Polygalae Radix Oligosaccharide Esters May Relieve Depressive-like Behavior in Rats with Chronic Unpredictable Mild Stress via Modulation of Gut Microbiota
Abstract
:1. Introduction
2. Results
2.1. Identification of Chemical Components in the PROEs
2.2. The Effect of PROEs on Depression Rat
2.3. The Effect of PROEs on Monoamine Neurotransmitters and Their Metabolites in the Hippocampus
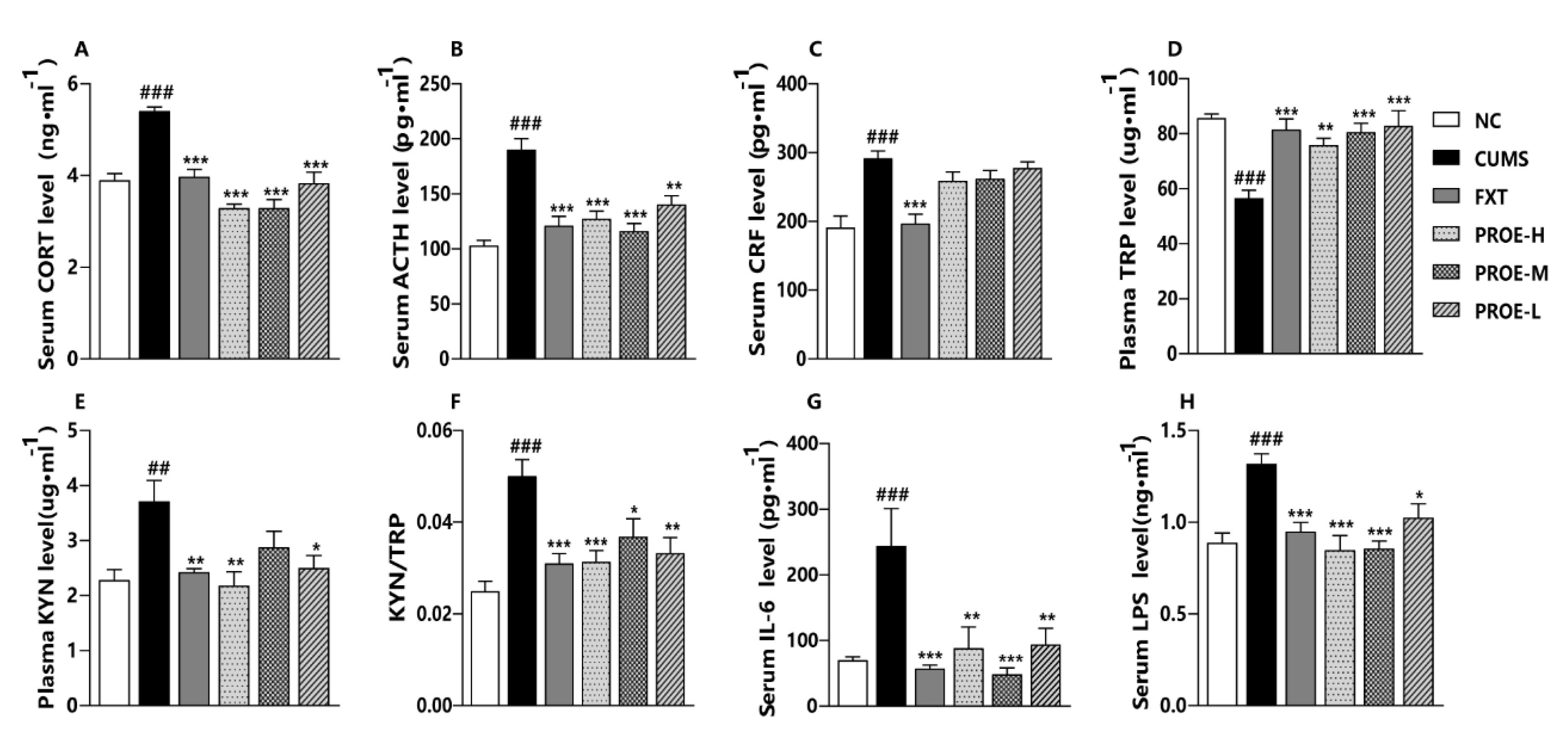
2.4. The Effect of PROEs on HPA Axis
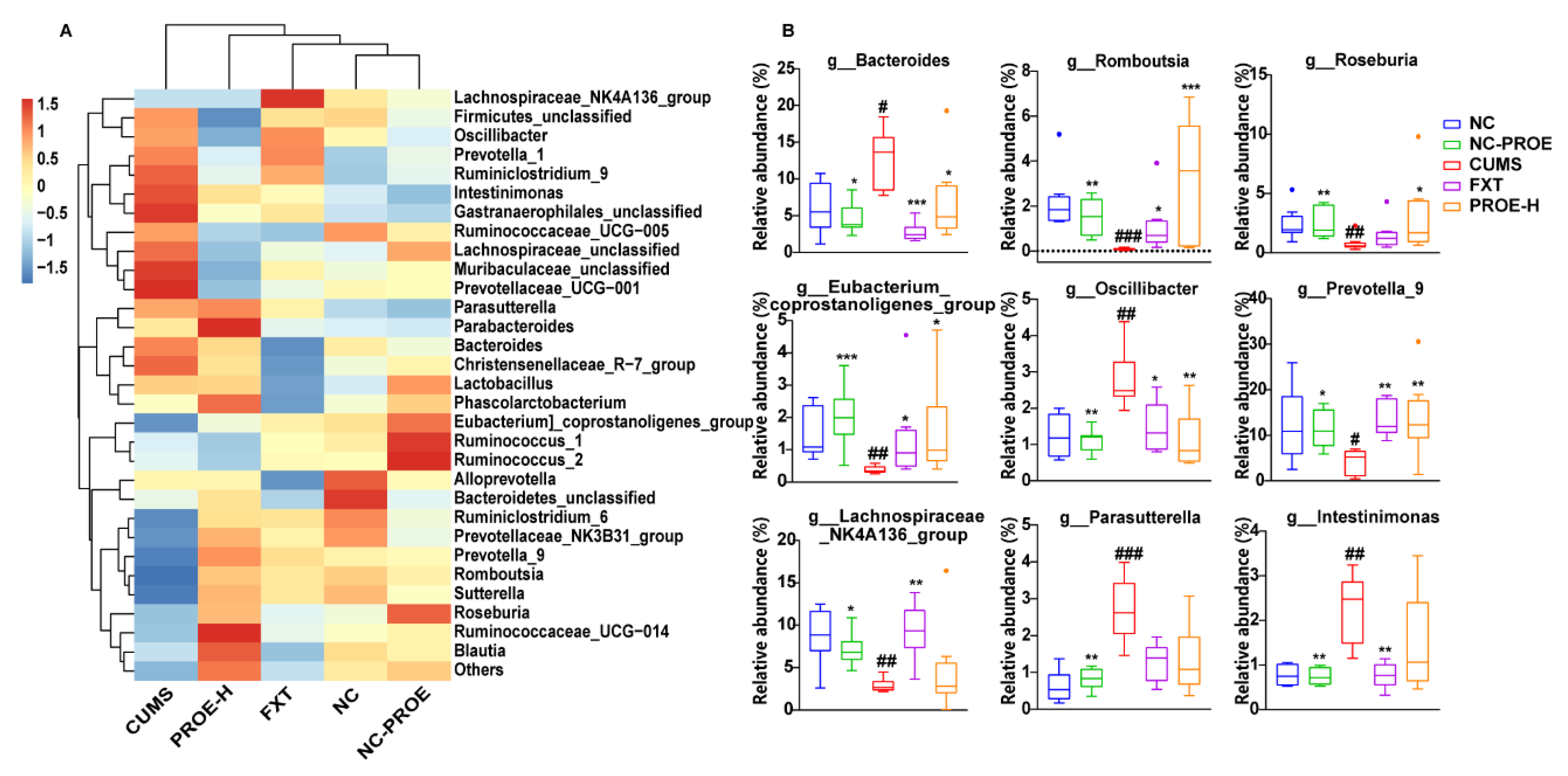
2.5. The Effects of PROEs on Gut Microbiota Composition
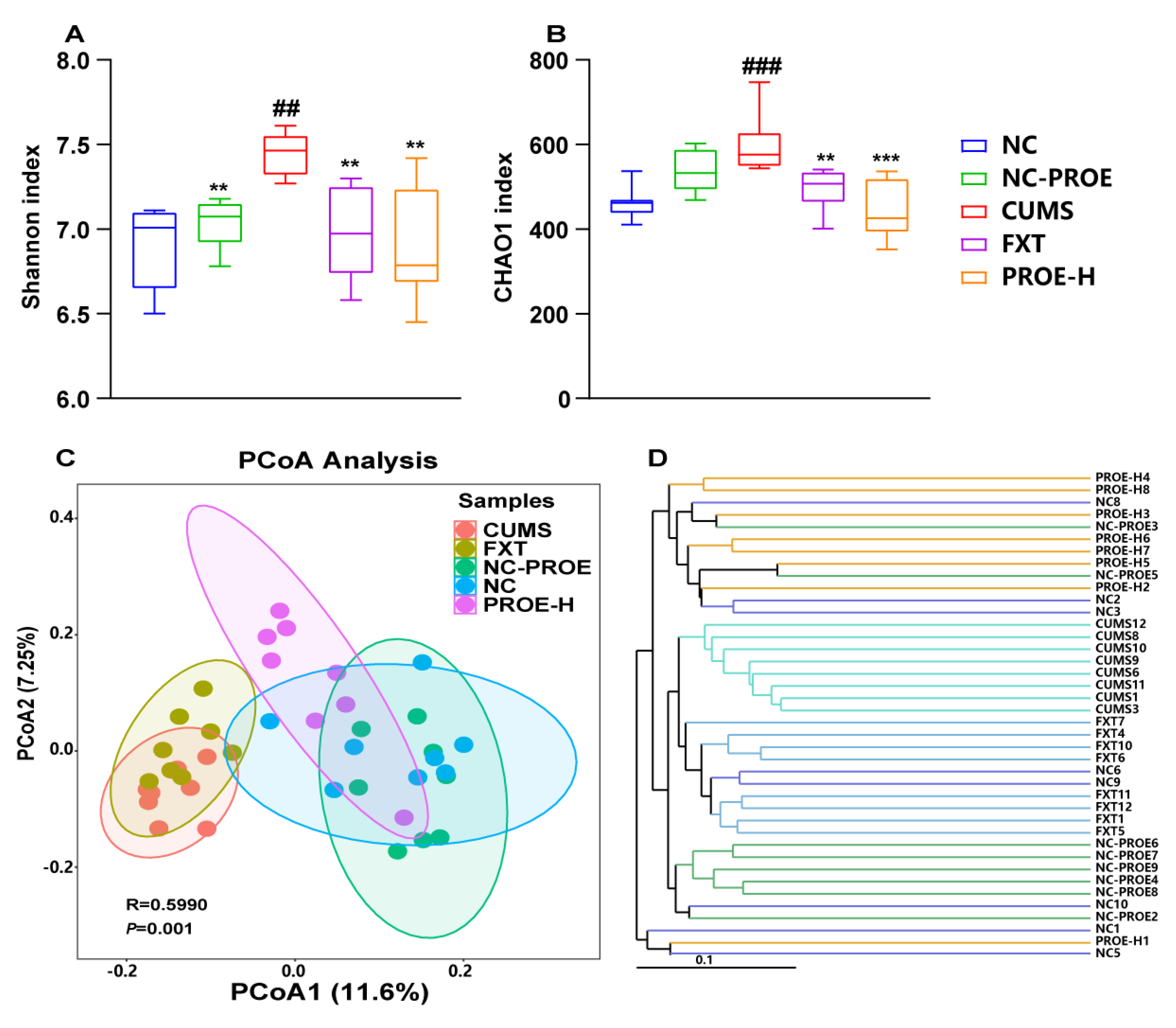
2.5.1. Changes in Gut Microbiota Diversity
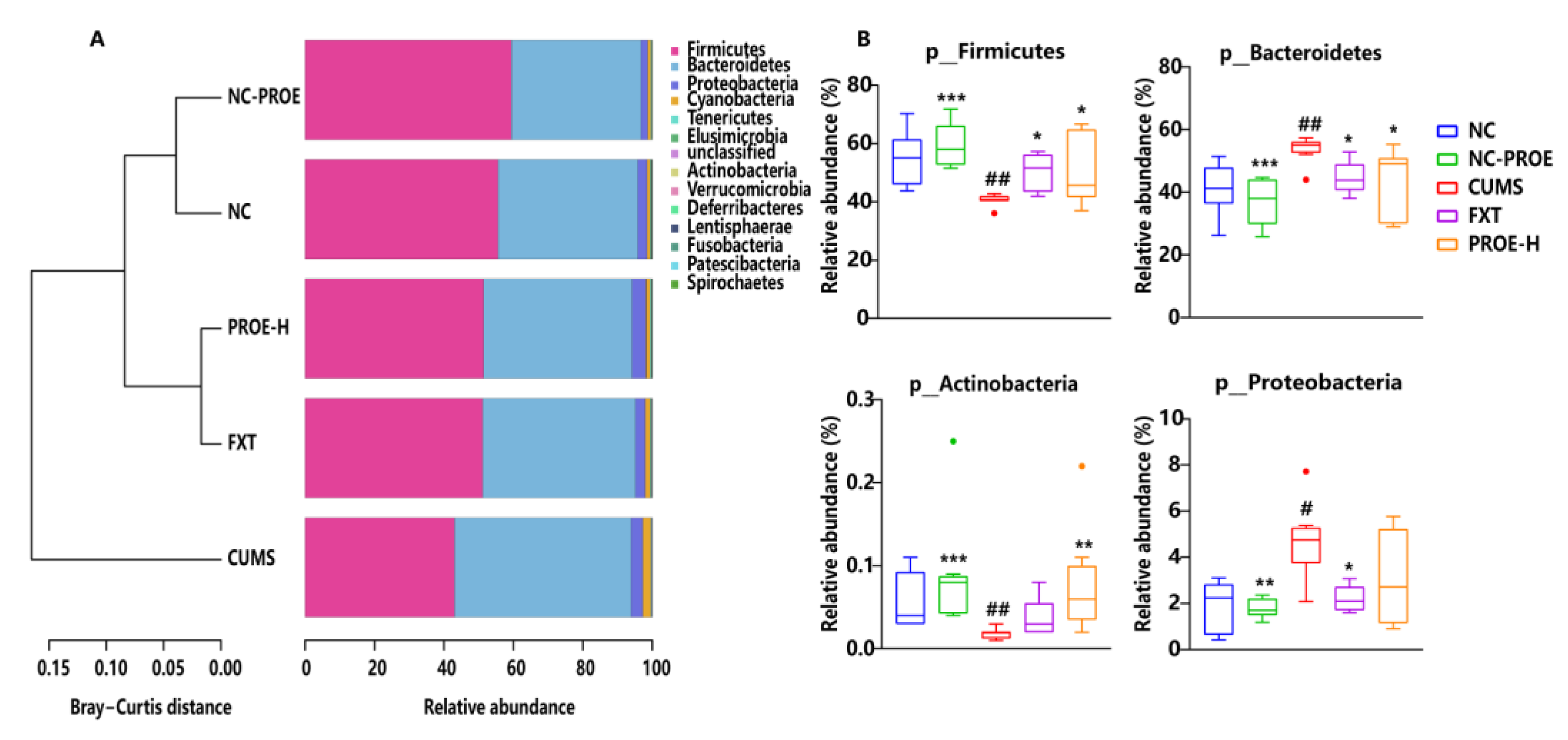
2.5.2. Changes in the Composition of Gut Microbiota
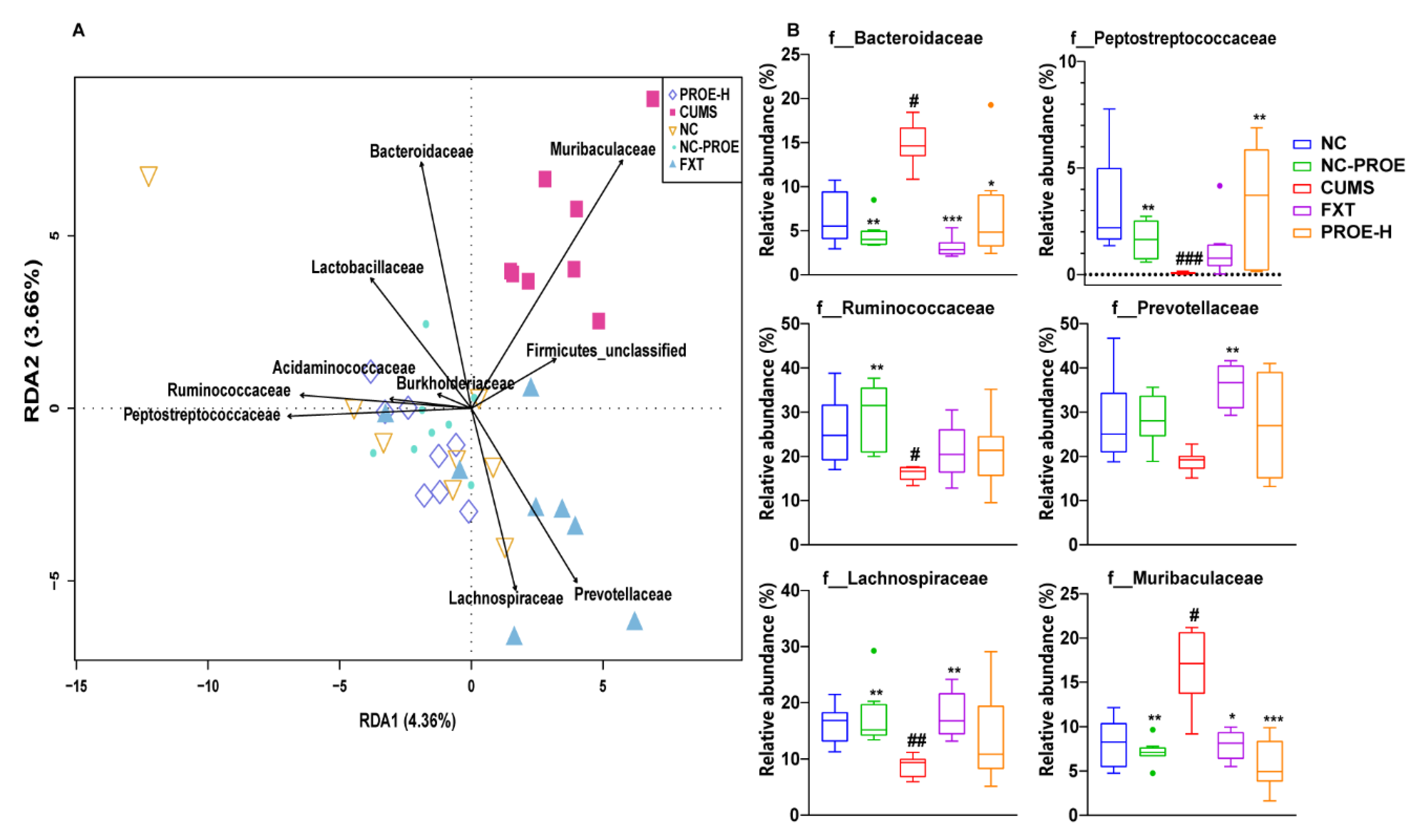
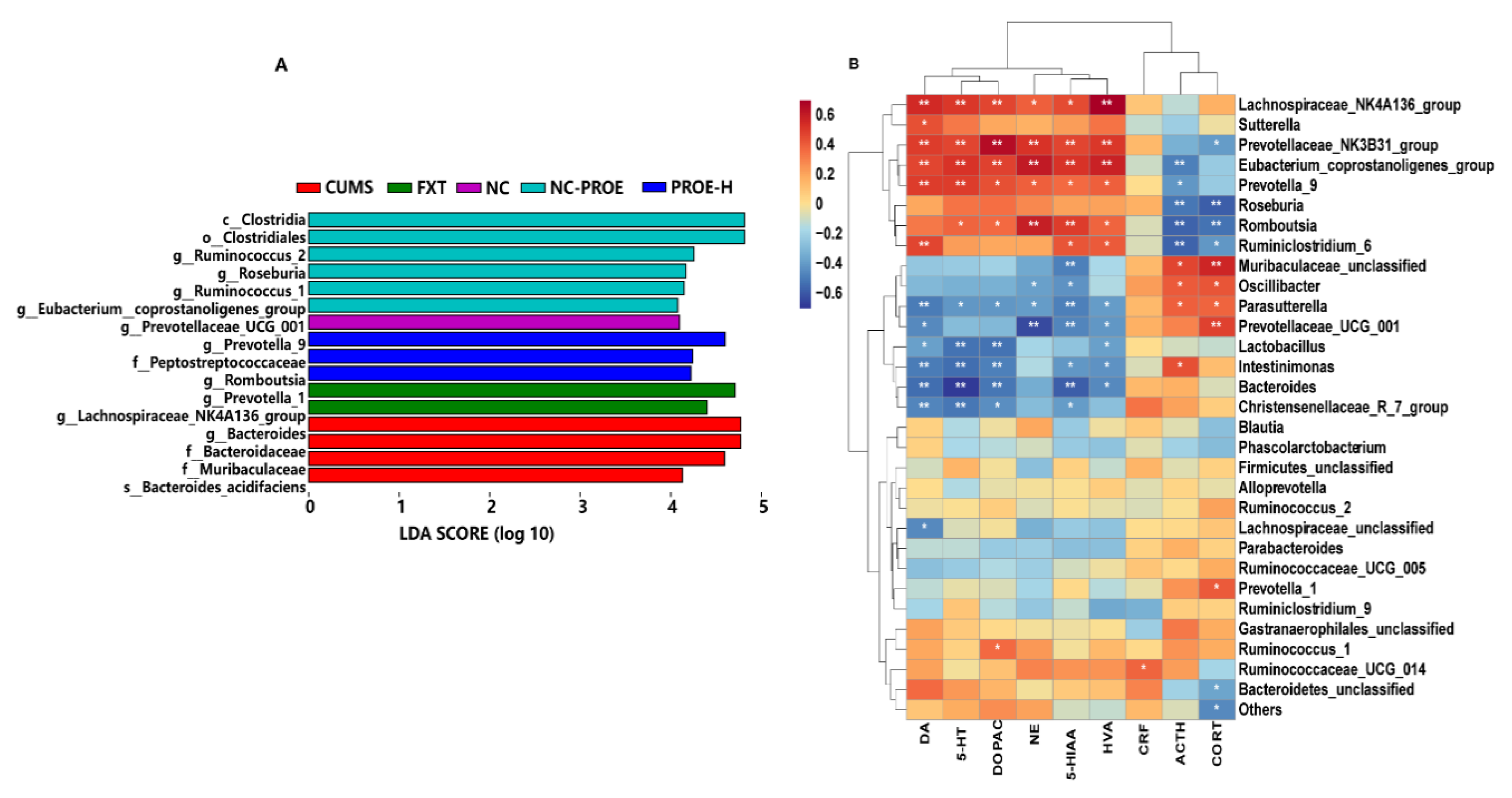
2.5.3. Taxonomic Biomarkers in Rat Gut Microbiota
2.5.4. Prediction of Metagenomic Functions
2.6. Correlation Analysis among Brain Neurotransmitters, HPA Axis Hormone, and Relative Abundances of Gut Microbiota
2.7. The Effect of PROEs on Plasma Tryptophan and Kynurenine Levels
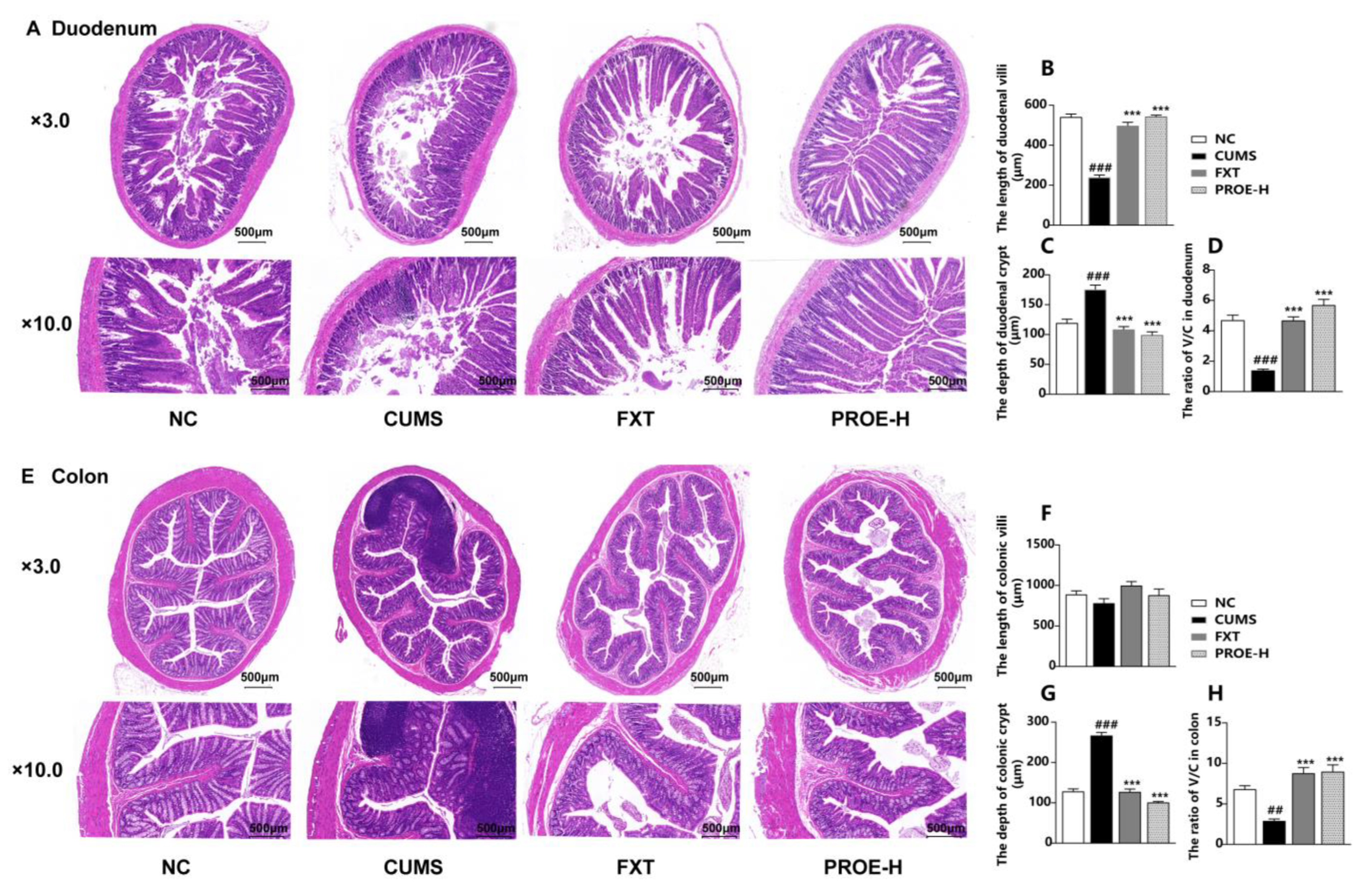
2.8. The Effects of PROEs on the Morphological Structure of the Intestinal Mucosa
2.8.1. The Effects of PROEs on Duodenum and Colon Histopathological Changes
2.8.2. Effects of PROEs on Duodenum and Colon Epithelium Ultrastructure
2.8.3. Effects of PROEs on the Protein Expression of Occludin in the Colon
2.9. The Effect of PROEs on Serum IL-6 and LPS Levels
2.10. The Effect of PROEs on the Expression of 5-HT1A, 5-HT2A, IDO1, and TNF-α mRNA
2.11. The Effect of PROEs on the Concentrations of SCFAs in Feces
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. PROEs Preparation
4.3. PROEs Ingredient Identification
4.4. Animals and Treatments
4.5. Behavioral Tests
4.6. ELISA Measurement
4.7. Detection of Monoamine Neurotransmitters and Their Metabolites
4.8. Detection of Tryptophan and Kynurenine in Plasma
4.9. Determination of Fecal Short-Chain Fatty Acids
4.10. Analysis of Intestinal Morphology
4.11. Western Blot Analysis
4.12. Quantitative Reverse-Transcription Polymerase Chain Reaction
4.13. DNA Extraction and 16S rRNA Gene Sequencing of Fecal Samples
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jie, W.; Liu, J.H.; Yang, J.M.; Gao, T.M. An astroglial basis of major depressive disorder? An overview. Glia 2017, 65, 1227–1250. [Google Scholar] [CrossRef]
