Blood ACE2 Protein Level Correlates with COVID-19 Severity
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
- HQM—Home Quarantined with Mild disease course.
- HMO—Hospitalized with Moderate course.
- HSV—Hospitalized oxygen-dependent patients with SeVere symptoms.
- HCR—Hospitalized Critical patients in ICU departments with artificial ventilation.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Del Valle, N.C.A.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. Long-COVID in Children and Adolescents: A Systematic Review and Meta-Analyses. Sci. Rep. 2022, 12, 9950. [Google Scholar] [CrossRef]
- Perlis, R.H.; Santillana, M.; Ognyanova, K.; Safarpour, A.; Trujillo, K.L.; Simonson, M.D.; Green, J.; Quintana, A.; Druckman, J.; Baum, M.A.; et al. Prevalence and Correlates of Long COVID Symptoms Among US Adults. JAMA Netw. Open 2022, 5, e2238804. [Google Scholar] [CrossRef]
- Tran, V.-T.; Porcher, R.; Pane, I.; Ravaud, P. Course of post COVID-19 disease symptoms over time in the ComPaRe long COVID prospective e-cohort. Nat. Commun. 2022, 13, 1812. [Google Scholar] [CrossRef]
- Grasselli, G.; Zangrillo, A.; Zanella, A.; Antonelli, M.; Cabrini, L.; Castelli, A.; Cereda, D.; Coluccello, A.; Foti, G.; Fumagalli, R.; et al. Baseline Characteristics and Outcomes of 1591 Patients Infected with SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA 2020, 323, 1574–1581. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Zhou, Y.; Chi, J.; Lv, W.; Wang, Y. Obesity and Diabetes as High-Risk Factors for Severe Coronavirus Disease 2019 (COVID-19). Diabetes/Metab. Res. Rev. 2020, 37, e3377. [Google Scholar] [CrossRef]
- Ahmed, S.; Zimba, O.; Gasparyan, A.Y. Thrombosis in Coronavirus Disease 2019 (COVID-19) through the Prism of Virchow’s Triad. Clin. Rheumatol. 2020, 39, 2529–2543. [Google Scholar] [CrossRef]
- Su, H.; Yang, M.; Wan, C.; Yi, L.-X.; Tang, F.; Zhu, H.-Y.; Yi, F.; Yang, H.-C.; Fogo, A.B.; Nie, X.; et al. Renal Histopathological Analysis of 26 Postmortem Findings of Patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef]
- Li, X.; Ma, X. Acute Respiratory Failure in COVID-19: Is it “Typical” ARDS? Crit. Care 2020, 24, 198. [Google Scholar] [CrossRef]
