1. Introduction
The 5-hydroxytryptamine (5-HT) was first isolated by Vittorio Erspamer from enterochromaffin cells of the intestine as a low-molecular substance that causes smooth muscle contraction [
1]. Later, 5-HT was isolated and crystallized from bovine blood serum, and its chemical structure has been established [
2]. It caused the contraction of an isolated blood vessel, the ear artery of a rabbit, so it was named serotonin. About 90% of the body’s 5-HT is produced in the digestive tract in the enterochromaffin cells [
3]. In the brain, 5-HT is synthesized in neurons in raphe nuclei of the brain stem [
4]. The 5-HT is also produced in the pulmonary endothelial [
5] and neuroendocrine [
6] cells, skin Merkel cells [
7], and taste buds [
8]. The action of 5-HT is mediated by seven types of 5-HT receptors (5-HTR), six of which are G-protein-coupled receptors (5-HT
1,2,4,5,6,7R), and one type (5-HT
3R) is a cationic channel [
9]. Within these types, there are subtypes of receptors, and the total number of genes coding 5-HTR is 18 (
https://www.guidetopharmacology.org, accessed on 20 May 2023). All subtypes of 5-HT receptors are expressed in the mammalian brain. Their functions in the central nervous system and involvement in neurological pathologies have been intensively studied for decades [
9]. Despite the discovery of 5-HT as a substance that causes vasoconstriction, the 5-HT receptors in blood vessels received less attention [
10].
The majority of 5-HT, which enters the bloodstream, is synthesized by the enterochromaffin cells of the human small intestinal mucosa and is transported by platelets. The average plasma concentration of 5-HT is very low; according to various estimates, it ranges from 1 to 100 nM [
10]. However, in some pathological conditions, it can increase greatly [
10,
11]. In particular, patients with COVID-19 had significantly increased plasma 5-HT and 5-hydroxyindoleacetic acid (5-HIAA) levels compared with healthy donors [
12]. The 5-HT and 5-HIAA plasma concentrations increased with higher severity of symptoms. It has been hypothesized that 5-HT is a putative mediator linking systemic manifestations, such as pulmonary, intestinal, and cardiac, which characterize the severe course of COVID-19 in individuals with diabetes and obesity [
13]. Altered concentrations of circulating 5-HT are implicated in several other pathologic conditions, including atherosclerosis and primary and secondary hypertension [
14].
Violations of 5-HT metabolism in pathologies are accompanied by changes in the functional activity of 5-HT receptors. Normally, 5-HT contracts blood vessels via 5-HT
2AR and 5-HT
1BR localized in smooth muscle cells [
10,
15]. However, in the hypertension [
16,
17] and diabetes, 5-HT
2BR is also involved in the 5-HT-induced vasoconstriction [
18]. Elevated vasoconstriction in deoxycorticosterone acetate (DOCA)-salt and N(omega)-nitro-L-arginine (L-NAME) hypertensive rats correlates with the elevation of 5-HT
2BR expression [
16,
17]. On the contrary, enhanced contraction of arteries from diabetics to 5-HT via 5-HT
2BR in a mouse model of type II diabetes mellitus is not accompanied by an increase in their expressions [
18]. Oxidative stress and the generation of excessive reactive oxygen species (ROS) are known to be associated with the development of metabolic diseases, including diabetes [
19]. It can be suggested that, in this case, ROS affects the sensitivity of cells to serotonin. We have demonstrated that in smooth muscle cells (SMCs) from rat aorta, there are functionally inactive, “silent” 5-HT
2BR, which, under conditions of artificially induced oxidative stress, acquire the ability to stimulate a rise in [Ca
2+]
i level in SMCs [
20]. Under the influence of oxidative stress, the isolated rat mesenteric artery and aorta became able to contract in response to the activation of 5-HT
2BR by its agonist BW723C86.
Thus, the above data indicate the existence of a link between the state of 5-HT
2BR and the development of several vascular pathologies. For a more complete understanding of the mechanisms of pathogenesis of these diseases, a detailed study of the regulation of the [Ca
2+]
i level by 5-HT
2BR and other 5-HT receptors in vascular cells is required. Endothelial cells (EC) are the first target of 5-HT in blood vessels and are in the frontline protecting vascular wall from elevated concentrations of 5-HT in plasma. However, little is known about the effect of 5-HT on [Ca
2+]
i in EC and the role of the individual receptors in this process. Vascular EC express 5-HT
2BR coupled with G
q/11 protein and phospholipase C, 5-HT
1BR coupled with Gi protein and adenylyl cyclase (AC) inhibition, and 5-HT
4 receptors coupled with Gs protein and AC activation [
10]. Information on the effect of 5-HT on calcium metabolism in EC is fragmentary and somewhat contradictory. According to [
21], 5-HT at a concentration of up to 100 μM does not induce [Ca
2+]
i elevation in HUVEC. The authors suggested that 5-HT-induced secretion of von Willebrand factor in HUVEC is mediated by a decrease in cAMP level due to activation of 5-HT
1BR. On the other hand, it has been shown that in EC from human coronary arteries, activation of 5-HT
1BR and 5-HT
2BR causes an increase in [Ca
2+]
i [
22]. Ullmer et al. [
23] demonstrated that the 5-HT
2BR agonist BW723C86 causes an increase in [Ca
2+]
i in human pulmonary artery EC by activating ryanodine-sensitive reticulum channels, while 5-HT
1BR is not involved in the regulation of calcium metabolism [
23]. According to our data, both 5-HT
1BR and 5-HT
2BR in the presence of hydrogen peroxide markedly elevate [Ca
2+]
i in HUVEC [
24]. On the contrary, H
2O
2 attenuates histamine-induced [Ca
2+]
i rise in these cells [
25].
The aim of this work was to further explore the regulation of [Ca2+]i via 5-HT1BR and 5-HT2BR in HUVEC and to compare the effects of their agonists with the effects of histamine and agonists of other receptors that cause an increase in [Ca2+]i in EC. CGS12066B and BW723C86 were used to stimulate 5-HT1BR and 5-HT2BR, respectively. Since both 5-HT1BR and 5-HT2BR might be involved in the action of 5-HT on calcium metabolism in HUVEC, we investigated what would be the changes in [Ca2+]i when they are simultaneously activated by their selective agonists. Surprisingly, it has been found that their action on [Ca2+]i in HUVEC exhibits a strong synergy. Registration of the responses of individual cells has demonstrated that simultaneous stimulation of 5-HT1BR and 5-HT2BR results in an increase in the number of the cells in which [Ca2+]i rise occurs and initiates the bursts of [Ca2+]i oscillations in addition to single oscillations. We have also demonstrated that 5-HT induces very weak [Ca2+]i rise in HUVEC via 5-HT2BR, and 5-HT1BR agonist CGS12066B greatly enhances the 5-HT effect.
