Various LncRNA Mechanisms in Gene Regulation Involving miRNAs or RNA-Binding Proteins in Non-Small-Cell Lung Cancer: Main Signaling Pathways and Networks
Abstract
:1. Introduction
2. Mechanism of Competing Endogenous LncRNAs in Gene Expression Regulation in NSCLC
2.1. Potential lncRNA/miRNA/mRNA Interactome Axes
2.2. LncRNAs Involved in Multiple ceRNA Axes
2.2.1. Oncogenic lncRNA XIST
2.2.2. Oncogenic lncRNA MALAT1
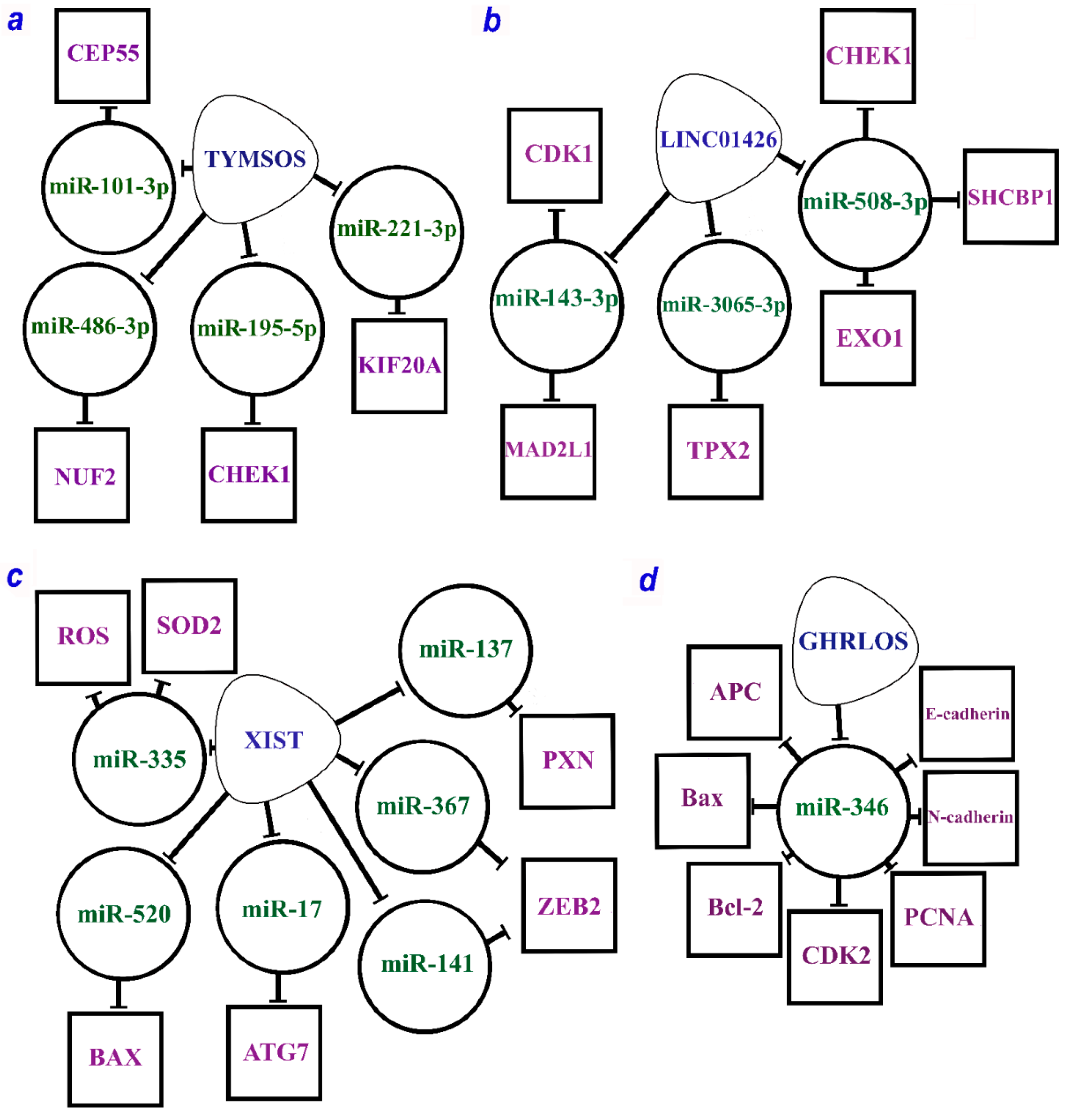
2.2.3. Oncogenic lncRNAs TYMSOS and LINC01426
2.2.4. Suppressor of lncRNA GHRLOS
3. Alternative Mechanisms of lncRNAs in Regulation of Target Genes in NSCLC
3.1. Effects of lncRNAs on Target mRNAs via Direct Binding
3.1.1. Downregulation of Target mRNAs
| Mechanisms, Axes | LncRNAs/Axes in Processes, Pathways, Prognosis, Survival, and Drug Resistance | Ref. |
|---|---|---|
| LncRNA/mRNA, protein | ||
| HIF2PUT↓ov-ex/HIF-2a↓mRNA, protein | Inhibits NSCLC proliferation, invasion | [118] |
| HOTAIR↑/CCL22↓mRNA, protein →CCL22-sign↓→Treg↓ | Promotes invasion, immune evasion; poor prognosis | [119] |
| HOXA-AS3↑/HOXA3↓mRNA, protein | Enhances EMT, drug resistance in vitro/in vivo | [117] |
| NBR2↓ov-ex/Notch1↓mRNA, protein | Inhibits EMT, progression, Notch1 sign | [120] |
| lncRNA-NEF↓ov-ex/GLUT1↓mRNA, protein | Inhibits cell proliferation, glucose uptake | [121] |
| STXBP5-AS1↓ov-ex/STXBP5↓mRNA, protein; STXBP5-AS1↓ov-ex/AKT1↓mRNA, protein | Inhibits cell proliferation, migration, invasion, and PI3K/AKT p-w | [122] |
| TUSC8↓ov-ex/VEGFA↓(3′UTR) mRNA | Inhibits NSCLC; better OS, cisplatin sensitivity | [116] |
| WT1-AS↓ov-ex/TGF-β1↓mRNA, protein | Inhibits cancer cell stemness; improves survival | [123] |
| LncRNA→mRNA, proteins | ||
| AWPPH↑→TGF-β1↑mRNA (in blood) | Promotes cell migration, invasion, and distant recurrence | [124] |
| BLACAT1↑→Cyclin D1↑protein | Enhances cisplatin resistance | [125] |
| CASC2↑→PERK↑mRNAstab, protein /eIF2α↓protein(phosph)→CHOP↑protein | Inhibition of NSCLC, promotion of radiosensitivity, and endoplasmic reticulum stress p-w in irradiated NSCLC cells | [126] |
| DSCAM-AS1↑→BCL11A↑mRNA, protein | Promotes cell migration, invasion, and poor OS | [127] |
| FEZF-AS1↑→FEZF1↑mRNA | Correlation with advanced stages | [128] |
| HOXA-AS2↑→IGF2↑mRNA, protein | Promotes cell migration, invasion, and metastasis | [129] |
| HOXC-AS2↑↔HOXC13↑mRNA | Enhances proliferation, migration, and EMT | [130] |
| LALTOP↑→Top2α↑mRNAstab | Enhances NSCLC progression, cell migration | [131] |
| LINC01288↑→IL-6↑mRNAstab→pSTAT3↑protein | Promotes migration, metastasis in vitro/ in vivo, STAT3 sign | [132] |
| MALAT1↑→SOX9↑mRNA, protein | Enhances chemoresistance; poor OS | [133] |
| NORAD↑→CXCR4↑CXCL12↑protein→ RHOA,ROCK1,ROCK2,LIMK1,LIMK2,P-CFL↑ | Activates proliferation, migration, invasion, RhoA/ROCK sign, in vitro/in vivo | [134] |
| SENCR↑→FLI1↑mRNA, protein | Promotes tumor growth, cisplatin resistance | [135] |
| SFTA1P↑↔TAZ↑mRNA,protein↔YAP-TAZ-TEAD↑ | Promotes proliferation in vitro/in vivo; Hippo-YAP/TAZ sign p-w | [136] |
| SNHG7↑→MRD1, BCRP↑mRNA,protein; SNHG7↑→P-gp, PI3K,AKT,mTOR↑protein | Induces cisplatin resistance, PI3K/AKT/mTOR sign p-w | [137] |
| TMPO-AS1↑→TMPO↑mRNAstab | Promotes NSCLC progression in vitro/in vivo | [138] |
| IL-6↑mRNA→ZEB2-AS1↑→pSTAT1↑protein | Promotes migration, metastasis, and poor OS | [139] |
| ZNF205-AS1(pr)↑↔EGR4↑mRNA, stab | Promotes tumor cell growth; poor prognosis | [140] |
3.1.2. Upregulation of Target mRNAs
3.2. Action of lncRNAs Mediated by RNA-Binding Proteins (RBPs)
3.2.1. IGF2BP1/2/3 as RBP Mediator
3.2.2. HuR/ELAVL1 as RBP-Mediator
3.2.3. Heterogeneous Nuclear Ribonucleoproteins with RBP Function
3.2.4. Other RBPs (FBL, EIF4A3, UPF1, WDR5, YTHDF1/2/3) as Mediators of lncRNAs
3.2.5. LncRNAs Mediated by Both miRNA and RBP
4. Major Signaling Pathways and Networks Involving lncRNAs in NSCLC
- The oncogenic and oncosuppressive proteins crucial for NSCLC development regulated by lncRNA only moderately overlapped with the major proteins from the NSCLC-related signaling pathways.
- The oncogenic and oncosuppressive proteins crucial for NSCLC development affected by lncRNA only moderately overlap with the proteins encoded by genes most frequently mutated or undergoing methylation changes in NSCLC.
