In Vitro Evaluation of Biological and Anticancer Activities Exhibited by Five Varieties of Vitis vinifera L.
Abstract
:1. Introduction
2. Results
2.1. Qualitative Phytochemical Analysis for VVM Extracts
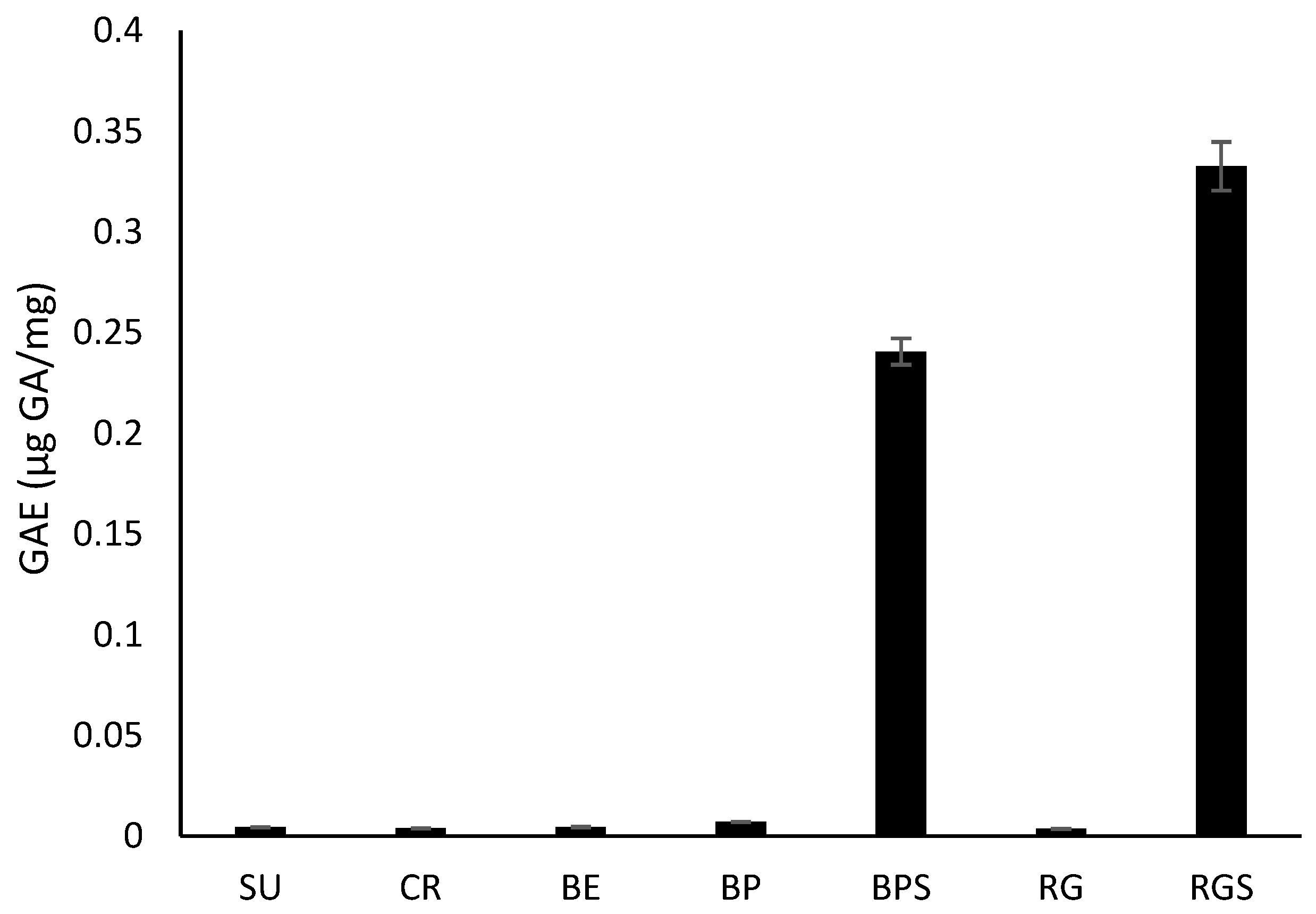
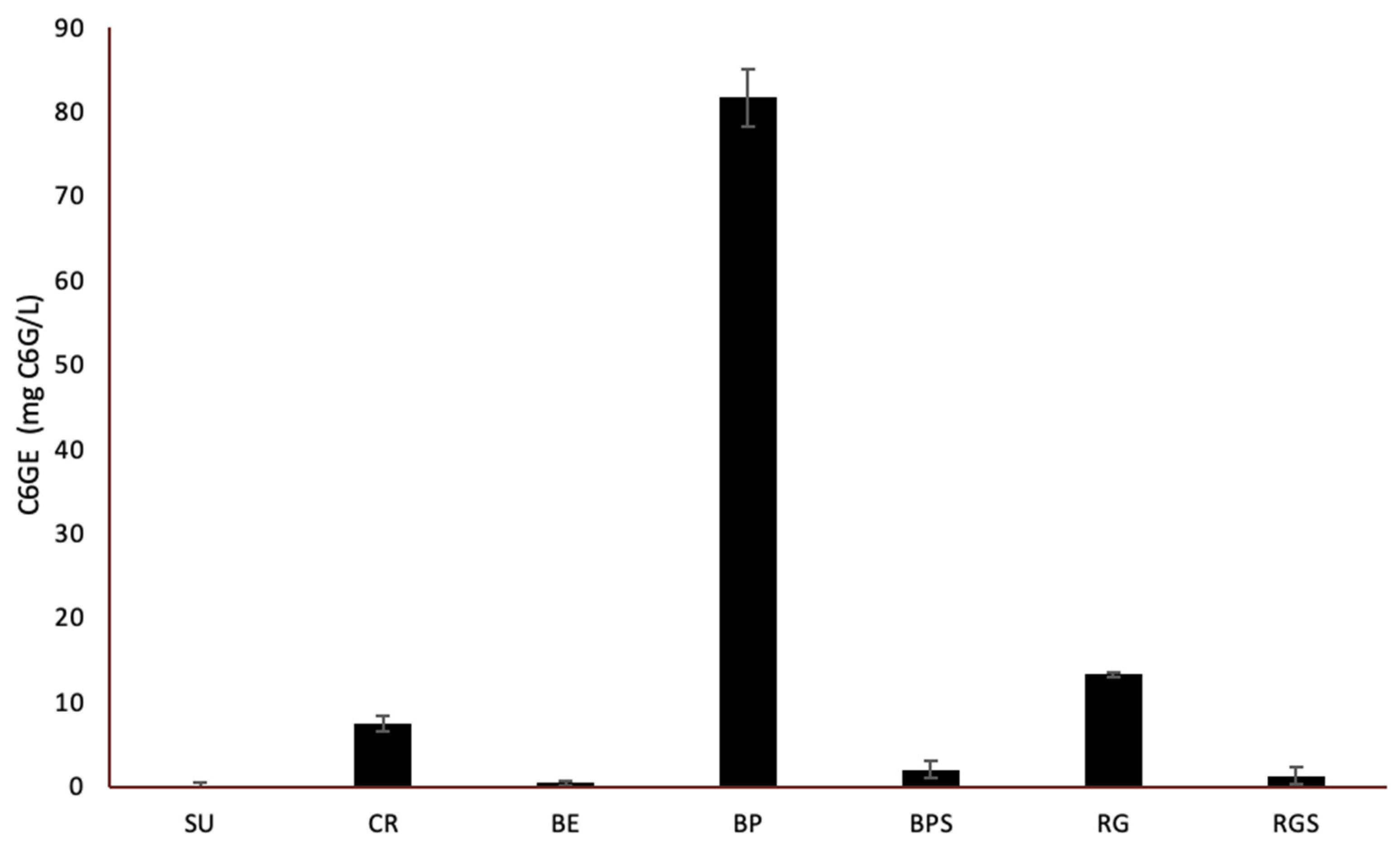
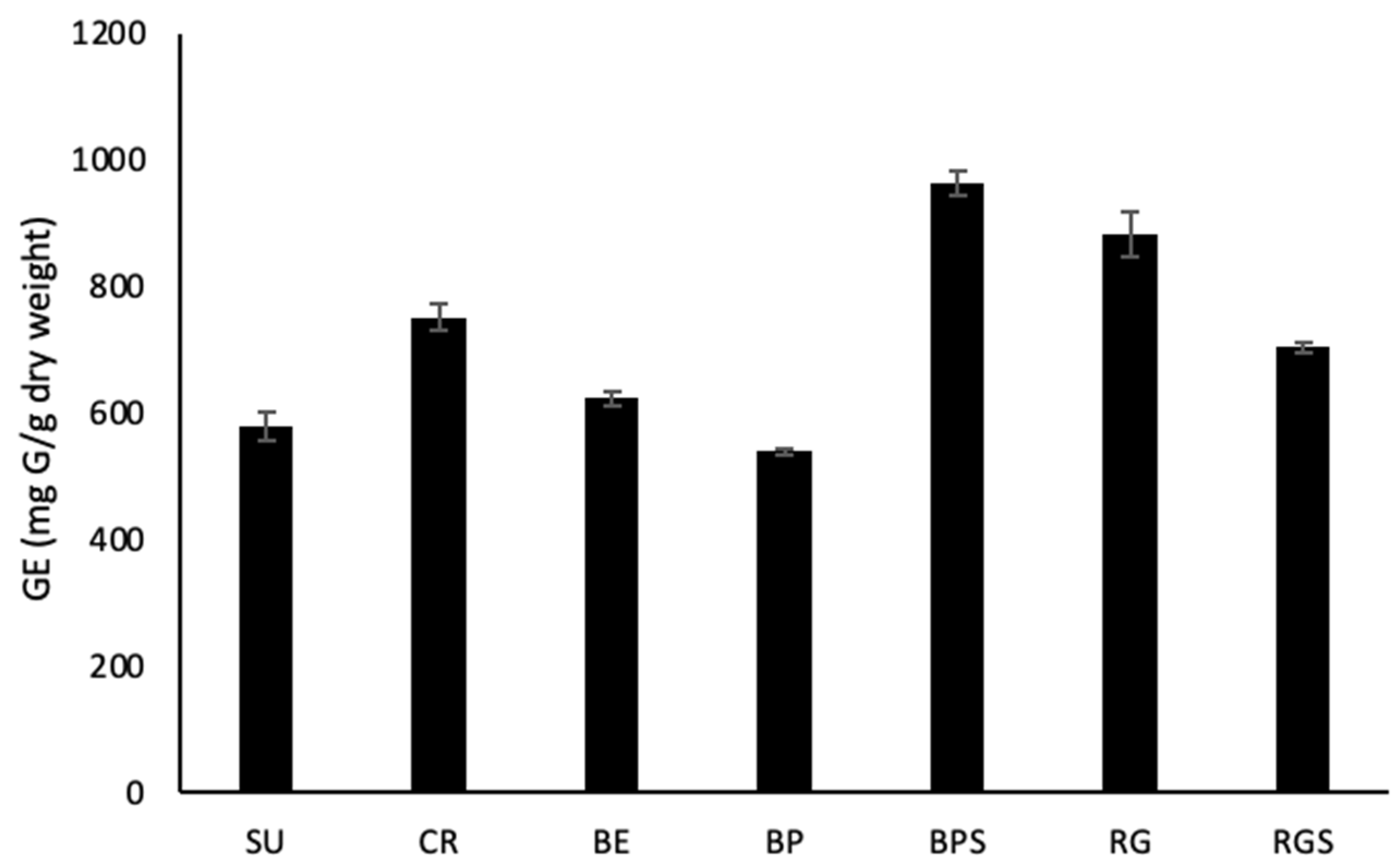
