The Comparison of Serum Exosome Protein Profile in Diagnosis of NSCLC Patients
Abstract
1. Introduction
2. Results
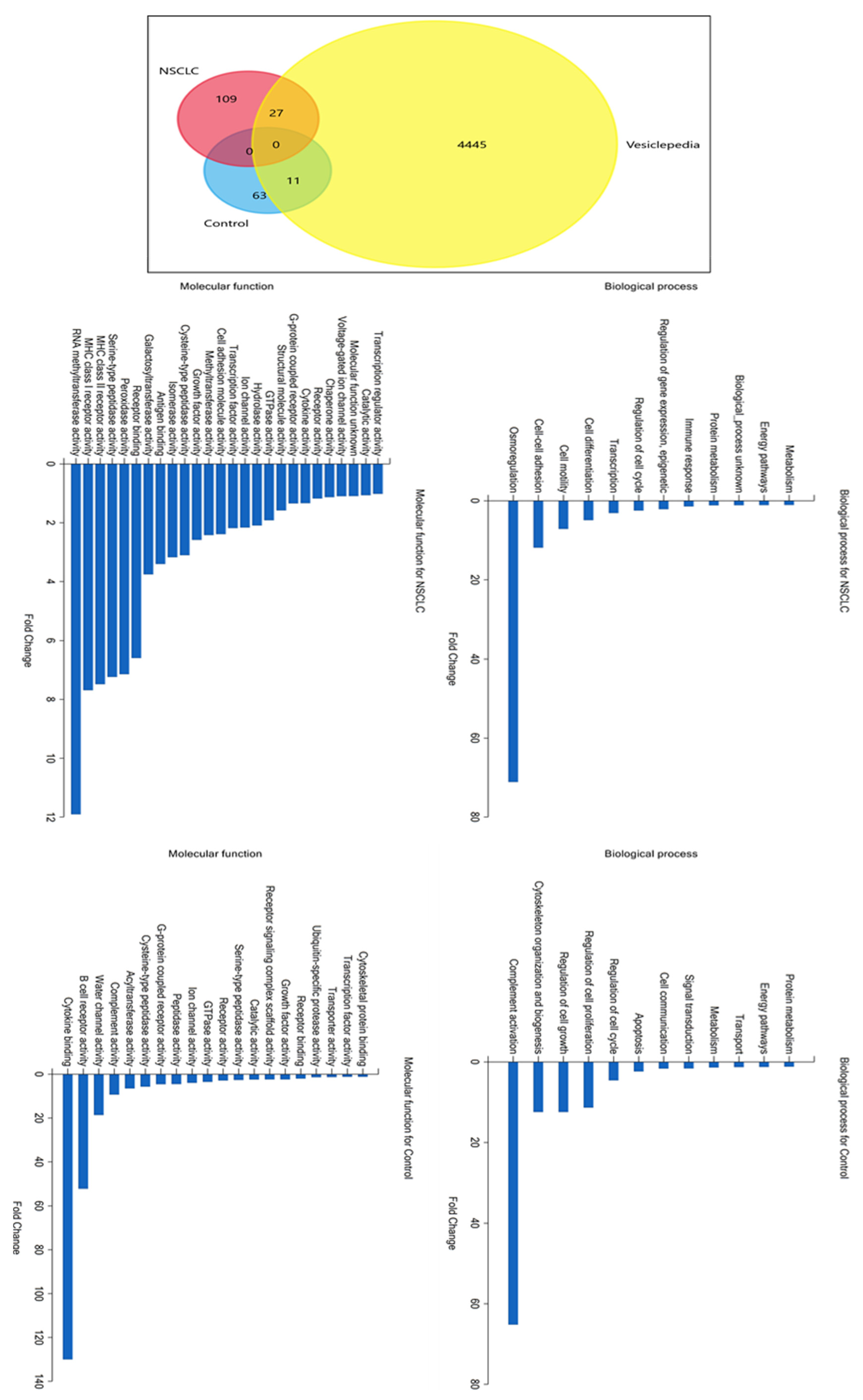
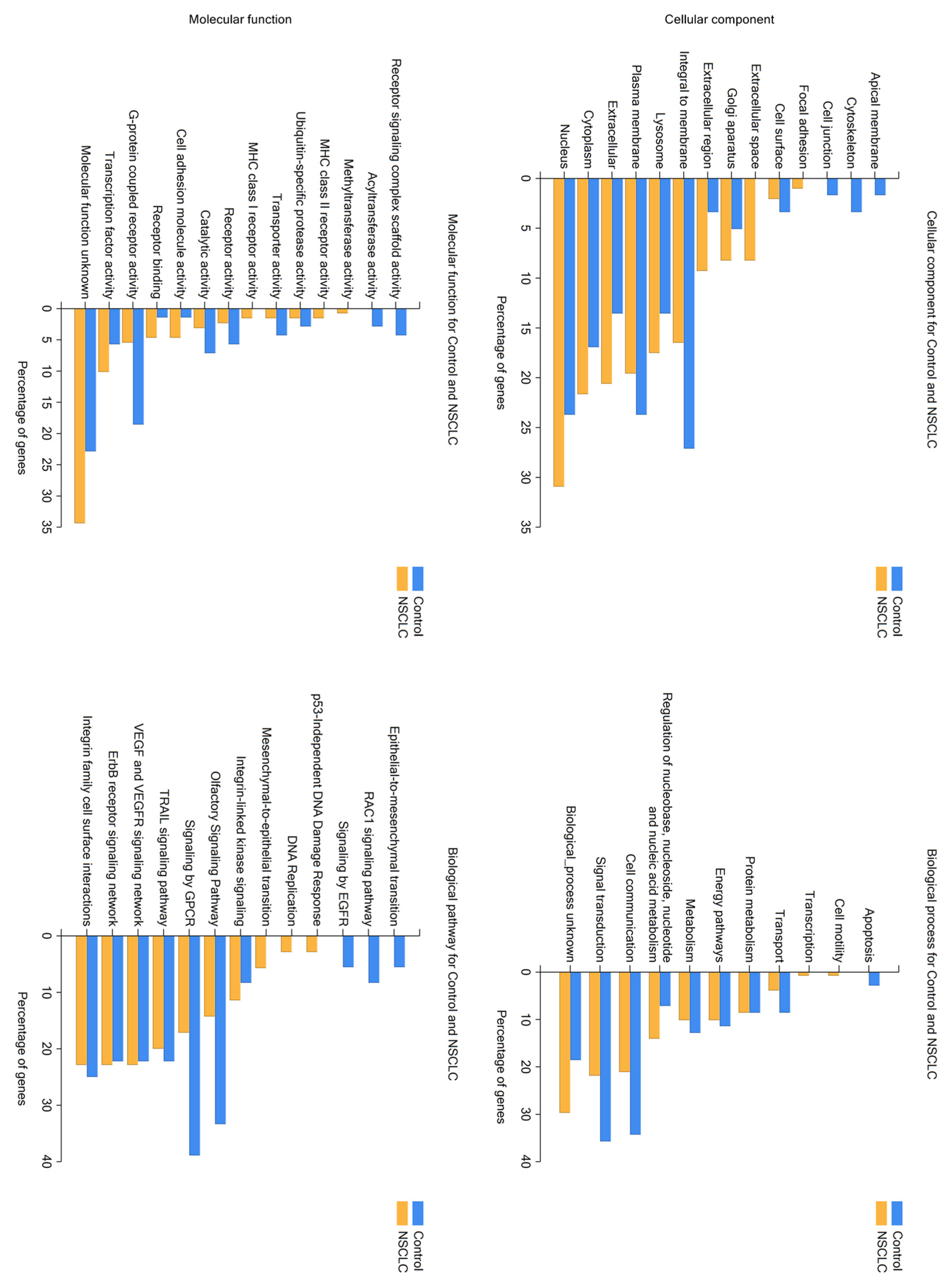
2.1. LC/MS Analysis of Protein Profiles of Serum Exosomes of NSCLC Patients vs. Controls
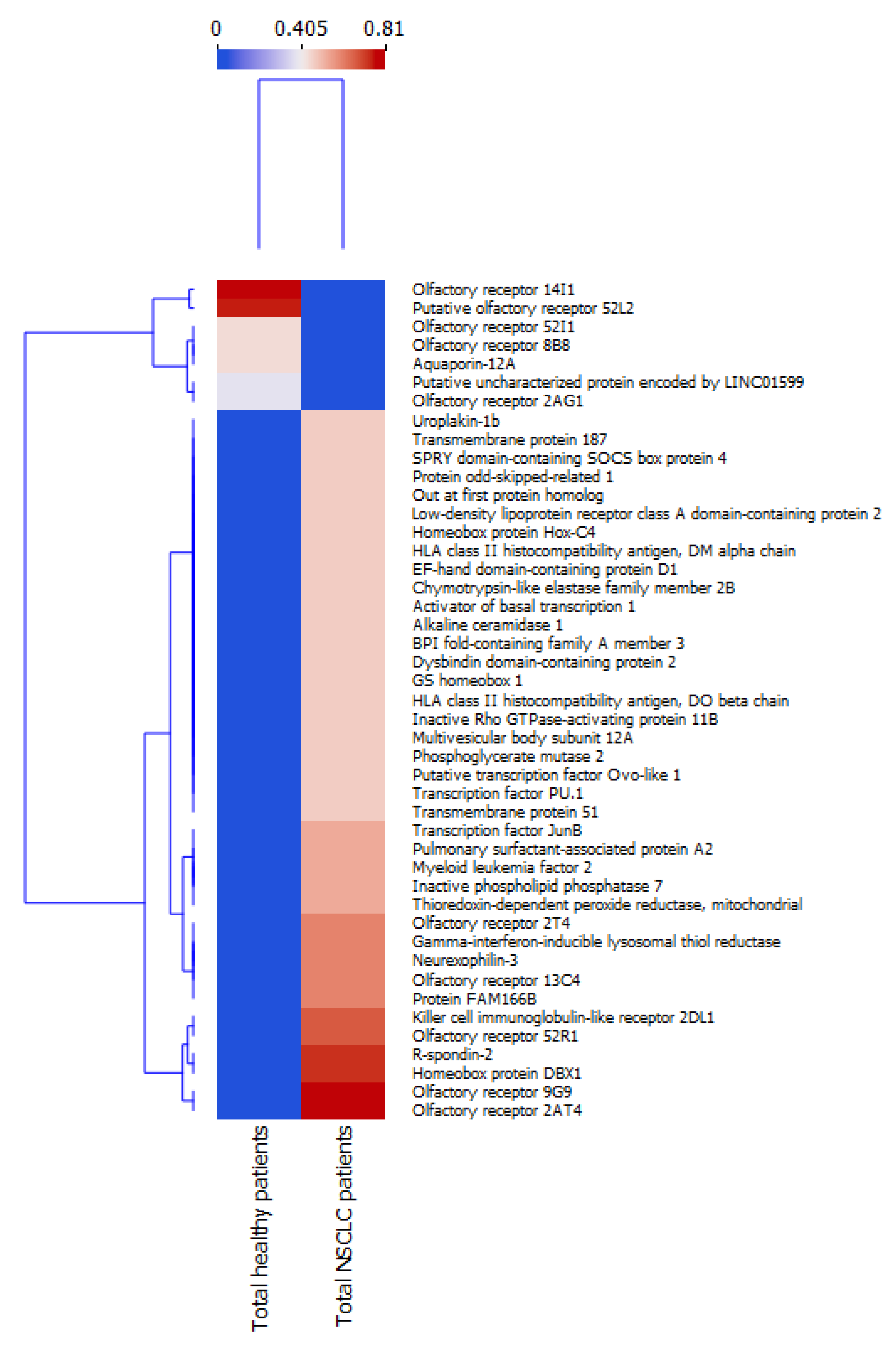
2.2. Analysis of Hierarchical Clustering
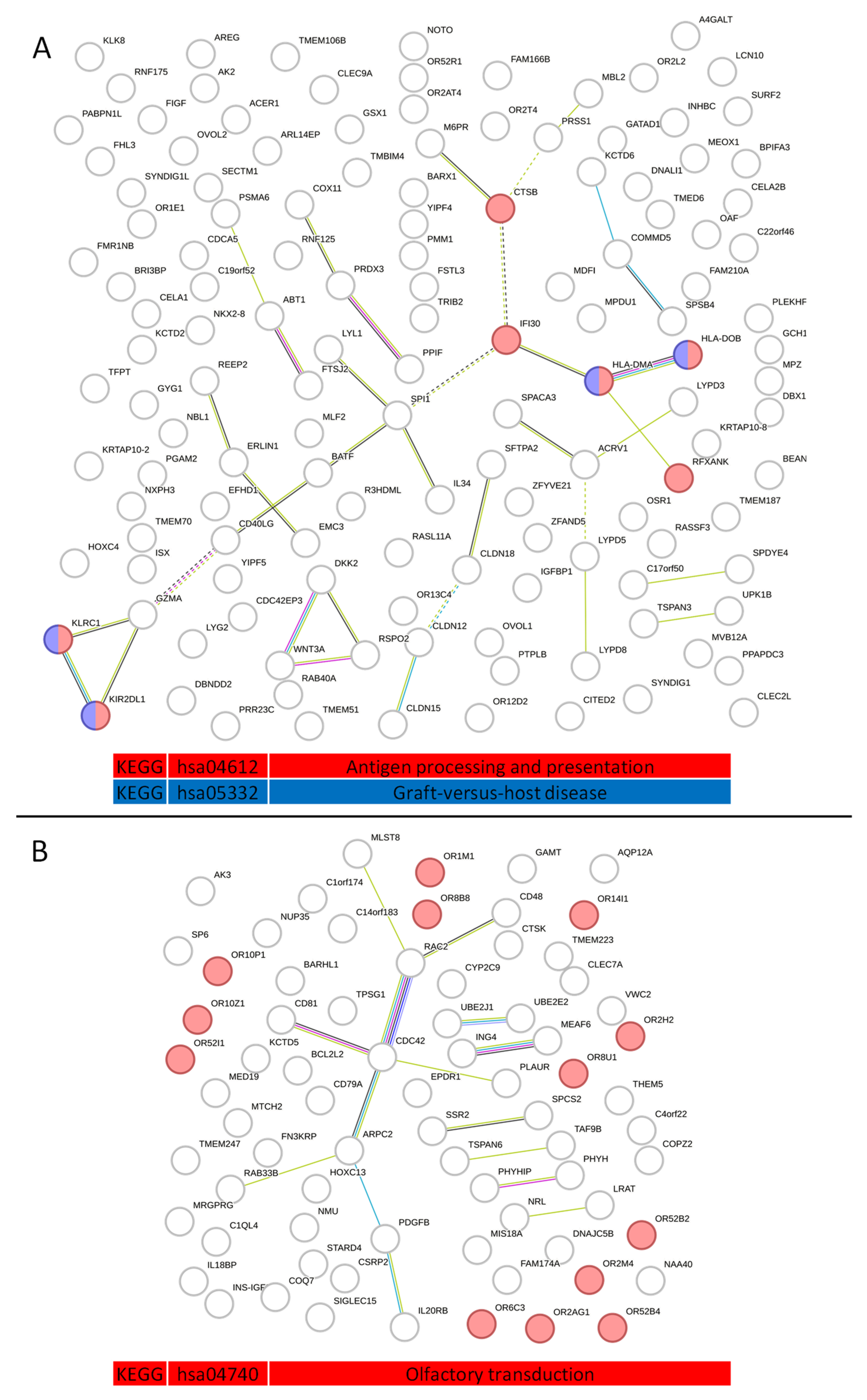
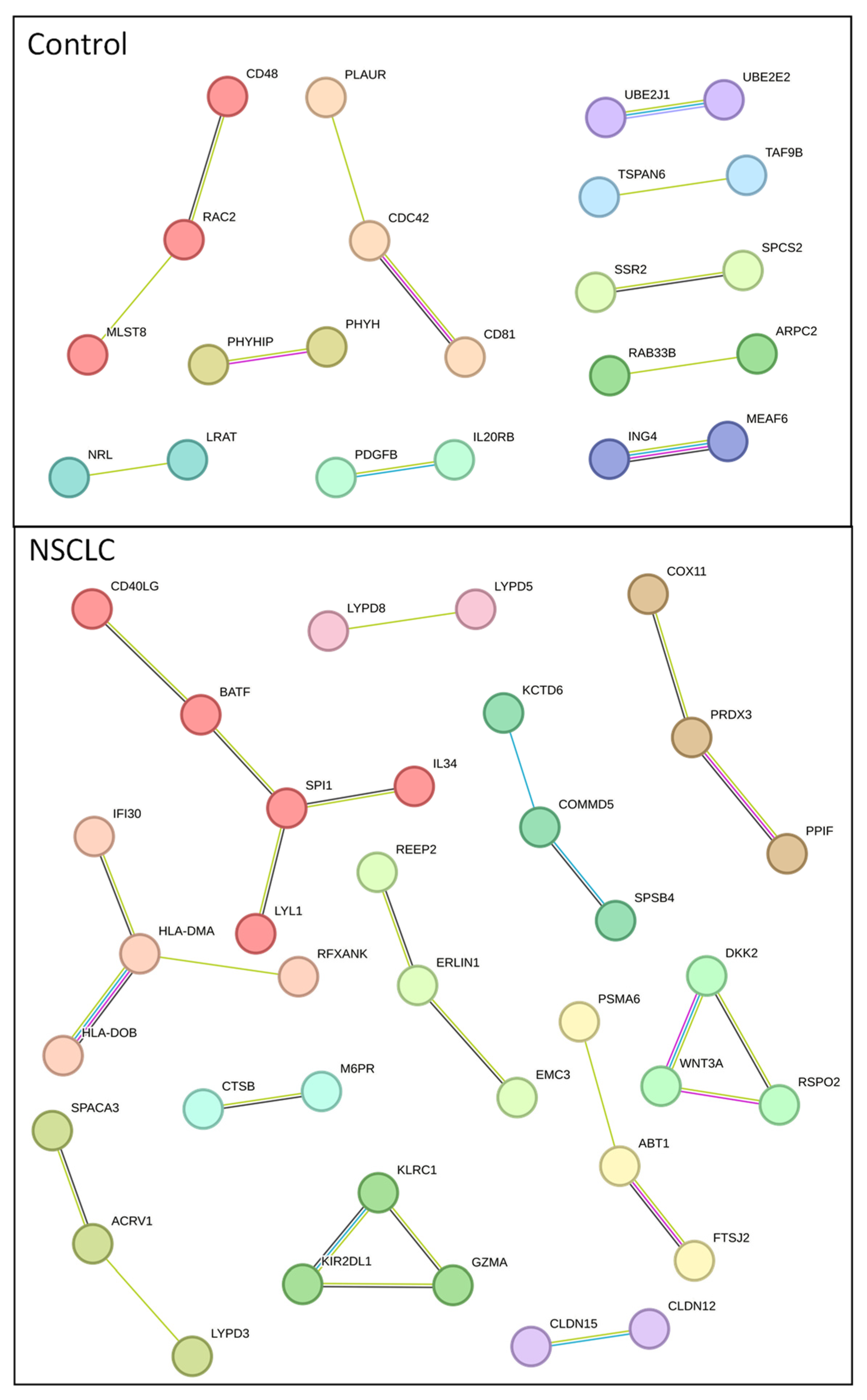
2.3. Analysis of Protein–Protein Interactions in Serum Exosome-Derived Proteins
2.4. Analysis of Serum Exosome-Derived Protein Hub Clusters
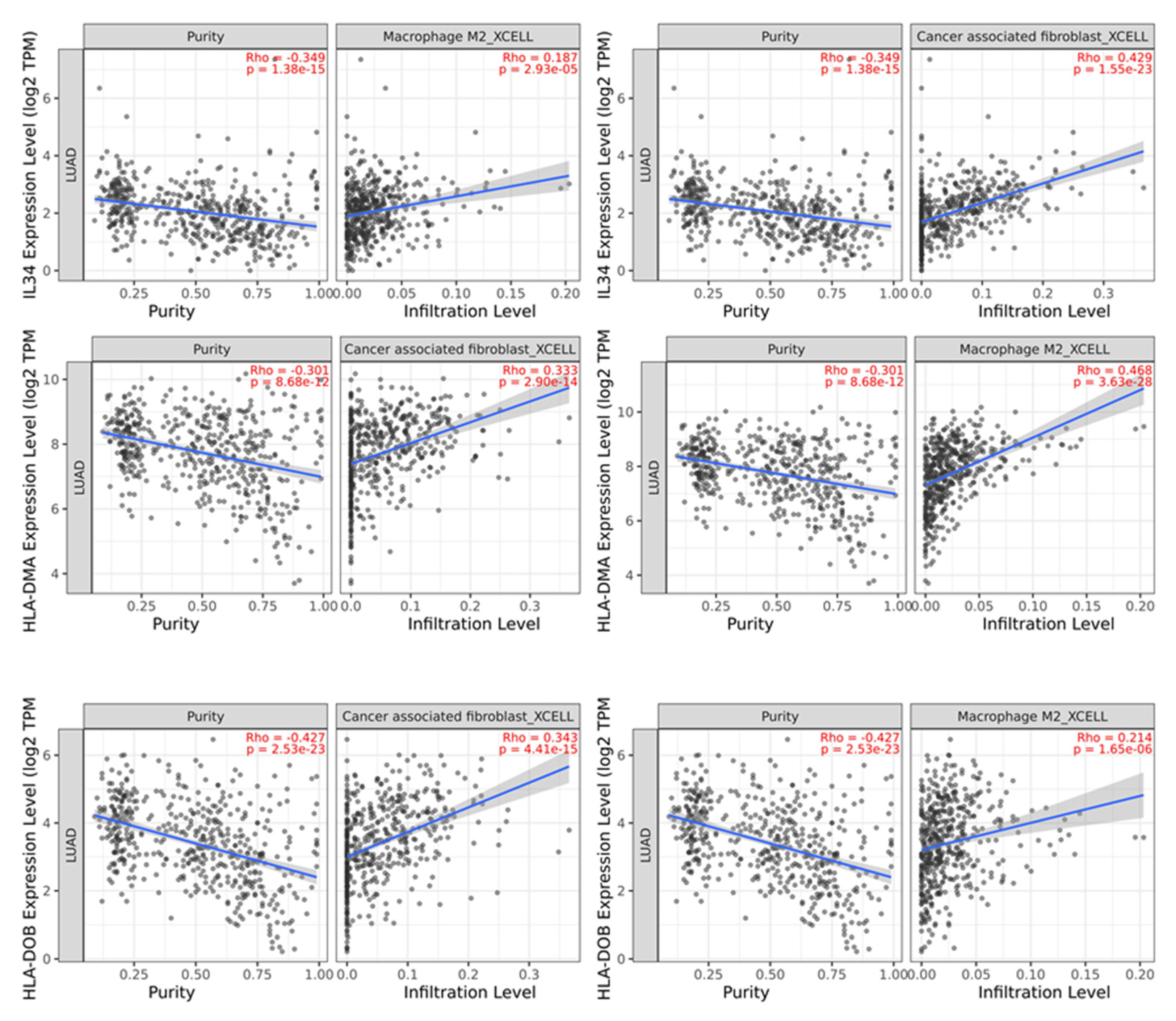
2.5. Analysis of the Impact of NSCLC Patients Serum Exosome-Derived Proteins on Immune Cell Infiltration
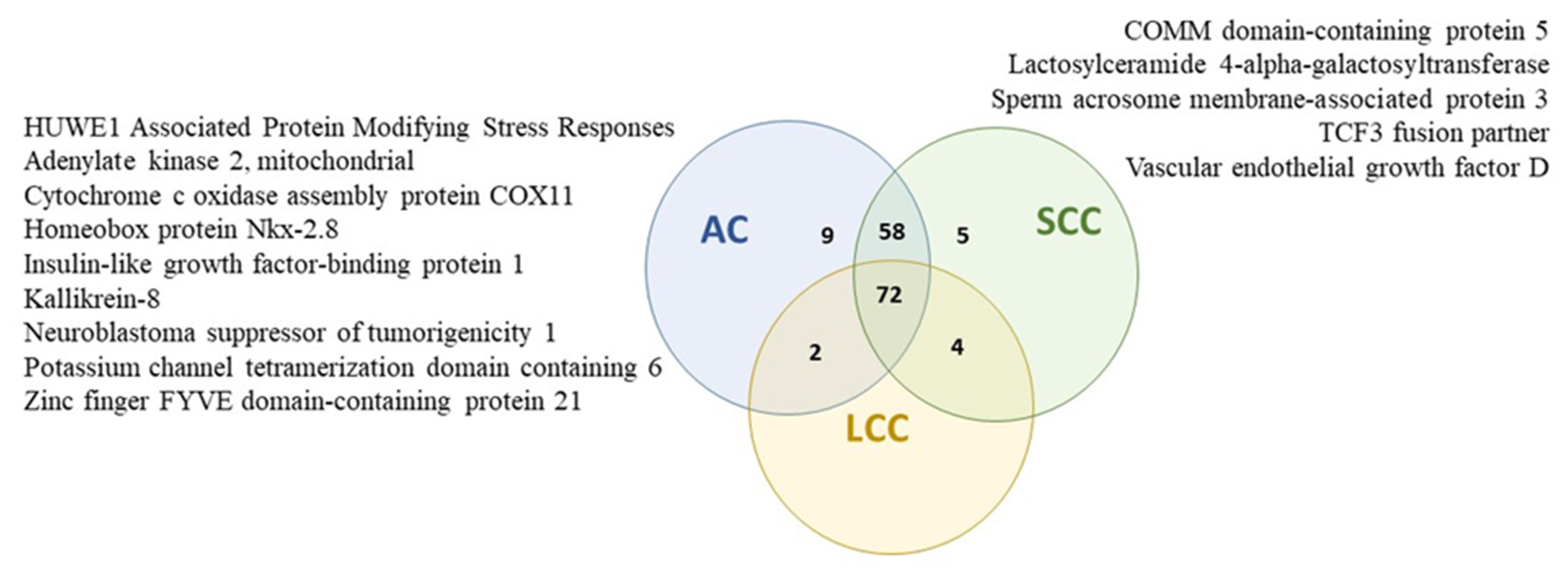
2.6. Protein Profiles of Serum Exosomes of NSCLC Patients According to the Histopathological Subtype of Tumor
2.7. Protein Profiles of Serum Exosomes of NSCLC Patients According to the Tumor Size
2.8. Protein Profiles of Serum Exosomes of NSCLC Patients According to the State of Regional Lymph Nodes
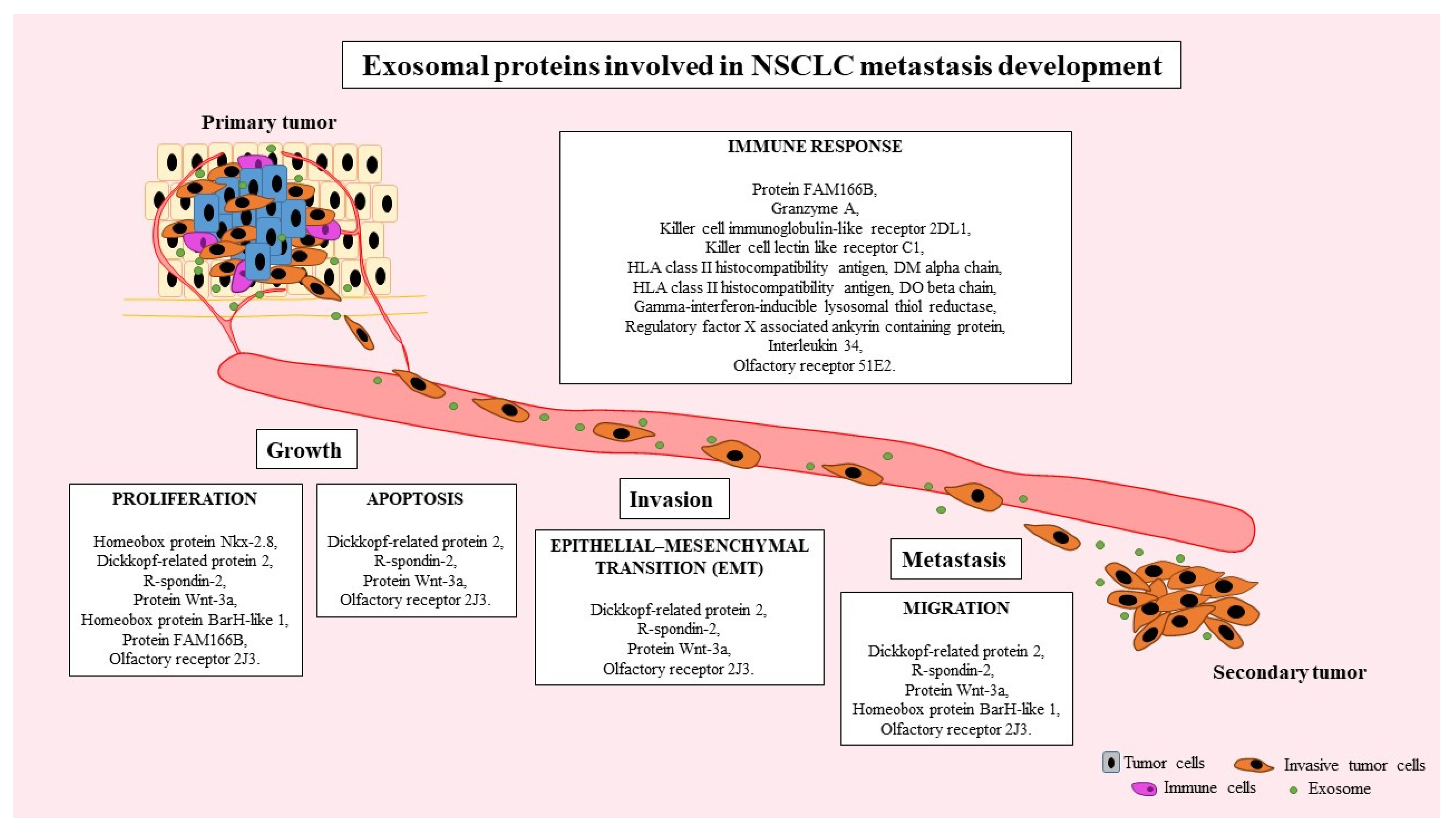
3. Discussion
4. Materials and Methods
4.1. Patient Characteristics
4.2. Serum Collection
4.3. Isolation of Proteins from Serum Exosomes
4.4. LC/MS Analysis of Exosomal Proteins
4.5. Hierarchical Clustering
4.6. Protein–Protein Interaction Network
4.7. Analysis of Cancer-Associated Fibroblasts (CAFs) and M2 Macrophages Infiltration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancer Today. Available online: http://gco.iarc.fr/today/home (accessed on 9 August 2023).
- Yang, T.; Xiong, Y.; Zeng, Y.; Wang, Y.; Zeng, J.; Liu, J.; Xu, S.; Li, L.-S. Current status of immunotherapy for non-small cell lung cancer. Front. Pharmacol. 2022, 13, 989461. [Google Scholar] [CrossRef] [PubMed]
- Osmani, L.; Askin, F.; Gabrielson, E.; Li, Q.K. Current WHO guidelines and the critical role of immunohistochemical markers in the subclassification of non-small cell lung carcinoma (NSCLC): Moving from targeted therapy to immunotherapy. Semin. Cancer Biol. 2018, 52, 103–109. [Google Scholar] [CrossRef] [PubMed]
- SEER Cancer Statistics Review, 1975–2016, SEER. Available online: https://seer.cancer.gov/csr/1975_2016/index.html (accessed on 9 August 2023).
- Castro-Giner, F.; Gkountela, S.; Donato, C.; Alborelli, I.; Quagliata, L.; Ng, C.K.Y.; Piscuoglio, S.; Aceto, N. Cancer Diagnosis Using a Liquid Biopsy: Challenges and Expectations. Diagnostics 2018, 8, 31. [Google Scholar] [CrossRef] [PubMed]