- Osadchiy, V.; Martin, C.R.; Mayer, E.A. The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin. Gastroenterol. Hepatol. 2019, 17, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D. Human gut microbiome: Hopes, threats and promises. Gut 2018, 67, 1716–1725. [Google Scholar] [CrossRef]
- Bercik, P.; Verdu, E.F.; Foster, J.A.; Macri, J.; Potter, M.; Huang, X.; Malinowski, P.; Jackson, W.; Blennerhassett, P.; Neufeld, K.A.; et al. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 2010, 139, 2102–2112.e1. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Zheng, P.; Zeng, B.; Zhou, C.; Liu, M.; Fang, Z.; Xu, X.; Zeng, L.; Chen, J.; Fan, S.; Du, X.; et al. Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol. Psychiatry 2016, 21, 786–796. [Google Scholar] [CrossRef]
- Liu, P.; Hu, Y.; Guo, D.H.; Wang, D.X.; Tu, H.H.; Ma, L.; Xie, T.T.; Kong, L.Y. Potential antidepressant properties of Radix Polygalae (Yuan Zhi). Phytomedicine 2010, 17, 794–799. [Google Scholar] [CrossRef]
- Qu, S.; Liu, M.; Cao, C.; Wei, C.; Meng, X.E.; Lou, Q.; Wang, B.; Li, X.; She, Y.; Wang, Q.; et al. Chinese Medicine Formula Kai-Xin-San Ameliorates Neuronal Inflammation of CUMS-Induced Depression-like Mice and Reduces the Expressions of Inflammatory Factors via Inhibiting TLR4/IKK/NF-κB Pathways on BV2 Cells. Front. Pharmacol. 2021, 12, 626949. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, X.; Jiang, X.W.; Yao, D.; Zhou, L.J.; Xu, Z.H.; Wang, N.; Zhao, Q.C.; Zhang, Z. Yuan-Zhi decoction in the treatment of Alzheimer’s disease: An integrated approach based on chemical profiling, network pharmacology, molecular docking and experimental evaluation. Front. Pharmacol. 2022, 13, 893244. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, L.; Li, Y.; Zhu, Q.; Liu, D.; Xue, W.; Cao, B.; Liu, Y.; Wang, X. Clinical treatment of depressive patients with anshendingzhi decoction. J. Tradit. Chin. Med. 2014, 34, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Cui, Y.; Wu, P.; Zhao, P.; Zhou, Q.; Zhang, Z.; Wang, Y.; Zhang, X. Polygalae Radix: A review of its traditional uses, phytochemistry, pharmacology, toxicology, and pharmacokinetics. Fitoterapia 2020, 147, 104759. [Google Scholar] [CrossRef]
- Liu, X.; Wang, D.; Zhao, R.; Dong, X.; Hu, Y.; Liu, P. Synergistic Neuroprotective Effects of Two Herbal Ingredients via CREB-Dependent Pathway. Front. Pharmacol. 2016, 7, 337. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, M.; Liu, P.; Guo, D.H.; Wei, R.B.; Rahman, K. Possible mechanism of the antidepressant effect of 3,6′-disinapoyl sucrose from Polygala tenuifolia Willd. J. Pharm. Pharmacol. 2011, 63, 869–874. [Google Scholar] [CrossRef]