2.2. Quantitative Phytochemical Analysis of VVM Pulp and Seed Extracts
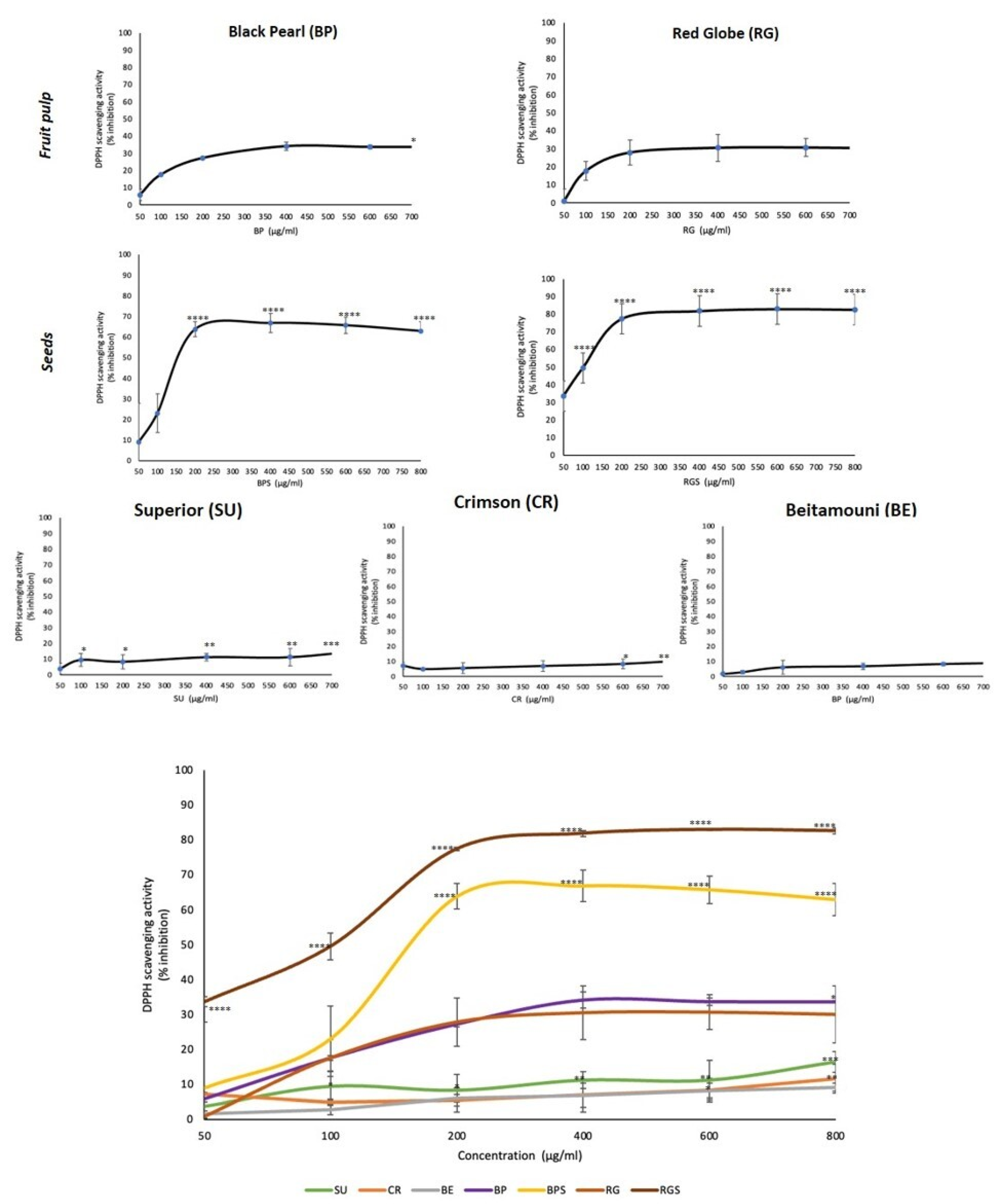
2.3. VVM Extracts Have Variable Antioxidant Activities
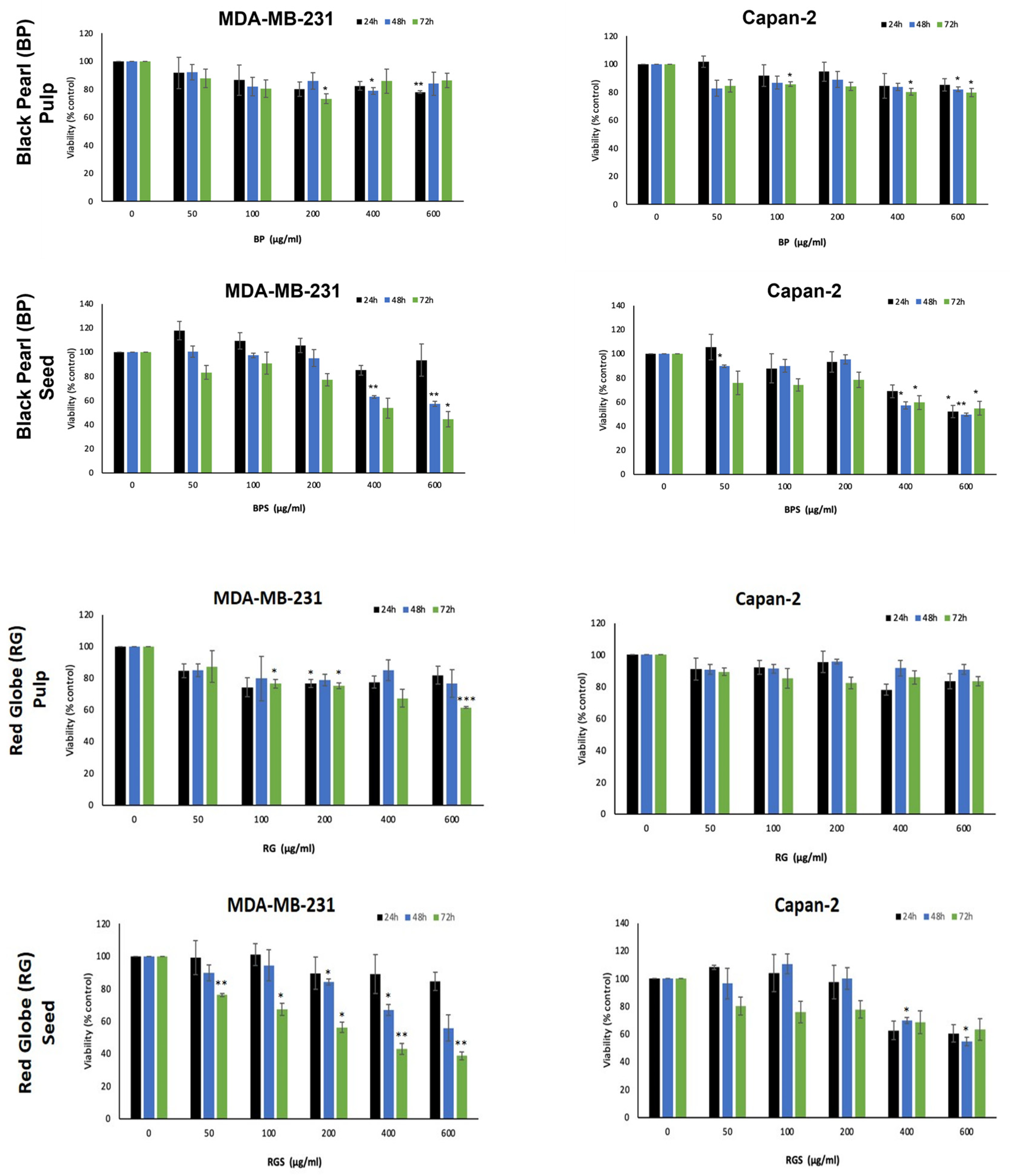
2.4. VVM Extracts Inhibit the Proliferation of Capan-2 and MDA-MB231 Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Preparation of Vitis vinifera Extracts
4.2. Phytochemical Tests
4.3. Total Phenolic Content (TPC)
4.4. Total Anthocyanin Content (TAC)
4.5. Total Reducing Sugar Content (RSC)
4.6. Assay of Antioxidant Activity
4.7. Culture of MDA-MB-231 and Capan-2 Cell Lines
4.8. Assay of Cell Viability
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torregrosa, L.; Vialet, S.; Adivèze, A.; Iocco-Corena, P.; Thomas, M.R. Grapevine (Vitis vinifera L.). Methods Mol. Biol. Clifton NJ 2015, 1224, 177–194. [Google Scholar] [CrossRef]
- Alston, J.; Sambucci, O. Grapes in the World Economy; Springer: Berlin/Heidelberg, Germany, 2019; pp. 1–24. [Google Scholar] [CrossRef]
- Alfrey, P. The Very Fine Grapevine—The Essential Guide to Everything You Need to Know about Growing Grapes. Medium. Available online: https://thepolycultureproject.medium.com/the-very-fine-grapevine-the-essential-guide-to-everything-you-need-to-know-about-growing-grapes-4e1dc06ff6a6 (accessed on 5 June 2023).
