Abstract
Copper (Cu) is an essential trace element for maintaining normal homeostasis in living organisms. Yet, an elevated level of Cu beyond homeostatic capacity may lead to oxidative damage of cellular components in several organs, including the lungs. This work investigated the effects of curcumin (Curc) and nano-curcumin (nCurc) against Cu-induced lung injury, accenting the roles of oxidative stress, inflammation, and the nuclear factor erythroid 2-related factor/heme oxygenase-1 Nrf2/HO-1 pathway. Rats were challenged with 100 mg/kg of copper sulfate (CuSO4) while being treated with Curc or nCurc for 7 days. Cu-triggered lung oxidative stress detected as dysregulation of oxidative/antioxidant markers, a downregulation of Nrf-2/HO-1 signaling, and an increase in the inflammatory markers interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and intracellular adhesion molecule-1 (ICAM-1). Additionally, it decreased the expression of lung-specific proteins, surfactant protein-C (SP-C), and mucin-1 (MUC-1), induced apoptosis, and caused changes in lung histology. Curc and nCurc alleviated CuSO4-induced lung injury by suppressing oxidative damage and inflammation and activating Nrf-2/HO-1. They also prevented apoptosis and restored the normal expression of SP-C and MUC-1. We concluded that nCurc exhibited superior efficacy compared with Curc in mitigating CuSO4-induced lung injury. This was associated with reduced oxidative stress, inflammation, and apoptotic responses and increased Nrf2/HO-1 signaling and expression of SP-C and MUC-1.
Keywords:
curcumin; lung toxicity; inflammation; copper sulfate; oxidative stress; Nrf2/HO-1 pathway; ICAM-1 1. Introduction
Copper (Cu) is a crucial trace element and a cofactor of several redox enzymes. It is involved in numerous biological functions, including blood coagulation, neurotransmitter synthesis, antioxidant defense, energy production, and cellular metabolism [1,2]. The maintenance of Cu homeostasis is tightly regulated via the balancing of its absorption, excretion, and circulating levels. Cu possesses the potential for toxicity owing to its chemical redox potential and capacity to engage in free radical reactions [3]. It is widely used in many industries to synthesize electronics, building materials, wood protection, and pesticides. Cu toxicity may result from acute or chronic exposure to excess Cu due to accidents, occupational hazards, and environmental pollution. It can also be accumulated due to genetic defects, as in the case of Wilson’s disease, an inherited mutation in the ATP7B gene that encodes for a protein responsible for Cu excretion [4,5,6]. Such toxicity increases the risk of developing neurological, hepatic, and renal diseases, which are attributed primarily to oxidative stress, DNA damage, and cell apoptosis [4,7,8].
Copper sulfate (CuSO4) is an inorganic compound frequently used in agriculture, analytical, and tissue culture laboratories as it possesses pesticidal, redox potential, and antimicrobial actions, respectively. However, accidental or deliberate intoxication with Cu may cause multiorgan toxicity, which can be life-threatening [9,10]. The acute toxicity of CuSO4 can cause hepatitis, jaundice, intravascular hemolysis, methemoglobinemia, erosive gastritis, acute tubular necrosis, and rhabdomyolysis [11,12]. The toxic effect of CuSO4 ingestion on the lungs has been reported previously as it exhibits corrosive effects on mucous membranes [13]. Repeated exposure to CuSO4 pentahydrate via inhalation results in a dose-related pulmonary inflammatory response and increased lung weight due to epithelial hyperplasia in rats [14]. Chelating therapies, including D-penicillamine, trientine, and deferoxamine (DFO), are still used to control Cu toxicity, just like with other metals [15]. However, the adoption of safer substitutes is required owing to the partial efficacy and side effects of these agents.
Since the lungs are usually exposed to substantial oxygen levels, they possess a collection of enzymatic and non-enzymatic antioxidants that often function extensively to counter any potential oxidative assaults [16]. It was recently reported that an elevation in urinary Cu levels was directly correlated with a higher risk of lung fibrotic changes [17]. In addition, there is an association between environmental Cu exposure and the risk of developing lung cancer [18]. Copper oxide nanoparticles (CuO NPs) induce lung epithelial cell death and pulmonary fibrosis in mice [19]. Likewise, studies have revealed that Cu has the capacity to elicit oxidative stress and provoke an accumulation of reactive oxygen species (ROS) [20,21]. ROS incur cycles of catastrophic events that initiate several inflammatory responses and cytokine release [22,23]. Persistent release of these mediators can cause tissue injury by activating the nuclear factor kappa B (NF-κB) pathway, mitogen-activated protein kinases (MAPKs), and apoptosis [4,24].
Curcumin (Cur) is a natural phytochemical present in turmeric with substantial antioxidant, anti-inflammatory, immunomodulatory, antibacterial, and antiviral actions [25,26,27]. Curc exerts in vivo and in vitro antioxidant effects via multiple mechanisms as it neutralizes ROS and reactive nitrogen species (RNS) [28,29]. This neutralization is attributed to the abundance of conjugated double bonds in its structure which serve as efficient electron donors in the counteraction of reactive species in many redox reactions [30]. Curc has some therapeutic effects in acute and chronic lung diseases like acute lung injury (ALI), asthma, pneumonia, and chronic obstructive pulmonary disease (COPD) [31,32]. While there are existing studies that demonstrate its protective efficacy against viral-induced lung injury in acute respiratory distress syndrome in mice [33] and ventilator-induced lung injury (VILI) in rats [34], and reduced symptoms, hospital stay, and mortality in COVID-19 patients [35], the protective effect of Curc against lung injury caused by CuSO4 has not yet been studied. Some of the therapeutic potential of Curc is hindered by its inadequate solubility in water, limited absorption and systemic bioavailability, fast metabolism, physicochemical instability, and low effectiveness in penetrating and targeting specific sites [36]. Therefore, this study investigated the impact of CuSO4-induced lung injury in rats and explored the potential protective effects of Curc and its nanoformulation (nano-curcumin, nCurc) against such injury. These were achieved by assessing the pulmonary expression of the nuclear factor erythroid 2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) signaling pathway, inflammatory cytokines, and intracellular adhesion molecule-1 (ICAM-1) expression in rats.
2. Results
2.1. Curc and nCurc Mitigate Oxidative Stress after CuSO4-Induced Lung Injury
Lipid peroxidation, indicated by MDA, was significantly increased in the lung tissue homogenate of CuSO4-intoxicated rats relative to that of the control rats (p ≤ 0.01; Figure 1A). In contrast, the SOD activity (p ≤ 0.0001; Figure 1B), GSH level (p ≤ 0.01; Figure 1C), and GPX2 level (p ≤ 0.0001; Figure 1D) were reduced after such injury in comparison to those of the control group. The concurrent use of nCurc showed profound mitigation of pulmonary oxidative stress by lowering the MDA level and increasing SOD, GSH, and GPX2 relative to the CuSO4 group (p ≤ 0.01, p ≤ 0.05, p ≤ 0.01, and p ≤ 0.0001, respectively). Curc exhibited significant elevations in GSH and GPX-2 (p ≤ 0.05, p ≤ 0.0001), while DFO increased GPX2 (p ≤ 0.0001) only following CuSO4 overexposure.
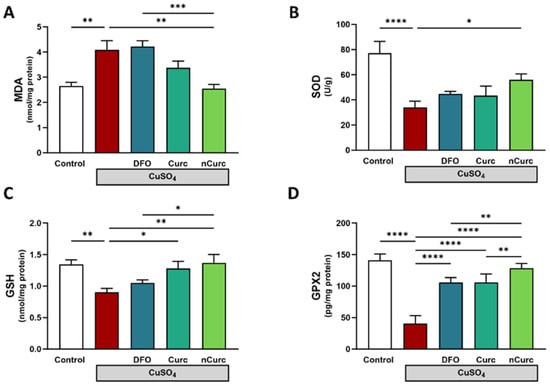
Figure 1.
Curc and nCurc mitigate oxidative stress after CuSO4-induced lung injury. Treatment with nCurc decreased (A) MDA and increased (B) SOD activity, (C) GSH, and (D) GPX2. Curc increased GSH and GPX2 after CuSO4 overexposure. Data are depicted as mean ± SEM (n = 7). * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, and **** p ≤ 0.0001.
