Drought Stress Tolerance in Vegetables: The Functional Role of Structural Features, Key Gene Pathways, and Exogenous Hormones
Abstract
:1. Introduction
| Vegetable Crop | Critical Stage of Water Requirement | Effect of Drought | Reference |
|---|---|---|---|
| Leafy vegetables | During the process of plant growth and development. | Leaf toughness, inadequate foliage development, and nitrate accumulation. | [9] |
| Potato | The process of tuber formation and the growth of tubers. | Inadequate tuber development and low yield, along with tuber splitting. | [11,12] |
| Pea | The process of flower formation and the filling of pods. | Decreased root nodulation and stunted plant growth, along with inadequate grain filling. | [13] |
| Lettuce | Consistently throughout the entire developmental process. | Leaf toughness, inadequate growth of plants, and tip burn. | [14] |
| Melons | The process of flowering and uniform fruit development throughout. | Muskmelons exhibit diminished fruit quality due to reduced total soluble solids (TSS), decreased sugar and ascorbic acid levels, and increased nitrate content in watermelon fruits. | [15] |
| Okra | Flowering and pod development. | Intensive decrease in the yield, fiber development, and potential infestation by mites. | [16] |
| Onion | Bulb enlargement and bulb formation. | Splitting and doubling of the bulb decrease the shelf life. | [17] |
| Cucumber | Across the flowering period and development of fruits. | Deformed and less vigorous pollens, bitterness in taste, and abnormal fruit shape and size. | [9] |
| Turnip, carrot, and radish | Development of roots. | Poor and distorted growth of roots, the production of harmful nitrates, and ultimately pungent odor of carrots. | [9] |
| Cabbage and cauliflower | Formation and enlargement of the head. | Tip burning of stiff leaves; browning and buttoning in cauliflower curd. | [15] |
| Eggplant | Flower development and fruit setting. | Poor development of fruit color with reduced yield. | |
| Chili and Capsicum | Development of fruits and fruit setting. | Shedding of juvenile flowers and fruits and reduced dry matter production and nutrient uptake. | [15] |
| Tomato | Period of flowering and fruits’ rapid enlargement. | Flower shedding hindered fertilization and decreased the size of fruits, and splitting disorders were attributed to calcium deficiency. | [18] |
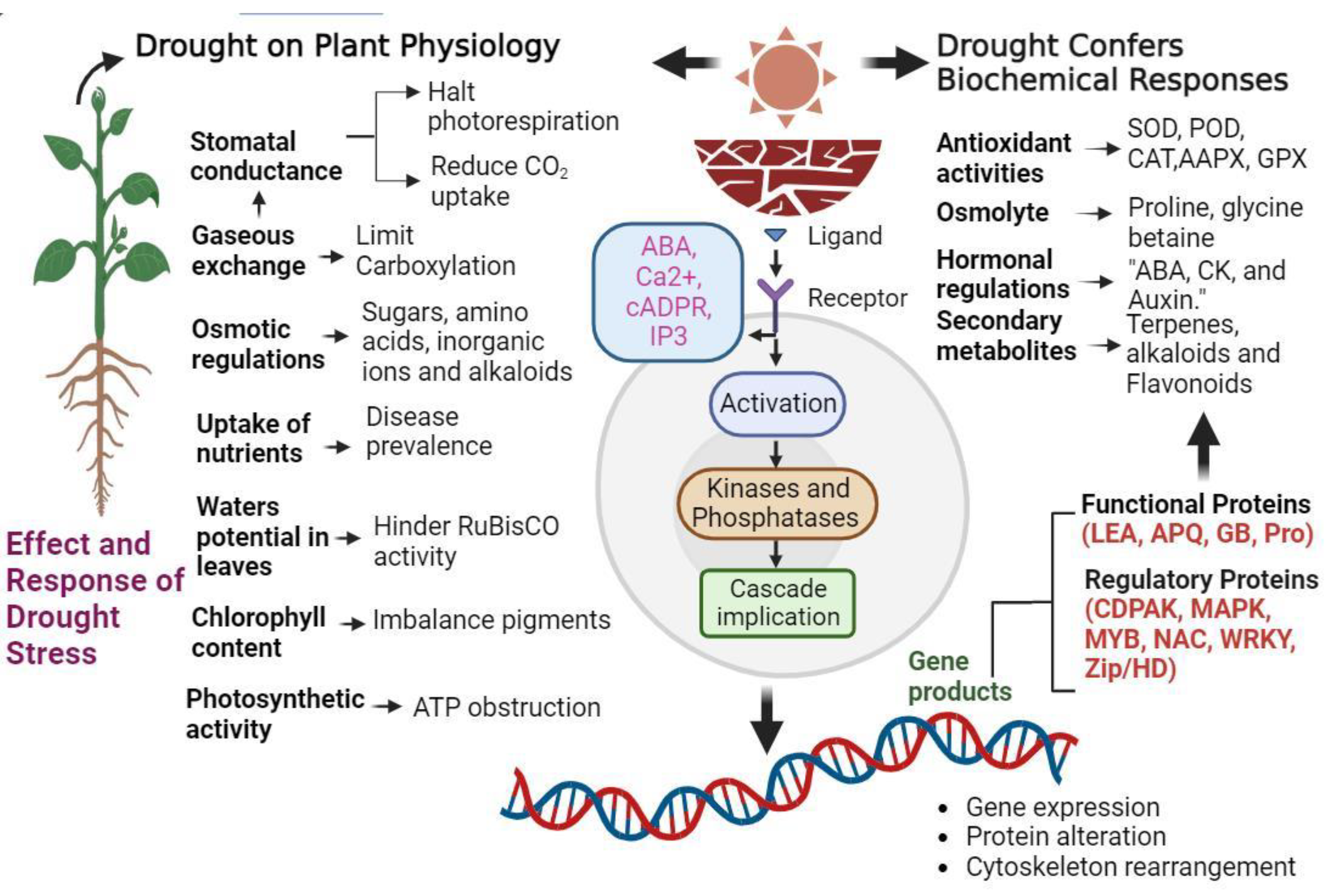
2. Drought Stress Impact on Morphological Traits in Relation to Tolerance in Vegetables
2.1. Drought Stress Impact on External Features in Relation to Tolerance
2.2. Drought Stress Impact on Internal Features in Relation to Tolerance
3. Drought Stress Impact on Physiological and ROS Metabolism in Relation to Tolerance in Vegetables
3.1. Drought Stress Impact on Physiological Response in Relation to Tolerance
| Vegetable Crops and Cultivation Condition | Drought Stress Treatment | Impact on Crop and Drought Stress Tolerance | References |
|---|---|---|---|
| Potato (Solanum tuberosum L. cultivars) in greenhouse | Irrigation interruption for 12–13 days before tuber formation. | Decrease in: relative water content (RWC); leaf osmotic potential. Elevation of: nitrogen (N) levels and augmented levels of proteins; proline within the leaves. | [69] |
| Lettuce (Lactuca sativa L.) Veneranda cultivar in greenhouse | Watering at 90% and 80% field capacity, followed by a 4-day irrigation pause before harvest (inducing acute stress). | Increase in: carotenoids; biomass; chlorophyll content; flavonoids; phenolic acids. | [14] |
| Lettuce (Lactuca sativa L.) butterhead (Aquino) and red butterhead (Barlach) cultivar in greenhouse | Soil water contents of 70% and 40% | Reduction in PSII efficiency; elevated biomass. | [70] |
| Eggplant (Solanum melongena L.) field | Seven regimes of irrigation. | Reduction in: fruit weight and firmness; total sugars; proteins. Increase in: CAT and APX activity; total phenols; flavonoids. | [71] |
| Amaranth (Amaranth tricolor; Amaranth cruentus) in greenhouse | Suspension of watering for 14 days. | Reduction in: plant height, leaves, roots, stem fresh and dry weight; leaf area; chlorophyll content. Increase in transpiration efficiency. | [72] |
| Wild asparagus (Asparagus acutifolius L.) in greenhouse | Leaf water potential of −1.4 MPa and −2.4 MPa over 6 days. | Decrease in net photosynthesis. | [65] |
| Common chicory (Cichorium intybus L.) in greenhouse | 80%, 60%, and 40% of field capacity. | Increase in: SOD and CAT activity; proline and ascorbic acid content; abscisic content in leaves. | [73] |
| Cassava (Manihot esculenta Crantz), cv. SC205, GR4, RS0I, and SC124 in glasshouse | 50% and 20% of field capacity. | Reduction in: chlorophyll content and RWC and plant height. Increase in: H2O2; malondialdehyde (MDA), ascorbic acid; glutathione; SOD and CAT activity; total phenols. Overexpression of Mn-SOD, CAT, and GR genes. | [27] |
| Cabbage (Brassica oleracea var. capital) in greenhouse | 80% and 60% of the field capacity. | Increase in: H2O2, lipid peroxidation, electrolyte leakage, proline content, and sucrose. Reduction in: biometric parameters (plant height, stem diameter, number of leaves, leaf area, fresh and dry shoot weights); photosynthesis; stomatal conductance and transpiration and chlorophyll content. | [25] |
| Tomato (Solanum lycopersicum L., cv. landrace Cietttaicale and Moneymaker) in growth chamber | Treatment irrigation with 50% of the field capacity every 48 days for twenty days. | Reduction in: osmotic potential, stomatal conductance, photochemical efficiency of PSII, leaf starch. Increase in: non-photochemical fluorescence quenching; ABA and IAA contents in leaves and roots; soluble sugars; lipid peroxidation; proline and antioxidant activity in roots. | [28] |
| Pepper (Capsicum annum cultivars (Nongchengjiao-2 and Shansshu-2001)) in greenhouse | Grown under four water regimes: 80, 60, 40, and 20 of field capacity for 6, 12, 18, and 24 days. | Reduction in RWC; increased proline content, total soluble proteins, and SOD, POD, and CAT activity at the onset of stress; decreased leaf area and fruit yield. | [74] |
| Sage (Salvia officinalis) in field | Stop irrigation for six weeks. | Hampers stomatal closure; reduction in CO2 assimilation; increase in NADPH. | [75,76,77] |
| Pepper (Capsicum chinense) (cultivars. Rex and Genesis), Capsicum annum cv. Padron)) in greenhouse | Restriction of water during the flowering stage for 7, 10, 14, 18, and 21 days. | Noticeable decrease in RWC, along with an increase in electrolyte leakage and proline content. | [57] |
| Soya bean (Glycine max L.) in field | Treatments applied to control drought at different reproductive phases. | Drought reduces the seed germination. | [78] |
| Okra (Abelmoschus esculentus L. Moench) in field experiment | Exposed to water deficit under various waters regimes for 5 or 10 days. | Waters restrictions exceeding ten days during the reproductive period result in diverse growth and yield effects. | [79] |
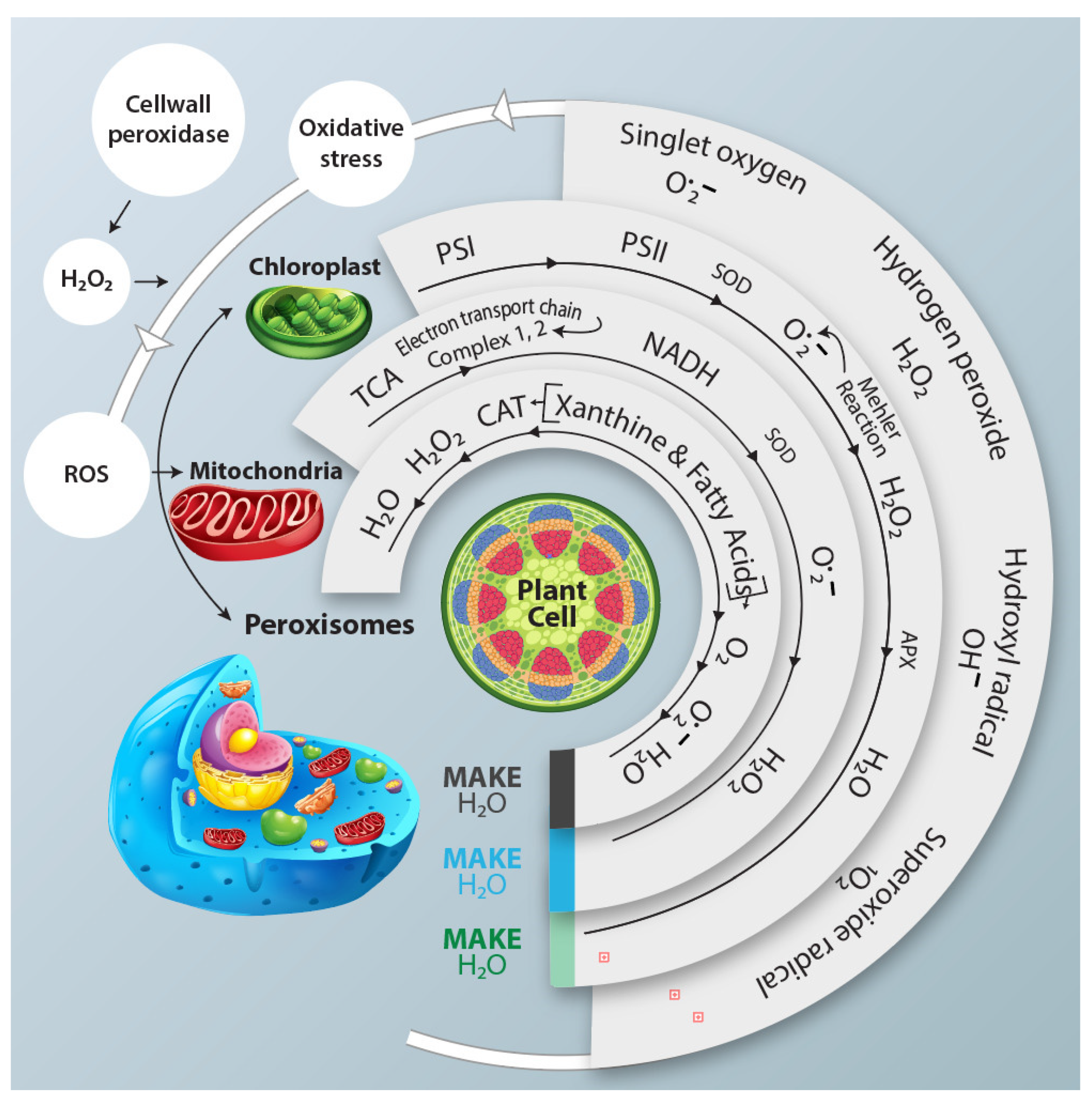
3.2. Drought Stress Impact on ROS Metabolism in Relation to Tolerance
4. Signaling Transmission and Transduction in Vegetable Plants under Drought Stress
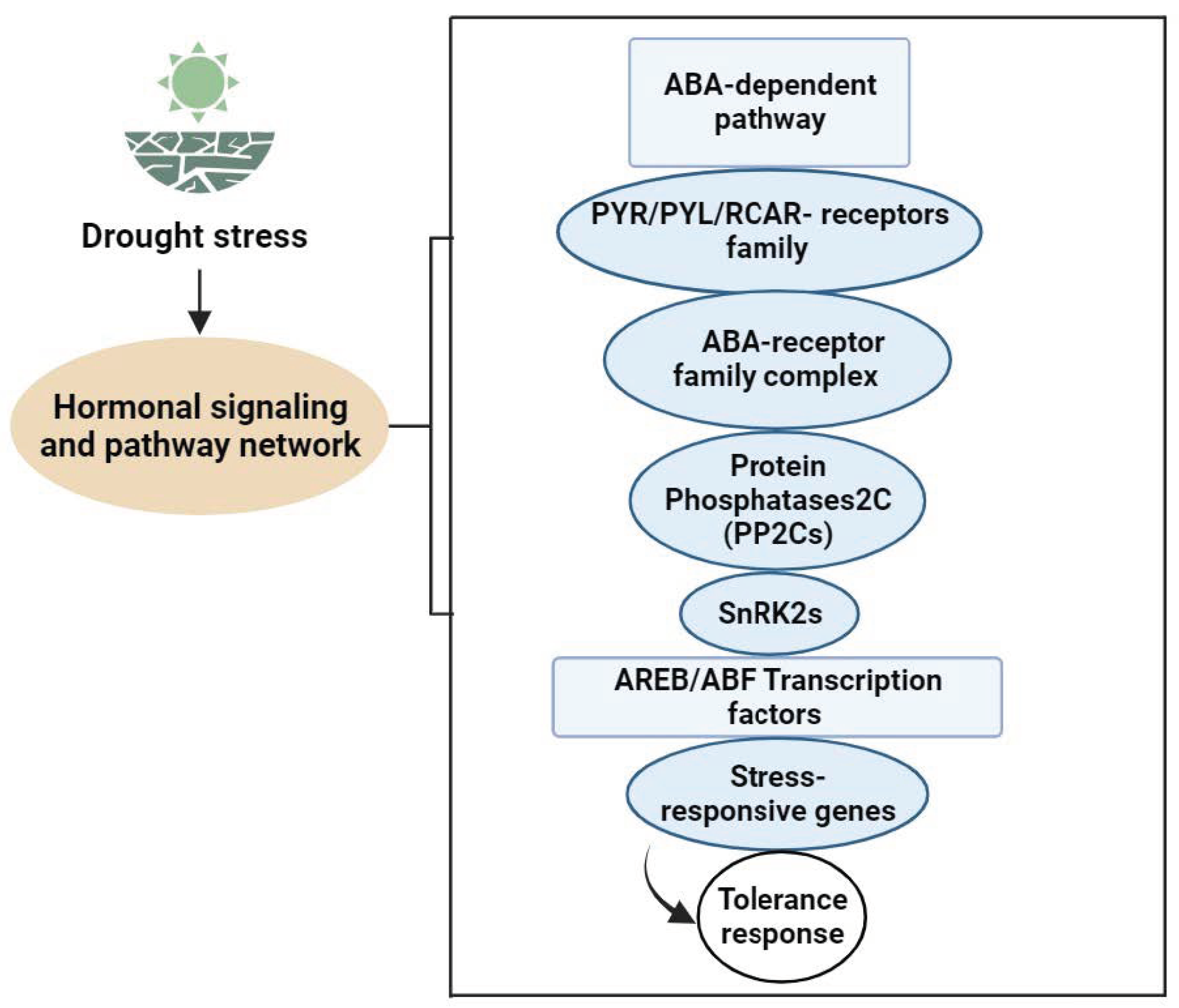
4.1. Signal Transmission in Vegetable Plants under Drought Stress
4.2. Signal Regulation Pathways in Vegetable Plants under Drought Stress
5. Drought Stress Tolerance-Related Functional and Regulatory Genes in Vegetables
5.1. Drought Stress Functional Genes
5.2. Drought Stress Regulatory Genes
6. Exogenous Hormonal Regulation in Enhancing Vegetable Drought Stress Tolerance
6.1. Exogenous ABA and JA in Vegetable Drought Tolerance
6.2. Exogenous SA and ET in Vegetable Drought Stress Tolerance
