Rhamnolipid 89 Biosurfactant Is Effective against Streptococcus oralis Biofilm and Preserves Osteoblast Behavior: Perspectives in Dental Implantology
Abstract
:1. Introduction
2. Results
2.1. Antibacterial Activity on Planktonic Cells
2.2. Effects on Bacterial Cell Surface Hydrophobicity and Membrane Permeability
2.3. Dislodging Activity on Pre-Formed Bacterial Biofilm
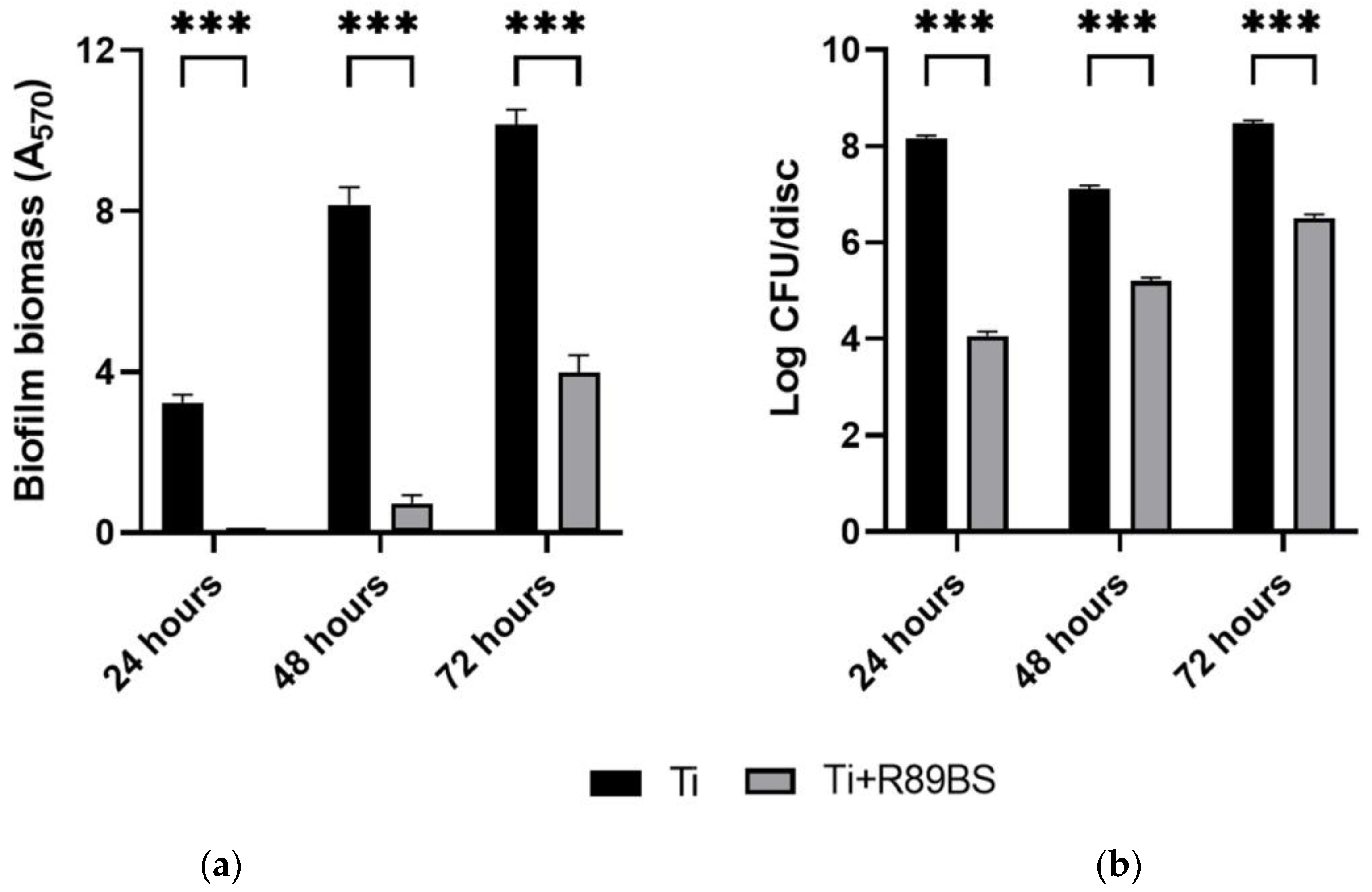
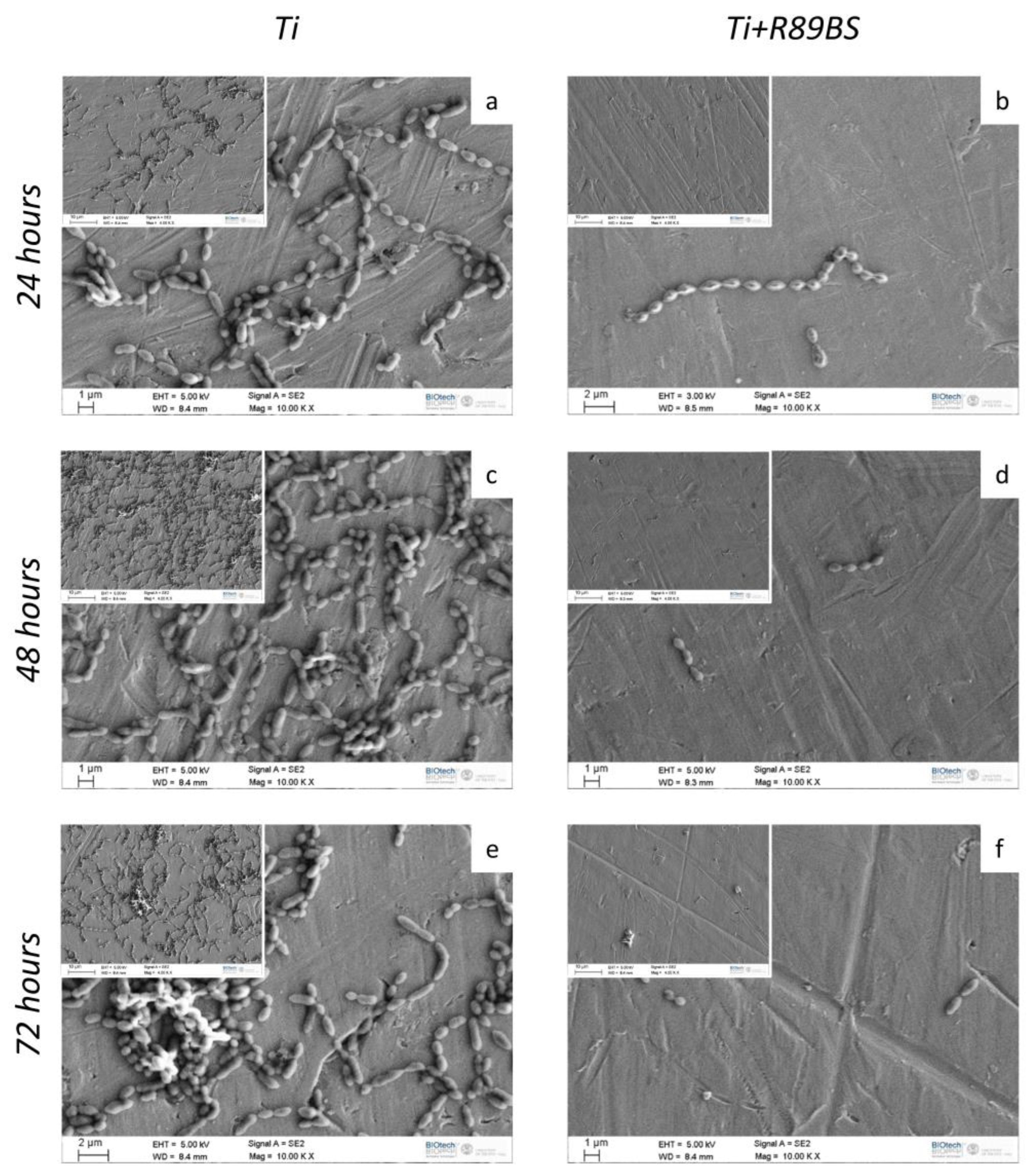
2.4. Inhibitory Activity on Bacterial Biofilm Formation
2.5. hOBs Adhesion and Proliferation
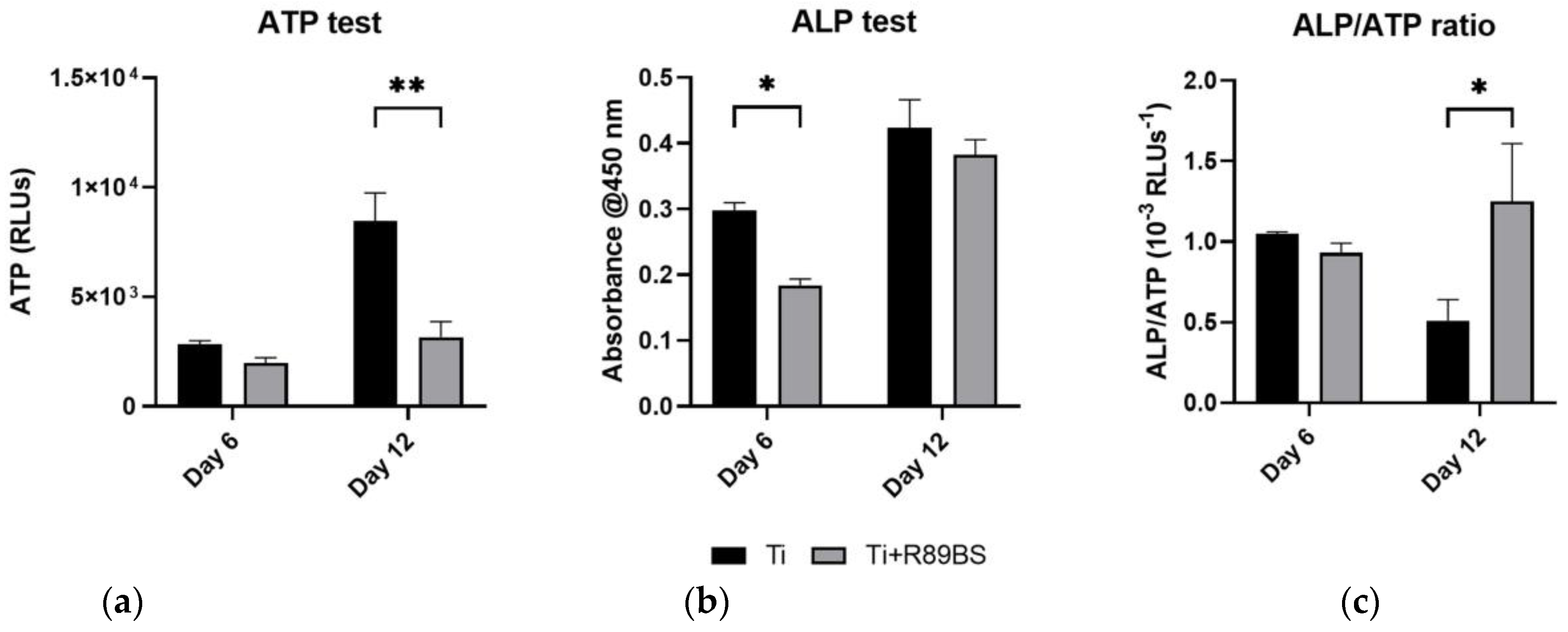
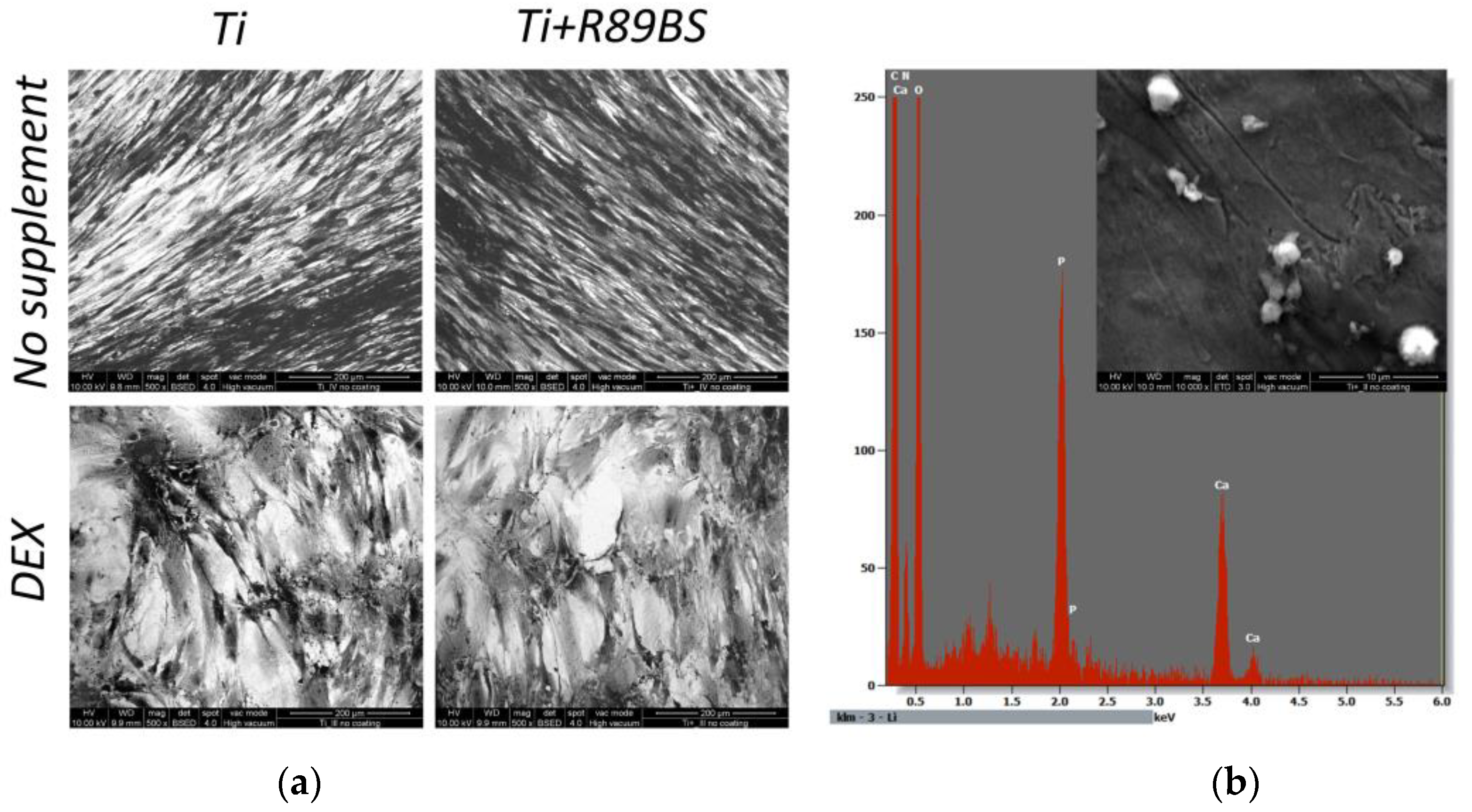
2.6. Cell Differentiation on R89BS-Coated and Uncoated TDs
3. Discussion
Study Limitations
4. Materials and Methods
4.1. Streptococcus oralis Growth Conditions
4.2. Biosurfactant Production
4.3. Antibacterial Activity of R89BS on Planktonic Cells
4.4. Effects of R89BS on Bacterial Cell Surface Hydrophobicity
4.5. Effects of R89BS on Bacterial Cell Membrane Permeability
4.6. Medical-Grade Titanium Discs Preparation
4.7. R89BS Dislodging Activity on Pre-Formed Bacterial Biofilm
4.8. Titanium Surface Coating Process
4.9. Antibiofilm Activity of R89BS-Coated TDs
4.10. Isolation of hOBs from Human Trabecular Bone Fragments
4.11. hOB Adhesion and Proliferation Tests
4.12. hOBs Differentiation Tests
4.13. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raikar, S.; Talukdar, P.; Kumari, S.; Panda, S.K.; Oommen, V.M.; Prasad, A. Factors Affecting the Survival Rate of Dental Implants: A Retrospective Study. J. Int. Soc. Prev. Community Dent. 2017, 7, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Parihar, A.S.; Vaibhav, V.; Kumar, K.; Singh, R.; Jerry, J.J. A 10 Years Retrospective Study of Assessment of Prevalence and Risk Factors of Dental Implants Failures. J. Family Med. Prim. Care 2020, 9, 1617–1619. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-Implant Diseases and Conditions: Consensus Report of Workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S286–S291. [Google Scholar] [CrossRef] [PubMed]
- Blank, E.; Grischke, J.; Winkel, A.; Eberhard, J.; Kommerein, N.; Doll, K.; Yang, I.; Stiesch, M. Evaluation of Biofilm Colonization on Multi-Part Dental Implants in a Rat Model. BMC Oral. Health 2021, 21, 313. [Google Scholar] [CrossRef]
- Salvi, G.E.; Cosgarea, R.; Sculean, A. Prevalence and Mechanisms of Peri-Implant Diseases. J. Dent. Res. 2017, 96, 31–37. [Google Scholar] [CrossRef]
- Varoni, E.M.; Rimondini, L. Oral Microbiome, Oral Health and Systemic Health: A Multidirectional Link. Biomedicines 2022, 10, 186. [Google Scholar] [CrossRef]