- Jin, Z.L.; Gao, N.; Zhang, J.R.; Li, X.R.; Chen, H.X.; Xiong, J.; Li, Y.F.; Tang, Y. The discovery of Yuanzhi-1, a triterpenoid saponin derived from the traditional Chinese medicine, has antidepressant-like activity. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 53, 9–14. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, X.; Pan, R.; Zhu, X.; Steinmetz, A.; Liao, Y.; Wang, N.; Peng, B.; Chang, Q. Intestinal transport of 3,6′-disinapoylsucrose, a major active component of Polygala tenuifolia, using Caco-2 cell monolayer and in situ rat intestinal perfusion models. Planta Med. 2013, 79, 1434–1439. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, P.; Guo, D.H.; Rahman, K.; Wang, D.X.; Xie, T.T. Antidepressant effects of the extract YZ-50 from Polygala tenuifolia in chronic mild stress treated rats and its possible mechanisms. Pharm. Biol. 2010, 48, 794–800. [Google Scholar] [CrossRef]
- Ling, Y.; Li, Z.; Chen, M.; Sun, Z.; Fan, M.; Huang, C. Analysis and detection of the chemical constituents of Radix Polygalae and their metabolites in rats after oral administration by ultra high-performance liquid chromatography coupled with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. J. Pharm. Biomed. Anal. 2013, 85, 1–13. [Google Scholar]
- Feng, G.F.; Liu, S.; Pi, Z.F.; Song, F.R.; Liu, Z.Q. Comprehensive characterization of in vivo metabolic profile of Polygalae radix based on ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2019, 165, 173–181. [Google Scholar] [CrossRef]
- Feng, G.F.; Liu, S.; Pi, Z.F.; Song, F.R.; Liu, Z.Q. Studies on the chemical and intestinal metabolic profiles of Polygalae Radix by using UHPLC-IT-MSn and UHPLC-Q-TOF-MS method coupled with intestinal bacteria incubation model in vitro. J. Pharm. Biomed. Anal. 2018, 148, 298–306. [Google Scholar] [CrossRef]
- Hill, M.N.; Hellemans, K.G.; Verma, P.; Gorzalka, B.B.; Weinberg, J. Neurobiology of chronic mild stress: Parallels to major depression. Neurosci. Biobehav. Rev. 2012, 36, 2085–2117. [Google Scholar] [CrossRef]
- Guo, Y.; Xie, J.P.; Deng, K.; Li, X.; Yuan, Y.; Xuan, Q.; Xie, J.; He, X.M.; Wang, Q.; Li, J.J.; et al. Prophylactic Effects of Bifidobacterium adolescentis on Anxiety and Depression-like Phenotypes after Chronic Stress: A Role of the Gut Microbiota-Inflammation Axis. Front. Behav. Neurosci. 2019, 13, 126. [Google Scholar] [CrossRef]
- Huang, N.; Hua, D.; Zhan, G.; Li, S.; Zhu, B.; Jiang, R.; Yang, L.; Bi, J.; Xu, H.; Hashimoto, K.; et al. Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression. Pharmacol. Biochem. Behav. 2019, 176, 93–100. [Google Scholar] [CrossRef]
