Ezetimibe Induces Vasodilation in Rat Mesenteric Resistance Arteries through Inhibition of Extracellular Ca2+ Influx
Abstract
1. Introduction
2. Results
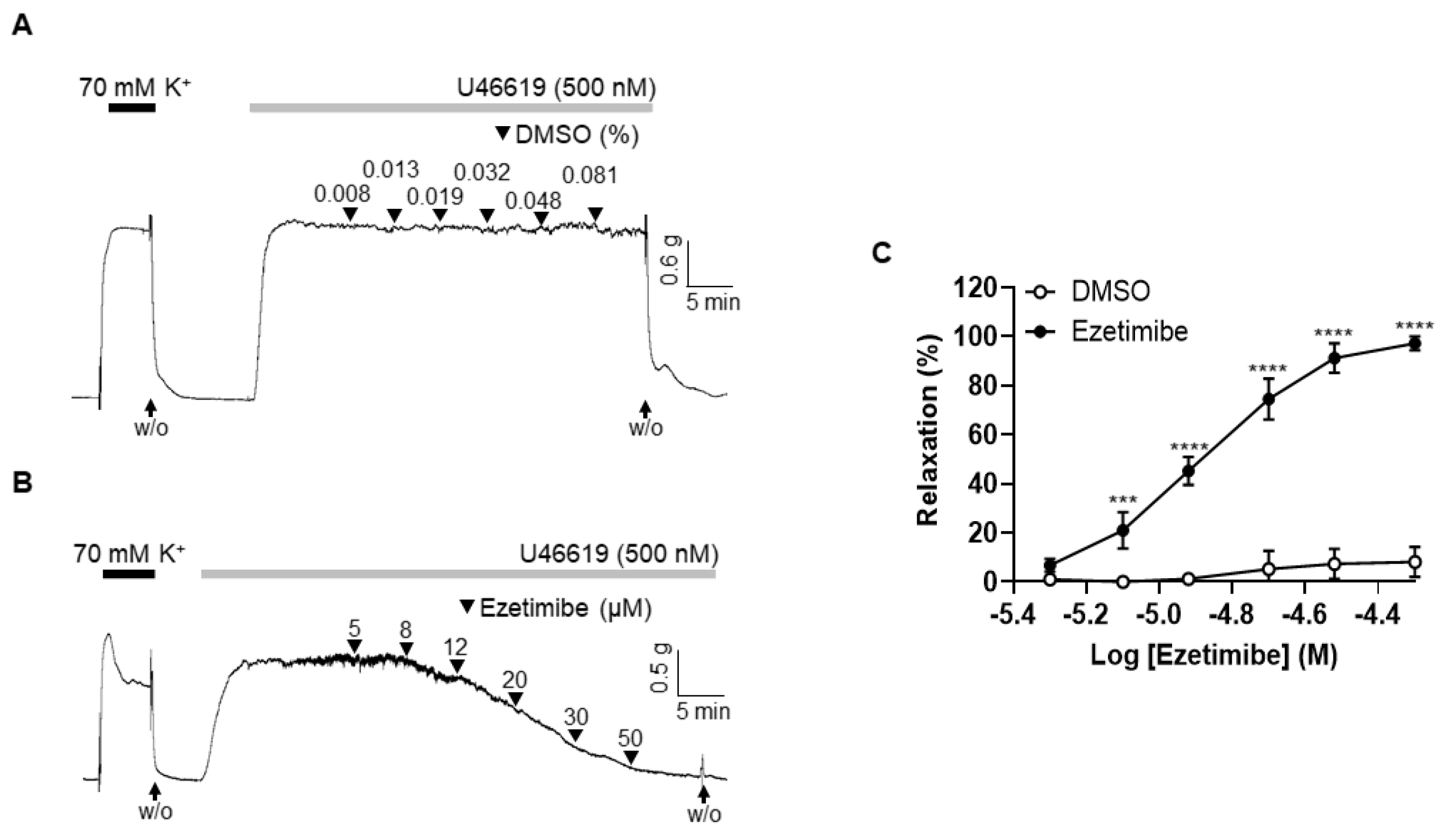
2.1. Effect of Ezetimibe on the Contraction Induced by Thromboxane Analogue, U46619
2.2. Endothelium-Independent Vasodilation Induced by Ezetimibe
2.3. Effect of Non-Specific K+ Channel Blocker, TEA, on the Vasodilation Induced by Ezetimibe
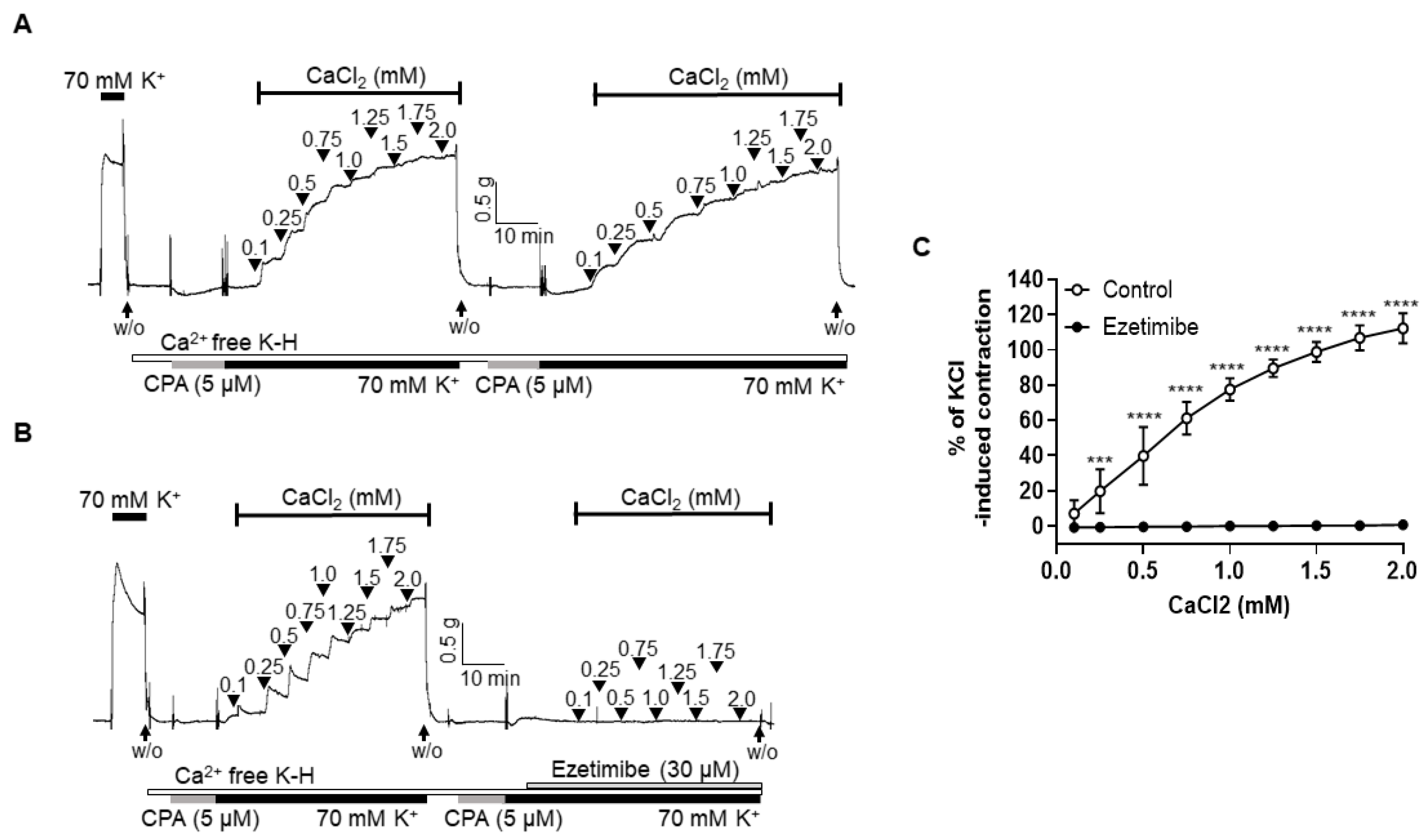
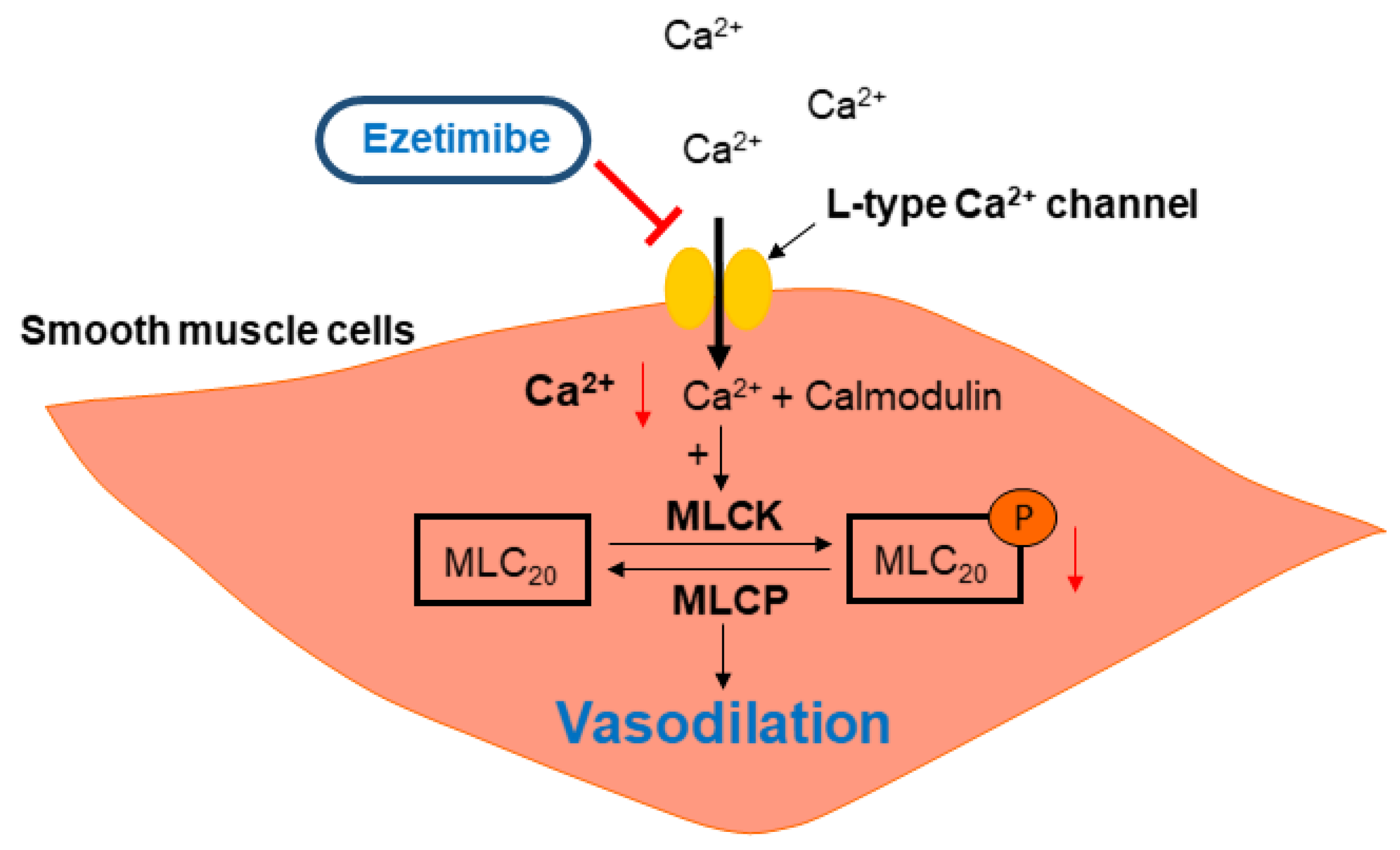
2.4. Effect of Ezetimibe on the Extracellular Ca2+-Induced Vascular Contraction
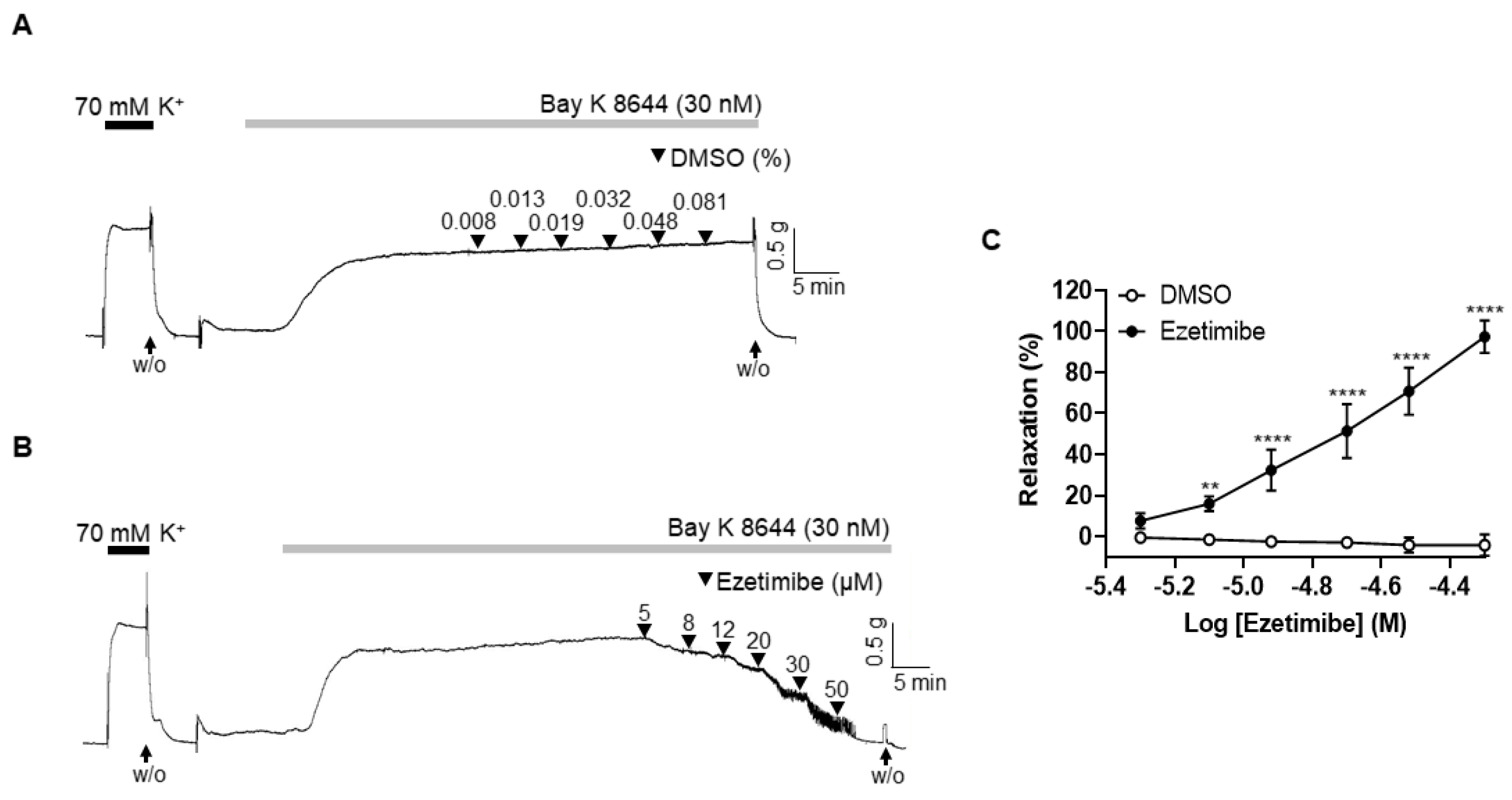
2.5. Effect of Ezetimibe on the BAY K 8644-Induced Contraction
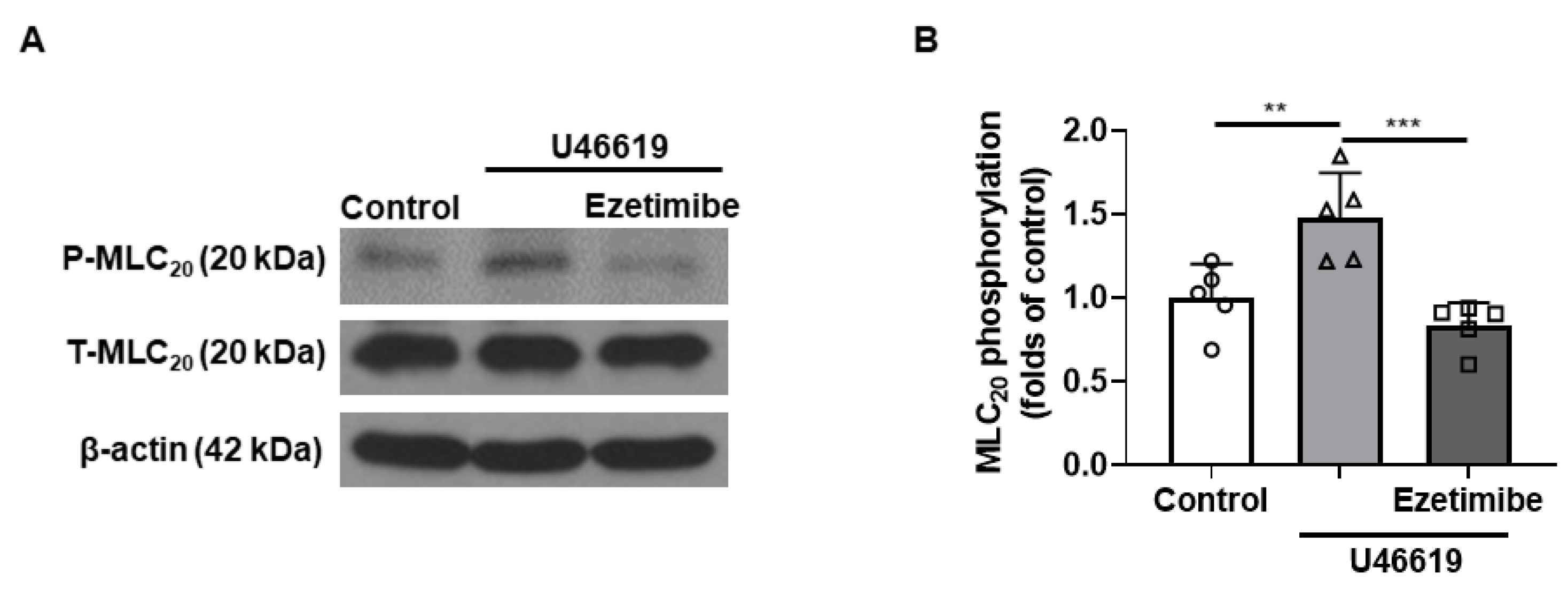
2.6. Effect of Ezetimibe on the Phosphorylation Level of MLC20 in VSMCs
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tissue Preparation
4.3. Measurement of Isometric Tension in Mesenteric Arteries
4.4. Experimental Protocols
4.5. Isolation and Culture of Vascular Smooth Muscle Cells
4.6. Western Blot Analysis
4.7. Chemicals and Reagents
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, C.O.; Johnson, G.; Addolorato, E.; Ammirati, L.M. Baddour. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Shepherd, j.; Cobbe, S.M.; Ford, I.; Isles, C.G.; Lorimer, A.R.; MacFarlane, P.W.; McKillop, J.H.; Packard, C.J. