Differential Effects of the Processed and Unprocessed Garlic (Allium sativum L.) Ethanol Extracts on Neuritogenesis and Synaptogenesis in Rat Primary Hippocampal Neurons
Abstract
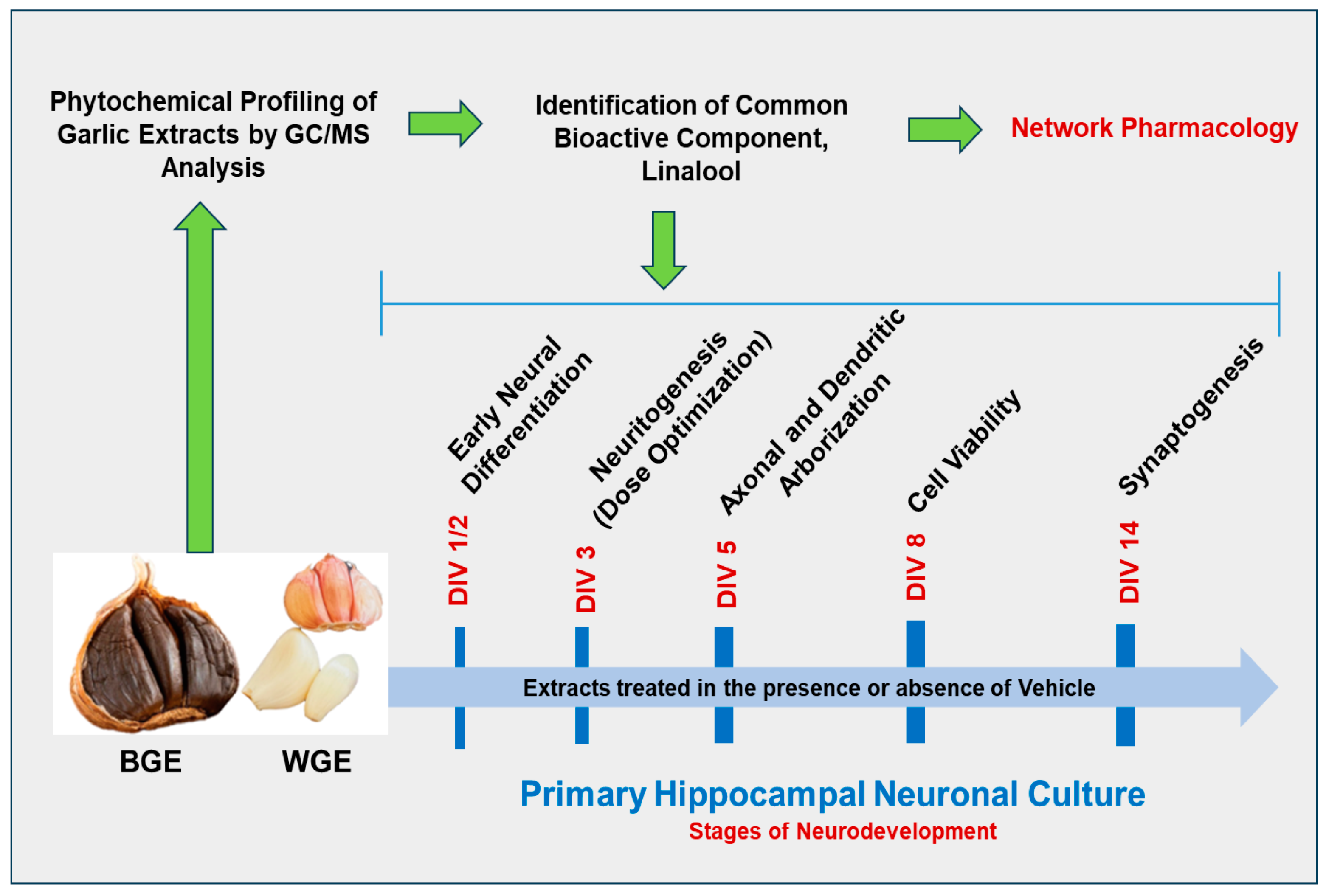
:1. Introduction
2. Results
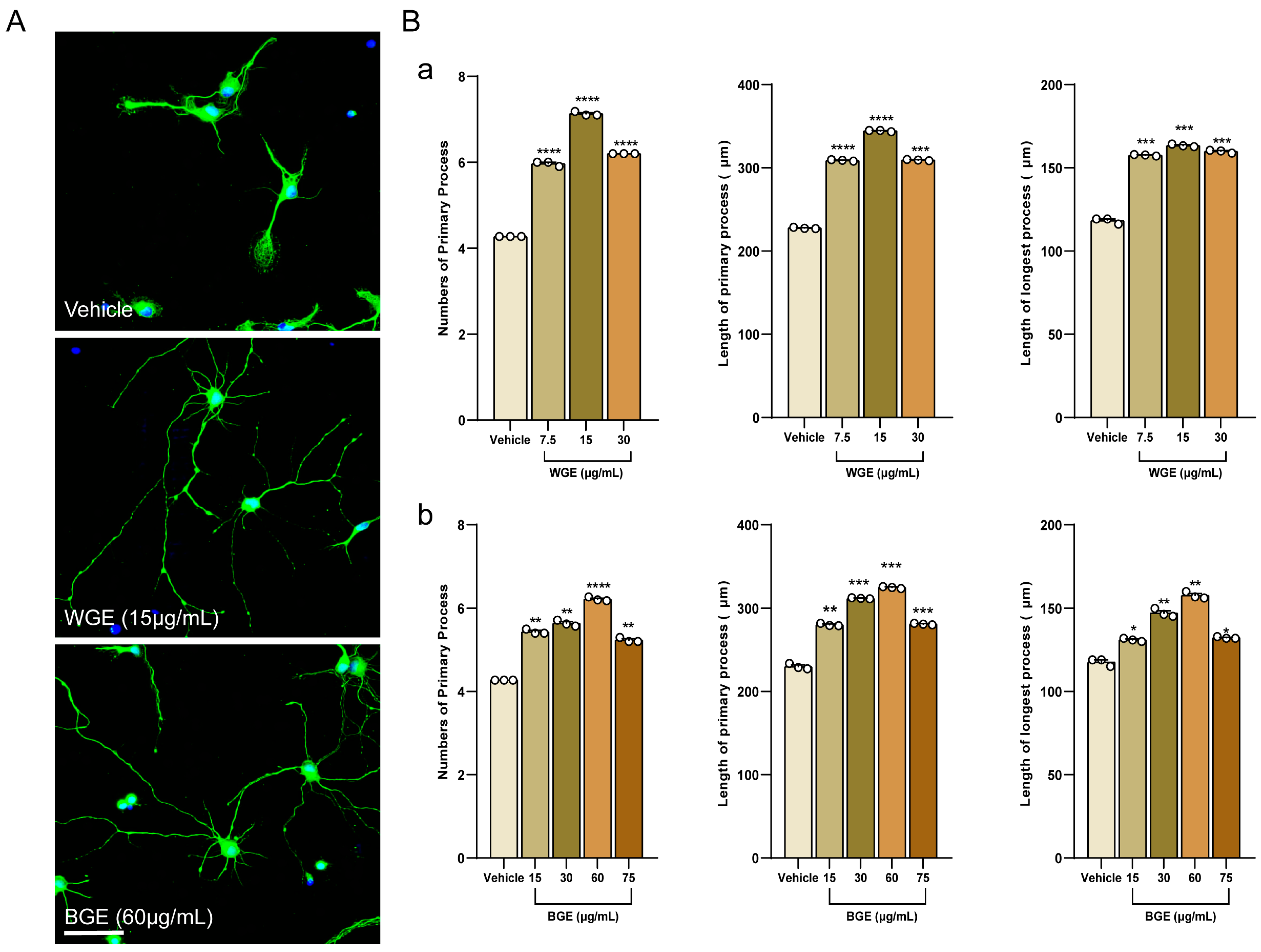
2.1. Dose Optimization of Garlic Ethanol Extract for Neurite Outgrowth Activity
2.2. Effects of BGE and WGE on Neuronal Viability
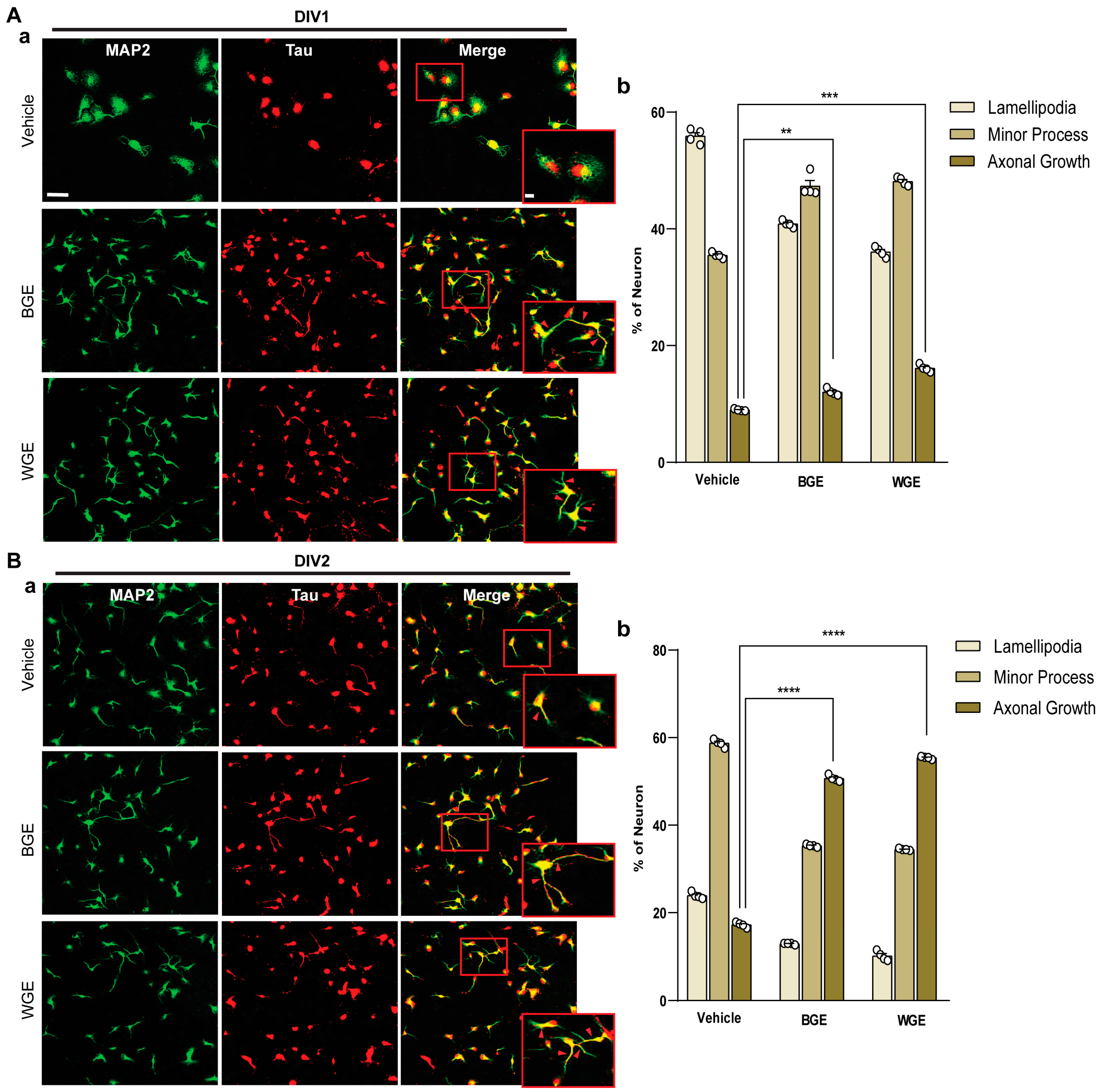
2.3. Early Neuritogenic Activity of BGE and WGE
2.4. Effect of BGE and WGE on Axonal Development
2.5. Effect of BGE and WGE on Dendritic Morphological Complexity
2.6. Effect of BGE and WGE on the Synaptic Formation
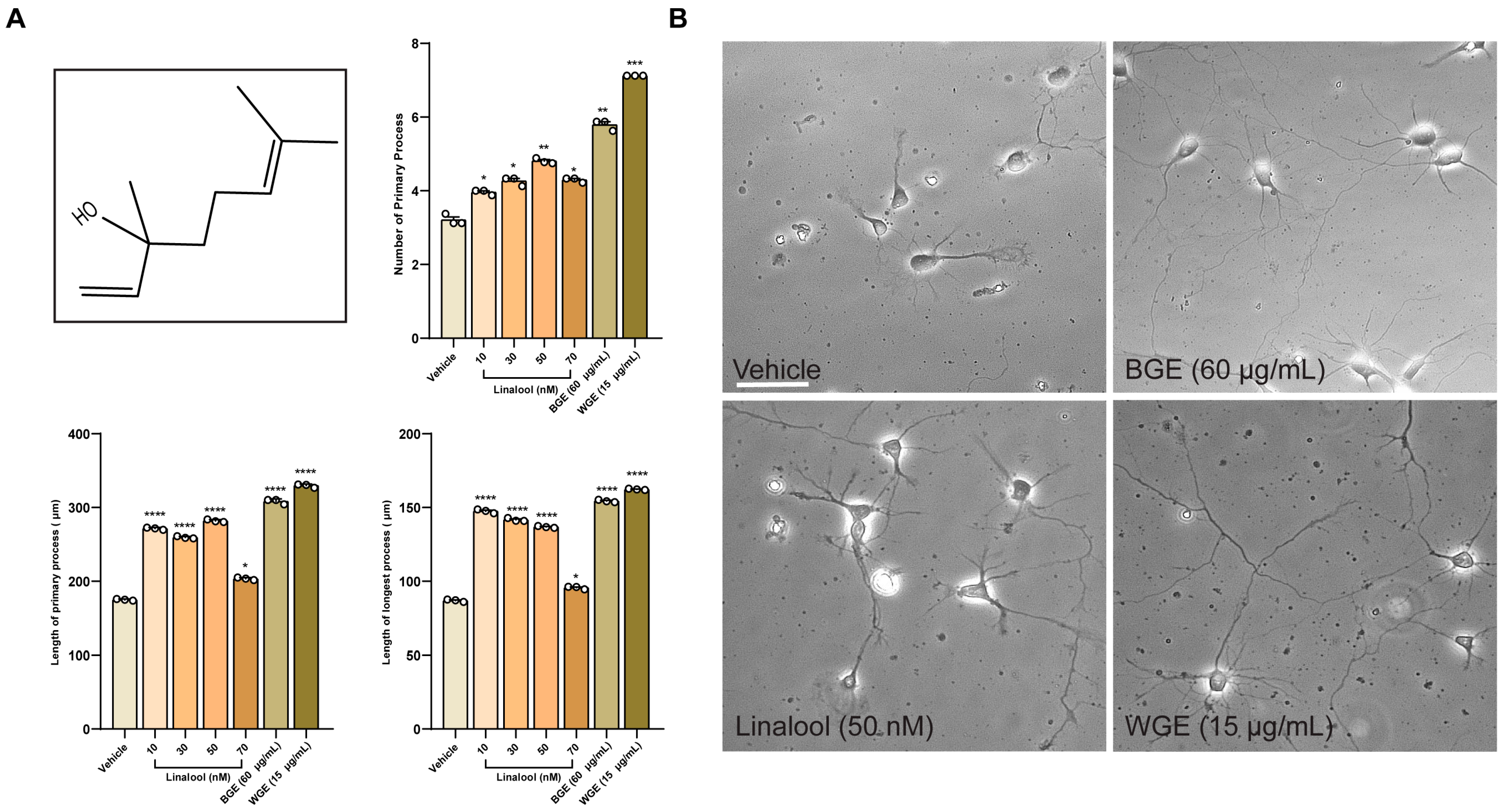
2.7. Phytochemical Profiling of Garlic Extracts by GC/MS Analysis
2.8. Linalool Stimulates Neurite Outgrowth in Hippocampal Neurons
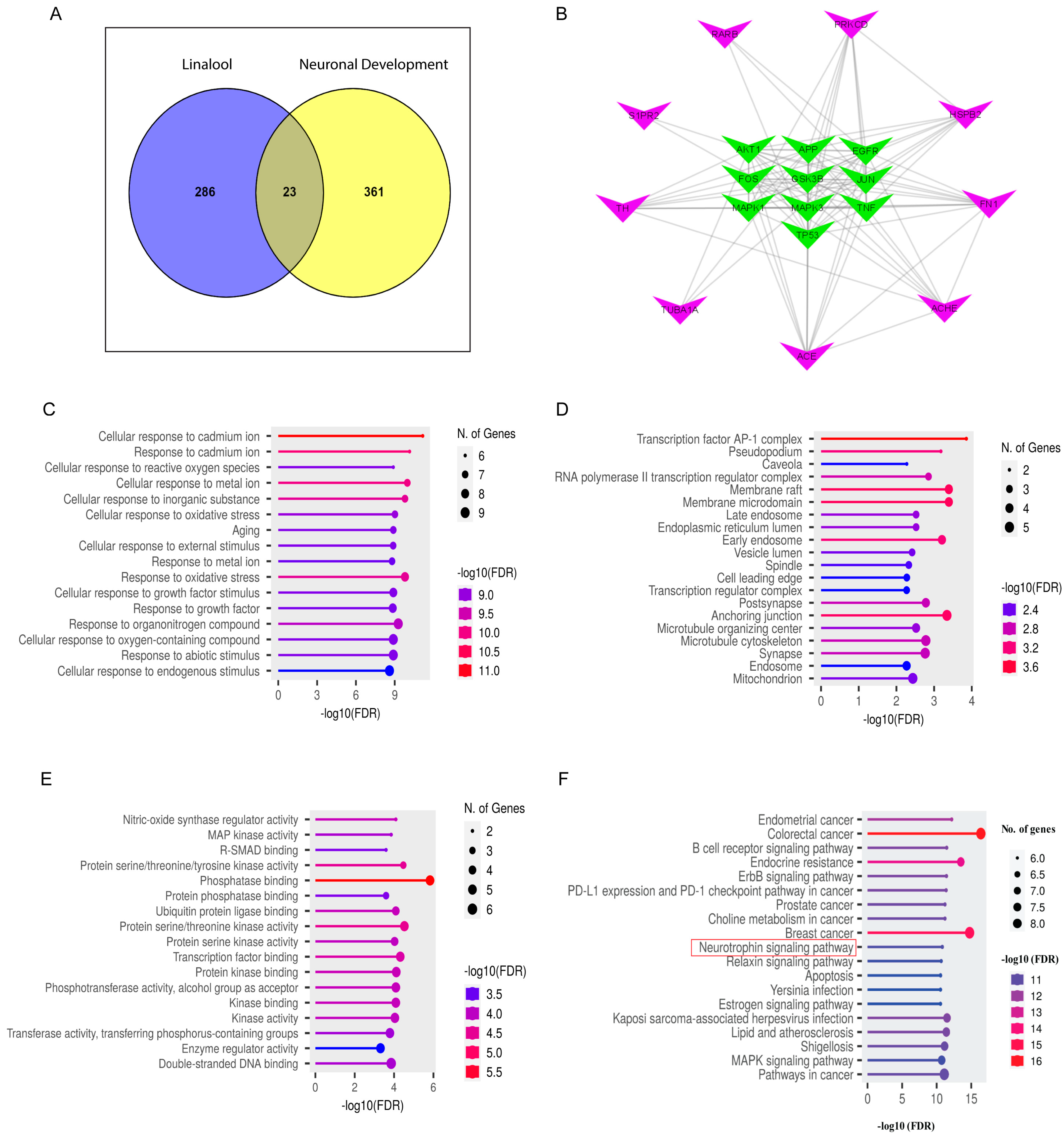
2.9. Network Pharmacology Analysis
2.10. Validation of Target Genes by Immunocytochemistry
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Collection and Extract Preparation of WGE and BGE
4.3. GC-MS Analysis
4.4. Primary Culture of Hippocampal Neurons and Extract/Compound Treatment
4.5. Neuronal Viability
4.6. Immunofluorescence Staining
4.7. Western Blotting
4.8. Image Acquisition, Analysis, and Quantification
4.9. In Silico Network Pharmacology
4.9.1. ADME/T Analysis of Linalool
4.9.2. Screening of the Common Target Genes
4.9.3. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment Analyses
