Expression Profiles of Long Non-Coding RNAs in the Articular Cartilage of Rats Exposed to T-2 Toxin
Abstract
1. Introduction
2. Results
2.1. Safranin O/Fast Green Staining of Articular Cartilage
2.2. Quality Control of RNA Sequencing Data and Read Mapping
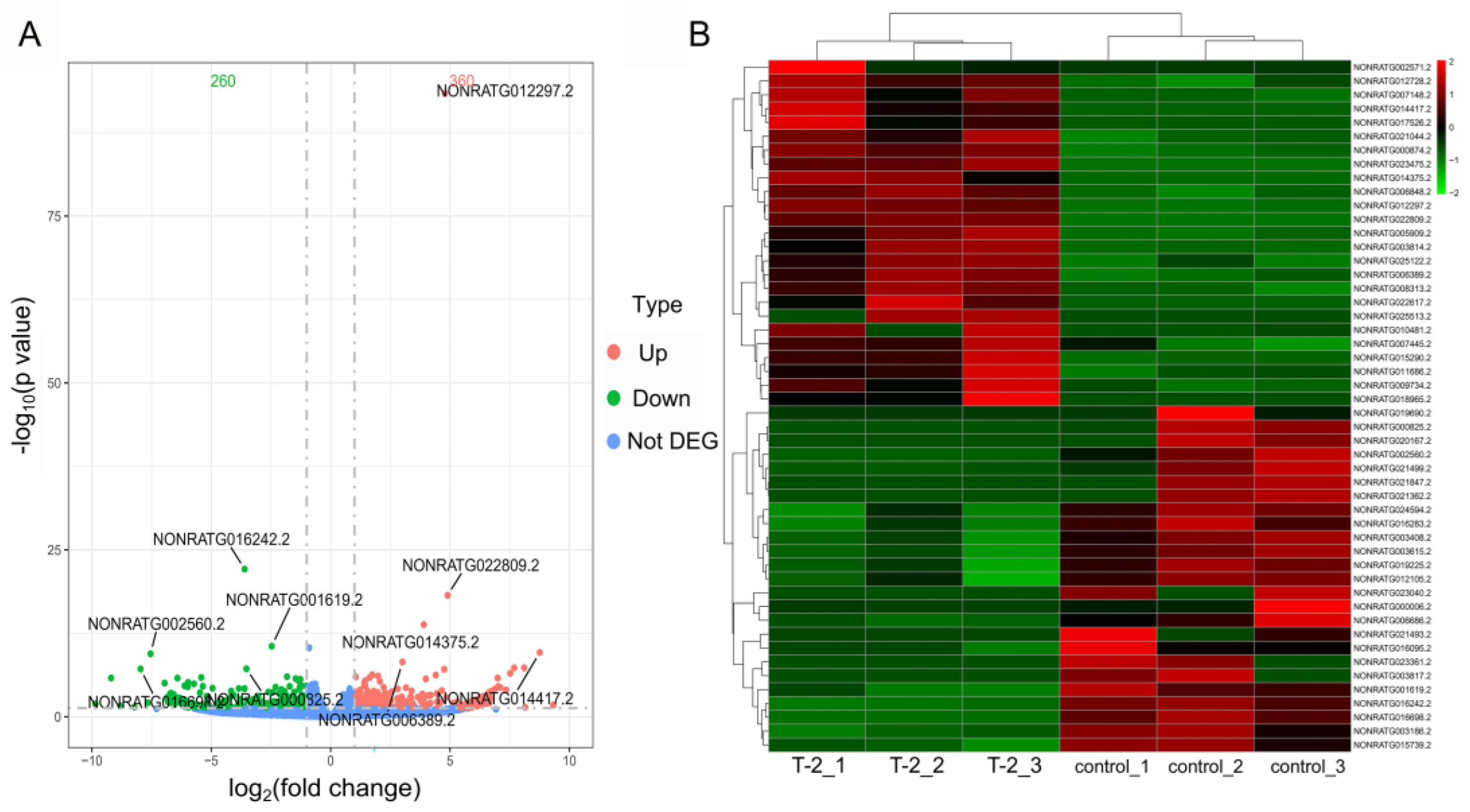
2.3. Identification of Differentially Expressed lncRNAs
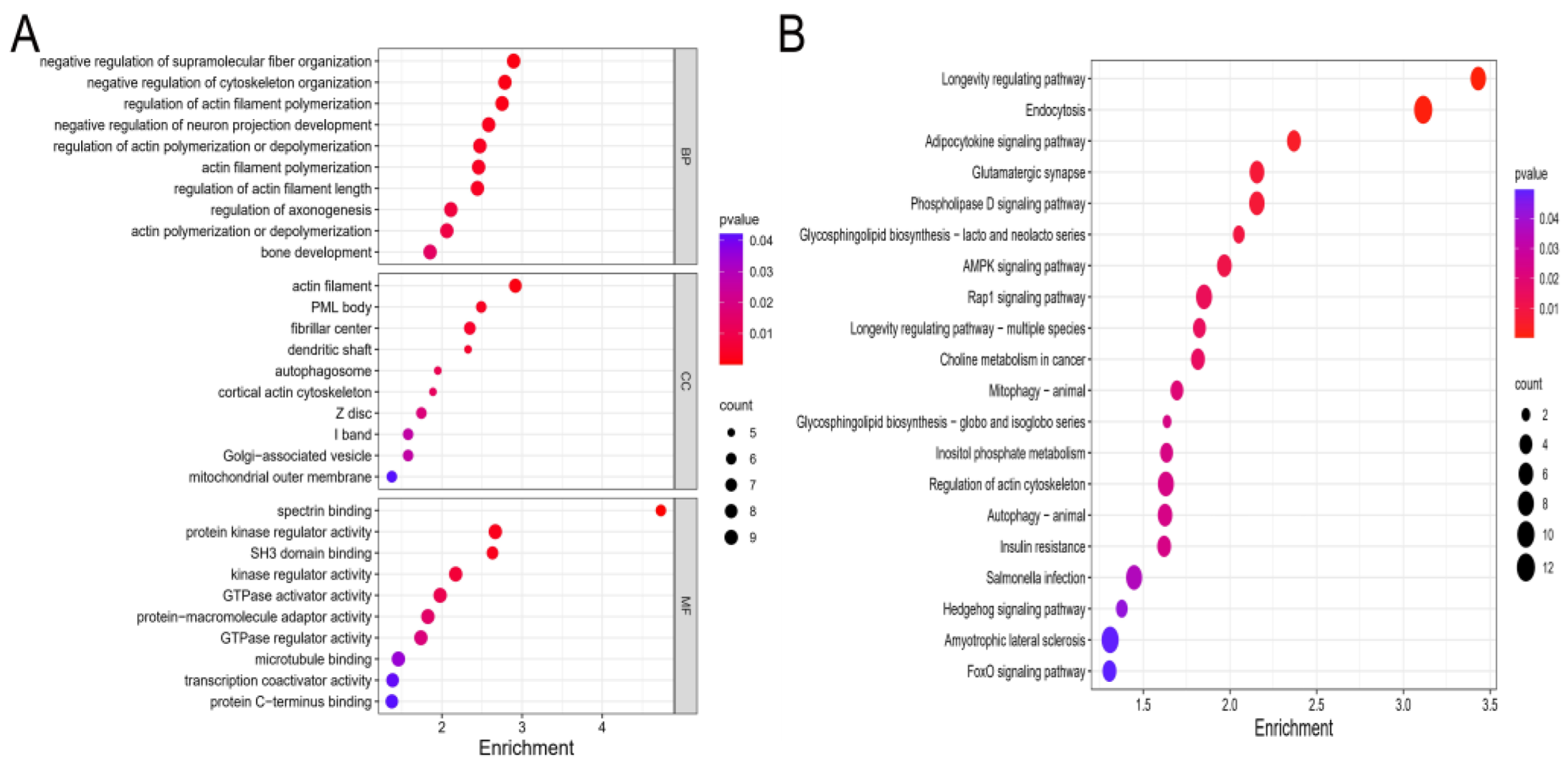
2.4. Functional Enrichment Analyses of Target Genes in the Cartilage Tissues Samples
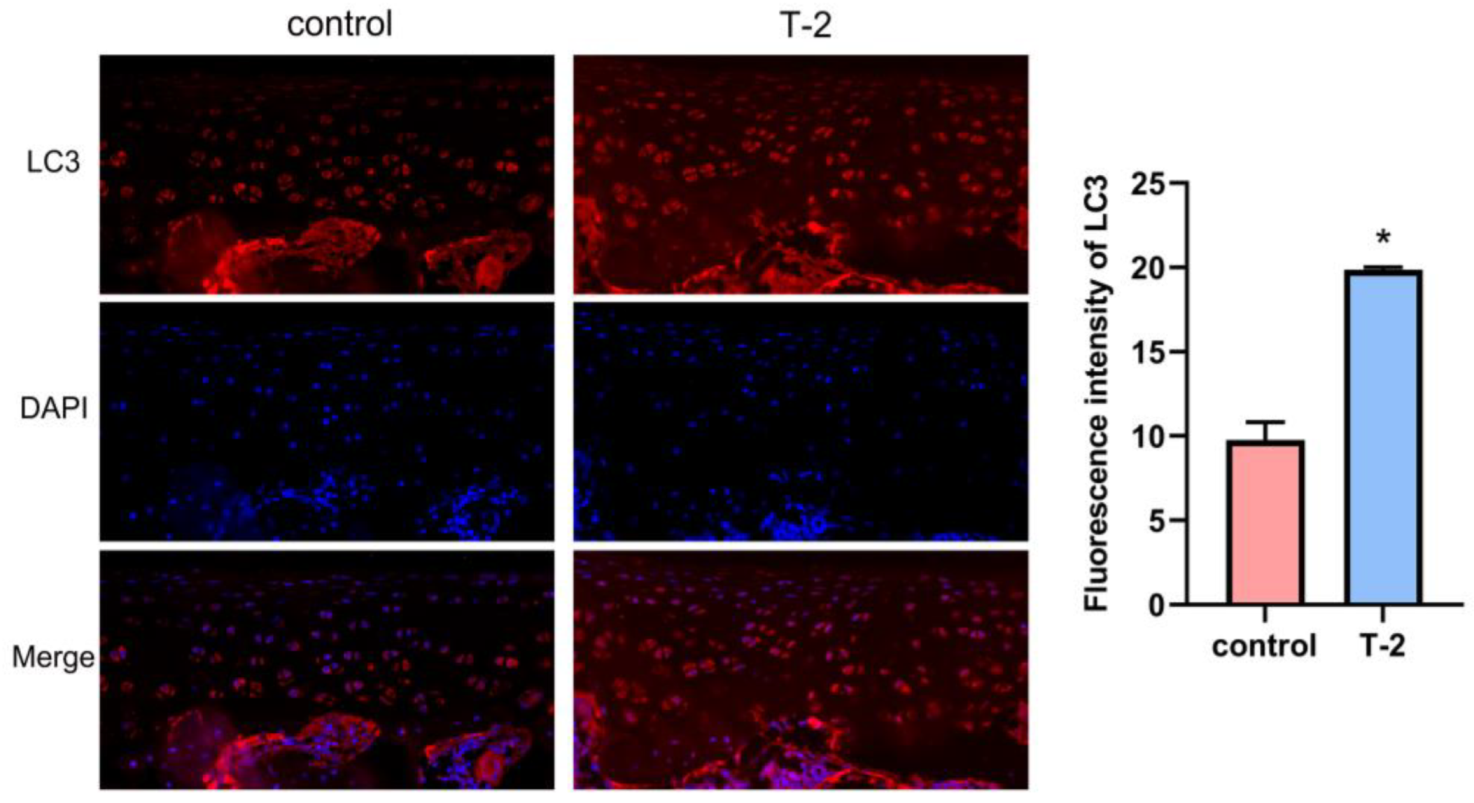
2.5. T-2 Toxin Triggered Autophagy in Articular Cartilage
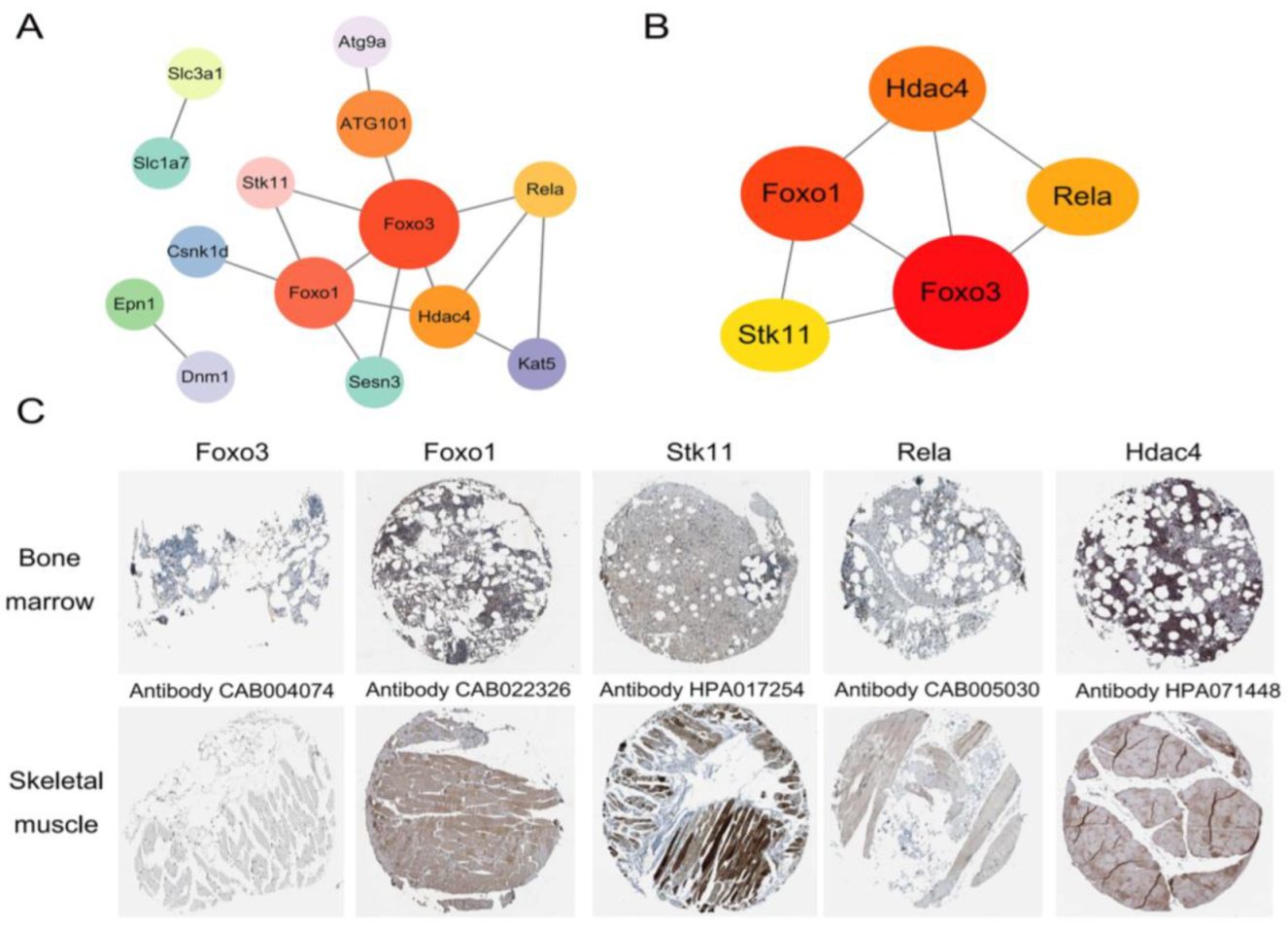
2.6. PPI Network Analysis and the Validation of Hub Genes
2.7. Prediction of Candidate Drugs
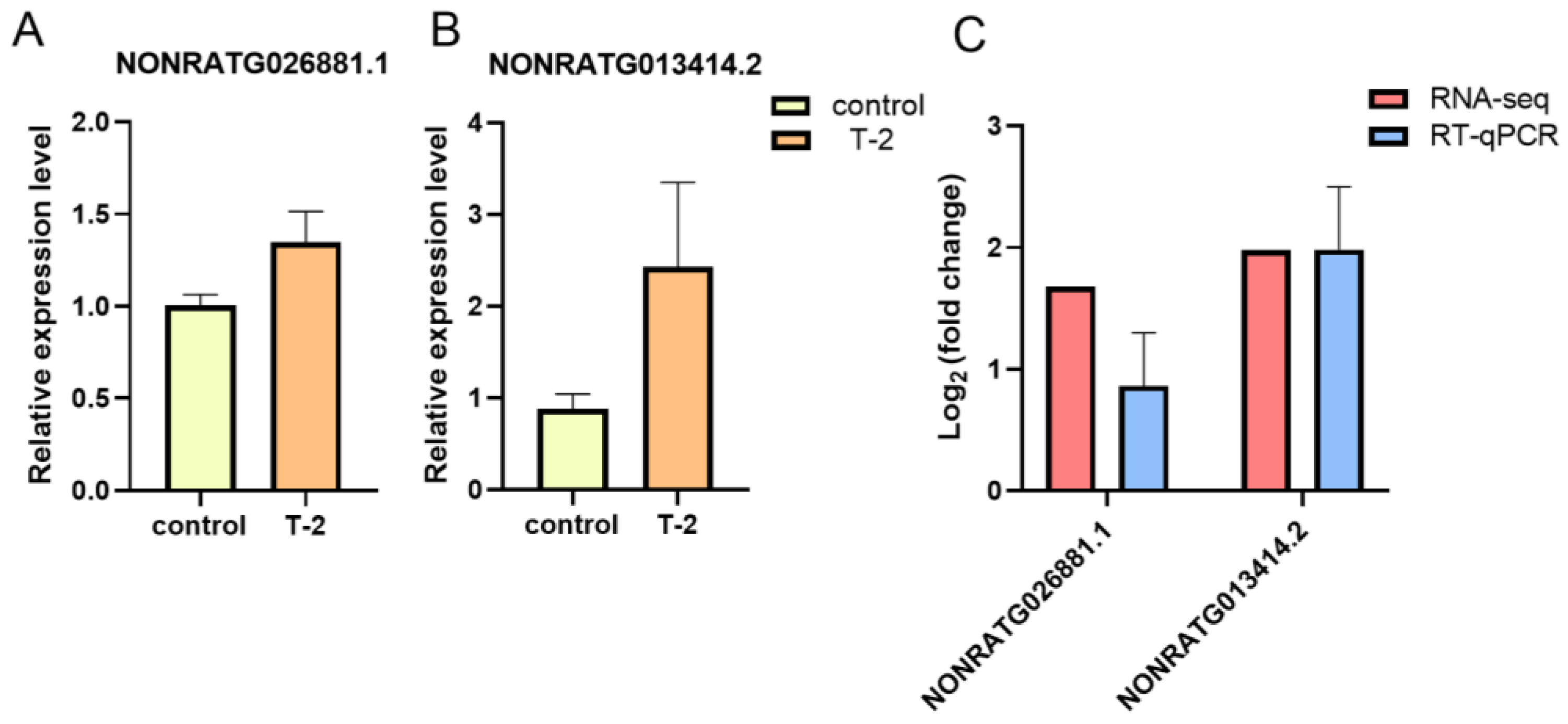
2.8. Validation of DE-lncRNAs via RT-qPCR
3. Discussion
4. Materials and Methods
4.1. Establishment of Animal Models Treated with T-2 Toxin
4.2. Safranin O/Fast Green Staining
4.3. Library Construction and RNA-Seq
4.4. Identification of Differentially Expressed lncRNAs
4.5. Target Gene Prediction
4.6. GO and KEGG Pathway Enrichment Analyses
4.7. Immunofluorescence Assay
4.8. PPI Network Construction and Validation of Hub Genes
4.9. Prediction of Candidate Pharmacological Targets
4.10. Verification Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Stela, M.; Bijak, M. T-2 toxin-the most toxic trichothecene mycotoxin: Metabolism, toxicity, and decontamination strategies. Molecules 2021, 26, 6868. [Google Scholar] [CrossRef] [PubMed]
- Haidukowski, M.; Somma, S.; Ghionna, V.; Cimmarusti, M.T.; Masiello, M.; Logrieco, A.F.; Moretti, A. Deoxynivalenol and T-2 Toxin as Major Concerns in Durum Wheat from Italy. Toxins 2022, 14, 627. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Han, S.; Chen, Y.; Wang, Y.; Li, D.; Zhu, Q. T-2 Toxin Induces Oxidative Stress, Apoptosis and Cytoprotective Autophagy in Chicken Hepatocytes. Toxins 2020, 12, 90. [Google Scholar] [CrossRef]
- Dai, C.; Das Gupta, S.; Wang, Z.; Jiang, H.; Velkov, T.; Shen, J. T-2 toxin and its cardiotoxicity: New insights on the molecular mechanism s and therapeutic implications. Food Chem. Toxicol. 2022, 167, 113262. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.-F.; Lin, X.-L.; Wang, X.; Ping, Z.-G.; Guo, X. Comparison of Apoptosis and Autophagy in Human Chondrocytes Induced by the T-2 and HT-2 Toxins. Toxins 2019, 11, 260. [Google Scholar] [CrossRef]
