Advancements in the Application of Nanomedicine in Alzheimer’s Disease: A Therapeutic Perspective
Abstract
:1. Introduction
2. Alzheimer’s Disease
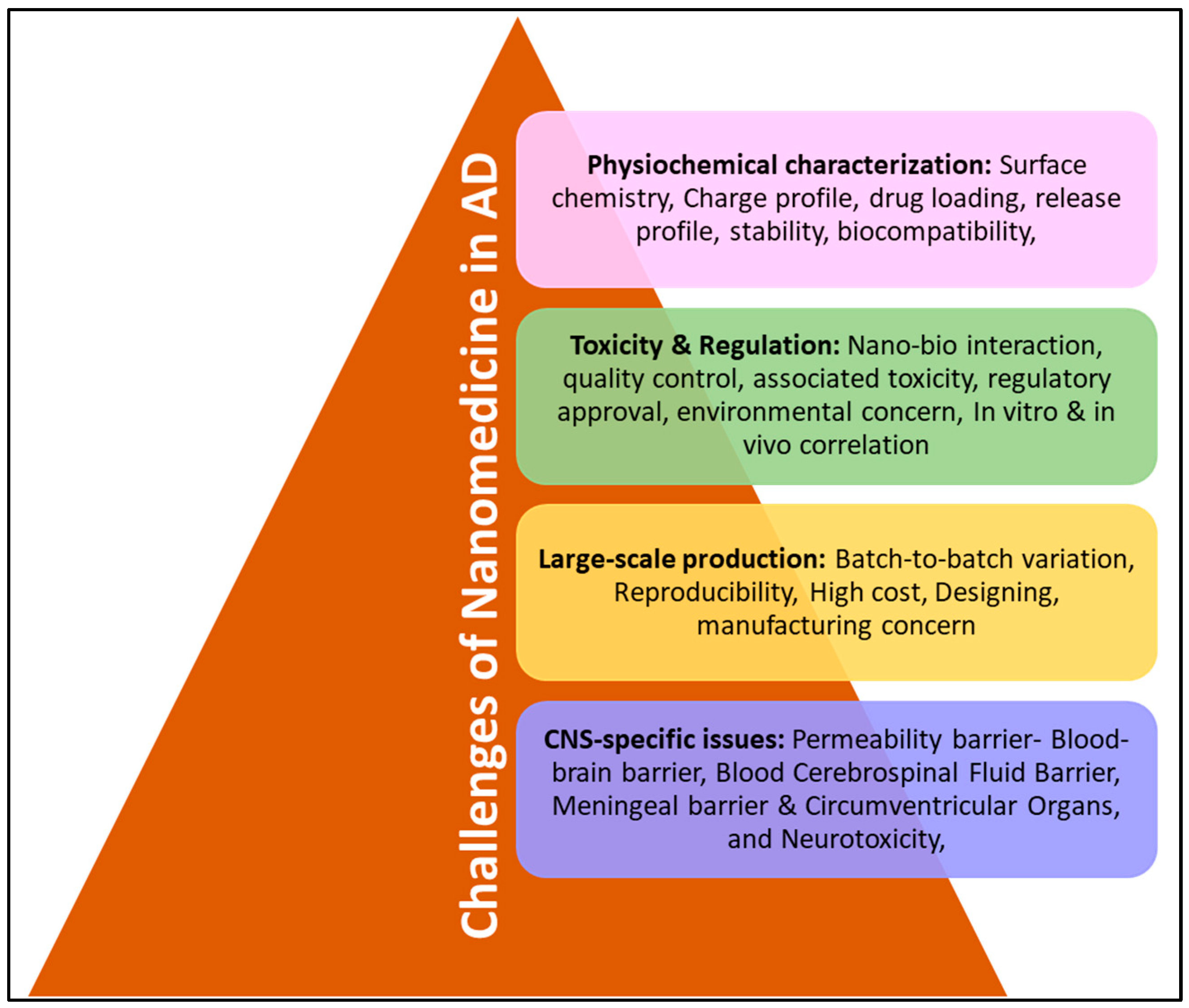
3. Challenges of Drug Designing for AD Treatment
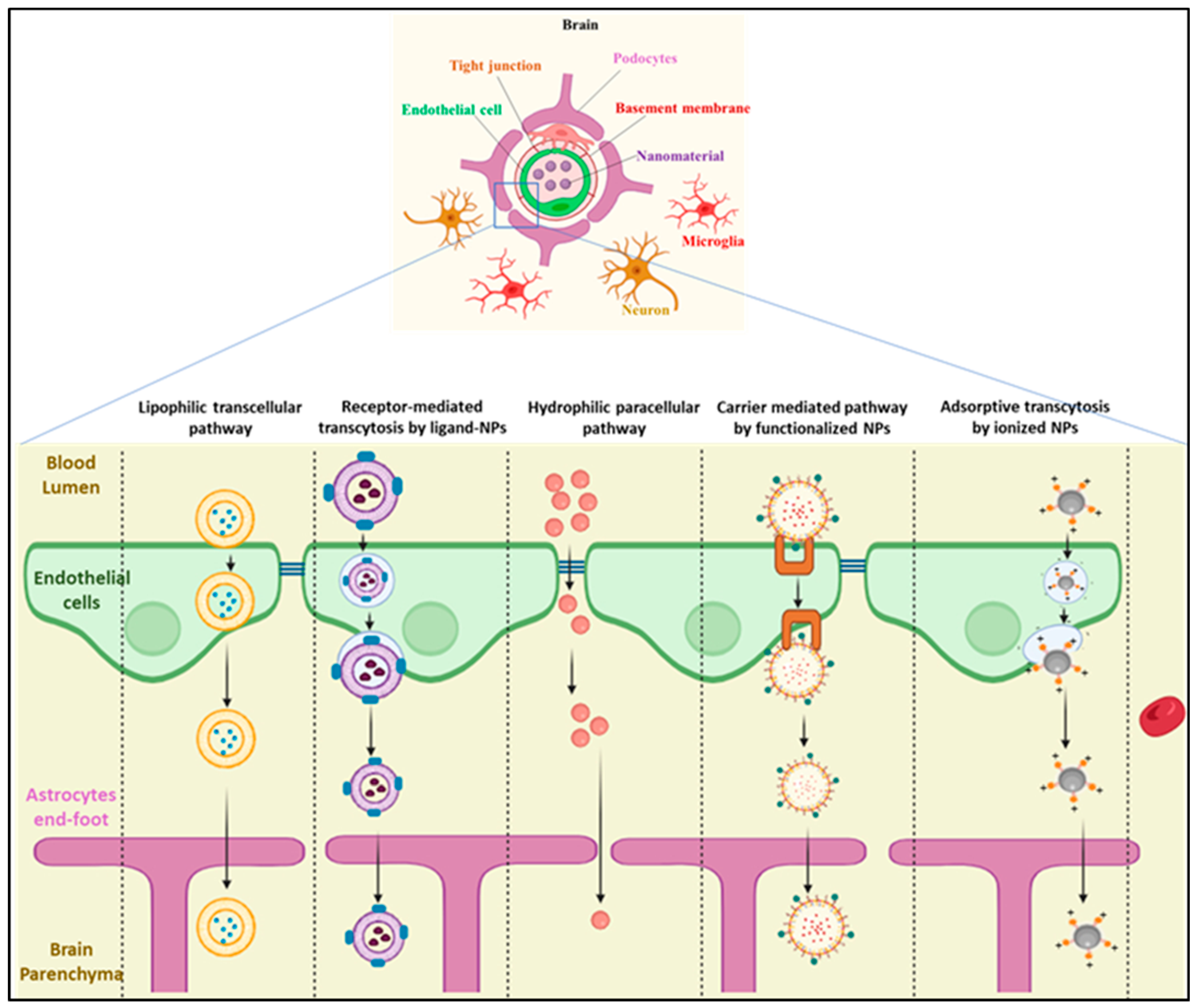
3.1. The Blood–Brain Barrier
3.2. The Blood–Cerebrospinal Fluid Barrier
3.3. Multidrug Resistance Proteins
4. Nanomedicine
4.1. Metallic/Inorganic Nanoparticles
4.2. Carbon-Based Nanoparticles
4.3. Lipid-Based Nanocarriers
4.4. Polymeric Nanoparticles
5. Discussion
6. Current Research and Future Directions of Nanomedicine
- Biodegradable nature of nanocarrier;
- Different kinds of functional groups;
- Different research study has different protocols and show different biodistribution of nanomedicine at different times;
- The ability of nanomedicine to control its morphological as well as chemical properties in the bloodstream/stability in blood;
- Nanomaterial does not show aggregation and is non-toxic, target-specific along with being pharmacodynamic and pharmacokinetic;
- In vivo condition;
- Reproducibility, predictability, accessibility, and cost-effectiveness;
- Should cross BBB and another barrier of CNS.