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ROS | Reactive oxygen species |
| ABA | Abscisic acid |
| JA | Jasmonic acid |
| RWC | Relative water content |
| OA | Osmotic adjustment |
| WUE | Water-use efficiency |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| GR | Glutathione reductase |
| APX | Ascorbate peroxidase |
| CAT | Catalase |
| IP3 | Inositol trisphosphate |
| MDA | Malondialdehyde |
| Ca2+ | Calcium ion |
| cADPR | Cyclic adenosine diphosphate ribose |
| CaM | Calmodulin |
| CBLs | Calcineurin B-like proteins |
| MAPK | Mitogen-activated protein kinase |
| P5CS | △-pyrroline-5-carboxylate synthetase |
| P5CR | Pyrroline-5-carboxylate reductase |
| ProDH | Proline dehydrogenase |
| Glu | Glutamic acid |
| Orn | Ornithine |
| P-MME | Phosphate-monomethyl-ethanolamine |
| CMO | Choline monoocygenase |
References
- Liu, R.H. Health-Promoting Components of Fruits and Vegetables in the Diet. Adv. Nutr. 2013, 4, 384S–392S. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Lloyd, B. Health Benefits of Fruits and Vegetables. Adv. Nutr. 2012, 3, 506–516. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Luo, Y.; Li, D.; Cao, S.; Xia, J.; Li, J.; Smith, M.D. Plant Growth and Mortality under Climatic Extremes: An Overview. Environ. Exp. Bot. 2014, 98, 13–19. [Google Scholar] [CrossRef]
- Li, J.; Abbas, K.; Wang, W.; Gong, B.; Wang, L.; Hou, S.; Xia, H.; Wu, X.; Chen, L.; Gao, H. Drought Tolerance Evaluation and Verification of Fifty Pakchoi (Brassica Rapa Ssp. Chinensis) Varieties under Water Deficit Condition. Agronomy 2023, 13, 2087. [Google Scholar] [CrossRef]
- Dhankher, O.P.; Foyer, C.H. Climate Resilient Crops for Improving Global Food Security and Safety. Plant. Cell Environ. 2018, 41, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Seymen, M. Comparative Analysis of the Relationship between Morphological, Physiological, and Biochemical Properties in Spinach (Spinacea oleracea L.) under Deficit Irrigation Conditions. Turkish J. Agric. For. 2021, 45, 55–67. [Google Scholar]
- Razi, K.; Muneer, S. Drought Stress-Induced Physiological Mechanisms, Signaling Pathways and Molecular Response of Chloroplasts in Common Vegetable Crops. Crit. Rev. Biotechnol. 2021, 41, 669–691. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Schwarz, D.; Franken, P.; Colla, G. Effects of Drought on Nutrient Uptake and Assimilation in Vegetable Crops. In Plant Responses to Drought Stress; Springer: Berlin/Heidelberg, Germany, 2012; pp. 171–195. [Google Scholar]
- Bahadur, A.; Chatterjee, A.; Kumar, R.; Singh, M.; Naik, P.S. Physiological and Biochemical Basis of Drought Tolerance in Vegetables. Veg. Sci. 2011, 38, 1–16. [Google Scholar]
- Cai, S.; Papanatsiou, M.; Blatt, M.R.; Chen, Z.-H. Speedy Grass Stomata: Emerging Molecular and Evolutionary Features. Mol. Plant 2017, 10, 912–914. [Google Scholar] [CrossRef]
- Gervais, T.; Creelman, A.; Li, X.-Q.; Bizimungu, B.; De Koeyer, D.; Dahal, K. Potato Response to Drought Stress: Physiological and Growth Basis. Front. Plant Sci. 2021, 12, 698060. [Google Scholar] [CrossRef]
- Obidiegwu, J.E.; Bryan, G.J.; Jones, H.G.; Prashar, A. Coping with Drought: Stress and Adaptive Responses in Potato and Perspectives for Improvement. Front. Plant Sci. 2015, 6, 542. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, J.N.; Edelenbos, M.; Wienberg, L. Drought Effects on Green Pea Texture and Related Physical-Chemical Properties at Comparable Maturity. J. Am. Soc. Hortic. Sci. 2003, 128, 128–135. [Google Scholar] [CrossRef]
- Paim, B.T.; Crizel, R.L.; Tatiane, S.J.; Rodrigues, V.R.; Rombaldi, C.V.; Galli, V. Mild Drought Stress Has Potential to Improve Lettuce Yield and Quality. Sci. Hortic. 2020, 272, 109578. [Google Scholar] [CrossRef]
- Kemble, J.K.; Sanders, D.C. Basics of Vegetable Crop Irrigation. In ANR-1169; Alabama Cooperative Extension: Tuscaloosa, AL, USA, 2000. [Google Scholar]
- Chaturvedi, A.K.; Surendran, U.; Gopinath, G.; Chandran, K.M.; Anjali, N.K.; Ct, M.F. Elucidation of Stage Specific Physiological Sensitivity of Okra to Drought Stress through Leaf Gas Exchange, Spectral Indices, Growth and Yield Parameters. Agric. Water Manag. 2019, 222, 92–104. [Google Scholar] [CrossRef]
- Pelter, G.Q.; Mittelstadt, R.; Leib, B.G.; Redulla, C.A. Effects of Water Stress at Specific Growth Stages on Onion Bulb Yield and Quality. Agric. Water Manag. 2004, 68, 107–115. [Google Scholar] [CrossRef]
- Bahadur, A.; Kumar, R.; Mishra, U.; Rai, A.; Singh, M. Physiological Approaches for Screening of Tomato Genotypes for Moisture Stress Tolerance. In Proceedings of the National Conference of Plant Physiology (NCPP-2010) BHU, Varanasi, India, 25–27 November 2010; pp. 25–27. [Google Scholar]
- Islam, M.M.; Kayesh, E.; Zaman, E.; Urmi, T.A.; Haque, M.M. Evaluation of Rice (Oryza sativa L.) Genotypes for Drought Tolerance at Germination and Early Seedling Stage. Agriculturists 2018, 16, 44–54. [Google Scholar] [CrossRef]
- Lei, C.; Bagavathiannan, M.; Wang, H.; Sharpe, S.M.; Meng, W.; Yu, J. Osmopriming with Polyethylene Glycol (PEG) for Abiotic Stress Tolerance in Germinating Crop Seeds: A Review. Agronomy 2021, 11, 2194. [Google Scholar] [CrossRef]
- AL-Quraan, N.A.; Al-Ajlouni, Z.I.; Qawasma, N.F. Physiological and Biochemical Characterization of the GABA Shunt Pathway in Pea (Pisum sativum L.) Seedlings under Drought Stress. Horticulturae 2021, 7, 125. [Google Scholar] [CrossRef]
- El Sabagh, A.; Hossain, A.; Barutçular, C.; Iqbal, M.A.; Islam, M.S.; Fahad, S.; Sytar, O.; Çiğ, F.; Meena, R.S.; Erman, M. Consequences of Salinity Stress on the Quality of Crops and Its Mitigation Strategies for Sustainable Crop Production: An Outlook of Arid and Semi-Arid Regions. In Environment, Climate, Plant and Vegetation Growth; Springer: Berlin/Heidelberg, Germany, 2020; pp. 503–533. [Google Scholar]
- Hashmat, S.; Shahid, M.; Tanwir, K.; Abbas, S.; Ali, Q.; Niazi, N.K.; Akram, M.S.; Saleem, M.H.; Javed, M.T. Elucidating Distinct Oxidative Stress Management, Nutrient Acquisition and Yield Responses of Pisum sativum L. Fertigated with Diluted and Treated Wastewater. Agric. Water Manag. 2021, 247, 106720. [Google Scholar] [CrossRef]
- Yang, X.; Lu, M.; Wang, Y.; Wang, Y.; Liu, Z.; Chen, S. Response Mechanism of Plants to Drought Stress. Horticulturae 2021, 7, 50. [Google Scholar] [CrossRef]
- Sahin, U.; Ekinci, M.; Ors, S.; Turan, M.; Yildiz, S.; Yildirim, E. Effects of Individual and Combined Effects of Salinity and Drought on Physiological, Nutritional and Biochemical Properties of Cabbage (Brassica oleracea Var. Capitata). Sci. Hortic. 2018, 240, 196–204. [Google Scholar] [CrossRef]
- Jamalluddin, N.; Massawe, F.J.; Mayes, S.; Ho, W.K.; Singh, A.; Symonds, R.C. Physiological Screening for Drought Tolerance Traits in Vegetable Amaranth (Amaranthus tricolor) Germplasm. Agriculture 2021, 11, 994. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, X.; Nawaz, G.; Yin, J.; Yang, J. Physiological and Biochemical Responses of Four Cassava Cultivars to Drought Stress. Sci. Rep. 2020, 10, 6968. [Google Scholar] [CrossRef] [PubMed]
- Moles, T.M.; Mariotti, L.; De Pedro, L.F.; Guglielminetti, L.; Picciarelli, P.; Scartazza, A. Drought Induced Changes of Leaf-to-Root Relationships in Two Tomato Genotypes. Plant Physiol. Biochem. 2018, 128, 24–31. [Google Scholar] [CrossRef]
- Werner, C.; Correia, O.; Beyschlag, W. Two Different Strategies of Mediterranean Macchia Plants to Avoid Photoinhibitory Damage by Excessive Radiation Levels during Summer Drought. Acta Oecologica 1999, 20, 15–23. [Google Scholar] [CrossRef]
- Frary, A. Plant Physiology and Development; Sinauer Associates Incorporated: Sunderland, MA, USA, 2015. [Google Scholar]
