Metabolic and Transcriptomic Analyses Reveal Different Metabolite Biosynthesis Profiles between Purple and Green Pak Choi
Abstract
:1. Introduction
2. Results
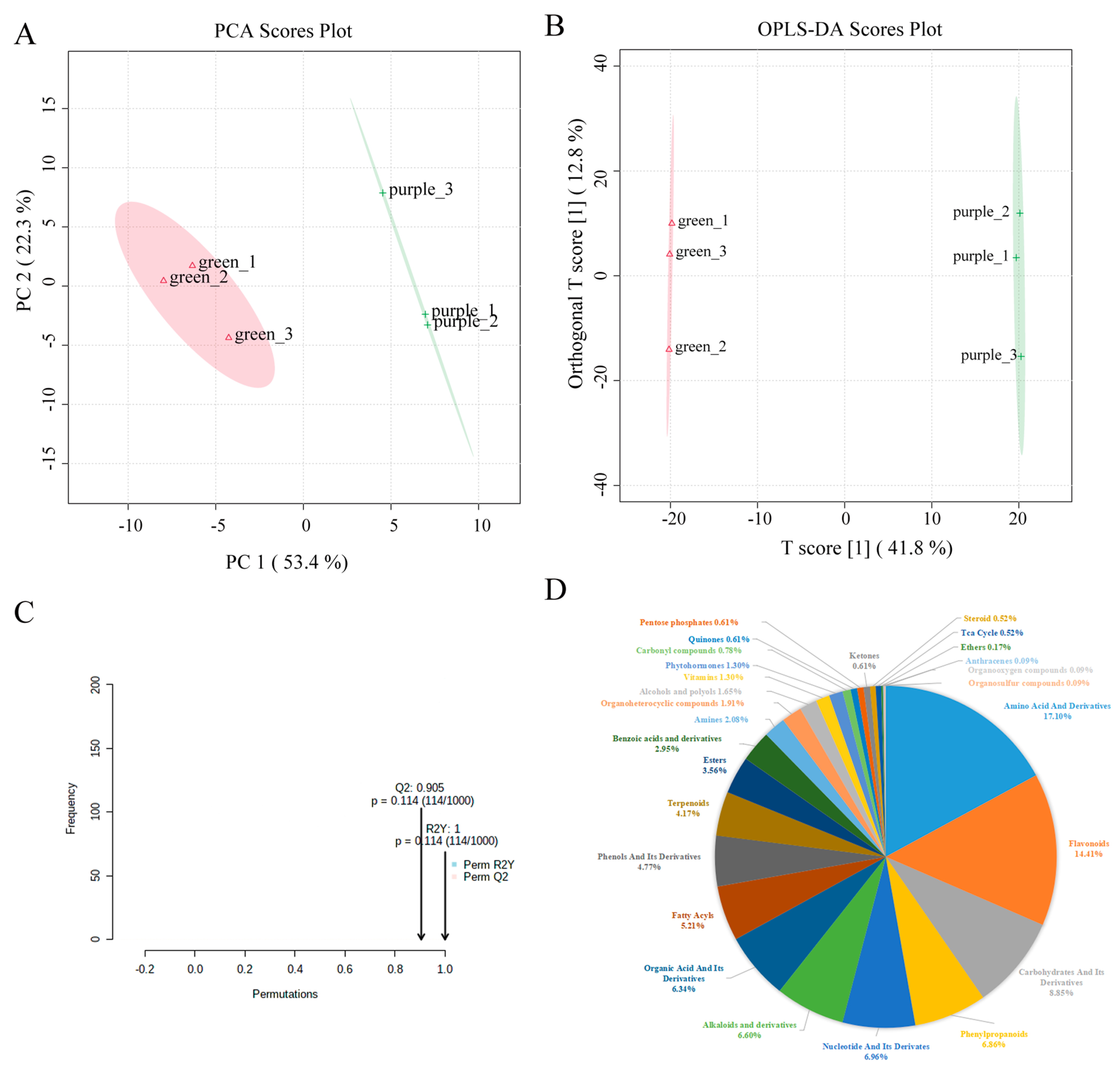
2.1. Metabolic Analysis of Pak Choi Leaves
2.2. Metabolomic Difference between Green and Purple Pak Choi Leaves
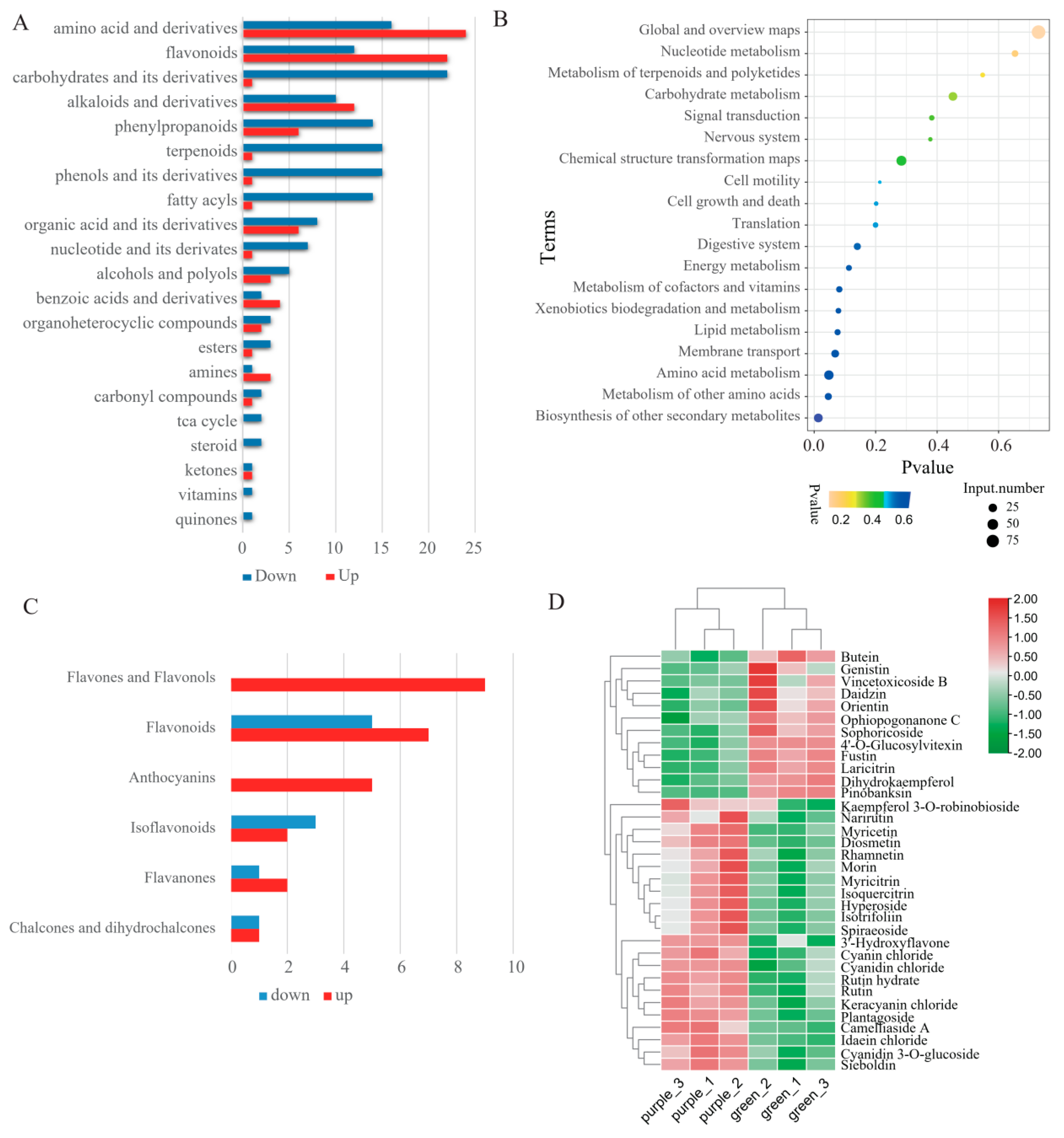
2.3. Differential Accumulation of Flavonoids between Green and Purple Pak Choi Leaves
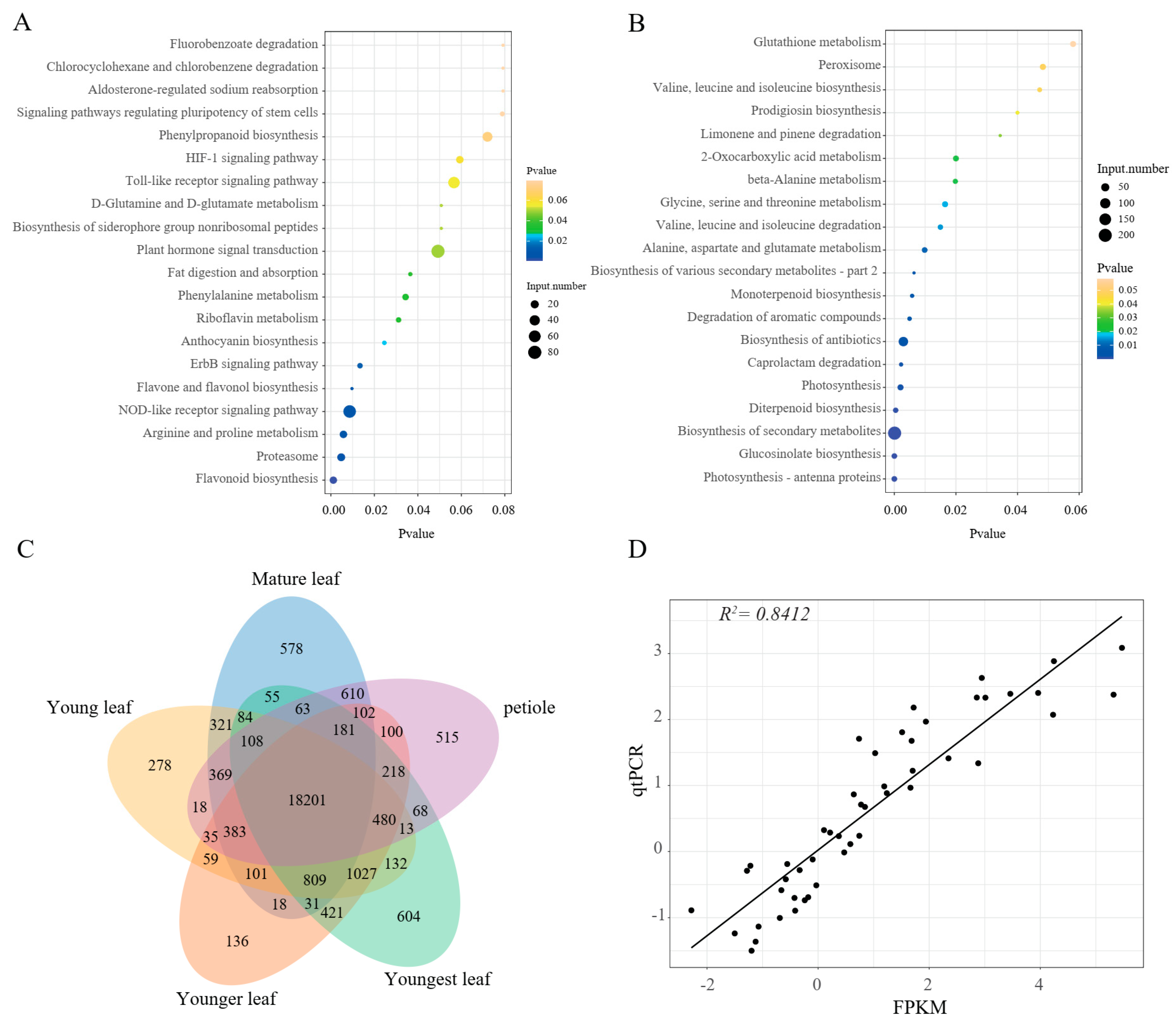
2.4. Transcriptome Profiles of Pak Choi Leaves
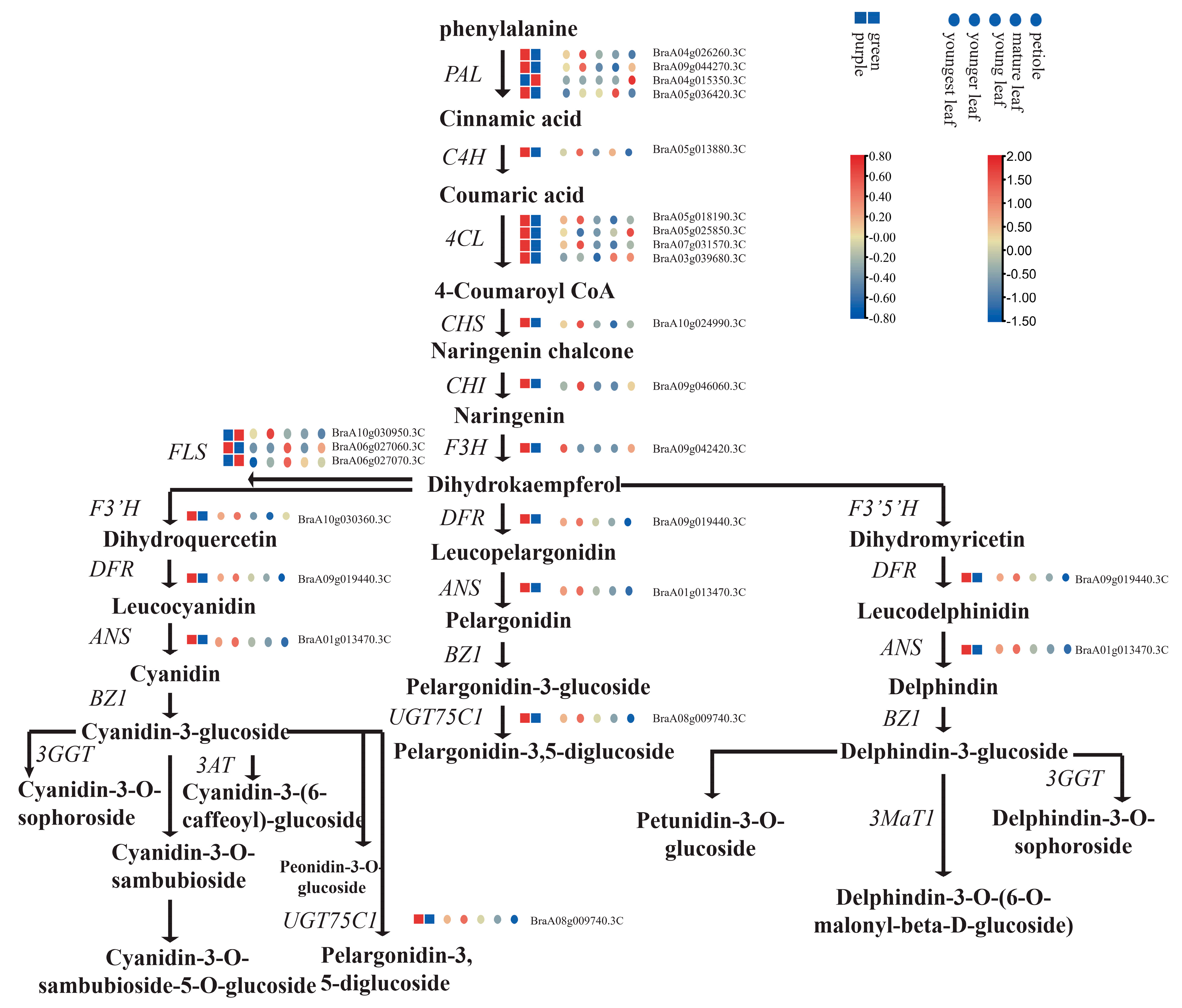
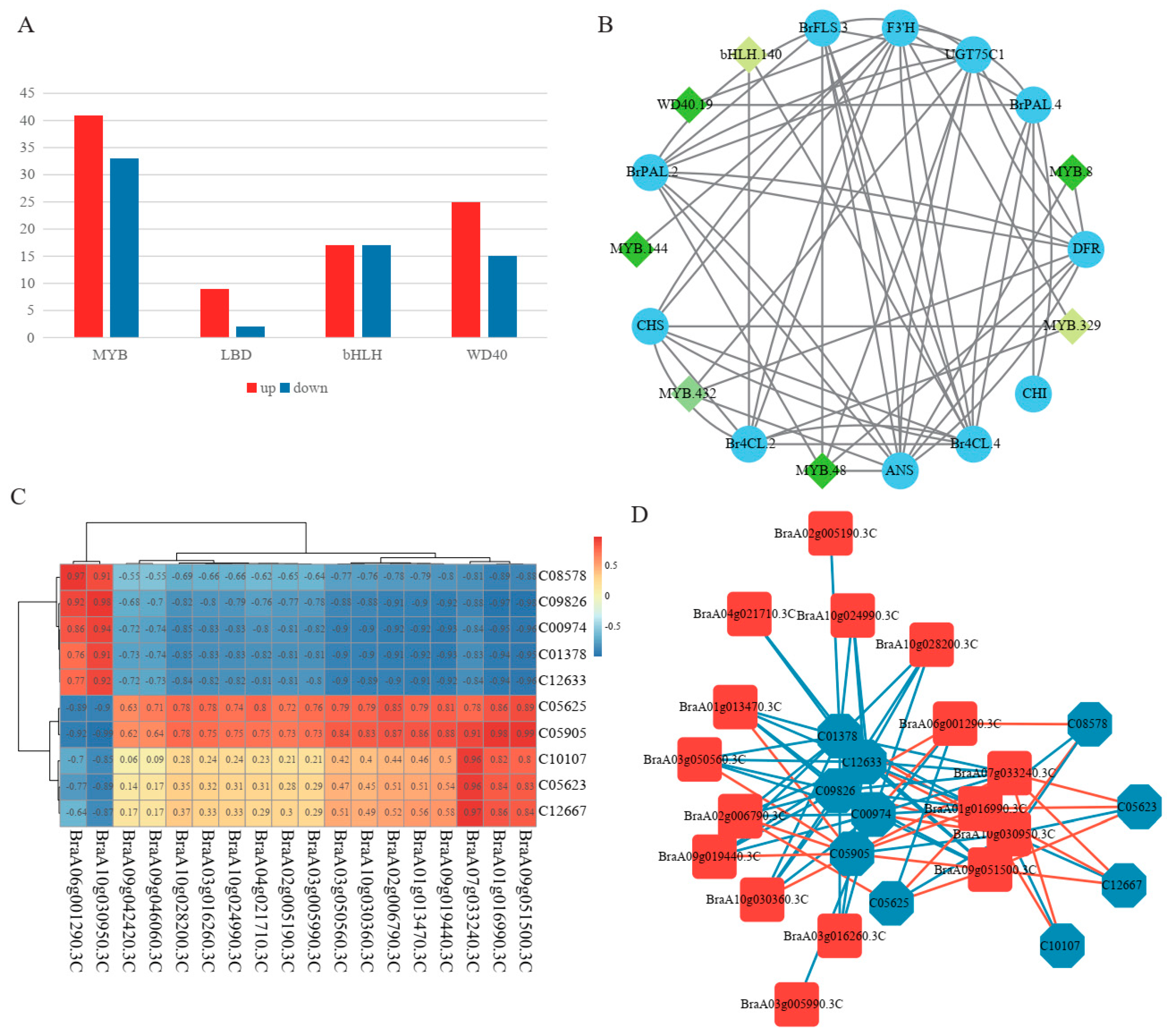
2.5. Differential Expression of Flavonoid Structural Genes
2.6. Analysis of Key Transcription Factors for Regulating Flavonoid Synthesis
2.7. Association Analysis of the Transcriptome and Metabolome
3. Discussion
4. Materials and Methods
4.1. Plant Material and Sampling
4.2. Sample Preparation and Metabolite Extraction
4.3. LC–MS/MS Analysis
4.4. Identification of Metabolites
4.5. Transcriptome Sequencing and Differentially Expressed Gene Analysis
4.6. Coexpression Network Analysis for the Construction of Modules
4.7. Gene Validation via Real-Time Quantitative PCR (qRT—PCR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solovyeva, A.E.; Shelenga, T.V.; Konarev, A.V.; Kurina, A.B.; Kornyukhin, D.L.; Fateev, D.A.; Artemyeva, A.M. Nutritional and biologically active compounds in Russian (VIR) Brassicaceae vegetable crops collection. Turk. J. Agric. For. 2021, 45, 541–556. [Google Scholar] [CrossRef]