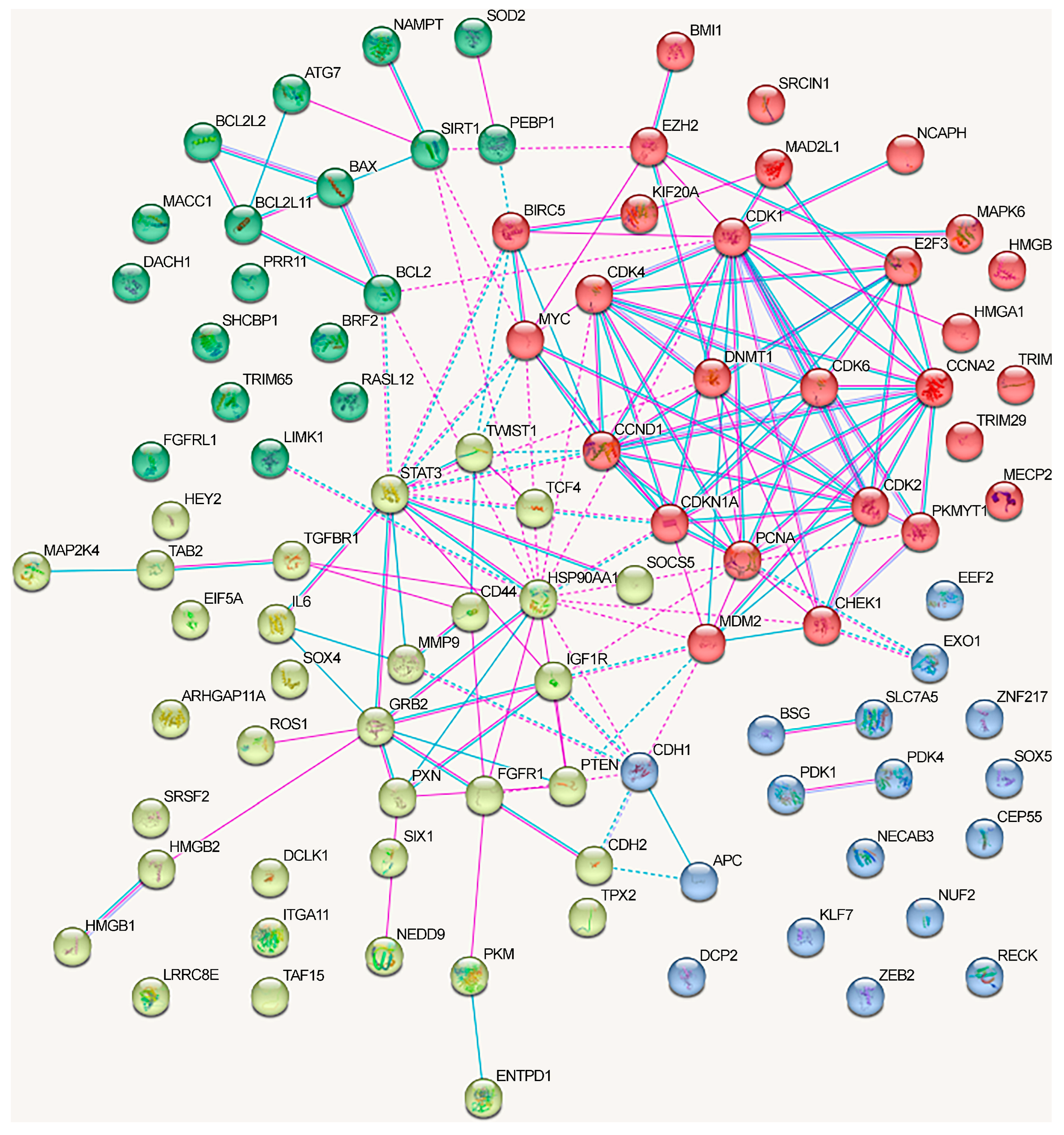
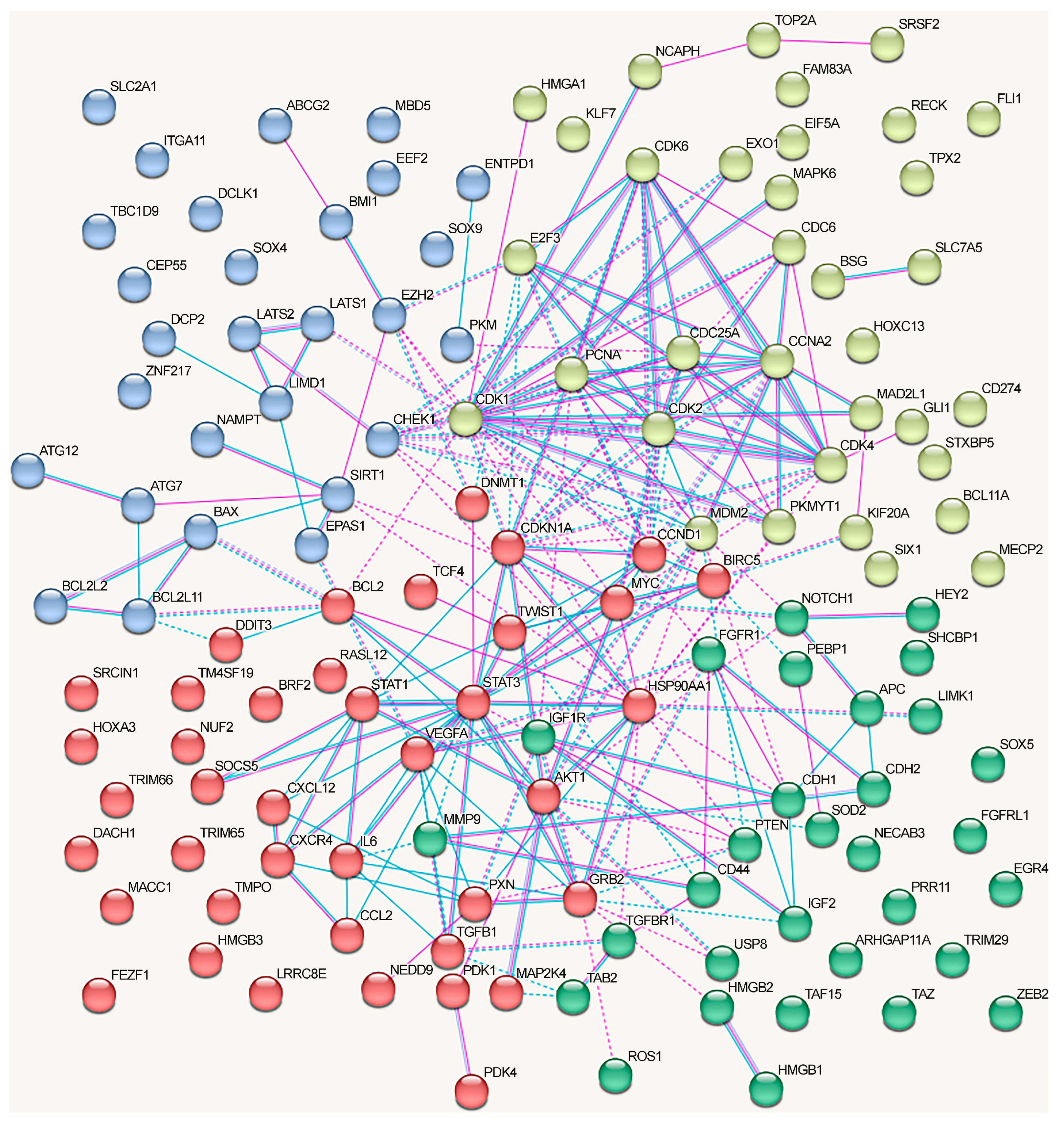
- Among the oncogenic and oncosuppressive proteins regulated by lncRNAs via the ceRNA mechanism, the overrepresented proteins are associated with the regulation of cell cycle and DNA damage response, the cytokine and immune systems, and the JAK-STAT and VEGFA-VEGFR2 signaling pathways.
- Among the oncogenic and oncosuppressive proteins regulated through lncRNAs via alternative mechanisms, the overrepresented proteins are associated with the cytokine system, the Hippo signaling pathway, and neovascularization.
- The effects of different lncRNAs on NSCLC are potentially cumulative since the affected proteins, jointly involved in such processes as cell cycle regulation, cytokine system, and the Hippo signaling pathway, may directly interact with each other.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ginn, L.; Shi, L.; Montagna, M.; Garofalo, M. LncRNAs in Non-Small-Cell Lung Cancer. Non-Coding RNA 2020, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Feng, J.; Tang, L. Competing endogenous RNAs in lung cancer. Cancer Biol. Med. 2021, 18, 1. [Google Scholar] [CrossRef] [PubMed]
- Djebali, S.; Davis, C.A.; Merkel, A.; Dobin, A.; Lassmann, T.; Mortazavi, A.; Tanzer, A.; Lagarde, J.; Lin, W.; Schlesinger, F.; et al. Landscape of transcription in human cells. Nature 2012, 489, 101–108. [Google Scholar] [CrossRef] [PubMed]
- FANTOM Consortium and the RIKEN PMI and CLST (DGT); Forrest, A.R.; Kawaji, H.; Rehli, M.; Baillie, J.K.; de Hoon, M.J.; Haberle, V.; Lassmann, T.; Kulakovskiy, I.V.; Lizio, M.; et al. A promoter-level mammalian expression atlas. Nature 2014, 507, 462–470. [Google Scholar] [CrossRef]
- Ling, H.; Girnita, L.; Buda, O.; Calin, G.A. Non-coding RNAs: The cancer genome dark matter that matters! Clin. Chem. Lab. Med. 2017, 55, 705–714. [Google Scholar] [CrossRef]
- Slack, F.J.; Chinnaiyan, A.M. The Role of Non-coding RNAs in Oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef]
- Kielbowski, K.; Ptaszynski, K.; Wojcik, J.; Wojtys, M.E. The role of selected non-coding RNAs in the biology of non-small cell lung cancer. Adv. Med. Sci. 2023, 68, 121–137. [Google Scholar] [CrossRef]
- Baek, D.; Villen, J.; Shin, C.; Camargo, F.D.; Gygi, S.P.; Bartel, D.P. The impact of microRNAs on protein output. Nature 2008, 455, 64–71. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Lee, Y.S.; Dutta, A. MicroRNAs in cancer. Annu. Rev. Pathol. 2009, 4, 199–227. [Google Scholar] [CrossRef]
- Sadakierska-Chudy, A. MicroRNAs: Diverse Mechanisms of Action and Their Potential Applications as Cancer Epi-Therapeutics. Biomolecules 2020, 10, 1285. [Google Scholar] [CrossRef] [PubMed]
- Gerstein, M.B.; Kundaje, A.; Hariharan, M.; Landt, S.G.; Yan, K.K.; Cheng, C.; Mu, X.J.; Khurana, E.; Rozowsky, J.; Alexander, R.; et al. Architecture of the human regulatory network derived from ENCODE data. Nature 2012, 489, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Iyer, M.K.; Niknafs, Y.S.; Malik, R.; Singhal, U.; Sahu, A.; Hosono, Y.; Barrette, T.R.; Prensner, J.R.; Evans, J.R.; Zhao, S.; et al. The landscape of long noncoding RNAs in the human transcriptome. Nat. Genet. 2015, 47, 199–208. [Google Scholar] [CrossRef]
- Al-Rugeebah, A.; Alanazi, M.; Parine, N.R. MEG3: An Oncogenic Long Non-coding RNA in Different Cancers. Pathol. Oncol. Res. 2019, 25, 859–874. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, F.; Li, W.; Song, G.; Kasim, V.; Wu, S. The Biological Roles and Molecular Mechanisms of Long Non-Coding RNA MEG3 in the Hallmarks of Cancer. Cancers 2022, 14, 6032. [Google Scholar] [CrossRef]
- Salmena, L.; Poliseno, L.; Tay, Y.; Kats, L.; Pandolfi, P.P. A ceRNA hypothesis: The Rosetta Stone of a hidden RNA language? Cell 2011, 146, 353–358. [Google Scholar] [CrossRef]
- Rajakumar, S.; Jamespaulraj, S.; Shah, Y.; Kejamurthy, P.; Jaganathan, M.K.; Mahalingam, G.; Ramya Devi, K.T. Long non-coding RNAs: An overview on miRNA sponging and its co-regulation in lung cancer. Mol. Biol. Rep. 2023, 50, 1727–1741. [Google Scholar] [CrossRef]
- Dreyfuss, G.; Kim, V.N.; Kataoka, N. Messenger-RNA-binding proteins and the messages they carry. Nat. Rev. Mol. Cell Biol. 2002, 3, 195–205. [Google Scholar] [CrossRef]
- Mitchell, S.F.; Parker, R. Principles and properties of eukaryotic mRNPs. Mol. Cell 2014, 54, 547–558. [Google Scholar] [CrossRef]
- Pereira, B.; Billaud, M.; Almeida, R. RNA-Binding Proteins in Cancer: Old Players and New Actors. Trends Cancer 2017, 3, 506–528. [Google Scholar] [CrossRef] [PubMed]
- Jonas, K.; Calin, G.A.; Pichler, M. RNA-Binding Proteins as Important Regulators of Long Non-Coding RNAs in Cancer. Int. J. Mol. Sci. 2020, 21, 2969. [Google Scholar] [CrossRef] [PubMed]
- Murtha, M.; Esteller, M. Extraordinary Cancer Epigenomics: Thinking Outside the Classical Coding and Promoter Box. Trends Cancer 2016, 2, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Gupta, S. 3D Modeling of Non-coding RNA Interactions. Adv. Exp. Med. Biol. 2022, 1385, 281–317. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.; Qu, Y.; Li, C.; Huang, A.; Tong, S.; Wu, C.; Fan, K. Deregulation of lncRNA-AC078883.3 and microRNA-19a is involved in the development of chemoresistance to cisplatin via modulating signaling pathway of PTEN/AKT. J. Cell Physiol. 2019, 234, 22657–22665. [Google Scholar] [CrossRef]
- Kong, X.; Hu, S.; Yuan, Y.; Du, Y.; Zhu, Z.; Song, Z.; Lu, S.; Zhao, C.; Yan, D. Analysis of lncRNA, miRNA and mRNA-associated ceRNA networks and identification of potential drug targets for drug-resistant non-small cell lung cancer. J. Cancer 2020, 11, 3357–3368. [Google Scholar] [CrossRef]
- Lang, N.; Wang, C.; Zhao, J.; Shi, F.; Wu, T.; Cao, H. Long non-coding RNA BCYRN1 promotes glycolysis and tumor progression by regulating the miR-149/PKM2 axis in non-small-cell lung cancer. Mol. Med. Rep. 2020, 21, 1509–1516. [Google Scholar] [CrossRef]
- Li, N.; Hao, W.; Yang, J.; Guo, Y.; Guo, Y.; Du, Y. Long non-coding RNA colon cancer-associated transcript-1 regulates tumor cell proliferation and invasion of non-small-cell lung cancer through suppressing miR-152. Geriatr. Gerontol. Int. 2020, 20, 629–636. [Google Scholar] [CrossRef]
- Wang, J.; Sun, N.; Han, W.; Tong, L.; Xu, T.; Li, G. Long non-coding RNA CCAT1 sponges miR-490 to enhance cell proliferation and migration of non-small cell lung cancer. Thorac. Cancer 2021, 12, 364–371. [Google Scholar] [CrossRef]
- Huang, Y.F.; Zhang, Y.; Fu, X. Long non-coding RNA DANCR promoted non-small cell lung cancer cells metastasis via modulating of miR-1225-3p/ErbB2 signal. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 758–769. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, G.; Chu, H.; Li, P.; Li, J. The positive feedback loop of lncRNA DANCR/miR-138/Sox4 facilitates malignancy in non-small cell lung cancer. Am. J. Cancer Res. 2019, 9, 270–284. [Google Scholar]
- Wang, R.; Dong, H.X.; Zeng, J.; Pan, J.; Jin, X.Y. LncRNA DGCR5 contributes to CSC-like properties via modulating miR-330-5p/CD44 in NSCLC. J. Cell Physiol. 2018, 233, 7447–7456. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, L.; Ge, T.; Zhu, F.; Liu, D.; Guo, H.; Qian, P.; Xu, N. LncRNA DLGAP1-AS2 regulates miR-503/cyclin D1 to promote cell proliferation in non-small cell lung cancer. BMC Pulm. Med. 2021, 21, 277. [Google Scholar] [CrossRef]
- Huang, Y.; Ni, R.; Wang, J.; Liu, Y. Knockdown of lncRNA DLX6-AS1 inhibits cell proliferation, migration and invasion while promotes apoptosis by downregulating PRR11 expression and upregulating miR-144 in non-small cell lung cancer. Biomed. Pharmacother. 2019, 109, 1851–1859. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Lin, W.; Fu, F. Long non-coding RNA DLX6-AS1 knockdown suppresses the tumorigenesis and progression of non-small cell lung cancer through microRNA-16-5p/BMI1 axis. Transl. Cancer Res. 2021, 10, 3772–3787. [Google Scholar] [CrossRef] [PubMed]