- Misra, V.; Solomon, S.; Mall, A.K.; Prajapati, C.P.; Hashem, A.; Abd_Allah, E.F.; Ansari, M.I. Morphological Assessment of Water Stressed Sugarcane: A Comparison of Waterlogged and Drought Affected Crop. Saudi J. Biol. Sci. 2020, 27, 1228–1236. [Google Scholar] [CrossRef]
- Patmi, Y.S.; Pitoyo, A. Effect of Drought Stress on Morphological, Anatomical, and Physiological Characteristics of Cempo Ireng Cultivar Mutant Rice (Oryza sativa L.) Strain 51 Irradiated by Gamma-Ray. J. Phys. Conf. Ser. IOP Publ. 2020, 1436, 12015. [Google Scholar] [CrossRef]
- Khaleghi, A.; Naderi, R.; Brunetti, C.; Maserti, B.E.; Salami, S.A.; Babalar, M. Morphological, Physiochemical and Antioxidant Responses of Maclura Pomifera to Drought Stress. Sci. Rep. 2019, 9, 19250. [Google Scholar] [CrossRef]
- Hosseini, F.; Mosaddeghi, M.R.; Dexter, A.R. Effect of the Fungus Piriformospora Indica on Physiological Characteristics and Root Morphology of Wheat under Combined Drought and Mechanical Stresses. Plant Physiol. Biochem. 2017, 118, 107–120. [Google Scholar] [CrossRef]
- Mishra, B.K.; Srivastava, J.P.; Lal, J.P. Drought Resistance in Lentil (Lens culinaris Medik.) in Relation to Morphological, Physiological Parameters and Phenological Developments. Int. J. Curr. Microbiol. Appl. Sci 2018, 7, 2288–2304. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Su, Y.; Lei, Y.; Mustafa, N.S.A.; Ahmad, P.; Zhang, L. Improved Drought Tolerance by AMF Inoculation in Maize (Zea mays) Involves Physiological and Biochemical Implications. Plants 2019, 8, 579. [Google Scholar] [CrossRef] [PubMed]
- Lobet, G.; Draye, X. Novel Scanning Procedure Enabling the Vectorization of Entire Rhizotron-Grown Root Systems. Plant Methods 2013, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Gowda, V.R.P.; Henry, A.; Yamauchi, A.; Shashidhar, H.E.; Serraj, R. Root Biology and Genetic Improvement for Drought Avoidance in Rice. F. Crop. Res. 2011, 122, 1–13. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, L.; Zhang, Y.; Sun, H.; Zhang, K.; Bai, Z.; Dong, H.; Li, C. Fine Root and Root Hair Morphology of Cotton under Drought Stress Revealed with RhizoPot. J. Agron. Crop Sci. 2020, 206, 679–693. [Google Scholar] [CrossRef]
- Benjamin, J.G.; Nielsen, D.C. Water Deficit Effects on Root Distribution of Soybean, Field Pea and Chickpea. F. Crop. Res. 2006, 97, 248–253. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of Drought Stress on Sugar Metabolism in Leaves and Roots of Soybean Seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef]
- Kosma, D.K.; Bourdenx, B.; Bernard, A.; Parsons, E.P.; Lu, S.; Joubes, J.; Jenks, M.A. The Impact of Water Deficiency on Leaf Cuticle Lipids of Arabidopsis. Plant Physiol. 2009, 151, 1918–1929. [Google Scholar] [CrossRef]
- Chen, M.; Zhu, X.; Zhang, Y.; Du, Z.; Chen, X.; Kong, X.; Sun, W.; Chen, C. Drought Stress Modify Cuticle of Tender Tea Leaf and Mature Leaf for Transpiration Barrier Enhancement through Common and Distinct Modes. Sci. Rep. 2020, 10, 6696. [Google Scholar] [CrossRef]
- Wu, H.; Liu, L.; Chen, Y.; Liu, T.; Jiang, Q.; Wei, Z.; Li, C.; Wang, Z. Tomato SlCER1–1 Catalyzes the Synthesis of Wax Alkanes, Increasing Drought Tolerance and Fruit Storability. Hortic. Res. 2022, 9, uhac004. [Google Scholar] [CrossRef]
- Liu, D.; Guo, W.; Guo, X.; Yang, L.; Hu, W.; Kuang, L.; Huang, Y.; Xie, J.; Liu, Y. Ectopic Overexpression of CsECR from Navel Orange Increases Cuticular Wax Accumulation in Tomato and Enhances Its Tolerance to Drought Stress. Front. Plant Sci. 2022, 13, 924552. [Google Scholar] [CrossRef]
- Chartzoulakis, K.; Patakas, A.; Kofidis, G.; Bosabalidis, A.; Nastou, A. Water Stress Affects Leaf Anatomy, Gas Exchange, Water Relations and Growth of Two Avocado Cultivars. Sci. Hortic. 2002, 95, 39–50. [Google Scholar] [CrossRef]
- Hughes, J.; Hepworth, C.; Dutton, C.; Dunn, J.A.; Hunt, L.; Stephens, J.; Waugh, R.; Cameron, D.D.; Gray, J.E. Reducing Stomatal Density in Barley Improves Drought Tolerance without Impacting on Yield. Plant Physiol. 2017, 174, 776–787. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, H.; Wu, W.; Li, W. Effect of Drought Stress on Physiological Changes and Leaf Surface Morphology in the Blackberry. Brazilian J. Bot. 2017, 40, 625–634. [Google Scholar] [CrossRef]
- Vincent, D.; Lapierre, C.; Pollet, B.; Cornic, G.; Negroni, L.; Zivy, M. Water Deficits Affect Caffeate O-Methyltransferase, Lignification, and Related Enzymes in Maize Leaves. A Proteomic Investigation. Plant Physiol. 2005, 137, 949–960. [Google Scholar] [CrossRef]
- Yin, N.; Li, J.; Liu, X.; Lian, J.; Fu, C.; Li, W.; Jiang, J.; Xue, Y.; Wang, J.; Chai, Y. Lignification Response and the Difference between Stem and Root of Brassica Napus under Heat and Drought Compound Stress. Acta Agron. Sin. 2017, 43, 1689–1695. [Google Scholar] [CrossRef]
- Pagès, L. Simulating the Diversity and Plasticity of Root Systems Using 3D Models of the Root System Architecture. In The Root Systems in Sustainable Agricultural Intensification; Wiley: Hoboken, NJ, USA, 2021; pp. 355–373. [Google Scholar]
- Gérard, F.; Blitz-Frayret, C.; Hinsinger, P.; Pagès, L. Modelling the Interactions between Root System Architecture, Root Functions and Reactive Transport Processes in Soil. Plant Soil 2017, 413, 161–180. [Google Scholar] [CrossRef]
- Koevoets, I.T.; Venema, J.H.; Elzenga, J.T.M.; Testerink, C. Roots Withstanding Their Environment: Exploiting Root System Architecture Responses to Abiotic Stress to Improve Crop Tolerance. Front. Plant Sci. 2016, 7, 1335. [Google Scholar] [CrossRef]
- Ranjan, A.; Sinha, R.; Lal, S.K.; Bishi, S.K.; Singh, A.K. Phytohormone Signalling and Cross-Talk to Alleviate Aluminium Toxicity in Plants. Plant Cell Rep. 2021, 40, 1331–1343. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Fujita, M. Drought Stress Responses in Plants, Oxidative Stress, and Antioxidant Defense. In Climate Change and Plant Abiotic Stress Tolerance; Wiley: Hoboken, NJ, USA, 2013; pp. 209–250. [Google Scholar]
- Goto, K.; Yabuta, S.; Ssenyonga, P.; Tamaru, S.; Sakagami, J.-I. Response of Leaf Water Potential, Stomatal Conductance and Chlorophyll Content under Different Levels of Soil Water, Air Vapor Pressure Deficit and Solar Radiation in Chili Pepper (Capsicum chinense). Sci. Hortic. 2021, 281, 109943. [Google Scholar] [CrossRef]
- Escalante-Magana, C.; Aguilar-Caamal, L.F.; Echevarría-Machado, I.; Medina-Lara, F.; Cach, L.S.; Martínez-Estévez, M. Contribution of Glycine Betaine and Proline to Water Deficit Tolerance in Pepper Plants. HortScience 2019, 54, 1044–1054. [Google Scholar] [CrossRef]
- Giordano, M.; Petropoulos, S.A.; Rouphael, Y. Response and Defence Mechanisms of Vegetable Crops against Drought, Heat and Salinity Stress. Agriculture 2021, 11, 463. [Google Scholar] [CrossRef]
- Chatterjee, A.; Solankey, S.S. Functional Physiology in Drought Tolerance of Vegetable Crops: An Approach to Mitigate Climate Change Impact. Clim. Dyn. Hortic. Sci 2015, 1, 149–171. [Google Scholar]
- Chen, J.; Chang, S.X.; Anyia, A.O. Gene Discovery in Cereals through Quantitative Trait Loci and Expression Analysis in Water-use Efficiency Measured by Carbon Isotope Discrimination. Plant. Cell Environ. 2011, 34, 2009–2023. [Google Scholar] [CrossRef] [PubMed]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Breeding for High Water-Use Efficiency. J. Exp. Bot. 2004, 55, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- Condon, A.G.; Richards, R.A.; Rebetzke, G.J.; Farquhar, G.D. Improving Intrinsic Water-use Efficiency and Crop Yield. Crop Sci. 2002, 42, 122–131. [Google Scholar]
- Hussain, I.S.A.; Prasad, T.G.; Wright, G.C.; Kumar, M.U.; Rao, R.C.N. Variation in Transpiration Efficiency and Carbon Isotope Discrimination in Cowpea. Funct. Plant Biol. 1999, 26, 503–510. [Google Scholar]