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fan, L.W.; Pang, Y. Dysregulation of neurogenesis by neuroinflammation: Key differences in neurodevelopmental and neurological disorders. Neural Regen. Res. 2017, 12, 366–371. [Google Scholar]
- Kovacs, G.G. Molecular pathology of neurodegenerative diseases: Principles and practice. J. Clin. Pathol. 2019, 72, 725–735. [Google Scholar]
- Sampaio, T.B.; Savall, A.S.; Gutierrez, M.E.Z.; Pinton, S. Neurotrophic factors in alzheimer’s and parkinson’s diseases: Implications for pathogenesis and therapy. Neural Regen. Res. 2017, 12, 549–557. [Google Scholar]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. Bdnf: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [PubMed]
- Schaeffer, E.L.; Novaes, B.A.; da Silva, E.R.; Skaf, H.D.; Mendes-Neto, A.G. Strategies to promote differentiation of newborn neurons into mature functional cells in alzheimer brain. Prog. Neuropsychopharmacol. Biol. Psychiatry 2009, 33, 1087–1102. [Google Scholar] [PubMed]
- Camuso, S.; Canterini, S. Brain-derived neurotrophic factor in main neurodegenerative diseases. Neural Regen. Res. 2023, 18, 554–555. [Google Scholar]
- Hannan, M.A.; Haque, M.N.; Dash, R.; Alam, M.; Moon, I.S. 3β, 6β-dichloro-5-hydroxy-5α-cholestane facilitates neuronal development through modulating trka signaling regulated proteins in primary hippocampal neuron. Sci. Rep. 2019, 9, 18919. [Google Scholar] [CrossRef] [PubMed]
- Bagli, E.; Goussia, A.; Moschos, M.M.; Agnantis, N.; Kitsos, G. Natural compounds and neuroprotection: Mechanisms of action and novel delivery systems. In Vivo 2016, 30, 535–547. [Google Scholar]
