Protective Effects of ζ-Carotene-like Compounds against Acute UVB-Induced Skin Damage
Abstract
:1. Introduction
2. Results
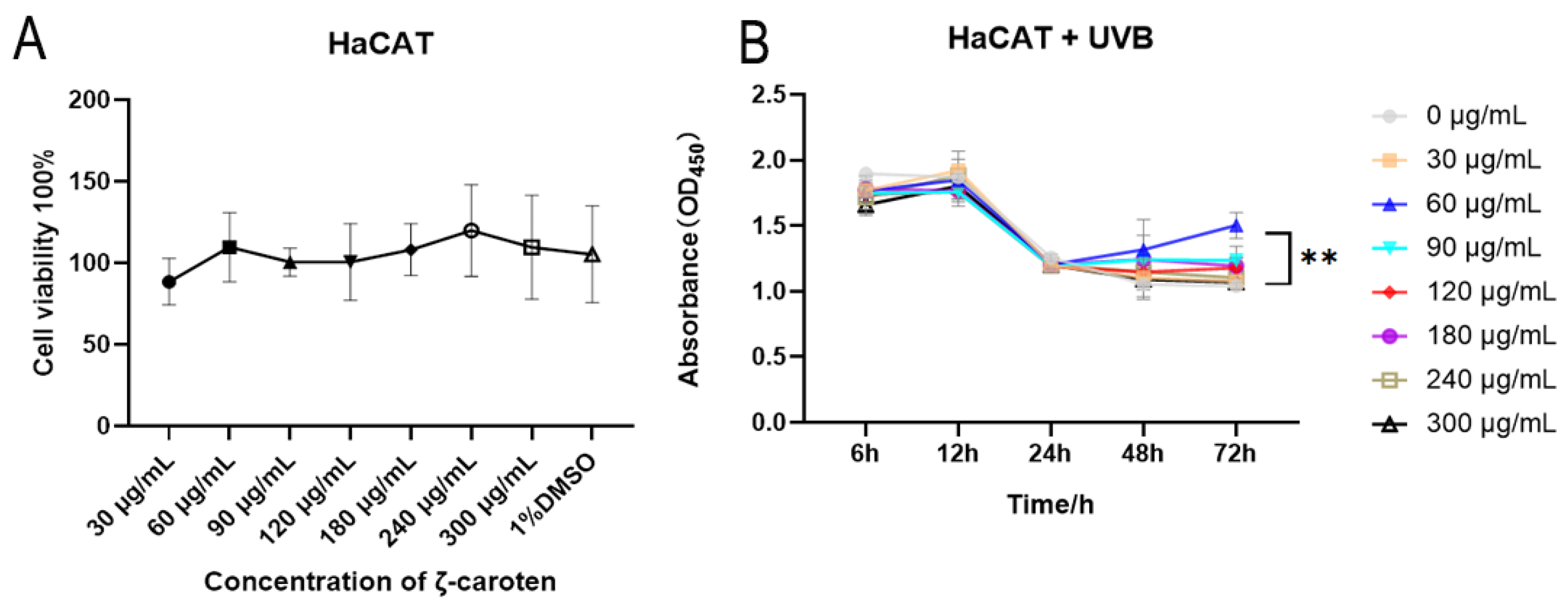
2.1. Cytotoxicity and Cell Growth Curves under UVB Conditions
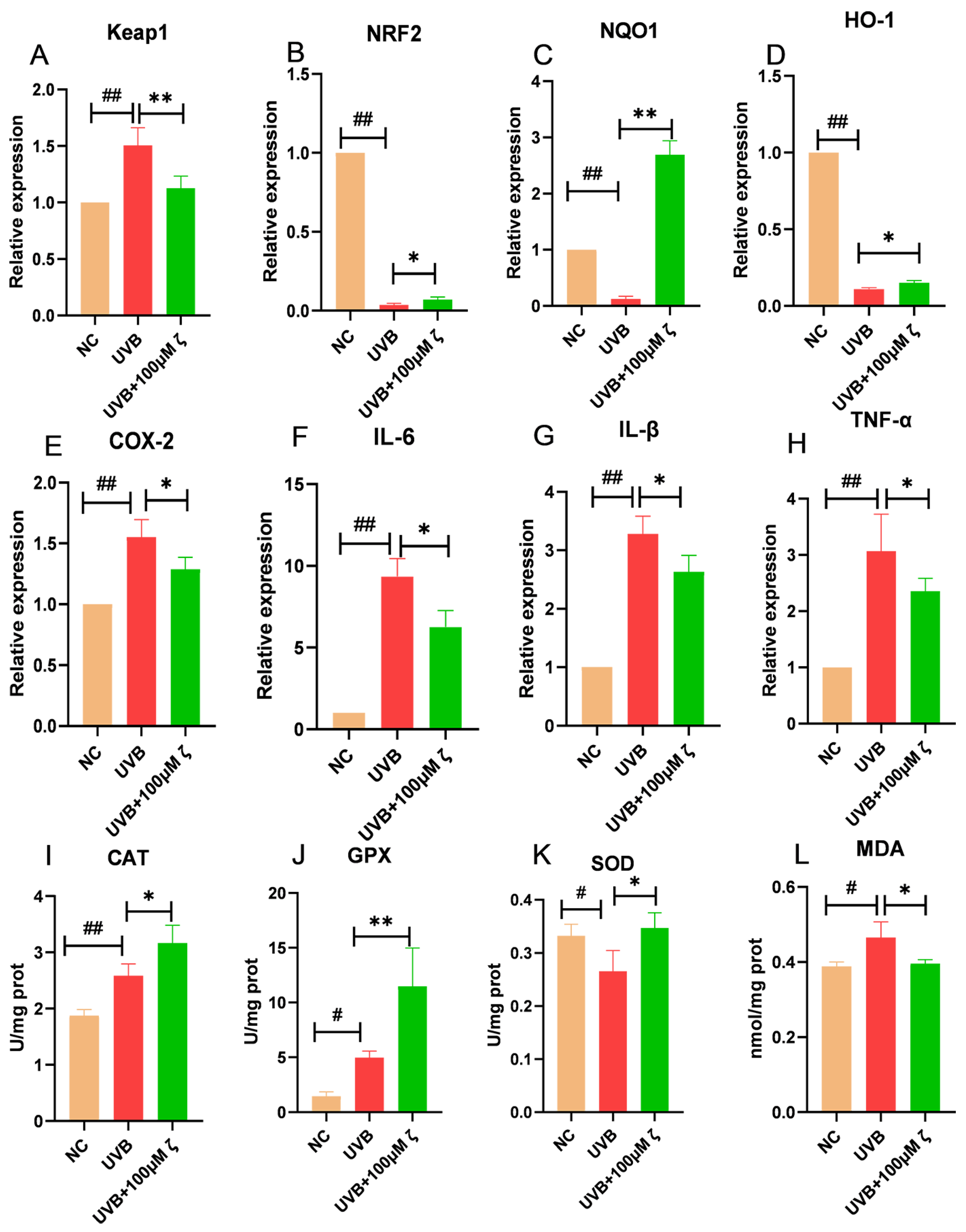
2.2. CLC Regulates the Keap1/Nrf2/ARE Pathway of HaCAT
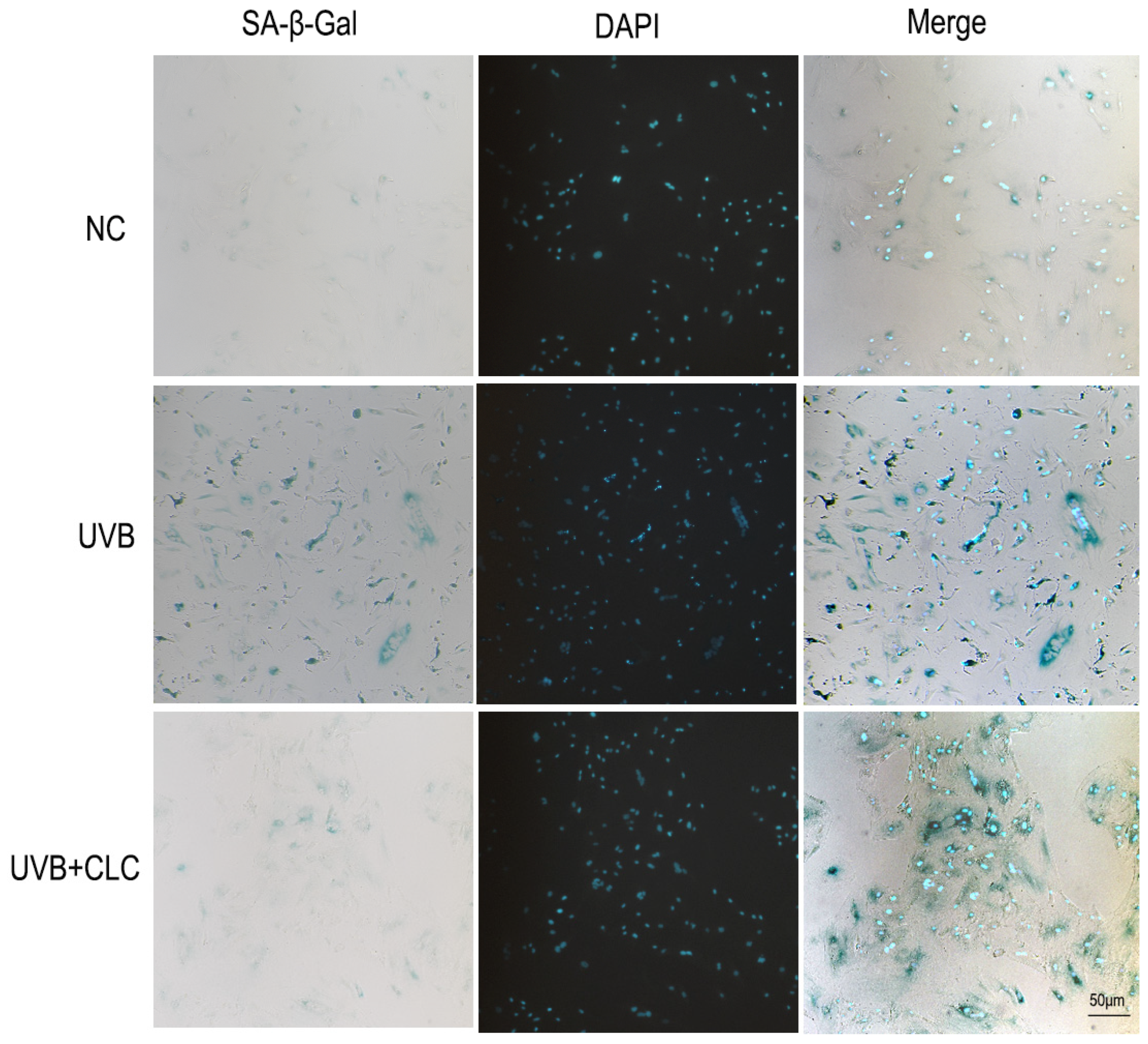
2.3. CLC Inhibits Apoptosis, CPD Formation and Aging in Cells
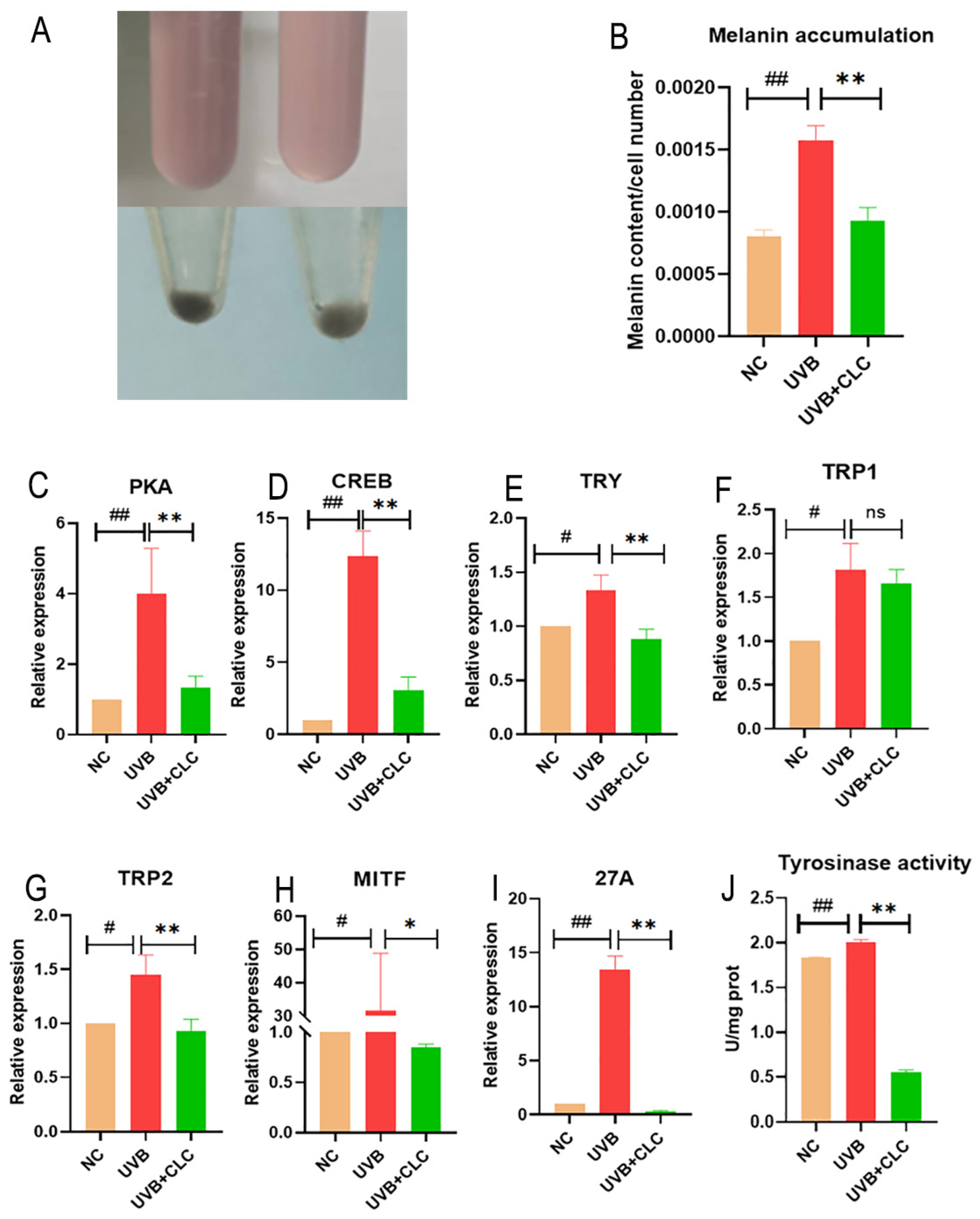
2.4. CLC Inhibits Melanogenesis in B16-F10 Cells
2.5. Changes in Body Weight and Food Intake in Mice during UVB Irradiation
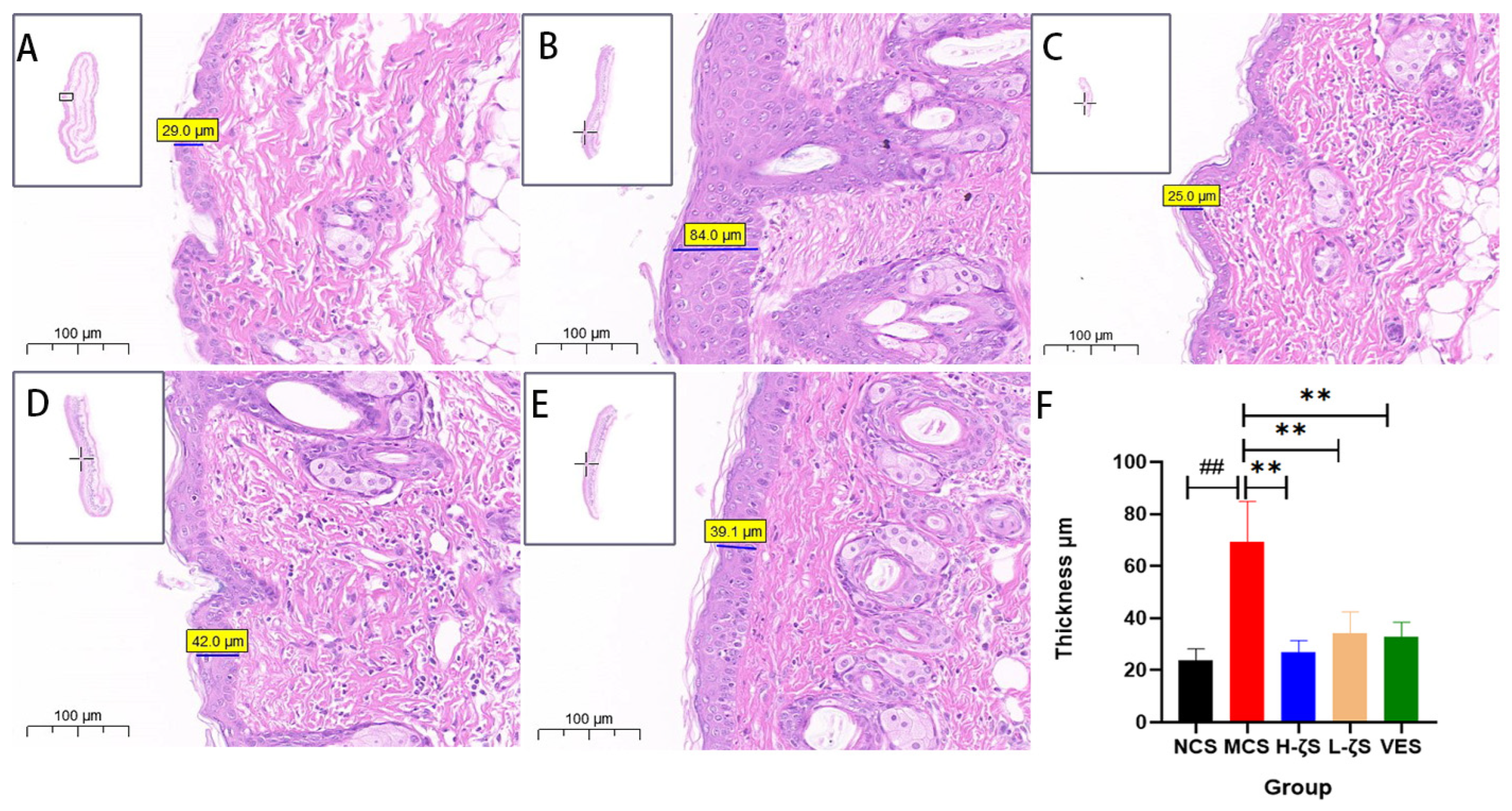
2.6. CLC Alleviates UVB-Induced Skin Photodamage in Mice
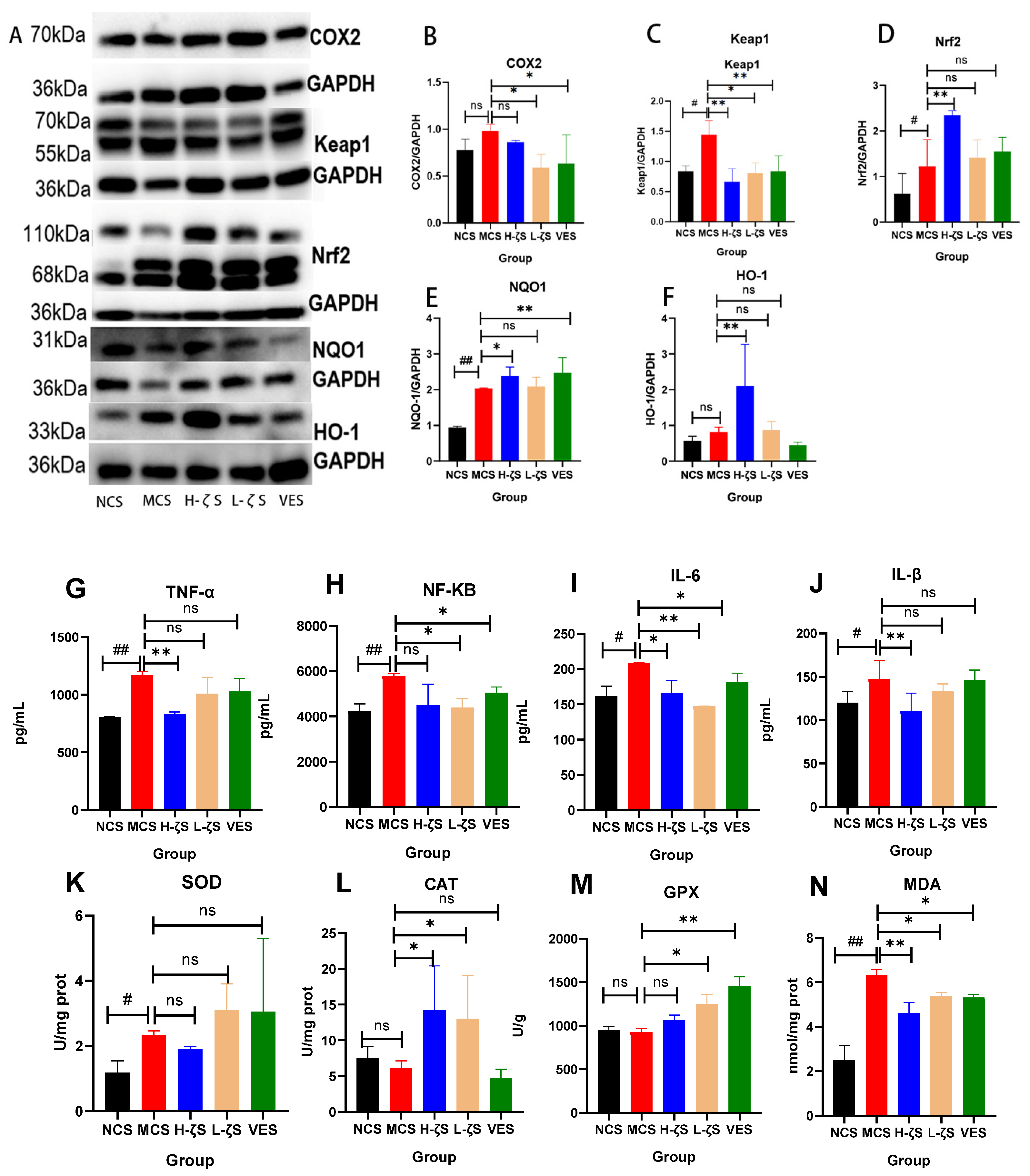
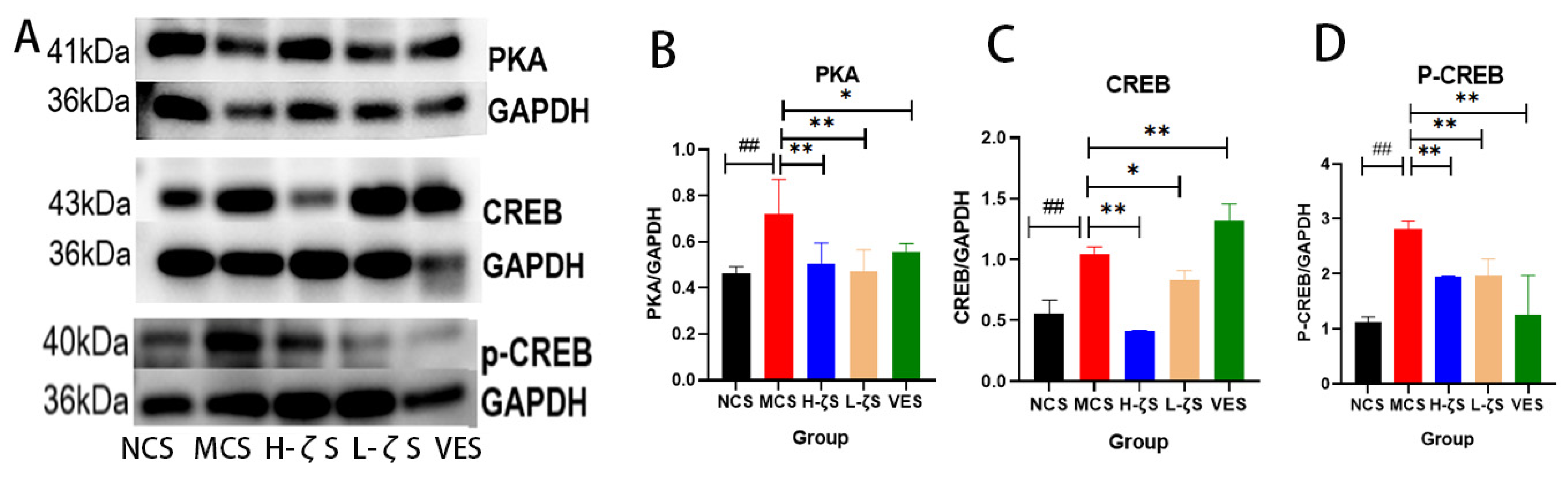
2.7. CLC Regulates the Keap1/Nrf2/ARE Pathway in Mouse Skin Tissue
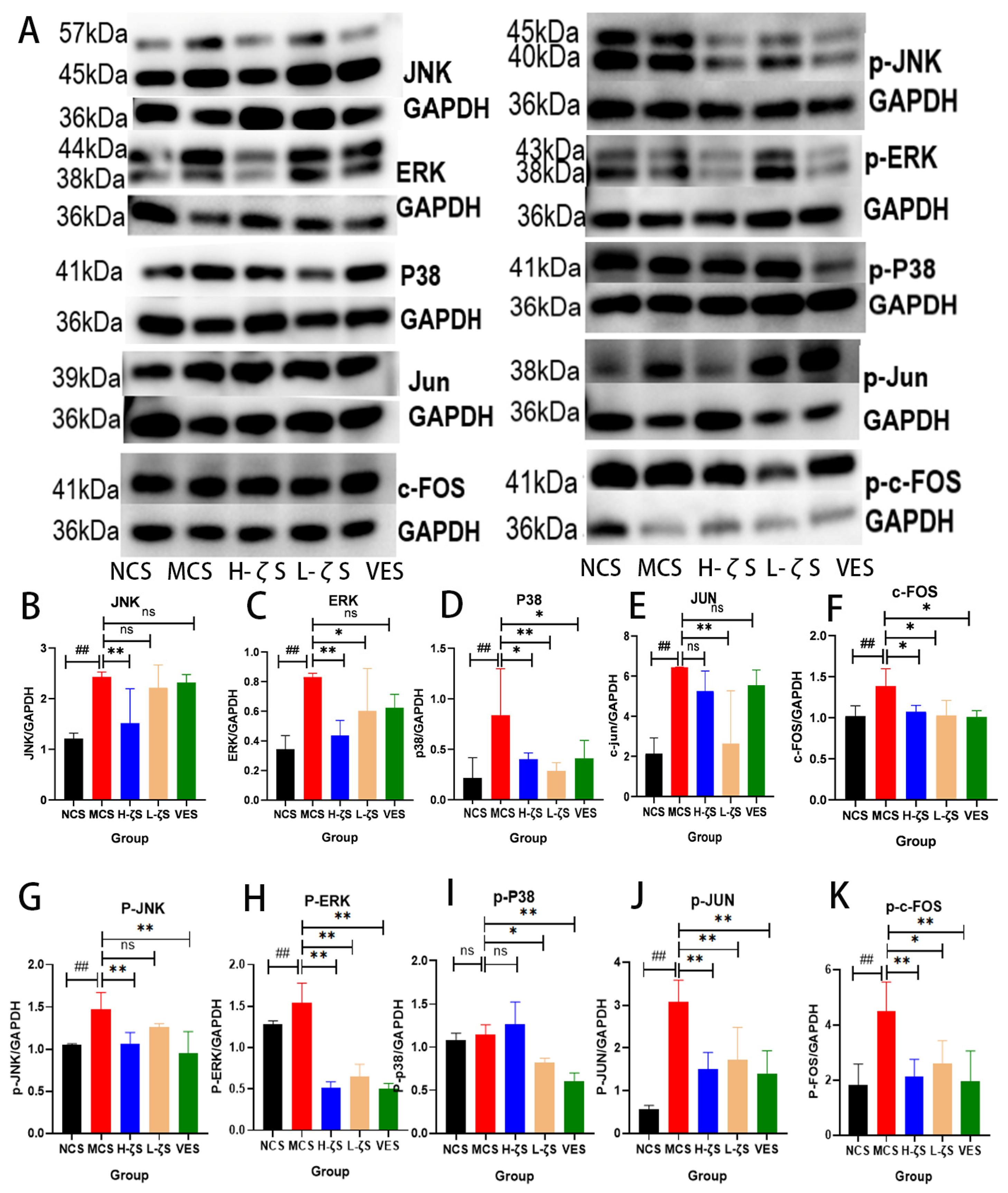
2.8. The Activation of the MAPK/AP-1 Pathway by CLC Reduces Skin Collagen Loss in Mice
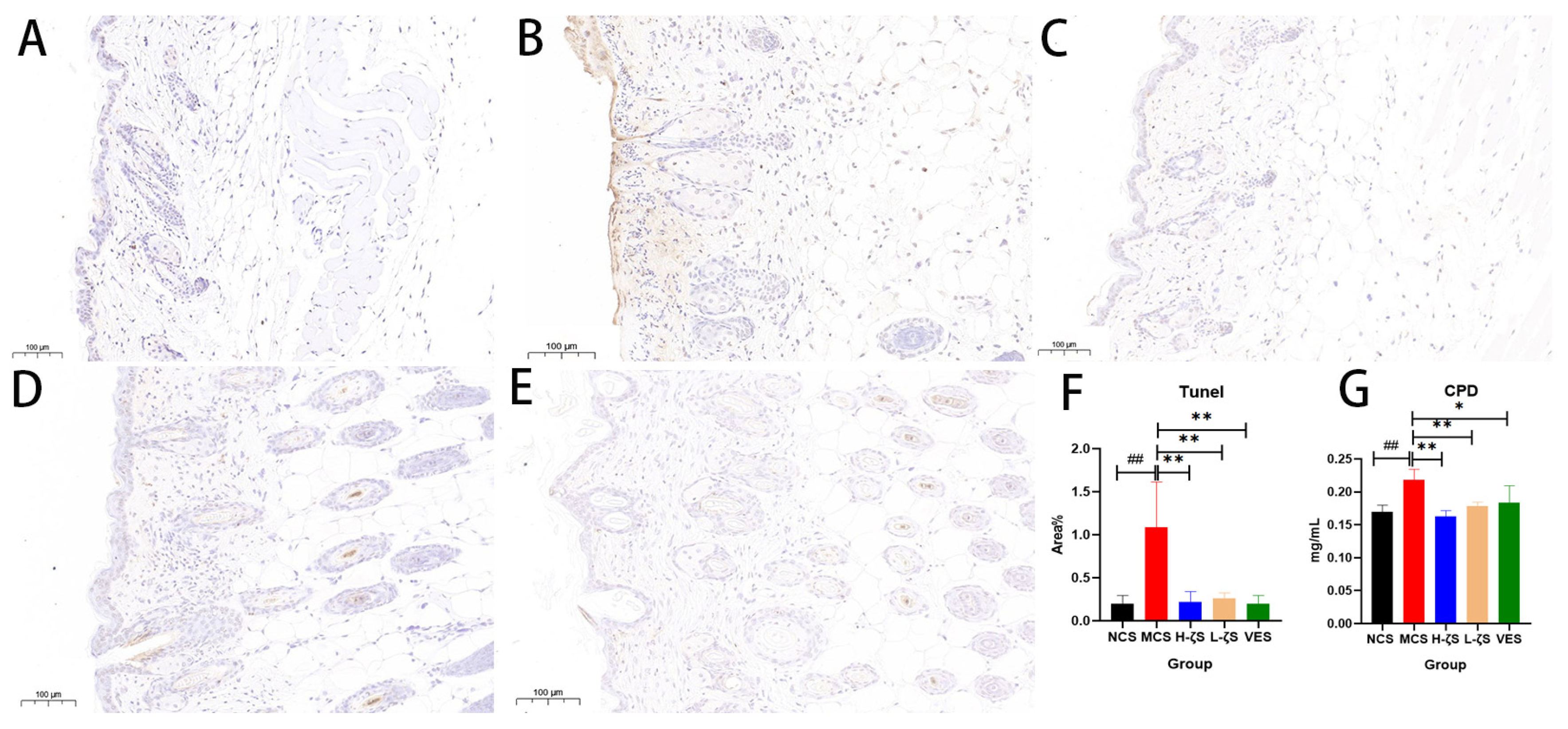
2.9. The Inhibitory Effect of CLC on CPD and Apoptosis in Mice
2.10. CLC Inhibits Melanogenesis in Mice
3. Discussion
4. Materials and Methods
4.1. Reagent Materials
4.2. Cell Culture and UVB Irradiation
4.3. CCK8 Assay
4.4. Antioxidant Enzyme Activities
4.5. Measurement of ROS Generation and Tunel
4.6. Staining of SA-β-Gal in HS68 Cells
4.7. Determination of Melanin Content and Tyrosinase Activity of B16-F10 Cells
4.8. Experimental Animals
4.9. Histological Observation
4.10. Tunel Staining and MMP-2 Immunohistochemistry
4.11. Biochemical Indexes of Skin Tissue
4.12. RNA Extraction and qRT-PCR
4.13. Western Blotting
