagy machinery controls nitrogen remobilization at the whole-plant level under both limiting and ample nitrate conditions in Arabidopsis. New Phytol. 2012, 194, 732–740. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.J.; Wang, K.X.; Xia, X.J.; Shi, K.; Zhou, Y.H.; Yu, J.Q.; Zhou, J. BZR1 Mediates Brassinosteroid-Induced Autophagy and Nitrogen Starvation in Tomato. Plant Physiol. 2019, 179, 671–685. [Google Scholar] [CrossRef]
- Liu, K.; Sutter, B.M.; Tu, B.P. Autophagy sustains glutamate and aspartate synthesis in Saccharomyces cerevisiae during nitrogen starvation. Nat. Commun. 2021, 12, 57. [Google Scholar] [CrossRef]
- Spitz, F.; Furlong, E.E. Transcription factors: From enhancer binding to developmental control. Nat. Rev. Genet. 2012, 13, 613–626. [Google Scholar] [CrossRef]
- Deng, S.; Zhang, J.; Su, J.; Zuo, Z.; Zeng, L.; Liu, K.; Zheng, Y.; Huang, X.; Bai, R.; Zhuang, L.; et al. RNA m(6)A regulates transcription via DNA demethylation and chromatin accessibility. Nat. Genet. 2022, 54, 1427–1437. [Google Scholar] [CrossRef]
- Bolt, S.; Zuther, E.; Zintl, S.; Hincha, D.K.; Schmulling, T. ERF105 is a transcription factor gene of Arabidopsis thaliana required for freezing tolerance and cold acclimation. Plant Cell Env. Environ. 2017, 40, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gao, Y.; Wang, H.L.; Kan, C.; Li, Z.; Yang, X.; Yin, W.; Xia, X.; Nam, H.G.; Li, Z.; et al. Verticillium dahliae secretory effector PevD1 induces leaf senescence by promoting ORE1-mediated ethylene biosynthesis. Mol. Plant 2021, 14, 1901–1917. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.L.; Chua, N.H. ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. Plant J. 2007, 49, 592–606. [Google Scholar] [CrossRef] [PubMed]
- Wyrzykowska, A.; Bielewicz, D.; Plewka, P.; Soltys-Kalina, D.; Wasilewicz-Flis, I.; Marczewski, W.; Jarmolowski, A.; Szweykowska-Kulinska, Z. The MYB33, MYB65, and MYB101 transcription factors affect Arabidopsis and potato responses to drought by regulating the ABA signaling pathway. Physiol. Plant 2022, 174, e13775. [Google Scholar] [CrossRef] [PubMed]
- Auler, P.A.; Nogueira do Amaral, M.; Rossatto, T.; Lopez Crizel, R.; Milech, C.; Clasen Chaves, F.; Maia Souza, G.; Bolacel Braga, E.J. Metabolism of abscisic acid in two contrasting rice genotypes submitted to recurrent water deficit. Physiol. Plant 2021, 172, 304–316. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zhao, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, P.; Tang, B.; Hu, Z.; Xie, Q.; Zhou, S.; Chen, G. The AP2/ERF transcription factor SlERF.F5 functions in leaf senescence in tomato. Plant Cell Rep. 2022, 41, 1181–1195. [Google Scholar] [CrossRef]
- Chung, K.; Nakano, T.; Fujiwara, S.; Mitsuda, N.; Otsuki, N.; Tsujimoto-Inui, Y.; Naito, Y.; Ohme-Takagi, M.; Suzuki, K. The ERF transcription factor EPI1 is a negative regulator of dark-induced and jasmonate-stimulated senescence in Arabidopsis. Plant Biotechnol. 2016, 33, 235–243. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Wang, H.; Fu, Z.; Liu, J.; Yu, Y. Identification and expression analysis of ERF transcription factor genes in petunia during flower senescence and in response to hormone treatments. J. Exp. Bot. 2011, 62, 825–840. [Google Scholar] [CrossRef]