- Hall, A.E.; Thiaw, S.; Ismail, A.M.; Ehlers, J.D. Water-Use Efficiency and Drought Adaptation of Cowpea. Adv. Cowpea Res. 1997, 87, 76–84. [Google Scholar]
- Mantovani, D.; Rosati, A.; Perrone, D. Photosynthetic Characterization and Response to Drought and Temperature in Wild Asparagus (Asparagus acutifolius L.). HortScience 2019, 54, 1039–1043. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Jo, J.S.; Song, J.W.; Lee, J.G. Effect of Drought Stress on Chlorophyll Fluorescence Parameters, Phytochemical Contents, and Antioxidant Activities in Lettuce Seedlings. Horticulturae 2021, 7, 238. [Google Scholar] [CrossRef]
- Liang, G.; Liu, J.; Zhang, J.; Guo, J. Effects of Drought Stress on Photosynthetic and Physiological Parameters of Tomato. J. Am. Soc. Hortic. Sci. 2020, 145, 12–17. [Google Scholar] [CrossRef]
- Murchie, E.H.; Lawson, T. Chlorophyll Fluorescence Analysis: A Guide to Good Practice and Understanding Some New Applications. J. Exp. Bot. 2013, 64, 3983–3998. [Google Scholar] [CrossRef] [PubMed]
- Meise, P.; Seddig, S.; Uptmoor, R.; Ordon, F.; Schum, A. Impact of Nitrogen Supply on Leaf Water Relations and Physiological Traits in a Set of Potato (Solanum tuberosum L.) Cultivars under Drought Stress. J. Agron. Crop Sci. 2018, 204, 359–374. [Google Scholar] [CrossRef]
- Sorrentino, M.; Colla, G.; Rouphael, Y.; Panzarová, K.; Trtílek, M. Lettuce Reaction to Drought Stress: Automated High-Throughput Phenotyping of Plant Growth and Photosynthetic Performance. In Proceedings of the ISHS Acta Horticulturae 1268: XI International Symposium on Protected Cultivation in Mild Winter Climates and I International Symposium on Nettings and Screens in Horticulture, Tenerife, Spain, 27–31 January 2019; pp. 133–142. [Google Scholar]
- Wakchaure, G.C.; Minhas, P.S.; Meena, K.K.; Kumar, S.; Rane, J. Effect of Plant Growth Regulators and Deficit Irrigation on Canopy Traits, Yield, Water Productivity and Fruit Quality of Eggplant (Solanum melongena L.) Grown in the Water Scarce Environment. J. Environ. Manag. 2020, 262, 110320. [Google Scholar] [CrossRef]
- Jamalluddin, N.; Massawe, F.J.; Symonds, R.C. Transpiration Efficiency of Amaranth (Amaranthus Sp.) in Response to Drought Stress. J. Hortic. Sci. Biotechnol. 2019, 94, 448–459. [Google Scholar] [CrossRef]
- Ghanaatiyan, K.; Sadeghi, H. Differential Responses of Chicory Ecotypes Exposed to Drought Stress in Relation to Enzymatic and Non-Enzymatic Antioxidants as Well as ABA Concentration. J. Hortic. Sci. Biotechnol. 2017, 92, 404–410. [Google Scholar] [CrossRef]
- Anjum, S.A.; Farooq, M.; Xie, X.; Liu, X.; Ijaz, M.F. Antioxidant Defense System and Proline Accumulation Enables Hot Pepper to Perform Better under Drought. Sci. Hortic. 2012, 140, 66–73. [Google Scholar] [CrossRef]
- Kleinwächter, M.; Selmar, D. New Insights Explain That Drought Stress Enhances the Quality of Spice and Medicinal Plants: Potential Applications. Agron. Sustain. Dev. 2015, 35, 121–131. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Influencing the Product Quality by Deliberately Applying Drought Stress during the Cultivation of Medicinal Plants. Ind. Crops Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Selmar, D.; Kleinwächter, M. Stress Enhances the Synthesis of Secondary Plant Products: The Impact of Stress-Related over-Reduction on the Accumulation of Natural Products. Plant Cell Physiol. 2013, 54, 817–826. [Google Scholar] [CrossRef]
- Heatherly, L.G. Drought Stress and Irrigation Effects on Germination of Harvested Soybean Seed. Crop Sci. 1993, 33, 777–781. [Google Scholar] [CrossRef]
- Adejumo, S.A.; Ezeh, O.S.; Mur, L.A.J. Okra Growth and Drought Tolerance When Exposed to Water Regimes at Different Growth Stages. Int. J. Veg. Sci. 2019, 25, 226–258. [Google Scholar] [CrossRef]
- Sun, C.; Li, X.; Hu, Y.; Zhao, P.; Xu, T.; Sun, J.; Gao, X. Proline, Sugars, and Antioxidant Enzymes Respond to Drought Stress in the Leaves of Strawberry Plants. Hortic. Sci. Technol. 2015, 33, 625–632. [Google Scholar] [CrossRef]
- Szabados, L.; Savouré, A. Proline: A Multifunctional Amino Acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Potters, G.; Horemans, N.; Jansen, M.A.K. The Cellular Redox State in Plant Stress Biology—A Charging Concept. Plant Physiol. Biochem. 2010, 48, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Sgherri, C.; Pinzino, C.; Quartacci, M.F. Reactive Oxygen Species and Photosynthetic Functioning: Past and Present. In Reactive Oxygen Species in Plants: Boon or Bane—Revisiting the Role of ROS; Wiley: Hoboken, NJ, USA, 2017; pp. 137–155. [Google Scholar]
- Khalid, M.F.; Vincent, C.; Morillon, R.; Anjum, M.A.; Ahmad, S.; Hussain, S. Different Strategies Lead to a Common Outcome: Different Water-Deficit Scenarios Highlight Physiological and Biochemical Strategies of Water-Deficit Tolerance in Diploid versus Tetraploid Volkamer Lemon. Tree Physiol. 2021, 41, 2359–2374. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Kong, L.; Yu, X.; Ottosen, C.-O.; Zhao, T.; Jiang, F.; Wu, Z. Oxidative Damage and Antioxidant Mechanism in Tomatoes Responding to Drought and Heat Stress. Acta Physiol. Plant. 2019, 41, 20. [Google Scholar] [CrossRef]
- Farzane, A.; Nemati, H.; Shoor, M.; Ansari, H. Foliar Application of Potassium on Antioxidant Enzyme Activities of Tomato Plants under Drought Stress. Adv. Hortic. Sci. 2021, 35, 3–9. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, E.; del Mar Rubio-Wilhelmi, M.; Blasco, B.; Leyva, R.; Romero, L.; Ruiz, J.M. Antioxidant Response Resides in the Shoot in Reciprocal Grafts of Drought-Tolerant and Drought-Sensitive Cultivars in Tomato under Water Stress. Plant Sci. 2012, 188, 89–96. [Google Scholar] [CrossRef]
- Maham, S.; Muhammad, S. Mitigation of Drought Stress-Induced Adverse Effects on Antioxidant System of Eggplant by Exogenous Application of Alpha-Tocopherol. Int. J. Agric. Biol. 2019, 21, 971–978. [Google Scholar]
- Abdelaal, K.A.; EL-Maghraby, L.M.; Elansary, H.; Hafez, Y.M.; Ibrahim, E.I.; El-Banna, M.; El-Esawi, M.; Elkelish, A. Treatment of Sweet Pepper with Stress Tolerance-Inducing Compounds Alleviates Salinity Stress Oxidative Damage by Mediating the Physio-Biochemical Activities and Antioxidant Systems. Agronomy 2019, 10, 26. [Google Scholar]
- Kopta, T.; Sekara, A.; Pokluda, R.; Ferby, V.; Caruso, G. Screening of Chilli Pepper Genotypes as a Source of Capsaicinoids and Antioxidants under Conditions of Simulated Drought Stress. Plants 2020, 9, 364. [Google Scholar] [CrossRef] [PubMed]
- Alabdallah, N.M.; Hasan, M.M.; Salih, A.M.; Roushdy, S.S.; Al-Shammari, A.S.; Alsanie, S.I.; El-Zaidy, M. Silver Nanoparticles Improve Growth and Protect against Oxidative Damage in Eggplant Seedlings under Drought Stress. Plant Soil Environ. 2021, 67, 617–624. [Google Scholar] [CrossRef]
- Mahmood, T.; Rana, R.M.; Ahmar, S.; Saeed, S.; Gulzar, A.; Khan, M.A.; Wattoo, F.M.; Wang, X.; Branca, F.; Mora-Poblete, F. Effect of Drought Stress on Capsaicin and Antioxidant Contents in Pepper Genotypes at Reproductive Stage. Plants 2021, 10, 1286. [Google Scholar]
- Semida, W.M.; Abdelkhalik, A.; Mohamed, G.F.; Abd El-Mageed, T.A.; Abd El-Mageed, S.A.; Rady, M.M.; Ali, E.F. Foliar Application of Zinc Oxide Nanoparticles Promotes Drought Stress Tolerance in Eggplant (Solanum melongena L.). Plants 2021, 10, 421. [Google Scholar]
- Liu, Z.; Guo, Y.; Lin, S.; Bai, J. Effects of Exogenous Hydrogen Peroxide on Ultrastructure of Chloroplasts and Activities of Antioxidant Enzymes in Greenhouse-Ecotype Cucumber under Drought Stress. Acta Hortic. Sin. 2009, 36, 1140–1146. [Google Scholar]