- Zhu, H.Z.; Liang, Y.D.; Ma, Q.Y.; Hao, W.Z.; Li, X.J.; Wu, M.S.; Deng, L.J.; Li, Y.M.; Chen, J.X. Xiaoyaosan improves depressive-like behavior in rats with chronic immobilization stress through modulation of the gut microbiota. Biomed. Pharmacother. 2019, 112, 108621. [Google Scholar] [CrossRef]
- Liu, P.H.; Zhang, K.R. Progress in research on the pathogenesis of gut microbiota alternation-induced depression. Chin. J. Microecol. 2019, 31, 475–479. [Google Scholar]
- Bai, Y.; Hu, Y.X.; Chen, J.W. Establishment of the theoretical system of bacteria brain gut axis. J. Southeast. Univ. (Med. Sci. Ed.) 2016, 35, 781–785. [Google Scholar]
- Mir, H.; Meena, A.S.; Chaudhry, K.K.; Shukla, P.K.; Gangwar, R.; Manda, B.; Padala, M.K.; Shen, L.; Turner, J.R.; Dietrich, P.; et al. Occludin deficiency promotes ethanol-induced disruption of colonic epithelial junctions, gut barrier dysfunction and liver damage in mice. Biochim. Biophys. Acta 2016, 1860, 765–774. [Google Scholar] [CrossRef]
- Ichikawa, H.; Shineha, R.; Satomi, S.; Sakata, T. Gastric or rectal instillation of short-chain fatty acids stimulates epithelial cell proliferation of small and large intestine in rats. Dig. Dis. Sci. 2002, 47, 1141–1146. [Google Scholar] [CrossRef]
- Lewis, K.; Lutgendorff, F.; Phan, V.; Söderholm, J.D.; Sherman, P.M.; McKay, D.M. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm. Bowel Dis. 2010, 16, 1138–1148. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, D.; Qiao, S.; Wu, X.; Cao, S.; Wang, L.; Su, X.; Li, L. Metabolic and microbial signatures in rat hepatocellular carcinoma treated with caffeic acid and chlorogenic acid. Sci. Rep. 2017, 7, 4508. [Google Scholar] [CrossRef]
- Tao, S.; Li, L.; Li, L.; Liu, Y.; Ren, Q.; Shi, M.; Liu, J.; Jiang, J.; Ma, H.; Huang, Z.; et al. Understanding the gut-kidney axis among biopsy-proven diabetic nephropathy, type 2 diabetes mellitus and healthy controls: An analysis of the gut microbiota composition. Acta Diabetol. 2019, 56, 581–592. [Google Scholar] [CrossRef]
- Kovatcheva-Datchary, P.; Nilsson, A.; Akrami, R.; Lee, Y.S.; De Vadder, F.; Arora, T.; Hallen, A.; Martens, E.; Björck, I.; Bäckhed, F. Dietary Fiber-Induced Improvement in Glucose Metabolism Is Associated with Increased Abundance of Prevotella. Cell. Metab. 2015, 22, 971–982. [Google Scholar] [CrossRef]
- Li, J.W.; Fang, B.; Pang, G.F.; Zhang, M.; Ren, F.Z. Age- and diet-specific effects of chronic exposure to chlorpyrifos on hormones, inflammation and gut microbiota in rats. Pestic. Biochem. Physiol. 2019, 159, 68–79. [Google Scholar] [CrossRef]