4.14. Statistics Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gupta, A.K.; Seth, K.; Maheshwari, K.; Baroliya, P.K.; Meena, M.; Kumar, A.; Vinayak, V. Harish Biosynthesis and extraction of high-value carotenoid from algae. Front. Biosci. 2021, 26, 171–190. [Google Scholar] [CrossRef]
- Honda, M.; Kageyama, H.; Hibino, T.; Ichihashi, K.; Takada, W.; Goto, M. Isomerization of Commercially Important Carotenoids (Lycopene, β-Carotene, and Astaxanthin) by Natural Catalysts: Isothiocyanates and Polysulfides. J. Agric. Food Chem. 2020, 68, 3228–3237. [Google Scholar] [CrossRef]
- El-Agamey, A.; Lowe, G.M.; McGarvey, D.J.; Mortensen, A.; Phillip, D.M.; Truscott, T.; Young, A.J. Carotenoid radical chemistry and antioxidant/pro-oxidant properties. Arch. Biochem. Biophys. 2004, 430, 37–48. [Google Scholar] [CrossRef]
- Sanches Silveira, J.E.; Myaki Pedroso, D.M. UV light and skin aging. Rev. Environ. Health 2014, 29, 243–254. [Google Scholar] [CrossRef]
- Perluigi, M.; Di Domenico, F.; Blarzino, C.; Foppoli, C.; Cini, C.; Giorgi, A.; Grillo, C.; De Marco, F.; Butterfield, D.A.; Schininà, M.E.; et al. Effects of UVB-induced oxidative stress on protein expression and specific protein oxidation in normal human epithelial keratinocytes: A proteomic approach. Proteome Sci. 2010, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Hwang, E.; Gao, W.; Xiao, Y.; Ngo, H.T.T.; Yi, T. Helianthus annuus L. flower prevents UVB-induced photodamage in human dermal fibroblasts by regulating the MAPK/AP-1, NFAT, and Nrf2 signaling pathways. J. Cell Biochem. 2018, 120, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Park, S.Y.; Hwang, E.; Zhang, M.; Seo, S.A.; Lin, P.; Yi, T. Thymus vulgaris alleviates UVB irradiation induced skin damage via inhibition of MAPK / AP -1 and activation of Nrf2- ARE antioxidant system. J. Cell Mol. Med. 2016, 21, 336–348. [Google Scholar] [CrossRef]