- Fan, H.F.; Ding, L.; Xu, Y.L.; Du, C.X. Antioxidant System and Photosynthetic Characteristics Responses to Short-Term PEG-Induced Drought Stress in Cucumber Seedling Leaves. Russ. J. Plant Physiol. 2017, 64, 162–173. [Google Scholar]
- Yasar, F.; Uzal, O.; Yasar, T.O.; Ozlem, Y. Investigation of the Relationship between the Tolerance to Drought Stress Levels and Antioxidant Enzyme Activities in Green Bean (Phaseolus vulgaris L.) Genotypes. Afr. J. Agric. Res. 2013, 8, 5759–5763. [Google Scholar]
- Mittler, R.; Zilinskas, B.A. Regulation of Pea Cytosolic Ascorbate Peroxidase and Other Antioxidant Enzymes during the Progression of Drought Stress and Following Recovery from Drought. Plant J. 1994, 5, 397–405. [Google Scholar] [CrossRef]
- Osman, H.S. Enhancing Antioxidant–Yield Relationship of Pea Plant under Drought at Different Growth Stages by Exogenously Applied Glycine Betaine and Proline. Ann. Agric. Sci. 2015, 60, 389–402. [Google Scholar]
- Guler, N.S.; Pehlivan, N. Exogenous Low-Dose Hydrogen Peroxide Enhances Drought Tolerance of Soybean (Glycine max L.) through Inducing Antioxidant System. Acta Biol. Hung. 2016, 67, 169–183. [Google Scholar] [PubMed]
- Patel, P.K.; Hemantaranjan, A.; Sarma, B.K.; Radha, S. Growth and Antioxidant System under Drought Stress in Chickpea (Cicer arietinum L.) as Sustained by Salicylic Acid. J. Stress Physiol. Biochem. 2011, 7, 130–144. [Google Scholar]
- Saglam, A.; Saruhan, N.; Terzi, R.; Kadioglu, A. The Relations between Antioxidant Enzymes and Chlorophyll Fluorescence Parameters in Common Bean Cultivars Differing in Sensitivity to Drought Stress. Russ. J. Plant Physiol. 2011, 58, 60–68. [Google Scholar]
- Jyoti, B.; Yadav, S.K. Comparative Study on Biochemical Parameters and Antioxidant Enzymes in a Drought Tolerant and a Sensitive Variety of Horsegram (Macrotyloma Uniflorum) under Drought Stress. Am. J. Plant Physiol. 2012, 7, 17–29. [Google Scholar]
- Davies, W.J.; Zhang, J. Root Signals and the Regulation of Growth and Development of Plants in Drying Soil. Annu. Rev. Plant Biol. 1991, 42, 55–76. [Google Scholar] [CrossRef]
- Chazen, O.; Neumann, P.M. Hydraulic Signals from the Roots and Rapid Cell-Wall Hardening in Growing Maize (Zea mays L.) Leaves Are Primary Responses to Polyethylene Glycol-Induced Water Deficits. Plant Physiol. 1994, 104, 1385–1392. [Google Scholar]
- Chen, Z.-H.; Chen, G.; Dai, F.; Wang, Y.; Hills, A.; Ruan, Y.-L.; Zhang, G.; Franks, P.J.; Nevo, E.; Blatt, M.R. Molecular Evolution of Grass Stomata. Trends Plant Sci. 2017, 22, 124–139. [Google Scholar]
- Xue, D.; Zhang, X.; Lu, X.; Chen, G.; Chen, Z.-H. Eco-Physiological and Molecular-Genetic Determinants of Plant Cuticle Function in Drought and Salt Stress Tolerance. Front. Plant Sci. 2017, 8, 621. [Google Scholar]
- Fromm, J.; Fei, H. Electrical Signaling and Gas Exchange in Maize Plants of Drying Soil. Plant Sci. 1998, 132, 203–213. [Google Scholar]
- Osakabe, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.P. ABA Control of Plant Macroelement Membrane Transport Systems in Response to Water Deficit and High Salinity. New Phytol. 2014, 202, 35–49. [Google Scholar]
- Malcheska, F.; Ahmad, A.; Batool, S.; Müller, H.M.; Ludwig-Müller, J.; Kreuzwieser, J.; Randewig, D.; Hänsch, R.; Mendel, R.R.; Hell, R. Drought-Enhanced Xylem Sap Sulfate Closes Stomata by Affecting ALMT12 and Guard Cell ABA Synthesis. Plant Physiol. 2017, 174, 798–814. [Google Scholar]
- Walker, J.C.; Willows, D.R. Mechanism and Regulation of Mg-Chelatase. Biochem. J. 1997, 327, 321–333. [Google Scholar]
- Mochizuki, N.; Brusslan, J.A.; Larkin, R.; Nagatani, A.; Chory, J. Arabidopsis Genomes Uncoupled 5 (GUN5) Mutant Reveals the Involvement of Mg-Chelatase H Subunit in Plastid-to-Nucleus Signal Transduction. Proc. Natl. Acad. Sci. USA 2001, 98, 2053–2058. [Google Scholar] [PubMed]
- Yin, P.; Fan, H.; Hao, Q.; Yuan, X.; Wu, D.; Pang, Y.; Yan, C.; Li, W.; Wang, J.; Yan, N. Structural Insights into the Mechanism of Abscisic Acid Signaling by PYL Proteins. Nat. Struct. Mol. Biol. 2009, 16, 1230–1236. [Google Scholar] [PubMed]
- Wang, W.-H.; Yi, X.-Q.; Han, A.-D.; Liu, T.-W.; Chen, J.; Wu, F.-H.; Dong, X.-J.; He, J.-X.; Pei, Z.-M.; Zheng, H.-L. Calcium-Sensing Receptor Regulates Stomatal Closure through Hydrogen Peroxide and Nitric Oxide in Response to Extracellular Calcium in Arabidopsis. J. Exp. Bot. 2012, 63, 177–190. [Google Scholar]
- Case, R.M.; Eisner, D.; Gurney, A.; Jones, O.; Muallem, S.; Verkhratsky, A. Evolution of Calcium Homeostasis: From Birth of the First Cell to an Omnipresent Signalling System. Cell Calcium 2007, 42, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Assmann, S.M.; Albert, R. Predicting Essential Components of Signal Transduction Networks: A Dynamic Model of Guard Cell Abscisic Acid Signaling. PLoS Biol. 2006, 4, e312. [Google Scholar]
- McAdam, S.A.M.; Brodribb, T.J. Separating Active and Passive Influences on Stomatal Control of Transpiration. Plant Physiol. 2014, 164, 1578–1586. [Google Scholar]
- Miller, G.; Shulaev, V.; Mittler, R. Reactive Oxygen Signaling and Abiotic Stress. Physiol. Plant. 2008, 133, 481–489. [Google Scholar]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd_Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of Subcellular ROS and NO Metabolism in Higher Plants: Multifunctional Signaling Molecules. Antioxidants 2019, 8, 641. [Google Scholar]
- Singh, A.; Kumar, A.; Yadav, S.; Singh, I.K. Reactive Oxygen Species-Mediated Signaling during Abiotic Stress. Plant Gene 2019, 18, 100173. [Google Scholar] [CrossRef]
- Pei, Z.-M.; Murata, Y.; Benning, G.; Thomine, S.; Klüsener, B.; Allen, G.J.; Grill, E.; Schroeder, J.I. Calcium Channels Activated by Hydrogen Peroxide Mediate Abscisic Acid Signalling in Guard Cells. Nature 2000, 406, 731–734. [Google Scholar] [CrossRef] [PubMed]
- Mori, I.C.; Schroeder, J.I. Reactive Oxygen Species Activation of Plant Ca2+ Channels. A Signaling Mechanism in Polar Growth, Hormone Transduction, Stress Signaling, and Hypothetically Mechanotransduction. Plant Physiol. 2004, 135, 702–708. [Google Scholar] [CrossRef]
- Yan, J.; Tsuichihara, N.; Etoh, T.; Iwai, S. Reactive Oxygen Species and Nitric Oxide Are Involved in ABA Inhibition of Stomatal Opening. Plant. Cell Environ. 2007, 30, 1320–1325. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Bhattacharjee, S.; Armin, S.-M.; Qian, P.; Xin, W.; Li, H.-Y.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Hydrogen Peroxide Priming Modulates Abiotic Oxidative Stress Tolerance: Insights from ROS Detoxification and Scavenging. Front. Plant Sci. 2015, 6, 420. [Google Scholar] [CrossRef]
- Delauney, A.J.; Verma, D.P.S. Proline Biosynthesis and Osmoregulation in Plants. Plant J. 1993, 4, 215–223. [Google Scholar] [CrossRef]
- Hua, X.-J.; Van de Cotte, B.; Van Montagu, M.; Verbruggen, N. Developmental Regulation of Pyrroline-5-Carboxylate Reductase Gene Expression in Arabidopsis. Plant Physiol. 1997, 114, 1215–1224. [Google Scholar] [CrossRef]
- Savouré, A.; Jaoua, S.; Hua, X.-J.; Ardiles, W.; Van Montagu, M.; Verbruggen, N. Isolation, Characterization, and Chromosomal Location of a Gene Encoding the Δ 1-pyrroline-5-carboxylate Synthetase in Arabidopsis Thaliana. FEBS Lett. 1995, 372, 13–19. [Google Scholar] [CrossRef]
- Hu, C.A.; Delauney, A.J.; Verma, D.P. A Bifunctional Enzyme (Delta 1-Pyrroline-5-Carboxylate Synthetase) Catalyzes the First Two Steps in Proline Biosynthesis in Plants. Proc. Natl. Acad. Sci. USA 1992, 89, 9354–9358. [Google Scholar] [CrossRef]
- LaRosa, P.C.; Rhodes, D.; Rhodes, J.C.; Bressan, R.A.; Csonka, L.N. Elevated Accumulation of Proline in NaCl-Adapted Tobacco Cells Is Not Due to Altered Δ1-Pyrroline-5-Carboxylate Reductase. Plant Physiol. 1991, 96, 245–250. [Google Scholar] [CrossRef]