2.2. Curc and nCurc Attenuate CuSO4-Induced Histopathological Changes in Lung Tissues
Histological staining was utilized to assess the pathological changes that occurred in the lungs after CuSO4 exposure and their response to treatments (Figure 2A–J). The control group showed a normal bronchus, alveoli with thin inter-alveolar septa, and type I and II pneumocytes. In contrast, CuSO4 induced some pathological changes which were identified as infiltration of lymphocytes, intra-alveolar hemorrhage, and ruptured alveoli. Treatment with DFO showed alveoli with thin inter-alveolar septa and mild lymphocyte infiltration. Curc and nCurc improved lung tissue appearance and reversed the pathological changes induced by CuSO4.
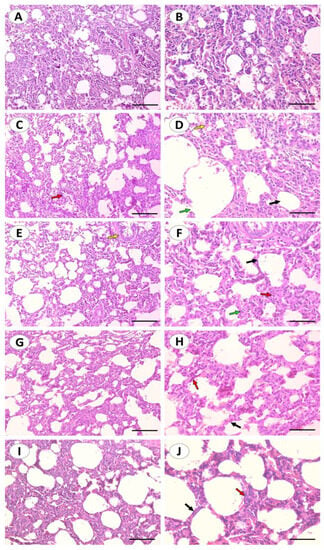
Figure 2.
Curc and nCurc improve tissue appearance and reverse CuSO4-induced lung injury. Representative images of H&E-stained lung sections of control rats (A,B) showing normal lung histology. CuSO4-challenged rats (C,D) showing inter-alveolar septum (black arrow), intra-alveolar hemorrhage and congestion (yellow arrow), bronchus (red arrow), ruptured alveolus (green arrow). Cu-challenged rats treated with DFO (E,F) showing alveoli with thin inter-alveolar septum (black arrow), mild infiltration of lymphocytes (red arrow), bronchus (yellow arrow), type I and type II pneumocytes are also seen (green arrow). CuSO4-challenged rats treated with Curc (G,H) and nCurc (I,J) showing alveoli with normal inter-alveolar septum (black arrow), type I and type II pneumocytes are also seen (red arrow). (200×: (A,C,E,G,I), scale bar = 200 µm, 400×: (B,D,F,H,J), scale bar = 100 µm).
2.3. Curc and nCurc Modulate Pulmonary Keap-1/Nrf-2/HO-1 Signaling after CuSO4-Induced Lung Injury
The protein expression of pulmonary Keap1 was significantly upregulated, while Nrf-2 and HO-1 were significantly downregulated in CuSO4-intoxicated rats, as compared with the control group (p ≤ 0.0001, as shown in Figure 3). However, treatment of those rats with DFO, Curc, and nCurc exhibited a significant decrease in Keap-1 expression and an increase in Nrf-2 and HO-1 expression (p ≤ 0.0001). Notably, nCurc upregulated HO-1 expression significantly compared with DFO or Curc (p ≤ 0.0001).
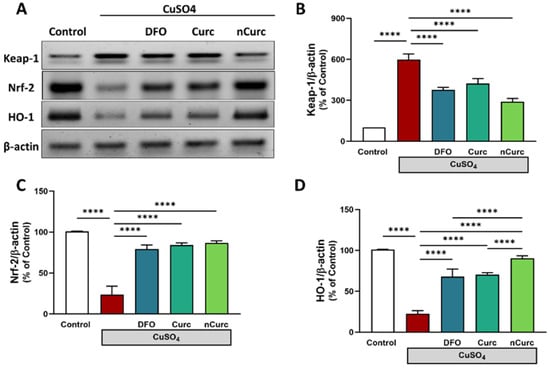
Figure 3.
Curc and nCurc modulate pulmonary Keap-1/Nrf-2/HO-1 signaling after CuSO4-induced lung injury (A–D). Exposure to CuSO4 caused an increase in Keap-1 and a decrease in Nrf-2 and HO-1 protein expression. Treatment with DFO, Curc, and nCurc improved the expression of these proteins. Data are depicted as mean ± SEM (n = 7). **** p ≤ 0.0001.
2.4. Curc and nCurc Ameliorate Inflammation after CuSO4-Induced Lung Injury
To confirm the lung-protective effects of DFO, Curc, and nCurc, we measured the levels of inflammatory biomarkers (IL-1β, TNF-α, and ICAM-1) in lung tissues. Rats exposed to CuSO4 demonstrated a significant increase in these markers (Figure 4) compared with the control group. Nevertheless, treatment with antioxidants effectively mitigated the levels of these inflammatory markers in rats administered CuSO4, as demonstrated in Figure 4.
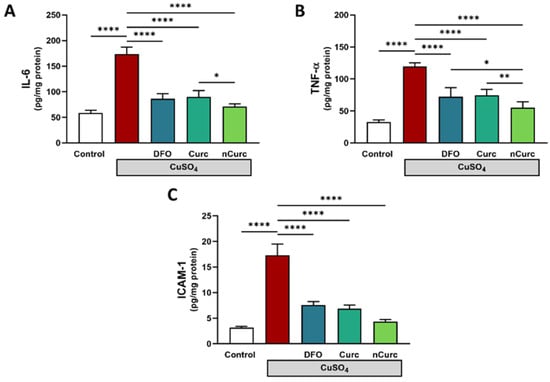
Figure 4.
Curc and nCurc ameliorate pulmonary inflammation after CuSO4-induced lung injury (A–C). Cu exposure caused an increase in inflammatory marker levels. Treatment with DFO, Curc, and nCurc ameliorated the inflammation. Data are depicted as mean ± SEM (n = 7). * p ≤ 0.05, ** p ≤ 0.01, **** p ≤ 0.0001.
2.5. Curc and nCurc Restore SP-C and MUC-1 Levels after CuSO4-Induced Lung Injury
To further confirm the protective effects of Curc and nCurc, we measured the levels of SP-C and MUC-1 in the lung tissues. CuSO4-administered rats demonstrated a significant decrease in these markers (Figure 5) compared with the control group. However, treatment with antioxidants effectively restored the levels of these inflammatory markers in rats administered CuSO4.
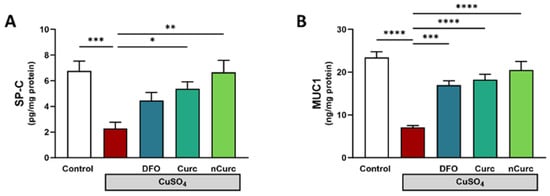
Figure 5.
Curc and nCurc restore the pulmonary expression of SP-C and MUC-1 after CuSO4-induced lung injury (A,B). Data are depicted as mean ± S.E.M. (n = 7). * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, **** p ≤ 0.0001.
2.6. Curc and nCurc Prevent Apoptosis by Regulating BAX and Bcl-2 Gene Expression Levels after CuSO4-Induced Lung Injury
The CuSO4-administered group showed a significant upregulation of BAX gene expression (p ≤ 0.001) and downregulation of Bcl-2 gene expression (p ≤ 0.001) compared with the control group, as depicted in Figure 6. Nevertheless, treatment with DFO, Curc, and nCurc significantly mitigated the effects of CuSO4 on gene expression (p ≤ 0.001) by restoring their average expression levels.
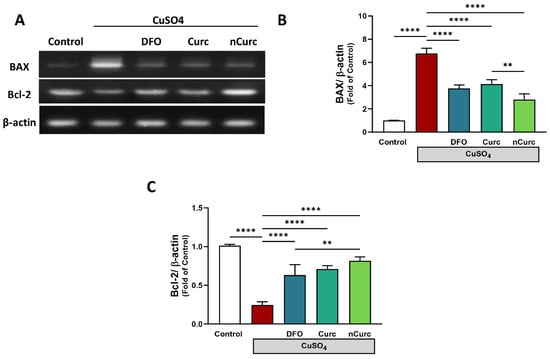
Figure 6.
Curc and nCurc prevent apoptosis by regulating BAX and Bcl-2 gene expression after CuSO4-induced lung injury (A–C). CuSO4 exposure caused an increase in BAX and a reduction in Bcl2 mRNA levels. Treatment with DFO, Curc, and nCurc improved the levels of apoptotic markers. Data are depicted as mean ± SEM (n = 7). ** p ≤ 0.01, **** p ≤ 0.0001.