- Gold, B.; Cankovic, M.; Furtado, L.V.; Meier, F.; Gocke, C.D. Do circulating tumor cells, exosomes, and circulating tumor nucleic acids have clinical utility? A report of the association for molecular pathology. J. Mol. Diagn. 2015, 17, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Boukouris, S.; Mathivanan, S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin. Appl. 2015, 9, 358–367. [Google Scholar] [CrossRef]
- Ge, Q.; Zhou, Y.; Lu, J.; Bai, Y.; Xie, X.; Lu, Z. miRNA in plasma exosome is stable under different storage conditions. Molecules 2014, 19, 1568–1575. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, N.; Hu, X.; Wang, H. Tumor-associated exosomes promote lung cancer metastasis through multiple mechanisms. Mol. Cancer 2021, 20, 117. [Google Scholar] [CrossRef]
- Sandúa, A.; Alegre, E.; González, Á. Exosomes in Lung Cancer: Actors and Heralds of Tumor Development. Cancers 2021, 13, 4330. [Google Scholar] [CrossRef]
- Yin, L.; Liu, X.; Shao, X.; Feng, T.; Xu, J.; Wang, Q.; Hua, S. The role of exosomes in lung cancer metastasis and clinical applications: An updated review. J. Transl. Med. 2021, 19, 312. [Google Scholar] [CrossRef]
- Wang, N.; Song, X.; Liu, L.; Niu, L.; Wang, X.; Song, X.; Xie, L. Circulating exosomes contain protein biomarkers of metastatic non-small-cell lung cancer. Cancer Sci. 2018, 109, 1701–1709. [Google Scholar] [CrossRef]
- Niu, L.; Song, X.; Wang, N.; Xue, L.; Song, X.; Xie, L. Tumor-derived exosomal proteins as diagnostic biomarkers in non-small cell lung cancer. Cancer Sci. 2019, 110, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tang, Y.; Song, X.; Xie, L.; Zhao, S.; Song, X. Tumor-Derived Exosomal miRNAs as Diagnostic Biomarkers in Non-Small Cell Lung Cancer. Front. Oncol. 2020, 10, 560025. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Huo, C.; Qiao, Z.; Shang, Z.; Uzzaman, A.; Liu, S.; Jiang, X.; Fan, L.-Y.; Ji, L.; Guan, X.; et al. Comparative Proteomic Analysis of Exosomes and Microvesicles in Human Saliva for Lung Cancer. J. Proteome Res. 2018, 17, 1101–1107. [Google Scholar] [CrossRef]
- Li, L.S.; Kim, H.; Rhee, H.; Kim, S.H.; Shin, D.H.; Chung, K.Y.; Park, K.-S.; Paik, Y.-K.; Chang, J.; Kim, H. Proteomic analysis distinguishes basaloid carcinoma as a distinct subtype of nonsmall cell lung carcinoma. Proteomics 2004, 4, 3394–3400. [Google Scholar] [CrossRef]
- Baran, K.; Brzeziańska-Lasota, E. Proteomic biomarkers of non-small cell lung cancer patients. Adv. Respir. Med. 2021, 89, 419–426. [Google Scholar] [CrossRef]