- Zhu, B.; Su, J.; Chang, M.; Verma, D.P.S.; Fan, Y.-L.; Wu, R. Overexpression of a Δ1-Pyrroline-5-Carboxylate Synthetase Gene and Analysis of Tolerance to Water-and Salt-Stress in Transgenic Rice. Plant Sci. 1998, 139, 41–48. [Google Scholar] [CrossRef]
- Yamchi, A.; Rastgar Jazii, F.; Mousavi, A.; Karkhane, A.A. Renu Proline Accumulation in Transgenic Tobacco as a Result of Expression of Arabidopsis Δ 1-Pyrroline-5-Carboxylate Synthetase (P5CS) during Osmotic Stress. J. Plant Biochem. Biotechnol. 2007, 16, 9–15. [Google Scholar] [CrossRef]
- Hmida-Sayari, A.; Gargouri-Bouzid, R.; Bidani, A.; Jaoua, L.; Savouré, A.; Jaoua, S. Overexpression of Δ1-Pyrroline-5-Carboxylate Synthetase Increases Proline Production and Confers Salt Tolerance in Transgenic Potato Plants. Plant Sci. 2005, 169, 746–752. [Google Scholar] [CrossRef]
- Zhang, G.-C.; Zhu, W.-L.; Gai, J.-Y.; Zhu, Y.-L.; Yang, L.-F. Enhanced Salt Tolerance of Transgenic Vegetable Soybeans Resulting from Overexpression of a Novel Δ 1-Pyrroline-5-Carboxylate Synthetase Gene from Solanum Torvum Swartz. Hortic. Environ. Biotechnol. 2015, 56, 94–104. [Google Scholar] [CrossRef]
- Hervieu, F.; Le Dily, F.; Billard, J.-P.; Huault, C. Effects of Water-Stress on Proline Content and Ornithine Aminotransferase Activity of Radish Cotyledons. Phytochemistry 1994, 37, 1227–1231. [Google Scholar] [CrossRef]
- Sharma, S.; Villamor, J.G.; Verslues, P.E. Essential Role of Tissue-Specific Proline Synthesis and Catabolism in Growth and Redox Balance at Low Water Potential. Plant Physiol. 2011, 157, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Rentsch, D.; Hirner, B.; Schmelzer, E.; Frommer, W.B. Salt Stress-Induced Proline Transporters and Salt Stress-Repressed Broad Specificity Amino Acid Permeases Identified by Suppression of a Yeast Amino Acid Permease-Targeting Mutant. Plant Cell 1996, 8, 1437–1446. [Google Scholar]
- Nuccio, M.L.; McNeil, S.D.; Ziemak, M.J.; Hanson, A.D.; Jain, R.K.; Selvaraj, G. Choline Import into Chloroplasts Limits Glycine Betaine Synthesis in Tobacco: Analysis of Plants Engineered with a Chloroplastic or a Cytosolic Pathway. Metab. Eng. 2000, 2, 300–311. [Google Scholar] [CrossRef]
- Brendza, K.M.; Haakenson, W.; Cahoon, R.E.; Hicks, L.M.; Palavalli, L.H.; Chiapelli, B.J.; McLaird, M.; McCarter, J.P.; Williams, D.J.; Hresko, M.C. Phosphoethanolamine N-Methyltransferase (PMT-1) Catalyses the First Reaction of a New Pathway for Phosphocholine Biosynthesis in Caenorhabditis Elegans. Biochem. J. 2007, 404, 439–448. [Google Scholar] [CrossRef]
- McNeil, S.D.; Nuccio, M.L.; Ziemak, M.J.; Hanson, A.D.; EMcNeil, S.D.; Nuccio, M.L.; Ziemak, M.J.; Hanson, A.D. Enhanced Synthesis of Choline and Glycine Betaine in Transgenic Tobacco Plants That Overexpress Phosphoethanolamine N-Methyltransferase. Proc. Natl. Acad. Sci. USA 2001, 98, 10001–10005. [Google Scholar] [CrossRef]
- Bhuiyan, N.H.; Hamada, A.; Yamada, N.; Rai, V.; Hibino, T.; Takabe, T. Regulation of Betaine Synthesis by Precursor Supply and Choline Monooxygenase Expression in Amaranthus Tricolor. J. Exp. Bot. 2007, 58, 4203–4212. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, A.; Murata, N. The Role of Glycine Betaine in the Protection of Plants from Stress: Clues from Transgenic Plants. Plant. Cell Environ. 2002, 25, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Rathinasabapathi, B.; Burnet, M.; Russell, B.L.; Gage, D.A.; Liao, P.-C.; Nye, G.J.; Scott, P.; Golbeck, J.H.; Hanson, A.D. Choline Monooxygenase, an Unusual Iron-Sulfur Enzyme Catalyzing the First Step of Glycine Betaine Synthesis in Plants: Prosthetic Group Characterization and CDNA Cloning. Proc. Natl. Acad. Sci. USA 1997, 94, 3454–3458. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, T.L.; Waters, D.L.E.; Henry, R.J. Betaine Aldehyde Dehydrogenase in Plants. Plant Biol. 2009, 11, 119–130. [Google Scholar] [CrossRef]
- Fujiwara, T.; Hori, K.; Ozaki, K.; Yokota, Y.; Mitsuya, S.; Ichiyanagi, T.; Hattori, T.; Takabe, T. Enzymatic Characterization of Peroxisomal and Cytosolic Betaine Aldehyde Dehydrogenases in Barley. Physiol. Plant. 2008, 134, 22–30. [Google Scholar] [CrossRef]
- Hussain Wani, S.; Brajendra Singh, N.; Haribhushan, A.; Iqbal Mir, J. Compatible Solute Engineering in Plants for Abiotic Stress Tolerance-Role of Glycine Betaine. Curr. Genom. 2013, 14, 157–165. [Google Scholar] [CrossRef]
- Mitsuya, S.; Kuwahara, J.; Ozaki, K.; Saeki, E.; Fujiwara, T.; Takabe, T. Isolation and Characterization of a Novel Peroxisomal Choline Monooxygenase in Barley. Planta 2011, 234, 1215–1226. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, M.; Zhang, H.; Zhang, P. Improved Tolerance to Various Abiotic Stresses in Transgenic Sweet Potato (Ipomoea Batatas) Expressing Spinach Betaine Aldehyde Dehydrogenase. PLoS ONE 2012, 7, e37344. [Google Scholar] [CrossRef]
- Shen, Y.-G.; Du, B.-X.; Zhang, W.-K.; Zhang, J.-S.; Chen, S.-Y. AhCMO, Regulated by Stresses in Atriplex Hortensis, Can Improve Drought Tolerance in Transgenic Tobacco. Theor. Appl. Genet. 2002, 105, 815–821. [Google Scholar] [CrossRef]
- Li, M.; Li, Z.; Li, S.; Guo, S.; Meng, Q.; Li, G.; Yang, X. Genetic Engineering of Glycine Betaine Biosynthesis Reduces Heat-Enhanced Photoinhibition by Enhancing Antioxidative Defense and Alleviating Lipid Peroxidation in Tomato. Plant Mol. Biol. Rep. 2014, 32, 42–51. [Google Scholar] [CrossRef]
- Ishitani, M.; Nakamura, T.; Han, S.Y.; Takabe, T. Expression of the Betaine Aldehyde Dehydrogenase Gene in Barley in Response to Osmotic Stress and Abscisic Acid. Plant Mol. Biol. 1995, 27, 307–315. [Google Scholar] [CrossRef]
- Luo, D.; Hou, X.; Zhang, Y.; Meng, Y.; Zhang, H.; Liu, S.; Wang, X.; Chen, R. CaDHN5, a Dehydrin Gene from Pepper, Plays an Important Role in Salt and Osmotic Stress Responses. Int. J. Mol. Sci. 2019, 20, 1989. [Google Scholar] [CrossRef]
- Zrenner, R.; Krause, K.; Apel, P.; Sonnewald, U. Reduction of the Cytosolic Fructose-1, 6-bisphosphatase in Transgenic Potato Plants Limits Photosynthetic Sucrose Biosynthesis with No Impact on Plant Growth and Tuber Yield. Plant J. 1996, 9, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Quoc, B.; N’Tchobo, H.; Foyer, C.H.; Yelle, S. Overexpression of Sucrose Phosphate Synthase Increases Sucrose Unloading in Transformed Tomato Fruit. J. Exp. Bot. 1999, 50, 785–791. [Google Scholar] [CrossRef]
- Ishimaru, K.; Hirotsu, N.; Kashiwagi, T.; Madoka, Y.; Nagasuga, K.; Ono, K.; Ohsugi, R. Overexpression of a Maize SPS Gene Improves Yield Characters of Potato under Field Conditions. Plant Prod. Sci. 2008, 11, 104–107. [Google Scholar] [CrossRef]
- Park, J.-Y.; Canam, T.; Kang, K.-Y.; Ellis, D.D.; Mansfield, S.D. Over-Expression of an Arabidopsis Family A Sucrose Phosphate Synthase (SPS) Gene Alters Plant Growth and Fibre Development. Transgenic Res. 2008, 17, 181–192. [Google Scholar] [CrossRef]
- Kumar, R.; Solankey, S.S.; Singh, M. Breeding for Drought Tolerance in Vegetables. Veg. Sci. 2012, 39, 1–15. [Google Scholar]
- Abebe, T.; Guenzi, A.C.; Martin, B.; Cushman, J.C. Tolerance of Mannitol-Accumulating Transgenic Wheat to Water Stress and Salinity. Plant Physiol. 2003, 131, 1748–1755. [Google Scholar] [CrossRef]
- Muñiz García, M.N.; Cortelezzi, J.I.; Fumagalli, M.; Capiati, D.A. Expression of the Arabidopsis ABF4 Gene in Potato Increases Tuber Yield, Improves Tuber Quality and Enhances Salt and Drought Tolerance. Plant Mol. Biol. 2018, 98, 137–152. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, L.; Shi, Y.; Su, D.; Lu, W.; Cheng, Y.; Li, Z. Stress-Responsive Tomato Gene SlGRAS4 Function in Drought Stress and Abscisic Acid Signaling. Plant Sci. 2021, 304, 110804. [Google Scholar] [CrossRef]