- Dong, K.K.; Damaghi, N.; Picart, S.D.; Markova, N.G.; Obayashi, K.; Okano, Y.; Masaki, H.; Grether-Beck, S.; Krutmann, J.; Smiles, K.A.; et al. UV-induced DNA damage initiates release of MMP-1 in human skin. Exp. Dermatol. 2008, 17, 1037–1044. [Google Scholar] [CrossRef]
- Bosch, R.; Philips, N.; Suárez-Pérez, J.A.; Juarranz, A.; Devmurari, A.; Chalensouk-Khaosaat, J.; González, S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants 2015, 4, 248–268. [Google Scholar] [CrossRef]
- Lu, Y.; Tonissen, K.F.; Di Trapani, G. Modulating skin colour: Role of the thioredoxin and glutathione systems in regulating melanogenesis. Biosci. Rep. 2021, 41, bsr20210427. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. β-Carotene and other carotenoids in protection from sunlight. Am. J. Clin. Nutr. 2012, 96, 1179S–1184S. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.-S. Microbial platforms to produce commercially vital carotenoids at industrial scale: An updated review of critical issues. J. Ind. Microbiol. Biotechnol. 2018, 46, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Cavinato, M.; Waltenberger, B.; Baraldo, G.; Grade, C.V.C.; Stuppner, H.; Jansen-Dürr, P. Plant extracts and natural compounds used against UVB-induced photoaging. Biogerontology 2017, 18, 499–516. [Google Scholar] [CrossRef]
- Zhang, L.P.; Wang, K.; Liang, S.X.; Cao, J.H.; Yao, M.K.; Qin, L.; Qu, C.F.; Miao, J.L. Beneficial effect of ζ-carotene-like compounds on acute UVB irradiation by alleviating inflammation and regulating intestinal flora. Food Funct. 2023. accepted. [Google Scholar] [CrossRef] [PubMed]