- Kao, W.-C.; Chen, J.-C.; Liu, P.-C.; Lu, C.-C.; Lin, S.-Y.; Chuang, S.-C.; Wu, S.-C.; Chang, L.-H.; Lee, M.-J.; Yang, C.-D.; et al. The Role of Autophagy in Osteoarthritic Cartilage. Biomolecules 2022, 12, 1357. [Google Scholar] [CrossRef]
- Glick, D.; Barth, S.; MacLeod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef]
- Li, W.; He, P.; Huang, Y.; Li, Y.-F.; Lu, J.; Li, M.; Kurihara, H.; Luo, Z.; Meng, T.; Onishi, M.; et al. Selective autophagy of intracellular organelles: Recent research advances. Theranostics 2021, 11, 222–256. [Google Scholar] [CrossRef]
- Yu, F.-F.; Zuo, J.; Sun, L.; Yu, S.-Y.; Lei, X.-L.; Zhu, J.-H.; Zhou, G.-Y.; Guo, X.; Ba, Y. Animal models of Kashin-Beck disease exposed to environmental risk factors: Methods and comparisons. Ecotoxicol. Environ. Saf. 2022, 234, 113419. [Google Scholar] [CrossRef]
- Jia, Y.; Shi, S.; Cheng, B.; Cheng, S.; Liu, L.; Meng, P.; Yang, X.; Chu, X.; Wen, Y.; Zhang, F.; et al. Fluorine impairs carboxylesterase 1-mediated hydrolysis of T-2 toxin and increases its chondrocyte toxicity. Front. Nutr. 2022, 9, 935112. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Ma, W.-J.; Zhang, F.; Ren, F.-L.; Qu, C.-J.; Lammi, M. Recent advances in the research of an endemic osteochondropathy in China: Kashin-Beck disease. Osteoarthr. Cartil. 2014, 22, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Yu, J.; Liu, H.; Liu, Y.; Liu, N.; Cao, Y.; Zhang, X.; Sun, D. Endemic Kashin-Beck disease: A food-sourced osteoarthropathy. Semin. Arthritis Rheum. 2020, 50, 366–372. [Google Scholar] [CrossRef]
- Li, S.-Y.; Cao, J.-L.; Shi, Z.-L.; Chen, J.-H.; Zhang, Z.-T.; Hughes, C.E.; Caterson, B. Promotion of the articular cartilage proteoglycan degradation by T-2 toxin and selenium protective effect. J. Zhejiang Univ. B 2008, 9, 22–33. [Google Scholar] [CrossRef]
- Tian, J.; Yan, J.; Wang, W.; Zhong, N.; Tian, L.; Sun, J.; Min, Z.; Ma, J.; Lu, S. T-2 toxin enhances catabolic activity of hypertrophic chondrocytes through ROS-NF-κB-HIF-2α pathway. Toxicol. Vitr. 2012, 26, 1106–1113. [Google Scholar] [CrossRef]