- Li, Z.; Lee, H.W.; Liang, X.; Liang, D.; Wang, Q.; Huang, D.; Ong, C.N. Profiling of phenolic compounds and antioxidant activity of 12 cruciferous vegetables. Molecules 2018, 23, 1139. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.; Lim, C.J.; Kim, J.K.; Park, S.U. Comparative metabolic profiling of green and purple pakchoi (Brassica Rapa Subsp. Chinensis). Molecules 2018, 23, 1613. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.A.; Hu, H.; Ann Cuin, T.; Hao, Y.; Ji, X.; Wang, J.; Hu, C. Untargeted metabolomics and comparative flavonoid analysis reveal the nutritional aspects of pak choi. Food Chem. 2022, 383, 132375. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, G.; Dong, T.; Pan, Y.; Zhao, Z.; Tian, S.; Hu, Z. Anthocyanin accumulation and transcriptional regulation of anthocyanin biosynthesis in purple bok choy (Brassica rapa var. chinensis). J. Agric. Food Chem. 2014, 62, 12366–12376. [Google Scholar]
- Gould, K.S. Muriel wheldale onslow and the rediscovery of anthocyanin function in plants. In Recent Advances in Polyphenol Research; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; Volume 2. [Google Scholar] [CrossRef]
- Grotewold, E. The genetics and biochemistry of floral pigments. Annu. Rev. Plant Biol. 2006, 57, 761–780. [Google Scholar] [CrossRef]
- Enaru, B.; Drețcanu, G.; Pop, T.D.; Stǎnilǎ, A.; Diaconeasa, Z. Anthocyanins: Factors affecting their stability and degradation. Antioxidants 2021, 10, 1967. [Google Scholar]
- Yeo, H.J.; Baek, S.A.; Sathasivam, R.; Kim, J.K.; Park, S.U. Metabolomic analysis reveals the interaction of primary and secondary metabolism in white, pale green, and green pak choi (Brassica rapa Subsp. Chinensis). Appl. Biol. Chem. 2021, 64, 3. [Google Scholar] [CrossRef]