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N. Engl. J. Med. 1995, 333, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- LIPID Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N. Engl. J. Med. 1998, 339, 1349–1357. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.A.; Goodwill, A.G.; James, M.E.; Brock, R.W.; Frisbee, J.C. Hypercholesterolemia and microvascular dysfunction: Interventional strategies. J. Inflamm. 2010, 7, 54. [Google Scholar] [CrossRef]
- Stapleton, P.A.; Goodwill, A.G.; James, M.E.; D’Audiffret, A.C.; Frisbee, J.C. Differential Impact of Familial Hypercholesterolemia and Combined Hyperlipidemia on Vascular Wall and Network Remodeling in Mice. Microcirculation 2010, 17, 47–58. [Google Scholar] [CrossRef]
- Mourikis, P.; Zako, S.; Dannenberg, L.; Nia, A.M.; Heinen, Y.; Busch, L.; Richter, H.; Hohlfel, T.; Zeus, T.; Kelm, T.; et al. Lipid lowering therapy in cardiovascular disease: From myth to molecular reality. Pharmacol. Ther. 2020, 213, 107592. [Google Scholar] [CrossRef]
- Vallejo-Vaz, A.J.; Robertson, M.; Catapano, A.L.; Watts, G.F.; Kastelein, J.J.; Packard, C.J.; Ray, K.K. Low-density lipoprotein cholesterol lowering for the primary prevention of cardiovascular disease among men with primary elevations of lowdensity lipoprotein cholesterol levels of 190 mg/dL or above: Analyses from the WOSCOPS (west of Scotland coronary prevention study) 5-year randomized trial and 20-year observational follow-up. Circulation 2017, 136, 1878–1891. [Google Scholar]