- Bridges, M.C.; Daulagala, A.C.; Kourtidis, A. Lnccation: LncRNA localization and function. J. Cell Biol. 2021, 220, e202009045. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Jiang, S.; Shang, J.; Jiang, Y.; Dai, Y.; Xu, B.; Yu, Y.; Liang, Z.; Yang, Y. LncRNA: Shedding light on mechanisms and opportunities in fibrosis and aging. Ageing Res. Rev. 2019, 52, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Murillo-Maldonado, J.M.; Riesgo-Escovar, J.R. The various and shared roles of lncRNAs during development. Dev. Dyn. 2019, 248, 1059–1069. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef]
- Constanty, F.; Shkumatava, A. lncRNAs in development and differentiation: From sequence motifs to functional characterization. Development 2021, 148, 182741. [Google Scholar] [CrossRef]
- Dai, Y.; Jian, C.; Wang, X.; Dai, X. Comprehensive expression profiles of mRNAs, lncRNAs and miRNAs in Kashin-Beck disease identified by RNA-sequencing. Mol. Omics 2022, 18, 154–166. [Google Scholar] [CrossRef]
- Zuo, Y.; Xiong, C.; Gan, X.; Xie, W.; Yan, X.; Chen, Y.; Li, X. LncRNA HAGLR silencing inhibits IL-1β-induced chondrocytes inflammatory injury via miR-130a-3p/JAK1 axis. J. Orthop. Surg. Res. 2023, 18, 203. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Huang, J.; Lai, J.; Zhou, Q.; Liu, T.; Xu, Q.; Ji, G.; Ye, Y. Long non-coding RNA hotairincreased mechanical stimulation-induced apoptosis by regulating microRNA-221/BBC3 axis in C28/I2 cells. Bioengineered 2021, 12, 10734–10744. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yan, J.; Lan, X.; Guo, Y.; Sun, M.; Zhao, Y.; Zhang, F.; Sun, J.; Lu, S. LncRNA WDR11-AS1 Promotes Extracellular Matrix Synthesis in Osteoarthritis by Directly Interacting with RNA-Binding Protein PABPC1 to Stabilize SOX9 Expression. Int. J. Mol. Sci. 2023, 24, 817. [Google Scholar] [CrossRef]
- Xu, S.; Wang, J.; Jiang, J.; Song, J.; Zhu, W.; Zhang, F.; Shao, M.; Xu, H.; Ma, X.; Lyu, F. TLR4 promotes microglial pyroptosis via lncRNA-F630028o10Rik by activating PI3K/AKT pathway after spinal cord injury. Cell Death Dis. 2020, 11, 693. [Google Scholar] [CrossRef]
- Li, S.-J.; Zhang, G.; Xue, B.; Ding, Q.; Han, L.; Huang, J.-C.; Wu, F.; Li, C.; Yang, C. Toxicity and detoxification of T-2 toxin in poultry. Food Chem. Toxicol. 2022, 169, 113392. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Liu, Q.; Yang, H.; Wang, Q.; Wang, J.; Fan, Y. Inflammation-related pathways involved in damaged articular cartilage of rats exposed to T-2 toxin based on RNA-sequencing analysis. Front. Genet. 2022, 13, 1079739. [Google Scholar] [CrossRef] [PubMed]