- Derks, J.; Tomasi, C. Peri-Implant Health and Disease. A Systematic Review of Current Epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S158–S171. [Google Scholar] [CrossRef]
- de Waal, Y.C.M.; Winning, L.; Stavropoulos, A.; Polyzois, I. Efficacy of Chemical Approaches for Implant Surface Decontamination in Conjunction with Sub-Marginal Instrumentation, in the Non-Surgical Treatment of Peri-Implantitis: A Systematic Review. J. Clin. Periodontol. 2023, 50 (Suppl. S26), 212–223. [Google Scholar] [CrossRef]
- Kotsailidi, E.A.; Michelogiannakis, D.; Al-Zawawi, A.S.; Javed, F. Surgical or Non-Surgical Treatment of Peri-Implantitis—What Is the Verdict? Surg. Pract. Sci. 2020, 1, 100010. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, Z.; Qiu, W.; Fang, F. Overview of Antibacterial Strategies of Dental Implant Materials for the Prevention of Peri-Implantitis. Bioconjug Chem. 2021, 32, 627–638. [Google Scholar] [CrossRef]
- Esteves, G.M.; Esteves, J.; Resende, M.; Mendes, L.; Azevedo, A.S. Antimicrobial and Antibiofilm Coating of Dental Implants-Past and New Perspectives. Antibiotics 2022, 11, 235. [Google Scholar] [CrossRef]
- Banat, I.M.; Franzetti, A.; Gandolfi, I.; Bestetti, G.; Martinotti, M.G.; Fracchia, L.; Smyth, T.J.; Marchant, R. Microbial Biosurfactants Production, Applications and Future Potential. Appl. Microbiol. Biotechnol. 2010, 87, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Holban, A.-M.; Farcasiu, C.; Andrei, O.-C.; Grumezescu, A.M.; Farcasiu, A.-T. Surface Modification to Modulate Microbial Biofilms-Applications in Dental Medicine. Materials 2021, 14, 6994. [Google Scholar] [CrossRef]
- Kligman, S.; Ren, Z.; Chung, C.-H.; Perillo, M.A.; Chang, Y.-C.; Koo, H.; Zheng, Z.; Li, C. The Impact of Dental Implant Surface Modifications on Osseointegration and Biofilm Formation. J. Clin. Med. 2021, 10, 1641. [Google Scholar] [CrossRef]
- Ceresa, C.; Fracchia, L.; Fedeli, E.; Porta, C.; Banat, I.M. Recent Advances in Biomedical, Therapeutic and Pharmaceutical Applications of Microbial Surfactants. Pharmaceutics 2021, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.K.F.; Rufino, R.D.; Luna, J.M.; Santos, V.A.; Sarubbo, L.A. Biosurfactants: Multifunctional Biomolecules of the 21st Century. Int. J. Mol. Sci. 2016, 17, 401. [Google Scholar] [CrossRef] [PubMed]
- Naughton, P.J.; Marchant, R.; Naughton, V.; Banat, I.M. Microbial Biosurfactants: Current Trends and Applications in Agricultural and Biomedical Industries. J. Appl. Microbiol. 2019, 127, 12–28. [Google Scholar] [CrossRef]
- Kumar, R.; Das, A.J. Application of Rhamnolipids in Medical Sciences. In Rhamnolipid Biosurfactant: Recent Trends in Production and Application; Kumar, R., Das, A.J., Eds.; Springer: Singapore, 2018; pp. 79–87. ISBN 9789811312892. [Google Scholar]