- Davis, H.R.; Compton, D.S.; Hoos, L.; Tetzloff, G. Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in ApoE knockout mice. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 2032–2038. [Google Scholar] [CrossRef]
- Amarenco, P.; Bogousslavsky, J.; Callahan, A.; Goldstein, L.B.; Hennerici, M.; Rudolph, A.E. Stroke Prevention by Aggressive Reduction in Cholesterol Levels I. High-dose atorvastatin after stroke or transient ischemic attack. N. Engl. J. Med. 2006, 355, 549–559. [Google Scholar]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Group ESCSD. ESC/EAS guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Pandor, A.; Ara, R.M.; Tumur, I.; Wilkinson, A.J.; Paisley, S.; Duenas, A.; Chilcott, J. Ezetimibe monotherapy for cholesterol lowering in 2722 people: Systematic review and meta-analysis of randomized controlled trials. J. Intern. Med. 2009, 265, 568–580. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.R. The pharmacology of statins. Pharmacol. Res. 2014, 88, 3–11. [Google Scholar] [CrossRef]
- Oesterle, A.; Laufs, U.; Liao, J.K. Pleiotropic effects of statins on the cardiovascular system. Circ. Res. 2017, 120, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E.; Stroes, E.; Dent-Acosta, R.E.; Rosenson, R.S.; Lehman, S.J.; Sattar, N. Efficacy and tolerability of evolocumab vs ezetimibe in patients with muscle-related statin intolerance: The GAUSS-3 randomized clinical trial. JAMA 2016, 315, 1580–1590. [Google Scholar] [CrossRef] [PubMed]