- Lauer, J.C.; Selig, M.; Hart, M.L.; Kurz, B.; Rolauffs, B. Articular Chondrocyte Phenotype Regulation through the Cytoskeleton and the Signaling Processes That Originate from or Converge on the Cytoskeleton: Towards a Novel Understanding of the Intersection between Actin Dynamics and Chondrogenic Function. Int. J. Mol. Sci. 2021, 22, 3279. [Google Scholar] [CrossRef]
- Juretschke, T.; Beli, P. Causes and consequences of DNA damage-induced autophagy. Matrix Biol. 2021, 100, 39–53. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, X.; Yuan, W.; Xiang, Y.; Guo, X.; Wei, W.; Soberón, M.; Bravo, A.; Liu, K. Bacillus thuringiensis cry toxin triggers autophagy activity that may enhance cell death. Pestic. Biochem. Physiol. 2021, 171, 104728. [Google Scholar] [CrossRef]
- Kajikawa, M.; Fukuzawa, H. Algal Autophagy Is Necessary for the Regulation of Carbon Metabolism Under Nutrient Deficiency. Front. Plant Sci. 2020, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; De Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Yue, L.; Wan, W.; Zhang, Y.; Zhang, B.; Otomo, C.; Li, Q.; Lin, T.; Hu, J.; Xu, P.; et al. Inhibition of autophagy by a small molecule through covalent modification of the LC3 protein. Angew. Chem. Int. Ed. Engl. 2021, 60, 26105–26114. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Q.; Li, M.; Tang, S.; Dai, C. T-2 Toxin Induces Apoptotic Cell Death and Protective Autophagy in Mouse Microglia BV2 Cells. J. Fungi 2022, 8, 761. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Jiang, W.S.; Su, Y.R.; Tu, K.W.; Zou, L.; Liao, C.R.; Wu, Q.; Wang, Z.H.; Zhong, Z.M.; Chen, J.T.; et al. PINK1/PARKIN-mediated mitophagy inhibits osteoblast apoptosis induced by advanced oxidation protein products. Cell Death Dis. 2023, 14, 88. [Google Scholar] [CrossRef]
- Dai, C.; Xiao, X.; Sun, F.; Zhang, Y.; Hoyer, D.; Shen, J.; Tang, S.; Velkov, T. T-2 toxin neurotoxicity: Role of oxidative stress and mitochondrial dysfunction. Arch. Toxicol. 2019, 93, 3041–3056. [Google Scholar] [CrossRef] [PubMed]
- Osman, S. PINK spots: Diseased mitochondria prepare for mitophagy. Nat. Struct. Mol. Biol. 2022, 29, 82. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Lee, S.; Dong, H.H. FoxO integration of insulin signaling with glucose and lipid metabolism. J. Endocrinol. 2017, 233, R67–R79. [Google Scholar] [CrossRef]