- Torrens-Spence, M.P.; Bobokalonova, A.; Carballo, V.; Glinkerman, C.M.; Pluskal, T.; Shen, A.; Weng, J.K. PBS3 and EPS1 Complete Salicylic Acid Biosynthesis from Isochorismate in Arabidopsis. Mol. Plant 2019, 12, 1577–1586. [Google Scholar] [CrossRef]
- Niu, F.; Cui, X.; Zhao, P.; Sun, M.; Yang, B.; Deyholos, M.K.; Li, Y.; Zhao, X.; Jiang, Y.Q. WRKY42 transcription factor positively regulates leaf senescence through modulating SA and ROS synthesis in Arabidopsis thaliana. Plant J. 2020, 104, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. Cellular senescence: The good, the bad and the unknown. Nat. Rev. Nephrol. 2022, 18, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Garcion, C.; Lohmann, A.; Lamodiere, E.; Catinot, J.; Buchala, A.; Doermann, P.; Metraux, J.P. Characterization and biological function of the ISOCHORISMATE SYNTHASE2 gene of Arabidopsis. Plant Physiol. 2008, 147, 1279–1287. [Google Scholar] [CrossRef]
- Wildermuth, M.C.; Dewdney, J.; Wu, G.; Ausubel, F.M. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 2001, 414, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 3, 10–12. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Krishnakumar, V.; Chan, A.P.; Thibaud-Nissen, F.; Schobel, S.; Town, C.D. Araport11: A complete reannotation of the Arabidopsis thaliana reference genome. Plant J. 2017, 89, 789–804. [Google Scholar] [CrossRef]
- Xi, Y.; Li, W. BSMAP: Whole genome bisulfite sequence MAPping program. BMC Bioinform. 2009, 10, 232. [Google Scholar] [CrossRef]
- Guo, W.; Zhu, P.; Pellegrini, M.; Zhang, M.Q.; Wang, X.; Ni, Z. CGmapTools improves the precision of heterozygous SNV calls and supports allele-specific methylation detection and visualization in bisulfite-sequencing data. Bioinformatics 2018, 34, 381–387. [Google Scholar] [CrossRef]
- Vandenbrink, J.P.; Herranz, R.; Poehlman, W.L.; Alex Feltus, F.; Villacampa, A.; Ciska, M.; Javier Medina, F.; Kiss, J.Z. RNA-seq analyses of Arabidopsis thaliana seedlings after exposure to blue-light phototropic stimuli in microgravity. Am. J. Bot. 2019, 106, 1466–1476. [Google Scholar] [CrossRef]
- Liu, N.; Cheng, F.; Zhong, Y.; Guo, X. Comparative transcriptome and coexpression network analysis of carpel quantitative variation in Paeonia rockii. BMC Genom. 2019, 20, 683. [Google Scholar] [CrossRef]
- Cai, B.D.; Zhu, J.X.; Gao, Q.; Luo, D.; Yuan, B.F.; Feng, Y.Q. Rapid and high-throughput determination of endogenous cytokinins in Oryza sativa by bare Fe3O4 nanoparticles-based magnetic solid-phase extraction. J. Chromatogr. A 2014, 1340, 146–150. [Google Scholar] [CrossRef]
- Flokova, K.; Tarkowska, D.; Miersch, O.; Strnad, M.; Wasternack, C.; Novak, O. UHPLC-MS/MS based target profiling of stress-induced phytohormones. Phytochemistry 2014, 105, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, C.; Yan, X.; Zhang, J.; Xu, J. Simultaneous analysis of ten phytohormones in Sargassum horneri by high-performance liquid chromatography with electrospray ionization tandem mass spectrometry. J. Sep. Sci. 2016, 39, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Simura, J.; Antoniadi, I.; Siroka, J.; Tarkowska, D.; Strnad, M.; Ljung, K.; Novak, O. Plant Hormonomics: Multiple Phytohormone Profiling by Targeted Metabolomics. Plant Physiol. 2018, 177, 476–489. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).