- Baenas, N.; García-Viguera, C.; Moreno, D.A. Elicitation: A tool for enriching the bioactive composition of foods. Molecules 2014, 19, 13541–13563. [Google Scholar]
- Arumugam, T.; Sona, C.L.; Maheswari, M.U. Fruits and vegetables as superfoods: Scope and demand. J. Pharm. Innov. 2021, 10, 119–129. [Google Scholar]
- Kakegawa, K.; Suda, J.; Sugiyama, M.; Komamine, A. Regulation of anthocyanin biosynthesis in cell suspension cultures of Vitis in relation to cell division. Physiol. Plant. 1995, 94, 661–666. [Google Scholar] [CrossRef]
- Kruger, M.J.; Davies, N.; Myburgh, K.H.; Lecour, S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014, 59, 41–52. [Google Scholar] [CrossRef]
- Solfanelli, C.; Poggi, A.; Loreti, E.; Alpi, A.; Perata, P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006, 140, 637–646. [Google Scholar] [PubMed]
- Roubelakis-Angelakis, K.A.; Kliewer, W.M. Effects of exogenous factors on phenylalanine ammonia-lyase activity and accumulation of anthocyanins and total phenolics in grape berries. Am. J. Enol. Vitic. 1986, 37, 275–280. [Google Scholar] [CrossRef]
- Pervaiz, T.; Songtao, J.; Faghihi, F.; Haider, M.S.; Fang, J. Naturally occurring anthocyanin, structure, functions and biosynthetic pathway in fruit plants. J. Plant Biochem. Biotechnol. 2017, 5, 2. [Google Scholar]