- Park, S.; Li, J.; Pittman, J.K.; Berkowitz, G.A.; Yang, H.; Undurraga, S.; Morris, J.; Hirschi, K.D.; Gaxiola, R.A. Up-Regulation of a H+-Pyrophosphatase (H+-PPase) as a Strategy to Engineer Drought-Resistant Crop Plants. Proc. Natl. Acad. Sci. USA 2005, 102, 18830–18835. [Google Scholar] [CrossRef]
- Laporte, M.M.; Shen, B.; Tarczynski, M.C. Engineering for Drought Avoidance: Expression of Maize NADP-malic Enzyme in Tobacco Results in Altered Stomatal Function. J. Exp. Bot. 2002, 53, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.-C.; Liao, P.-M.; Kuo, W.-W.; Lin, T.-P. The Arabidopsis Ethylene Response Factor1 Regulates Abiotic Stress-Responsive Gene Expression by Binding to Different Cis-Acting Elements in Response to Different Stress Signals. Plant Physiol. 2013, 162, 1566–1582. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, W.; Wan, L.; Li, F.; Dai, L.; Li, D.; Zhang, Z.; Huang, R. Functional Analyses of Ethylene Response Factor JERF3 with the Aim of Improving Tolerance to Drought and Osmotic Stress in Transgenic Rice. Transgenic Res. 2010, 19, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wan, L.; Zhang, L.; Zhang, Z.; Zhang, H.; Quan, R.; Zhou, S.; Huang, R. An Ethylene Response Factor OsWR1 Responsive to Drought Stress Transcriptionally Activates Wax Synthesis Related Genes and Increases Wax Production in Rice. Plant Mol. Biol. 2012, 78, 275–288. [Google Scholar] [CrossRef]
- Chen, K.; Song, M.; Guo, Y.; Liu, L.; Xue, H.; Dai, H.; Zhang, Z. Md MYB 46 Could Enhance Salt and Osmotic Stress Tolerance in Apple by Directly Activating Stress-responsive Signals. Plant Biotechnol. J. 2019, 17, 2341–2355. [Google Scholar] [CrossRef]
- Geng, D.; Chen, P.; Shen, X.; Zhang, Y.; Li, X.; Jiang, L.; Xie, Y.; Niu, C.; Zhang, J.; Huang, X. MdMYB88 and MdMYB124 Enhance Drought Tolerance by Modulating Root Vessels and Cell Walls in Apple. Plant Physiol. 2018, 178, 1296–1309. [Google Scholar] [CrossRef]
- Yan, H.; Jia, H.; Chen, X.; Hao, L.; An, H.; Guo, X. The Cotton WRKY Transcription Factor GhWRKY17 Functions in Drought and Salt Stress in Transgenic Nicotiana Benthamiana through ABA Signaling and the Modulation of Reactive Oxygen Species Production. Plant Cell Physiol. 2014, 55, 2060–2076. [Google Scholar] [CrossRef]
- Nakashima, K.; Ito, Y.; Yamaguchi-Shinozaki, K. Transcriptional Regulatory Networks in Response to Abiotic Stresses in Arabidopsis and Grasses. Plant Physiol. 2009, 149, 88–95. [Google Scholar] [CrossRef]
- Jogawat, A.; Yadav, B.; Chhaya; Lakra, N.; Singh, A.K.; Narayan, O.P. Crosstalk between Phytohormones and Secondary Metabolites in the Drought Stress Tolerance of Crop Plants: A Review. Physiol. Plant. 2021, 172, 1106–1132. [Google Scholar] [CrossRef]
- Binenbaum, J.; Weinstain, R.; Shani, E. Gibberellin Localization and Transport in Plants. Trends Plant Sci. 2018, 23, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, P.; Wang, G.; Augstein, F.; de Vries, J.; Carlsbecker, A. Continuous Root Xylem Formation and Vascular Acclimation to Water Deficit Involves Endodermal ABA Signalling via MiR165. Development 2018, 145, dev159202. [Google Scholar] [CrossRef] [PubMed]
- Fraudentali, I.; Ghuge, S.A.; Carucci, A.; Tavladoraki, P.; Angelini, R.; Rodrigues-Pousada, R.A.; Cona, A. Developmental, Hormone-and Stress-Modulated Expression Profiles of Four Members of the Arabidopsis Copper-Amine Oxidase Gene Family. Plant Physiol. Biochem. 2020, 147, 141–160. [Google Scholar] [CrossRef]
- Yoon, Y.; Seo, D.H.; Shin, H.; Kim, H.J.; Kim, C.M.; Jang, G. The Role of Stress-Responsive Transcription Factors in Modulating Abiotic Stress Tolerance in Plants. Agronomy 2020, 10, 788. [Google Scholar] [CrossRef]
- Chen, R.; Ma, J.; Luo, D.; Hou, X.; Ma, F.; Zhang, Y.; Meng, Y.; Zhang, H.; Guo, W. CaMADS, a MADS-Box Transcription Factor from Pepper, Plays an Important Role in the Response to Cold, Salt, and Osmotic Stress. Plant Sci. 2019, 280, 164–174. [Google Scholar] [CrossRef]
- Khan, A.; Pan, X.; Najeeb, U.; Tan, D.K.Y.; Fahad, S.; Zahoor, R.; Luo, H. Coping with Drought: Stress and Adaptive Mechanisms, and Management through Cultural and Molecular Alternatives in Cotton as Vital Constituents for Plant Stress Resilience and Fitness. Biol. Res. 2018, 51. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Khalid, S.; Abdullah, M.; Ahmed, Z.; Shah, M.K.N.; Ghafoor, A.; Du, X. Insights into Drought Stress Signaling in Plants and the Molecular Genetic Basis of Cotton Drought Tolerance. Cells 2019, 9, 105. [Google Scholar] [CrossRef]
- Haider, M.E. Advances in Transgenic Technology for Crop Cultivation and Stomatal Regulation as Potent Role in Agriculture. Sch. Int. J. Biochem. 2021, 4, 86–90. [Google Scholar]
- Wang, N.-N.; Xu, S.-W.; Sun, Y.-L.; Liu, D.; Zhou, L.; Li, Y.; Li, X.-B. The Cotton WRKY Transcription Factor (GhWRKY33) Reduces Transgenic Arabidopsis Resistance to Drought Stress. Sci. Rep. 2019, 9, 724. [Google Scholar] [CrossRef]
- Rehman, A.; Azhar, M.T.; Hinze, L.; Qayyum, A.; Li, H.; Peng, Z.; Qin, G.; Jia, Y.; Pan, Z.; He, S. Insight into Abscisic Acid Perception and Signaling to Increase Plant Tolerance to Abiotic Stress. J. Plant Interact. 2021, 16, 222–237. [Google Scholar] [CrossRef]
- Yang, W.H.; Lu, C.Z.; Chen, W.; Xu, H.Y. Reduction of Early Fruit Abscission by Main-Branch-Girdling in Macadamia Is Related to the Favorable Status of Carbohydrates and Endogenous Hormones. HortScience 2022, 57, 40–47. [Google Scholar] [CrossRef]
- Parveen, A.; Ahmar, S.; Kamran, M.; Malik, Z.; Ali, A.; Riaz, M.; Abbasi, G.H.; Khan, M.; Sohail, A.B.; Rizwan, M. Abscisic Acid Signaling Reduced Transpiration Flow, Regulated Na+ Ion Homeostasis and Antioxidant Enzyme Activities to Induce Salinity Tolerance in Wheat (Triticum aestivum L.) Seedlings. Environ. Technol. Innov. 2021, 24, 101808. [Google Scholar] [CrossRef]
- Chen, K.; Li, G.; Bressan, R.A.; Song, C.; Zhu, J.; Zhao, Y. Abscisic Acid Dynamics, Signaling, and Functions in Plants. J. Integr. Plant Biol. 2020, 62, 25–54. [Google Scholar] [CrossRef] [PubMed]
- Khatun, M.; Sarkar, S.; Era, F.M.; Islam, A.K.M.M.; Anwar, M.P.; Fahad, S.; Datta, R.; Islam, A.K.M.A. Drought Stress in Grain Legumes: Effects, Tolerance Mechanisms and Management. Agronomy 2021, 11, 2374. [Google Scholar] [CrossRef]
- Pál, M.; Tajti, J.; Szalai, G.; Peeva, V.; Végh, B.; Janda, T. Interaction of Polyamines, Abscisic Acid and Proline under Osmotic Stress in the Leaves of Wheat Plants. Sci. Rep. 2018, 8, 12839. [Google Scholar] [CrossRef]
- Ouledali, S.; Ennajeh, M.; Ferrandino, A.; Khemira, H.; Schubert, A.; Secchi, F. Influence of Arbuscular Mycorrhizal Fungi Inoculation on the Control of Stomata Functioning by Abscisic Acid (ABA) in Drought-Stressed Olive Plants. S. Afr. J. Bot. 2019, 121, 152–158. [Google Scholar] [CrossRef]
- Li, S.; Liu, J.; Liu, H.; Qiu, R.; Gao, Y.; Duan, A. Role of Hydraulic Signal and ABA in Decrease of Leaf Stomatal and Mesophyll Conductance in Soil Drought-Stressed Tomato. Front. Plant Sci. 2021, 12, 653186. [Google Scholar] [CrossRef]
- El-Yazied, A.A.; Ibrahim, M.F.M.; Ibrahim, M.A.R.; Nasef, I.N.; Al-Qahtani, S.M.; Al-Harbi, N.A.; Alzuaibr, F.M.; Alaklabi, A.; Dessoky, E.S.; Alabdallah, N.M. Melatonin Mitigates Drought Induced Oxidative Stress in Potato Plants through Modulation of Osmolytes, Sugar Metabolism, ABA Homeostasis and Antioxidant Enzymes. Plants 2022, 11, 1151. [Google Scholar] [CrossRef]
- Al-Abdallat, A.M.; Al-Debei, H.S.; Ayad, J.Y.; Hasan, S. Over-Expression of SlSHN1 Gene Improves Drought Tolerance by Increasing Cuticular Wax Accumulation in Tomato. Int. J. Mol. Sci. 2014, 15, 19499–19515. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Xu, C.; Ren, J.; Liu, X.; Black, K.; Gai, X.; Wang, Q.; Ren, H. Cucumber ECERIFERUM1 (CsCER1), Which Influences the Cuticle Properties and Drought Tolerance of Cucumber, Plays a Key Role in VLC Alkanes Biosynthesis. Plant Mol. Biol. 2015, 87, 219–233. [Google Scholar] [CrossRef]