- Cheng, Z. The FoxO-autophagy axis in health and disease. Trends Endocrinol. Metab. 2019, 30, 658–671. [Google Scholar] [CrossRef]
- Guo, X.; Li, Z.; Zhu, X.; Zhan, M.; Wu, C.; Ding, X.; Peng, K.; Li, W.; Ma, X.; Lv, Z.; et al. A coherent FOXO3-SNAI2 feed-forward loop in autophagy. Proc. Natl. Acad. Sci. USA 2022, 119, e2118285119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yang, J.; Liao, W.; Liu, X.; Zhang, H.; Wang, S.; Wang, D.; Feng, J.; Yu, L.; Zhu, W.-G. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat. Cell Biol. 2010, 12, 665–675. [Google Scholar] [CrossRef]
- Collier, J.J.; Suomi, F.; Oláhová, M.; McWilliams, T.G.; Taylor, R.W. Emerging roles of ATG7 in human health and disease. EMBO Mol. Med. 2021, 13, e14824. [Google Scholar] [CrossRef]
- Yue, J.; Aobulikasimu, A.; Sun, W.; Liu, S.; Xie, W.; Sun, W. Targeted regulation of FoxO1 in chondrocytes prevents age-related osteoarthritis via autophagy mechanism. J. Cell Mol. Med. 2022, 26, 3075–3082. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lin, Z.; Liu, X.; Ding, Q.; Cai, J.; Zhang, Z.; Rose, P.; Zhu, Y.Z. HDAC4 Inhibitors as Antivascular Senescence Therapeutics. Oxidative Med. Cell Longev. 2022, 2022, 3087916. [Google Scholar] [CrossRef] [PubMed]
- Hou, F.; Wei, W.; Qin, X.; Liang, J.; Han, S.; Han, A.; Kong, Q. The posttranslational modification of HDAC4 in cell biology: Mechanisms and potential targets. J. Cell Biochem. 2019, 121, 930–937. [Google Scholar] [CrossRef]
- Wani, A.; Al Rihani, S.B.; Sharma, A.; Weadick, B.; Govindarajan, R.; Khan, S.U.; Sharma, P.R.; Dogra, A.; Nandi, U.; Reddy, C.N.; et al. Crocetin promotes clearance of amyloid-β by inducing autophagy via the STK11/LKB1-mediated AMPK pathway. Autophagy 2021, 17, 3813–3832. [Google Scholar] [CrossRef]
- Liu, C.; Wang, X.; Wang, X.; Zhang, Y.; Min, W.; Yu, P.; Miao, J.; Shen, W.; Chen, S.; Zhou, S.; et al. A new LKB1 activator, piericidin analogue S14, retards renal fibrosis through promoting autophagy and mitochondrial homeostasis in renal tubular epithelial cells. Theranostics 2022, 12, 7158–7179. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, J.; Jiang, H.; Hu, Z.; He, L.; Yang, J.; Xie, Y.; Wu, D.; Li, H.-Y.; Zeng, K.; et al. Vangl2 suppresses NF-κB signaling and ameliorates sepsis by targeting p65 for ndp52-mediated autophagic degradation. bioRxiv 2023. [Google Scholar] [CrossRef]