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babazadeh, A.; Vahed, F.M.; Jafari, S.M. Nanocarrier-mediated brain delivery of bioactives for treatment/prevention of neurodegenerative diseases. J. Control Release 2020, 321, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in cns neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, A.; Norton, N.; Fast, T.; Frölich, L.; Georges, J.; Holzapfel, D.; Kirabali, T.; Krolak-Salmon, P.; Rossini, P.M.; Ferretti, M.T.; et al. Global estimates on the number of persons across the alzheimer’s disease continuum. Alzheimers Dement. 2023, 19, 658–670. [Google Scholar] [CrossRef] [PubMed]
- DeTure, M.A.; Dickson, D.W. The neuropathological diagnosis of alzheimer’s disease. Mol. Neurodegener. 2019, 14, 32. [Google Scholar] [CrossRef] [PubMed]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Singh Chandel, A.K. Bioavailability enhancement techniques for poorly aqueous soluble drugs and therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef]
- Khan, M.I.; Zahra, Q.u.A.; Batool, F.; Kalsoom, F.; Gao, S.; Ali, R.; Wang, W.; Kazmi, A.; Lianliang, L.; Wang, G.; et al. Trends in nanotechnology to improve therapeutic efficacy across special structures. OpenNano 2022, 7, 100049. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Yadav, D.; Koul, B.; Mohanta, Y.K.; Jin, J.O. Recent advances in nanotechnology: A novel therapeutic system for the treatment of alzheimer’s disease. Curr. Drug Metab. 2020, 21, 1144–1151. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Chu, K.S.; Schorzman, A.N.; Finniss, M.C.; Bowerman, C.J.; Peng, L.; Luft, J.C.; Madden, A.J.; Wang, A.Z.; Zamboni, W.C.; DeSimone, J.M. Nanoparticle drug loading as a design parameter to improve docetaxel pharmacokinetics and efficacy. Biomaterials 2013, 34, 8424–8429. [Google Scholar] [CrossRef]
- Wechsler, M.E.; Stephenson, R.E.; Murphy, A.C.; Oldenkamp, H.F.; Singh, A.; Peppas, N.A. Engineered microscale hydrogels for drug delivery, cell therapy, and sequencing. Biomed. Microdevices 2019, 21, 31. [Google Scholar] [CrossRef]
- Wilson, B.; Geetha, K.M. Neurotherapeutic applications of nanomedicine for treating alzheimer’s disease. J. Control Release 2020, 325, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Ettcheto, M.; Cano, A.; Busquets, O.; Manzine, P.R.; Sanchez-Lopez, E.; Castro-Torres, R.D.; Beas-Zarate, C.; Verdaguer, E.; García, M.L.; Olloquequi, J. A metabolic perspective of late onset alzheimer’s disease. Pharmacol. Res. 2019, 145, 104255. [Google Scholar] [CrossRef]
- Nation, D.A.; Sweeney, M.D.; Montagne, A.; Sagare, A.P.; D’Orazio, L.M.; Pachicano, M.; Sepehrband, F.; Nelson, A.R.; Buennagel, D.P.; Harrington, M.G. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat. Med. 2019, 25, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Cano, A.; Turowski, P.; Ettcheto, M.; Duskey, J.T.; Tosi, G.; Sánchez-López, E.; García, M.L.; Camins, A.; Souto, E.B.; Ruiz, A.; et al. Nanomedicine-based technologies and novel biomarkers for the diagnosis and treatment of alzheimer’s disease: From current to future challenges. J. Nanobiotechnol. 2021, 19, 122. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine III, H. Alzheimer’s disease and the amyloid-β peptide. J. Alzheimers Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef]
- Takahashi, R.H.; Nagao, T.; Gouras, G.K. Plaque formation and the intraneuronal accumulation of β-amyloid in alzheimer’s disease. Pathol. Int. 2017, 67, 185–193. [Google Scholar] [CrossRef]
- Khan, N.H.; Mir, M.; Ngowi, E.E.; Zafar, U.; Khakwani, M.; Khattak, S.; Zhai, Y.K.; Jiang, E.S.; Zheng, M.; Duan, S.F.; et al. Nanomedicine: A promising way to manage alzheimer’s disease. Front. Bioeng. Biotechnol. 2021, 9, 630055. [Google Scholar] [CrossRef]
- Slater, C.; Wang, Q. Alzheimer’s disease: An evolving understanding of noradrenergic involvement and the promising future of electroceutical therapies. Clin. Transl. Med. 2021, 11, e397. [Google Scholar] [CrossRef]
- Selman, A.; Burns, S.; Reddy, A.P.; Culberson, J.; Reddy, P.H. The role of obesity and diabetes in dementia. Int. J. Mol. Sci. 2022, 23, 9267. [Google Scholar] [CrossRef]
- Ezkurdia, A.; Ramírez, M.J.; Solas, M. Metabolic syndrome as a risk factor for alzheimer’s disease: A focus on insulin resistance. Int. J. Mol. Sci. 2023, 24, 4354. [Google Scholar] [CrossRef]
- Fan, Y.-C.; Hsu, J.-L.; Tung, H.-Y.; Chou, C.-C.; Bai, C.-H. Increased dementia risk predominantly in diabetes mellitus rather than in hypertension or hyperlipidemia: A population-based cohort study. Alzheimers Res. Ther. 2017, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A critical appraisal of amyloid-β-targeting therapies for alzheimer disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.; Faustino, C. Therapeutic strategies targeting amyloid-β in alzheimer’s disease. Curr. Alzheimer Res. 2019, 16, 418–452. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Park, S.; Allington, G.; Prelli, F.; Sun, Y.; Martá-Ariza, M.; Scholtzova, H.; Biswas, G.; Brown, B.; Verghese, P.B. Targeting apolipoprotein e/amyloid β binding by peptoid cpo_aβ17-21 p ameliorates alzheimer’s disease related pathology and cognitive decline. Sci. Rep. 2017, 7, 8009. [Google Scholar] [CrossRef]
- Xiao, X.; Jiao, B.; Liao, X.; Zhang, W.; Yuan, Z.; Guo, L.; Wang, X.; Zhou, L.; Liu, X.; Yan, X. Association of genes involved in the metabolic pathways of amyloid-β and tau proteins with sporadic late-onset alzheimer’s disease in the southern han chinese population. Front. Aging Neurosci. 2020, 12, 584801. [Google Scholar] [CrossRef]
- Gauthier, S.; Boxer, A.; Knopman, D.; Sims, J.; Doody, R.; Aisen, P.; Iwatsubo, T.; Bateman, R.; Vellas, B. Therapeutic targets for alzheimer’s disease: Amyloid vs. Non-amyloid. Where does consensus lie today? An ctad task force report. J. Prev. Alzheimers Dis. 2022, 9, 231–235. [Google Scholar] [CrossRef]
- Novak, P.; Kovacech, B.; Katina, S.; Schmidt, R.; Scheltens, P.; Kontsekova, E.; Ropele, S.; Fialova, L.; Kramberger, M.; Paulenka-Ivanovova, N. Adamant: A placebo-controlled randomized phase 2 study of aadvac1, an active immunotherapy against pathological tau in alzheimer’s disease. Nat. Aging 2021, 1, 521–534. [Google Scholar] [CrossRef]
- Coman, H.; Nemeş, B. New therapeutic targets in alzheimer’s disease. Int. J. Gerontol. 2017, 11, 2–6. [Google Scholar] [CrossRef]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for alzheimer disease. Nat. Rev. Neurol. 2018, 14, 399–415. [Google Scholar] [CrossRef]
- Wang, J.; Chen, G.J. Mitochondria as a therapeutic target in alzheimer’s disease. Genes Dis. 2016, 3, 220–227. [Google Scholar] [CrossRef]
- Mary, A.; Eysert, F.; Checler, F.; Chami, M. Mitophagy in alzheimer’s disease: Molecular defects and therapeutic approaches. Mol. Psychiatry 2023, 28, 202–216. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D.; Reddy, P.H. Dynamics of dynamin-related protein 1 in alzheimer’s disease and other neurodegenerative diseases. Cells 2019, 8, 961. [Google Scholar] [CrossRef]
- Cenini, G.; Voos, W. Mitochondria as potential targets in alzheimer disease therapy: An update. Front. Pharmacol. 2019, 10, 902. [Google Scholar] [CrossRef] [PubMed]
- Pritam, P.; Deka, R.; Bhardwaj, A.; Srivastava, R.; Kumar, D.; Jha, A.K.; Jha, N.K.; Villa, C.; Jha, S.K. Antioxidants in alzheimer’s disease: Current therapeutic significance and future prospects. Biology 2022, 11, 212. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.P.; de Castro, A.A.; Soares, F.V.; da Cunha, E.F.F.; Ramalho, T.C. Future therapeutic perspectives into the alzheimer’s disease targeting the oxidative stress hypothesis. Molecules 2019, 24, 4410. [Google Scholar] [CrossRef]
- Cassidy, L.; Fernandez, F.; Johnson, J.B.; Naiker, M.; Owoola, A.G.; Broszczak, D.A. Oxidative stress in alzheimer’s disease: A review on emergent natural polyphenolic therapeutics. Complement. Ther. Med. 2020, 49, 102294. [Google Scholar] [CrossRef]
- Galasko, D.R.; Peskind, E.; Clark, C.M.; Quinn, J.F.; Ringman, J.M.; Jicha, G.A.; Cotman, C.; Cottrell, B.; Montine, T.J.; Thomas, R.G. Antioxidants for alzheimer disease: A randomized clinical trial with cerebrospinal fluid biomarker measures. Arch. Neurol. 2012, 69, 836–841. [Google Scholar] [CrossRef]
- Cenini, G.; Rüb, C.; Bruderek, M.; Voos, W. Amyloid β-peptides interfere with mitochondrial preprotein import competence by a coaggregation process. Mol. Biol. Cell 2016, 27, 3257–3272. [Google Scholar] [CrossRef]
- Si, Z.-Z.; Zou, C.-J.; Mei, X.; Li, X.-F.; Luo, H.; Shen, Y.; Hu, J.; Li, X.-X.; Wu, L.; Liu, Y. Targeting neuroinflammation in alzheimer’s disease: From mechanisms to clinical applications. Neural Regen. Res. 2023, 18, 708–715. [Google Scholar]
- Li, J.J.; Wang, B.; Kodali, M.C.; Chen, C.; Kim, E.; Patters, B.J.; Lan, L.; Kumar, S.; Wang, X.; Yue, J.; et al. In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J. Neuroinflammation 2018, 15, 8. [Google Scholar] [CrossRef]
- Bronzuoli, M.R.; Facchinetti, R.; Valenza, M.; Cassano, T.; Steardo, L.; Scuderi, C. Astrocyte function is affected by aging and not alzheimer’s disease: A preliminary investigation in hippocampi of 3xtg-ad mice. Front. Pharmacol. 2019, 10, 644. [Google Scholar] [CrossRef] [PubMed]