- Winkel-Shirley, B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001, 126, 485–493. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, C.; Huang, Z.; Lyu, L.; Li, W.; Wu, W. Integrative analysis of the metabolome and transcriptome provides insights into the mechanisms of flavonoid biosynthesis in blackberry. Food Res. Int. 2022, 153, 110948. [Google Scholar] [CrossRef]
- Zhao, Y.; Qi, X.; Liu, Z.; Zheng, W.; Guan, J.; Liu, Z.; Ren, J.; Feng, H.; Zhang, Y. Transcriptome and metabolome profiling to explore the causes of purple leaves formation in non-Heading chinese cabbage (Brassica rapa L. ssp. chinensis Makino var. mutliceps Hort.). Foods 2022, 11, 1787. [Google Scholar]
- Xi, D.; Gao, L.; Li, X.; Yang, X.; Zhu, Y.; Zhu, H. Metabolome and transcriptome revealed genes associated with anthocyanin accumulation in purple Caitai (Brassica compestris. var. tsai-tai Hort.). Sci. Hortic. 2022, 303, 111171. [Google Scholar] [CrossRef]
- He, Q.; Xue, Y.; Wang, Y.; Zhang, N.; Zhang, L. Metabolic profiling and transcriptomic data providing critical flavonoid biosynthesis mechanisms disclose color differences of purple heading Chinese cabbages (Brassica rapa L.). Food Sci. Technol. 2022, 168, 113885. [Google Scholar]
- Park, C.H.; Bong, S.J.; Lim, C.J.; Kim, J.K.; Park, S.U. Transcriptome analysis and metabolic profiling of green and red mizuna (Brassica rapa L. var. japonica). Foods 2020, 9, 1079. [Google Scholar] [CrossRef]
- Guo, J.; Wu, T.; Fu, M.; Li, G.; Luo, W.; Kang, Y.; Wang, T. An Integrated analysis of transcriptome and metabolism reveals an inhibitory effect of low light on anthocyanin biosynthesis in purple cai-tai (Brassica rapa L. var. purpurea). Horticulturae 2022, 8, 566. [Google Scholar] [CrossRef]