- Coperchini, F.; Chiovato, L.; Croce, L.; Magri, F.; Rotondi, M. The Cytokine Storm in COVID-19: An Overview of the Involvement of the Chemokine/Chemokine-Receptor System. Cytokine Growth Factor Rev. 2020, 53, 25–32. [Google Scholar] [CrossRef] [PubMed]
- McGonagle, D.; Sharif, K.; O’Regan, A.; Bridgewood, C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun. Rev. 2020, 19, 102537. [Google Scholar] [CrossRef] [PubMed]
- Kai, H.; Kai, M. Interactions of Coronaviruses with ACE2, Angiotensin II, and RAS Inhibitors—Lessons from Available Evidence and Insights into COVID-19. Hypertens. Res. 2020, 43, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Pons, S.; Fodil, S.; Azoulay, E.; Zafrani, L. The Vascular Endothelium: The Cornerstone of Organ Dysfunction in Severe SARS-CoV-2 Infection. Crit. Care 2020, 24, 353. [Google Scholar] [CrossRef]
- Amraei, R.; Rahimi, N. COVID19, Renin-Angiotensin System and Endothelial Dysfunction. Cells 2020, 9, 1652. [Google Scholar] [CrossRef]
- Long, B.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020, 38, 1504–1507. [Google Scholar] [CrossRef]
- Guzik, T.J.; Mohiddin, S.A.; DiMarco, A.; Patel, V.; Savvatis, K.; Marelli-Berg, F.M.; Madhur, M.S.; Tomaszewski, M.; Maffia, P.; D’Acquisto, F.; et al. COVID-19 and the Cardiovascular System: Implications for Risk Assessment, Diagnosis, and Treatment Options. Cardiovasc. Res. 2020, 116, 1666–1687. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of Comorbidities and Its Effects in Coronavirus Disease 2019 Patients: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Parit, R.; Jayavel, S. Association of ACE Inhibitors and Angiotensin Type II Blockers with ACE2 Overexpression in COVID-19 Comorbidities: A Pathway-Based Analytical Study. Eur. J. Pharmacol. 2021, 896, 173899. [Google Scholar] [CrossRef]
- South, A.M.; Tomlinson, L.; Edmonston, D.; Hiremath, S.; Sparks, M.A. Controversies of Renin–Angiotensin System Inhibition during the COVID-19 Pandemic. Nat. Rev. Nephrol. 2020, 16, 305–307. [Google Scholar] [CrossRef]
- Chary, M.A.; Barbuto, A.F.; Izadmehr, S.; Hayes, B.D.; Burns, M.M. COVID-19: Therapeutics and Their Toxicities. J. Med. Toxicol. 2020, 16, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Khashkhusha, T.R.; Chan, J.S.K.; Harky, A. ACE Inhibitors and COVID-19: We Don’t Know Yet. J. Card. Surg. 2020, 35, 1172–1173. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Theodoridou, K.; Poland, G. Influenza immunization and COVID-19. Vaccine 2020, 38, 6078–6079. [Google Scholar] [CrossRef]