2. Results
Previous PCR analyses demonstrated the expression of mRNA of 5-HT
1BR and 5-HT
2BR in HUVEC [
26]. Using quantitative PCR, we also showed the presence of mRNA encoding 5-HT
1BR and 5-HT
2BR in these cells (
Supplementary Figure S1). The amounts of 5-HT
1BR and 5-HT
2BR mRNAs were nearly equal. In HUVEC, a low level of expression of mRNA encoding of 5-HT
2CR was revealed. The amount of 5-HT
2CR mRNA was less than 0.5% of 5-HT
1BR or 5-HT
2BR mRNA. HT
2AR mRNA is absent in HUVEC. The presence of 5-HT
1BR and 5-HT
2BR proteins in HUVEC was demonstrated by Western blot hybridization (
Supplementary Figure S2) and immunofluorescent staining. To determine whether 5-HT
1BR and 5-HT
2BR are localized not only in the plasma membrane but also within HUVEC, the cells were stained with antibodies in the absence and presence of Triton X-100. It was found that 5-HT
1BR and 5-HT
2BR were expressed both on the cell surface (
Supplementary Figure S3) and inside the cells (
Supplementary Figure S4). We determined intracellular localization of 5-HT
1BR and 5-HT
2BR in HUVEC at higher resolution. As shown in
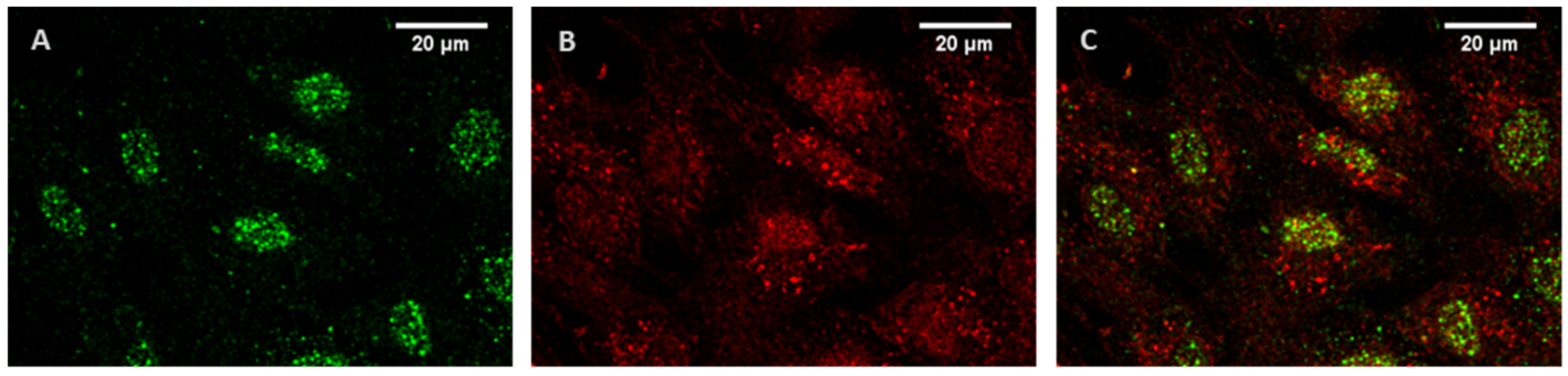
Figure 1, the majority of 5-HT
1BR is localized in the nuclear region, while 5-HT
2BR is distributed relatively evenly in the cells.
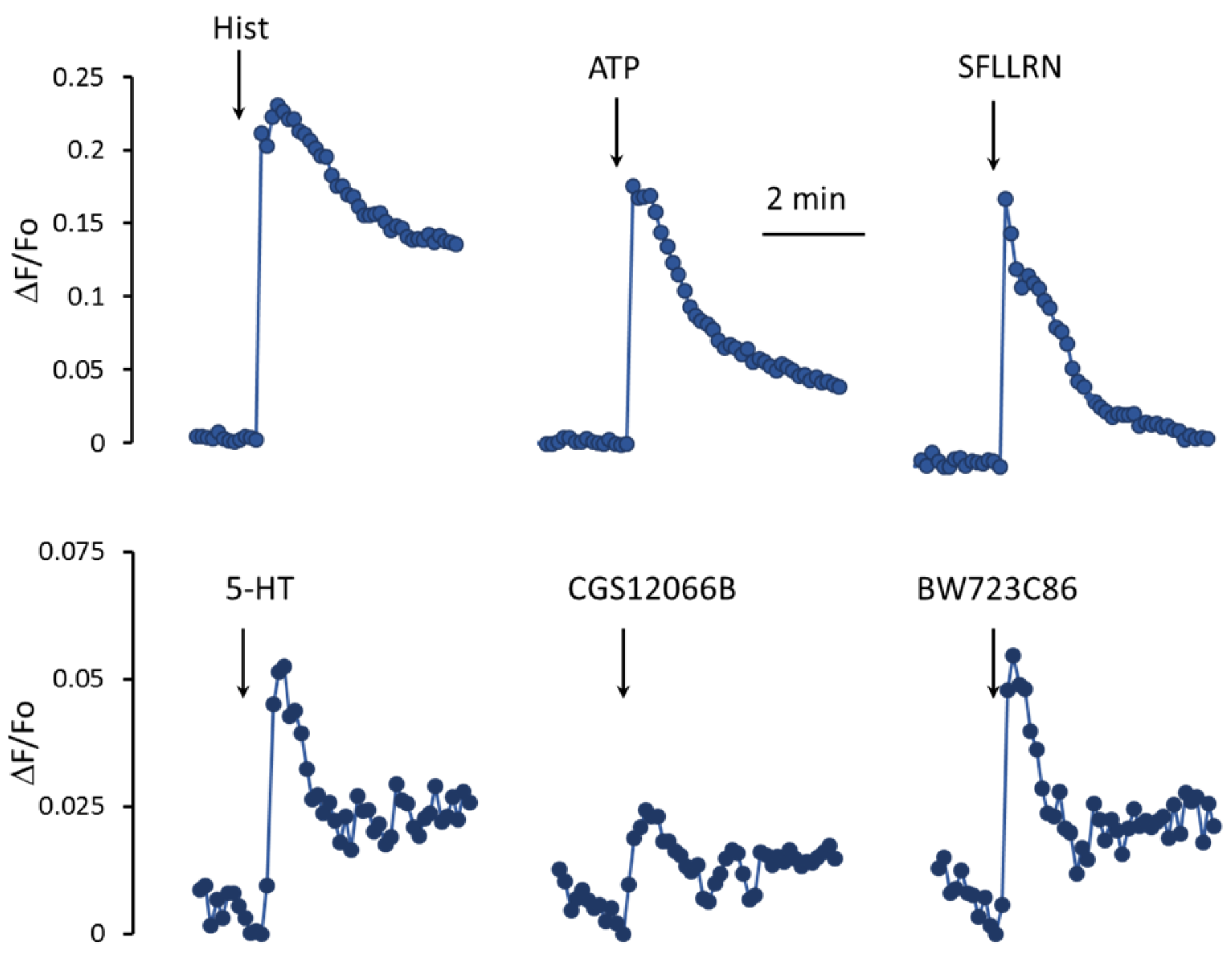
In EC, the metabolism of calcium ions is activated by histamine, ATP, thrombin, and a number of other factors [
27,
28]. As shown in
Figure 2, histamine, an agonist of protease-activated receptors type 1 SFLLRN, and ATP cause a rapid increase in mean [Ca
2+]
i levels in a population of HUVEC. Unlike histamine and other agonists, 5-HT acts much weaker. In response to 100 μM 5-HT, the increase in [Ca
2+]
i is three–five times lower. In addition, the rate of rise in the [Ca
2+]
i level in the cell population in response to 5-HT is also significantly slower than in response to histamine, SFLLRN, and ATP. To elucidate the role of 5-HT
1BR and 5-HT
2BR in the regulation of [Ca
2+]
i in HUVEC, we used their agonists, CGS12066B [
29] and BW723C86 [
30], respectively. The 5-HT
1BR agonist CGS12066B caused a weaker increase in mean [Ca
2+]
i levels in the HUVEC compared to 5-HT. The 5-HT
2BR agonist BW723C86 at a concentration of 50 µM caused an increase in [Ca
2+]
i comparable to that of 5-HT by magnitude and rate.