- Kuber, B.; Fadnavis, M.; Chatterjee, B. Role of angiotensin receptor blockers in the context of alzheimer’s disease. Fundam. Clin. Pharmacol. 2023, 37, 429–445. [Google Scholar] [CrossRef] [PubMed]
- Royea, J.; Hamel, E. Brain angiotensin ii and angiotensin iv receptors as potential alzheimer’s disease therapeutic targets. GeroScience 2020, 42, 1237–1256. [Google Scholar] [CrossRef] [PubMed]
- Gebre, A.K.; Altaye, B.M.; Atey, T.M.; Tuem, K.B.; Berhe, D.F. Targeting renin-angiotensin system against alzheimer’s disease. Front. Pharmacol. 2018, 9, 440. [Google Scholar] [CrossRef] [PubMed]
- Wharton, W.; Goldstein, F.C.; Zhao, L.; Steenland, K.; Levey, A.I.; Hajjar, I. Modulation of renin-angiotensin system may slow conversion from mild cognitive impairment to alzheimer’s disease. J. Am. Geriatr. Soc. 2015, 63, 1749–1756. [Google Scholar] [CrossRef]
- Mogi, M.; Iwanami, J.; Horiuchi, M. Roles of brain angiotensin ii in cognitive function and dementia. Int. J. Hypertens. 2012, 2012, 169649. [Google Scholar] [CrossRef]
- Soto, M.E.; Abellan van Kan, G.; Nourhashemi, F.; Gillette-Guyonnet, S.; Cesari, M.; Cantet, C.; Rolland, Y.; Vellas, B. Angiotensin-converting enzyme inhibitors and alzheimer’s disease progression in older adults: Results from the réseau sur la maladie d’alzheimer français cohort. J. Am. Geriatr. Soc. 2013, 61, 1482–1488. [Google Scholar] [CrossRef]
- Menting, K.W.; Claassen, J.A. Β-secretase inhibitor; a promising novel therapeutic drug in alzheimer’s disease. Front. Aging Neurosci. 2014, 6, 165. [Google Scholar] [CrossRef]
- De Strooper, B.; Vassar, R.; Golde, T. The secretases: Enzymes with therapeutic potential in alzheimer disease. Nat. Rev. Neurol. 2010, 6, 99–107. [Google Scholar] [CrossRef]
- MacLeod, R.; Hillert, E.-K.; Cameron, R.T.; Baillie, G.S. The role and therapeutic targeting of α-, β-and γ-secretase in alzheimer’s disease. Future Sci. OA 2015, 1. [Google Scholar] [CrossRef]
- Maia, M.A.; Sousa, E. Bace-1 and γ-secretase as therapeutic targets for alzheimer’s disease. Pharmaceuticals 2019, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- Kurz, C.; Walker, L.; Rauchmann, B.S.; Perneczky, R. Dysfunction of the blood–brain barrier in alzheimer’s disease: Evidence from human studies. Neuropathol. Appl. Neurobiol. 2022, 48, e12782. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood-brain barrier and delivery of protein and gene therapeutics to brain. Front. Aging Neurosci. 2020, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- Rofo, F.; Metzendorf, N.G.; Saubi, C.; Suominen, L.; Godec, A.; Sehlin, D.; Syvänen, S.; Hultqvist, G. Blood–brain barrier penetrating neprilysin degrades monomeric amyloid-beta in a mouse model of alzheimer’s disease. Alzheimers Res. Ther. 2022, 14, 180. [Google Scholar] [CrossRef]
- Mufson, E.J.; Counts, S.E.; Perez, S.E.; Ginsberg, S.D. Cholinergic system during the progression of alzheimer’s disease: Therapeutic implications. Expert Rev. Neurother. 2008, 8, 1703–1718. [Google Scholar] [CrossRef]
- H Ferreira-Vieira, T.; M Guimaraes, I.; R Silva, F.; M Ribeiro, F. Alzheimer’s disease: Targeting the cholinergic system. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef]
- Hanif, S.; Muhammad, P.; Chesworth, R.; Rehman, F.U.; Qian, R.-j.; Zheng, M.; Shi, B.-y. Nanomedicine-based immunotherapy for central nervous system disorders. Acta Pharmacol. Sin. 2020, 41, 936–953. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, H.; Zhao, J.; Lee, J.; Low, L.E.; Gong, L.; Chen, Y.; Wang, N.; Zhu, C.; Lin, P. Dynamic nanoassemblies for imaging and therapy of neurological disorders. Adv. Drug Deliv. Rev. 2021, 175, 113832. [Google Scholar] [CrossRef]
- Tian, X.; Fan, T.; Zhao, W.; Abbas, G.; Han, B.; Zhang, K.; Li, N.; Liu, N.; Liang, W.; Huang, H.; et al. Recent advances in the development of nanomedicines for the treatment of ischemic stroke. Bioact. Mater. 2021, 6, 2854–2869. [Google Scholar] [CrossRef]
- Mittal, K.R.; Pharasi, N.; Sarna, B.; Singh, M.; Haider, S.; Singh, S.K.; Dua, K.; Jha, S.K.; Dey, A.; Ojha, S. Nanotechnology-based drug delivery for the treatment of cns disorders. Transl. Neurosci. 2022, 13, 527–546. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Dengra, B.; González-Álvarez, I.; Bermejo, M.; González-Álvarez, M. Access to the cns: Strategies to overcome the bbb. Int. J. Pharm. 2023, 636, 122759. [Google Scholar] [CrossRef] [PubMed]
- Jagaran, K.; Singh, M. Nanomedicine for neurodegenerative disorders: Focus on alzheimer’s and parkinson’s diseases. Int. J. Mol. Sci. 2021, 22, 9082. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Zhang, W.; Park, H.-B.; Yadav, D.; Jeon, Y.H.; Jin, J.-O. Escherichia coli adhesin protein-conjugated thermal responsive hybrid nanoparticles for photothermal and immunotherapy against cancer and its metastasis. J. Immunother. Cancer 2021, 9, e002666. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Kwak, M.; Chauhan, P.S.; Puranik, N.; Lee, P.C.; Jin, J.-O. Cancer Immunotherapy by Immune Checkpoint Blockade and Its Advanced Application Using Bio-Nanomaterials; Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Koul, B.; Poonia, A.K.; Yadav, D.; Jin, J.O. Microbe-mediated biosynthesis of nanoparticles: Applications and future prospects. Biomolecules 2021, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Puranik, N.; Yadav, D.; Jin, J.-O.; Lee, P.C. Lipid nanocarrier-based drug delivery systems: Therapeutic advances in the treatment of lung cancer. Int. J. Nanomed. 2023, 2023, 2659–2676. [Google Scholar] [CrossRef]
- Mukherjee, S.; Madamsetty, V.S.; Bhattacharya, D.; Roy Chowdhury, S.; Paul, M.K.; Mukherjee, A. Recent advancements of nanomedicine in neurodegenerative disorders theranostics. Adv. Funct. Mater. 2020, 30, 2003054. [Google Scholar] [CrossRef]
- Ghalamfarsa, G.; Hojjat-Farsangi, M.; Mohammadnia-Afrouzi, M.; Anvari, E.; Farhadi, S.; Yousefi, M.; Jadidi-Niaragh, F. Application of nanomedicine for crossing the blood–brain barrier: Theranostic opportunities in multiple sclerosis. J. Immunotoxicol. 2016, 13, 603–619. [Google Scholar] [CrossRef]
- Maiti, S. Nanometric biopolymer devices for oral delivery of macromolecules with clinical significance. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 109–138. [Google Scholar]
- Zhu, X.; Jin, K.; Huang, Y.; Pang, Z. Brain drug delivery by adsorption-mediated transcytosis. In Brain Targeted Drug Delivery System; Elsevier: Amsterdam, The Netherlands, 2019; pp. 159–183. [Google Scholar]
- Pulgar, V.M. Transcytosis to cross the blood brain barrier, new advancements and challenges. Front. Neurosci. 2019, 12, 1019. [Google Scholar] [CrossRef]
- Khan, N.U.; Miao, T.; Ju, X.; Guo, Q.; Han, L. Carrier-mediated transportation through bbb. In Brain Targeted Drug Delivery System; Elsevier: Amsterdam, The Netherlands, 2019; pp. 129–158. [Google Scholar]
- Hervé, F.; Ghinea, N.; Scherrmann, J.-M. Cns delivery via adsorptive transcytosis. AAPS J. 2008, 10, 455–472. [Google Scholar] [CrossRef]
- Hersh, D.S.; Wadajkar, A.S.; Roberts, N.; Perez, J.G.; Connolly, N.P.; Frenkel, V.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Evolving drug delivery strategies to overcome the blood brain barrier. Curr. Pharm. Des. 2016, 22, 1177–1193. [Google Scholar] [CrossRef] [PubMed]
- Pawar, B.; Vasdev, N.; Gupta, T.; Mhatre, M.; More, A.; Anup, N.; Tekade, R.K. Current update on transcellular brain drug delivery. Pharmaceutics 2022, 14, 2719. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.-H.; Khattak, S.; Rauf, M.A.; Ansari, M.A.; Alomary, M.N.; Razak, S.; Yang, C.-Y.; Wu, D.-D.; Ji, X.-Y. Role of nanomedicine-based therapeutics in the treatment of cns disorders. Molecules 2023, 28, 1283. [Google Scholar] [CrossRef] [PubMed]
- Dabbagh, F.; Schroten, H.; Schwerk, C. In vitro models of the blood-cerebrospinal fluid barrier and their applications in the development and research of (neuro)pharmaceuticals. Pharmaceutics 2022, 14, 1729. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.; Mischeck, U.; Veyhl, M.; Henzel, K.; Galla, H.J. Blood-brain barrier characteristic enzymatic properties in cultured brain capillary endothelial cells. Brain Res. 1990, 514, 305–309. [Google Scholar] [CrossRef]
- Macdonald, J.A.; Murugesan, N.; Pachter, J.S. Endothelial cell heterogeneity of blood-brain barrier gene expression along the cerebral microvasculature. J. Neurosci. Res. 2010, 88, 1457–1474. [Google Scholar] [CrossRef]
- Vašková, J.; Kočan, L.; Vaško, L.; Perjési, P. Glutathione-related enzymes and proteins: A review. Molecules 2023, 28, 1447. [Google Scholar] [CrossRef]
- Lye, P.; Bloise, E.; Matthews, S.G. Effects of bacterial and viral pathogen-associated molecular patterns (pamps) on multidrug resistance (mdr) transporters in brain endothelial cells of the developing human blood–brain barrier. Fluids Barriers CNS 2023, 20, 8. [Google Scholar] [CrossRef]
- Yalamarty, S.S.K.; Filipczak, N.; Li, X.; Subhan, M.A.; Parveen, F.; Ataide, J.A.; Rajmalani, B.A.; Torchilin, V.P. Mechanisms of resistance and current treatment options for glioblastoma multiforme (gbm). Cancers 2023, 15, 2116. [Google Scholar] [CrossRef]