- Li, D.-J.; Deng, G.; Xiao, Z.-Q.; Yao, H.-X.; Li, C.; Peng, F.; Li, M.-Y.; Zhang, P.-F.; Chen, Y.-H.; Chen, Z.-C. Identificating 14-3-3 sigma as a lymph node metastasis-related protein in human lung squamous carcinoma. Cancer Lett. 2009, 279, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-H.; Hsu, C.-W.; Hsueh, C.; Wang, C.-L.; Wu, Y.-C.; Wu, C.-C.; Liu, C.-C.; Yu, J.-S.; Chang, Y.-S.; Yu, C.-J. Identification and Characterization of Potential Biomarkers by Quantitative Tissue Proteomics of Primary Lung Adenocarcinoma. Mol. Cell. Proteomics 2016, 15, 2396–2410. [Google Scholar] [CrossRef]
- Bergman, L.R.; Magnusson, D. Person-centered Research. In The International Encyclopedia of the Social and Behavioral Sciences: Logic of Inquiry and Research Design; Elsevier: Amsterdam, The Netherlands, 2001; pp. 11333–11339. [Google Scholar] [CrossRef]
- Shokati, E.; Safari, E. The immunomodulatory role of exosomal microRNA networks in the crosstalk between tumor-associated myeloid-derived suppressor cells and tumor cells. Int. Immunopharmacol. 2023, 120, 110267. [Google Scholar] [CrossRef]
- Zhang, H.; Jiang, H.; Zhu, L.; Li, J.; Ma, S. Cancer-associated fibroblasts in non-small cell lung cancer: Recent advances and future perspectives. Cancer Lett. 2021, 514, 38–47. [Google Scholar] [CrossRef]
- Bonanno, L.; Dal Maso, A.; Pavan, A.; Zulato, E.; Calvetti, L.; Pasello, G.; Guarneri, V.; Conte, P.; Indraccolo, S. Liquid biopsy and non-small cell lung cancer: Are we looking at the tip of the iceberg? Br. J. Cancer 2022, 127, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Vanderlaan, P.A.; Yamaguchi, N.; Folch, E.; Boucher, D.H.; Kent, M.S.; Gangadharan, S.P.; Majid, A.; Goldstein, M.A.; Huberman, M.S.; Kocher, O.N.; et al. Success and failure rates of tumor genotyping techniques in routine pathological samples with non-small-cell lung cancer. Lung Cancer 2014, 84, 39–44. [Google Scholar] [CrossRef]
- Schwartzberg, L.S.; Horinouchi, H.; Chan, D.; Chernilo, S.; Tsai, M.L.; Isla, D.; Escriu, C.; Bennett, J.P.; Clark-Langone, K.; Svedman, C.; et al. Liquid biopsy mutation panel for non-small cell lung cancer: Analytical validation and clinical concordance. NPJ Precis. Oncol. 2020, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Palmirotta, R.; Lovero, D.; Cafforio, P.; Felici, C.; Mannavola, F.; Pellè, E.; Quaresmini, D.; Tucci, M.; Silvestris, F. Liquid biopsy of cancer: A multimodal diagnostic tool in clinical oncology. Ther. Adv. Med. Oncol. 2018, 10, 1758835918794630. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hui, Y.; Zhang, Y.; Zhang, M.; Ji, B.; Tian, G.; Guo, Y.; Tang, M.; Li, L.; Guo, B.; et al. Application of Circulating Tumor DNA as a Biomarker for Non-Small Cell Lung Cancer. Front. Oncol. 2021, 11, 725938. [Google Scholar] [CrossRef]