- Du, L.J.; Mao, L.J.; Jing, R.J. Long noncoding RNA DNAH17-AS1 promotes tumorigenesis and metastasis of non-small cell lung cancer via regulating miR-877-5p/CCNA2 pathway. Biochem. Biophys. Res. Commun. 2020, 533, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Li, H.; Ding, X.; Li, M.; Shen, H.; Li, Y.; Zhang, X.; Xing, L. Long non-coding RNA FEZF1-AS1 facilitates non-small cell lung cancer progression via the ITGA11/miR-516b-5p axis. Int. J. Oncol. 2020, 57, 1333–1347. [Google Scholar] [CrossRef]
- Fan, Y.; Li, H.; Yu, Z.; Dong, W.; Cui, X.; Ma, J.; Li, S. Long non-coding RNA FGD5-AS1 promotes non-small cell lung cancer cell proliferation through sponging hsa-miR-107 to up-regulate FGFRL1. Biosci. Rep. 2020, 40, BSR20193309. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Li, Q.; Ma, R.; Wang, Z.; Yu, Y.; Liu, H.; Miao, Y.; Jiang, S. Long Noncoding RNA FGD5-AS1 Knockdown Decrease Viability, Migration, and Invasion of Non-Small Cell Lung Cancer (NSCLC) Cells by Regulating the MicroRNA-944/MACC1 Axis. Technol. Cancer Res. Treat. 2021, 20, 1–13. [Google Scholar] [CrossRef]
- Ge, P.; Cao, L.; Yao, Y.J.; Jing, R.J.; Wang, W.; Li, H.J. lncRNA FOXD2-AS1 confers cisplatin resistance of non-small-cell lung cancer via regulation of miR185-5p-SIX1 axis. OncoTargets Ther. 2019, 12, 6105–6117. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhao, G.; Zhu, H.; Nie, L.; He, L.; Liu, J.; Li, R.; Xiao, S.; Hua, G. LncRNA FOXD3-AS1 promoted chemo-resistance of NSCLC cells via directly acting on miR-127-3p/MDM2 axis. Cancer Cell Int. 2020, 20, 350. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Lei, W.; Hu, H.B.; Zhang, H.; Zhu, Y. H19 promotes non-small-cell lung cancer (NSCLC) development through STAT3 signaling via sponging miR-17. J. Cell Physiol. 2018, 233, 6768–6776. [Google Scholar] [CrossRef]
- Li, C.; Lei, Z.; Peng, B.; Zhu, J.; Chen, L. LncRNA HCP5 Stimulates the Proliferation of Non-Small Cell Lung Cancer Cells by Up-Regulating Survivin through the Down-Regulation of miR-320. Cancer Manag. Res. 2020, 12, 1129–1134. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Du, Y.; Mi, Y.; Wang, L. The HNF1A-AS1/miR-92a-3p axis affects the radiosensitivity of non-small cell lung cancer by competitively regulating the JNK pathway. Cell Biol. Toxicol. 2021, 37, 715–729. [Google Scholar] [CrossRef]
- Chen, S.S.; Peng, M.; Zhou, G.Z.; Pu, Y.C.; Yi, M.C.; Zhu, Y.; Jiang, B. Long non-coding RNA HOTAIR regulates the development of non-small cell lung cancer through miR-217/DACH1 signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 670–678. [Google Scholar] [CrossRef]
- Shi, J.; Wang, H.; Feng, W.; Huang, S.; An, J.; Qiu, Y.; Wu, K. Long non-coding RNA HOTTIP promotes hypoxia-induced glycolysis through targeting miR-615-3p/HMGB3 axis in non-small cell lung cancer cells. Eur. J. Pharmacol. 2019, 862, 172615. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Lang, L.; Zhao, W.; Niu, R. Long Non-Coding RNA HOXA11-AS Promotes Non-Small Cell Lung Cancer Tumorigenesis through microRNA-148a-3p/DNMT1 Regulatory Axis. OncoTargets Ther. 2019, 12, 11195–11206. [Google Scholar] [CrossRef]
- Xia, H.; Jing, H.; Li, Y.; Lv, X. Long noncoding RNA HOXD-AS1 promotes non-small cell lung cancer migration and invasion through regulating miR-133b/MMP9 axis. Biomed. Pharmacother. 2018, 106, 156–162. [Google Scholar] [CrossRef]
- Dong, Z.; Yang, P.; Qiu, X.; Liang, S.; Guan, B.; Yang, H.; Li, F.; Sun, L.; Liu, H.; Zou, G.; et al. KCNQ1OT1 facilitates progression of non-small-cell lung carcinoma via modulating miRNA-27b-3p/HSP90AA1 axis. J. Cell Physiol. 2019, 234, 11304–11314. [Google Scholar] [CrossRef]
- Hu, H.; Chen, C.; Chen, F.; Sun, N. LINC00152 knockdown suppresses tumorigenesis in non-small cell lung cancer via sponging miR-16-5p. J. Thorac. Dis. 2022, 14, 614–624. [Google Scholar] [CrossRef]
- Feng, X.; Yang, S. Long non-coding RNA LINC00243 promotes proliferation and glycolysis in non-small cell lung cancer cells by positively regulating PDK4 through sponging miR-507. Mol. Cell Biochem. 2020, 463, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lin, B.; Liu, Y.; Huang, T.; Chen, M.; Lian, D.; Deng, S.; Zhuang, C. LINC00324 affects non-small cell lung cancer cell proliferation and invasion through regulation of the miR-139-5p/IGF1R axis. Mol. Cell Biochem. 2020, 473, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhou, F.; Liu, H.; Wang, Y.; Wang, J.; Ren, F.; Xu, S. Knockdown of LINC00511 decreased cisplatin resistance in non-small cell lung cancer by elevating miR-625 level to suppress the expression of leucine rich repeat containing eight volume-regulated anion channel subunit E. Hum. Exp. Toxicol. 2022, 41, 1–10. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Yi, H.; Liu, Z. IncRNA Linc00511 Upregulation Elevates TGFBR1 and Participates in the Postoperative Distant Recurrence of Non-Small-Cell Lung Cancer by Targeting miR-98-5p. Crit. Rev. Eukaryot. Gene Expr. 2022, 32, 1–10. [Google Scholar] [CrossRef]
- Han, X.; Wu, J.; Zhang, Y.; Song, J.; Shi, Z.; Chang, H. LINC00518 Promotes Cell Proliferation by Regulating the Cell Cycle of Lung Adenocarcinoma through miR-185-3p Targeting MECP2. Front. Oncol. 2021, 11, 646559. [Google Scholar] [CrossRef] [PubMed]
- An, Y.X.; Shang, Y.J.; Xu, Z.W.; Zhang, Q.C.; Wang, Z.; Xuan, W.X.; Zhang, X.J. STAT3-induced long noncoding RNA LINC00668 promotes migration and invasion of non-small cell lung cancer via the miR-193a/KLF7 axis. Biomed. Pharmacother. 2019, 116, 109023. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Jin, M.; Mi, B.; Xu, F.; Li, T.; Zhao, L.; Liu, J.; Huang, G. LINC01123, a c-Myc-activated long non-coding RNA, promotes proliferation and aerobic glycolysis of non-small cell lung cancer through miR-199a-5p/c-Myc axis. J. Hematol. Oncol. 2019, 12, 91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jin, Z.; Zhang, H. LINC01207 promotes the progression of non-small cell lung cancer via regulating ARHGAP11A by sponging miR-525-5p. Cancer Biomark. Sect. A Dis. Markers 2022, 33, 401–414. [Google Scholar] [CrossRef]
- Xu, L.; Wei, B.; Hui, H.; Sun, Y.; Liu, Y.; Yu, X.; Dai, J. Positive feedback loop of lncRNA LINC01296/miR-598/Twist1 promotes non-small cell lung cancer tumorigenesis. J. Cell Physiol. 2019, 234, 4563–4571. [Google Scholar] [CrossRef]
- Ji, L.; Yang, T.; Liu, M.; Li, J.; Si, Q.; Wang, Y.; Liu, J.; Dai, L. Construction of lncRNA TYMSOS/hsa-miR-101-3p/CEP55 and TYMSOS/hsa-miR-195-5p/CHEK1 Axis in Non-small Cell Lung Cancer. Biochem. Genet. 2023, 61, 995–1014. [Google Scholar] [CrossRef]
- Tan, Y.; Xu, F.; Xu, L.; Cui, J. Long non-coding RNA LINC01748 exerts carcinogenic effects in non-small cell lung cancer cell lines by regulating the microRNA-520a-5p/HMGA1 axis. Int. J. Mol. Med. 2022, 49, 22. [Google Scholar] [CrossRef]
- Yu, X.; Liu, D.; Wang, L.; Wang, L. LncRNA LOC285758 Induced Non-Small Cell Lung Cancer Development through Up-Regulating CDK6 by Sponge Adsorption of miRNA-204. Iran. J. Public Health 2022, 51, 2117–2127. [Google Scholar] [CrossRef]
- Rong, F.; Liu, L.; Zou, C.; Zeng, J.; Xu, Y. MALAT1 Promotes Cell Tumorigenicity through Regulating miR-515-5p/EEF2 Axis in Non-Small Cell Lung Cancer. Cancer Manag. Res. 2020, 12, 7691–7701. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Q. Long Noncoding RNA MALAT1 Knockdown Inhibits Proliferation, Migration, and Invasion and Promotes Apoptosis in Non-Small-Cell Lung Cancer Cells through Regulating miR-515-3p/TRIM65 Axis. Cancer Biother. Radiopharm. 2020; ahead of print. [Google Scholar] [CrossRef]
- Yu, W.; Ding, J.; He, M.; Chen, Y.; Wang, R.; Han, Z.; Xing, E.Z.; Zhang, C.; Yeh, S. Estrogen receptor beta promotes the vasculogenic mimicry (VM) and cell invasion via altering the lncRNA-MALAT1/miR-145-5p/NEDD9 signals in lung cancer. Oncogene 2019, 38, 1225–1238. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mei, Z.; Hu, H.B.; Zhang, X. The lncRNA MALAT1 contributes to non-small cell lung cancer development via modulating miR-124/STAT3 axis. J. Cell Physiol. 2018, 233, 6679–6688. [Google Scholar] [CrossRef]