- Jin, S.-W.; Rahim, M.A.; Afrin, K.S.; Park, J.-I.; Kang, J.-G.; Nou, I.-S. Transcriptome profiling of two contrasting ornamental cabbage (Brassica oleracea var. acephala) lines provides insights into purple and white inner leaf pigmentation. BMC Genom. 2018, 19, 797. [Google Scholar] [CrossRef]
- Jeon, J.; Kim, J.K.; Kim, H.; Kim, Y.J.; Park, Y.J.; Kim, S.J.; Kim, C.; Park, S.U. Transcriptome analysis and metabolic profiling of green and red kale (Brassica oleracea var. acephala) seedlings. Food Chem. 2018, 241, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Tang, Y.; Zhang, C.; Yin, N.; Mao, Y.; Sun, F.; Chen, S.; Hu, R.; Liu, X.; Shang, G.; et al. Metabolite profiling and transcriptome analysis provide insight into seed coat color in Brassica juncea. Int. J. Mol. Sci. 2021, 22, 7215. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wei, Q.; Wang, W.; Hu, H.; Yan, Y.; Wang, Y.; Li, Y.; Jiang, Y.; Wu, G.; Hu, T.; et al. Understanding the nutraceutical diversity through a comparative analysis of the taproot metabolomes of different edible radish types via UHPLC-Q-TOF-MS. Food Chem. 2023, 403, 134469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, Z.; Zhu, M.; Zhu, Z.; Wang, Z.; Tian, S.; Chen, G. Anthocyanin accumulation and molecular analysis of correlated genes in purple kohlrabi (Brassica oleracea var. gongylodes L.). J. Agric. Food Chem. 2015, 63, 4160–4169. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Y.; Wan, H.; Tian, Z.; Xin, S.; Zhu, P. The dihydroflavonol 4-reductase BoDFR1 drives anthocyanin accumulation in pink-leaved ornamental kale. Theor. Appl. Genet. 2021, 134, 159–169. [Google Scholar] [CrossRef]