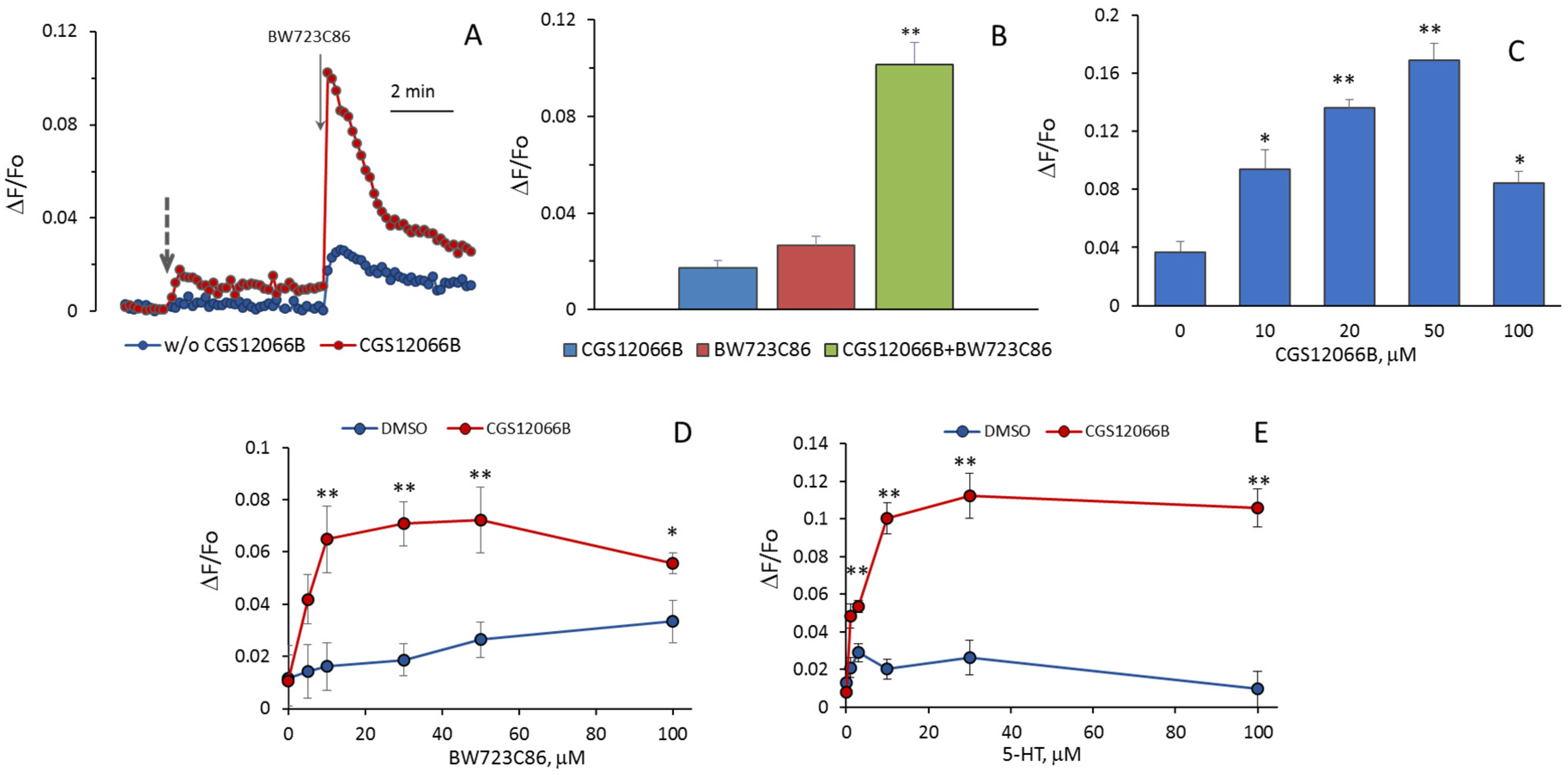
It could be expected that 5-HT increases [Ca
2+]
i as a result of activation of 5-HT
1BR and 5-HT
2BR. We assumed that an additive effect would be observed when agonists of these receptors were added to HUVEC, but it turned out that CGS12066B and BW723C86 have a synergistic effect on [Ca
2+]
i. After preincubation with 50 µM CGS12066B, the rise in [Ca
2+]
i in HUVEC in response to 30 µM BW723C86 increased three–five times (
Figure 3A,B). In a separate experiment, the cells were preincubated with different concentrations of CGS12066B for 5 min, and after that, we determined the response to 30 μM BW723C86 (
Figure 3C). CGS12066B potentiated the calcium response to BW723C86 starting from a concentration of 10 μM; the maximum enhancement occurred at a CGS12066B concentration of about 50 μM, and this effect was weakened at 100 μM. We have studied whether and how 5-HT
1BR activation would affect the concentration dependence of the increase in [Ca
2+]
i in response to BW723C86 and 5-HT. After preincubation with 50 μM CGS12066B, BW723C86 causes an increase in [Ca
2+]
i already at 5 μM, and at 10 μM, the effect of BW723C86 reaches its maximum (
Figure 3D). In the presence of CGS12066B [Ca
2+]
i, elevation occurs at 1–3 μM of 5-HT and further increases at 10–100 μM (
Figure 3E). Without CGS12066B, the effect of 5-HT on [Ca
2+]
i was either rather small (
Figure 2) or almost absent (
Figure 3E).