- Wine & Spirit Education Trust. Wines and Spirits: Understanding Style and Quality; University College Prague; University of International Relations; Institute of Hospitality Management and Economics, Ltd.: Prague, Czech, 2012. [Google Scholar]
- Miraldi, E.; Baini, G. Medicinal Plants and Health in Human History: From Empirical Use to Modern Phytotherapy. J. Siena Acad. Sci. 2018, 10. [Google Scholar] [CrossRef]
- Sharma, A.; Sabharwal, P.; Dada, R. Chapter 1—Herbal Medicine—An Introduction to Its History. In Herbal Medicine in Andrology; Henkel, R., Agarwal, A., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 1–8. [Google Scholar] [CrossRef]
- Wachtel-Galor, S.; Benzie, I.F.F. Herbal Medicine: An Introduction to Its History, Usage, Regulation, Current Trends, and Research Needs. In Herbal Medicine: Biomolecular and Clinical Aspects; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- National Institutes of Health (US); Biological Sciences Curriculum Study. Understanding Cancer. In NIH Curriculum Supplement Series [Internet]; National Institutes of Health (US): Bethesda, MD, USA, 2007. [Google Scholar]
- Nagai, H.; Kim, Y.H. Cancer Prevention from the Perspective of Global Cancer Burden Patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef]
- Tavakoli, J.; Miar, S.; Majid Zadehzare, M.; Akbari, H. Evaluation of Effectiveness of Herbal Medication in Cancer Care: A Review Study. Iran. J. Cancer Prev. 2012, 5, 144–156. [Google Scholar] [PubMed]
- Pott, D.M.; Osorio, S.; Vallarino, J.G. From Central to Specialized Metabolism: An Overview of Some Secondary Compounds Derived From the Primary Metabolism for Their Role in Conferring Nutritional and Organoleptic Characteristics to Fruit. Front. Plant Sci. 2019, 10, 835. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, W. Pancreatic Cancer: A Review of Risk Factors, Diagnosis, and Treatment. Technol. Cancer Res. Treat. 2020, 19, 1533033820962117. [Google Scholar] [CrossRef] [PubMed]
- Łukasiewicz, S.; Czeczelewski, M.; Forma, A.; Baj, J.; Sitarz, R.; Stanisławek, A. Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review. Cancers 2021, 13, 4287. [Google Scholar] [CrossRef]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and Its Bioactive Constituents: An Update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef]
- Aghbali, A.; Hosseini, S.V.; Delazar, A.; Gharavi, N.K.; Shahneh, F.Z.; Orangi, M.; Bandehagh, A.; Baradaran, B. Induction of Apoptosis by Grape Seed Extract (Vitis vinifera) in Oral Squamous Cell Carcinoma. Bosn. J. Basic Med. Sci. 2013, 13, 186–191. [Google Scholar] [CrossRef]
- Derry, M.; Raina, K.; Agarwal, R.; Agarwal, C. Differential Effects of Grape Seed Extract against Human Colorectal Cancer Cell Lines: The Intricate Role of Death Receptors and Mitochondria. Cancer Lett. 2013, 334, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Homayoun, M.; Ghasemnezhad Targhi, R.; Soleimani, M. Anti-Proliferative and Anti-Apoptotic Effects of Grape Seed Extract on Chemo-Resistant OVCAR-3 Ovarian Cancer Cells. Res. Pharm. Sci. 2020, 15, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Sahpazidou, D.; Geromichalos, G.D.; Stagos, D.; Apostolou, A.; Haroutounian, S.A.; Tsatsakis, A.M.; Tzanakakis, G.N.; Hayes, A.W.; Kouretas, D. Anticarcinogenic Activity of Polyphenolic Extracts from Grape Stems against Breast, Colon, Renal and Thyroid Cancer Cells. Toxicol. Lett. 2014, 230, 218–224. [Google Scholar] [CrossRef]