- Miao, J.; Gao, Y.; Guan, W.; Yu, X.; Wang, Y.; Jiang, P.; Yang, L.; Xu, L.; You, W. High level of LncRNA MAPKAPK5-AS1 predicts poor prognosis and contributes to the malignant proliferation and EMT of non-small cell lung cancer via sponging miR-490-3p from HMGB2. Genes Genomics 2023, 45, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Li, J.; Tao, K.; Jiang, Y. Long non-coding RNA MCM3AP antisense RNA 1 promotes non-small cell lung cancer progression through targeting microRNA-195-5p. Bioengineered 2021, 12, 3525–3538. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Shang, P.; Song, X. LncRNA MEG8 promotes tumor progression of non-small cell lung cancer via regulating miR-107/CDK6 axis. Anti-Cancer Drugs 2020, 31, 1065–1073. [Google Scholar] [CrossRef]
- Xu, J.; Wang, H.; Shi, B.; Li, N.; Xu, G.; Yan, X.; Xu, L. Exosomal MFI2-AS1 sponge miR-107 promotes non-small cell lung cancer progression through NFAT5. Cancer Cell Int. 2023, 23, 51. [Google Scholar] [CrossRef]
- Wang, J.; Ding, M.; Zhu, H.; Cao, Y.; Zhao, W. Up-regulation of long noncoding RNA MINCR promotes non-small cell of lung cancer growth by negatively regulating miR-126/SLC7A5 axis. Biochem. Biophys. Res. Commun. 2019, 508, 780–784. [Google Scholar] [CrossRef]
- Xiong, Y.; Yang, C.; Yang, X.; Ding, C.; Wang, Q.; Zhu, H. LncRNA MIR9-3HG enhances LIMK1 mRNA and protein levels to contribute to the carcinogenesis of lung squamous cell carcinoma via sponging miR-138-5p and recruiting TAF15. Pathol. Res. Pract. 2022, 237, 153941. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zhang, Y.; Zhang, X.; Liu, Y.; Xu, Y.; Cui, S.; Li, G.; Wang, J. LncRNA MNX1-AS1 contributes to lung adenocarcinoma progression by targeting the miR-34a/SIRT1 axis. Am. J. Transl. Res. 2022, 14, 4977–4989. [Google Scholar]
- Liu, H.; Han, L.; Liu, Z.; Gao, N. Long noncoding RNA MNX1-AS1 contributes to lung cancer progression through the miR-527/BRF2 pathway. J. Cell Physiol. 2019, 234, 13843–13850. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, T.; Ding, X.; Ma, C.; Cheng, L.; Sheng, L.; Du, X. lncRNA MRUL Suppressed Non-Small Cell Lung Cancer Cells Proliferation and Invasion by Targeting miR-17-5p/SRSF2 Axis. BioMed Res. Int. 2020, 2020, 9567846. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhou, J.; Quan, L.; Liang, Y.; Huang, P.; Chen, F.; Liu, S. LncRNA NCK1-AS1 promotes non-small cell lung cancer progression via regulating miR-512-5p/p21 axis. Pathol. Res. Pract. 2020, 216, 153157. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Mo, Q.; Wan, X.; Dan, J.; Hu, H. NEAT1/hsa-mir-98-5p/MAPK6 axis is involved in non-small-cell lung cancer development. J. Cell Biochem. 2019, 120, 2836–2846. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Niu, N.; Li, P.; Zhai, L.; Xiao, K.; Chen, W.; Zhuang, X. LncRNA OGFRP1 acts as an oncogene in NSCLC via miR-4640-5p/eIF5A axis. Cancer Cell Int. 2021, 21, 425. [Google Scholar] [CrossRef]
- Geng, W.; Qiu, M.; Zhang, D.; Li, P.; Sun, G.; Zhou, X. LncRNA PCAT7 promotes non-small cell lung cancer progression by activating miR-486-5p/CDK4 axis-mediated cell cycle. Am. J. Transl. Res. 2022, 14, 3003–3016. [Google Scholar]
- He, Y.; Jiang, X.; Duan, L.; Xiong, Q.; Yuan, Y.; Liu, P.; Jiang, L.; Shen, Q.; Zhao, S.; Yang, C.; et al. LncRNA PKMYT1AR promotes cancer stem cell maintenance in non-small cell lung cancer via activating Wnt signaling pathway. Mol. Cancer 2021, 20, 156. [Google Scholar] [CrossRef]
- Cheng, D.; Bao, C.; Zhang, X.; Lin, X.; Huang, H.; Zhao, L. LncRNA PRNCR1 interacts with HEY2 to abolish miR-448-mediated growth inhibition in non-small cell lung cancer. Biomed. Pharmacother. 2018, 107, 1540–1547. [Google Scholar] [CrossRef]
- Ma, Q.; Niu, R.; Huang, W.; Da, L.; Tang, Y.; Jiang, D.; Xi, Y.; Zhang, C. Long Noncoding RNA PTPRG Antisense RNA 1 Reduces Radiosensitivity of Nonsmall Cell Lung Cancer Cells Via Regulating MiR-200c-3p/TCF4. Technol. Cancer Res. Treat. 2020, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, Z.; Dai, L.; Jiang, T.; Li, P.; Jia, L.; Jing, X.; An, L.; Liu, M.; Wu, S.; et al. LncRNA PVT1 Facilitates Proliferation, Migration and Invasion of NSCLC Cells via miR-551b/FGFR1 Axis. OncoTargets Ther. 2021, 14, 3555–3565. [Google Scholar] [CrossRef] [PubMed]
- Su, X.H.; Zhu, Y.R.; Hou, Y.J.; Li, K.; Dong, N.H. PVT1 induces NSCLC cell migration and invasion by regulating IL-6 via sponging miR-760. Mol. Cell Probes 2020, 54, 101652. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wang, J. E2F1-Induced Overexpression of Long Noncoding RNA SBF2-AS1 Promotes Non-Small-Cell Lung Cancer Metastasis through Regulating miR-362-3p/GRB2 Axis. DNA Cell Biol. 2020, 39, 1290–1298. [Google Scholar] [CrossRef]
- Ge, P.; Cao, L.; Zheng, M.; Yao, Y.; Wang, W.; Chen, X. LncRNA SNHG1 contributes to the cisplatin resistance and progression of NSCLC via miR-330-5p/DCLK1 axis. Exp. Mol. Pathol. 2021, 120, 104633. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Lu, X.; Xu, C.; Zhang, F.; Zhang, G. SNHG11 contributes to NSCLC cell growth and migration by targeting miR-485-5p/BSG axis. Biomed. Pharmacother. 2020, 128, 110324. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Wang, S.; Zhang, P.; Peng, C.; Wu, T. LncRNA SNHG12 Decreases Non-Small Cell Lung Cancer Cell Sensitivity to Cisplatin by Repressing miR-525-5p and Promoting XIAP. Ann. Clin. Lab. Sci. 2023, 53, 64–75. [Google Scholar]
- Jiao, P.; Hou, J.; Yao, M.; Wu, J.; Ren, G. SNHG14 silencing suppresses the progression and promotes cisplatin sensitivity in non-small cell lung cancer. Biomed. Pharmacother. 2019, 117, 109164. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.R.; Xu, Y.L.; Qian, J.; Wang, Y. Long non-coding RNA SNHG15 accelerates the progression of non-small cell lung cancer by absorbing miR-211-3p. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1536–1544. [Google Scholar] [CrossRef]
- Wang, L.; Shang, X.; Feng, Q. LncRNA TATDN1 contributes to the cisplatin resistance of non-small cell lung cancer through TATDN1/miR-451/TRIM66 axis. Cancer Biol. Ther. 2019, 20, 261–271. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, Q.; Huang, Y.; Wen, C.; Li, Y.; Bao, M.; Wu, L. Lnc-TMEM132D-AS1 as a potential therapeutic target for acquired resistance to osimertinib in non-small-cell lung cancer. Mol. Omics 2023, 19, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Shen, M.; Chen, H.; Li, W.; Chen, C. Long non-coding RNA TP73-AS1 accelerates the progression and cisplatin resistance of non-small cell lung cancer by upregulating the expression of TRIM29 via competitively targeting microRNA-34a-5p. Mol. Med. Rep. 2020, 22, 3822–3832. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fang, F.; He, X. Long noncoding RNA TP73-AS1 promotes non-small cell lung cancer progression by competitively sponging miR-449a/EZH2. Biomed. Pharmacother. 2018, 104, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Zhang, C.; Cheng, Y.; Wang, S.; Lin, H.; Zhang, H. LncRNA UCC promotes epithelial-mesenchymal transition via the miR-143-3p/SOX5 axis in non-small-cell lung cancer. Lab. Investig. 2021, 101, 1153–1165. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, Y.F.; Lv, Q.; Li, Y.J.; Wang, R.R.; Sun, G.B.; Pan, L.; Hu, J.X.; Xie, N.; Zhang, C.; et al. miR-4293 upregulates lncRNA WFDC21P by suppressing mRNA-decapping enzyme 2 to promote lung carcinoma proliferation. Cell Death Dis. 2021, 12, 735. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Li, Z.J.; Shi, Y.; Deng, J.; Bai, J.; Ma, L.; Zeng, X.X.; Feng, S.S.; Ren, J.L.; et al. Unravelling the Role of LncRNA WT1-AS/miR-206/NAMPT Axis as Prognostic Biomarkers in Lung Adenocarcinoma. Biomolecules 2021, 11, 203. [Google Scholar] [CrossRef]
- Sun, W.; Zu, Y.; Fu, X.; Deng, Y. Knockdown of lncRNA-XIST enhances the chemosensitivity of NSCLC cells via suppression of autophagy. Oncol. Rep. 2017, 38, 3347–3354. [Google Scholar] [CrossRef]