In additional experiments, we determined the effects of 5-HT
1BR and 5-HT
2BR antagonists on the [Ca
2+]
i rise induced by CGS12066B and BW723C86 in HUVEC. As shown in
Figure 4A,B, the 5-HT
1BR antagonist methiothepin [
31] at a concentration of 10 μM abolished potentiation by CGS12066B of BW723C86-induced Ca
2+ mobilization. According to [
32], methiothepin also binds to 5-HT
2BR. In our experiments, methiothepin did not suppress the rise in [Ca
2+]
i induced by BW723C86 in the absence of CGS12066B. This indicates that methiothepin affects 5-HT
2BR in HUVEC. We explored the effect of another 5-HT
1BR antagonist, SB216641 [
33]. SB216641 at concentrations of 10 to 50 μM was added 5 min before CGS12066B (50 μM). Then, [Ca
2+]
i responses to BW723C86 (30 μM) were recorded. As shown in
Figure 4C, SB216641 caused a concentration-dependent decrease in [Ca
2+]
i rise. The 5-HT
2BR antagonist RS127445 [
34] suppressed calcium response to 30 µM BW723C86 (
Figure 5A,B), but the blocking effect of RS127445 disappeared after a subsequent increase in the concentration of BW723C86 to 100 µM. This indicates a competition between these two compounds for binding to the 5-HT
2BR. CGS12066B strongly increases the response to 5-HT (
Figure 5C), which is suppressed by the 5-HT
2BR antagonist RS127445.
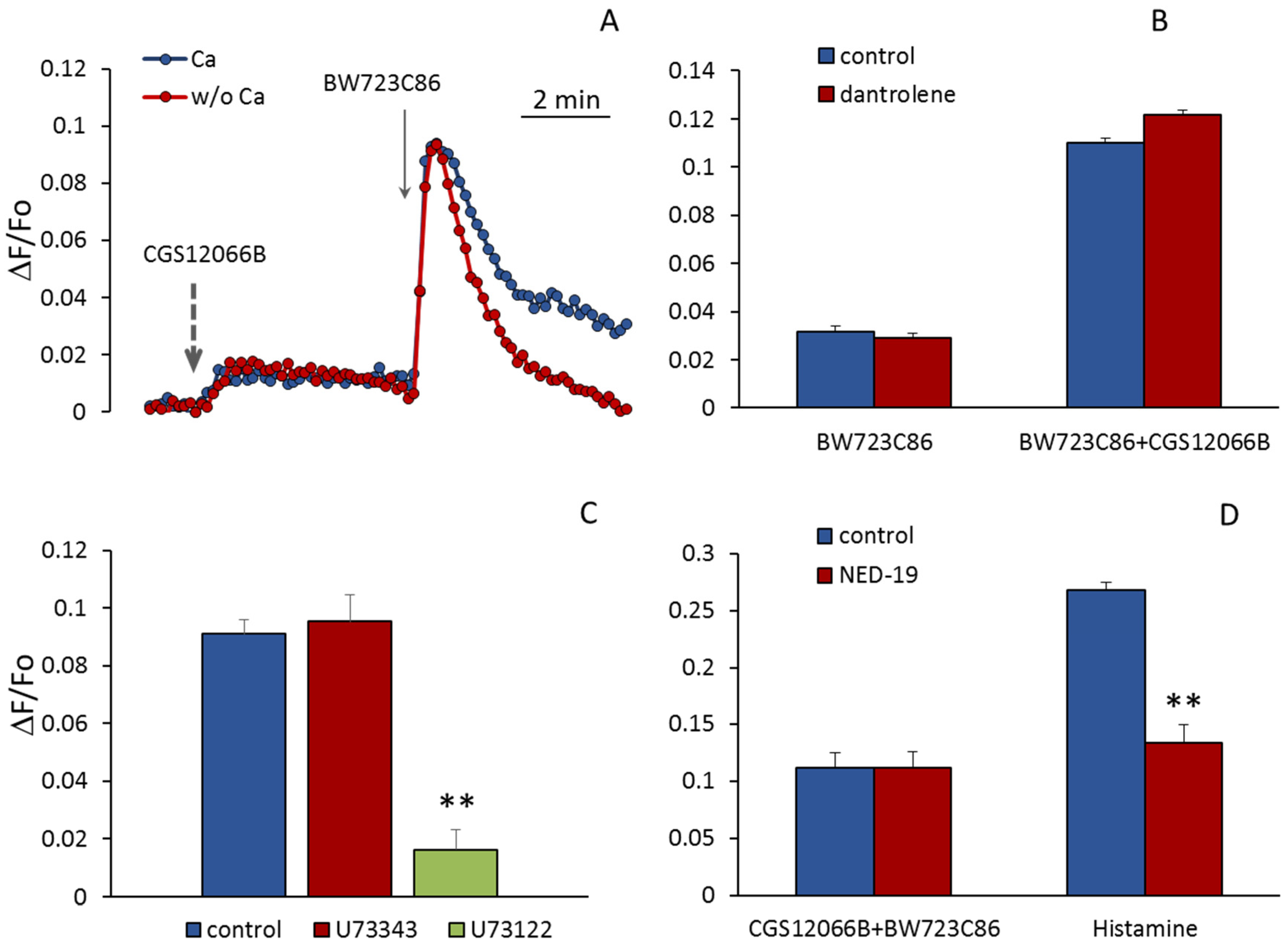
According to Ullmer et al. [
23], upon activation of 5-HT
2BR in human pulmonary artery EC, an increase in [Ca
2+]
i occurs as a result of the release of Ca
2+ from the reticulum through ryanodine-sensitive channels (RyR). It could be assumed that CGS12066 potentiates the EC response to BW723C86 due to the influx of Ca
2+ ions from the outside and triggering Ca
2+-induced Ca
2+-release (CICR). However, as shown in
Figure 6A, the removal of calcium ions from the extracellular environment does not reduce the rise in [Ca
2+]
i induced by BW723C86 in the presence of added CGS12066B. Dependence on extracellular calcium manifests itself in the second slow phase of the response to BW723C86, which indicates an inward current of Ca
2+, apparently by the mechanism of store-operated calcium entry. It has been shown that EC of the human mesenteric artery and immortalized EA.hy926 endothelial-derived cells express RyR3, whereas RyR1 and RyR2 were not detected in these cells [
35]. RyR3 is known to be inhibited by dantrolene [
36]. We used this compound to find out if RyRs in HUVEC are involved in the potentiation of the response to BW723C86 by CGS12066B. In our experiments, dantrolene at a concentration of 50 μM did not reduce the rise in [Ca
2+]
i in HUVEC in response to BW723C86 alone and together with CGS12066B (
Figure 6B). In contrast, inhibition of phospholipase C activity by U73122 almost completely abolished the calcium signal from 5-HT
2BR (
Figure 6C). We hypothesized that potentiation of the BW723C886-induced [Ca
2+]
i increase by CGS12066B could be due to the release of calcium ions from acidic endolysosomal vesicles via two-pore channels (TPC) activated by NAADP. One of the functions of these channels is to trigger global calcium release by recruiting CICR channels at lysosomal–endoplasmic reticulum (ER) junctions [
37]. The structural analog of NAADP substance NED-19 is a blocker of these channels [
38]. NAADP-activated channels have been shown to be involved in histamine-induced Ca increase in HUVEC [
39]. To find out if these channels are activated under the influence of CGS12066B and, thus, potentiate the action of BW723C86, we used NED-19 and revealed that it did not affect the rise in [Ca
2+]
i under the influence of CGS12066B and BW723C86, although attenuated histamine-induced [Ca
2+]
i elevation (
Figure 6D).