- Chen, J.; Wu, Q.; Hua, Y.; Chen, J.; Zhang, H.; Wang, H. Potential Applications of Biosurfactant Rhamnolipids in Agriculture and Biomedicine. Appl. Microbiol. Biotechnol. 2017, 101, 8309–8319. [Google Scholar] [CrossRef]
- Thakur, P.; Saini, N.K.; Thakur, V.K.; Gupta, V.K.; Saini, R.V.; Saini, A.K. Rhamnolipid the Glycolipid Biosurfactant: Emerging Trends and Promising Strategies in the Field of Biotechnology and Biomedicine. Microb. Cell Fact. 2021, 20, 1. [Google Scholar] [CrossRef]
- Tambone, E.; Bonomi, E.; Ghensi, P.; Maniglio, D.; Ceresa, C.; Agostinacchio, F.; Caciagli, P.; Nollo, G.; Piccoli, F.; Caola, I.; et al. Rhamnolipid Coating Reduces Microbial Biofilm Formation on Titanium Implants: An in Vitro Study. BMC Oral. Health 2021, 21, 49. [Google Scholar] [CrossRef]
- Elshikh, M.; Marchant, R.; Banat, I.M. Biosurfactants: Promising Bioactive Molecules for Oral-Related Health Applications. FEMS Microbiol. Lett. 2016, 363, fnw213. [Google Scholar] [CrossRef] [PubMed]
- Elshikh, M.; Moya-Ramírez, I.; Moens, H.; Roelants, S.; Soetaert, W.; Marchant, R.; Banat, I.M. Rhamnolipids and Lactonic Sophorolipids: Natural Antimicrobial Surfactants for Oral Hygiene. J. Appl. Microbiol. 2017, 123, 1111–1123. [Google Scholar] [CrossRef] [PubMed]
- Elshikh, M.; Funston, S.; Chebbi, A.; Ahmed, S.; Marchant, R.; Banat, I.M. Rhamnolipids from Non-Pathogenic Burkholderia Thailandensis E264: Physicochemical Characterization, Antimicrobial and Antibiofilm Efficacy against Oral Hygiene Related Pathogens. N. Biotechnol. 2017, 36, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Tahmourespour, A.; Kasra-Kermanshahi, R.; Salehi, R. Lactobacillus Rhamnosus Biosurfactant Inhibits Biofilm Formation and Gene Expression of Caries-Inducing Streptococcus Mutans. Dent. Res. J. 2019, 16, 87–94. [Google Scholar] [CrossRef]
- Tambone, E.; Marchetti, A.; Ceresa, C.; Piccoli, F.; Anesi, A.; Nollo, G.; Caola, I.; Bosetti, M.; Fracchia, L.; Ghensi, P.; et al. Counter-Acting Candida Albicans-Staphylococcus Aureus Mixed Biofilm on Titanium Implants Using Microbial Biosurfactants. Polymers 2021, 13, 2420. [Google Scholar] [CrossRef]
- Allegrone, G.; Ceresa, C.; Rinaldi, M.; Fracchia, L. Diverse Effects of Natural and Synthetic Surfactants on the Inhibition of Staphylococcus Aureus Biofilm. Pharmaceutics 2021, 13, 1172. [Google Scholar] [CrossRef]
- Ceresa, C.; Tessarolo, F.; Maniglio, D.; Tambone, E.; Carmagnola, I.; Fedeli, E.; Caola, I.; Nollo, G.; Chiono, V.; Allegrone, G.; et al. Medical-Grade Silicone Coated with Rhamnolipid R89 Is Effective against Staphylococcus Spp. Biofilms. Molecules 2019, 24, 3843. [Google Scholar] [CrossRef]