- Verzella, D.; Pescatore, A.; Capece, D.; Vecchiotti, D.; Ursini, M.V.; Franzoso, G.; Alesse, E.; Zazzeroni, F. Life, death, and autophagy in cancer: NF-κB turns up everywhere. Cell Death Dis. 2020, 11, 210. [Google Scholar] [CrossRef]
- Kluska, M.; Woźniak, K. Natural Polyphenols as Modulators of Etoposide Anti-Cancer Activity. Int. J. Mol. Sci. 2021, 22, 6602. [Google Scholar] [CrossRef]
- Ehl, S.; Astigarraga, I.; Greenwood, T.v.B.; Hines, M.; Horne, A.; Ishii, E.; Janka, G.; Jordan, M.B.; La Rosée, P.; Lehmberg, K.; et al. Recommendations for the Use of Etoposide-Based Therapy and Bone Marrow Transplantation for the Treatment of HLH: Consensus Statements by the HLH Steering Committee of the Histiocyte Society. J. Allergy Clin. Immunol. Pract. 2018, 6, 1508–1517. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Song, Z.; Cai, F.; Ruan, L.; Jiang, R. Chemistry and Biological Activities of Naturally Occurring and Structurally Modified Podophyllotoxins. Molecules 2022, 28, 302. [Google Scholar] [CrossRef]
- Ward, D.B.; Brown, K.C.; Valentovic, M.A. Radiocontrast Agent Diatrizoic Acid Induces Mitophagy and Oxidative Stress via Calcium Dysregulation. Int. J. Mol. Sci. 2019, 20, 4074. [Google Scholar] [CrossRef] [PubMed]
- Yanina, I.Y.; Tanikawa, Y.; Genina, E.A.; Dyachenko, P.A.; Tuchina, D.K.; Bashkatov, A.N.; Dolotov, L.E.; Tarakanchikova, Y.V.; Terentuk, G.S.; Navolokin, N.A.; et al. Immersion optical clearing of adipose tissue in rats: Ex vivo and in vivo studies. J. Biophotonics 2022, 15, e202100393. [Google Scholar] [CrossRef] [PubMed]
- Tuchin, V.V.; Genina, E.A.; Tuchina, E.S.; Svetlakova, A.V.; Svenskaya, Y.I. Optical clearing of tissues: Issues of antimicrobial phototherapy and drug delivery. Adv. Drug Deliv. Rev. 2022, 180, 114037. [Google Scholar] [CrossRef]
- Wu, R.; Geng, L.; Zhao, Z.; Liao, D.; He, B.; Hu, H.; Lin, Y.; Li, M.; Xiang, M.; Zhang, Y.; et al. Clinical Application of Oral Meglumine Diatrizoate Esophagogram in Screening for Esophageal Fistula During Radiotherapy or Chemoradiotherapy for Esophageal Cancer. Front. Oncol. 2020, 10, 562147. [Google Scholar] [CrossRef]