- Zhuge, A.; Li, B.; Yuan, Y.; Lv, L.; Li, Y.; Wu, J.; Yang, L.; Bian, X.; Wang, K.; Wang, Q.; et al. Lactobacillus salivarius LI01 encapsulated in alginate-pectin microgels ameliorates D-galactosamine-induced acute liver injury in rats. Appl. Microbiol. Biotechnol. 2020, 104, 7437–7455. [Google Scholar] [CrossRef]
- Peng, C.; Xu, X.; Li, Y.; Li, X.; Yang, X.; Chen, H.; Zhu, Y.; Lu, N.; He, C. Sex-specific association between the gut microbiome and high-fat diet-induced metabolic disorders in mice. Biol. Sex. Differ. 2020, 11, 5. [Google Scholar] [CrossRef]
- Stilling, R.M.; van de Wouw, M.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem. Int. 2016, 99, 110–132. [Google Scholar] [CrossRef]
- Xia, T.; Zhang, B.; Li, Y.; Fang, B.; Zhu, X.; Xu, B.; Zhang, J.; Wang, M.; Fang, J. New insight into 20(S)-ginsenoside Rh2 against T-cell acute lymphoblastic leukemia associated with the gut microbiota and the immune system. Eur. J. Med. Chem. 2020, 203, 112582. [Google Scholar] [CrossRef]
- Cheng, D.; Chang, H.; Ma, S.; Guo, J.; She, G.; Zhang, F.; Li, L.; Li, X.; Lu, Y. Tiansi Liquid Modulates Gut Microbiota Composition and Tryptophan⁻Kynurenine Metabolism in Rats with Hydrocortisone-Induced Depression. Molecules 2018, 23, 2832. [Google Scholar] [CrossRef]
- Ye, J.Z.; Li, Y.T.; Wu, W.R.; Shi, D.; Fang, D.Q.; Yang, L.Y.; Bian, X.Y.; Wu, J.J.; Wang, Q.; Jiang, X.W.; et al. Dynamic alterations in the gut microbiota and metabolome during the development of methionine-choline-deficient diet-induced nonalcoholic steatohepatitis. World J. Gastroenterol. 2018, 24, 2468–2481. [Google Scholar] [CrossRef]
- Barandouzi, Z.A.; Starkweather, A.R.; Henderson, W.A.; Gyamfi, A.; Cong, X.S. Altered Composition of Gut Microbiota in Depression: A Systematic Review. Front. Psychiatry 2020, 11, 541. [Google Scholar] [CrossRef] [PubMed]
- Rong, H.; Xie, X.H.; Zhao, J.; Lai, W.T.; Wang, M.B.; Xu, D.; Liu, Y.H.; Guo, Y.Y.; Xu, S.X.; Deng, W.F.; et al. Similarly in depression, nuances of gut microbiota: Evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. J. Psychiatr. Res. 2019, 113, 90–99. [Google Scholar] [CrossRef]
- Lukiw, W.J. The microbiome, microbial-generated proinflammatory neurotoxins, and Alzheimer’s disease. J. Sport Health Sci. 2016, 5, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Sudo, N.; Chida, Y.; Aiba, Y.; Sonoda, J.; Oyama, N.; Yu, X.N.; Kubo, C.; Koga, Y. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J. Physiol. 2004, 558, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yan, M.; Pan, R.; Wang, Z.; Tao, X.; Li, C.; Xia, T.; Liu, X.; Chang, Q. Radix Polygalae extract exerts antidepressant effects in behavioral despair mice and chronic restraint stress-induced rats probably by promoting autophagy and inhibiting neuroinflammation. J. Ethnopharmacol. 2021, 265, 113317. [Google Scholar] [CrossRef]