- Liu, T.T.; Li, R.; Liu, X.; Zhou, X.J.; Huo, C.; Li, J.P.; Qu, Y.Q. LncRNA XIST acts as a MicroRNA-520 sponge to regulate the Cisplatin resistance in NSCLC cells by mediating BAX through CeRNA network. Int. J. Med. Sci. 2021, 18, 419–431. [Google Scholar] [CrossRef]
- Jiang, H.; Zhang, H.; Hu, X.; Li, W. Knockdown of long non-coding RNA XIST inhibits cell viability and invasion by regulating miR-137/PXN axis in non-small cell lung cancer. Int. J. Biol. Macromol. 2018, 111, 623–631. [Google Scholar] [CrossRef]
- Liu, J.; Yao, L.; Zhang, M.; Jiang, J.; Yang, M.; Wang, Y. Downregulation of LncRNA-XIST inhibited development of non-small cell lung cancer by activating miR-335/SOD2/ROS signal pathway mediated pyroptotic cell death. Aging 2019, 11, 7830–7846. [Google Scholar] [CrossRef]
- Li, C.; Wan, L.; Liu, Z.; Xu, G.; Wang, S.; Su, Z.; Zhang, Y.; Zhang, C.; Liu, X.; Lei, Z.; et al. Long non-coding RNA XIST promotes TGF-beta-induced epithelial-mesenchymal transition by regulating miR-367/141-ZEB2 axis in non-small-cell lung cancer. Cancer Lett. 2018, 418, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Zhang, Y.; Wang, Z.; Hou, Z.; Gao, X.; Zhang, X. FOXD3-AS1 suppresses the progression of non-small cell lung cancer by regulating miR-150/SRCIN1axis. Cancer Biomark. Sect. A Dis. Markers 2020, 29, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Q.; Long, X.R.; Zhou, N.N.; Chen, D.N.; Zhang, M.Y.; Wen, Z.S.; Zhang, L.J.; He, F.Z.; Zhou, Z.L.; Mai, S.J.; et al. Lnc-GAN1 expression is associated with good survival and suppresses tumor progression by sponging mir-26a-5p to activate PTEN signaling in non-small cell lung cancer. J. Exp. Clin. Cancer Res. CR 2021, 40, 9. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Chen, X.; Zhang, Y.; Liu, C.; Wang, Z.; Xu, X.; Zhu, J.; Xue, T. LncRNA GATA6-AS1 Inhibits the Progression of Non-Small Cell Lung Cancer via Repressing microRNA-543 to Up-Regulating RKIP. Cancer Manag. Res. 2020, 12, 9327–9338. [Google Scholar] [CrossRef]
- Ren, K.; Sun, J.; Liu, L.; Yang, Y.; Li, H.; Wang, Z.; Deng, J.; Hou, M.; Qiu, J.; Zhao, W. TP53-Activated lncRNA GHRLOS Regulates Cell Proliferation, Invasion, and Apoptosis of Non-Small Cell Lung Cancer by Modulating the miR-346/APC Axis. Front. Oncol. 2021, 11, 676202. [Google Scholar] [CrossRef]
- Fan, G.; Jiao, J.; Shen, F.; Ren, Q.; Wang, Q.; Chu, F. Long non-coding RNA HCG11 sponging miR-522-3p inhibits the tumorigenesis of non-small cell lung cancer by upregulating SOCS5. Thorac. Cancer 2020, 11, 2877–2886. [Google Scholar] [CrossRef]
- Tao, X.; Li, Y.; Fan, S.; Wu, L.; Xin, J.; Su, Y.; Xian, X.; Huang, Y.; Huang, R.; Fang, W.; et al. Downregulation of Linc00173 increases BCL2 mRNA stability via the miR-1275/PROCA1/ZFP36L2 axis and induces acquired cisplatin resistance of lung adenocarcinoma. J. Exp. Clin. Cancer Res. CR 2023, 42, 12. [Google Scholar] [CrossRef]
- Ding, D.; Zhang, J.; Luo, Z.; Wu, H.; Lin, Z.; Liang, W.; Xue, X. Analysis of the lncRNA-miRNA-mRNA Network Reveals a Potential Regulatory Mechanism of EGFR-TKI Resistance in NSCLC. Front. Genet. 2022, 13, 851391. [Google Scholar] [CrossRef]
- Dong, J.; Li, B.; Lin, D.; Lu, D.; Liu, C.; Lu, X.; Tang, X.; Li, L.; Zhu, D.; Liu, J.; et al. LincRNA00494 Suppresses Non-small Cell Lung Cancer Cell Proliferation by Regulating SRCIN1 Expression as a ceRNA. Front. Oncol. 2020, 10, 79. [Google Scholar] [CrossRef]
- Sui, Y.; Chi, W.; Feng, L.; Jiang, J. LncRNA MAGI2-AS3 is downregulated in non-small cell lung cancer and may be a sponge of miR-25. BMC Pulm. Med. 2020, 20, 59. [Google Scholar] [CrossRef]
- Ma, J.; Yan, H.; Zhang, J.; Tan, Y.; Gu, W. Long-Chain Non-Coding RNA (lncRNA) MT1JP Suppresses Biological Activities of Lung Cancer by Regulating miRNA-423-3p/Bim Axis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 5114–5126. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, Z.; Xu, S.; Zhang, Q. LncRNA SOX2-OT/miR-30d-5p/PDK1 Regulates PD-L1 Checkpoint through the mTOR Signaling Pathway to Promote Non-small Cell Lung Cancer Progression and Immune Escape. Front. Genet. 2021, 12, 674856. [Google Scholar] [CrossRef]
- Xiao, H.; Liu, Y.; Liang, P.; Wang, B.; Tan, H.; Zhang, Y.; Gao, X.; Gao, J. TP53TG1 enhances cisplatin sensitivity of non-small cell lung cancer cells through regulating miR-18a/PTEN axis. Cell Biosci. 2018, 8, 23. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Wang, Z.; Feng, Y.; Zhu, H.; Yang, M.; Zhang, S.; Wang, X. lncRNA TPTEP1 competitively sponges miR-328-5p to inhibit the proliferation of non-small cell lung cancer cells. Oncol. Rep. 2020, 43, 1606–1618. [Google Scholar] [CrossRef] [PubMed]
- Shi, N.; Feng, D.; Gu, Y.; Zheng, C.; Miao, M. TUSC8 enhances cisplatin sensitivity of NSCLC cells through regulating VEGFA. J. BUON 2021, 26, 336–344. [Google Scholar]
- Lin, S.; Zhang, R.; An, X.; Li, Z.; Fang, C.; Pan, B.; Chen, W.; Xu, G.; Han, W. LncRNA HOXA-AS3 confers cisplatin resistance by interacting with HOXA3 in non-small-cell lung carcinoma cells. Oncogenesis 2019, 8, 60. [Google Scholar] [CrossRef]
- Wang, J.; Tian, Y.; Yu, M.; Ma, M.; Gao, Y. Overexpression of the Long Noncoding RNA HIF2PUT Inhibits Non-Small Cell Lung Cancer Cell Proliferation and Invasion through HIF-2a Pathway. Cancer Biother. Radiopharm. 2023, 38, 275–281. [Google Scholar] [CrossRef]
- Liang, H.; Peng, J. LncRNA HOTAIR promotes proliferation, invasion and migration in NSCLC cells via the CCL22 signaling pathway. PLoS ONE 2022, 17, e0263997. [Google Scholar] [CrossRef]
- Gao, Y.P.; Li, Y.; Li, H.J.; Zhao, B. LncRNA NBR2 inhibits EMT progression by regulating Notch1 pathway in NSCLC. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 7950–7958. [Google Scholar] [CrossRef]
- Chang, L.; Xu, W.; Zhang, Y.; Gong, F. Long non-coding RNA-NEF targets glucose transportation to inhibit the proliferation of non-small-cell lung cancer cells. Oncol. Lett. 2019, 17, 2795–2801. [Google Scholar] [CrossRef]
- Huang, J.; Xie, N.; Huang, H.; Yao, J.; Hu, W. Long noncoding RNA STXBP5-AS1 inhibits cell proliferation, migration, and invasion via preventing the PI3K/AKT against STXBP5 expression in non-small-cell lung carcinoma. J. Cell Biochem. 2019, 120, 7489–7498. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Wang, J.; Fang, L. LncRNA WT1-AS over-expression inhibits non-small cell lung cancer cell stemness by down-regulating TGF-beta1. BMC Pulm. Med. 2020, 20, 113. [Google Scholar] [CrossRef]
- Tang, L.; Wang, T.; Zhang, Y.; Zhang, J.; Zhao, H.; Wang, H.; Wu, Y.; Liu, K. Long Non-Coding RNA AWPPH Promotes Postoperative Distant Recurrence in Resected Non-Small Cell Lung Cancer by Upregulating Transforming Growth Factor beta 1 (TGF-beta1). Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 2535–2541. [Google Scholar] [CrossRef]
- Ju, Z.S.; Sun, B.; Bao, D.; Zhang, X.F. Effect of lncRNA-BLACAT1 on drug resistance of non-small cell lung cancer cells in DDP chemotherapy by regulating cyclin D1 expression. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9465–9472. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Kang, J.; Yang, Y. Long non-coding RNA CASC2 enhances irradiation-induced endoplasmic reticulum stress in NSCLC cells through PERK signaling. 3 Biotech 2020, 10, 449. [Google Scholar] [CrossRef]
- Liao, J.; Xie, N. Long noncoding RNA DSCAM-AS1 functions as an oncogene in non-small cell lung cancer by targeting BCL11A. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Gong, W.; Cao, Y.; Wang, Y.; Yang, L.; Su, W.; Qiu, F.; Datta, S.; Rao, B.; Xian, J.; Lin, M.; et al. Upregulation of LncRNA FEZF-AS1 is associated with advanced clinical stages and family history of cancer in patients with NSCLC. Pathol. Res. Pract. 2018, 214, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.X.; Wang, X.Q.; Zheng, W.X.; Zhao, J. Long noncoding RNA HOXA-AS2 promotes cell migration and invasion via upregulating IGF-2 in non-small cell lung cancer as an oncogene. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 4793–4799. [Google Scholar] [CrossRef]