- Aryal, M.; Fischer, K.; Gentile, C.; Gitto, S.; Zhang, Y.Z.; McDannold, N. Effects on p-glycoprotein expression after blood-brain barrier disruption using focused ultrasound and microbubbles. PLoS ONE 2017, 12, e0166061. [Google Scholar] [CrossRef]
- Asil, S.M.; Ahlawat, J.; Barroso, G.G.; Narayan, M. Nanomaterial based drug delivery systems for the treatment of neurodegenerative diseases. Biomater. Sci. 2020, 8, 4109–4128. [Google Scholar] [CrossRef]
- Thoe, E.S.; Fauzi, A.; Tang, Y.Q.; Chamyuang, S.; Chia, A.Y.Y. A review on advances of treatment modalities for alzheimer’s disease. Life Sci. 2021, 276, 119129. [Google Scholar] [CrossRef] [PubMed]
- Moss, D.E.; Perez, R.G.; Kobayashi, H. Cholinesterase inhibitor therapy in alzheimer’s disease: The limits and tolerability of irreversible cns-selective acetylcholinesterase inhibition in primates. J. Alzheimers Dis. 2017, 55, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Pathak, M.; Singhal, R. Therapeutic and diagnostic applications of nanocomposites in the treatment alzheimer’s disease studies. Biointerface Res. Appl. Chem. 2022, 12, 940–960. [Google Scholar]
- Yadav, D.; Wairagu, P.M.; Kwak, M.; Jin, J.O. Nanoparticle-based inhalation therapy for pulmonary diseases. Curr. Drug Metab. 2022, 23, 882–896. [Google Scholar] [CrossRef] [PubMed]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a unique position in medicine. Nanomaterials 2023, 13, 574. [Google Scholar] [CrossRef]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid nanoparticles─from liposomes to mrna vaccine delivery, a landscape of research diversity and advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef]
- Feng, L.; Wang, H.; Xue, X. Recent progress of nanomedicine in the treatment of central nervous system diseases. Adv. Ther. 2020, 3, 1900159. [Google Scholar] [CrossRef]
- Saffari, P.M.; Alijanpour, S.; Takzaree, N.; Sahebgharani, M.; Etemad-Moghadam, S.; Noorbakhsh, F.; Partoazar, A. Metformin loaded phosphatidylserine nanoliposomes improve memory deficit and reduce neuroinflammation in streptozotocin-induced alzheimer’s disease model. Life Sci. 2020, 255, 117861. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; El-Shabasy, R.M.; Khalifa, S.A.; Saeed, A.; Shah, A.; Shah, R.; Iftikhar, F.J.; Abdel-Daim, M.M.; Omri, A.; Hajrahand, N.H. Metal nanoparticles fabricated by green chemistry using natural extracts: Biosynthesis, mechanisms, and applications. RSC Adv. 2019, 9, 24539–24559. [Google Scholar] [CrossRef]
- Zhang, W.; Taheri-Ledari, R.; Ganjali, F.; Afruzi, F.H.; Hajizadeh, Z.; Saeidirad, M.; Qazi, F.S.; Kashtiaray, A.; Sehat, S.S.; Hamblin, M.R.; et al. Nanoscale bioconjugates: A review of the structural attributes of drug-loaded nanocarrier conjugates for selective cancer therapy. Heliyon 2022, 8, e09577. [Google Scholar] [CrossRef] [PubMed]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Yan, C.; Wang, C.; Wang, C.; Cao, Y.; Zhou, Y.; Guan, P.; Hu, X.; Zhu, W.; Ding, S. Advanced nanomaterials for modulating alzheimer’s related amyloid aggregation. Nanoscale Adv. 2023, 5, 46–80. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Guan, Y.; Li, Z.; Guo, X.; Zhang, M.; Wang, D.; Tang, J. Aptamer conjugated polydopamine-coated gold nanoparticles as a dual-action nanoplatform targeting β-amyloid peptide for alzheimer’s disease therapy. J. Mater. Chem. B Mater. Biol. Med. 2022, 10, 8525–8534. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Sun, H.; Dong, K.; Ren, J.; Qu, X. Gold-nanoparticle-based multifunctional amyloid-β inhibitor against alzheimer’s disease. Chemistry 2015, 21, 829–835. [Google Scholar] [CrossRef]
- Dos Santos Tramontin, N.; da Silva, S.; Arruda, R.; Ugioni, K.S.; Canteiro, P.B.; de Bem Silveira, G.; Mendes, C.; Silveira, P.C.L.; Muller, A.P. Gold nanoparticles treatment reverses brain damage in alzheimer’s disease model. Mol. Neurobiol. 2020, 57, 926–936. [Google Scholar] [CrossRef]
- Sanati, M.; Aminyavari, S.; Khodagholi, F.; Hajipour, M.J.; Sadeghi, P.; Noruzi, M.; Moshtagh, A.; Behmadi, H.; Sharifzadeh, M. Pegylated superparamagnetic iron oxide nanoparticles (spions) ameliorate learning and memory deficit in a rat model of alzheimer’s disease: Potential participation of stims. NeuroToxicology 2021, 85, 145–159. [Google Scholar] [CrossRef]
- Yin, T.; Yang, L.; Liu, Y.; Zhou, X.; Sun, J.; Liu, J. Sialic acid (sa)-modified selenium nanoparticles coated with a high blood–brain barrier permeability peptide-b6 peptide for potential use in alzheimer’s disease. Acta Biomater. 2015, 25, 172–183. [Google Scholar] [CrossRef]
- Nazıroğlu, M.; Muhamad, S.; Pecze, L. Nanoparticles as potential clinical therapeutic agents in alzheimer’s disease: Focus on selenium nanoparticles. Expert Rev. Clin. Pharmacol. 2017, 10, 773–782. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Zhang, W.; Sigdel, G.; Mintz, K.J.; Seven, E.S.; Zhou, Y.; Wang, C.; Leblanc, R.M. Carbon dots: A future blood–brain barrier penetrating nanomedicine and drug nanocarrier. Int. J. Nanomedicine 2021, 16, 5003–5016. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liyanage, P.Y.; Devadoss, D.; Guevara, L.R.R.; Cheng, L.; Graham, R.M.; Chand, H.S.; Al-Youbi, A.O.; Bashammakh, A.S.; El-Shahawi, M.S. Nontoxic amphiphilic carbon dots as promising drug nanocarriers across the blood–brain barrier and inhibitors of β-amyloid. Nanoscale 2019, 11, 22387–22397. [Google Scholar] [CrossRef] [PubMed]
- Seven, E.S.; Sharma, S.K.; Meziane, D.; Zhou, Y.; Mintz, K.J.; Pandey, R.R.; Chusuei, C.C.; Leblanc, R.M. Close-packed langmuir monolayers of saccharide-based carbon dots at the air–subphase interface. Langmuir 2019, 35, 6708–6718. [Google Scholar] [CrossRef]
- Li, J.; Tian, M.; Cui, L.; Dwyer, J.; Fullwood, N.J.; Shen, H.; Martin, F.L. Low-dose carbon-based nanoparticle-induced effects in a549 lung cells determined by biospectroscopy are associated with increases in genomic methylation. Sci. Rep. 2016, 6, 20207. [Google Scholar] [CrossRef]
- Zhang, W.J.; Li, D.N.; Lian, T.H.; Guo, P.; Zhang, Y.N.; Li, J.H.; Guan, H.Y.; He, M.Y.; Zhang, W.J.; Zhang, W.J.; et al. Clinical features and potential mechanisms relating neuropathological biomarkers and blood-brain barrier in patients with alzheimer’s disease and hearing loss. Front. Aging Neurosci. 2022, 14, 911028. [Google Scholar] [CrossRef]
- Chung, Y.J.; Lee, B.I.; Park, C.B. Multifunctional carbon dots as a therapeutic nanoagent for modulating cu (ii)-mediated β-amyloid aggregation. Nanoscale 2019, 11, 6297–6306. [Google Scholar] [CrossRef]
- Yan, C.; Wang, C.; Shao, X.; Teng, Y.; Chen, P.; Hu, X.; Guan, P.; Wu, H. Multifunctional carbon-dot-photosensitizer nanoassemblies for inhibiting amyloid aggregates, suppressing microbial infection, and overcoming the blood–brain barrier. ACS Appl. Mater. Interfaces 2022, 14, 47432–47444. [Google Scholar] [CrossRef]
- Chung, Y.J.; Lee, C.H.; Lim, J.; Jang, J.; Kang, H.; Park, C.B. Photomodulating carbon dots for spatiotemporal suppression of alzheimer’s β-amyloid aggregation. ACS Nano 2020, 14, 16973–16983. [Google Scholar] [CrossRef]
- Gil, H.M.; Price, T.W.; Chelani, K.; Bouillard, J.-S.G.; Calaminus, S.D.J.; Stasiuk, G.J. Nir-quantum dots in biomedical imaging and their future. iScience 2021, 24, 102189. [Google Scholar] [CrossRef]
- Hasannejadasl, B.; Janbaz, F.P.; Choupani, E.; Fadaie, M.; Hamidinejad, M.A.; Ahmadvand, D. Quantum dots application in neurodegenerative diseases. Thrita 2020, 9. [Google Scholar] [CrossRef]
- Tak, K.; Sharma, R.; Dave, V.; Jain, S.; Sharma, S. Clitoria ternatea mediated synthesis of graphene quantum dots for the treatment of alzheimer’s disease. ACS Chem. Neurosci. 2020, 11, 3741–3748. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Wang, L.; Chen, L.; Peng, C.; Luo, B.; Mo, J.; Chen, W. Intranasal administration of dauricine loaded on graphene oxide: Multi-target therapy for alzheimer’s disease. Drug Deliv. 2021, 28, 580–593. [Google Scholar] [CrossRef]
- Liu, C.; Luo, X. Potential molecular and graphene oxide chelators to dissolve amyloid-β plaques in alzheimer’s disease: A density functional theory study. J. Mater. Chem. B Mater. Biol. Med. 2021, 9, 2736–2746. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, K.; Chu, F.; Huang, J.; Yang, Z. Graphene oxide enhances β-amyloid clearance by inducing autophagy of microglia and neurons. Chem. Biol. Interact. 2020, 325, 109126. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Wang, X.; Shi, J.; Wu, D. Preparation of electrochemical sensor based on β-cyclodextrin/carbon nanotube nanocomposite for donepezil hydrochloride as drug for treatment of alzheimer’s disease. Int. J. Electrochem. Sci. 2022, 17, 220119. [Google Scholar] [CrossRef]
- Caffo, M.; Curcio, A.; Rajiv, K.; Caruso, G.; Venza, M.; Germanò, A. Potential role of carbon nanomaterials in the treatment of malignant brain gliomas. Cancers 2023, 15, 2575. [Google Scholar] [CrossRef]
- Cacciatore, I.; Ciulla, M.; Fornasari, E.; Marinelli, L.; Di Stefano, A. Solid lipid nanoparticles as a drug delivery system for the treatment of neurodegenerative diseases. Expert Opin. Drug Deliv. 2016, 13, 1121–1131. [Google Scholar] [CrossRef]