- Madronich, S.; McKenzie, R.; Björn, L.; Caldwell, M. Changes in biologically active ultraviolet radiation reaching the Earth’s surface. J. Photochem. Photobiol. B Biol. 1998, 46, 5–19. [Google Scholar] [CrossRef]
- Han, J.H.; Bang, J.S.; Choi, Y.J.; Choung, S.-Y. Anti-melanogenic effects of oyster hydrolysate in UVB-irradiated C57BL/6J mice and B16F10 melanoma cells via downregulation of cAMP signaling pathway. J. Ethnopharmacol. 2018, 229, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Arulmozhi, V.; Pandian, K.; Mirunalini, S. Ellagic acid encapsulated chitosan nanoparticles for drug delivery system in human oral cancer cell line (KB). Colloids Surfaces B Biointerfaces 2013, 110, 313–320. [Google Scholar] [CrossRef]
- Tan, K.; Zhang, H.; Lim, L.-S.; Ma, H.; Li, S.; Zheng, H. Roles of Carotenoids in Invertebrate Immunology. Front. Immunol. 2019, 10, 3041. [Google Scholar] [CrossRef]
- Hwang, E.; Park, S.-Y.; Lee, H.J.; Lee, T.Y.; Sun, Z.-W.; Yi, T.H. Gallic Acid Regulates Skin Photoaging in UVB-exposed Fibroblast and Hairless Mice. Phytother. Res. 2014, 28, 1778–1788. [Google Scholar] [CrossRef]
- Resnick-Silverman, L. Using TUNEL Assay to Quantitate p53-Induced Apoptosis in Mouse Tissues. Methods Mol Biol. 2021, 2267, 181–190. [Google Scholar] [CrossRef]
- Baswan, S.M.; Klosner, A.E.; Weir, C.; Salter-Venzon, D.; Gellenbeck, K.W.; Leverett, J.; Krutmann, J. Role of ingestible carotenoids in skin protection: A review of clinical evidence. Photodermatol. Photoimmunol. Photomed. 2021, 37, 490–504. [Google Scholar] [CrossRef] [PubMed]