- Bao, M.; Huang, Y.; Lang, Z.; Zhao, H.; Saito, Y.; Nagano, T.; Kawagoe, I.; Divisi, D.; Hu, X.; Jiang, G. Proteomic analysis of plasma exosomes in patients with non-small cell lung cancer. Transl. Lung Cancer Res. 2022, 11, 1434–1452. [Google Scholar] [CrossRef]
- Kuang, M.; Tao, X.; Peng, Y.; Zhang, W.; Pan, Y.; Cheng, L.; Yuan, C.; Zhao, Y.; Mao, H.; Zhuge, L.; et al. Proteomic analysis of plasma exosomes to differentiate malignant from benign pulmonary nodules. Clin. Proteomics 2019, 16, 5. [Google Scholar] [CrossRef]
- Alipoor, S.D.; Mortaz, E.; Varahram, M.; Movassaghi, M.; Kraneveld, A.D.; Garssen, J.; Adcock, I.M. The Potential Biomarkers and Immunological Effects of Tumor-Derived Exosomes in Lung Cancer. Front. Immunol. 2018, 9, 819. [Google Scholar] [CrossRef]
- Dilsiz, N. Role of exosomes and exosomal microRNAs in cancer. Future Sci. OA 2020, 6, FSO465. [Google Scholar] [CrossRef]
- Ranzani, M.; Iyer, V.; Ibarra-Soria, X.; Del Castillo Velasco-Herrera, M.; Garnett, M.; Logan, D.; Adams, D.J. Revisiting olfactory receptors as putative drivers of cancer. Wellcome Open Res. 2017, 2, 9. [Google Scholar] [CrossRef]
- Giandomenico, V.; Cui, T.; Grimelius, L.; Öberg, K.; Pelosi, G.; Tsolakis, A.V. Olfactory receptor 51E1 as a novel target for diagnosis in somatostatin receptor-negative lung carcinoids. J. Mol. Endocrinol. 2013, 51, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Kalbe, B.; Schulz, V.M.; Schlimm, M.; Philippou, S.; Jovancevic, N.; Jansen, F.; Scholz, P.; Lübbert, H.; Jarocki, M.; Faissner, A.; et al. Helional-induced activation of human olfactory receptor 2J3 promotes apoptosis and inhibits proliferation in a non-small-cell lung cancer cell line. Eur. J. Cell Biol. 2017, 96, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Vadevoo, S.M.P.; Gunassekaran, G.R.; Lee, C.; Lee, N.; Lee, J.; Chae, S.; Park, J.-Y.; Koo, J.; Lee, B. The macrophage odorant receptor Olfr78 mediates the lactate-induced M2 phenotype of tumor-associated macrophages. Proc. Natl. Acad. Sci. USA 2021, 118, e2102434118. [Google Scholar] [CrossRef]