- Cacciatore, I.; Baldassarre, L.; Fornasari, E.; Mollica, A.; Pinnen, F. Recent advances in the treatment of neurodegenerative diseases based on gsh delivery systems. Oxidative Med. Cell. Longev. 2012, 2012, 240146. [Google Scholar] [CrossRef]
- Vakilinezhad, M.A.; Amini, A.; Akbari Javar, H.; Baha’addini Beigi Zarandi, B.F.; Montaseri, H.; Dinarvand, R. Nicotinamide loaded functionalized solid lipid nanoparticles improves cognition in alzheimer’s disease animal model by reducing tau hyperphosphorylation. DARU J. Pharm. Sci. 2018, 26, 165–177. [Google Scholar] [CrossRef]
- Arora, D.; Bhatt, S.; Kumar, M.; Verma, R.; Taneja, Y.; Kaushal, N.; Tiwari, A.; Tiwari, V.; Alexiou, A.; Albogami, S. Qbd-based rivastigmine tartrate loaded solid lipid nanoparticles for enhanced intranasal delivery to the brain for alzheimer’s therapeutics. Front. Aging Neurosci. 2022, 14, 960246. [Google Scholar] [CrossRef]
- Pinheiro, R.; Granja, A.; Loureiro, J.A.; Pereira, M.; Pinheiro, M.; Neves, A.; Reis, S. Rvg29-functionalized lipid nanoparticles for quercetin brain delivery and alzheimer’s disease. Pharm. Res. 2020, 37, 139. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.; Taylor, M.; Fullwood, N.; Allsop, D. Liposome delivery systems for the treatment of alzheimer’s disease. Int. J. Nanomed. 2018, 13, 8507–8522. [Google Scholar] [CrossRef] [PubMed]
- Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Transferrin-functionalized liposomes loaded with vitamin vb12 for alzheimer’s disease therapy. Int. J. Pharm. 2022, 626, 122167. [Google Scholar] [CrossRef]
- Andrade, S.; Pereira, M.C.; Loureiro, J.A. Caffeic acid loaded into engineered lipid nanoparticles for alzheimer’s disease therapy. Colloids Surf. B Biointerfaces 2023, 225, 113270. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.S. Dendrimers for drug delivery. Molecules 2018, 23, 938. [Google Scholar] [CrossRef]
- Fazil, M.; Md, S.; Haque, S.; Kumar, M.; Baboota, S.; Sahni, J.K.; Ali, J. Development and evaluation of rivastigmine loaded chitosan nanoparticles for brain targeting. Eur. J. Pharm. Sci. 2012, 47, 6–15. [Google Scholar] [CrossRef]
- Sharma, A.K.; Gupta, L.; Sahu, H.; Qayum, A.; Singh, S.K.; Nakhate, K.T.; Gupta, U. Chitosan engineered pamam dendrimers as nanoconstructs for the enhanced anti-cancer potential and improved in vivo brain pharmacokinetics of temozolomide. Pharm. Res. 2018, 35, 9. [Google Scholar] [CrossRef]
- Singh, A.K.; Mishra, S.K.; Mishra, G.; Maurya, A.; Awasthi, R.; Yadav, M.K.; Atri, N.; Pandey, P.K.; Singh, S.K. Inorganic clay nanocomposite system for improved cholinesterase inhibition and brain pharmacokinetics of donepezil. Drug Dev. Ind. Pharm. 2020, 46, 8–19. [Google Scholar] [CrossRef]
- Igartúa, D.E.; Martinez, C.S.; Del, V.A.S.; Prieto, M.J. Combined therapy for alzheimer’s disease: Tacrine and pamam dendrimers co-administration reduces the side effects of the drug without modifying its activity. AAPS PharmSciTech 2020, 21, 110. [Google Scholar] [CrossRef]
- Igartúa, D.E.; Martinez, C.S.; Temprana, C.F.; Alonso, S.D.V.; Prieto, M.J. Pamam dendrimers as a carbamazepine delivery system for neurodegenerative diseases: A biophysical and nanotoxicological characterization. Int. J. Pharm. 2018, 544, 191–202. [Google Scholar] [CrossRef]
- Witika, B.A.; Poka, M.S.; Demana, P.H.; Matafwali, S.K.; Melamane, S.; Malungelo Khamanga, S.M.; Makoni, P.A. Lipid-based nanocarriers for neurological disorders: A review of the state-of-the-art and therapeutic success to date. Pharmaceutics 2022, 14, 836. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mehta, A.; Tong, Z.; Esser, L.; Voelcker, N.H. Development of polymeric nanoparticles for blood-brain barrier transfer-strategies and challenges. Adv. Sci. 2021, 8, 2003937. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Fukuda, T.; Nagaoka, Y.; Hasumura, T.; Morimoto, H.; Yoshida, Y.; Maekawa, T.; Venugopal, K.; Kumar, D.S. Curcumin loaded-plga nanoparticles conjugated with tet-1 peptide for potential use in alzheimer’s disease. PLoS ONE 2012, 7, e32616. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Lu, S.; Liu, X.-G.; Zhu, J.; Wang, Y.-J.; Liu, R.-T. Plga nanoparticles modified with a bbb-penetrating peptide co-delivering aβ generation inhibitor and curcumin attenuate memory deficits and neuropathology in alzheimer’s disease mice. Oncotarget 2017, 8, 81001. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Ettcheto, M.; Egea, M.A.; Espina, M.; Calpena, A.C.; Folch, J.; Camins, A.; García, M.L. New potential strategies for alzheimer’s disease prevention: Pegylated biodegradable dexibuprofen nanospheres administration to appswe/ps1de9. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1171–1182. [Google Scholar] [CrossRef]
- Liu, X.G.; Zhang, L.; Lu, S.; Liu, D.Q.; Huang, Y.R.; Zhu, J.; Zhou, W.W.; Yu, X.L.; Liu, R.T. Superparamagnetic iron oxide nanoparticles conjugated with aβ oligomer-specific scfv antibody and class a scavenger receptor activator show therapeutic potentials for alzheimer’s disease. J. Nanobiotechnology 2020, 18, 160. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Liu, X.; Zhang, Y.; Liu, Y.; Wang, W.; Su, R.; Sun, Y.; Huang, Y.; Song, D. Ratiometric fluorescence and colorimetric dual-mode sensing platform based on carbon dots for detecting copper (ii) ions and d-penicillamine. Anal. Bioanal. Chem. 2022, 414, 1651–1662. [Google Scholar] [CrossRef]
- Li, C.; Xiang, Y.; Wang, Y.; Li, P. Study on nano drug particles in the diagnosis and treatment of alzheimer’s disease in the elderly. Bioinorg. Chem. Appl. 2022, 2022, 3335581. [Google Scholar] [CrossRef]
- Han, Y.; Chu, X.; Cui, L.; Fu, S.; Gao, C.; Li, Y.; Sun, B. Neuronal mitochondria-targeted therapy for alzheimer’s disease by systemic delivery of resveratrol using dual-modified novel biomimetic nanosystems. Drug Deliv. 2020, 27, 502–518. [Google Scholar] [CrossRef]
- Agwa, M.M.; Abdelmonsif, D.A.; Khattab, S.N.; Sabra, S. Self-assembled lactoferrin-conjugated linoleic acid micelles as an orally active targeted nanoplatform for alzheimer’s disease. Int. J. Biol. Macromol. 2020, 162, 246–261. [Google Scholar] [CrossRef]
- Nanaki, S.G.; Spyrou, K.; Bekiari, C.; Veneti, P.; Baroud, T.N.; Karouta, N.; Grivas, I.; Papadopoulos, G.C.; Gournis, D.; Bikiaris, D.N. Hierarchical porous carbon-plla and plga hybrid nanoparticles for intranasal delivery of galantamine for alzheimer’s disease therapy. Pharmaceutics 2020, 12, 227. [Google Scholar] [CrossRef] [PubMed]
- Sintov, A.C. Amylolipid nanovesicles: A self-assembled lipid-modified starch hybrid system constructed for direct nose-to-brain delivery of curcumin. Int. J. Pharm. 2020, 588, 119725. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Zhao, J.; Wang, H.; Li, B.; Li, K.; Shi, X.; Wan, K.; Ai, J.; Lv, J.; Wang, D. Chiral gold nanoparticles enantioselectively rescue memory deficits in a mouse model of alzheimer’s disease. Nat. Commun. 2020, 11, 4790. [Google Scholar] [CrossRef] [PubMed]
- Gothwal, A.; Kumar, H.; Nakhate, K.T.; Ajazuddin; Dutta, A.; Borah, A.; Gupta, U. Lactoferrin coupled lower generation pamam dendrimers for brain targeted delivery of memantine in aluminum-chloride-induced alzheimer’s disease in mice. Bioconjug. Chem. 2019, 30, 2573–2583. [Google Scholar] [CrossRef]
- Dara, T.; Vatanara, A.; Meybodi, M.N.; Vakilinezhad, M.A.; Malvajerd, S.S.; Vakhshiteh, F.; Shamsian, A.; Sharifzadeh, M.; Kaghazian, H.; Mosaddegh, M.H. Erythropoietin-loaded solid lipid nanoparticles: Preparation, optimization, and in vivo evaluation. Colloids Surf. B Biointerfaces 2019, 178, 307–316. [Google Scholar] [CrossRef]
- Silva-Abreu, M.; Espinoza, L.C.; Halbaut, L.; Espina, M.; García, M.L.; Calpena, A.C. Comparative study of ex vivo transmucosal permeation of pioglitazone nanoparticles for the treatment of alzheimer’s disease. Polymers 2018, 10, 316. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, A.; Hua, H.; Jiang, Y.; Wang, Y.; Mu, H.; Wu, Z.; Sun, K. Intranasal delivery of huperzine a to the brain using lactoferrin-conjugated n-trimethylated chitosan surface-modified plga nanoparticles for treatment of alzheimer’s disease. Int. J. Nanomed. 2018, 13, 705–718. [Google Scholar] [CrossRef]
- Muller, A.P.; Ferreira, G.K.; Pires, A.J.; de Bem Silveira, G.; de Souza, D.L.; Brandolfi, J.A.; de Souza, C.T.; Paula, M.M.S.; Silveira, P.C.L. Gold nanoparticles prevent cognitive deficits, oxidative stress and inflammation in a rat model of sporadic dementia of alzheimer’s type. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 476–483. [Google Scholar] [CrossRef]
- Sun, D.; Li, N.; Zhang, W.; Zhao, Z.; Mou, Z.; Huang, D.; Liu, J.; Wang, W. Design of plga-functionalized quercetin nanoparticles for potential use in alzheimer’s disease. Colloids Surf. B Biointerfaces 2016, 148, 116–129. [Google Scholar] [CrossRef]
- Misra, S.; Chopra, K.; Sinha, V.R.; Medhi, B. Galantamine-loaded solid-lipid nanoparticles for enhanced brain delivery: Preparation, characterization, in vitro and in vivo evaluations. Drug Deliv. 2016, 23, 1434–1443. [Google Scholar] [CrossRef]