- Stahl, W.; Sies, H. Photoprotection by dietary carotenoids: Concept, mechanisms, evidence and future development. Mol. Nutr. Food Res. 2012, 56, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Ludwiczuk, A.; Asakawa, Y. Bryophytes as a source of bioactive volatile terpenoids—A review. Food Chem. Toxicol. 2019, 132, 110649. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Ma, Q. Role of Nrf2 in Oxidative Stress and Toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Nguyen, T.; Sherratt, P.J.; Pickett, C.B. Regulatory Mechanisms Controlling Gene Expression Mediated by the Antioxidant Response Element. Annu. Rev. Pharmacol. Toxicol. 2003, 43, 233–260. [Google Scholar] [CrossRef]
- Talalay, P.; Dinkova-Kostova, A.T.; Holtzclaw, W. Importance of phase 2 gene regulation in protection against electrophile and reactive oxygen toxicity and carcinogenesis. Adv. Enzym. Regul. 2003, 43, 121–134. [Google Scholar] [CrossRef]
- Yang, M.; Huang, C.-Z. Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World J. Gastroenterol. 2015, 21, 11673–11679. [Google Scholar] [CrossRef]
- Han, S.H.; Ballinger, E.; Choung, S.-Y.; Kwon, J.Y. Anti-Photoaging Effect of Hydrolysates from Pacific Whiting Skin via MAPK/AP-1, NF-κB, TGF-β/Smad, and Nrf-2/HO-1 Signaling Pathway in UVB-Induced Human Dermal Fibroblasts. Mar. Drugs 2022, 20, 308. [Google Scholar] [CrossRef]
- Qu, C.; Li, N.; Liu, T.; He, Y.; Miao, J. Preparation of CPD Photolyase Nanoliposomes Derived from Antarctic Microalgae and Their Effect on UVB-Induced Skin Damage in Mice. Int. J. Mol. Sci. 2022, 23, 15148. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Guo, Y.; Wu, W.-J.; Man, M.-Q.; Tu, Y.; He, L. UVB-Induced Secretion of IL-1β Promotes Melanogenesis by Upregulating TYR/TRP-1 Expression In Vitro. BioMed Res. Int. 2022, 2022, 8230646. [Google Scholar] [CrossRef]