- Ceresa, C.; Rinaldi, M.; Tessarolo, F.; Maniglio, D.; Fedeli, E.; Tambone, E.; Caciagli, P.; Banat, I.M.; Diaz De Rienzo, M.A.; Fracchia, L. Inhibitory Effects of Lipopeptides and Glycolipids on C. Albicans–Staphylococcus Spp. Dual-Species Biofilms. Front. Microbiol. 2021, 11, 545654. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L. Microbial Complexes in Subgingival Plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Jenkinson, H.F. Beyond the Oral Microbiome. Environ. Microbiol. 2011, 13, 3077–3087. [Google Scholar] [CrossRef]
- Baker, J.L.; Bor, B.; Agnello, M.; Shi, W.; He, X. Ecology of the Oral Microbiome: Beyond Bacteria. Trends Microbiol. 2017, 25, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Kommerein, N.; Doll, K.; Stumpp, N.S.; Stiesch, M. Development and Characterization of an Oral Multispecies Biofilm Implant Flow Chamber Model. PLoS ONE 2018, 13, e0196967. [Google Scholar] [CrossRef] [PubMed]
- Ingendoh-Tsakmakidis, A.; Eberhard, J.; Falk, C.S.; Stiesch, M.; Winkel, A. In Vitro Effects of Streptococcus Oralis Biofilm on Peri-Implant Soft Tissue Cells. Cells 2020, 9, 1226. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z.; Chen, Z. Tuning Surface Properties of Bone Biomaterials to Manipulate Osteoblastic Cell Adhesion and the Signaling Pathways for the Enhancement of Early Osseointegration. Colloids Surf. B Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef]
- de Freitas Ferreira, J.; Vieira, E.A.; Nitschke, M. The Antibacterial Activity of Rhamnolipid Biosurfactant Is PH Dependent. Food Res. Int. 2019, 116, 737–744. [Google Scholar] [CrossRef]
- Singh, N.; Pemmaraju, S.C.; Pruthi, P.A.; Cameotra, S.S.; Pruthi, V. Candida Biofilm Disrupting Ability of Di-Rhamnolipid (RL-2) Produced from Pseudomonas Aeruginosa DSVP20. Appl. Biochem. Biotechnol. 2013, 169, 2374–2391. [Google Scholar] [CrossRef]
- Das, P.; Yang, X.-P.; Ma, L.Z. Analysis of Biosurfactants from Industrially Viable Pseudomonas Strain Isolated from Crude Oil Suggests How Rhamnolipids Congeners Affect Emulsification Property and Antimicrobial Activity. Front. Microbiol. 2014, 5, 696. [Google Scholar] [CrossRef]
- Diaz De Rienzo, M.A.; Stevenson, P.S.; Marchant, R.; Banat, I.M. Effect of Biosurfactants on Pseudomonas Aeruginosa and Staphylococcus Aureus Biofilms in a BioFlux Channel. Appl. Microbiol. Biotechnol. 2016, 100, 5773–5779. [Google Scholar] [CrossRef]
- Wilensky, A.; Shapira, L.; Limones, A.; Martin, C. The Efficacy of Implant Surface Decontamination Using Chemicals during Surgical Treatment of Peri-Implantitis: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2023, 50 (Suppl. 26), 336–358. [Google Scholar] [CrossRef]