- Meng, F.; Asghar, S.; Xu, Y.; Wang, J.; Jin, X.; Wang, Z.; Wang, J.; Ping, Q.; Zhou, J.; Xiao, Y. Design and evaluation of lipoprotein resembling curcumin-encapsulated protein-free nanostructured lipid carrier for brain targeting. Int. J. Pharm. 2016, 506, 46–56. [Google Scholar] [CrossRef]
- Shah, B.; Khunt, D.; Bhatt, H.; Misra, M.; Padh, H. Application of quality by design approach for intranasal delivery of rivastigmine loaded solid lipid nanoparticles: Effect on formulation and characterization parameters. Eur. J. Pharm. Sci. 2015, 78, 54–66. [Google Scholar] [CrossRef]
- Yin, H.; Si, J.; Xu, H.; Dong, J.; Zheng, D.; Lu, X.; Li, X. Resveratrol-loaded nanoparticles reduce oxidative stress induced by radiation or amyloid-in transgenic caenorhabditis elegans. J. Biomed. Nanotechnol. 2014, 10, 1536–1544. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, X.; Kang, T.; Jiang, M.; Miao, D.; Gu, G.; Hu, Q.; Song, Q.; Yao, L.; Tu, Y.; et al. B6 peptide-modified peg-pla nanoparticles for enhanced brain delivery of neuroprotective peptide. Bioconjug. Chem. 2013, 24, 997–1007. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Zhang, Y.Q.; Wang, Z.Z.; Wu, K.; Lou, J.N.; Qi, X.R. Enhanced brain distribution and pharmacodynamics of rivastigmine by liposomes following intranasal administration. Int. J. Pharm. 2013, 452, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, J.; Feng, C.; Shao, X.; Liu, Q.; Zhang, Q.; Pang, Z.; Jiang, X. Intranasal nanoparticles of basic fibroblast growth factor for brain delivery to treat alzheimer’s disease. Int. J. Pharm. 2014, 461, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Luppi, B.; Bigucci, F.; Corace, G.; Delucca, A.; Cerchiara, T.; Sorrenti, M.; Catenacci, L.; Di Pietra, A.M.; Zecchi, V. Albumin nanoparticles carrying cyclodextrins for nasal delivery of the anti-alzheimer drug tacrine. Eur. J. Pharm. Sci. 2011, 44, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Canovi, M.; Markoutsa, E.; Lazar, A.N.; Pampalakis, G.; Clemente, C.; Re, F.; Sesana, S.; Masserini, M.; Salmona, M.; Duyckaerts, C. The binding affinity of anti-aβ1-42 mab-decorated nanoliposomes to aβ1-42 peptides in vitro and to amyloid deposits in post-mortem tissue. Biomaterials 2011, 32, 5489–5497. [Google Scholar] [CrossRef] [PubMed]
- Wilson, B.; Samanta, M.K.; Santhi, K.; Kumar, K.P.; Paramakrishnan, N.; Suresh, B. Poly(n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat alzheimer’s disease. Brain Res. 2008, 1200, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Li, X.-t.; Ni, Y.-n.; Xiao, H.-h.; Yao, Y.-j.; Wang, Y.-y.; Ju, R.-j.; Li, H.-y.; Liu, J.-j.; Fu, M. Transferrin-modified osthole pegylated liposomes travel the blood-brain barrier and mitigate alzheimer’s disease-related pathology in app/ps-1 mice. Int. J. Nanomed. 2020, 15, 2841–2858. [Google Scholar] [CrossRef]
- Rotman, M.; Welling, M.M.; Bunschoten, A.; de Backer, M.E.; Rip, J.; Nabuurs, R.J.; Gaillard, P.J.; van Buchem, M.A.; van der Maarel, S.M.; van der Weerd, L. Enhanced glutathione pegylated liposomal brain delivery of an anti-amyloid single domain antibody fragment in a mouse model for alzheimer’s disease. J. Control. Release 2015, 203, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Layek, B.; Singh, J. Design and validation of liposomal apoe2 gene delivery system to evade blood–brain barrier for effective treatment of alzheimer’s disease. Mol. Pharm. 2020, 18, 714–725. [Google Scholar] [CrossRef] [PubMed]
- Ruff, J.; Hassan, N.; Morales-Zavala, F.; Steitz, J.; Araya, E.; Kogan, M.J.; Simon, U. Clpffd–peg functionalized nir-absorbing hollow gold nanospheres and gold nanorods inhibit β-amyloid aggregation. J. Mater. Chem. B Mater. Biol. Med. 2018, 6, 2432–2443. [Google Scholar] [CrossRef]
- Li, M.; Xu, C.; Wu, L.; Ren, J.; Wang, E.; Qu, X. Self-assembled peptide-polyoxometalate hybrid nanospheres: Two in one enhances targeted inhibition of amyloid β-peptide aggregation associated with alzheimer’s disease. Small 2013, 9, 3455–3461. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Guo, Z.; Zhang, Y.; Li, C.; Zhang, Y.; Guo, Q.; Chen, Q.; Chen, X.; He, X.; Liu, L.; et al. Microenvironment remodeling micelles for alzheimer’s disease therapy by early modulation of activated microglia. Adv. Sci. 2019, 6, 1801586. [Google Scholar] [CrossRef]
- Gothwal, A.; Lamptey, R.N.L.; Singh, J. Multifunctionalized cationic chitosan polymeric micelles polyplexed with pvgf for noninvasive delivery to the mouse brain through the intranasal route for developing therapeutics for alzheimer’s disease. Mol. Pharm. 2023, 20, 3009–3019. [Google Scholar] [CrossRef]
- Parikh, A.; Kathawala, K.; Li, J.; Chen, C.; Shan, Z.; Cao, X.; Zhou, X.-F.; Garg, S. Curcumin-loaded self-nanomicellizing solid dispersion system: Part ii: In vivo safety and efficacy assessment against behavior deficit in alzheimer disease. Drug Deliv. Transl. Res. 2018, 8, 1406–1420. [Google Scholar] [CrossRef]
- Elnaggar, Y.S.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Intranasal piperine-loaded chitosan nanoparticles as brain-targeted therapy in alzheimer’s disease: Optimization, biological efficacy, and potential toxicity. J. Pharm. Sci. 2015, 104, 3544–3556. [Google Scholar] [CrossRef]
- Jiang, Z.; Dong, X.; Sun, Y. Charge effects of self-assembled chitosan-hyaluronic acid nanoparticles on inhibiting amyloid β-protein aggregation. Carbohydr. Res. 2018, 461, 11–18. [Google Scholar] [CrossRef]
- Kuang, Y.; Zhang, J.; Xiong, M.; Zeng, W.; Lin, X.; Yi, X.; Luo, Y.; Yang, M.; Li, F.; Huang, Q. A novel nanosystem realizing curcumin delivery based on fe3o4@ carbon dots nanocomposite for alzheimer’s disease therapy. Front. Bioeng. Biotechnol. 2020, 8, 614906. [Google Scholar] [CrossRef]
- De la Torre, C.; Ceña, V. The delivery challenge in neurodegenerative disorders: The nanoparticles role in alzheimer’s disease therapeutics and diagnostics. Pharmaceutics 2018, 10, 190. [Google Scholar] [CrossRef]
- Lamptey, R.N.L.; Chaulagain, B.; Trivedi, R.; Gothwal, A.; Layek, B.; Singh, J. A review of the common neurodegenerative disorders: Current therapeutic approaches and the potential role of nanotherapeutics. Int. J. Mol. Sci. 2022, 23, 1851. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Harashima, H. Current status and challenges associated with cns-targeted gene delivery across the bbb. Pharmaceutics 2020, 12, 1216. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Jiang, F.; Wang, M.; Hu, H.; Zhang, B.; Chen, L.; Dai, F. Increased cross-linking micelle retention in the brain of alzheimer’s disease mice by elevated asparagine endopeptidase protease responsive aggregation. Biomater. Sci. 2020, 8, 6533–6544. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Khan, A.I.; Cai, X.; Song, Y.; Lyu, Z.; Du, D.; Dutta, P.; Lin, Y. Overcoming blood-brain barrier transport: Advances in nanoparticle-based drug delivery strategies. Mater. Today 2020, 37, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.M.; Alomari, S.; Tyler, B.M. Crossing the blood-brain barrier: Advances in nanoparticle technology for drug delivery in neuro-oncology. Int. J. Mol. Sci. 2022, 23, 4153. [Google Scholar] [CrossRef]
- Saucier-Sawyer, J.K.; Deng, Y.; Seo, Y.E.; Cheng, C.J.; Zhang, J.; Quijano, E.; Saltzman, W.M. Systemic delivery of blood-brain barrier-targeted polymeric nanoparticles enhances delivery to brain tissue. J. Drug Target. 2015, 23, 736–749. [Google Scholar] [CrossRef]
- Ling, T.S.; Chandrasegaran, S.; Xuan, L.Z.; Suan, T.L.; Elaine, E.; Nathan, D.V.; Chai, Y.H.; Gunasekaran, B.; Salvamani, S. The potential benefits of nanotechnology in treating alzheimer’s disease. Biomed. Res. Int. 2021, 2021, 5550938. [Google Scholar] [CrossRef]
- Bashir, W.; Shahzadi, S. Nanoparticles–a novel theranostic approach to treat alzheimer’s disease. J. Appl. Biotechnol. Bioeng. 2022, 9, 216–220. [Google Scholar] [CrossRef]
- Harish, V.; Tewari, D.; Gaur, M.; Yadav, A.B.; Swaroop, S.; Bechelany, M.; Barhoum, A. Review on nanoparticles and nanostructured materials: Bioimaging, biosensing, drug delivery, tissue engineering, antimicrobial, and agro-food applications. Nanomaterials 2022, 12, 457. [Google Scholar] [CrossRef]
- Chakraborty, A.; Mohapatra, S.S.; Barik, S.; Roy, I.; Gupta, B.; Biswas, A. Impact of nanoparticles on amyloid beta-induced alzheimer’s disease, tuberculosis, leprosy and cancer: A systematic review. Biosci. Rep. 2023, 43, BSR20220324. [Google Scholar] [CrossRef] [PubMed]
- Kulavi, S.; Kaur, R.; Iyer, K.; Bandyopadhyay, J.; Sengupta, T. A smart & precise approach with nanoparticles-based therapeutic intervention in neurodegenerative diseases. Nanomed. J. 2023, 10, 96–106. [Google Scholar]
- Pardridge, W.M. Csf, blood-brain barrier, and brain drug delivery. Expert Opin. Drug Deliv. 2016, 13, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Kung, Y.; Chen, K.-Y.; Liao, W.-H.; Hsu, Y.-H.; Wu, C.-H.; Hsiao, M.-Y.; Huang, A.P.H.; Chen, W.-S. Facilitating drug delivery in the central nervous system by opening the blood-cerebrospinal fluid barrier with a single low energy shockwave pulse. Fluids Barriers CNS 2022, 19, 3. [Google Scholar] [CrossRef] [PubMed]