- AlTurki, A.; Marafi, M.; Dawas, A.; Dube, M.P.; Vieira, L.; Sherman, M.H.; Huynh, T. Meta-analysis of randomized controlled trials assessing the impact of proprotein convertase subtilisin/kexin type 9 antibodies on mortality and cardiovascular outcomes. Am. J. Cardiol. 2019, 124, 1869–1875. [Google Scholar] [CrossRef]
- Jalloh, M.A.; Doroudgar, S.; Ip, E.J. What is the impact of the 2017 cochrane systematic review and meta-analysis that evaluated the use of PCSK9 inhibitors for lowering cardiovascular disease and mortality? Expert. Opin. Pharmacother. 2018, 19, 739–741. [Google Scholar] [CrossRef]
- Nakano, T.; Inoue, I.; Takenaka, Y.; Ono, H.; Katayama, S.; Awata, T.; Murakoshi, T. Ezetimibe Promotes Brush Border Membrane-to-Lumen Cholesterol Efflux in the Small Intestine. PLoS ONE 2016, 11, e0152207. [Google Scholar] [CrossRef]
- Bass, A.; Hinderliter, A.L.; Lee, C.R. The impact of ezetimibe on endothelial function and other markers of cardiovascular risk. Ann. Pharmacother. 2009, 43, 2021–2030. [Google Scholar] [CrossRef]
- Tamaki, N.; Ueno, H.; Morinaga, Y.; Shiiya, T.; Nakazato, M. Ezetimibe ameliorates atherosclerotic and inflammatory markers, atherogenic lipid profiles, insulin sensitivity, and liver dysfunction in Japanese patients with hypercholesterolemia. J. Atheroscler. Thromb. 2012, 19, 532–538. [Google Scholar] [CrossRef]