- Wu, C.; Chen, X.; Cai, Y.; Xia, J.; Zhou, X.; Xu, S.; Huang, H.; Zhang, L.; Zhou, X.; Du, C.; et al. Risk Factors Associated with Acute Respiratory Distress Syndrome and Death in Patients with Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020, 180, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Verma, R.; Lohana, P.; Lohana, A.; Ramphul, K. Acute Myocardial Infarction in COVID-19 Patients. A Review of Cases in the Literature. Arch. Med. Sci.–Atheroscler. Dis. 2021, 6, 169–175. [Google Scholar] [CrossRef]
- Rojas-García, M.; Vázquez, B.; Torres-Poveda, K.; Madrid-Marina, V. Lethality Risk Markers by Sex and Age-Group for COVID-19 in Mexico: A Cross-Sectional Study Based on Machine Learning Approach. BMC Infect. Dis. 2023, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Hamer, M.; Gale, C.R.; Kivimäki, M.; Batty, G.D. Overweight, Obesity, and Risk of Hospitalization for COVID-19: A Community-Based Cohort Study of Adults in the United Kingdom. Proc. Natl. Acad. Sci. USA 2020, 117, 21011–21013. [Google Scholar] [CrossRef]
- Bastolla, U.; Chambers, P.; Abia, D.; Garcia-Bermejo, M.-L.; Fresno, M. Is Covid-19 Severity Associated with ACE2 Degradation? Front. Drug Discov. 2022, 1, 789710. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Y.; Ke, Q.; Wang, Y.; Gong, Z.; Chen, X.; Cai, Y.; Li, S.; Sun, Y.; Peng, X.; et al. ApoE4 associated with severe COVID-19 outcomes via downregulation of ACE2 and imbalanced RAS pathway. J. Transl. Med. 2023, 21, 103. [Google Scholar] [CrossRef]
- Lee, S.H.; Lee, Y.H.; Jung, S.W.; Kim, D.J.; Park, S.H.; Song, S.J.; Jeong, K.H.; Moon, J.Y.; Ihm, C.-G.; Lee, T.W.; et al. Sex-related differences in the intratubular renin-angiotensin system in two-kidney, one-clip hypertensive rats. Am. J. Physiol. Physiol. 2019, 317, F670–F682. [Google Scholar] [CrossRef]
- Pouremamali, A.; Babaei, A.; Malekshahi, S.S.; Abbasi, A.; Rafiee, N. Understanding the Pivotal Roles of ACE2 in SARS-CoV-2 Infection: From Structure/Function to Therapeutic Implication. Egypt. J. Med. Hum. Genet. 2022, 23, 103. [Google Scholar] [CrossRef] [PubMed]