For a better understanding of how the calcium signal of HUVEC is enhanced with simultaneous activation of 5-HT
1BR and 5-HT
2BR, we registered changes in [Ca
2+]
i in single cells. Fluorescence measurement was performed using a Leica DMI6000 microscope in the second–fourth passages of HUVEC cultured in 24-well plates. It has been previously shown that in response to the activation of membrane receptors in single EC [Ca
2+]
i, changes occur in the form of oscillations [
40].
Figure 7 shows characteristic oscillations when BW723C86 alone was added to HUVEC, when BW723C86 was added to HUVEC 5 min after CGS12066B, and when histamine was added to the cells. The fluorescence of CalciumGreen was recorded entirely from the whole cell. Processing of fluorescent signals from each cell in the field of view was carried out.
Theoretically observed potentiation of the response to BW723C86 in a population of HUVEC can occur for several reasons: as a result of an increase in the number of responding cells; synchronization of [Ca
2+]
i; rises in individual cells in the population; an increase in maximal [Ca
2+]
i; rise at oscillation; and the appearance of secondary oscillations. It can be seen from the graphs in
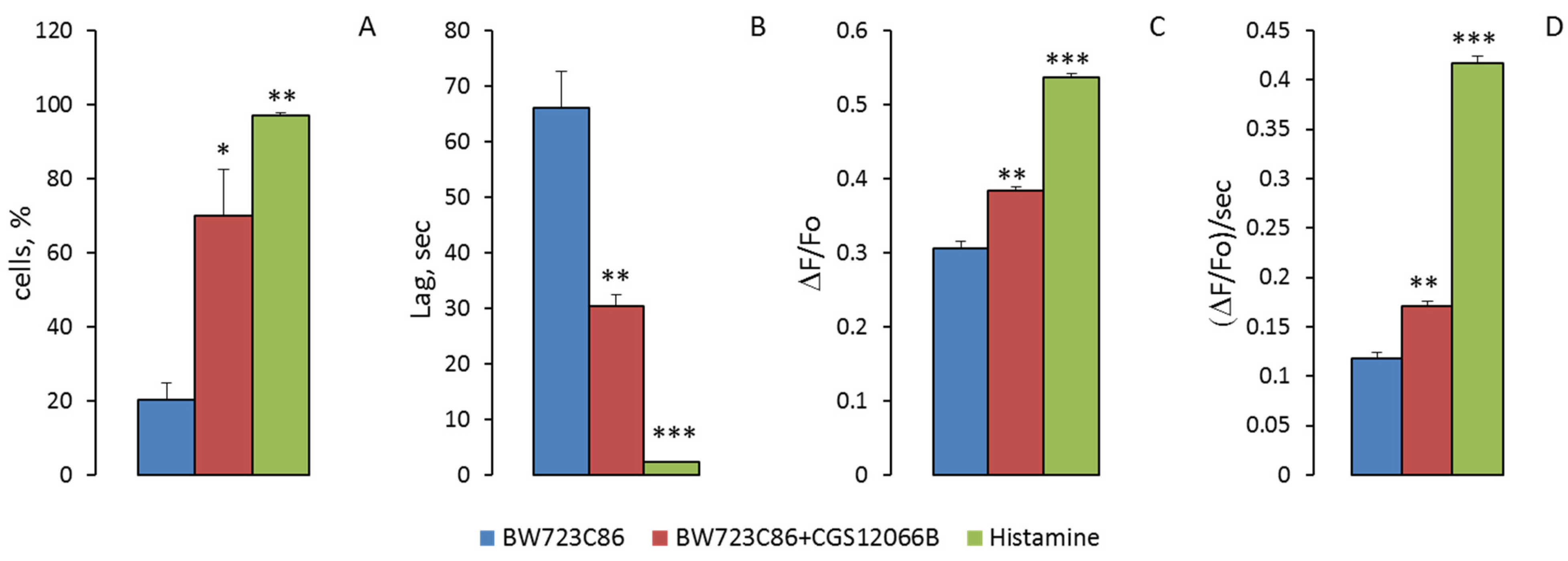
Figure 7 that the responses of individual cells to BW712C86 in the absence and presence of CGS12066B sharply differ. When only BW723C86 was added to HUVEC, they responded with single oscillations. The relative number of cells in which [Ca
2+]
i oscillations occurred was 19.8 ± 4.2% (
n = 3) (
Figure 8A). Measurements of [Ca
2+]
i were performed in three wells with the average number of HUVEC in the field of view 116 ± 3 (mean ± SEM). Within 5 min after the addition of BW723C86, secondary single oscillations of [Ca
2+]
i occurred in 2.3 ± 0.5% (
n = 3) of cells. After preincubation with CGS12066B, the cells responded to BW723C86 not only with single oscillations but also with bursts of oscillations. Responses to BW723C86 occurred in 69.7 ± 12.4% (
n = 3) cells. Secondary single oscillations or bursts of oscillations in response to BW723C86 added after CGS12066B occurred in 39.7 ± 13.2% (
n = 3) of HUVEC. Calcium responses of HUVEC to BW723C86 after preincubation with CGS12066B resembled those to histamine (
Figure 7). However, histamine calcium response was much stronger—it caused bursts of oscillation and secondary oscillations in 98 ± 1.4% (
n = 3) of HUVEC.
The responses of single cells to BW723C86 in the form of oscillations occurred with a much longer delay than responses to BW723C86 added after CGS12066B or responses to histamine (
Figure 7). The average times (means ± SEM) to reach the peak of the oscillation after adding BW723C86 and BW723C86 in the presence of CGS12066B or histamine were 66.1 ± 4.3 s (
n = 71; 95% CI for mean 53.0 to 79.1 s), 30.5 ± 1.9 s (
n = 227; 95% CI for mean 26.8 to 34.2 s), and 2.31 ± 0.04 s (
n = 292; 95% CI for means 2.24 to 2.38 s), respectively. This indicates that CGS12066B partially synchronizes the calcium responses of a single HUVEC.