3. Discussion
Cu is a pivotal micromineral for many physiological processes in the body, such as cellular respiration, enzyme activation, immune responses, antioxidant defenses, and energy homeostasis [1]. Despite its importance, excess Cu can cause serious multiorgan toxicities if absorbed systemically through the lungs, skin, and gastrointestinal tract [9]. Extensive occupational exposure to Cu can cause severe lung diseases primarily from inhaled particulates from mining or metal fumes from smelting, welding, agriculture, or other related enterprises [5,37]. Cu accumulation is a risk for multiple tissue damage and fibrosis, including in the lungs [38]. Limited research has been conducted regarding pulmonary toxicity due to copper exposure. One case report revealed that the ingestion of CuSO4 caused severe pulmonary toxicity manifested by acute, bilateral pulmonary infiltrates and hypoxemia [13]. Yet, the deleterious effects and mechanistic consequences of this metal on the lungs are less addressed. Therefore, we investigated the role of Curc and its nanoform against CuSO4-induced pulmonary injury in rats, pointing towards the changes in inflammation, the Nrf2/HO-1 pathway, and the lung-functioning proteins SP-C and MUC-1.
The present study demonstrated that exposure to CuSO4 resulted in pulmonary oxidative stress, as evidenced by an elevation in lipid peroxidation levels and a reduction in SOD activity, GSH, and GPX2 levels. Cu has a redox catalytic reactivity that is crucial for many biological reactions. On the contrary, when its concentration surpasses a certain limit, this metal generates free radicals that are extremely reactive and toxic [39]. Cu can also alter the activity of electron respiratory chain proteins, which are crucial for releasing energy in the mitochondria, and produce excess ROS [40]. ROS are strong oxidizers that enhance lipid peroxidation, protein damage, DNA fragmentation, and cell death [4,41]. In addition, aberrations in the oxidant/antioxidant balance caused by Cu result in the oxidation of proteins’ sulfhydryl groups, thereby depleting GSH stores and reducing SOD activity in the lungs and other organs, as previously reported [42,43,44,45]. MDA is the most prominent byproduct of lipid peroxidation, and it crosslinks to tissue DNA or protein to form adducts, resulting in biomolecular damage [46]. GSH is a non-enzymatic antioxidant defense that acts directly on free radicals by quenching their reactivity, thus protecting cells from their destructive consequences, or indirectly by activating detoxification enzymes such as glutathione peroxidases, glutathione S-transferases, and glyoxalases [47,48,49]. SOD is an enzymatic antioxidant found in most organs, including the brain, liver, thyroid, and lungs. This enzyme converts toxic superoxide radicals to less reactive dioxygen and hydrogen peroxide [50,51]. Moreover, Cu causes downregulation of some antioxidant genes including GPX2, GSR., and KEAP1, in A549 lung epithelial cells [48]. It competes with other metals inside the cells, displaces them from their metal binding sites, and further impairs cellular health and survival [52].
Considering the contribution of oxidative stress in the mechanism of Cu-induced lung injury, Curc can potentially mitigate such damage because of its antioxidant action and modulation of the Keap-1/Nrf-2/HO-1 signaling pathway. In the present study, rats that received Curc or nCurc concurrently with CuSO4 exhibited a notable reversal in pulmonary oxidative stress markers by decreasing MDA levels and increasing GSH, GPX2, and SOD activity. Previous studies reported that Curc possessed antioxidant effects against animal models of lung toxicity or injury induced by nicotine [53], elastase and cigarette smoke [54], bleomycin [55], cyclophosphamide [25], and even radiation [56]; it decreased MDA and enhanced GSH and antioxidant enzymes that parallel with our findings. The rats that received nCurc displayed more significant antioxidant effects than those observed in rats administered the native form of Curc. In the same context, the protective findings for Curc and nCurc were analyzed using histopathological observations of rat lungs after Cu injury. The histopathological examination of the lung tissue of rats exposed to CuSO4 demonstrated degenerative changes that supported the previous biochemical results. These changes included pulmonary congestion, intra-alveolar hemorrhage, ruptured alveoli, and lymphocyte infiltration. The antioxidant agents almost restored normal lung tissue architecture with no sign of ruptured alveoli or lymphocyte infiltration.
Keap-1/Nrf-2/HO-1 is a prominent pathway of cytoprotective responses to both endogenous and exogenous stresses resulting from ROS. An essential signaling protein within this pathway is the transcription factor Nrf2. The significance of Nrf2 in the pulmonary system has emerged via knockout experiments in which mice lacking the Nrf2 gene exhibit increased susceptibility to various chemically induced pulmonary toxicities and pathologies [57]. The disruption of the Nrf2 gene in mice results in prompt and severe emphysema upon exposure to cigarette smoke [58]. Moreover, Nrf2−/− mice exhibited severe lung damage characterized by increased protein permeability, macrophage inflammation, and epithelial injury after hyperoxia exposure [59]. Previous studies have reported that Cu toxicity can cause downregulation of Nrf2 and HO-1 in the liver [60], brain [61,62], and testes [45], which supports our findings.
The beneficial effects of Curc and nCurc against CuSO4-induced oxidative lung injury might be attributed to the activation of Nrf2/HO-1 signaling and induction of detoxifying enzymes. Of note, nCurc was more effective than Curc in upregulating pulmonary HO-1 expression. In consistence with the results, Curc upregulated the Nrf2/HO-1 pathway and attenuated oxidative stress in lipopolysaccharide (LPS)-induced lung injury [60]. It also regulates this pathway in several other organs, either in vitro or in vivo as reviewed in [60,63,64]. Nrf2, a redox-sensitive factor, regulates cellular antioxidant responses against oxidative stress by controlling the transcription of antioxidant genes [65]. Nrf2 expression is low under basal conditions and sequestered by its repressor Keap-1. During oxidative stress conditions such as Cu toxicity, however, Nrf2 expression is strikingly elevated due to its dissociation from Keap-1. Nrf2 then migrates to the nucleus and attaches to the antioxidant response element (ARE) sequence to upregulate the expression of some genes encoding antioxidant proteins, including HO-1 [57,60]. HO-1 is responsible for heme degradation and has antioxidant, cytoprotective, and anti-inflammatory properties [31]. The anti-inflammatory properties of Curc are mediated by the activation of this enzyme via the Nrf2 and p38 MAPK signaling pathways. The capacity of Curc to reduce inflammation is abolished when HO-1 is inhibited in vascular endothelial cells [66]. In addition, Curc protects against H2O2-induced damage in lung mesenchymal stem cells via the Akt/Nrf2/HO-1 signaling pathway [67].
In light of the association between CuSO4 pulmonary toxicity and oxidative stress, inflammation is another logical consequence of this stress. Cu-induced lung tissue inflammation is a characteristic of augmented pathological tissue injury. Several studies reported that Cu overload induced a significant immune response and elevated IL-6 and TNF-α levels in murine bronchoalveolar lavage fluid [68] and rat liver tissue [24]. The generation of ROS by Cu is the key step behind inflammation development. Our findings are supported by the study of Kim et al. [69] who reported that excess ROS production can induce upregulation of ICAM-1 via the activation of many signaling molecules, including NF-κB. Similarly, a study by Gosens et al. showed pulmonary toxicity after inhalation of CuO NPs induced interstitial and alveolar inflammation accompanied by abundant macrophages and/or granulocytes at higher doses of CuO NPs [70]. In this regard, lung tissue inflammation was denoted by the elevated levels of pro-inflammatory cytokines, IL-6, and TNF-α and upregulation of ICAM-1 in CuSO4-treated rats. ICAM-1 is a cell surface glycoprotein that is normally expressed at low levels in immune, endothelial, and epithelial cells, but its expression can be upregulated by inflammatory cytokines [71]. The pro-inflammatory effects of TNF-α are exerted in part by promoting monocyte adhesion to the pulmonary epithelium and upregulation of ICAM-1 expression in an NF-κB-dependent manner [72].