- Nochioka, K.; Tanaka, S.; Miura, M.; Zhulanqiqige, D.E.; Fukumoto, Y.; Shiba, N.; Shimokawa, H. Ezetimibe improves endothelial function and inhibits Rho-kinase activity associated with inhibition of cholesterol absorption in humans. Circ. J. 2012, 76, 2023–2030. [Google Scholar] [CrossRef]
- Pajkowski, M.; Dudziak, M.; Chlebus, K.; Hellmann, M. Assessment of microvascular function and pharmacological regulation in genetically confirmed familial hypercholesterolemia. Microvasc. Res. 2021, 138, 104216. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Gong, K.; Xu, S.; Zhang, F.; Meng, X.; Han, J. Regulation of cholesterol homeostasis in health and diseases: From mechanisms to targeted therapeutics. Signal Transduct. Target Ther. 2022, 7, 265. [Google Scholar] [CrossRef]
- Stapleton, P.A.; Goodwill, A.G.; James, M.E.; Frisbee, J.C. Altered mechanisms of endothelium-dependent dilation in skeletal muscle arterioles with genetic hypercholesterolemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1110–R1119. [Google Scholar] [CrossRef]
- Jiang, F.; Gibson, A.P.; Dusting, G.J. Endothelial dysfunction induced by oxidized low-density lipoproteins in isolated mouse aorta: A comparison with apolipoprotein-E deficient mice. Eur. J. Pharmacol. 2001, 424, 141–149. [Google Scholar] [CrossRef]