- Pierani, A.; Moran-Rivard, L.; Sunshine, M.J.; Littman, D.R.; Goulding, M.; Jessell, T.M. Control of Interneuron Fate in the Developing Spinal Cord by the Progenitor Homeodomain Protein Dbx1. Neuron 2001, 29, 367–384. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, M.; Zhang, W.; Li, Y.; Zhu, J.; Zhang, X.; Zhao, L.; Zhu, S.; Chen, B. Downregulation of BarH-like homeobox 2 promotes cell proliferation, migration and aerobic glycolysis through Wnt/β-catenin signaling, and predicts a poor prognosis in non-small cell lung carcinoma. Thorac. Cancer 2018, 9, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Kendall, J.; Liu, Q.; Bakleh, A.; Krasnitz, A.; Nguyen, K.C.Q.; Lakshmi, B.; Gerald, W.L.; Powers, S.; Mu, D. Oncogenic cooperation and coamplification of developmental transcription factor genes in lung cancer. Proc. Natl. Acad. Sci. USA 2007, 104, 16663–16668. [Google Scholar] [CrossRef]
- Hsu, D.S.; Acharya, C.R.; Balakumaran, B.S.; Riedel, R.F.; Kim, M.K.; Stevenson, M.; Tuchman, S.; Mukherjee, S.; Barry, W.; Dressman, H.K.; et al. Characterizing the developmental pathways TTF-1, NKX2–8, and PAX9 in lung cancer. Proc. Natl. Acad. Sci. USA 2009, 106, 5312–5317. [Google Scholar] [CrossRef]
- Johnson, A.M.; Bullock, B.L.; Neuwelt, A.J.; Poczobutt, J.M.; Kaspar, R.E.; Li, H.Y.; Kwak, J.W.; Hopp, K.; Weiser-Evans, M.C.M.; Heasley, L.E.; et al. Cancer Cell–Intrinsic Expression of MHC Class II Regulates the Immune Microenvironment and Response to Anti–PD-1 Therapy in Lung Adenocarcinoma. J. Immunol. 2020, 204, 2295–2307. [Google Scholar] [CrossRef]
- Axelrod, M.L.; Cook, R.S.; Johnson, D.B.; Balko, J.M. Biological Consequences of MHC-II Expression by Tumor Cells in Cancer. Clin. Cancer Res. 2019, 25, 2392–2402. [Google Scholar] [CrossRef]
- Kim, S.H.; Bianco, N.R.; Shufesky, W.J.; Morelli, A.E.; Robbins, P.D. MHC Class II+ Exosomes in Plasma Suppress Inflammation in an Antigen-Specific and Fas Ligand/Fas-Dependent Manner1. J. Immunol. 2007, 179, 2235–2241. [Google Scholar] [CrossRef]
- Wang, W.; Li, Q.; Yamada, T.; Matsumoto, K.; Matsumoto, I.; Oda, M.; Watanabe, G.; Kayano, Y.; Nishioka, Y.; Sone, S.; et al. Crosstalk to stromal fibroblasts induces resistance of lung cancer to epidermal growth factor receptor tyrosine kinase inhibitors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2009, 15, 6630–6638. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, L.; Wang, H.; Liu, B.; Zhang, Q.; Meng, Z.; Wu, X.; Zhou, Q.; Xu, K. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget 2017, 8, 76116–76128. [Google Scholar] [CrossRef] [PubMed]
- Gunaydin, G. CAFs Interacting With TAMs in Tumor Microenvironment to Enhance Tumorigenesis and Immune Evasion. Front. Oncol. 2021, 11, 668349. [Google Scholar] [CrossRef]