On the other hand, Curc can reduce inflammation and protect the lungs from damage via other mechanisms. Curc downregulated the expression of ICAM-1 induced by NF-κB and TNF-α in lung epithelial cells [34,73,74,75]. The suppression of ICAM-1 production by Curc may have a protective effect on the integrity of the epithelial-endothelial cells by changing the interactions between their surface molecules and thereby limiting neutrophil adherence to endothelial cell monolayers in vivo [31]. Curc confers immunomodulatory actions by regulating the activation of various immune cells: T-cells, B-cells, macrophages, neutrophils, natural killer, and dendritic cells [76]. Furthermore, the anti-inflammatory activity of Curc can be partly credited to the upregulation of the Nrf2/HO-1 pathway as confirmed in Nrf2-knockout macrophages [77].
In the inflammation context, CuSO4 reduced the SP-C level in rat lungs, possibly due to tissue inflammation and lymphocyte infiltration. It has been reported that SP-C deficiency in human and animal models is correlated with increased inflammation and delayed healing. This protein can inhibit inflammation by decreasing JAK/STAT activation during lung repair [78]. SP-C consists of a complex mixture of phospholipids that coats the surfaces of the alveoli of the lungs where gas exchange takes place to maintain alveolar integrity and lower the surface tension that is essential for normal respiratory function. Numerous studies showed that TNF-α caused negative effects on surfactant synthesis in the lungs [79,80,81]. Furthermore, some studies revealed a strong association between surfactant C and lung diseases; for example, Stephan et al. demonstrated that the absence of SP-C or pro-SP-C is directly linked with the pathogenesis of interstitial lung disease in mice [82]. SP-C is dramatically decreased in different lung injuries and is frequently associated with apoptosis in type II alveolar epithelial cells. Inhibiting SP-C in these cells may enhance CXCL1 and 2, as well as their receptor CXCR2 and ICAM-1 expression, indicating an inflammatory response [83]. In the current study, the use of Curc or nCur almost restored the normal expression of SP-C. Guzel et al. reported that Curc significantly reduced the severity of intestinal ischemia/reperfusion injury by decreasing the activity of inducible nitric oxide synthase and increasing SP-D expression in lung tissue [84].
In addition, exposure to CuSO4 reduced the expression of MUC-1 in rat lungs, while Curc and nCurc restored the normal protein expression. MUC-1 is a membrane-bound glycoprotein expressed on the surfaces of all epithelial cells that line mucosal surfaces. Under physiological conditions, MUC-1 is vital in lubrication, preventing dehydration, and providing protection from degradative enzymes and microorganisms [85]. However, during exposure to pathogenic stimuli, MUC-1 exerts anti-inflammatory effects mediated by the inhibition of Toll-like receptor 5 (TLR-5) signaling [86]. MUC-1-knockout mice had more inflammation in response to flagellin, a TLR5 agonist, than wild-type mice, confirming that MUC-1 has an anti-inflammatory function during airway infection. Also, the knockdown of MUC-1 in normal human bronchial epithelial cells can induce the release of IL-8 after TLR5 agonist addition [86,87]. On the contrary, other studies reported that TNF upregulated MUC-1 gene expression [88] or neutrophil elastase [89], suggesting that the expression of the MUC-1 gene increased in response to inflammatory mediators to control inflammation. However, data showing the role of Cu in IL-8 and TLR expression and their association with MUC-1 are lacking; thus, future studies are needed.
Moreover, apoptosis regulatory genes were measured in order to determine the effect of CuSO4 in lung tissue. Cu-induced cell death might be directly related to the induced oxidative stress and inflammatory response. As expected, apoptotic cell death was found in the lungs of CuSO4-challenged rats, in which Bax and Bcl-2 gene expression was increased and decreased, respectively. These findings align with prior research indicating that overexposure to CuSO4 elicits cellular apoptosis in several organs [43,44,45]. ROS and pro-inflammatory mediators induce the pro-apoptotic Bax, thus disturbing the outer mitochondrial membrane. Consequently, cytochrome c can leak out into the cytoplasm and trigger the activation of several caspases, including caspase-3, the main executioner enzyme of cell apoptosis [90]. Excessive Cu exposure induced autophagic gene expression such as Beclin1, reduced mitochondrial membrane potential (ΔΨm), and increased the number of dead cells as reported by TUNEL assay [91]. The anti-apoptotic BCL-2, however, inhibits the release of cytochrome c by dimerization with BAX, controlling Ca2+ and suppressing caspases, and thus interferes with apoptosis [42]. In agreement with previous studies [92,93], Curc showed anti-apoptotic effects in the lungs after CuSO4 exposure by restoring the regular expression of BAX and Bcl-2 and controlling Bax/Bcl-2-mediated cell death. Nevertheless, the potency of nCurc was superior to its native form in attenuating apoptosis.
4. Materials and Methods
4.1. Chemicals
CuSO4, Cur, carboxymethylcellulose (CMC), trichloroacetic acid, thiobarbituric acid, reduced glutathione (GSH), pyrogallol, hematoxylin and eosin (H&E), acrylamide, agarose, and primers were procured from Sigma (St. Louis, MO, USA). Deferoxamine (DFO) and nCurc were obtained from Novartis Pharma AG (Rotkreuz, Switzerland) and Lipolife (LLT1, Essex, UK), respectively. The nCurc was encapsulated liposomal nano-curcumin to enhance the pharmacokinetic properties and systemic bioavailability by up to 98%. It was manufactured in a European laboratory registered under the Hazard Analysis Critical Control Point (HACCP) system. IL-6 and TNF-α ELISA kits were obtained from R&D Systems (Minneapolis, MN, USA), while other ELISA kits for glutathione peroxidase 2 (GPX2), ICAM-1, mucin-1 (MUC-1), and surfactant protein C (SP-C) were purchased from MyBioSource (San Diego, CA, USA). Antibodies targeting Keap1, Nrf2, HO-1, and β-actin were bought from Novus Biologicals (Centennial, CO, USA).
4.2. Animals and Experimental Design
A total of forty male Wistar Albino rats, weighing between 180 g and 200 g, were obtained from the Animals Research Centre at King Saud University (KSU). The experiments complied with the regulations set forth by the research ethics committee of KSU, as indicated by the ethical approval no. SE-19-129. The rats were placed in standard cages, divided into five groups with eight rats in each, and allowed to acclimate for one week. They were provided free access to water and food and kept under standard temperature, humidity, and a 12-h light/dark cycle. After one week of acclimation, the control group, designated as Group I, was given 1% C.M.C. orally. All rats in the remaining groups received 100 mg/kg CuSO4 [94,95], but only groups III, IV, and V were treated with daily doses of 23 mg/kg DFO [96], 80 mg/kg Curc [9,96], and 80 mg/kg nCurc [9,96], respectively. All treatments were suspended in 1% C.M.C. and administered orally for 7 days. Twenty-four hours post-treatment, the rats were sacrificed under anesthesia, blood samples were collected to obtain sera, and the lungs were harvested and rinsed with cold phosphate-buffered saline (PBS). Some tissue was snap-frozen in liquid nitrogen for RNA isolation and Western blotting experiments. The remaining tissues were preserved in 4% neutral-buffered formaldehyde for histopathological experiments and some were homogenized in Tris-HCl buffer (10% w/v, pH 7.4) and centrifuged to collect supernatants for biochemical assays.
4.3. Measuring Oxidative Stress Markers
The lung tissue supernatants were used to measure malondialdehyde (MDA), GSH, and SOD activity based on colorimetric methods as previously described in Ohkawa et al. [97], Ellman [98], and Marklund and Marklund [99], respectively.
4.4. Histological Evaluation
The fixed lung tissues were processed, embedded in paraffin wax, and then sliced into 5 µm thick sections. The sections were subsequently stained using H&E stains and visualized using light microscopy.
4.5. Determination of Inflammatory and Lung-Specific Biomarkers
IL-6 and TNF-α were measured using R&D Systems ELISA kits (R&D Systems, Minneapolis, MN, USA), whereas other markers, GPX2, ICAM-1, MUC-1, and SP-C, were determined using MyBioSource ELISA kits (MyBioSource, San Diego, CA, USA). All assays were carried out in compliance with the manufacturer’s protocols.