- Begley, T.P.; Kinsland, C.; Mehl, R.A.; Osterman, A.; Dorrestein, P. The biosynthesis of nicotinamide adenine dinucleotides in bacteria. Vitam. Horm. 2001, 61, 103–119. [Google Scholar]
- Kuc, D.; Zgrajka, W.; Parada-Turska, J.; Urbanik-Sypniewska, T.; Turski, W.A. Micromolar concentration of kynurenic acid in rat small intestine. Amino Acids 2008, 35, 503–505. [Google Scholar] [CrossRef]
- Siopi, E.; Chevalier, G.; Katsimpardi, L.; Saha, S.; Bigot, M.; Moigneu, C.; Eberl, G.; Lledo, P.M. Changes in Gut Microbiota by Chronic Stress Impair the Efficacy of Fluoxetine. Cell Rep. 2020, 30, 3682–3690.e6. [Google Scholar] [CrossRef]
- Oxenkrug, G.F. Metabolic syndrome, age-associated neuroendocrine disorders, and dysregulation of tryptophan-kynurenine metabolism. Ann. N. Y. Acad. Sci. 2010, 1199, 1–14. [Google Scholar] [CrossRef]
- Pertovaara, M.; Raitala, A.; Uusitalo, H.; Pukander, J.; Helin, H.; Oja, S.S.; Hurme, M. Mechanisms dependent on tryptophan catabolism regulate immune responses in primary Sjögren’s syndrome. Clin. Exp. Immunol. 2005, 142, 155–161. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Carvalho, A.; Caso, J.R.; Sanz, Y.; Walder, K.; Maes, M. The Role of the Microbial Metabolites Including Tryptophan Catabolites and Short Chain Fatty Acids in the Pathophysiology of Immune-Inflammatory and Neuroimmune Disease. Mol. Neurobiol. 2017, 54, 4432–4451. [Google Scholar] [CrossRef]
- Martin-Gallausiaux, C.; Larraufie, P.; Jarry, A.; Béguet-Crespel, F.; Marinelli, L.; Ledue, F.; Reimann, F.; Blottière, H.M.; Lapaque, N. Butyrate Produced by Commensal Bacteria Down-Regulates Indolamine 2,3-Dioxygenase 1 (IDO-1) Expression via a Dual Mechanism in Human Intestinal Epithelial Cells. Front. Immunol. 2018, 9, 2838. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Gui, X.; Shi, X.; Bao, Z.; Han, H.; Li, M.D. Updated review of research on the gut microbiota and their relation to depression in animals and human beings. Mol. Psychiatry 2020, 25, 2759–2772. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.S.; Kim, T.W.; Lee, J.M.; Sung, Y.H.; Lim, B.V. Treadmill exercise alleviates depressive symptoms in rotenone-induced Parkinson disease rats. J. Exerc. Rehabil. 2017, 13, 124–129. [Google Scholar] [CrossRef]