- Stokes, K.Y.; Cooper, D.; Tailor, A.; Granger, D.N. Hypercholesterolemia promotes inflammation and microvascular dysfunction: Role of nitric oxide and superoxide. Free Radic. Biol. Med. 2002, 33, 1026–1036. [Google Scholar] [CrossRef]
- Stokes, K.Y.; Calahan, L.; Russell, J.M.; Gurwara, S.; Granger, D.N. Role of platelets in hypercholesterolemia-induced leukocyte recruitment and arteriolar dysfunction. Microcirculation 2006, 13, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Van Heek, M.; France, C.F.; Compton, D.S.; McLeod, R.L.; Yumibe, N.P.; Alton, K.B.; Sybertz, E.J.; Davis, H.R. In vivo metabolism-based discovery of a potent cholesterol absorption inhibitor, SCH58235, in the rat and rhesus monkey through the identification of the active metabolites of SCH48461. J. Pharmacol. Exp. Ther. 1997, 283, 157–163. [Google Scholar]
- Genest, J. Combination of statin and ezetimibe for the treatment of dyslipidemias and the prevention of coronary artery disease. Can. J. Cardiol. 2006, 22, 863–868. [Google Scholar] [CrossRef]
- Takase, S.; Matoba, T.; Nakashiro, S.; Mukai, Y.; Inoue, S.; Oi, K. Ezetimibe in Combination with Statins Ameliorates Endothelial Dysfunction in Coronary Arteries After Stenting: The CuVIC Trial (Effect of Cholesterol Absorption Inhibitor Usage on Target Vessel Dysfunction After Coronary Stenting), a Multicenter Randomized Controlled Trial. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 350–358. [Google Scholar]