- Skogman, M.E.; Vuorela, P.M.; Fallarero, A. Combining Biofilm Matrix Measurements with Biomass and Viability Assays in Susceptibility Assessments of Antimicrobials against Staphylococcus Aureus Biofilms. J. Antibiot. 2012, 65, 453–459. [Google Scholar] [CrossRef]
- Kuang, Z.; Yang, X.; Cao, Z.; Li, Y.; Hu, J.; Hong, X.; Li, B.; Wu, C.; Qi, Q.; Liu, X.; et al. Surfactin Suppresses Osteoclastogenesis via the NF-ΚB Signaling Pathway, Promotes Osteogenic Differentiation in Vitro, and Inhibits Oestrogen Deficiency-Induced Bone Loss in Vivo. Int. Immunopharmacol. 2023, 117, 109884. [Google Scholar] [CrossRef] [PubMed]
- Cichoń, E.; Czechowska, J.P.; Krok-Borkowicz, M.; Allinson, S.L.; Stępień, K.; Smith, A.; Pamuła, E.; Douglas, T.E.L.; Zima, A. Biosurfactants as Foaming Agents in Calcium Phosphate Bone Cements. Biomater. Adv. 2023, 145, 213273. [Google Scholar] [CrossRef] [PubMed]
- Orshesh, Z.; Borhan, S.; Kafashan, H. Physical, Mechanical and in Vitro Biological Evaluation of Synthesized Biosurfactant-Modified Silanated-Gelatin/Sodium Alginate/45S5 Bioglass Bone Tissue Engineering Scaffolds. J. Biomater. Sci. Polym. Ed. 2020, 31, 93–109. [Google Scholar] [CrossRef]
- Anselme, K.; Bigerelle, M. On the Relation between Surface Roughness of Metallic Substrates and Adhesion of Human Primary Bone Cells. Scanning 2014, 36, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Pardhi, D.S.; Panchal, R.R.; Raval, V.H.; Joshi, R.G.; Poczai, P.; Almalki, W.H.; Rajput, K.N. Microbial Surfactants: A Journey from Fundamentals to Recent Advances. Front. Microbiol. 2022, 13, 982603. [Google Scholar] [CrossRef]
- Lian, J.B.; Stein, G.S. Development of the Osteoblast Phenotype: Molecular Mechanisms Mediating Osteoblast Growth and Differentiation. Iowa Orthop. J. 1995, 15, 118–140. [Google Scholar]
- Huang, W.; Yang, S.; Shao, J.; Li, Y.-P. Signaling and Transcriptional Regulation in Osteoblast Commitment and Differentiation. Front. Biosci. 2007, 12, 3068–3092. [Google Scholar] [CrossRef]
- Owen, T.A.; Aronow, M.; Shalhoub, V.; Barone, L.M.; Wilming, L.; Tassinari, M.S.; Kennedy, M.B.; Pockwinse, S.; Lian, J.B.; Stein, G.S. Progressive Development of the Rat Osteoblast Phenotype in Vitro: Reciprocal Relationships in Expression of Genes Associated with Osteoblast Proliferation and Differentiation during Formation of the Bone Extracellular Matrix. J. Cell Physiol. 1990, 143, 420–430. [Google Scholar] [CrossRef]
- Kilian, K.A.; Bugarija, B.; Lahn, B.T.; Mrksich, M. Geometric Cues for Directing the Differentiation of Mesenchymal Stem Cells. Proc. Natl. Acad. Sci. USA 2010, 107, 4872–4877. [Google Scholar] [CrossRef]