- Duangjan, C.; Rangsinth, P.; Zhang, S.; Wink, M.; Tencomnao, T. Anacardium occidentale l. Leaf extracts protect against glutamate/H2O2-induced oxidative toxicity and induce neurite outgrowth: The involvement of sirt1/nrf2 signaling pathway and teneurin 4 transmembrane protein. Front. Pharmacol. 2021, 12, 627738. [Google Scholar] [CrossRef]
- Mitra, S.; Munni, Y.A.; Dash, R.; Sultana, A.; Moon, I.S. Unveiling the effect of withania somnifera on neuronal cytoarchitecture and synaptogenesis: A combined in vitro and network pharmacology approach. Phytother. Res. 2022, 36, 2524–2541. [Google Scholar] [CrossRef]
- Munni, Y.A.; Dash, R.; Mitra, S.; Dash, N.; Shima, M.; Moon, I.S. Mechanistic study of Coriandrum sativum on neuritogenesis and synaptogenesis based on computationally guided in vitro analyses. J. Ethnopharmacol. 2023, 306, 116165. [Google Scholar] [CrossRef]
- Petrovska, B.B.; Cekovska, S. Extracts from the history and medical properties of garlic. Pharmacogn. Rev. 2010, 4, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Gaafar, M.R. Efficacy of Allium sativum (garlic) against experimental cryptosporidiosis. Alexandria J. Med. 2012, 48, 59–66. [Google Scholar] [CrossRef]
- Wijayanto, D.; Nugroho, R.A.; Kurohman, F.; Nursanto, D.B. The effect of garlic (Allium sativum) supplementation in feed on the growth, survival and profits of asian seabass (lates calcarifer) cultivation reared in freshwater media. Aquac. Aquar. Conserv. Legis.-Int. J. Bioflux Soc. 2022, 15, 1882–1890. [Google Scholar]
- Yusuf, A.; Fagbuaro, S.S.; Fajemilehin, S.O.K. Chemical composition, phytochemical and mineral profile of garlic (Allium sativum). J. Biosci. Biotechnol. Discov. 2018, 3, 105–109. [Google Scholar] [CrossRef]