- Chiu, L.W.; Li, L. Characterization of the regulatory network of BoMYB2 in controlling anthocyanin biosynthesis in purple cauliflower. Planta 2012, 236, 1153–1164. [Google Scholar] [CrossRef]
- Chiu, L.W.; Zhou, X.; Burke, S.; Wu, X.; Prior, R.L.; Li, L. The purple cauliflower arises from activation of a MYB transcription factor. Plant Physiol. 2010, 154, 1470–1480. [Google Scholar] [CrossRef]
- Song, H.; Yi, H.; Lee, M.; Han, C.T.; Lee, J.; Kim, H.; Park, J.I.; Nou, I.S.; Kim, S.J.; Hur, Y. Purple Brassica oleracea var. capitata F. rubra is due to the loss of BoMYBL2-1 expression. BMC Plant Biol. 2018, 18, 82. [Google Scholar] [CrossRef]
- He, Q.; Wu, J.; Xue, Y.; Zhao, W.; Li, R.; Zhang, L. The novel gene BrMYB2, located on chromosome A07, with a short intron 1 controls the purple-head trait of Chinese cabbage (Brassica rapa L.). Hortic. Res. 2020, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, K.; Wu, J.; Guo, N.; Liang, J.; Wang, X.; Cheng, F. QTL-seq and sequence assembly rapidly mapped the gene BrMYBL2.1 for the purple trait in Brassica rapa. Sci. Rep. 2020, 10, 2328. [Google Scholar] [CrossRef]
- Kahie, M.A.; Wang, Y.; Fang, P.; Qi, J.; Lei, R.; Xu, J.; Lin, L.; Zhang, L.; Zhang, J.; Tao, A. Evolution and expression analysis of the caffeoyl-CoA 3-O-methyltransferase (CCoAOMT) gene family in jute (Corchorus L.). BMC Genom. 2023, 24, 204. [Google Scholar] [CrossRef]
- Shaipulah, N.F.; Muhlemann, J.K.; Woodworth, B.D.; Van Moerkercke, A.; Verdonk, J.C. CCoAOMT Down-regulation activates anthocyanin biosynthesis in Petunia. Plant Physiol. 2016, 170, 717–731. [Google Scholar] [CrossRef]