- Alves, C.P.; Yokoyama, S.; Goedert, L.; Pontes, C.L.; Sousa, J.F.; Fisher, D.E.; Espreafico, E.M. MYO5A Gene Is a Target of MITF in Melanocytes. J. Investig. Dermatol. 2016, 137, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.Y.; Jeong, D.; Park, S.H.; Shin, K.K.; Hong, Y.H.; Kim, E.; Yu, Y.-G.; Kim, T.-R.; Kim, H.; Lee, J.; et al. Antiwrinkle and Antimelanogenesis Effects of Tyndallized Lactobacillus acidophilus KCCM12625P. Int. J. Mol. Sci. 2020, 21, 1620. [Google Scholar] [CrossRef]
- Kumar, K.J.S.; Vani, M.G.; Wang, S.-Y.; Liao, J.-W.; Hsu, L.-S.; Yang, H.-L.; Hseu, Y.-C. In vitro and in vivo studies disclosed the depigmenting effects of gallic acid: A novel skin lightening agent for hyperpigmentary skin diseases. BioFactors 2013, 39, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Huang, J.; Pei, S.; Ouyang, Y.; Ding, Y.; Jiang, L.; Lu, J.; Kang, L.; Huang, L.; Xiang, H.; et al. Ganoderma lucidum polysaccharide inhibits UVB-induced melanogenesis by antagonizing cAMP/PKA and ROS/MAPK signaling pathways. J. Cell Physiol. 2018, 234, 7330–7340. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Wang, G.; Chen, L.; Jiang, J.; Liu, L.; Li, N.; Zhao, J.; Sun, X.; Zhou, P. Genome-wide analysis of long non-coding RNAs at early stage of skin pigmentation in goats (Capra hircus). BMC Genom. 2016, 17, 67. [Google Scholar] [CrossRef]
- Liu, S.-J.; Meng, M.-Y.; Han, S.; Gao, H.; Zhao, Y.-Y.; Yang, Y.; Lin, Z.-Y.; Yang, L.-R.; Zhu, K.; Han, R.; et al. Umbilical Cord Mesenchymal Stem Cell-Derived Exosomes Ameliorate HaCaT Cell Photo-Aging. Rejuvenation Res. 2021, 24, 283–293. [Google Scholar] [CrossRef]