- Teodoro, A.J. Bioactive Compounds of Food: Their Role in the Prevention and Treatment of Diseases. Oxid. Med. Cell. Longev. 2019, 2019, e3765986. [Google Scholar] [CrossRef] [PubMed]
- Balea, Ş.S.; Pârvu, A.E.; Pârvu, M.; Vlase, L.; Dehelean, C.A.; Pop, T.I. Antioxidant, Anti-Inflammatory and Antiproliferative Effects of the Vitis vinifera L. Var. Fetească Neagră and Pinot Noir Pomace Extracts. Front. Pharmacol. 2020, 11, 990. [Google Scholar] [CrossRef]
- Insanu, M.; Karimah, H.; Pramastya, H.; Fidrianny, I. Phytochemical Compounds and Pharmacological Activities of Vitis vinifera L.: An Updated Review. Biointerface Res. Appl. Chem. 2021, 11, 13829–13849. [Google Scholar] [CrossRef]
- Baer-Dubowska, W.; Szaefer, H.; Majchrzak-Celińska, A.; Krajka-Kuźniak, V. Tannic Acid: Specific Form of Tannins in Cancer Chemoprevention and Therapy-Old and New Applications. Curr. Pharmacol. Rep. 2020, 6, 28–37. [Google Scholar] [CrossRef]
- Xu, X.-H.; Li, T.; Fong, C.M.V.; Chen, X.; Chen, X.-J.; Wang, Y.-T.; Huang, M.-Q.; Lu, J.-J. Saponins from Chinese Medicines as Anticancer Agents. Molecules 2016, 21, 1326. [Google Scholar] [CrossRef]
- Dimas, K.S.; Pantazis, P.; Ramanujam, R. Chios Mastic Gum: A Plant-Produced Resin Exhibiting Numerous Diverse Pharmaceutical and Biomedical Properties. In Vivo 2012, 26, 777–785. [Google Scholar]
- Lu, J.-J.; Bao, J.-L.; Wu, G.-S.; Xu, W.-S.; Huang, M.-Q.; Chen, X.-P.; Wang, Y.-T. Quinones Derived from Plant Secondary Metabolites as Anti-Cancer Agents. Anticancer Agents Med. Chem. 2013, 13, 456–463. [Google Scholar]
- Kamran, S.; Sinniah, A.; Abdulghani, M.A.M.; Alshawsh, M.A. Therapeutic Potential of Certain Terpenoids as Anticancer Agents: A Scoping Review. Cancers 2022, 14, 1100. [Google Scholar] [CrossRef]
- Kepp, O.; Menger, L.; Vacchelli, E.; Adjemian, S.; Martins, I.; Ma, Y.; Sukkurwala, A.Q.; Michaud, M.; Galluzzi, L.; Zitvogel, L.; et al. Anticancer Activity of Cardiac Glycosides. Oncoimmunology 2012, 1, 1640–1642. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, P.R.; Vail, D.R.; Wobbe, K.K.; Weathers, P.J. Effect of Sugars on Artemisinin Production in Artemisia Annua L.: Transcription and Metabolite Measurements. Molecules 2010, 15, 2302–2318. [Google Scholar] [CrossRef] [PubMed]
- Rolland, F.; Moore, B.; Sheen, J. Sugar Sensing and Signaling in Plants. Plant Cell 2002, 14, s185–s205. [Google Scholar] [CrossRef] [PubMed]
- Akšić, M.F.; Tosti, T.; Sredojević, M.; Milivojević, J.; Meland, M.; Natić, M. Comparison of Sugar Profile between Leaves and Fruits of Blueberry and Strawberry Cultivars Grown in Organic and Integrated Production System. Plants 2019, 8, 205. [Google Scholar] [CrossRef] [PubMed]
- Khatri, D.; Chhetri, S.B.B. Reducing Sugar, Total Phenolic Content, and Antioxidant Potential of Nepalese Plants. BioMed Res. Int. 2020, 2020, 7296859. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, M.; Zhao, J.; Li, Z.; Xing, B.; Li, X.; Kong, M.; Li, L.; Zhang, Q.; Liu, Y.; et al. An Overview of Plant Phenolic Compounds and Their Importance in Human Nutrition and Management of Type 2 Diabetes. Molecules 2016, 21, 1374. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Annunziata, G.; Jimenez-García, M.; Tejada, S.; Moranta, D.; Arnone, A.; Ciampaglia, R.; Tenore, G.C.; Sureda, A.; Novellino, E.; Capó, X. Grape Polyphenols Ameliorate Muscle Decline Reducing Oxidative Stress and Oxidative Damage in Aged Rats. Nutrients 2020, 12, 1280. [Google Scholar] [CrossRef]