- Jiang, T.; Ge, H.; Sun, J.; Li, R.; Han, R.; Liu, J. Relationship between Occupational Stress, 5-HT2A Receptor Polymorphisms and Mental Health in Petroleum Workers in the Xinjiang Arid Desert: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2017, 14, 402. [Google Scholar] [CrossRef] [PubMed]
- Lacivita, E.; Leopoldo, M.; Berardi, F.; Perrone, R. 5-HT1A receptor, an old target for new therapeutic agents. Curr. Top. Med. Chem. 2008, 8, 1024–1034. [Google Scholar] [CrossRef]
- Homberg, J.R. Serotonergic modulation of conditioned fear. Scientifica 2012, 2012, 821549. [Google Scholar] [CrossRef] [PubMed]
- Costall, B.; Naylor, R.J. Behavioural interactions between 5-hydroxytryptophan, neuroleptic agents and 5-HT receptor antagonists in modifying rodent responding to aversive situations. Br. J. Pharmacol. 1995, 116, 2989–2999. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.G.; Bartoszyk, G.D.; Edwards, E.; Ashby, C.R., Jr. The highly selective 5-hydroxytryptamine (5-HT)2A receptor antagonist, EMD 281014, significantly increases swimming and decreases immobility in male congenital learned helpless rats in the forced swim test. Synapse 2004, 52, 73–75. [Google Scholar] [CrossRef]
- Fung, T.C.; Olson, C.A.; Hsiao, E.Y. Interactions between the microbiota, immune and nervous systems in health and disease. Nat. Neurosci. 2017, 20, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Martin-Subero, M.; Anderson, G.; Kanchanatawan, B.; Berk, M.; Maes, M. Comorbidity between depression and inflammatory bowel disease explained by immune-inflammatory, oxidative, and nitrosative stress; tryptophan catabolite; and gut-brain pathways. CNS Spectr. 2016, 21, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhou, N.; Ma, W.; Gu, X.; Chen, B.; Zeng, Y.; Yang, L.; Zhou, M. Modulation of gut microbiota by chlorogenic acid pretreatment on rats with adrenocorticotropic hormone induced depression-like behavior. Food Funct. 2019, 10, 2947–2957. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.Y.; Duan, C.L.; Fan, C.X.; Liu, Q.; Zhang, T.; Yang, H. A Method for Simultaneous Detection of 8 Kinds of Monoamine Neurotransmitter in Biological Samples Using High Perfomance Liquid Chromatography with Electrochemical Detection. Chin. J. Anal. Chem. 2009, 37, 330–334. [Google Scholar]





| Groups | NE (ng/g) | 5-HT (ng/g) | DA (ng/g) | HVA (ng/g) | DOPAC (ng/g) | 5-HIAA (ng/g) |
|---|---|---|---|---|---|---|
| NC | 151.22 ± 17.07 | 110.66 ± 11.74 | 103.56 ± 11.99 | 101.95 ± 9.99 | 229.90 ± 17.71 | 215.39 ± 7.42 |