4.6. Gene Expression
In this study, we employed quantitative polymerase chain reaction (qPCR) to ascertain whether CuSO4 and treating agents changed the expression of B cell lymphoma-2 (BCL-2) and BCL-2-associated X protein (BAX). In brief, RNA extraction was performed using TRIzol reagent (Invitrogen, Waltham, MA, USA) and then the RNA concentrations were measured using Nanodrop. High-quality RNA samples were reverse-transcribed to cDNA using a high-capacity cDNA reverse transcription kit (ThermoFisher Scientific, Waltham, MA, USA). The PCR mixture contained cDNA, SYBR Green PCR master mix (ThermoFisher Scientific, Waltham, MA, USA), and the primer pairs listed in Table 1. The cycling conditions began with 10 min denaturation at 95 °C, then 40 cycles at 95 °C for 15 s, and 60 °C for 1 min. The obtained data were analyzed using the 2−ΔΔCt method [100], normalized to the reference gene β-actin, and the gene expression of samples was presented as a fold change relative to controls [101].

Table 1.
Primers used for gene expression assay.
4.7. Western Blotting
Changes in the protein expression of Keap1, Nrf2, HO-1, and β-actin were determined in lung homogenates in which the tissue was lysed by the addition of RIPA buffer and Protease Inhibitor CocktailX (Sigma, St. Louis, MO, USA). A Bradford protein assay kit (BioBasic, Markham, ON, Canada) was used to determine protein concentrations. A total of 50 µg of protein lysate was separated using 10% SDS-PAGE and transferred onto PVDF membranes (Millipore, Bedford, MA, USA). Following a 1 h incubation at room temperature with 5% milk, the membranes were incubated overnight at 4 °C with primary antibodies. On the next day, the membranes were thoroughly washed with TBST and then incubated with HRP-labeled secondary antibody. The signal was developed using Pierce™ ECL Western Blotting Substrate (ThermoFisher Scientific, Waltham, MA, USA), and the protein bands were acquired using ImageQuant LAS 4000 and quantified using Image J software.
4.8. Statistical Analysis
The data analysis and comparison of means were carried out using one-way ANOVA and Tukey’s post hoc test using GraphPad Prism 10 software (GraphPad, San Diego, CA, USA), and the data were presented as means ± standard errors of the mean (SEM). The mean differences were deemed statistically significant if the p-values ≤ 0.05.
5. Conclusions
To sum up, by addressing oxidative stress, the Nrf-2/HO-1 pathway, and inflammation in the lungs, we indicate the mechanisms underlying CuSO4-induced lung injury in rats and determine the efficacy of Curc and nCurc in preventing such toxicity. These beneficial effects were accompanied by the suppression of apoptotic markers and restoration of SP-C and MUC-1 expression. The enhanced Curc bioavailability in its nanoform improved lung protection against CuSO4. These results shed new light on Curc and nCurc and their lung-protective effects against CuSO4-induced lung injury, but further research is needed to investigate other associated mechanisms.
Author Contributions
Conceptualization, W.S.S. and A.M.A.; methodology, W.S.S., A.M.A. and I.H.H.; validation, W.S.S.; formal analysis, W.S.S. and I.H.H.; investigation, W.S.S., A.M.A., H.K.A., J.S.A. and I.H.H.; resources, W.S.S. and A.M.A.; data curation, W.S.S., A.M.A. and I.H.H.; writing—original draft preparation, W.S.S.; writing—review and editing, W.S.S., I.H.H., A.M.A., H.K.A. and J.S.A.; visualization, W.S.S.; supervision, W.S.S.; project administration, W.S.S. and A.M.A.; funding acquisition, W.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Researchers Supporting Project number (RSPD2023R778), King Saud University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
The experiment was carried out in accordance with the guidelines outlined in the National Institutes of Health (NIH) publication No. 85-23, revised in 2011. Additionally, it received approval from the research ethics committee at King Saud University, with an ethical reference number of SE-19-129.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data analyzed or generated during this study are included in this manuscript.
Acknowledgments
The authors would like to extend their gratitude to Researchers Supporting Project number (RSPD2023R778), King Saud University, Riyadh, Saudi Arabia for funding this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Uriu-Adams, J.Y.; Keen, C.L. Copper, oxidative stress, and human health. Mol. Asp. Med. 2005, 26, 268–298. [Google Scholar] [CrossRef]
- Scheiber, I.; Dringen, R.; Mercer, J.F. Copper: Effects of deficiency and overload. Met. Ions Life Sci. 2013, 13, 359–387. [Google Scholar] [CrossRef] [PubMed]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: ‘Copper That Cancer’. Met. Integr. Biometal Sci. 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Gaetke, L.M.; Chow-Johnson, H.S.; Chow, C.K. Copper: Toxicological relevance and mechanisms. Arch. Toxicol. 2014, 88, 1929–1938. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhury, S.; Nath, S.; Massanyi, P.; Stawarz, R.; Kacaniova, M.; Kolesarova, A. Copper-induced changes in reproductive functions: In vivo and in vitro effects. Physiol. Res. 2016, 65, 11–22. [Google Scholar] [CrossRef]
- Gosens, I.; Cassee, F.R.; Zanella, M.; Manodori, L.; Brunelli, A.; Costa, A.L.; Bokkers, B.G.H.; de Jong, W.H.; Brown, D.; Hristozov, D.; et al. Organ burden and pulmonary toxicity of nano-sized copper (II) oxide particles after short-term inhalation exposure. Nanotoxicology 2016, 10, 1084–1095. [Google Scholar] [CrossRef]
- Oe, S.; Miyagawa, K.; Honma, Y.; Harada, M. Copper induces hepatocyte injury due to the endoplasmic reticulum stress in cultured cells and patients with Wilson disease. Exp. Cell Res. 2016, 347, 192–200. [Google Scholar] [CrossRef]
- Lamtai, M.; Zghari, O.; Ouakki, S.; Marmouzi, I.; Mesfioui, A.; El Hessni, A.; Ouichou, A. Chronic copper exposure leads to hippocampus oxidative stress and impaired learning and memory in male and female rats. Toxicol. Res. 2020, 36, 359–366. [Google Scholar] [CrossRef]
- Hashish, E.A.; Elgaml, S.A. Hepatoprotective and Nephroprotective Effect of Curcumin against Copper Toxicity in Rats. Indian J. Clin. Biochem. IJCB 2016, 31, 270–277. [Google Scholar] [CrossRef]
- Motlhatlhedi, K.; Firth, J.A.; Setlhare, V.; Kaguamba, J.K.; Mmolaatshepe, M. A novel and fatal method of copper sulphate poisoning. Afr. J. Emerg. Med. 2014, 4, e23–e25. [Google Scholar] [CrossRef]
- Franchitto, N.; Gandia-Mailly, P.; Georges, B.; Galinier, A.; Telmon, N.; Ducassé, J.L.; Rougé, D. Acute copper sulphate poisoning: A case report and literature review. Resuscitation 2008, 78, 92–96. [Google Scholar] [CrossRef]