- Esfahanian, Z.; Behbahani, M.; Shanehsaz, M.; Hessami, M.J.; Nejatian, M.A. Evaluation of Anticancer Activity of Fruit and Leave Extracts from Virus Infected and Healthy Cultivars of Vitis vinifera. Cell J. Yakhteh 2013, 15, 116–123. [Google Scholar]
- Belwal, T.; Nabavi, S.; Habtemariam, S. Dietary Anthocyanins and Insulin Resistance: When Food Becomes a Medicine. Nutrients 2017, 9, 1111. [Google Scholar] [CrossRef] [PubMed]
- Moraes-de-Souza, R.A.; Oldoni, T.L.C.; Regitano-d’Arce, M.A.B.; Alencar, S.M. Antioxidant Activity and Phenolic Composition of Herbal Infusions Consumed in Brazil Activid Ad Antioxidante Y Compuestos Fenólicos En Infusiones Herbarias Consumid as En Brasil. Cienc. Tecnol. Aliment. 2008, 6, 41–47. [Google Scholar] [CrossRef]

| SU | CR | BE | BP | BPS | RG | RGS | |
|---|---|---|---|---|---|---|---|
| Tannins | - | - | - | - | + | - | + |
| Resins | - | - | - | - | - | - | + |
| Saponins | - | - | - | - | + | - | + |
| Phenols | + | + | + | + | + | + | + |
| Quinones | + | + | + | + | + | + | + |
| Steroids | - | - | - | - | - | - | - |
| Cardiac Glycosides | - | + | - | + | + | - | - |
| Terpenoids | + | + | + | + | + | + | + |
| Anthraquinones | - | - | - | - | - | - | - |
| Anthocyanins | + | + | + | + | + | + | + |
| Reducing sugar | + | + | + | + | + | + | + |
| Phytoconstituents | Added Reagent | Expected Result |
|---|---|---|
| Reducing sugar | Drops of Fehling (A + B) | Brick-red precipitate |
| Anthraquinones | HCl (10%) | Precipitate |
| Tannins | Ferric chloride FeCl3 (10%) | Blue color |
| Resins | Acetone + water | Turbidity |
| Terpenoids | Chloroform + concentrated sulfuric acid | Reddish brown color on the surface |
| Quinones | HCl concentrated | Precipitate or yellow color |
| Sterols & steroids | Chloroform + concentrated sulfuric acid | Red color of the upper layer+ greenish yellow fluorescence in the acid layer |
| Anthocyanins | NaOH (10%) | Blue color |
| Cardiac glycosides | Acetic acid glacial + ferric chloride FeCl3 (5%) + concentrated sulfuric acid | Purple ring + Brown ring + Green ring |
| Saponins | Vigorous shaking | Layer of foam |
| Phenols | FeCl3 (1%) + K3(Fe(CN)6) (1%) | Greenish blue color |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwiche, L.; Mesmar, J.; Baydoun, E.; El Kayal, W. In Vitro Evaluation of Biological and Anticancer Activities Exhibited by Five Varieties of Vitis vinifera L. Int. J. Mol. Sci. 2023, 24, 13440. https://doi.org/10.3390/ijms241713440
Darwiche L, Mesmar J, Baydoun E, El Kayal W. In Vitro Evaluation of Biological and Anticancer Activities Exhibited by Five Varieties of Vitis vinifera L. International Journal of Molecular Sciences. 2023; 24(17):13440. https://doi.org/10.3390/ijms241713440
Chicago/Turabian StyleDarwiche, Linda, Joelle Mesmar, Elias Baydoun, and Walid El Kayal. 2023. "In Vitro Evaluation of Biological and Anticancer Activities Exhibited by Five Varieties of Vitis vinifera L." International Journal of Molecular Sciences 24, no. 17: 13440. https://doi.org/10.3390/ijms241713440
APA StyleDarwiche, L., Mesmar, J., Baydoun, E., & El Kayal, W. (2023). In Vitro Evaluation of Biological and Anticancer Activities Exhibited by Five Varieties of Vitis vinifera L. International Journal of Molecular Sciences, 24(17), 13440. https://doi.org/10.3390/ijms241713440