- Kosuge, Y. Neuroprotective mechanisms of s-allyl-l-cysteine in neurological disease. Exp. Ther. Med. 2020, 19, 1565–1569. [Google Scholar] [CrossRef]
- Mocayar Marón, F.J.; Camargo, A.B.; Manucha, W. Allicin pharmacology: Common molecular mechanisms against neuroinflammation and cardiovascular diseases. Life Sci. 2020, 249, 117513. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.S.; Kazmi, I.; Ullah, I.; Muhammad, K.; Anwar, F. Allicin, an antioxidant and neuroprotective agent, ameliorates cognitive impairment. Antioxidants 2021, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Ahangar-Sirous, R.; Poudineh, M.; Ansari, A.; Nili, A.; Dana, S.; Nasiri, Z.; Hosseini, Z.; Karami, D.; Mokhtari, M.; Deravi, N. Pharmacotherapeutic potential of garlic in age-related neurological disorders. CNS Neurol. Disord. Drug Targets 2022, 21, 377–398. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Mossine, V.V.; Cui, J.; Sun, G.Y.; Gu, Z. Protective effects of age and its components on neuroinflammation and neurodegeneration. Neuromol. Med. 2016, 18, 474–482. [Google Scholar] [CrossRef]
- Sripanidkulchai, B. Benefits of aged garlic extract on alzheimer’s disease: Possible mechanisms of action. Exp. Ther. Med. 2020, 19, 1560–1564. [Google Scholar] [CrossRef]
- Flynn, K.C. The cytoskeleton and neurite initiation. Bioarchitecture 2013, 3, 86–109. [Google Scholar] [CrossRef]
- Dotti, C.G.; Sullivan, C.A.; Banker, G.A. The establishment of polarity by hippocampal neurons in culture. J. Neurosci. 1988, 8, 1454–1468. [Google Scholar] [CrossRef]