- Liu, B.; Li, J.; Li, J.M.; Liu, G.Y.; Wang, Y.S. HOXC-AS2 mediates the proliferation, apoptosis, and migration of non-small cell lung cancer by combining with HOXC13 gene. Cell Cycle 2021, 20, 236–246. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, Y.; Wang, Q.; Yang, X.; Ding, C.; Xiong, Y. Long non-coding RNA LALTOP promotes non-small cell lung cancer progression by stabilizing topoisomerase IIalpha mRNA. Biochem. Biophys. Res. Commun. 2021, 574, 56–62. [Google Scholar] [CrossRef]
- Bian, C.; Yuan, L.; Gai, H. A long non-coding RNA LINC01288 facilitates non-small cell lung cancer progression through stabilizing IL-6 mRNA. Biochem. Biophys. Res. Commun. 2019, 514, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, W.; Chen, S.; Zhang, L.; Guo, Z.; Wang, L.; Wang, J.; Wan, Z.; Hong, Y.; Yu, L. Expression and correlation of MALAT1 and SOX9 in non-small cell lung cancer. Clin. Respir. J. 2018, 12, 2284–2291. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shen, Q.W.; Niu, Y.X.; Chen, X.Y.; Liu, H.W.; Shen, X.Y. LncNORAD interference inhibits tumor growth and lung cancer cell proliferation, invasion and migration by down-regulating CXCR4 to suppress RhoA/ROCK signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5446–5455. [Google Scholar] [CrossRef]
- Shen, Q.; Zhou, H.; Zhang, M.; Wu, R.; Wang, L.; Wang, Y.; Chen, J. Super enhancer-LncRNA SENCR promoted cisplatin resistance and growth of NSCLC through upregulating FLI1. J. Clin. Lab. Anal. 2022, 36, e24460. [Google Scholar] [CrossRef]
- Zhu, B.; Finch-Edmondson, M.; Leong, K.W.; Zhang, X.; Mitheera, V.; Lin, Q.X.; Lee, Y.; Ng, W.T.; Guo, H.; Wan, Y.; et al. LncRNA SFTA1P mediates positive feedback regulation of the Hippo-YAP/TAZ signaling pathway in non-small cell lung cancer. Cell Death Discov. 2021, 7, 369. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Abuduwufuer, A.; Zhang, H.; Luo, L.; Suotesiyali, M.; Zou, Y. SNHG7 mediates cisplatin-resistance in non-small cell lung cancer by activating PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 6935–6943. [Google Scholar] [CrossRef]
- Qin, Z.; Zheng, X.; Fang, Y. Long noncoding RNA TMPO-AS1 promotes progression of non-small cell lung cancer through regulating its natural antisense transcript TMPO. Biochem. Biophys. Res. Commun. 2019, 516, 486–493. [Google Scholar] [CrossRef]
- Chen, T.; Li, J.; Zhou, M.H.; Xu, L.J.; Pan, T.C. IL-6 stimulates lncRNA ZEB2-AS1 to aggravate the progression of non-small cell lung cancer through activating STAT1. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 3734–3740. [Google Scholar] [CrossRef]
- He, S.; Lin, J.; Xu, Y.; Lin, L.; Feng, J. A positive feedback loop between ZNF205-AS1 and EGR4 promotes non-small cell lung cancer growth. J. Cell Mol. Med. 2019, 23, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
- Mercer, T.R.; Dinger, M.E.; Mattick, J.S. Long non-coding RNAs: Insights into functions. Nat. Rev. Genet. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Ulitsky, I. Evolution to the rescue: Using comparative genomics to understand long non-coding RNAs. Nat. Rev. Genet. 2016, 17, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qian, X.; Qiu, Q.; Xu, L.; Pan, M.; Li, J.; Ren, J.; Lu, B.; Qiu, T.; Chen, E.; et al. LCAT1 is an oncogenic LncRNA by stabilizing the IGF2BP2-CDC6 axis. Cell Death Dis. 2022, 13, 877. [Google Scholar] [CrossRef] [PubMed]
- Brownmiller, T.; Juric, J.A.; Ivey, A.D.; Harvey, B.M.; Westemeier, E.S.; Winters, M.T.; Stevens, A.M.; Stanley, A.N.; Hayes, K.E.; Sprowls, S.A.; et al. Y Chromosome LncRNA Are Involved in Radiation Response of Male Non-Small Cell Lung Cancer Cells. Cancer Res. 2020, 80, 4046–4057. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liu, Y.; Tang, H.; Wang, P. FOXP3 activated-LINC01232 accelerates the stemness of non-small cell lung carcinoma by activating TGF-beta signaling pathway and recruiting IGF2BP2 to stabilize TGFBR1. Exp. Cell Res. 2022, 413, 113024. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Lei, G.; Chen, Z.; Zhang, Y.; Huang, C.; Chen, W. IGF2BP2 Regulates MALAT1 by Serving as an N6-Methyladenosine Reader to Promote NSCLC Proliferation. Front. Mol. Biosci. 2021, 8, 780089. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhang, C.; Qu, T.; Lu, X.; He, X.; Li, W.; Yin, D.; Han, L.; Guo, R.; Zhang, E. MNX1-AS1 Promotes Phase Separation of IGF2BP1 to Drive c-Myc-Mediated Cell-Cycle Progression and Proliferation in Lung Cancer. Cancer Res. 2022, 82, 4340–4358. [Google Scholar] [CrossRef]
- Jiao, P.F.; Tang, P.J.; Chu, D.; Li, Y.M.; Xu, W.H.; Ren, G.F. Long Non-Coding RNA THOR Depletion Inhibits Human Non-Small Cell Lung Cancer Cell Growth. Front. Oncol. 2021, 11, 756148. [Google Scholar] [CrossRef]
- Gong, F.; Dong, D.; Zhang, T.; Xu, W. Long non-coding RNA FENDRR attenuates the stemness of non-small cell lung cancer cells via decreasing multidrug resistance gene 1 (MDR1) expression through competitively binding with RNA binding protein HuR. Eur. J. Pharmacol. 2019, 853, 345–352. [Google Scholar] [CrossRef]
- Shan, K.Z.; Yang, S.F.; Deng, Y.J.; Yue, P.Y.; Du, Z.Q. E2F1-induced long non-coding RNA MCF2L-AS1 modulates Cyclin D1 mRNA stability through ELAVL1 to induce Gefitinib resistance in non-small cell lung cancer. Acta Biochim. Pol. 2022, 69, 795–804. [Google Scholar] [CrossRef]
- Huang, Y.; Xia, L.; Tan, X.; Zhang, J.; Zeng, W.; Tan, B.; Yu, X.; Fang, W.; Yang, Z. Molecular mechanism of lncRNA SNHG12 in immune escape of non-small cell lung cancer through the HuR/PD-L1/USP8 axis. Cell Mol. Biol. Lett. 2022, 27, 43. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Niu, S.; Wang, J.; Wu, J.; Li, S.; Yi, X. SChLAP1 contributes to non-small cell lung cancer cell progression and immune evasion through regulating the AUF1/PD-L1 axis. Autoimmunity 2021, 54, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wu, J.; Zhong, W.; Zhao, Z.; He, W. DNA-methylation-induced silencing of DIO3OS drives non-small cell lung cancer progression via activating hnRNPK-MYC-CDC25A axis. Mol. Ther. Oncolytics 2021, 23, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Tang, Y.; Liu, S.; Li, L.; Yu, B.; Lu, Y.; Wang, Y. LIMD1-AS1 suppressed non-small cell lung cancer progression through stabilizing LIMD1 mRNA via hnRNP U. Cancer Med. 2020, 9, 3829–3839. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, Z.; Xu, C.; Li, C.; Ding, C.; Chen, J.; Chen, T.; Zhao, J. LncRNA FAM83A-AS1 promotes lung adenocarcinoma progression by enhancing the pre-mRNA stability of FAM83A. Thorac. Cancer 2021, 12, 1495–1502. [Google Scholar] [CrossRef]
- Yang, H.; Yang, W.; Dai, W.; Ma, Y.; Zhang, G. LINC00667 promotes the proliferation, migration, and pathological angiogenesis in non-small cell lung cancer through stabilizing VEGFA by EIF4A3. Cell Biol. Int. 2020, 44, 1671–1680. [Google Scholar] [CrossRef]
- Wang, X.; Yu, X.; Wei, W.; Liu, Y. Long noncoding RNA MACC1-AS1 promotes the stemness of nonsmall cell lung cancer cells through promoting UPF1-mediated destabilization of LATS1/2. Environ. Toxicol. 2020, 35, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Xie, L.; Su, Y.; Zhang, K.; Liang, R.; Ma, Z.; Li, Y. TM4SF19-AS1 facilitates the proliferation of lung squamous cell carcinoma by recruiting WDR5 to mediate TM4SF19. Mol. Cell Probes 2022, 65, 101849. [Google Scholar] [CrossRef]
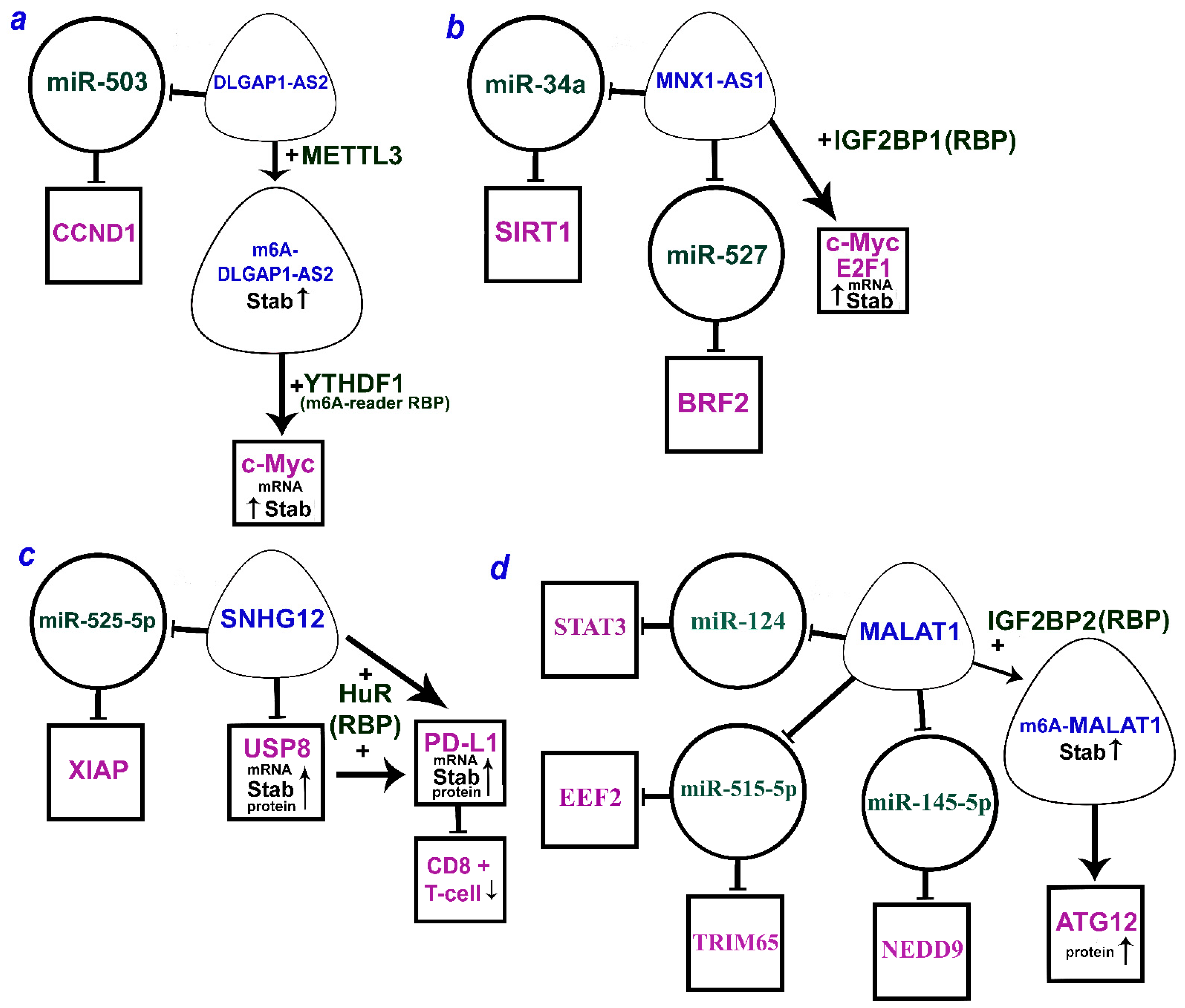
- Zhang, Q.; Zhang, Y.; Chen, H.; Sun, L.N.; Zhang, B.; Yue, D.S.; Wang, C.L.; Zhang, Z.F. METTL3-induced DLGAP1-AS2 promotes non-small cell lung cancer tumorigenesis through m6A/c-Myc-dependent aerobic glycolysis. Cell Cycle 2022, 21, 2602–2614. [Google Scholar] [CrossRef]
- Meyer, K.D.; Jaffrey, S.R. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat. Rev. Mol. Cell Biol. 2014, 15, 313–326. [Google Scholar] [CrossRef]
- Zaccara, S.; Ries, R.J.; Jaffrey, S.R. Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Biol. 2019, 20, 608–624. [Google Scholar] [CrossRef]