- Sodhi, C.P.; Wohlford-Lenane, C.; Yamaguchi, Y.; Prindle, T.; Fulton, W.B.; Wang, S.; McCray, P.B., Jr.; Chappell, M.; Hackam, D.J.; Jia, H.; et al. Attenuation of Pulmonary ACE2 Activity Impairs Inactivation of Des-Arg9 Bradykinin/BKB1R Axis and Facilitates LPS-Induced Neutrophil Infiltration. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 314, L17–L31. [Google Scholar] [CrossRef] [PubMed]
- Verdecchia, P.; Cavallini, C.; Spanevello, A.; Angeli, F. The Pivotal Link between ACE2 Deficiency and SARS-CoV-2 Infection. Eur. J. Intern. Med. 2020, 76, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Abbas, M.; Verma, S.; Khan, F.H.; Raza, S.T.; Siddiqi, Z.; Ahmad, I.; Mahdi, F. Impact of I/D Polymorphism of Angiotensin-Converting Enzyme 1 (ACE1) Gene on the Severity of COVID-19 Patients. Infect. Genet. Evol. 2021, 91, 104801. [Google Scholar] [CrossRef] [PubMed]
- Mortaz, E.; Jamaati, H.; Roofchayee, N.D.; Sheikhzade, H.; Mirenayat, M.; Sadeghi, M.; Lookzadeh, S.; Dezfuli, N.K.; Folkerts, G.; Mumby, S.; et al. Decreased Serum Levels of Angiotensin Converting Enzyme (ACE)2 and Enhanced Cytokine Levels with Severity of COVID-19: Normalisation upon Disease Recovery. Heliyon 2022, 8, e08957. [Google Scholar] [CrossRef]
- Maza, M.d.C.; Úbeda, M.; Delgado, P.; Horndler, L.; Llamas, M.A.; Van Santen, H.M.; Alarcón, B.; Abia, D.; García-Bermejo, L.; Serrano-Villar, S.; et al. ACE2 Serum Levels as Predictor of Infectability and Outcome in COVID-19. Front. Immunol. 2022, 13, 836516. [Google Scholar] [CrossRef]
- Florescu, S.; Stanciu, D.; Zaharia, M.; Kosa, A.; Codreanu, D.; Fareed, K.; Kidwai, A.; Kaye, C.; Coutts, A.; MacKay, L.; et al. Effect of Angiotensin-Converting Enzyme Inhibitor and Angiotensin Receptor Blocker Initiation on Organ Support–Free Days in Patients Hospitalized with COVID-19: A Randomized Clinical Trial. JAMA 2023, 329, 1183–1196. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; An, Y. ACE2 Shedding and the Role in COVID-19. Front. Cell. Infect. Microbiol. 2022, 11, 789180. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Salah, H.M.; Mehta, J.L. Hypothesis: Sex-Related Differences in ACE2 Activity May Contribute to Higher Mortality in Men Versus Women with COVID-19. J. Cardiovasc. Pharmacol. Ther. 2020, 26, 114–118. [Google Scholar] [CrossRef]