| CUMS | 58.02 ± 3.19 ### | 18.52 ± 2.47 ### | 14.97 ± 3.54 ## | 15.91 ± 2.20 ### | 52.85 ± 5.22 ## | 113.96 ± 3.34 ## |
| FXT | 180.78 ± 11.40 *** | 109.55 ± 17.42 *** | 97.13 ± 12.06 ** | 102.83 ± 7.07 *** | 212.69 ± 15.70 ** | 206.62 ± 12.60 ** |
| PROE-H | 208.76 ± 13.46 *** | 80.76 ± 14.46 ** | 98.03 ± 25.22 ** | 82.89 ± 21.88 ** | 215.91 ± 50.60 ** | 199.57 ± 17.74 * |
| PROE-M | 122.08 ± 17.13 * | 66.81 ± 6.30 * | 74.18 ± 13.81 | 87.93 ± 9.51 ** | 206.01 ± 27.01 * | 198.18 ± 14.91 * |
| PROE-L | 168.83 ± 8.92 *** | 81.51 ± 3.26 ** | 38.45 ± 16.21 | 62.67 ± 6.39 | 180.39 ± 35.47 * | 195.92 ± 30.79 * |
| Gene | Primer | Primer Sequence (5′ to 3′) | Product Size (bp) |
|---|---|---|---|
| GAPDH | Forward primer | 5′CTGGAGAAACCTGCCAAGTATG3′ | 138 |
| Reverse primer | 5′GGTGGAAGAATGGGAGTTGCT3′ | ||
| TNF-α | Forward primer | 5′CCAGGTTCTCTTCAAGGGACAA3′ | 80 |
| Reverse primer | 5′GGTATGAAATGGCAAATCGGCT3′ | ||
| 5-HT1A | Forward primer | 5′ACTTGGCTCATTGGCTTTCTCA3′ | 119 |
| Reverse primer | 5′GAGTAGATGGTGTAGCCGTGGTC3′ | ||
| 5-HT2A | Forward primer | 5′TATGCTGCTGGGTTTCCTTGTC3′ | 201 |
| Reverse primer | 5′TTGAAGCGGCTGTGGTGAAT3′ | ||
| IDO1 | Forward primer | 5′GATGAAGATGTGGGCTTTGCT3′ | 285 |
| Reverse primer | 5′GCAGTAGGGAACGGCAAGA3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Jia, T.; Wu, X.; Chen, X.; Wang, J.; Ba, Y. Polygalae Radix Oligosaccharide Esters May Relieve Depressive-like Behavior in Rats with Chronic Unpredictable Mild Stress via Modulation of Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 13877. https://doi.org/10.3390/ijms241813877
Chen Q, Jia T, Wu X, Chen X, Wang J, Ba Y. Polygalae Radix Oligosaccharide Esters May Relieve Depressive-like Behavior in Rats with Chronic Unpredictable Mild Stress via Modulation of Gut Microbiota. International Journal of Molecular Sciences. 2023; 24(18):13877. https://doi.org/10.3390/ijms241813877
Chicago/Turabian StyleChen, Qijun, Tanrong Jia, Xia Wu, Xiaoqing Chen, Jiajia Wang, and Yinying Ba. 2023. "Polygalae Radix Oligosaccharide Esters May Relieve Depressive-like Behavior in Rats with Chronic Unpredictable Mild Stress via Modulation of Gut Microbiota" International Journal of Molecular Sciences 24, no. 18: 13877. https://doi.org/10.3390/ijms241813877
APA StyleChen, Q., Jia, T., Wu, X., Chen, X., Wang, J., & Ba, Y. (2023). Polygalae Radix Oligosaccharide Esters May Relieve Depressive-like Behavior in Rats with Chronic Unpredictable Mild Stress via Modulation of Gut Microbiota. International Journal of Molecular Sciences, 24(18), 13877. https://doi.org/10.3390/ijms241813877