- Brennan, C.M.; Steitz, J.A. HuR and mRNA stability. Cell Mol. Life Sci. CMLS 2001, 58, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, S.; Jens, M.; Theil, K.; Schwanhausser, B.; Selbach, M.; Landthaler, M.; Rajewsky, N. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol. Cell 2011, 43, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Geuens, T.; Bouhy, D.; Timmerman, V. The hnRNP family: Insights into their role in health and disease. Hum. Genet. 2016, 135, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.W.; Flynn, R.A.; Chen, Y.; Qu, K.; Wan, B.; Wang, K.C.; Lei, M.; Chang, H.Y. Essential role of lncRNA binding for WDR5 maintenance of active chromatin and embryonic stem cell pluripotency. eLife 2014, 3, e02046. [Google Scholar] [CrossRef]
- Zhu, Y.; Ren, C.; Yang, L. Effect of eukaryotic translation initiation factor 4A3 in malignant tumors. Oncol. Lett. 2021, 21, 358. [Google Scholar] [CrossRef] [PubMed]
- Sakellariou, D.; Frankel, L.B. EIF4A3: A gatekeeper of autophagy. Autophagy 2021, 17, 4504–4505. [Google Scholar] [CrossRef]
- Tang, Y.; Feinberg, T.; Keller, E.T.; Li, X.Y.; Weiss, S.J. Snail/Slug binding interactions with YAP/TAZ control skeletal stem cell self-renewal and differentiation. Nat. Cell Biol. 2016, 18, 917–929. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Z.; Yu, X.; Huang, X.; Liu, Z.; Chai, Y.; Yang, L.; Wang, Q.; Li, M.; Zhao, J.; et al. Unbalanced YAP-SOX9 circuit drives stemness and malignant progression in esophageal squamous cell carcinoma. Oncogene 2019, 38, 2042–2055. [Google Scholar] [CrossRef]
- Patil, D.P.; Pickering, B.F.; Jaffrey, S.R. Reading m(6)A in the Transcriptome: M(6)A-Binding Proteins. Trends Cell Biol. 2018, 28, 113–127. [Google Scholar] [CrossRef]
- Guttman, M.; Donaghey, J.; Carey, B.W.; Garber, M.; Grenier, J.K.; Munson, G.; Young, G.; Lucas, A.B.; Ach, R.; Bruhn, L.; et al. lincRNAs act in the circuitry controlling pluripotency and differentiation. Nature 2011, 477, 295–300. [Google Scholar] [CrossRef]
- Herbst, R.S.; Morgensztern, D.; Boshoff, C. The biology and management of non-small cell lung cancer. Nature 2018, 553, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Kakumani, P.K. AGO-RBP crosstalk on target mRNAs: Implications in miRNA-guided gene silencing and cancer. Transl. Oncol. 2022, 21, 101434. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, L.; Wen, K. Interactions between long non-coding RNAs and RNA-binding proteins in cancer. Oncol. Rep. 2021, 46, 256. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.T.; Yang, Y.M.; Sun, M.M.; He, Y.; Liao, L.; Chen, K.S.; Li, B. New insights into the interplay between long non-coding RNAs and RNA-binding proteins in cancer. Cancer Commun. 2022, 42, 117–140. [Google Scholar] [CrossRef]

| lncRNA/miRNA/mRNA Axes | Regulated Processes and Signaling Pathways; Role in Cancer Prognosis, Survival, and Drug Resistance | Ref. |
|---|---|---|
| AC078883.3/miR-19a/PTEN | Sensitivity to cisplatin | [25] |
| ATP2B1/miR-222-5p/TAB2 | Associated with survival and chemosensitivity | [26] |
| BCYRN1/miR-149/PKM2 | Cell glycolysis, proliferation, and invasion | [27] |
| CCAT1/miR-152 | Promoted cell proliferation, cell invasion, and EMT of NSCLC cell lines and metastasis | [28] |
| CCAT1/miR-490 | [29] | |
| DANCR/miR-1225-3p/ErbB2 | NSCLC cell migration and invasion; enhanced growth and metastasis, larger tumor size, advanced TNM stage, lymph node metastasis, predicted poor prognoses | [30] |
| DANCR/miR-138/Sox4 | [31] | |
| DGCR5/miR-330-5p/CD44 | Promoted lung cancer progression | [32] |
| DLGAP1-AS2/miR-503/cyclin D1 | Increased cell proliferation | [33] |
| DLX6-AS1/miR-144/PRR11 | Promoted cell proliferation, migration, invasion, EMT, and inhibited apoptosis | [34] |
| DLX6-AS1/miR-16-5p/BMI1 | [35] | |
| DNAH17-AS1/miR-877-5p/CCNA2 | Proliferation, migration, invasion of H1299 and 95D cell lines, and inhibition of apoptosis | [36] |
| FEZF1-AS1/miR-516b-5p/ITGA11 | Cell proliferation and migration, preventing cell cycle arrest at the G2/M phase | [37] |
| FGD5-AS1/miR-107/FGFRL1 | Increased the proliferation, viability, migration, and invasion of NSCLC cells | [38] |
| FGD5-AS1/miR-944/MACC1 | [39] | |
| FOXD2-AS1/miR185-5p/SIX1 | Promoted colony formation, cell proliferation, migration, invasion, and drug resistance | [40] |
| FOXD3-AS1/miR-127-3p/MDM2 | Promoted drug resistance in NSCLC cells | [41] |
| H19/miR-17/STAT3 | Promoted growth, migration, and invasion | [42] |
| HCP5/miR-320/survivin | Predicted poor survival | [43] |
| HNF1AS1/miR-92a-3p/MAP2K4 | Enhanced proliferation, inhibited apoptosis, and reduced radiotherapy sensitivity | [44] |
| HOTAIR/miR-217/DACH1 | Promoted cell migration, invasion, and proliferation | [45] |
| HOTTIP/miR-615-3p/HMGB3 | Hypoxia-induced glycolysis | [46] |
| HOXA11-AS/miR-148a-3p/DNMT1 | Increasing cell proliferation and inhibition of cell apoptosis | [47] |
| HOXD-AS1/miR-133a/MMP-9 | Proliferation, migration, and invasion of NSCLC cells | [48] |
| HUWE1/miR-222-5p/TAB2 | Associated with survival and chemosensitivity | [26] |
| KCNQ1OT1/miR-27b-3p/HSP90AA1 | Proliferation, migration, and invasion of H460 cells | [49] |
| LINC00152/miR-16-5p/BCL2L2 | The migration and invasion ability of NSCLC cells and inhibition of apoptosis | [50] |
| LINC00243/miR-507/PDK4 | Proliferation and glycolysis of NSCLC cells | [51] |
| LINC00324/miR-139-5p/IGF1R | Promoted cell proliferation and invasion | [52] |
| LINC00511/miR-625/LRRC8E | Increase in cell proliferation, invasion, and migration, postoperative distant recurrence growth, IC50 value, and metastasis in DDP resistance | [53] |
| LINC00511/miR-98-5p/TGFBR1 | [54] | |
| LINC00518/miR-185-3p/MECP2 | Promoted cell growth by regulating the cell cycle | [55] |
| STAT3/LINC00668/miR-193a/KLF7 | NSCLC cell proliferation, migration, invasion, and inhibition of apoptosis | [56] |
| LINC01123/miR-199a-5p/c-Myc | Promoted NSCLC cell proliferation and aerobic glycolysis | [57] |
| LINC01207/miR-525-5p/ARHGAP11A | Proliferation, migration, invasion of cancer cells, and inhibition of cell apoptosis | [58] |
| LINC01296/miR-598/Twist1 | Accelerated proliferation, inhibited apoptosis in vitro, and promoted tumor growth in vivo | [59] |
| LINC01426/hsa-miR-143-3P/CDK1 | Progression and development of NSCLC | [60] |
| LINC01426/hsa-miR-143-3P/MAD2L1 | ||
| LINC01426/hsa-miR-3065-3p/TPX2 | ||
| LINC01426/hsa-miR-508-3p/CHEK1 | ||
| LINC01426/hsa-miR-508-3p/SHCBP1 | ||
| LINC01426/hsa-miR-508-3p/EXO1 | ||
| LINC01748/miR-520a-5p/HMGA1 | Cell proliferation, migration, and invasion, inhibition of cell apoptosis in vitro, and increased tumor growth in vivo | [61] |
| LOC285758/miRNA-204/CDK6 | Promoted cell survival, migration and invasion | [62] |
| ERβ/MALAT1/miR-515-5p/EEF2 | High levels of axosomal MALAT1 increase cell proliferation, migration, invasion, colony formation, inhibit cell apoptosis in vitro, increase tumor growth in vivo in NSCLC | [63] |
| ERβ/MALAT1/miR-515-3p/TRIM65 | [64] | |
| ERβ/MALAT1/miR145-5p/NEDD9 | [65] | |
| ERβ/MALAT1/miR-124/STAT3 | [66] | |
| MAPKAPK5-AS1/miR-490-3p/HMGB2 | Promoted proliferation and EMT and induced apoptosis | [67] |
| MCM3AP-AS1/miR-195-5p/E2F3 | Proliferation, migration, and invasion of NSCLC cells | [68] |
| MEG8/miR-107/CDK6 | Cell proliferation, migration, and invasion | [69] |
| MFI2-AS1/miR-107/NFAT5 | Mediated proliferation, migration, invasion, angiogenesis, and metastasis | [70] |
| MINCR/miR-126/SLC7A5 | NSCLC cell proliferation, migration, and inhibition of cell apoptosis | [71] |
| MIR9-3HG/miR-138-5p/LIMK1 | Promoted proliferation, migration, invasion, EMT, inhibited cell apoptosis in lung squamous cell carcinoma | [72] |
| MIR9-3HG/miR-138-5p/TAF15 | ||
| MNX1-AS1/miR-34a/SIRT1 | Promoted proliferation, migration, invasion, lymph node metastasis, and poor prognosis | [73] |
| MNX1-AS1/miR-527/BRF2 | [74] | |
| MRUL/miR-17-5p/SRSF2 | Promoted cell proliferation, migration, and invasion and is correlated with poor prognosis | [75] |
| NCK1-AS1/miR-512-5p/p21 | Shorter overall survival time and faster progression | [76] |