- La Vignera, S.; Cannarella, R.; Condorelli, R.A.; Torre, F.; Aversa, A.; Calogero, A.E. Sex-Specific SARS-CoV-2 Mortality: Among Hormone-Modulated ACE2 Expression, Risk of Venous Thromboembolism and Hypovitaminosis D. Int. J. Mol. Sci. 2020, 21, 2948. [Google Scholar] [CrossRef] [PubMed]
- Bienvenu, L.A.; Noonan, J.; Wang, X.; Peter, K. Higher Mortality of COVID-19 in Males: Sex Differences in Immune Response and Cardiovascular Comorbidities. Cardiovasc. Res. 2020, 116, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, H.C.; Sukumaran, V.; Al-Ruweidi, M.K.A.A.; Shurbaji, S. Do Changes in ACE-2 Expression Affect SARS-CoV-2 Virulence and Related Complications: A Closer Look into Membrane-Bound and Soluble Forms. Int. J. Mol. Sci. 2021, 22, 6703. [Google Scholar] [CrossRef] [PubMed]
- Radzikowska, U.; Ding, M.; Tan, G.; Zhakparov, D.; Peng, Y.; Wawrzyniak, P.; Wang, M.; Li, S.; Morita, H.; Altunbulakli, C.; et al. Distribution of ACE2, CD147, CD26, and Other SARS-CoV-2 Associated Molecules in Tissues and Immune Cells in Health and in Asthma, COPD, Obesity, Hypertension, and COVID-19 Risk Factors. Allergy 2020, 75, 2829–2845. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-Y.; Li, L.; Zhang, Y.; Wang, X.-S. Expression of the SARS-CoV-2 Cell Receptor Gene ACE2 in a Wide Variety of Human Tissues. Infect. Dis. Poverty 2020, 9, 45. [Google Scholar] [CrossRef]
- Friske, M.M.; Giannone, F.; Senger, M.; Seitz, R.; Hansson, A.C.; Spanagel, R. Chronic Alcohol Intake Regulates Expression of SARS-CoV2 Infection-Relevant Genes in an Organ-Specific Manner. Alcohol. Clin. Exp. Res. 2023, 47, 76–86. [Google Scholar] [CrossRef]
- Solopov, P.A.; Biancatelli, R.M.C.; Catravas, J.D. Alcohol Increases Lung Angiotensin-Converting Enzyme 2 Expression and Exacerbates Severe Acute Respiratory Syndrome Coronavirus 2 Spike Protein Subunit 1–Induced Acute Lung Injury in K18-hACE2 Transgenic Mice. Am. J. Pathol. 2022, 192, 990–1000. [Google Scholar] [CrossRef]
- Althobaiti, Y.S.; Alzahrani, M.A.; Alsharif, N.A.; Alrobaie, N.S.; Alsaab, H.O.; Uddin, M.N. The Possible Relationship between the Abuse of Tobacco, Opioid, or Alcohol with COVID-19. Healthcare 2020, 9, 2. [Google Scholar] [CrossRef]