- Komohara, Y.; Takeya, M. CAFs and TAMs: Maestros of the tumour microenvironment. J. Pathol. 2017, 241, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, A.; Tousif, S.; Wang, Y.; Hough, K.; Khan, S.; Strenkowski, J.; Chacko, B.K.; Darley-Usmar, V.M.; Deshane, J.S. Lung Tumor Cell-Derived Exosomes Promote M2 Macrophage Polarization. Cells 2020, 9, 1303. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.S.; Kanugula, S.S.; Sudhir, S.; Pereira, M.P.; Jain, S.; Aghi, M.K. The Role of Cancer-Associated Fibroblasts in Tumor Progression. Cancers 2021, 13, 1399. [Google Scholar] [CrossRef]
- Ping, Q.; Yan, R.; Cheng, X.; Wang, W.; Zhong, Y.; Hou, Z.; Shi, Y.; Wang, C.; Li, R. Cancer-associated fibroblasts: Overview, progress, challenges, and directions. Cancer Gene Ther. 2021, 28, 984–999. [Google Scholar] [CrossRef]
- Sedighzadeh, S.S.; Khoshbin, A.P.; Razi, S.; Keshavarz-Fathi, M.; Rezaei, N. A narrative review of tumor-associated macrophages in lung cancer: Regulation of macrophage polarization and therapeutic implications. Transl. Lung Cancer Res. 2021, 10, 1889–1916. [Google Scholar] [CrossRef]
- Xu, C.; Song, L.; Yang, Y.; Liu, Y.; Pei, D.; Liu, J.; Guo, J.; Liu, N.; Li, X.; Liu, Y.; et al. Clinical M2 Macrophage-Related Genes Can Serve as a Reliable Predictor of Lung Adenocarcinoma. Front. Oncol. 2022, 12, 919899. [Google Scholar] [CrossRef]
- Raslan, A.A.; Oh, Y.J.; Jin, Y.R.; Yoon, J.K. R-Spondin2, a Positive Canonical WNT Signaling Regulator, Controls the Expansion and Differentiation of Distal Lung Epithelial Stem/Progenitor Cells in Mice. Int. J. Mol. Sci. 2022, 23, 3089. [Google Scholar] [CrossRef]
- Mei, X.D.; Su, H.; Song, J.; Dong, L. Prognostic significance of β-catenin expression in patients with non-small cell lung cancer: A meta-analysis. Biosci. Trends 2013, 7, 42–49. [Google Scholar] [CrossRef][Green Version]
- Giralt, I.; Gallo-Oller, G.; Navarro, N.; Zarzosa, P.; Pons, G.; Magdaleno, A.; Segura, M.F.; Sánchez de Toledo, J.; Moreno, L.; Gallego, S.; et al. Dickkopf Proteins and Their Role in Cancer: A Family of Wnt Antagonists with a Dual Role. Pharmaceuticals 2021, 14, 810. [Google Scholar] [CrossRef] [PubMed]
- Tam, W.L.; Weinberg, R.A. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat. Med. 2013, 19, 1438–1449. [Google Scholar] [CrossRef]
- Xiao, Q.; Wu, J.; Wang, W.-J.; Chen, S.; Zheng, Y.; Yu, X.; Meeth, K.; Sahraei, M.; Bothwell, A.L.M.; Chen, L.; et al. DKK2 imparts tumor immunity evasion through β-catenin-independent suppression of cytotoxic immune-cell activation. Nat. Med. 2018, 24, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Delpire, E.; Gagnon, K.B. Water Homeostasis and Cell Volume Maintenance and Regulation. Curr. Top. Membr. 2018, 81, 3–52. [Google Scholar] [CrossRef]