- Ramanlal, R.; Gupta, V. Physiology, Vasodilation; StatPearls: Treasure Island, FL, USA, 2023. [Google Scholar]
- Pi, X.; Xie, L.; Patterson, C. Emerging Roles of Vascular Endothelium in Metabolic Homeostasis. Circ. Res. 2018, 123, 477–494. [Google Scholar] [CrossRef]
- Sandoo, A.; van Zanten, J.J.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar]
- Cooke, J.P. The 1998 Nobel prize in Medicine: Clinical implications for 1999 and beyond. Vasc. Med. 1999, 4, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, T.; Komori, K.; Morisaki, K.; Itoh, T. Ezetimibe reduces intimal hyperplasia in rabbit jugular vein graft. J. Vasc. Surg. 2012, 56, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.S.; Kim, Y.B.; Lee, Y.H.; Kang, B.S.; Kang, D.H. Fatty acids directly increase the activity of Ca2+-activated K+ channels in rabbit coronary smooth muscle cells. Yonsei Med. J. 1994, 35, 10–24. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.F. Potassium Channels in Regulation of Vascular Smooth Muscle Contraction and Growth. Adv. Pharmacol. 2017, 78, 89–144. [Google Scholar]
- Kamm, K.E.; Stull, J.T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu. Rev. Pharmacol. Toxicol. 1985, 25, 593–620. [Google Scholar] [CrossRef]