| NEAT1/hsa-mir-98-5p/MAPK6 | Progression of NSCLC cells (growth, migration, invasion) | [77] |
| OGFRP1/miR-4640-5p/eIF5A | Promoted proliferation, migration, and invasion | [78] |
| PCAT7/miR-486-5p/CDK4 | Promoted the development of NSCLC | [79] |
| PKMYT1AR/miR-485-5p/PKMYT1 | Promoted cancer stem cells, tumor cell proliferation, migration, and xenograft tumor formation | [80] |
| PRNCR1/miR-488/HEY2 | Cell proliferation, migration, and invasion, and EMT | [81] |
| PTPRG-AS1/miR-200c-3p/TCF4 | Promoted viability and enhanced radioresistance | [82] |
| PVT1/miR-551b/FGFR1 | Promoted proliferation, migration, and invasion | [83] |
| PVT1/miR-760/IL-6 | [84] | |
| E2F1/SBF2-AS1/miR-362-3p/GRB2 | Tumor growth in vivo and cell proliferation, migration, and invasion in vitro | [85] |
| SNHG1/miR-330-5p/DCLK1 | Progression and chemoresistance of NSCLC | [86] |
| SNHG11/miR-485-5p/BSG | Promoted growth, migration, and EMT | [87] |
| SNHG12/miR-525-5p/XIAP | Promoted proliferation and enhanced DDP resistance | [88] |
| SNHG14/miR-34a/HMGB1 | Migration, invasion, and inhibition of apoptosis | [89] |
| SNHG15/miR-211-3p/ZNF217 | Proliferation and migration of NSCLC cells | [90] |
| TATDN1/miR-451/TRIM66 | Promoted cell proliferation and inhibited cell apoptosis | [91] |
| TMEM132D-AS1/miR-766-5p/ENTPD1 | Increased cell proliferation | [92] |
| TP73-AS1/miR-34a-5p/TRIM29 | Cell proliferation, migration, invasion, tumor growth, cycle progression, cisplatin resistance, and inhibition of apoptosis | [93] |
| TP73-AS1/miR-449a/EZH2 | [94] | |
| TYMSOS/hsa-miR-195-5p/CHEK1 | Progression and development of NSCLC | [60] |
| TYMSOS/hsa-miR-221-3p/KIF20A | ||
| TYMSOS/hsa-miR-486-3p/NUF2 | ||
| TYMSOS/hsa-miR-101-3p/CEP55 | ||
| UCC/miR-143-3p/SOX5 | Promoted EMT | [95] |
| VPS9D1-AS1/hsa-miR-548p/NCAPH | Progression and development of NSCLC | [60] |
| WFDC21P/MIR4293/DCP2 | Promoted tumor cell proliferation and metastasis but suppressed apoptosis | [96] |
| WT1-AS/miR-206/NAMPT | Associated with shortened survival | [97] |
| XIST/miR-17/ATG7 | Promoted cell proliferation, cell viability, migration, and invasion, inhibited apoptosis, increased autophagy, TGF-β-induced EMT, and pulmonary metastasis of NSCLC | [98] |
| XIST/miR-520/BAX | [99] | |
| XIST/miR-137/PXN | [100] | |
| XIST/miR-335/SOD2/ROS | [101] | |
| XIST/miR-367/miR-141/ZEB2 | [102] |
| lncRNA/miRNA/mRNA Axes | Regulated Processes and Signaling Pathways; Role in Cancer Prognosis, Survival, and Drug Resistance | Ref. |
|---|---|---|
| FOXD3-AS1/miR-150/SRCIN1 | Inhibited the proliferation and invasion of H1299 cell lines | [103] |
| GAN1/miR-26a-5p/PTEN | Suppressed cell proliferation, colony formation, and cell cycle progression and induced apoptosis | [104] |
| GATA6-AS1/miR-543/RKIP | Inhibited proliferation, migration, invasion, and EMT of NSCLC cells | [105] |
| TP53/GHRLOS/miR-346/APC | Suppressed cancer cell proliferation and invasion and promoted cell apoptosis | [106] |
| TP53/GHRLOS/miR-346/Bax | ||
| TP53/GHRLOS/miR-346/Bcl-2 | ||
| TP53/GHRLOS/miR-346/CDK2 | ||
| TP53/GHRLOS/miR-346/E-cadherin | ||
| TP53/GHRLOS/miR-346/N-cadherin | ||
| TP53/GHRLOS/miR-346/PCNA | ||
| HCG11/miR-522-3p/SOCS5 | Inhibition of cell viability, migration, and invasion | [107] |
| c-Myc/LINC00173/miR-1275/PROCA1, ZFP36L2, and BCL2 | Cisplatin chemosensitivity, apoptosis | [108] |
| LINC01128/miR-25-3p/PTEN | Promoted EGFR-TKI resistance | [109] |
| LINC00494/miR-150-3p/SRCIN1 | Inhibited NSCLC cell proliferation, tumor growth in vivo | [110] |
| MAGI2-AS3/miR-25/RECK | Decreased NSCLC cell invasion and migration | [111] |
| MT1JP/miRNA-423-3p/Bim | Suppressed cell proliferation and increased cell apoptosis | [112] |
| SOX2-OT/miR-30d-5p/PDK1 | Could inhibit the proliferation, migration, and invasion of NSCLC cells and promote cell apoptosis | [113] |
| TP53TG1/miR-18a/PTEN | Cisplatin sensitivity and apoptosis of A549/DDP cells | [114] |
| TPTEP1/miR-328-5p/SRCIN1 | Inhibited cell proliferation and induced apoptosis | [115] |
| Mechanisms, Axes | LncRNAs/Axes in Processes, Pathways, Prognosis, Survival, and Drug Resistance | Ref. |
|---|---|---|
| IGF2BP1/2/3 as an RNA-binding protein (RBP) | ||
| LCAT1↑→m6A-IGF2BP2(RBP)↑stab →m6A-CDC6↑mRNAstab | Promotes NSCLC cell growth, migration, and poor patient survival | [143] |
| Linc-SPRY3-2/3/4↓ov-ex+IGF2BP3(RBP) /HMGA2, c-MYC↓mRNAstab | Suppresses NSCLC and enhances cell radiation response | [144] |
| FOXP3→(pr)LINC01232↑+IGF2BP2(RBP)→TGFBR1↑stab | Promotes TGF-β signaling, NSCLC cell stemness | [145] |
| IGF2BP2(RBP)↑→m6A-MALAT1↑stab→ATG12↑protein | Promotes NSCLC proliferation; reduces OS, DFS | [146] |
| c-Myc→MNX1-AS1↑+IGF2BP1(RBP) ↔c-Myc, E2F1↑mRNAstab→c-Myc-sign | Promotes cell cycle progression, proliferation in vitro, in vivo; poor clinical outcomes | [147] |
| lnc-THOR↑→IGF2BP1(RBP)↑ →IGF2, Gli1, Myc, SOX9↑mRNAstab | Enhances NSCLC cell proliferation, migration, and invasion | [148] |
| HuR/ELAVL1 as an RNA-binding protein (RBP) | ||
| FENDRR↓ov-ex/MDR1↓mRNAstab ↔MDR1↑3’UTR← HuR(RBP) | Suppresses NSCLC cell stemness | [149] |
| E2F1→MCF2L-AS1↑+HuR(RBP)→CCND1↑mRNAstab | Drives NSCLC cell growth and induces gefitinib resistance | [150] |
| SNHG12↑+HuR(RBP)→USP8↑mRNAstab,protein →PD-L1↑mRNAstab, protein↑/CD8+T-cell↓ | Promotes proliferation, migration, invasion, and immune escape in vitro/in vivo | [151] |
| Heterogeneous nuclear ribonucleoproteins (hnRNPs) with RBP function | ||
| SChLAP1↑/hnRNPD(AUF1, RBP)↓/PDL1↑mRNAstab | Enhances proliferation, immune evasion | [152] |
| DNA-meth/DIO3OS↓ov-ex/hnRNPK↓ /MYC,DNA,mRNA↓/CDC25A↓ | Ectopic expression of DIO3OS suppresses NSCLC tumorigenesis, metastasis in vivo | [153] |
| LIMD1-AS1↓ov-ex+hnRNPU→LIMD1↑mRNAstab | Suppresses NSCLC progression | [154] |
| Other RNA-binding proteins (RBP) as mediators of lncRNAs | ||
| FAM83A-AS1↑+FBL(RBP)→FAM83A↑pre-mRNAstab | Promotes LUAC metastasis, ERK signaling; low OS, PFS | [155] |
| LINC00667↑+EIF4A3(RBP)→VEGFA↑mRNAstab | Promotes proliferation, migration, and angiogenesis | [156] |
| MACC1-AS1+UPF1(RBP)→LATS1/2↓mRNAdestab | Drives NSCLC cell stemness through inhibition of the Hippo pathway | [157] |
| TM4SF19-AS1↑+WDR5(RBP)→TM4SF19(pr-WDR5) →(DNA-demeth-pr) TM4SF19↑mRNA | Facilitates proliferation, adhesion of lung squamous cell carcinoma | [158] |
| METTL3→m6A-DLGAP1-AS2↑stab +YTHDF1(m6A-reader RBP)→c-Myc↑mRNAstab | Promotes aerobic glycolysis; correlated with advanced stages, poor prognosis | [159] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga, E.A.; Fridman, M.V.; Burdennyy, A.M.; Loginov, V.I.; Dmitriev, A.A.; Pronina, I.V.; Morozov, S.G. Various LncRNA Mechanisms in Gene Regulation Involving miRNAs or RNA-Binding Proteins in Non-Small-Cell Lung Cancer: Main Signaling Pathways and Networks. Int. J. Mol. Sci. 2023, 24, 13617. https://doi.org/10.3390/ijms241713617
Braga EA, Fridman MV, Burdennyy AM, Loginov VI, Dmitriev AA, Pronina IV, Morozov SG. Various LncRNA Mechanisms in Gene Regulation Involving miRNAs or RNA-Binding Proteins in Non-Small-Cell Lung Cancer: Main Signaling Pathways and Networks. International Journal of Molecular Sciences. 2023; 24(17):13617. https://doi.org/10.3390/ijms241713617
Chicago/Turabian StyleBraga, Eleonora A., Marina V. Fridman, Alexey M. Burdennyy, Vitaly I. Loginov, Alexey A. Dmitriev, Irina V. Pronina, and Sergey G. Morozov. 2023. "Various LncRNA Mechanisms in Gene Regulation Involving miRNAs or RNA-Binding Proteins in Non-Small-Cell Lung Cancer: Main Signaling Pathways and Networks" International Journal of Molecular Sciences 24, no. 17: 13617. https://doi.org/10.3390/ijms241713617
APA StyleBraga, E. A., Fridman, M. V., Burdennyy, A. M., Loginov, V. I., Dmitriev, A. A., Pronina, I. V., & Morozov, S. G. (2023). Various LncRNA Mechanisms in Gene Regulation Involving miRNAs or RNA-Binding Proteins in Non-Small-Cell Lung Cancer: Main Signaling Pathways and Networks. International Journal of Molecular Sciences, 24(17), 13617. https://doi.org/10.3390/ijms241713617