- Heijink, I.H.; Hackett, T.; Pouwels, S.D. Effects of Cigarette Smoking on SARS-CoV-2 Receptor ACE2 Expression in the Respiratory Epithelium†. J. Pathol. 2020, 253, 351–354. [Google Scholar] [CrossRef]
- Fernandes, S.; Sosa-Napolskij, M.; Lobo, G.; Silva, I. Impact of the COVID-19 Pandemic in the Portuguese Population: Consumption of Alcohol, Stimulant Drinks, Illegal Substances, and Pharmaceuticals. PLoS ONE 2021, 16, e0260322. [Google Scholar] [CrossRef]
- Barbosa, C.; Cowell, A.J.; Dowd, W.N. Alcohol Consumption in Response to the COVID-19 Pandemic in the United States. J. Addict. Med. 2021, 15, 341. [Google Scholar] [CrossRef] [PubMed]
- Patanavanich, R.; Glantz, S.A. Smoking is Associated with COVID-19 Progression: A Meta-Analysis. Nicotine Tob. Res. 2020, 22, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Mahamat-Saleh, Y.; Fiolet, T.; Rebeaud, M.E.; Mulot, M.; Guihur, A.; El Fatouhi, D.; Laouali, N.; Peiffer-Smadja, N.; Aune, D.; Severi, G. Diabetes, Hypertension, Body Mass Index, Smoking and COVID-19-Related Mortality: A Systematic Review and Meta-Analysis of Observational Studies. BMJ Open 2021, 11, e052777. [Google Scholar] [CrossRef] [PubMed]
- Emilsson, V.; Gudmundsson, E.F.; Aspelund, T.; Jonsson, B.G.; Gudjonsson, A.; Launer, L.J.; Lamb, J.R.; Gudmundsdottir, V.; Jennings, L.L.; Gudnason, V. Serum Levels of ACE2 are Higher in Patients with Obesity and Diabetes. Obes. Sci. Pr. 2020, 7, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Hrebenyk, M.; Maslii, S.; Shevchuk, O.; Korda, M.; Vari, G.S. Impact of High Blood Pressure and Antihypertensive Treatment on COVID-19 Severity (Retrospective Observational Study in Ternopil Region, Ukraine). In Proceedings of the Materials of 17th RECOOP Bridges in Life Sciences, Video Conference, Prague, Check Republic, 8 April 2022; pp. 72–73. [Google Scholar]
| 0–45 Days after the Last Negative PCR (59.6%) | 46–90 Days after the Last Negative PCR (40.4%) | |||
|---|---|---|---|---|
| 344 | 233 | |||
| Total Number | Females (66.9%) | Males (33.1%) | Average Age | Seronegative Patients (Control) |
| 577 | 386 | 191 | 50.63 ± 13.08 years | 30 |
| Patients based on COVID-19 severity were divided into groups | ||||
| HQM (45.6%) | HMO (37.3%) | HSV (14.2%) | HCR (2.9%) | |