- Kumar, K.S.; Yang, H.-L.; Tsai, Y.-C.; Hung, P.-C.; Chang, S.-H.; Lo, H.-W.; Shen, P.-C.; Chen, S.-C.; Wang, H.-M.; Wang, S.-Y.; et al. Lucidone protects human skin keratinocytes against free radical-induced oxidative damage and inflammation through the up-regulation of HO-1/Nrf2 antioxidant genes and down-regulation of NF-κB signaling pathway. Food Chem. Toxicol. 2013, 59, 55–66. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, K.; Zhang, D.; Duan, H.; Liu, Y.; Guo, M. Metabolite accumulation and metabolic network in developing roots of Rehmannia glutinosa reveals its root developmental mechanism and quality. Sci. Rep. 2018, 8, 14127–14138. [Google Scholar] [CrossRef]
- Tanaka, Y.; Uchi, H.; Furue, M. Antioxidant cinnamaldehyde attenuates UVB-induced photoaging. J. Dermatol. Sci. 2019, 96, 151–158. [Google Scholar] [CrossRef]
- Afaq, F.; Syed, D.N.; Malik, A.; Hadi, N.; Sarfaraz, S.; Kweon, M.-H.; Khan, N.; Abu Zaid, M.; Mukhtar, H. Delphinidin, an anthocyanidin in pigmented fruits and vegetables, protects human HaCat keratinocytes and mouse skin against UVB-mediated oxidative stress and apoptosis. J. Investig. Dermatol. 2007, 127, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Yamano, N.; Kunisada, M.; Nishiaki-Sawada, A.; Ohashi, H.; Igarashi, T.; Nishigori, C. Evaluation of Acute Reactions on Mouse Skin Irradiated with 222 and 235 nm UV-C. Photochem. Photobiol. 2021, 97, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Liu, L.; Wang, E.-J.; Xiao, H.-T.; Cai, C.-Z.; Wang, J.; Su, H.; Wang, Y.; Tan, J.; Zhang, Z.; et al. PI3KC3 complex subunit NRBF2 is required for apoptotic cell clearance to restrict intestinal inflammation. Autophagy 2020, 17, 1096–1111. [Google Scholar] [CrossRef] [PubMed]