- Dorkhan, M.; Svensäter, G.; Davies, J.R. Salivary Pellicles on Titanium and Their Effect on Metabolic Activity in Streptococcus Oralis. BMC Oral. Health 2013, 13, 32. [Google Scholar] [CrossRef]
- Martínez-Hernández, M.; Reyes-Grajeda, J.P.; Hannig, M.; Almaguer-Flores, A. Salivary Pellicle Modulates Biofilm Formation on Titanium Surfaces. Clin. Oral. Investig. 2023. [Google Scholar] [CrossRef] [PubMed]
- Neilands, J.; Svensäter, G.; Boisen, G.; Robertsson, C.; Wickström, C.; Davies, J.R. Formation and Analysis of Mono-Species and Polymicrobial Oral Biofilms in Flow-Cell Models. Methods Mol. Biol. 2023, 2674, 33–54. [Google Scholar] [CrossRef]
- Brown, J.L.; Butcher, M.C.; Veena, C.L.R.; Chogule, S.; Johnston, W.; Ramage, G. Generation of Multispecies Oral Bacteria Biofilm Models. Methods Mol. Biol. 2023, 2588, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Prado, M.M.; Figueiredo, N.; Pimenta, A.d.L.; Miranda, T.S.; Feres, M.; Figueiredo, L.C.; de Almeida, J.; Bueno-Silva, B. Recent Updates on Microbial Biofilms in Periodontitis: An Analysis of In Vitro Biofilm Models. Adv. Exp. Med. Biol. 2022, 1373, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.C.; Llama-Palacios, A.; Blanc, V.; León, R.; Herrera, D.; Sanz, M. Structure, Viability and Bacterial Kinetics of an in Vitro Biofilm Model Using Six Bacteria from the Subgingival Microbiota. J. Periodontal Res. 2011, 46, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.C.; Alonso-Español, A.; Ribeiro-Vidal, H.; Alonso, B.; Herrera, D.; Sanz, M. Relevance of Biofilm Models in Periodontal Research: From Static to Dynamic Systems. Microorganisms 2021, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra, S.S.; Wright, P.; Han, P.; Abdal-Hay, A.; Lee, R.S.B.; Ivanovski, S. Evaluating Models and Assessment Techniques for Understanding Oral Biofilm Complexity. Microbiologyopen 2023, 12, e1377. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. Effects of Titanium Surface Topography on Bone Integration: A Systematic Review. Clin. Oral. Implants Res. 2009, 20 (Suppl. 4), 172–184. [Google Scholar] [CrossRef]
- Rodriguez-González, R.; Monsalve-Guil, L.; Jimenez-Guerra, A.; Velasco-Ortega, E.; Moreno-Muñoz, J.; Nuñez-Marquez, E.; Pérez, R.A.; Gil, J.; Ortiz-Garcia, I. Relevant Aspects of Titanium Topography for Osteoblastic Adhesion and Inhibition of Bacterial Colonization. Materials 2023, 16, 3553. [Google Scholar] [CrossRef]