| 263 | 215 | 82 | 17 | |
| Groups | ACE2, ng/mL | |
|---|---|---|
| n = 440 | ||
| n | Me [Lq; Uq] | |
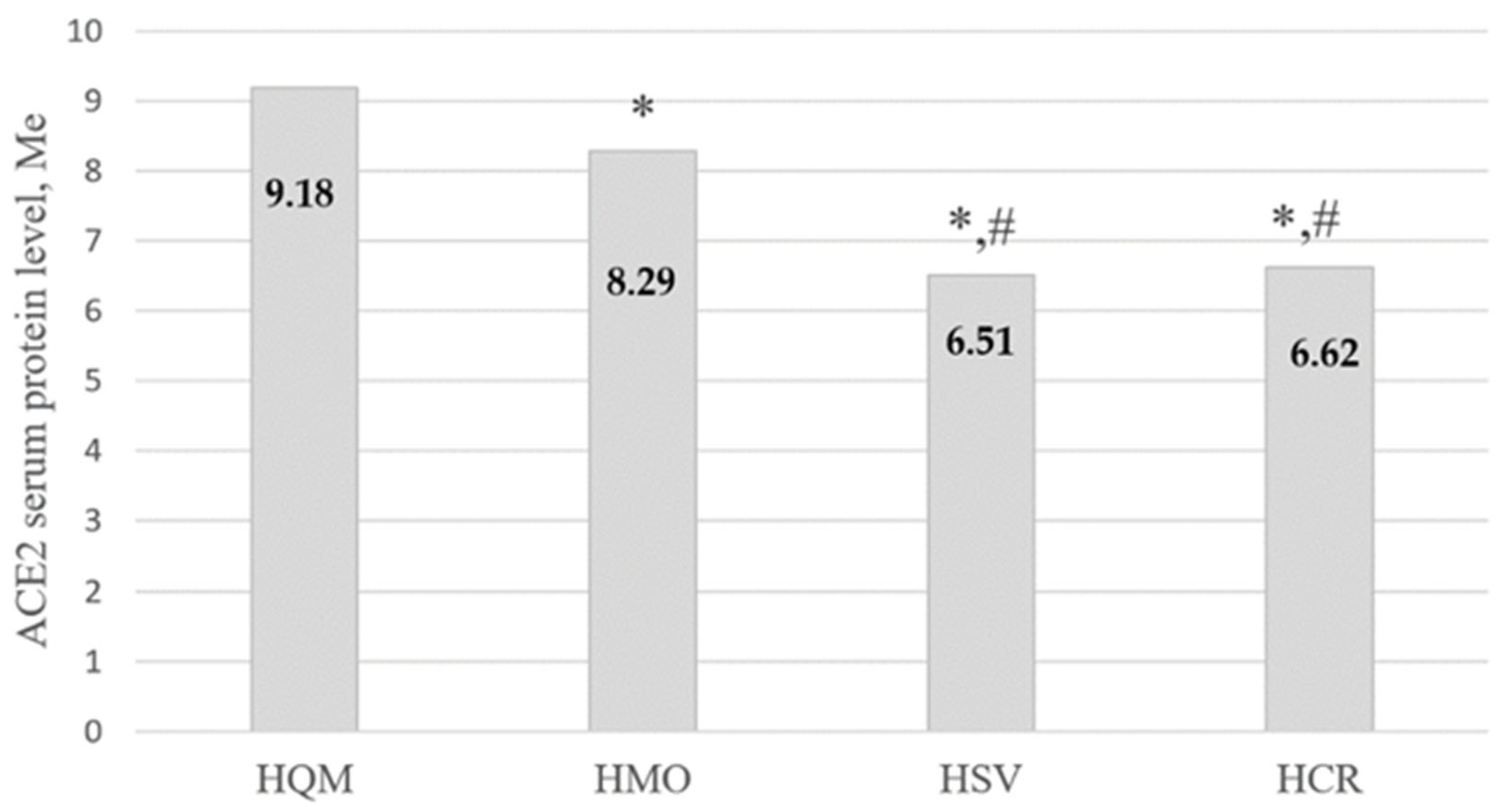
| HQM (1) | 155 | 9.19 [8.36; 10.05] |
| HMO (2) | 156 | 7.37 [5.75; 9.32] |
| HSV (3) | 82 | 6.49 [5.28; 7.25] |
| HCR (4) | 17 | 6.65 [5.35; 7.35] |
| Control group (5) | 30 | 7.69 [6.82; 9.09] |
| Kruskal-Wallis test | H = 104.53; p < 0.001 * | |
| Multiple groups comparisons | p1–2, 1–3, 1–4, 1–5 < 0.001*, p2–3 < 0.05 *, p3–5 < 0.05 * | |
| ACE2, ng/mL; n = 410 | |||
|---|---|---|---|
| Group | M | F | |
| HQM (1) | n | 54 | 101 |
| Me | 9.05 | 9.22 | |
| [Lq; Uq] | [8.19; 10.70] | [8.53; 9.94] | |
| HMO (2) | n | 60 | 96 |
| Me | 7.65 | 6.93 | |
| [Lq; Uq] | [6.32; 9.53] | [5.61; 9.04] | |
| HSV (3) | n | 41 | 41 |
| Me | 6.67 | 6.05 | |
| [Lq; Uq] | [4.95; 7.25] | [5.33; 7.17] | |
| HCR (4) | n | 9 | 8 |
| Me | 6.60 | 7.05 | |
| [Lq; Uq] | [5.35; 6.88] | [5.47; 7.41] | |
| Kruskal-Wallis test | H = 41.35, | H = 60.93, | |
| p < 0.001 * | p < 0.001 * | ||
| Multiple groups comparisons | p1–2, 1–3, 1–4, 2–3 < 0.05 * | p1–2, 1–3, 1–4 < 0.05 * | |
| Group | 0–45 Days | 46–90 Days |
|---|---|---|
| HQM | 9.41 [8.85; 10.88] | 8.94 [7.43; 9.54] * |
| HCR | 5.16 [4.75; 6.34] | 6.92 [6.60; 7.41] * |
| Parameters | HQM (1) | HMO (2) | HSV (3) | HCR (4) | Statistical Significance |
|---|---|---|---|---|---|
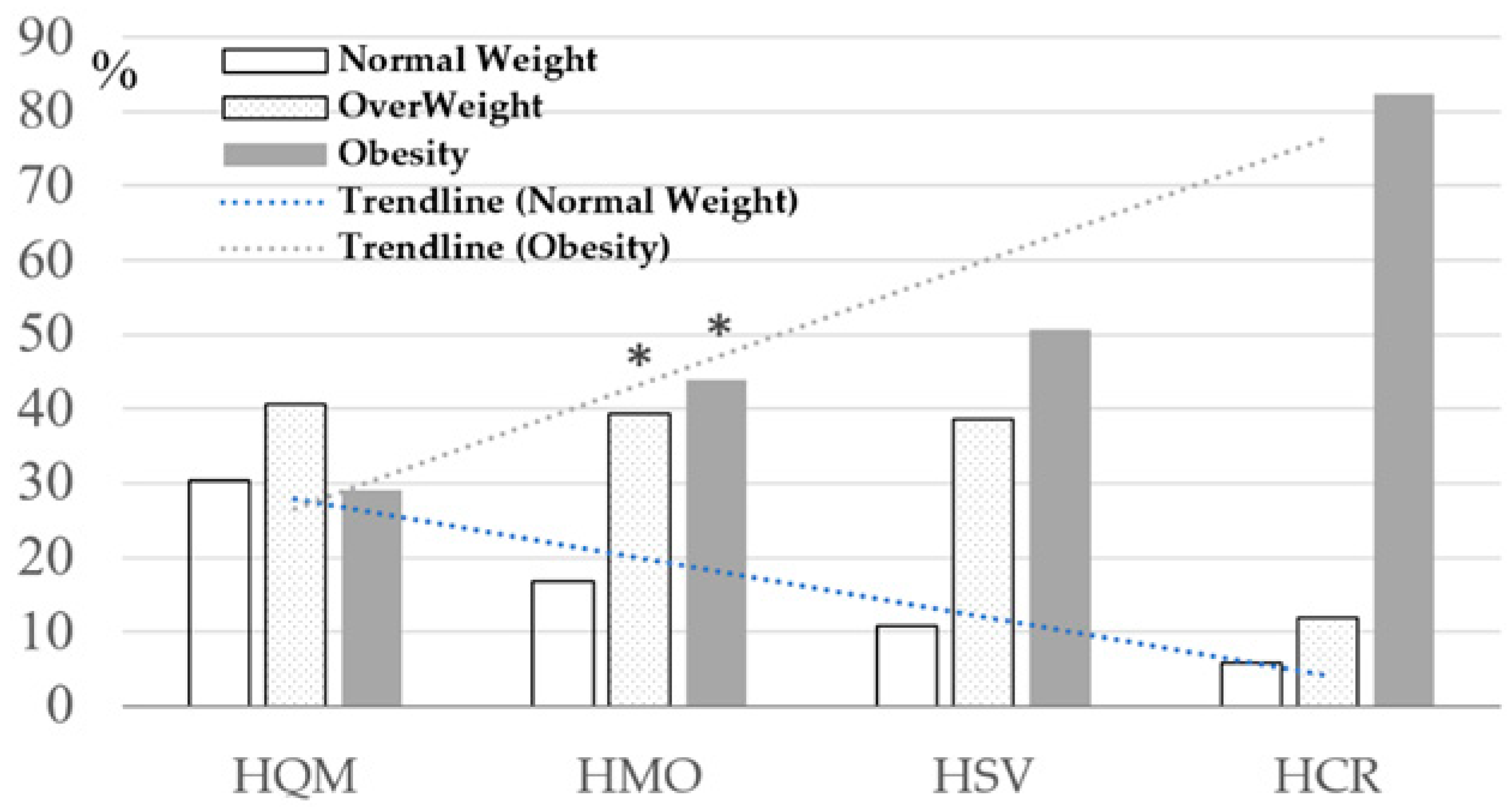
| Weight, kg | 76.02 ±17.31 | 83.24 # ±14.88 | 88.70 # ±18.04 | 93.41 # ±15.40 | pANOVA < 0.001 * p1–2; p1–3; p1–4 < 0.001 * |
| Height, cm | 166.43 ±13.35 | 168.25 ±9.26 | 168.99 ±9.67 | 168.76 ±9.58 | pANOVA = 0.186 |
| BMI, kg/m2 | 27.17 ±5.68 | 29.45 # ±4.76 | 31.01 # ±5.29 | 32.80 # ±4.91 | pANOVA < 0.001 * p1–2; p1–3; p1–4 < 0.001 * |
| WC, cm | 90.91 ±14.92 | 99.66 # ±13.49 | 106.63 # ±12.38 | 109.29 # ±9.58 | pANOVA < 0.001 * p1–2; p1–3; p1–4 < 0.001 * p2–3 < 0.001 * p2–4 = 0.031 * |
| HC, cm | 105.24 ±11.46 | 109.12 # ±9.48 | 111.35 # ±10.33 | 114.06 # ±11.71 | pANOVA < 0.001 * p1–2; p1–3; p1–4 < 0.01 * |
| WHtR | 0.54 ±0.09 | 0.59 # ±0.08 | 0.63 # ±0.07 | 0.65 # ±0.06 | pANOVA < 0.001 * p1–2; p1–3; p1–4 < 0.001* p2–3 = 0.002 * p2–4 = 0.038 * |
| WHR | 0.86 ±0.10 | 0.91 ±0.09 | 0.96 ±0.08 | 0.96 ±0.09 | pANOVA < 0.001 * p1–2; p1–3; p1–4 < 0.001* p2–3 = 0.001 * |