- Dotti, C.G.; De Anda, F.C.; Gärtner, A. Control of axon and dendrite identity and structure. In Reference Module in Biomedical Research; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Lim, S.; Kaiser, M. Developmental time windows for axon growth influence neuronal network topology. Biol. Cybern. 2015, 109, 275–286. [Google Scholar] [CrossRef]
- Marshall, K.L.; Farah, M.H. Axonal regeneration and sprouting as a potential therapeutic target for nervous system disorders. Neural Regen. Res. 2021, 16, 1901–1910. [Google Scholar] [PubMed]
- Kennedy, M.B. Synaptic signaling in learning and memory. Cold Spring Harb. Perspect. Biol. 2013, 8, a016824. [Google Scholar] [CrossRef]
- Neves, G.; Cooke, S.F.; Bliss, T.V. Synaptic plasticity, memory and the hippocampus: A neural network approach to causality. Nat. Rev. Neurosci. 2008, 9, 65–75. [Google Scholar] [CrossRef]
- Franchini, L.; Carrano, N.; Di Luca, M.; Gardoni, F. Synaptic glun2a-containing nmda receptors: From physiology to pathological synaptic plasticity. Int. J. Mol. Sci. 2020, 21, 1538. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.G. Nmda receptors and memory encoding. Neuropharmacology 2013, 74, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sids, O. Ethylbenzene: Sids Initial Assessment Report for Siam 14; UNEP Publications: Paris, France, 2002; Volume 7, pp. 1–177. [Google Scholar]