- Xing, X.; Cao, C.; Xu, Z.; Qi, Y.; Fei, T.; Jiang, H.; Wang, X. Reduced Soybean Water Stress Tolerance by MiR393a-Mediated Repression of GmTIR1 and Abscisic Acid Accumulation. J. Plant Growth Regul. 2023, 42, 1067–1083. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, H.; Zhu, H.; Tao, Y.; Liu, C.; Liu, J.; Yang, F.; Li, M. Exogenous ABA Enhances the Antioxidant Defense System of Maize by Regulating the AsA-GSH Cycle under Drought Stress. Sustainability 2022, 14, 3071. [Google Scholar] [CrossRef]
- Khosravi-Nejad, F.; Khavari-Nejad, R.A.; Moradi, F.; Najafi, F. Cytokinin and Abscisic Acid Alleviate Drought Stress through Changing Organic Acids Profile, Ion Immolation, and Fatty Acid Profile to Improve Yield of Wheat (Triticum aestivum L.) Cultivars. Physiol. Mol. Biol. Plants 2022, 28, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Yari Kamrani, Y.; Shomali, A.; Aliniaeifard, S.; Lastochkina, O.; Moosavi-Nezhad, M.; Hajinajaf, N.; Talar, U. Regulatory Role of Circadian Clocks on ABA Production and Signaling, Stomatal Responses, and Water-Use Efficiency under Water-Deficit Conditions. Cells 2022, 11, 1154. [Google Scholar] [CrossRef]
- Rehman, R.S.; Ali, M.; Ali Zafar, S.; Hussain, M.; Pasha, A.; Saqib Naveed, M.; Ahmad, M.; Waseem, M. Abscisic Acid Mediated Abiotic Stress Tolerance in Plants. Asian J. Res. Crop Sci. 2022, 7, 1–17. [Google Scholar] [CrossRef]
- Chung, K.; Demianski, A.J.; Harrison, G.A.; Laurie-Berry, N.; Mitsuda, N.; Kunkel, B.N. Jasmonate Hypersensitive 3 Negatively Regulates Both Jasmonate and Ethylene-Mediated Responses in Arabidopsis. J. Exp. Bot. 2022, 73, 5067–5083. [Google Scholar] [CrossRef]
- Kazan, K. Diverse Roles of Jasmonates and Ethylene in Abiotic Stress Tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Plant Response to Jasmonates: Current Developments and Their Role in Changing Environment. Bull. Natl. Res. Cent. 2019, 43, 153. [Google Scholar] [CrossRef]
- Ghorbel, M.; Brini, F.; Sharma, A.; Landi, M. Role of Jasmonic Acid in Plants: The Molecular Point of View. Plant Cell Rep. 2021, 40, 1471–1494. [Google Scholar] [CrossRef]
- Ali, M.S.; Baek, K.-H. Jasmonic Acid Signaling Pathway in Response to Abiotic Stresses in Plants. Int. J. Mol. Sci. 2020, 21, 621. [Google Scholar] [CrossRef]
- Arvin, M.J. The Effect of Sodium Silicate and Methyl Jasmonate on Pigments and Antioxidant Activity of Tomato (Solanum lycopersicum L.) Under Salinity Stress. J. Agric. Sci. 2020, 26, 479–487. [Google Scholar] [CrossRef]
- Per, T.S.; Khan, M.I.R.; Anjum, N.A.; Masood, A.; Hussain, S.J.; Khan, N.A. Jasmonates in Plants under Abiotic Stresses: Crosstalk with Other Phytohormones Matters. Environ. Exp. Bot. 2018, 145, 104–120. [Google Scholar] [CrossRef]
- Mir, R.A.; Bhat, B.A.; Yousuf, H.; Islam, S.T.; Raza, A.; Rizvi, M.A.; Charagh, S.; Albaqami, M.; Sofi, P.A.; Zargar, S.M. Multidimensional Role of Silicon to Activate Resilient Plant Growth and to Mitigate Abiotic Stress. Front. Plant Sci. 2022, 13, 819658. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Fatma, M.; Gautam, H.; Sehar, Z.; Rasheed, F.; Khan, M.I.R.; Sofo, A.; Khan, N.A. Salicylic Acid Increases Photosynthesis of Drought Grown Mustard Plants Effectively with Sufficient-N via Regulation of Ethylene, Abscisic Acid, and Nitrogen-Use Efficiency. J. Plant Growth Regul. 2022, 41, 1966–1977. [Google Scholar] [CrossRef]
- Yadav, B.; Jogawat, A.; Gnanasekaran, P.; Kumari, P.; Lakra, N.; Lal, S.K.; Pawar, J.; Narayan, O.P. An Overview of Recent Advancement in Phytohormones-Mediated Stress Management and Drought Tolerance in Crop Plants. Plant Gene 2021, 25, 100264. [Google Scholar]
- Signorelli, S.; Tarkowski, Ł.P.; Van den Ende, W.; Bassham, D.C. Linking Autophagy to Abiotic and Biotic Stress Responses. Trends Plant Sci. 2019, 24, 413–430. [Google Scholar] [CrossRef]
- Hassanein, R.A.; Amin, A.A.E.; Rashad, E.-S.M.; Ali, H. Effect of Thiourea and Salicylic Acid on Antioxidant Defense of Wheat Plants under Drought Stress. Int. J. ChemTech Res. 2015, 7, 346–354. [Google Scholar]
- Khalvandi, M.; Siosemardeh, A.; Roohi, E.; Keramati, S. Salicylic Acid Alleviated the Effect of Drought Stress on Photosynthetic Characteristics and Leaf Protein Pattern in Winter Wheat. Heliyon 2021, 7, e05908. [Google Scholar] [CrossRef]
- Garg, N.; Bharti, A. Salicylic Acid Improves Arbuscular Mycorrhizal Symbiosis, and Chickpea Growth and Yield by Modulating Carbohydrate Metabolism under Salt Stress. Mycorrhiza 2018, 28, 727–746. [Google Scholar] [CrossRef]
- Bandurska, H. Salicylic Acid: An Update on Biosynthesis and Action in Plant Response to Water Deficit and Performance under Drought. In Salicylic Acid; Springer: Dordrecht, The Netherlands, 2013; pp. 1–14. [Google Scholar]
- La, V.H.; Lee, B.-R.; Zhang, Q.; Park, S.-H.; Islam, M.T.; Kim, T.-H. Salicylic Acid Improves Drought-Stress Tolerance by Regulating the Redox Status and Proline Metabolism in Brassica Rapa. Hortic. Environ. Biotechnol. 2019, 60, 31–40. [Google Scholar] [CrossRef]
- Torun, H. Time-course Analysis of Salicylic Acid Effects on ROS Regulation and Antioxidant Defense in Roots of Hulled and Hulless Barley under Combined Stress of Drought, Heat and Salinity. Physiol. Plant. 2019, 165, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Chandwani, S.; Amaresan, N. Role of ACC Deaminase Producing Bacteria for Abiotic Stress Management and Sustainable Agriculture Production. Environ. Sci. Pollut. Res. 2022, 29, 22843–22859. [Google Scholar] [CrossRef] [PubMed]
- Gautam, H.; Fatma, M.; Sehar, Z.; Iqbal, N.; Albaqami, M.; Khan, N.A. Exogenously-Sourced Ethylene Positively Modulates Photosynthesis, Carbohydrate Metabolism, and Antioxidant Defense to Enhance Heat Tolerance in Rice. Int. J. Mol. Sci. 2022, 23, 1031. [Google Scholar] [CrossRef] [PubMed]
- Meena, S.; Taria, S.; Nagar, S.; Yadav, S. Phytohormone Engineering: A Potential Approach for Inducing Abiotic Stress Tolerance in Crop Plants. Multidisciplinary 2022, 2022, 35. [Google Scholar]
- Houben, M.; Van de Poel, B. 1-Aminocyclopropane-1-Carboxylic Acid Oxidase (ACO): The Enzyme That Makes the Plant Hormone Ethylene. Front. Plant Sci. 2019, 10, 695. [Google Scholar] [CrossRef]
- Pérez-Pérez, J.G.; Puertolas, J.; Albacete, A.; Dodd, I.C. Alternation of Wet and Dry Sides during Partial Rootzone Drying Irrigation Enhances Leaf Ethylene Evolution. Environ. Exp. Bot. 2020, 176, 104095. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, K.; Li, J.; Gong, B.; Lu, Y.; Wu, X.; Lü, G.; Gao, H. Drought Stress Tolerance in Vegetables: The Functional Role of Structural Features, Key Gene Pathways, and Exogenous Hormones. Int. J. Mol. Sci. 2023, 24, 13876. https://doi.org/10.3390/ijms241813876
Abbas K, Li J, Gong B, Lu Y, Wu X, Lü G, Gao H. Drought Stress Tolerance in Vegetables: The Functional Role of Structural Features, Key Gene Pathways, and Exogenous Hormones. International Journal of Molecular Sciences. 2023; 24(18):13876. https://doi.org/10.3390/ijms241813876
Chicago/Turabian StyleAbbas, Kumail, Jingrui Li, Binbin Gong, Yusong Lu, Xiaolei Wu, Guiyun Lü, and Hongbo Gao. 2023. "Drought Stress Tolerance in Vegetables: The Functional Role of Structural Features, Key Gene Pathways, and Exogenous Hormones" International Journal of Molecular Sciences 24, no. 18: 13876. https://doi.org/10.3390/ijms241813876
APA StyleAbbas, K., Li, J., Gong, B., Lu, Y., Wu, X., Lü, G., & Gao, H. (2023). Drought Stress Tolerance in Vegetables: The Functional Role of Structural Features, Key Gene Pathways, and Exogenous Hormones. International Journal of Molecular Sciences, 24(18), 13876. https://doi.org/10.3390/ijms241813876