- Guo, Z.; Chilufya, M.M.; Deng, H.; Qiao, L.; Liu, J.; Xiao, X.; Zhao, Y.; Lin, X.; Liu, H.; Xiang, R.; et al. Single and Combined Effects of Short-Term Selenium Deficiency and T-2 Toxin-Induced Kidney Pathological Injury through the MMPs/TIMPs System. Biol. Trace Element Res. 2023, 201, 4850–4860. [Google Scholar] [CrossRef]
- Chen, J.-H.; Xue, S.; Li, S.; Wang, Z.-L.; Yang, H.; Wang, W.; Song, D.; Zhou, X.; Chen, C. Oxidant damage in Kashin-Beck disease and a rat Kashin-Beck disease model by employing T-2 toxin treatment under selenium deficient conditions. J. Orthop. Res. 2012, 30, 1229–1237. [Google Scholar] [CrossRef]
- Shi, Y.; Tu, H.; Chen, X.; Zhang, Y.; Chen, L.; Liu, Z.; Sheng, J.; Han, S.; Yin, J.; Peng, B.; et al. The long non-coding RNA expression profile of Coxsackievirus A16 infected RD cells identified by RNA-seq. Virol. Sin. 2016, 31, 131–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Homma, K.; Suzuki, K.; Sugawara, H. The Autophagy Database: An all-inclusive information resource on autophagy that provides nourishment for research. Nucleic Acids Res. 2011, 39, D986–D990. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fu, X.; Du, Y.; Feng, Z.; Liu, X.; Li, Z.; Yu, F.; Zhou, G.; Ba, Y. In Silico Analysis of Ferroptosis-Related Genes and Its Implication in Drug Prediction against Fluorosis. Int. J. Mol. Sci. 2023, 24, 4221. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Duan, M.; Zhang, C.; Wang, K.; Feng, L.; Song, L.; Wu, S.; Chen, X. Identification of Potential Biomarkers Associated with Acute Myocardial Infarction by Weighted Gene Coexpression Network Analysis. Oxidative Med. Cell Longev. 2021, 2021, 5553811. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90–W97. [Google Scholar] [CrossRef]
- Gu, Y.; Ma, X.; Li, J.; Ma, Y.; Zhang, Y. Identification of candidate targets for the diagnosis and treatment of atherosclerosis by bioinformatics analysis. Am. J. Transl. Res. 2021, 13, 4137–4151. [Google Scholar]


| Samples | Raw Data | Clean Data | Clean Reads (%) | Total Mapped Reads (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Reads | Total Raw Bases (Gb) | Q30 (%) | Reads | Total Clean Bases (Gb) | Q30 (%) | |||
| T-2_1 | 47,053,795 | 13.0 | 95.1 | 46,831,502 | 12.9 | 95.4 | 99.5 | 90.4 |