- Puris, E.; Gynther, M.; Auriola, S.; Huttunen, K.M. L-type amino acid transporter 1 as a target for drug delivery. Pharm. Res. 2020, 37, 88. [Google Scholar] [CrossRef] [PubMed]
- Mir, S.A.; Hamid, L.; Bader, G.N.; Shoaib, A.; Rahamathulla, M.; Alshahrani, M.Y.; Alam, P.; Shakeel, F. Role of nanotechnology in overcoming the multidrug resistance in cancer therapy: A review. Molecules 2022, 27, 6608. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, X.; Zhang, H.; Han, X.; Li, B.; Yang, R.; Zhou, X. Recent progress of novel nanotechnology challenging the multidrug resistance of cancer. Front. Pharmacol. 2022, 13, 776895. [Google Scholar] [CrossRef]
- Wei, M.; Yang, Z.; Li, S.; Le, W. Nanotherapeutic and stem cell therapeutic strategies in neurodegenerative diseases: A promising therapeutic approach. Int. J. Nanomedicine 2023, 18, 611–626. [Google Scholar] [CrossRef]
- Richardson, J.J.; Caruso, F. Nanomedicine toward 2040; ACS Publications: Washington, DC, USA, 2020; Volume 20, pp. 1481–1482. [Google Scholar]
- Poudel, P.; Park, S. Recent advances in the treatment of alzheimer’s disease using nanoparticle-based drug delivery systems. Pharmaceutics 2022, 14, 835. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Gremião, M.P.D.; Chorilli, M. Nanotechnology-based drug delivery systems for the treatment of alzheimer’s disease. Int. J. Nanomedicine 2015, 10, 4981–5003. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of the MDPI and/or editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
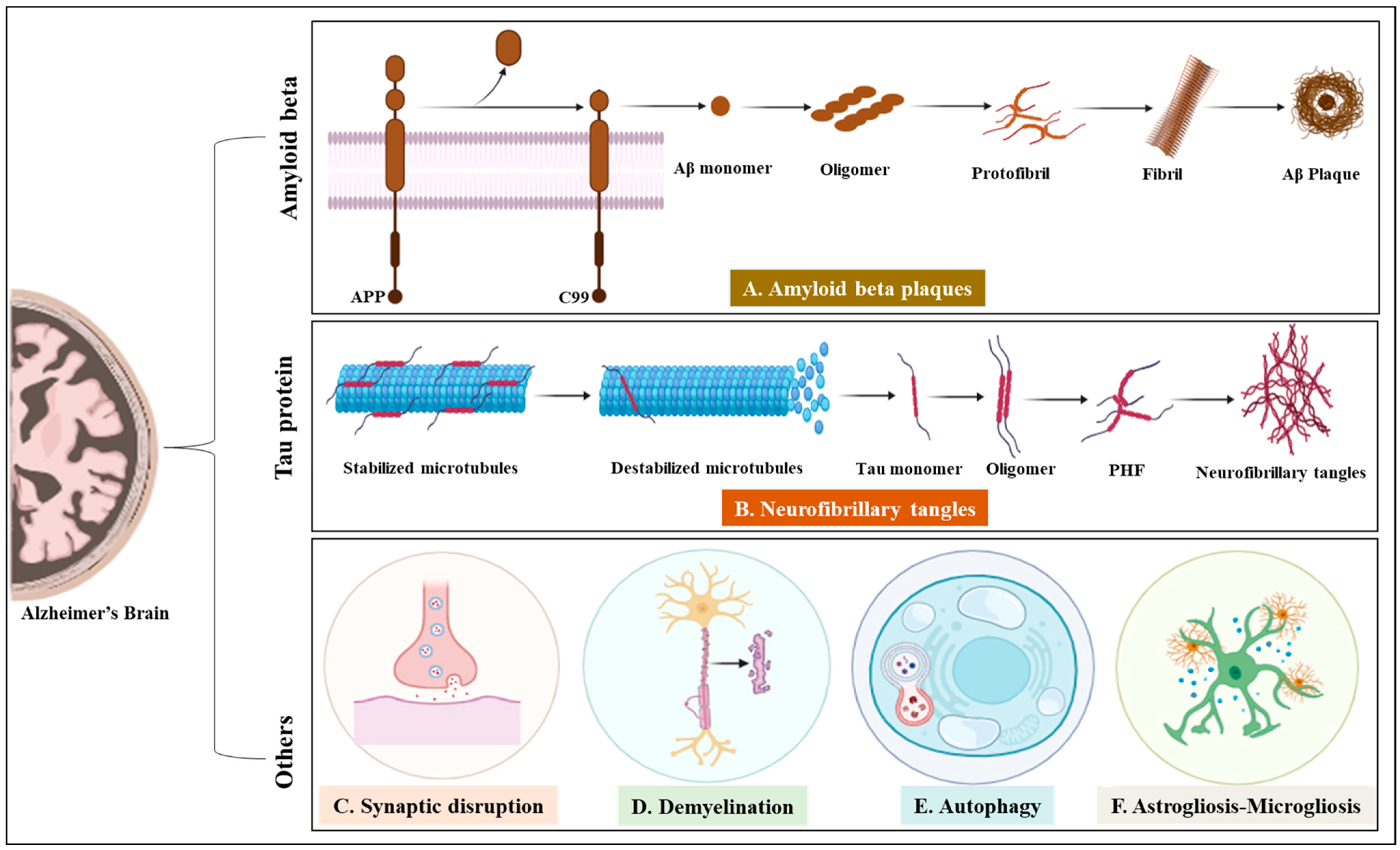


| Target and Its Structure | Role in AD | Therapeutic Strategies | Common Drugs | References |
|---|---|---|---|---|
Amyloid beta protein | Increases in Aβ concentration, forms oligomers and leads to neurotoxicity | Aβ-protein-targeted (Aβ oligomers treatment) therapeutic strategies for treating AD | Aducanumab, CPO-Aβ17–21 peptide, Huperzine A | [22,23,24,25] |
Tau protein | The misfolded Tau is self-assembled, forming a Tau oligomer, and then, Tau inclusions lead to AD | Reduction in toxic Tau gain of function may be an effective therapeutic strategy for AD | Gosuranemab, lonafarnib tilavonemab, semorinemab, zagotenemab, | [26,27] |
Neurofibrillary tangles | Excessive Tau phosphorylation and aggregation are the key processes in the formation of NFT that cause neuron death and lead to AD | Prevent phosphatase activity; promote microtubule stability; reduce Tau aggregate and formation of NFT | Memantine, AADvac1, ACI-35; Epithilone D | [28,29] |
Mitochondria | Mitochondrial dysfunction cause AD, which involves OS-induced respiratory chain dysfunction, loss of mitochondrial biogenesis, and mitochondrial DNA mutations | Therapeutics based on antioxidants, anti-apoptotic agents, and molecules that enhance glucose metabolism and mitochondrial bioenergetics | Kaempferol (flavonoid) and rhapontigenin (stilbenoid), P110 and mdivi-1, selenium, SkQ1, MitoApo, astaxanthin | [30,31,32,33] |
Oxidative stress | Neuronal cell abnormalities; apoptosis of neurons, cognitive dysfunction that leads to dementia | Antioxidant drug therapy has been investigated as a potential AD treatment | Vitamin C, Vitamin E, -lipoic acid, CoQ10, Curcumin; coenzyme Q and glutathione; melatonin, α-lipoic acid (LA), N-Acetyl-cysteine (NAC) | [34,35,36,37,38] |
Neuroinflammation | Neuroinflammation leading to memory loss and cognitive decline | Neuroprotective therapeutics using nonsteroidal anti-inflammatory drugs | Pterostilbene, Sulforaphane, Artemisinin | [39,40,41] |
Angiotensin receptors | Their role in blood pressure regulation and hypertension leads to neural injury, neuroinflammation, and cognitive function | Therapeutic agents that inhibit RAS, Angiotensin-converting enzymes, ARBs, and block Angiotensin Receptor | Ramipril (ACE inhibitor), ARBs, ACE-Is, RAS-Ms, telmisartan, candesartan, valsartan | [42,43,44,45,46,47] |
Secretase receptors | Caused AD by changes in Aβ stability and aggregation | Therapeutic agents that cleaved APP α-secretase have potential to treat AD | MK-8931 (inhibitor of β-site APP cleaving enzyme 1), PRX-03,140 (α-Secretase inhibitors), Begacestat (γ-Secretase inhibitors), CTS-2166 (β-Secretase inhibitors) | [48,49,50,51] |
Blood–brain barrier | BBB characterizes a link between ND, vascular damage, and neuro-inflammation | Drugs that can penetrate the BBB | ApoE-modifying agents; Neprilysin | [52,53,54] |
Cholinergic insufficiency | Reduced choline acetyltransferase (ChAT) and AChE activity in the cortex in AD | Cholinesterase inhibitors | Physostigmine, Aricept (donepezil), Exelon (rivastigmine), and Metrifonate | [55,56,57] |
| Nanocarrier Material | Modifications/ Functionalized by | Therapeutic Agent | Experimental Model | Route of Administration | Experimental Doses and Periods | Result | Limitations and Possibilities | Reference |
|---|---|---|---|---|---|---|---|---|
| Solid Lipid Nanoparticles | Polysorbate 80 | Rivastigmine tartrate | Sheep mucosa | Intranasal | 0.178 mg/mL 6 months | Formulation shows safe intranasal delivery without any toxicity | An in vivo study is needed to see the safety and effectiveness of formulation | [124] |
| Superparamagnetic Iron Oxide NPs | - | AβOs Antibody and Class A scavenger receptor activator | Mice | Intravenously | 1 mg/day 28 days | Formulation targeting the AβOs improved the uptake of AβOs by microglia | The distribution and the half-life of formulation will be studies in brains for long time benefits | [140] |
| Carbon Dots | - | Memantine | Wild-type zebrafish | Intravascularly | - | Cross the BBB and inhibes the tau aggregation | This study shows the significant therapeutic potential in AD treatment | [141] |
| Nano drug particles | - | Drug | AD patients | Oral | - 45 days | Have high stability and less toxicity | A large level study is needed to conclude the role of nanodrug in AD treatment | [142] |
| Graphene oxide | - | Dauricine | Mice | Intranasal | 1 µg/µL 21 days | The formulation protects against oxidative damage and apoptosis | Graphene oxide-dauricine crosses the BBB, enters the brain and shows potential therpeutic agent in AD treatment | [116] |
| Nanostructured lipid carriers | RBC membrane vesicles | Glycoprotein of Rabies virus and triphenylphosphine cation | APP/PS1 mice | Intravenously | 2 mg/kg/every 2 days 30 days | Conquered the ROS-induced mitochondrial dysfunction | Mitochondria-targeted nanosystems showing a capable therapeutic candidate for AD treatment | [143] |
| Graphene Quantum Dots | - | Flower Clitoria ternatea extract | Rat | - | 3 mg/kg 7 days | Improved cognitive behavior and memory capacity in experimental model | QDs significantly crossed the BBB and significantly reduced the Alzheimer-like symptoms in rats; shows potential as therapeutic DDS | [115] |
| Linoleic acid micelles | - | lactoferrin | Wistar rats | Oral | 500 mg/Kg 60 days (AlCl3 induction) and +30 days (treatment regimen) | Enhanced cognitive capabilities, reduced brain OS, apoptosis, inflammation, and AchE activity | Multiple functions of formulation show a greater potential in AD treatment and can be further evaluated at large level | [144] |
| PLLA/PLGA Hybrid NPs | Hierarchical porous carbon (HPC) | Galantamine | Wistar rats | Intranasal | 3 mg/kg 24–48 h (clinical evaluation) | Successful delivery to the hippocampus area | Further evaluation in rodent models of AD is needed | [145] |
| Amylo Lipid Nanovesicles | Lipid-modified starch hybrid | Curcumin | Rat | Intranasal | 160 μg/kg 24 h | Curcumin targeting specifically to the brain | Result shows potential of mylo lipid nanovesicles in drug delivery to brain | [146] |
| Chiral AuNPs | L- and D-glutathione | NA | KM mice | Intravenous | 25 mg/kg 6 days | Formulation crossed the BBB and inhibited Aβ42 aggregation | The formulation shows a promising therapeutic strategy for AD by significantly rescueing the spatial learning and memory impairments in AD model | [147] |
| PAMAM Dendrimers | Lactoferrin coupled | Memantine | Mice | Intravenous | mg/kg 8 weeks | The formulation enhances the memory improvement | PAMAM dendrimers shows a significant impact on the memory aspects in AD-induced mice and could be further evaluated in other AD model | [148] |
| SLNPs | - | Erythropoietin | Wistar rats | Injected in CA1 Region of hippocampus | 1250 and 2500 U/kg 28 days | Formulation reduced the OS, ADP/ATP ratio and Aβ plaque deposition | The result of in vivo study is encouraging for further evaluations | [149] |
| Polymeric PLGA NPs | PEG linked | Pioglitazone (PGZ) | Buccal and nasal mucosa | Oral | 110 µg/mL 6 h | Cross BBB and drug delivered to the brain | In vivo study is required to see the actual result | [150] |
| SLNPs | Polysorbate 80, phosphatidylserine or phosphatidic acid | Nicotinamide | Rat | Intravenous and Intraperitoneal | 200 mg/kg 4 days | Improving cognition, protecting the neuronal cells and suppress Tau hyperphosphorylation | Not sufficient in improving the AD at early stages | [123] |