- Rompen, E.; Domken, O.; Degidi, M.; Farias Pontes, A.E.; Piattelli, A. The Effect of Material Characteristics, of Surface Topography and of Implant Components and Connections on Soft Tissue Integration: A Literature Review. Clin. Oral. Implant. Res. 2006, 17, 55–67. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and Broth Dilution Methods to Determine the Minimal Inhibitory Concentration (MIC) of Antimicrobial Substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.; Gutnick, D.; Rosenberg, E. Adherence of Bacteria to Hydrocarbons: A Simple Method for Measuring Cell-Surface Hydrophobicity. FEMS Microbiol. Lett. 1980, 9, 29–33. [Google Scholar] [CrossRef]
- Sana, S.; Datta, S.; Biswas, D.; Sengupta, D. Assessment of Synergistic Antibacterial Activity of Combined Biosurfactants Revealed by Bacterial Cell Envelop Damage. Biochim. Biophys. Acta Biomembr. 2018, 1860, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Little, B.; Wagner, P.; Ray, R.; Pope, R.; Scheetz, R. Biofilms: An ESEM Evaluation of Artifacts Introduced during SEM Preparation. J. Ind. Microbiol. 1991, 8, 213–221. [Google Scholar] [CrossRef]
- Hrubanova, K.; Krzyzanek, V.; Nebesarova, J.; Ruzicka, F.; Pilat, Z.; Samek, O. Monitoring Candida Parapsilosis and Staphylococcus Epidermidis Biofilms by a Combination of Scanning Electron Microscopy and Raman Spectroscopy. Sensors 2018, 18, 4089. [Google Scholar] [CrossRef]
- Bressan, E.; Tessarolo, F.; Sbricoli, L.; Caola, I.; Nollo, G.; Di Fiore, A. Effect of Chlorhexidine in Preventing Plaque Biofilm on Healing Abutment: A Crossover Controlled Study. Implant. Dent. 2014, 23, 64–68. [Google Scholar] [CrossRef]
- Signoretto, C.; Marchi, A.; Bertoncelli, A.; Burlacchini, G.; Tessarolo, F.; Caola, I.; Pezzati, E.; Zaura, E.; Papetti, A.; Lingström, P.; et al. Effects of Mushroom and Chicory Extracts on the Physiology and Shape of Prevotella Intermedia, a Periodontopathogenic Bacterium. J. Biomed. Biotechnol. 2011, 2011, 635348. [Google Scholar] [CrossRef]
- Council of Europe, Committee of Ministers. Recommendation Rec(2006)4 of the Committee of Ministers to Member States on Research on Biological Materials of Human Origin. Jahrb. Für Wiss. Und Ethik 2006, 11, 387–394. [Google Scholar] [CrossRef]
- Bosetti, M.; Fusaro, L.; Nicolì, E.; Borrone, A.; Aprile, S.; Cannas, M. Poly-L-Lactide Acid-Modified Scaffolds for Osteoinduction and Osteoconduction. J. Biomed. Mater. Res. A 2014, 102, 3531–3539. [Google Scholar] [CrossRef]
- Bosetti, M.; Santin, M.; Lloyd, A.W.; Denyer, S.P.; Sabbatini, M.; Cannas, M. Cell Behaviour on Phospholipids-Coated Surfaces. J. Mater. Sci. Mater. Med. 2007, 18, 611–617. [Google Scholar] [CrossRef]