- Seitz, R.; Weber, G.; Albrecht, S.; Fuchshofer, R.; Tamm, E.R.; Ohlmann, A. Cross-Inhibition of Norrin and TGF-β Signaling Modulates Development of Retinal and Choroidal Vasculature. Investig. Opthalmology Vis. Sci. 2018, 59, 2240–2251. [Google Scholar] [CrossRef]
- Staniforth, V.; Huang, W.-C.; Aravindaram, K.; Yang, N.-S. Ferulic acid, a phenolic phytochemical, inhibits UVB-induced matrix metalloproteinases in mouse skin via posttranslational mechanisms. J. Nutr. Biochem. 2012, 23, 443–451. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liang, S.; Zhang, Z.; Wang, K.; Cao, J.; Yao, M.; Qin, L.; Qu, C.; Miao, J. Protective Effects of ζ-Carotene-like Compounds against Acute UVB-Induced Skin Damage. Int. J. Mol. Sci. 2023, 24, 13970. https://doi.org/10.3390/ijms241813970
Zhang L, Liang S, Zhang Z, Wang K, Cao J, Yao M, Qin L, Qu C, Miao J. Protective Effects of ζ-Carotene-like Compounds against Acute UVB-Induced Skin Damage. International Journal of Molecular Sciences. 2023; 24(18):13970. https://doi.org/10.3390/ijms241813970
Chicago/Turabian StyleZhang, Liping, Shaoxin Liang, Zhi Zhang, Kai Wang, Junhan Cao, Mengke Yao, Ling Qin, Changfeng Qu, and Jinlai Miao. 2023. "Protective Effects of ζ-Carotene-like Compounds against Acute UVB-Induced Skin Damage" International Journal of Molecular Sciences 24, no. 18: 13970. https://doi.org/10.3390/ijms241813970
APA StyleZhang, L., Liang, S., Zhang, Z., Wang, K., Cao, J., Yao, M., Qin, L., Qu, C., & Miao, J. (2023). Protective Effects of ζ-Carotene-like Compounds against Acute UVB-Induced Skin Damage. International Journal of Molecular Sciences, 24(18), 13970. https://doi.org/10.3390/ijms241813970