- Gamakaranage, C.S.; Rodrigo, C.; Weerasinghe, S.; Gnanathasan, A.; Puvanaraj, V.; Fernando, H. Complications and management of acute copper sulphate poisoning; a case discussion. J. Occup. Med. Toxicol. 2011, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Moon, J.M.; Jeong, Y.H.; Lee, D.H.; Chun, B.J. Successful extracorporeal life support in respiratory failure after copper sulphate ingestion. Natl. Med. J. India 2018, 31, 83–85. [Google Scholar] [CrossRef] [PubMed]
- Poland, C.A.; Hubbard, S.A.; Levy, L.; Mackie, C. Inhalation toxicity of copper compounds: Results of 14-day range finding study for copper sulphate pentahydrate and dicopper oxide and 28-day subacute inhalation exposure of dicopper oxide in rats. Toxicology 2022, 474, 153221. [Google Scholar] [CrossRef] [PubMed]
- Lawson, M.K.; Valko, M.; Cronin, M.T.D.; Jomová, K. Chelators in Iron and Copper Toxicity. Curr. Pharmacol. Rep. 2016, 2, 271–280. [Google Scholar] [CrossRef]
- Rahman, I.; Biswas, S.K.; Kode, A. Oxidant and antioxidant balance in the airways and airway diseases. Eur. J. Pharmacol. 2006, 533, 222–239. [Google Scholar] [CrossRef]
- Chiou, H.C.; Wang, C.W.; Chen, S.C.; Tsai, M.L.; Lin, M.H.; Hung, C.H.; Kuo, C.H. Copper Exposure Induces Epithelial-Mesenchymal Transition-Related Fibrotic Change via Autophagy and Increase Risk of Lung Fibrosis in Human. Antioxidants 2023, 12, 532. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Q. Association between serum copper levels and lung cancer risk: A meta-analysis. J. Int. Med. Res. 2018, 46, 4863–4873. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Zhao, H.; Zhang, Y.; Guo, K.; Xu, Y.; Chen, S.; Zhang, J. Intranasal Delivery of Copper Oxide Nanoparticles Induces Pulmonary Toxicity and Fibrosis in C57BL/6 mice. Sci. Rep. 2018, 8, 4499. [Google Scholar] [CrossRef]
- Liu, H.; Lai, W.; Liu, X.; Yang, H.; Fang, Y.; Tian, L.; Li, K.; Nie, H.; Zhang, W.; Shi, Y.; et al. Exposure to copper oxide nanoparticles triggers oxidative stress and endoplasmic reticulum (ER)-stress induced toxicology and apoptosis in male rat liver and BRL-3A cell. J. Hazard. Mater. 2021, 401, 123349. [Google Scholar] [CrossRef]
- Zhang, Z.; Weichenthal, S.; Kwong, J.C.; Burnett, R.T.; Hatzopoulou, M.; Jerrett, M.; van Donkelaar, A.; Bai, L.; Martin, R.V.; Copes, R.; et al. A Population-Based Cohort Study of Respiratory Disease and Long-Term Exposure to Iron and Copper in Fine Particulate Air Pollution and Their Combined Impact on Reactive Oxygen Species Generation in Human Lungs. Environ. Sci. Technol. 2021, 55, 3807–3818. [Google Scholar] [CrossRef]
- Yang, F.; Liao, J.; Yu, W.; Pei, R.; Qiao, N.; Han, Q.; Hu, L.; Li, Y.; Guo, J.; Pan, J.; et al. Copper induces oxidative stress with triggered NF-κB pathway leading to inflammatory responses in immune organs of chicken. Ecotoxicol. Environ. Saf. 2020, 200, 110715. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. CB 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Patwa, J.; Flora, S.J.S. MiADMSA abrogates chronic copper-induced hepatic and immunological changes in Sprague Dawley rats. Food Chem. Toxicol. 2020, 145, 111692. [Google Scholar] [CrossRef] [PubMed]
- Saghir, S.; Alharbi, S.; Al-garadi, M.; Al-Gabri, N.; Rady, H.; Olama, N.; Abdulghani, M.; Alhroob, A.; Almaiman, A.; Bin-Jumah, M.; et al. Curcumin Prevents Cyclophosphamide-Induced Lung Injury in Rats by Suppressing Oxidative Stress and Apoptosis. Processes 2020, 8, 127. [Google Scholar] [CrossRef]
- Pizzo, P.; Scapin, C.; Vitadello, M.; Florean, C.; Gorza, L. Grp94 acts as a mediator of curcumin-induced antioxidant defence in myogenic cells. J. Cell Mol. Med. 2010, 14, 970–981. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Yonar, M.E.; Mişe Yonar, S.; İspir, Ü.; Ural, M. Effects of curcumin on haematological values, immunity, antioxidant status and resistance of rainbow trout (Oncorhynchus mykiss) against Aeromonas salmonicida subsp. achromogenes. Fish Shellfish Immunol. 2019, 89, 83–90. [Google Scholar] [CrossRef]
- Al-Dossari, M.H.; Fadda, L.M.; Attia, H.A.; Hasan, I.H.; Mahmoud, A.M. Curcumin and Selenium Prevent Lipopolysaccharide/Diclofenac-Induced Liver Injury by Suppressing Inflammation and Oxidative Stress. Biol. Trace Elem. Res. 2020, 196, 173–183. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Venkatesan, N.; Punithavathi, D.; Babu, M. Protection from acute and chronic lung diseases by curcumin. Adv. Exp. Med. Biol. 2007, 595, 379–405. [Google Scholar] [CrossRef] [PubMed]
- Suresh, M.V.; Francis, S.; Aktay, S.; Kralovich, G.; Raghavendran, K. Therapeutic potential of curcumin in ARDS and COVID-19. Clin. Exp. Pharmacol. Physiol. 2023, 50, 267–276. [Google Scholar] [CrossRef]
- Avasarala, S.; Zhang, F.; Liu, G.; Wang, R.; London, S.D.; London, L. Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral-induced acute respiratory distress syndrome. PLoS ONE 2013, 8, e57285. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; An, X.; Wang, X.; Bao, C.; Li, J.; Yang, D.; Bai, C. Curcumin ameliorated ventilator-induced lung injury in rats. Biomed. Pharmacother. 2018, 98, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Vahedian-Azimi, A.; Abbasifard, M.; Rahimi-Bashar, F.; Guest, P.C.; Majeed, M.; Mohammadi, A.; Banach, M.; Jamialahmadi, T.; Sahebkar, A. Effectiveness of Curcumin on Outcomes of Hospitalized COVID-19 Patients: A Systematic Review of Clinical Trials. Nutrients 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Flora, G.; Gupta, D.; Tiwari, A. Nanocurcumin: A promising therapeutic advancement over native curcumin. Crit. Rev. Ther. Drug Carr. Syst. 2013, 30, 331–368. [Google Scholar] [CrossRef]
- Vázquez-Blanco, R.; Arias-Estévez, M.; Bååth, E.; Fernández-Calviño, D. Comparison of Cu salts and commercial Cu based fungicides on toxicity towards microorganisms in soil. Environ. Pollut. 2020, 257, 113585. [Google Scholar] [CrossRef]
- Janssen, R.; de Brouwer, B.; von der Thüsen, J.H.; Wouters, E.F.M. Copper as the most likely pathogenic divergence factor between lung fibrosis and emphysema. Med. Hypotheses 2018, 120, 49–54. [Google Scholar] [CrossRef]
- Gunther, M.R.; Hanna, P.M.; Mason, R.P.; Cohen, M.S. Hydroxyl Radical Formation from Cuprous Ion and Hydrogen Peroxide: A Spin-Trapping Study. Arch. Biochem. Biophys. 1995, 316, 515–522. [Google Scholar] [CrossRef]
- Husain, N.; Mahmood, R. Copper(II) generates ROS and RNS, impairs antioxidant system and damages membrane and DNA in human blood cells. Environ. Sci. Pollut. Res. Int. 2019, 26, 20654–20668. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxidative Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Pei, R.; Zhang, Z.; Liao, J.; Yu, W.; Qiao, N.; Han, Q.; Li, Y.; Hu, L.; Guo, J.; et al. Copper induces oxidative stress and apoptosis through mitochondria-mediated pathway in chicken hepatocytes. Toxicol. Vitr. 2019, 54, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Sarawi, W.S.; Alhusaini, A.M.; Fadda, L.M.; Alomar, H.A.; Albaker, A.B.; Aljrboa, A.S.; Alotaibi, A.M.; Hasan, I.H.; Mahmoud, A.M. Curcumin and Nano-Curcumin Mitigate Copper Neurotoxicity by Modulating Oxidative Stress, Inflammation, and Akt/GSK-3β Signaling. Molecules 2021, 26, 5591. [Google Scholar] [CrossRef] [PubMed]