- Lusvardi, G.; Malavasi, G.; Menabue, L.; Menziani, M.C.; Pedone, A.; Segre, U.; Aina, V.; Perardi, A.; Morterra, C.; Boccafoschi, F.; et al. Properties of Zinc Releasing Surfaces for Clinical Applications. J. Biomater. Appl. 2008, 22, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Nation, J.L. A New Method Using Hexamethyldisilazane for Preparation of Soft Insect Tissues for Scanning Electron Microscopy. Stain. Technol. 1983, 58, 347–351. [Google Scholar] [CrossRef] [PubMed]


| R89BS (µg/mL) | Planktonic Cells Concentration OD at 600 nm (Mean ± SD) | Growth Inhibition (%) |
|---|---|---|
| 0.0 | 0.668 ± 0.031 | - |
| 3.8 | 0.568 ± 0.030 | 15 |
| 7.5 | 0.442 ± 0.029 | 34 |
| 15 | 0.176 ± 0.028 | 74 |
| 30 | 0.007 ± 0.001 | 99 |
| R89BS (µg/mL) | 4 h | 24 h | ||
|---|---|---|---|---|
| Biofilm Biomass (Mean ± SD) | Removal Efficacy (%) | Biofilm Biomass (Mean ± SD) | Removal Efficacy (%) | |
| 0.0 | 5.77 ± 0.38 | - | 8.82 ± 0.73 | - |
| 30 | 3.67 ± 0.40 | 36 | 3.26 ± 0.63 | 63 |
| 60 | 3.60 ± 0.67 | 38 | 2.86 ± 0.44 | 68 |
| 120 | 2.38 ± 0.39 | 59 | 1.20 ± 0.30 | 86 |
| Incubation Time (h) | Inhibition (%) | |
|---|---|---|
| Biomass | Cell Viability | |
| 24 | 99.4 ± 0.2 | 99.99 ± 0.01 |
| 48 | 91.0 ± 2.5 | 98.79 ± 0.17 |
| 72 | 60.8 ± 4.2 | 98.84 ± 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tambone, E.; Ceresa, C.; Marchetti, A.; Chiera, S.; Anesi, A.; Nollo, G.; Caola, I.; Bosetti, M.; Fracchia, L.; Ghensi, P.; et al. Rhamnolipid 89 Biosurfactant Is Effective against Streptococcus oralis Biofilm and Preserves Osteoblast Behavior: Perspectives in Dental Implantology. Int. J. Mol. Sci. 2023, 24, 14014. https://doi.org/10.3390/ijms241814014
Tambone E, Ceresa C, Marchetti A, Chiera S, Anesi A, Nollo G, Caola I, Bosetti M, Fracchia L, Ghensi P, et al. Rhamnolipid 89 Biosurfactant Is Effective against Streptococcus oralis Biofilm and Preserves Osteoblast Behavior: Perspectives in Dental Implantology. International Journal of Molecular Sciences. 2023; 24(18):14014. https://doi.org/10.3390/ijms241814014
Chicago/Turabian StyleTambone, Erica, Chiara Ceresa, Alice Marchetti, Silvia Chiera, Adriano Anesi, Giandomenico Nollo, Iole Caola, Michela Bosetti, Letizia Fracchia, Paolo Ghensi, and et al. 2023. "Rhamnolipid 89 Biosurfactant Is Effective against Streptococcus oralis Biofilm and Preserves Osteoblast Behavior: Perspectives in Dental Implantology" International Journal of Molecular Sciences 24, no. 18: 14014. https://doi.org/10.3390/ijms241814014
APA StyleTambone, E., Ceresa, C., Marchetti, A., Chiera, S., Anesi, A., Nollo, G., Caola, I., Bosetti, M., Fracchia, L., Ghensi, P., & Tessarolo, F. (2023). Rhamnolipid 89 Biosurfactant Is Effective against Streptococcus oralis Biofilm and Preserves Osteoblast Behavior: Perspectives in Dental Implantology. International Journal of Molecular Sciences, 24(18), 14014. https://doi.org/10.3390/ijms241814014