We determined the parameters of [Ca
2+]
i oscillations arising in response to VW723C86, to BW723C86 in the presence of CGS12066B, and to histamine. The values of maximal [Ca
2+]
i rise at the peaks of oscillations (max ΔF/Fo), and the maximum rate of [Ca
2+]
i increase (ΔF/Fo per s) during oscillations were calculated (
Figure 8). The mean values of max ΔF/Fo (mean ± SEM) under the action of BW723C86 and BW723C86 in the presence of CGS12066B or histamine were 0.306 ± 0.009 (
n = 71; 95% CI for the mean 0.2878 to 0.3248), 0.414 ± 0.0678 (
n = 227; 95% CI for the mean 0.3719 to 0.3951), and 0.5369 ± 0.0054 (
n = 292; 95% CI for the mean 0.5262 to 0.5475). The maximum rates of [Ca
2+]
i rise (ΔF/Fo per second) were 0.118 ± 0.0061 s
−1 for BW723C86 (
n = 71; 95% CI for the mean 0.1058 to 0.1302), 0.171 ± 0.004 s
−1 (
n = 227; 95% CI for the mean 0.1616 to 0.1802) for BW723C86 with CGS12066B, and for histamine 0.4165 ± 0.0078 s
−1 (
n = 292; 95% CI for means 0.4010 to 0.4320). The presented data indicate that the increase in [Ca
2+]
i under the combined action of CGS12066B and BW723C86 occurs as a result of an increase in the number of reacting cells, synchronization of their responses, the appearance of bursts of oscillations and repeated oscillations, and an increase in the rate of [Ca
2+]
i rise.
3. Discussion
In this work, we have demonstrated that 5-HT
1BR and 5-HT
2BR in HUVEC are localized both in the plasma membrane and inside the cells. Intracellular 5-HT
1BR is located predominantly in the nuclear region, while 5-HT
2BR is distributed rather evenly in the cells. Expression in the nuclear membrane has been shown for different G-protein-coupled receptors (GPCRs) [
41,
42]. Various nuclear receptors activate G-proteins and stimulate the formation of second messengers (cAMP, IP3, etc.). With regard to 5-HT and other classical neurotransmitters, the idea of the existence of intracellular receptors functioning in cells at the pre-nervous stages of development was proposed many years ago [
43]. It has been shown that endogenous 5-HT is formed in cells already at the stage of cleavage divisions [
44]. An argument in favor of the existence of intracellular 5-HT receptors was the ability of lipophilic 5-HT derivatives to influence cleavage divisions and the absence of such an effect in hydrophilic 5-HT derivatives [
45].
We have found that the activation of 5-HT
1BR by CGS12066B by itself causes very slight changes in [Ca
2+]
i but increases the calcium signal from 5-HT
2BR in HUVEC several-fold. The potentiating effect of CGS12066B begins to manifest itself at concentrations of up to 10 µM, and at 50 µM, the effect of CGS12066B reaches its maximum. According to previously published data [
46], CGS12066A activates the binding of [
35S]GTPγS to rat striatal membranes with an EC50 of 1.2 µM and has a maximum effect at concentrations of 20 µM. These values are close to concentrations of CGS12066B, potentiating the calcium response to BW723C86 in HUVEC. Of note, the IC50 value for CGS12066B at the 5HT
1BR recognition site is 51 nM, as determined using the binding of [3H]5HT in the presence of 1 μM spiperone [
29]. A possible explanation for the quantitative discrepancy in the data on CGS12066B binding and on its functional effects may be the difference in the affinity for the agonist of the inactive G-protein uncoupled and the active G-protein coupled receptors.
To activate 5-HT
2BR, its agonist BW723C86 was used. It was demonstrated that this ligand could bind to all three types of 5-HT
2 receptors [
32]. As it was shown in [
26] and in our work, 5-HT
2ARs are not expressed in HUVEC. We have also demonstrated that the content of 5-HT
2CR mRNA in HUVEC is very low. Involvement of 5-HT
2CR [Ca
2+]
i rise in response to BW723C86 or 5-HT can be excluded since it is suppressed by 5-HT
2BR antagonist RS127445. We hypothesize that CGS12066B enhances the response to BW723C86 and 5-HT by activating intracellular 5-HT
1BR. CGS12066B is a lipophilic compound capable of diffusing through the plasma membrane and crossing the blood–brain barrier [
29]. We suggest that the lack of 5-HT action through 5-HT
1BR can be explained by the fact that 5-HT, unlike CGS12066B, cannot freely pass through the plasma membrane and bind to 5-HT
1BR inside the cell.
The next question concerns the mechanism by which 5-HT
2BR activates the rise in [Ca
2+]
i and how the response to BW723C86 is potentiated. It has been shown previously that in EC from the human pulmonary artery, this compound via 5-HT
2BR stimulates calcium release from intracellular stores through a pathway, which involves activation of ryanodine receptors and is independent of PI-hydrolysis [
23]. In contrast, in our experiments, blocking the activity of phospholipase C in HUVEC by 2 μM U73122 almost completely suppresses the rise in [Ca
2+]
i in response to BW723C86, while the RyR inhibitor dantrolene does not affect it. This suggests that 5-HT
2BR activates the release of calcium ions through InsP3-activated channels (InsP3R). InsP3R is a calcium ion-permeable channel formed by four protein subunits that, similar to RyR, are additionally activated by calcium ions [
47]. In the cytoplasmic part of the InsP3R monomers, there are high-affinity Ca
2+-binding sites that modulate the activity of the channel. According to [
48], the activation of these channels occurs as follows: the binding of inositol-1,4,5-trisphosphate to InsP3R causes the formation of clusters of single channels, and elevation of [Ca
2+]
i above the basal level increases the probability of channel open state, reduces the time of the closed state, and ensures their simultaneous opening in the cluster. Thus, there is an increase both in the intensity and duration of the current of calcium ions through the cluster formed by InsP3Rs. We suggest that the potentiation of the calcium signal from 5-HT
2BR under the action of the 5-HT
1BR agonist CGS12066B can be mediated by Ca
2+-induced activation of InsP3R. This hypothesis is consistent with our data on an increase in the [Ca
2+]
i rise rate and peak height of BW723C86-induced calcium oscillations in single cells under the influence of CGS12066B. At the first stage of the calcium response to BW723C86 added after CGS1206B, [Ca
2+]
i elevates due to its mobilization from intracellular depots since the maximal rise and the rate of [Ca
2+]
i elevation does not depend on the presence of calcium ions in the extracellular medium. After emptying the intracellular depots, the entry of calcium ions from the outside by the SOCE mechanism, the refilling of the reticulum, and repeated oscillations appear. We assume that CGS12066B locally elevates [Ca
2+]
i and, in this way, potentiates InsP3-dependent Ca
2+ release from the reticulum induced by BW723C86. The presence of a lag phase before the onset of Ca
2+ oscillations in HUVEC in response to activation of 5-HT
2BR can apparently be explained by a slow increase in the local [Ca
2+]
i to the threshold level required to open InsP
3-sensitive channels. CGS12066B causes a slight increase in [Ca
2+]
i in HUVEC. The intracellular source from which calcium ions are released in response to CGS12066B has not been established. We have shown that NED-19, a blocker of NAADP-stimulated two-pore channels, does not reduce the effect of CGS12066B, suggesting that acid endolysosomal vesicles are not involved in this process. The question of how the synergism is realized with the simultaneous activation of 5-HT
1BR and 5-HT
2BR needs further investigation.