- Sarawi, W.S.; Alhusaini, A.M.; Fadda, L.M.; Alomar, H.A.; Albaker, A.B.; Aljrboa, A.S.; Alotaibi, A.M.; Hasan, I.H.; Mahmoud, A.M. Nano-Curcumin Prevents Cardiac Injury, Oxidative Stress and Inflammation, and Modulates TLR4/NF-kappaB and MAPK Signaling in Copper Sulfate-Intoxicated Rats. Antioxidants 2021, 10, 1414. [Google Scholar] [CrossRef] [PubMed]
- Sarawi, W.S.; Alhusaini, A.M.; Fadda, L.M.; Alomar, H.A.; Albaker, A.B.; Alghibiwi, H.K.; Aljrboa, A.S.; Alotaibi, A.M.; Hasan, I.H.; Mahmoud, A.M. Nano-Curcumin Prevents Copper Reproductive Toxicity by Attenuating Oxidative Stress and Inflammation and Improving Nrf2/HO-1 Signaling and Pituitary-Gonadal Axis in Male Rats. Toxics 2022, 10, 356. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxidative Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef]
- Averill-Bates, D.A. The antioxidant glutathione. Vitam. Horm. 2023, 121, 109–141. [Google Scholar] [CrossRef]
- Hufnagel, M.; Neuberger, R.; Wall, J.; Link, M.; Friesen, A.; Hartwig, A. Impact of Differentiated Macrophage-Like Cells on the Transcriptional Toxicity Profile of CuO Nanoparticles in Co-Cultured Lung Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 5044. [Google Scholar] [CrossRef]
- Kwon, J.T.; Kim, Y.; Choi, S.; Yoon, B.L.; Kim, H.S.; Shim, I.; Sul, D. Pulmonary Toxicity and Proteomic Analysis in Bronchoalveolar Lavage Fluids and Lungs of Rats Exposed to Copper Oxide Nanoparticles. Int. J. Mol. Sci. 2022, 23, 13265. [Google Scholar] [CrossRef]
- Valko, M.; Jomova, K.; Rhodes, C.J.; Kuca, K.; Musilek, K. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch. Toxicol. 2016, 90, 1–37. [Google Scholar] [CrossRef]
- Valko, M.; Morris, H.; Cronin, M.T. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Witkowska, D.; Słowik, J.; Chilicka, K. Heavy Metals and Human Health: Possible Exposure Pathways and the Competition for Protein Binding Sites. Molecules 2021, 26, 6060. [Google Scholar] [CrossRef] [PubMed]
- Kalpana, C.; Menon, V.P. Curcumin ameliorates oxidative stress during nicotine-induced lung toxicity in Wistar rats. Ital. J. Biochem. 2004, 53, 82–86. [Google Scholar] [PubMed]
- Suzuki, M.; Betsuyaku, T.; Ito, Y.; Nagai, K.; Odajima, N.; Moriyama, C.; Nasuhara, Y.; Nishimura, M. Curcumin attenuates elastase- and cigarette smoke-induced pulmonary emphysema in mice. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2009, 296, L614–L623. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, C.; Yang, C.; Liu, R.J.; Wang, J.; Niu, J.; Brömme, D. Antifibrotic effects of curcumin are associated with overexpression of cathepsins K and L in bleomycin treated mice and human fibroblasts. Respir. Res. 2011, 12, 154. [Google Scholar] [CrossRef]
- Lee, J.C.; Kinniry, P.A.; Arguiri, E.; Serota, M.; Kanterakis, S.; Chatterjee, S.; Solomides, C.C.; Javvadi, P.; Koumenis, C.; Cengel, K.A.; et al. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat. Res. 2010, 173, 590–601. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Rangasamy, T.; Cho, C.Y.; Thimmulappa, R.K.; Zhen, L.; Srisuma, S.S.; Kensler, T.W.; Yamamoto, M.; Petrache, I.; Tuder, R.M.; Biswal, S. Genetic ablation of Nrf2 enhances susceptibility to cigarette smoke-induced emphysema in mice. J. Clin. Investig. 2004, 114, 1248–1259. [Google Scholar] [CrossRef]
- Cho, H.Y.; Jedlicka, A.E.; Reddy, S.P.; Kensler, T.W.; Yamamoto, M.; Zhang, L.Y.; Kleeberger, S.R. Role of NRF2 in protection against hyperoxic lung injury in mice. Am. J. Respir. Cell Mol. Biol. 2002, 26, 175–182. [Google Scholar] [CrossRef]
- Garcia-Nino, W.R.; Pedraza-Chaverri, J. Protective effect of curcumin against heavy metals-induced liver damage. Food Chem. Toxicol. 2014, 69, 182–201. [Google Scholar] [CrossRef]
- Jiang, W.D.; Liu, Y.; Hu, K.; Jiang, J.; Li, S.H.; Feng, L.; Zhou, X.Q. Copper exposure induces oxidative injury, disturbs the antioxidant system and changes the Nrf2/ARE (CuZnSOD) signaling in the fish brain: Protective effects of myo-inositol. Aquat. Toxicol. 2014, 155, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhang, Y.; Zhao, C.; Zhang, H.; Pu, Y.; Yin, L. Copper induces oxidative stress and apoptosis of hippocampal neuron via pCREB/BDNF/and Nrf2/HO-1/NQO1 pathway. J. Appl. Toxicol. JAT 2022, 42, 694–705. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection against Oxidative Injury. Curr. Mol. Med. 2020, 20, 116–133. [Google Scholar] [CrossRef] [PubMed]
- Shahcheraghi, S.H.; Salemi, F.; Peirovi, N.; Ayatollahi, J.; Alam, W.; Khan, H.; Saso, L. Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects. Molecules 2021, 27, 167. [Google Scholar] [CrossRef] [PubMed]
- Satta, S.; Mahmoud, A.M.; Wilkinson, F.L.; Yvonne Alexander, M.; White, S.J. The Role of Nrf2 in Cardiovascular Function and Disease. Oxidative Med. Cell. Longev. 2017, 2017, 9237263. [Google Scholar] [CrossRef]
- Xiao, Y.; Xia, J.; Wu, S.; Lv, Z.; Huang, S.; Huang, H.; Su, X.; Cheng, J.; Ke, Y. Curcumin Inhibits Acute Vascular Inflammation through the Activation of Heme Oxygenase-1. Oxidative Med. Cell. Longev. 2018, 2018, 3295807. [Google Scholar] [CrossRef]
- Ke, S.; Zhang, Y.; Lan, Z.; Li, S.; Zhu, W.; Liu, L. Curcumin protects murine lung mesenchymal stem cells from H(2)O(2) by modulating the Akt/Nrf2/HO-1 pathway. J. Int. Med. Res. 2020, 48, 300060520910665. [Google Scholar] [CrossRef]
- Pietrofesa, R.A.; Park, K.; Mishra, O.P.; Johnson-McDaniel, D.; Myerson, J.W.; Shuvaev, V.V.; Arguiri, E.; Chatterjee, S.; Moorthy, G.S.; Zuppa, A.; et al. Copper Oxide Nanoparticle-Induced Acute Inflammatory Response and Injury in Murine Lung Is Ameliorated by Synthetic Secoisolariciresinol Diglucoside (LGM2605). Int. J. Mol. Sci. 2021, 22, 9477. [Google Scholar] [CrossRef]
- Kim, S.R.; Bae, Y.H.; Bae, S.K.; Choi, K.S.; Yoon, K.H.; Koo, T.H.; Jang, H.O.; Yun, I.; Kim, K.W.; Kwon, Y.G.; et al. Visfatin enhances ICAM-1 and VCAM-1 expression through ROS-dependent NF-kappaB activation in endothelial cells. Biochim. Biophys. Acta 2008, 1783, 886–895. [Google Scholar] [CrossRef]
- Gosens, I.; Costa, P.M.; Olsson, M.; Stone, V.; Costa, A.L.; Brunelli, A.; Badetti, E.; Bonetto, A.; Bokkers, B.G.H.; de Jong, W.H.; et al. Pulmonary toxicity and gene expression changes after short-term inhalation exposure to surface-modified copper oxide nanoparticles. NanoImpact 2021, 22, 100313. [Google Scholar] [CrossRef]