- Yuan, C.; Shin, M.; Park, Y.; Choi, B.; Jang, S.; Lim, C.; Yun, H.S.; Lee, I.S.; Won, S.Y.; Cho, K.S. Linalool alleviates aβ42-induced neurodegeneration via suppressing ros production and inflammation in fly and rat models of alzheimer’s disease. Oxidative Med. Cell. Longev. 2021, 2021, 8887716. [Google Scholar] [CrossRef]
- Park, H.; Seol, G.H.; Ryu, S.; Choi, I.Y. Neuroprotective effects of (−)-linalool against oxygen-glucose deprivation-induced neuronal injury. Arch. Pharm. Res. 2016, 39, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, T.; Matsuura, H.; Kodera, Y.; Itakura, Y.; Katsuki, H.; Saito, H.; Nishiyama, N. Neurotrophic activity of organosulfur compounds having a thioallyl group on cultured rat hippocampal neurons. Neurochem. Res. 1997, 22, 1449–1452. [Google Scholar] [CrossRef]
- Zaidi, A.A.; Khan, M.A.; Shahreyar, Z.A.; Ahmed, H. Lauric acid: Its role in behavioral modulation, neuro-inflammatory and oxidative stress markers in haloperidol induced parkinson’s disease. Pak. J. Pharm. Sci. 2020, 33 (Suppl. S2), 755–763. [Google Scholar]
- Song, H.; Cui, J.; Mossine, V.V.; Greenlief, C.M.; Fritsche, K.; Sun, G.Y.; Gu, Z. Bioactive components from garlic on brain resiliency against neuroinflammation and neurodegeneration. Exp. Ther. Med. 2020, 19, 1554–1559. [Google Scholar] [CrossRef]
- Kim, Y.T.; Hur, E.M.; Snider, W.D.; Zhou, F.Q. Role of gsk3 signaling in neuronal morphogenesis. Front. Mol. Neurosci. 2011, 4, 48. [Google Scholar] [CrossRef] [PubMed]
- Hur, E.M.; Zhou, F.Q. Gsk3 signalling in neural development. Nat. Rev. Neurosci. 2010, 11, 539–551. [Google Scholar] [CrossRef]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (gsk3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Cruz, C.D.; Cruz, F. The erk 1 and 2 pathway in the nervous system: From basic aspects to possible clinical applications in pain and visceral dysfunction. Curr. Neuropharmacol. 2007, 5, 244–252. [Google Scholar] [CrossRef]