An increase in [Ca
2+]
i is a trigger of many physiological and pathological processes. It has been shown that, normally, 5-HT through 5-HT
2BR regulates differentiation and proliferation during development as well as cardiac structure and function in adults [
49]. Changes in the functional activity and expression of 5-HT
2BR can cause disturbances in the regulation by 5-HT of normal physiological processes and contribute to the development of pathologies. Increased activation of 5-HT
2BR by the fenfluramines due to the off-target effect cause valvulopathy [
50]. According to [
51], the initial steps of mitral valve remodeling involved the mobilization of bone marrow-derived endothelial progenitor cells by 5-HT
2BR stimulation. The 5-HT is implicated in the growth of various malignant cell types [
52]. There is evidence of the involvement of 5-HT
1BR and 5-HT
2BR in these pathological processes [
53,
54]. Primary pulmonary hypertension is associated with a substantial increase in 5-HT
2BR expression in pulmonary arteries [
55]. At the same time, in pulmonary hypertension, the synthesis of 5-HT in EC increases [
5], which can cause activation of intracellular 5HT
1BR. As a result, 5-HT, as we assume, may induce a sharp increase in [Ca
2+]
i in the EC, leading to their damage. The risk of pulmonary hypertension in humans is increased under the action of dexfenfluramine, which is an active metabolite and an agonist of 5-HT
2BR. Bloodworth et al. [
56] demonstrated that bone marrow-derived proangiogenic cells contribute to experimental pulmonary hypertension in a 5-HT
2BR signaling-dependent manner. It is worth noting that SB216641, a 5-HT
1BR antagonist, prevented the development of pulmonary hypertension in a ROS-dependent manner [
57]. Dysregulation of intracellular calcium ions is a factor causing hypertension and related pathologies. In cultured smooth muscle cells from rat aorta, the Ca
2+-mobilizing activity of 5-HT
2BR is elevated under the action of the compounds that cause the generation of ROS [
20]. We have previously shown that ROS also affects the serotonergic regulation of calcium metabolism in HUVEC: the rise in [Ca
2+]
i caused by the activation of 5-HT
1BR, and 5-HT
2BR is significantly increased by the addition of H
2O
2 [
24], and, conversely, hydrogen peroxide inhibited the rise in [Ca
2+]
i induced by histamine [
25]. In the present work, we have found another feature in serotoninergic calcium regulation in these cells—the synergistic interaction of 5-HT
1BR and 5-HT
2BR upon activation of the rise in [Ca
2+]
i. In the vascular bed, a potent vasoconstrictor and an activator of smooth muscle cell proliferation endothelin-1 (ET-1) is produced by EC and released preferentially to the basal side of endothelium [
58]. One can speculate that an excessive rise in [Ca
2+]
i leads to endothelial hypersecretion of ET-1, and this may be an additional factor in the pathogenesis of hypertension. The increase in the calcium response of EC to 5-HT via 5-HT
2BR upon activation of 5-HT
1BR should apparently be taken into account as a potential cause of 5-HT undesirable effects.
4. Materials and Methods
4.1. Reagents
CGS12066B, BW723C86, RS127445, and SB216649 were from Tocris Bioscience (Bristol, UK); 5-HT, histamine, ATP, SFLLRN, U73122, U73343, and dantrolene were from Sigma-Aldrich (St. Louis, MO, USA); CalciumGreen/AM was from Thermo Fischer Scientific (Waltham, MA, USA). The stock solutions of CGS12066B, BW723C86, RS127445, SB216649, dantrolene, U73122, and U73343 were prepared in Dimethylsulfoxide (DMSO). Before being added to the cells, they were dissolved to the required concentrations in a physiological salt solution (PSS). DMSO at appropriate concentrations was used as a vehicle control. PSS was added as a vehicle control for 5-HT, ATP, histamine, and SFLLRN.
4.2. Cell Culture
HUVEC were isolated, according to [
59]. The cells were grown in plastic flasks pre-coated with gelatin, using M199 medium with Earl’s salts and 20 mM HEPES containing 20% fetal calf serum (Sigma-Aldrich), 300 µg/mL endothelial growth supplement, isolated from rabbit brain, 100 µg/mL heparin, and gentamicin. We used the cells on early passages (2–5). Accutase
® was applied for passaging the cells (Sigma-Aldrich).
4.3. Immunofluorescence
To determine the expression of 5-HT1BR and 5-HT2BR in the plasma membrane and inside HUVEC, the cells grown in a 48-well plate were fixed in 4% paraformaldehyde in 0.01 M phosphate-buffered saline (PBS) for 20 min. Fixed cells were incubated in PBS with 1% BSA without Triton X-100 or in the presence of 0.1% Triton X-100 for 20 min at 4 °C. The non-permeabilized cells were incubated overnight at +4 °C with HTR1B rabbit pAB (ABclonal, Woburn, MA, USA, A18285) or with HTR2B rabbit pAB (ABclonal, A5670), each diluted 1:100. Then, the cells were stained with donkey anti-rabbit Alexa Fluor™ 647 (Thermo Fischer Scientific, A-31573) diluted 1:500. Permeabilized with 0.1% Triton X-100 HUVEC were incubated with goat anti-5-HT1BR (MyBioSource, San Diego, CA, USA MBS420311) at a concentration of 1 μg/mL in PBS and HTR2B rabbit pAB (ABclonal, A5670), diluted 1:100. The cells were stained with a mixture of chicken anti-goat Alexa Fluor™ 488 (Thermo Fischer Scientific, A-21467) and donkey anti-rabbit Alexa Fluor™ 647 (Thermo Fischer Scientific, A-31573). The nuclei were stained with 10 μg/mL Hoechst 33342 (Biotium Inc., Fremont, CA, USA, #40044). The preparations of intact and permeabilized HUVEC were analyzed using a Leica THUNDER fluorescent microscope (Leica, Wetzlar, Germany), HC PL FLUOTAR L 20×/0.40 lens, and Quad Filter Block DFT51010.
In order to obtain a higher resolution of 5-HT1BR and 5-HT2BR, HUVEC were grown on a glass coverslip. The cells were fixed in 4% paraformaldehyde in 0.01 M phosphate-buffered saline (PBS) for 20 min. After washing three times in PBS, the cells were permeabilized in a 0.1% solution of Triton X-100 in PBS with 1% bovine serum albumin (BSA) for 10 min. After that, the cells were incubated for 24 h at +4 °C in a mixture of primary antibodies: goat anti-5-HT1BR (MyBioSource, San Diego, CA, USA MBS420311) and rabbit anti-5-HT2BR (BIOSSUSA, Woburn, MA, USA, bs-1892R) at a concentration of 1 μg/mL in PBS with 1% BSA, washed in PBS and stained with a mixture of secondary antibodies: chicken anti-goat Alexa Fluor™ 488 (Thermo Fischer Scientific, A-21467) and donkey anti-Rabbit Alexa Fluor™ 594 (Thermo Fischer Scientific, A-21207) diluted 1:500 12 h at +4 °C. The preparations were analyzed using a Leica DMI 6000 fluorescent microscope (Leica, Germany) using an HCX PL APO CS 40.0 × 1.25 OIL UV lens (Leica, Germany), diode illuminators with wavelengths of 488 and 594 nm and fluorescent filter cubes L5 ET, and TX2 ET (Leica, Germany), respectively.