- Want, E.J.; O’maille, G.; Smith, C.A.; Brandon, T.R.; Uritboonthai, W.; Qin, C.; Trauger, S.A.; Siuzdak, G. Solvent-dependent metabolite distribution, clustering, and protein extraction for serum profiling with mass spectrometry. Anal. Chem. 2006, 78, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Dai, W.; Yin, P.; Zeng, Z.; Kong, H.; Zhou, L.; Wang, X.; Chen, S.; Lu, X.; Xu, G. Multiple reaction monitoring-ion pair finder: A systematic approach to transform nontargeted mode to pseudotargeted mode for metabolomics study based on liquid chromatography-mass spectrometry. Anal. Chem. 2015, 87, 5050–5055. [Google Scholar]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef] [PubMed]
- Sud, M.; Fahy, E.; Cotter, D.; Brown, A.; Dennis, E.A.; Glass, C.K.; Merrill, A.H., Jr.; Murphy, R.C.; Raetz, C.R.; Russell, D.W.; et al. LMSD: LIPID MAPS structure database. Nucleic Acids Res. 2007, 35, D527–D532. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, X.; Wu, J.; Liu, M.; Grob, S.; Cheng, F. Improved Brassica rapa reference genome by single-molecule sequencing and chromosome conformation capture technologies. Hortic. Res. 2018, 5, 50. [Google Scholar] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar]
- Kolde, R.; Kolde, M.R. Package ‘Pheatmap’. R Package 2015, 1, 790. [Google Scholar]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for visualization and analysis of biological networks. Data mining in proteomics: From standards to applications. Methods Mol. Biol. 2011, 696, 291–303. [Google Scholar] [PubMed]