- Hubbard, A.K.; Rothlein, R. Intercellular adhesion molecule-1 (ICAM-1) expression and cell signaling cascades. Free Radic. Biol. Med. 2000, 28, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hwang, J.S.; Woo, C.H.; Kim, E.Y.; Kim, T.H.; Cho, K.J.; Kim, J.H.; Seo, J.M.; Lee, S.S. TNF-alpha-induced up-regulation of intercellular adhesion molecule-1 is regulated by a Rac-ROS-dependent cascade in human airway epithelial cells. Exp. Mol. Med. 2008, 40, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Subramaniyan, D.; Ali, B.H. Protective effect of curcumin on pulmonary and cardiovascular effects induced by repeated exposure to diesel exhaust particles in mice. PLoS ONE 2012, 7, e39554. [Google Scholar] [CrossRef] [PubMed]
- Youn, H.; Jeong, J.C.; Jeong, Y.S.; Kim, E.J.; Um, S.J. Quercetin potentiates apoptosis by inhibiting nuclear factor-kappaB signaling in H460 lung cancer cells. Biol. Pharm. Bull. 2013, 36, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Yen, F.L.; Tsai, M.H.; Yang, C.M.; Liang, C.J.; Lin, C.C.; Chiang, Y.C.; Lee, H.C.; Ko, H.H.; Lee, C.W. Curcumin nanoparticles ameliorate ICAM-1 expression in TNF-α-treated lung epithelial cells through p47 (phox) and MAPKs/AP-1 pathways. PLoS ONE 2013, 8, e63845. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef]
- Boyanapalli, S.S.; Paredes-Gonzalez, X.; Fuentes, F.; Zhang, C.; Guo, Y.; Pung, D.; Saw, C.L.; Kong, A.N. Nrf2 knockout attenuates the anti-inflammatory effects of phenethyl isothiocyanate and curcumin. Chem. Res. Toxicol. 2014, 27, 2036–2043. [Google Scholar] [CrossRef]
- Jin, H.; Ciechanowicz, A.K.; Kaplan, A.R.; Wang, L.; Zhang, P.X.; Lu, Y.C.; Tobin, R.E.; Tobin, B.A.; Cohn, L.; Zeiss, C.J.; et al. Surfactant protein C dampens inflammation by decreasing JAK/STAT activation during lung repair. Am. J. Physiology. Lung Cell. Mol. Physiol. 2018, 314, L882–L892. [Google Scholar] [CrossRef]
- Arias-Díaz, J.; Vara, E.; García, C.; Balibrea, J.L. Tumor necrosis factor-alpha-induced inhibition of phosphatidylcholine synthesis by human type II pneumocytes is partially mediated by prostaglandins. J. Clin. Investig. 1994, 94, 244–250. [Google Scholar] [CrossRef]
- Wispé, J.R.; Clark, J.C.; Warner, B.B.; Fajardo, D.; Hull, W.E.; Holtzman, R.B.; Whitsett, J.A. Tumor necrosis factor-alpha inhibits expression of pulmonary surfactant protein. J. Clin. Investig. 1990, 86, 1954–1960. [Google Scholar] [CrossRef]
- Pryhuber, G.S.; Khalak, R.; Zhao, Q. Regulation of surfactant proteins A and B by TNF-alpha and phorbol ester independent of NF-kappa B. Am. J. Physiol. 1998, 274, L289–L295. [Google Scholar] [CrossRef] [PubMed]
- Glasser, S.W.; Detmer, E.A.; Ikegami, M.; Na, C.L.; Stahlman, M.T.; Whitsett, J.A. Pneumonitis and emphysema in sp-C gene targeted mice. J. Biol. Chem. 2003, 278, 14291–14298. [Google Scholar] [CrossRef] [PubMed]
- Puthusseri, B.; Marudamuthu, A.; Tiwari, N.; Fu, J.; Idell, S.; Shetty, S. Regulation of p53-mediated changes in the uPA-fibrinolytic system and in lung injury by loss of surfactant protein C expression in alveolar epithelial cells. Am. J. Physiology. Lung Cell. Mol. Physiol. 2017, 312, L783–L796. [Google Scholar] [CrossRef] [PubMed]
- Guzel, A.; Kanter, M.; Guzel, A.; Yucel, A.F.; Erboga, M. Protective effect of curcumin on acute lung injury induced by intestinal ischaemia/reperfusion. Toxicol. Ind. Health 2013, 29, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Kumar, S.; Bafna, S.; Rachagani, S.; Wagner, K.U.; Jain, M.; Batra, S.K. Genetically engineered mucin mouse models for inflammation and cancer. Cancer Metastasis Rev. 2015, 34, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Lillehoj, E.P. MUC1 mucin: A peacemaker in the lung. Am. J. Respir. Cell Mol. Biol. 2008, 39, 644–647. [Google Scholar] [CrossRef]
- Lu, W.; Hisatsune, A.; Koga, T.; Kato, K.; Kuwahara, I.; Lillehoj, E.P.; Chen, W.; Cross, A.S.; Gendler, S.J.; Gewirtz, A.T.; et al. Cutting edge: Enhanced pulmonary clearance of Pseudomonas aeruginosa by Muc1 knockout mice. J. Immunol. 2006, 176, 3890–3894. [Google Scholar] [CrossRef]
- Koga, T.; Kuwahara, I.; Lillehoj, E.P.; Lu, W.; Miyata, T.; Isohama, Y.; Kim, K.C. TNF-alpha induces MUC1 gene transcription in lung epithelial cells: Its signaling pathway and biological implication. Am. J. Physiology. Lung Cell. Mol. Physiol. 2007, 293, L693–L701. [Google Scholar] [CrossRef]
- Kuwahara, I.; Lillehoj, E.P.; Hisatsune, A.; Lu, W.; Isohama, Y.; Miyata, T.; Kim, K.C. Neutrophil elastase stimulates MUC1 gene expression through increased Sp1 binding to the MUC1 promoter. Am. J. Physiology. Lung Cell. Mol. Physiol. 2005, 289, L355–L362. [Google Scholar] [CrossRef]
- Shi, Y.; Chen, J.; Weng, C.; Chen, R.; Zheng, Y.; Chen, Q.; Tang, H. Identification of the protein-protein contact site and interaction mode of human VDAC1 with Bcl-2 family proteins. Biochem. Biophys. Res. Commun. 2003, 305, 989–996. [Google Scholar] [CrossRef]
- Li, Y.; Chen, H.; Liao, J.; Chen, K.; Javed, M.T.; Qiao, N.; Zeng, Q.; Liu, B.; Yi, J.; Tang, Z.; et al. Long-term copper exposure promotes apoptosis and autophagy by inducing oxidative stress in pig testis. Environ. Sci. Pollut. Res. 2021, 28, 55140–55153. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, S.; Geng, J.X.; Hu, X.Y. Curcumin inhibits human non-small cell lung cancer A549 cell proliferation through regulation of Bcl-2/Bax and cytochrome C. Asian Pac. J. Cancer Prev. 2013, 14, 4599–4602. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Long, M.; Li, X.; Zhu, S.; Zhang, M.; Yang, Z. Curcumin activates autophagy and attenuates oxidative damage in EA.hy926 cells via the Akt/mTOR pathway. Mol. Med. Rep. 2016, 13, 2187–2193. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Kalita, J.; Misra, U.K.; Bora, H.K. A study of dose response and organ susceptibility of copper toxicity in a rat model. J. Trace Elem. Med. Biol. 2015, 29, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Alhusaini, A.; Hasan, I.H.; Aldowsari, N.; Alsaadan, N. Prophylactic Administration of Nanocurcumin Abates the Incidence of Liver Toxicity Induced by an Overdose of Copper Sulfate: Role of CYP4502E1, NF-kappaB and Bax Expressions. Dose-Response A Publ. Int. Hormesis Soc. 2018, 16, 1559325818816284. [Google Scholar] [CrossRef]
- Alhusaini, A.; Fadda, L.; Hassan, I.; Ali, H.M.; Alsaadan, N.; Aldowsari, N.; Aldosari, A.; Alharbi, B. Liposomal Curcumin Attenuates the Incidence of Oxidative Stress, Inflammation, and DNA Damage Induced by Copper Sulfate in Rat Liver. Dose-Response A Publ. Int. Hormesis Soc. 2018, 16, 1559325818790869. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.L. Superoxide dismutase isoenzymes in tissues and plasma from New Zealand black mice, nude mice and normal BALB/c mice. Mutat. Res. 1985, 148, 129–134. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, F.F.; Peng, W.; Sweeney, J.A.; Jia, Z.Y.; Gong, Q.Y. Brain structure alterations in depression: Psychoradiological evidence. CNS Neurosci. Ther. 2018, 24, 994–1003. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).