- Choi, S.K.; Ahn, D.S.; Lee, Y.H. Comparison of contractile mechanisms of sphingosylphosphorylcholine and sphingosine-1-phosphate in rabbit coronary artery. Cardiovasc. Res. 2009, 82, 324–332. [Google Scholar] [CrossRef][Green Version]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, E.Y.; Haam, C.E.; Choi, S.; Byeon, S.; Choi, S.-K.; Lee, Y.-H. Ezetimibe Induces Vasodilation in Rat Mesenteric Resistance Arteries through Inhibition of Extracellular Ca2+ Influx. Int. J. Mol. Sci. 2023, 24, 13992. https://doi.org/10.3390/ijms241813992
Oh EY, Haam CE, Choi S, Byeon S, Choi S-K, Lee Y-H. Ezetimibe Induces Vasodilation in Rat Mesenteric Resistance Arteries through Inhibition of Extracellular Ca2+ Influx. International Journal of Molecular Sciences. 2023; 24(18):13992. https://doi.org/10.3390/ijms241813992
Chicago/Turabian StyleOh, Eun Yi, Chae Eun Haam, Sooyeon Choi, Seonhee Byeon, Soo-Kyoung Choi, and Young-Ho Lee. 2023. "Ezetimibe Induces Vasodilation in Rat Mesenteric Resistance Arteries through Inhibition of Extracellular Ca2+ Influx" International Journal of Molecular Sciences 24, no. 18: 13992. https://doi.org/10.3390/ijms241813992
APA StyleOh, E. Y., Haam, C. E., Choi, S., Byeon, S., Choi, S.-K., & Lee, Y.-H. (2023). Ezetimibe Induces Vasodilation in Rat Mesenteric Resistance Arteries through Inhibition of Extracellular Ca2+ Influx. International Journal of Molecular Sciences, 24(18), 13992. https://doi.org/10.3390/ijms241813992