- Shorthouse, D.; Riedel, A.; Kerr, E.; Pedro, L.; Bihary, D.; Samarajiwa, S.; Martins, C.P.; Shields, J.; Hall, B.A. Exploring the role of stromal osmoregulation in cancer and disease using executable modelling. Nat. Commun. 2018, 9, 3011. [Google Scholar] [CrossRef]
- Okada, Y.; Maeno, E. Apoptosis, cell volume regulation and volume-regulatory chloride channels. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2001, 130, 377–383. [Google Scholar] [CrossRef]
- Martial, S. Involvement of ion channels and transporters in carcinoma angiogenesis and metastasis. Am. J. Physiol.-Cell Physiol. 2016, 310, C710–C727. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, H.; He, G.; Zhang, H.; Cheng, X.; Liu, X. Overexpressed FAM166B Predicts Favorable Prognosis and Associated with Metabolic Pathways and Tumor Immune Infiltrates in BRC. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Phan, L.M.; Yeung, S.-C.J.; Lee, M.-H. Cancer metabolic reprogramming: Importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 2014, 11, 1–19. [Google Scholar] [CrossRef]
- Mandelboim, O.; Reyburn, H.T.; Sheu, E.G.; Vales-Gomez, M.; Davis, D.M.; Pazmany, L.; Strominger, J.L. The binding site of NK receptors on HLA-C molecules. Immunity 1997, 6, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Goding, S.R.; Hokland, M.E.; Basse, P.H. Antitumor activity of NK cells. Immunol. Res. 2006, 36, 13–25. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Bunn, P.A.; Zhou, C.; Chan, D. KIR 2D (L1, L3, L4, S4) and KIR 3DL1 protein expression in non-small cell lung cancer. Oncotarget 2016, 7, 82104–82111. [Google Scholar] [CrossRef] [PubMed]
- Boyiadzis, M.; Hong, C.S.; Whiteside, T.L. Natural Killer Cell Derived Exosomes As a Novel Therapeutic for Acute Myeloid Leukemia. Blood 2018, 132, 5226. [Google Scholar] [CrossRef]
- Mirsadraee, S.; Oswal, D.; Alizadeh, Y.; Caulo, A.; van Beek, E.J. The 7th lung cancer TNM classification and staging system: Review of the changes and implications. World J. Radiol. 2012, 4, 128–134. [Google Scholar] [CrossRef]
- Kryczka, J.; Boncela, J. Integrated Bioinformatics Analysis of the Hub Genes Involved in Irinotecan Resistance in Colorectal Cancer. Biomedicines 2022, 10, 1720. [Google Scholar] [CrossRef]