- Guégan, J.P.; Frémin, C.; Baffet, G. The mapk mek1/2-erk1/2 pathway and its implication in hepatocyte cell cycle control. Int. J. Hepatol. 2012, 2012, 328372. [Google Scholar] [CrossRef]
- Li, Z.; Theus, M.H.; Wei, L. Role of erk 1/2 signaling in neuronal differentiation of cultured embryonic stem cells. Dev. Growth Differ. 2006, 48, 513–523. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Lu, X.; Liu, P.; Qiao, X. Effects of temperature on the quality of black garlic. J. Sci. Food Agric. 2016, 96, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, K.; Nabila, A.; Aktar, A.; Farahnaky, A. Bioactive variability and in vitro and in vivo antioxidant activity of unprocessed and processed flour of nine cultivars of australian lupin species: A comprehensive substantiation. Antioxidants 2020, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Haque, M.N.; Munni, Y.A.; Oktaviani, D.F.; Timalsina, B.; Dash, R.; Afrin, T.; Moon, I.S. Centella asiatica promotes early differentiation, axodendritic maturation and synaptic formation in primary hippocampal neurons. Neurochem. Int. 2021, 144, 104957. [Google Scholar] [CrossRef] [PubMed]
- Moon, I.S.; Apperson, M.L.; Kennedy, M.B. The major tyrosine-phosphorylated protein in the postsynaptic density fraction is n-methyl-d-aspartate receptor subunit 2b. Proc. Natl. Acad. Sci. USA 1994, 91, 3954–3958. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munni, Y.A.; Dash, R.; Choi, H.J.; Mitra, S.; Hannan, M.A.; Mazumder, K.; Timalsina, B.; Moon, I.S. Differential Effects of the Processed and Unprocessed Garlic (Allium sativum L.) Ethanol Extracts on Neuritogenesis and Synaptogenesis in Rat Primary Hippocampal Neurons. Int. J. Mol. Sci. 2023, 24, 13386. https://doi.org/10.3390/ijms241713386
Munni YA, Dash R, Choi HJ, Mitra S, Hannan MA, Mazumder K, Timalsina B, Moon IS. Differential Effects of the Processed and Unprocessed Garlic (Allium sativum L.) Ethanol Extracts on Neuritogenesis and Synaptogenesis in Rat Primary Hippocampal Neurons. International Journal of Molecular Sciences. 2023; 24(17):13386. https://doi.org/10.3390/ijms241713386
Chicago/Turabian StyleMunni, Yeasmin Akter, Raju Dash, Ho Jin Choi, Sarmistha Mitra, Md. Abdul Hannan, Kishor Mazumder, Binod Timalsina, and Il Soo Moon. 2023. "Differential Effects of the Processed and Unprocessed Garlic (Allium sativum L.) Ethanol Extracts on Neuritogenesis and Synaptogenesis in Rat Primary Hippocampal Neurons" International Journal of Molecular Sciences 24, no. 17: 13386. https://doi.org/10.3390/ijms241713386
APA StyleMunni, Y. A., Dash, R., Choi, H. J., Mitra, S., Hannan, M. A., Mazumder, K., Timalsina, B., & Moon, I. S. (2023). Differential Effects of the Processed and Unprocessed Garlic (Allium sativum L.) Ethanol Extracts on Neuritogenesis and Synaptogenesis in Rat Primary Hippocampal Neurons. International Journal of Molecular Sciences, 24(17), 13386. https://doi.org/10.3390/ijms241713386