- Paul, P.; Chhapekar, S.S.; Rameneni, J.J.; Oh, S.H.; Dhandapani, V.; Subburaj, S.; Shin, S.Y.; Ramchiary, N.; Shin, C.; Choi, S.R.; et al. MiR1885 regulates disease tolerance genes in Brassica rapa during early infection with Plasmodiophora Brassicae. Int. J. Mol. Sci. 2021, 22, 9433. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Hu, T.; Wang, Y.; Wang, W.; Hu, H.; Wei, Q.; Yan, Y.; Bao, C. Metabolic and Transcriptomic Analyses Reveal Different Metabolite Biosynthesis Profiles between Purple and Green Pak Choi. Int. J. Mol. Sci. 2023, 24, 13781. https://doi.org/10.3390/ijms241813781
Wang J, Hu T, Wang Y, Wang W, Hu H, Wei Q, Yan Y, Bao C. Metabolic and Transcriptomic Analyses Reveal Different Metabolite Biosynthesis Profiles between Purple and Green Pak Choi. International Journal of Molecular Sciences. 2023; 24(18):13781. https://doi.org/10.3390/ijms241813781
Chicago/Turabian StyleWang, Jinglei, Tianhua Hu, Yidi Wang, Wuhong Wang, Haijiao Hu, Qingzhen Wei, Yaqin Yan, and Chonglai Bao. 2023. "Metabolic and Transcriptomic Analyses Reveal Different Metabolite Biosynthesis Profiles between Purple and Green Pak Choi" International Journal of Molecular Sciences 24, no. 18: 13781. https://doi.org/10.3390/ijms241813781
APA StyleWang, J., Hu, T., Wang, Y., Wang, W., Hu, H., Wei, Q., Yan, Y., & Bao, C. (2023). Metabolic and Transcriptomic Analyses Reveal Different Metabolite Biosynthesis Profiles between Purple and Green Pak Choi. International Journal of Molecular Sciences, 24(18), 13781. https://doi.org/10.3390/ijms241813781