| AO (WC ≥ 94 cm [M], ≥ 80 cm [W]) * | 179 (69.65%) | 186 (88.57%) | 76 (96.20%) | 17 (100.00%) | χ2 = 45.79; p < 0.001 * |
| AO (WHtR ≥ 0.5) | 175 (67.83%) | 185 (88.94%) | 77 (97.47%) | 17 (100.00%) | χ2 = 55.02; p < 0.001 * |
| AO (WHR ≥0.90 [M]; ≥0.85 [W]) | 137 (53.31%) | 158 (75.24%) | 72 (91.14%) | 17 (100.00%) | χ2 = 58.18; p < 0.001 * |
| Group | Diabetes Mellitus | ||
|---|---|---|---|
| Absent | Present | ||
| n | 150 | 5 | |
| HQM (1) | Me [Lq; Uq] | 9.18 [8.35; 9.9]) | 11.16 # [9.53; 12.77] |
| n | 138 | 18 | |
| HMO (2) | Me [Lq; Uq] | 7.11 [5.57; 9.26] | 8.21 # [7.19; 9.57] |
| n | 62 | 20 | |
| HSV (3) | Me [Lq; Uq] | 6.20 [4.90; 7.06] | 7.14 # [5.67; 8.96] |
| n | 13 | 4 | |
| HCR (4) | Me [Lq; Uq] | 6.34 [4.97; 6.88] | 7.37 # [7.26; 7.41] |
| Kruskal-Wallis test | H = 98.02 p < 0.001* | H = 11.92 p = 0.008 * | |
| Multiple groups comparisons | p1–2, 1–3, 1–4, 2–3 < 0.05 * | p1–3 < 0.05 * | |
| Groups | 1—HP, n = 338 | 2—HP + DM, n = 88 | 3—DM, n = 17 | 4—No HP, No DM, n = 500 | p < 0.05 |
|---|---|---|---|---|---|
| COVID-19 Diagnosis Verification, days | 5.58 ± 0.42 | 4.80 ± 0.38 | 8.33 ± 1.60 | 5.01 ± 0,34 | p1–3 = 0.037, p2–3 = 0.0003, p3–4 = 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shevchuk, O.; Pak, A.; Palii, S.; Ivankiv, Y.; Kozak, K.; Korda, M.; Vari, S.G. Blood ACE2 Protein Level Correlates with COVID-19 Severity. Int. J. Mol. Sci. 2023, 24, 13957. https://doi.org/10.3390/ijms241813957
Shevchuk O, Pak A, Palii S, Ivankiv Y, Kozak K, Korda M, Vari SG. Blood ACE2 Protein Level Correlates with COVID-19 Severity. International Journal of Molecular Sciences. 2023; 24(18):13957. https://doi.org/10.3390/ijms241813957
Chicago/Turabian StyleShevchuk, Oksana, Anastasia Pak, Svitlana Palii, Yana Ivankiv, Kateryna Kozak, Mykhaylo Korda, and Sandor G. Vari. 2023. "Blood ACE2 Protein Level Correlates with COVID-19 Severity" International Journal of Molecular Sciences 24, no. 18: 13957. https://doi.org/10.3390/ijms241813957
APA StyleShevchuk, O., Pak, A., Palii, S., Ivankiv, Y., Kozak, K., Korda, M., & Vari, S. G. (2023). Blood ACE2 Protein Level Correlates with COVID-19 Severity. International Journal of Molecular Sciences, 24(18), 13957. https://doi.org/10.3390/ijms241813957