| Polylactide-coglycoside NPs | Lactoferrin-conjugated N-trimethylated chitosan | Huperzine A | KM mice | Intranasal | 1 µg/mL 12 h | Have a prolonged release time of the drug and target specific delivery | Evaluation of therapeutic efficacy for formulation in animal AD models is needed | [151] |
| AuNPs | - | Streptozotocin | Wistar male rats | Intraperitoneal | 2.5 mg/kg body weight 21 days | Prevent mitochondrially ATP production, neuroinflammation, and OS | AuNPs treatment prevented the pathological events of brain during AD pathogenesis possibly use for treatment of AD | [152] |
| PLGA-NPs | PVA | Quercetin | AD control mice | Intravenous | 20 mg/kg body Weight 30 days | Significantly improved the spatial memory | The formulation have high therapeutic index and reduced the side effects | [153] |
| SLNPs | - | Galantamine hydrobromide | Wistar rats | Intraperitoneal | 5 mg/kg 26 days | Significant restored memory capability in cognitive deficit Rats | High bioavailability of drug in brain shows potential therapeutic role of formulation | [154] |
| Nanostructured lipid carrier (NLC) | Lactoferrin modified | Curcumin | Rats | Intraperitoneal | 10 mg/kg body Weight 24 h | Penetrate BBB and release the drug in the brain | The in vitro and in vivo results shows the safe and biocompatible nanoformulation and could be used for brain targeted DDS | [155] |
| SLNPs | - | Rivastigmine | Goat nasal mucosa | Incubated with formulation | 10 mg/mL 9 h | Showed intact nasal mucosa with RHT SLN indicating the safety of RHT SLN | In vivo study is needed to observe the actual biodistribution and release profile of drug | [156] |
| Polyethyleneglycol-poly-caprolactone (mPEG-PCL) | - | Resveratrol | Transgenic Caenorhabditis elegans (C. elegans) (CL4176 strains) | Through culture media | 0–100 µg/mL 50 days | The formulation shows protection against OS in C. elegans induced by both γ-ray radiation and amyloid- peptide, confirming the successful development of antioxidant NPs. | Further study in large animals is suggested for confirmation of potential DDS | [157] |
| Poly(lactic acid) NPs | PEG-coated | B6 peptide (CGHKAKGPRK) | Mice | Intravenous | 1 mg/kg 30 days | Showed excellent amelioration in learning impairments, cholinergic disruption | In vivo imaging results show a good biodistribution profile of formulation with significant level of accumulation in the brain | [158] |
| Liposomes | Modified by adding Cell-penetrating peptide | Rivastigmine | Male rats | Intranasal | 1 g/kg body Weight 7 days | Enhance the pharmacological properties of drug and BBB penetration | Liposomes improve the drug delivery to brain by penetrating BBB and decrease the hepatic first pass metabolism and gastrointestinal adversative effects | [159] |
| Polyethylene glycol-polylactide-polyglycolide NPs | Lectins modified | Fibroblast growth factor | Rat | Intranasal | 20 and 40 µg/kg/d 17 days | Significantly improved spatial learning and memory in experimental rats | The formulation has a promising DDS for peptide and protein drugs for CNS and play the therapeutic role in AD | [160] |
| Albumin NPs | Cyclodextrins | Tacrine hydrochloride | Sheep nasal mucosa/Ex vivo | Nasal delivery | 2 mg/mL 24 h | Modified NPs enhanced the drug loading and permeation | In vivo study is required | [161] |
| Liposomes | Biotin-coated | Aβ-mAb | Post-mortem AD tissue | Incubation | 10 pMoles/mg Lipid 30 days | Aβ deposits in post-mortem AD brain | Present study shows the diagnostic potential only | [162] |
| Poly(n-butylcyanoacrylate) NPs | Coated with polysorbate 80 | Rivastigmine | Wistar rats | _ | 273.0 and 408.2 ng/mL 24 h | Enhanced drug delivery 3.82-fold comparatively without nano-formulations | In vitro release studies were performed; however, in vivo drug release studies were not performed | [163] |
| Nanoliposomes | - | Metformin | Rats | i.c.v injection | 50 mg/kg 21 days | Improve memory deficit and reduce neuroinflammation in streptozotocin-induced AD model | Nanoliposome conjugated metformin enhanced the learning and memory impairment in patients suffering from AD and also suppressed the neuroinflammation | [93] |
| PEGylated Liposomes | Transferrin | Osthole | APP/PS-1 Mice | Intravenous | 10 mg/kg 48 h | The formulation exert a protective effect in animal by targeting the Osthole to brain tissue and accumulating Osthole in the brain for long time of period | The in vivo study along with pharmacodynamic and pharmacokinetic confirmed that liposome-based delivery of osthole has long-term circulation, controlled release, and target-specific release and effects | [164] |
| PEGylated Liposomes | Glutathione | Anti-amyloid single domain antibody fragment | APPswe/PS1dE9 mice | Intravenous | 5 μg/body weight 24 h | Liposome-based delivery of antibody fragments cross the BBB and reach into the brain | Pharmacokinetics and biodistribution studies of formulation confirmed the potential of liposome as DDS | [165] |
| Liposomes | CPP | ApoE2 Gene | C57BL/6 mice | Intravenous | 1 μg pApoE2/g body weight 5 days | Modified liposomes crossed the BBB and delivered the target gene to brain | Liposome-based gene delivery is safe and have potential in AD treatment | [166] |
| Gold nanospheres and gold nanorods | Polyethylene glycol | CLPFFD peptide | In vitro/SH-SY5Y cells | - | 20 μM to 1.4 nM 2 h | Conjugates inhibits Aβ-fibrillation | An in vivo/ex vivo study is needed | [167] |
| Polyoxometalate Nanospheres | - | Aβ peptide | In vitro/PC12 cells | - | 10 μM 48 h | Inhibits the aggregation of amyloid β-peptide in AD model | An in vivo/ex vivo study is needed | [168] |
| Polymeric micelle | PEG | Curcumin | APPswe/PSEN1dE9 model mice | Intravenous | 5 mg/kg 38 weeks | Decreased Aβ plaque accumulation and enhanced cognitive behavior in mice | Formulation shows targeted multiple target strategies that are more effective than single target strategies | [169] |
| Linoleic acid micelles | - | Lactoferrin | Wistar rats | Orally | AlCl3—100 mg/kg—30 days LF-CLA micelles-500 mg/kg 30 days | Formulation enhanced cognitive capabilities, reduced brain oxidative stress, inflammation | Additional animal studies are essential to reconnoiter the potential effect of formulated micelles in AD prevention | [144] |
| Polymeric Micelles | Cationic Chitosan | pVGF | C57BL6/J mice | Intranasal | 1 mg/kg body weight 7 days | Formulation effectively deliver pVGF gene to the brain | Formulation shows the potential of a nonviral gene delivery system for brain-targeted gene | [170] |
| Nanomicellizing solid dispersion | - | Curcumin | APPSwe/PS1deE9 | Orally | 47 mg/kg 17 months | Formulation shows potential therapeutic candidate | This study of the effect on other organ demonstrates that the formulation is safe and have a promising potential as a therapeutic candidate for AD | [171] |
| Chitosan Nanoparticles | _ | Piperine | Wistar rats | Intranasal | 0.250 mg/kg/day 22 days | Targated delivery and showing via anti-apoptosis and anti-inflammatory effects | The formulation shows a tremendous efficiency as therpeutic agent, and could be further studied at large level | [172] |
| Chitosan | - | Hyaluronic acid | SH-SY5Y cells | - | 25 µM 48 h | Inhibited Aβ aggregation | An in vivo/ex vivo study is needed to understand the impact of negative and positive surface charges of nano-inhibitors | [173] |
| Selenium (Se) nanoparticles | Sialic acid | Peptide-B6 peptide | PC12 cells. | - | 10 μM 4 h | High permeability and cross the BBB | An in vivo/ex vivo study is needed | [102] |
| PLGA Nanoparticles | Curcumin | Tet-1 Peptide | GI-1 glioma cells | - | 0.75 μg/mL, 1.5 μg/mL and 3 μg/mL 72 h | The formulation destroy amyloid aggregates and exhibit anti-oxidative properties | An in vivo/ex vivo study is needed | [137] |
| Poly(lactic-co-glycolic) | Pegylated | Dexibuprofen | APPswe/PS1dE9 | Oral | 39 μg/mL–5290 μg/mL 30 min | It reduces the memory impairment and decrease brain inflammation | The accumulation of drug can be observed on the liver and will be studied for safety purpose | [139] |
| Fe3O4@Carbon Dots | Fe3O4 | Curcumin | PC12 cells | - | 0.4 mg/mL 24–28 h | Inhibits the extracellular Aβ fibrillation | An in vivo/ex vivo study is needed | [174] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puranik, N.; Yadav, D.; Song, M. Advancements in the Application of Nanomedicine in Alzheimer’s Disease: A Therapeutic Perspective. Int. J. Mol. Sci. 2023, 24, 14044. https://doi.org/10.3390/ijms241814044
Puranik N, Yadav D, Song M. Advancements in the Application of Nanomedicine in Alzheimer’s Disease: A Therapeutic Perspective. International Journal of Molecular Sciences. 2023; 24(18):14044. https://doi.org/10.3390/ijms241814044
Chicago/Turabian StylePuranik, Nidhi, Dhananjay Yadav, and Minseok Song. 2023. "Advancements in the Application of Nanomedicine in Alzheimer’s Disease: A Therapeutic Perspective" International Journal of Molecular Sciences 24, no. 18: 14044. https://doi.org/10.3390/ijms241814044
APA StylePuranik, N., Yadav, D., & Song, M. (2023). Advancements in the Application of Nanomedicine in Alzheimer’s Disease: A Therapeutic Perspective. International Journal of Molecular Sciences, 24(18), 14044. https://doi.org/10.3390/ijms241814044