- Pathan, M.; Keerthikumar, S.; Ang, C.-S.; Gangoda, L.; Quek, C.Y.J.; Williamson, N.A.; Mouradov, D.; Sieber, O.M.; Simpson, R.J.; Salim, A.; et al. FunRich: An open access standalone functional enrichment and interaction network analysis tool. Proteomics 2015, 15, 2597–2601. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef]
- Li, T.; Fan, J.; Wang, B.; Traugh, N.; Chen, Q.; Liu, J.S.; Li, B.; Liu, X.S. TIMER: A Web Server for Comprehensive Analysis of Tumor-Infiltrating Immune Cells. Cancer Res. 2017, 77, e108–e110. [Google Scholar] [CrossRef]
- Li, B.; Severson, E.; Pignon, J.-C.; Zhao, H.; Li, T.; Novak, J.; Jiang, P.; Shen, H.; Aster, J.C.; Rodig, S.; et al. Comprehensive analyses of tumor immunity: Implications for cancer immunotherapy. Genome Biol. 2016, 17, 174. [Google Scholar] [CrossRef]

| Tumor Features | Number of Cases (n) | Total Percentage of Cases |
|---|---|---|
| Histopathological subtype of NSCLC | ||
| SCC a | 6 | 40% |
| AC b | 8 | 53% |
| LCC c | 1 | 7% |
| Stage of cancer development | ||
| according to AJCC d staging system | ||
| Stage I | 3 | 20% |
| Stage II | 6 | 40% |
| Stage III | 6 | 40% |
| Presence of lymph node metastasis | ||
| according to the pTNM e staging system | ||
| N0 | 5 | 33% |
| N1 | 6 | 40% |
| N2 | 4 | 27% |
| Tumor size according to the pTNM staging system | ||
| T1a + T1b | 6 | 40% |
| T2a + T2b | 5 | 33% |
| T3 + T4 | 4 | 27% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baran, K.; Waśko, J.; Kryczka, J.; Boncela, J.; Jabłoński, S.; Kolesińska, B.; Brzeziańska-Lasota, E.; Kordiak, J. The Comparison of Serum Exosome Protein Profile in Diagnosis of NSCLC Patients. Int. J. Mol. Sci. 2023, 24, 13669. https://doi.org/10.3390/ijms241813669
Baran K, Waśko J, Kryczka J, Boncela J, Jabłoński S, Kolesińska B, Brzeziańska-Lasota E, Kordiak J. The Comparison of Serum Exosome Protein Profile in Diagnosis of NSCLC Patients. International Journal of Molecular Sciences. 2023; 24(18):13669. https://doi.org/10.3390/ijms241813669
Chicago/Turabian StyleBaran, Kamila, Joanna Waśko, Jakub Kryczka, Joanna Boncela, Sławomir Jabłoński, Beata Kolesińska, Ewa Brzeziańska-Lasota, and Jacek Kordiak. 2023. "The Comparison of Serum Exosome Protein Profile in Diagnosis of NSCLC Patients" International Journal of Molecular Sciences 24, no. 18: 13669. https://doi.org/10.3390/ijms241813669
APA StyleBaran, K., Waśko, J., Kryczka, J., Boncela, J., Jabłoński, S., Kolesińska, B., Brzeziańska-Lasota, E., & Kordiak, J. (2023). The Comparison of Serum Exosome Protein Profile in Diagnosis of NSCLC Patients. International Journal of Molecular Sciences, 24(18), 13669. https://doi.org/10.3390/ijms241813669