4.4. Western Blot
HUVEC grown in a 25 cm2 flask were lysed in RIPA-buffer (Thermo Fisher Scientific, Waltham, MA, USA, #89901) containing protease inhibitor cocktail (Thermo Fischer Scientific, #78430) with 5 mM EDTA. The proteins (35 μg per lane) were separated on 10% PAAG in Tris-Glycine buffer (25 mM TRIS-base, 250 mM Glycine, 0.1% SDS) and electroblotted onto Immun-Blot PVDF membrane (BioRad Laboratories Inc., Hercules, CA, USA, #162-0177). After transfer, the membrane was blocked with 5% non-fat dry milk in TTBS (20 mM tris-Cl, pH 7.4, 0.5 M NaCl, 0.1% Tween 20) for 2 h at room temperature, cut into strips, and left to incubate with 5-HT1BR goat pAb (MyBioSource, MBS420311) at 1:150 dilution or HTR2B Rabbit pAb (Abclonal, A5670) at 1:1000 dilution in blocking buffer overnight at 4 °C. Then, the membrane strips were washed three times and incubated with secondary antibodies: HRP-conjugated goat anti-rabbit (BioRad #170-6515) or donkey anti-goat (R&D Systems, Minneapolis, MN, USA, #HAF109) at 1:1000 dilution for 1 h at room temperature. After incubation, the strips were washed three times and developed with Clarity MaxTM Western ECL Substrate #1705062. Luminescence was detected at the C-Digit reader manufactured by LI-COR Biosciences (Omaha, NE, USA).
4.5. Measurement of Free Cytoplasmic Calcium Concentration in HUVEC
HUVEC grown in 96-well plates were loaded with 1 µM CalciumGreen/AM dissolved with 0.02% Pluronic F-127 in M199 during 1 h at 37 °C in a CO2 incubator (New Brunswick Scientific, Edison, NJ, USA). Measurement of [Ca2+]i was performed in physiological salt solution (PSS) containing NaCl (145 mM), KCl (5 mM), MgCl2 (1 mM), CaCl2 (1 mM), HEPES (5 mM), and D-glucose (10 mM) at pH 7.4. Fluorescence was registered at 485 nm (excitation) and 530 nm (emission) at 25 °C using a Synergy 4 Microplate Reader (BioTek, Winooski, Vermont, USA). The changes in [Ca2+]i in HUVEC are presented as the ratio (F − Fo)/Fo or ΔF/Fo, where Fo is the basal fluorescence, and F is the fluorescence at time points during recording. Each curve in the graph is a superposition of five to ten curves recorded independently.
Measurement of [Ca
2+]
i in single cells was carried out using a Leica DMI 6000 fluorescent microscope (Leica, Germany) using an HCX PL FLUOTAR L 20.0 × 0.40 DRY objective (Leica, Germany), diode illuminator with wavelengths of 480/40 nm and fluorescent filter L5 ET (Leica, Germany). HUVEC were grown in 24-well plates and loaded with 1 µM CalciumGreen/AM, as described above. Fluorescence was recorded from the area of 334 × 447 microns in the center of the well. Fluorescence measurements were taken at 1 s intervals. The resulting images were converted to tiff files and analyzed using the CellProfiler program [
60] free at
http://cellprofiler.org/releases (accessed on 20 May 2023). Cells were isolated as separate objects, in each of which the kinetics of fluorescence changes were determined. The results are plotted as the ratio (F−Fo)/Fo or ΔF/Fo, where Fo is the fluorescence of a single cell before the addition of BW723C86 and F is the fluorescence at each time point during the recording. The maximum rate of increase in [Ca
2+]
i during the oscillation was determined from the increase in ΔF/Fo during one second during the development of the oscillation using Microsoft Excel 2010.
4.6. Measurement of mRNA Coding of 5-HT1BR, 5-HT2AR, 5-HT2BR, 5-HT2CR, and hEF1A in HUVEC
Total RNA was isolated from HUVEC using the Aurum™ Total RNA Mini Kit (Bio-Rad Laboratories, Hercules, CA, USA). The purity and concentration of RNA were assessed by absorption at 260 and 280 nm using a NanoDrop 8000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). For reverse transcription, a High-Capacity RNA-to-cDNA Kit (Thermo Fisher Scientific, Waltham, MA, USA) was used. Real-time PCR was performed on a 7500 Real-Time PCR System (Thermo Fisher Scientific, USA) with reagent mixture qPCRmix-HS LowROX (Eurogen, Moscow, Russia). Ct values for 5-HTRs genes and Ct values for human elongation factor 1A (EF1A) were determined in the same cDNA preparation. The relative amounts of mRNAs for 5-HTRs were calculated according to the following equation: fold gene expression = 2
−(∆∆Ct) [
61]. The content of 5-HT2BR mRNA was the highest; therefore, it was taken as a calibrator.
The following TaqMan probes and primers specific to human 5-HT1BR, 5-HT2AR, 5-HT2BR, and 5-HT2CR were used.
5-HT1BR Sense CTTCTGGCGTCAGGCTAAGG
5-HT1BR Antisense GAGTAGACCGTGTAGAGGATGTG
5-HT1BR Probe FAM-TCACCACGCATTCCGACACCTCCT-BHQ1
5-HT2AR Sense ATCTCGCTGGACCGCTACG
5-HT2AR Anti-sense CAACTCCCCTCCTTAAAGACCTTC
5-HT2ARProbe FAM-CCAGAATCCCATCCACCACAGCCGC-BHQ1
5-HT2BR Sense TGCTGACAAAGGAACGTTTTGG
5-HT2BRAntisense TTAGGCGTTGAGGTGGCTTG
5-HT2BR Probe FAM-TGCTCTTTGGCTCACTGGCTGCCT-BHQ1
5-HT2CR Sense CGCCGACAAGCTTTGATGTTAC
5-HT2CR Antisense CTTGCAGCACTTCAGGAAATCC
5-HT2CR Probe FAM-CCACACCGAGGAACCGCCTGGACT-BHQ1
hEF1A Sense CCATGTGTGTTGAGAGCTTCTCA
hEF1A Antisense CTTGTCCACTGCTTTGATGACAC
hEF1A Probe FAM-CTATCCACCTTTGGGTCGCTTTGCT-BHQ1
4.7. Statistics
Data are presented as mean ± SEM of 5 to 10 measurements. In each case, at least three independent experiments were performed with different cell preparations in which similar results were obtained. Statistical significance was calculated using the unpaired Student’s t-test (Microsoft Excel 2010) and one-way ANOVA, according to the Student–Newman–Keuls test (MedCalc, Version 14.8.1, Ostend, Belgium).