| T-2_2 | 50,495,169 | 14.0 | 94.9 | 50,276,942 | 14.0 | 95.1 | 99.6 | 90.8 |
| T-2_3 | 51,064,323 | 14.2 | 95.1 | 50,818,840 | 14.1 | 95.4 | 99.5 | 90.7 |
| control_1 | 49,245,939 | 13.7 | 95.5 | 49,020,806 | 13.6 | 95.7 | 99.5 | 90.2 |
| control_2 | 47,073,831 | 13.1 | 95.4 | 46,802,405 | 13.0 | 95.7 | 99.4 | 89.7 |
| control_3 | 45,589,580 | 12.6 | 95.4 | 45,383,773 | 12.6 | 95.5 | 99.5 | 90.0 |
| Name of Drugs | p-Value | Adjusted p-Value | Genes |
|---|---|---|---|
| etoposide CTD 00005948 | 1.37 × 10−6 | 7.65 × 10−4 | HDAC4, FOXO3, FOXO1, RELA |
| diatrizoic acid CTD 00005787 | 2.75 × 10−6 | 7.68 × 10−4 | FOXO3, RELA |
| mollugin CTD 00002058 | 7.64 × 10−6 | 7.99 × 10−4 | STK11, RELA |
| metformin BOSS | 8.19 × 10−6 | 7.99 × 10−4 | STK11, FOXO3, FOXO1 |
| 2-Butanone BOSS | 9.14 × 10−6 | 7.99 × 10−4 | STK11, FOXO3, FOXO1 |
| rapamycin BOSS | 9.42 × 10−6 | 7.99 × 10−4 | STK11, FOXO3, FOXO1 |
| dexamethasone BOSS | 1.00 × 10−5 | 7.99 × 10−4 | FOXO3, FOXO1, RELA |
| resveratrol BOSS | 1.38 × 10−5 | 8.81 × 10−4 | FOXO3, FOXO1, RELA |
| LY 294002 CTD 00003061 | 1.42 × 10−5 | 8.81 × 10−4 | FOXO3, FOXO1, RELA |
| rapamycin CTD 00007350 | 1.83 × 10−5 | 1.02 × 10−3 | FOXO3, FOXO1, RELA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, F.; Wang, M.; Luo, K.; Sun, L.; Yu, S.; Zuo, J.; Wang, Y. Expression Profiles of Long Non-Coding RNAs in the Articular Cartilage of Rats Exposed to T-2 Toxin. Int. J. Mol. Sci. 2023, 24, 13703. https://doi.org/10.3390/ijms241813703
Yu F, Wang M, Luo K, Sun L, Yu S, Zuo J, Wang Y. Expression Profiles of Long Non-Coding RNAs in the Articular Cartilage of Rats Exposed to T-2 Toxin. International Journal of Molecular Sciences. 2023; 24(18):13703. https://doi.org/10.3390/ijms241813703
Chicago/Turabian StyleYu, Fangfang, Miao Wang, Kangting Luo, Lei Sun, Shuiyuan Yu, Juan Zuo, and Yanjie Wang. 2023. "Expression Profiles of Long Non-Coding RNAs in the Articular Cartilage of Rats Exposed to T-2 Toxin" International Journal of Molecular Sciences 24, no. 18: 13703. https://doi.org/10.3390/ijms241813703
APA StyleYu, F., Wang, M., Luo, K., Sun, L., Yu, S., Zuo, J., & Wang, Y. (2023). Expression Profiles of Long Non-Coding RNAs in the Articular Cartilage of Rats Exposed to T-2 Toxin. International Journal of Molecular Sciences, 24(18), 13703. https://doi.org/10.3390/ijms241813703





