Vascular Endothelial Growth Factor-D (VEGF-D): An Angiogenesis Bypass in Malignant Tumors
Abstract
:1. Introduction
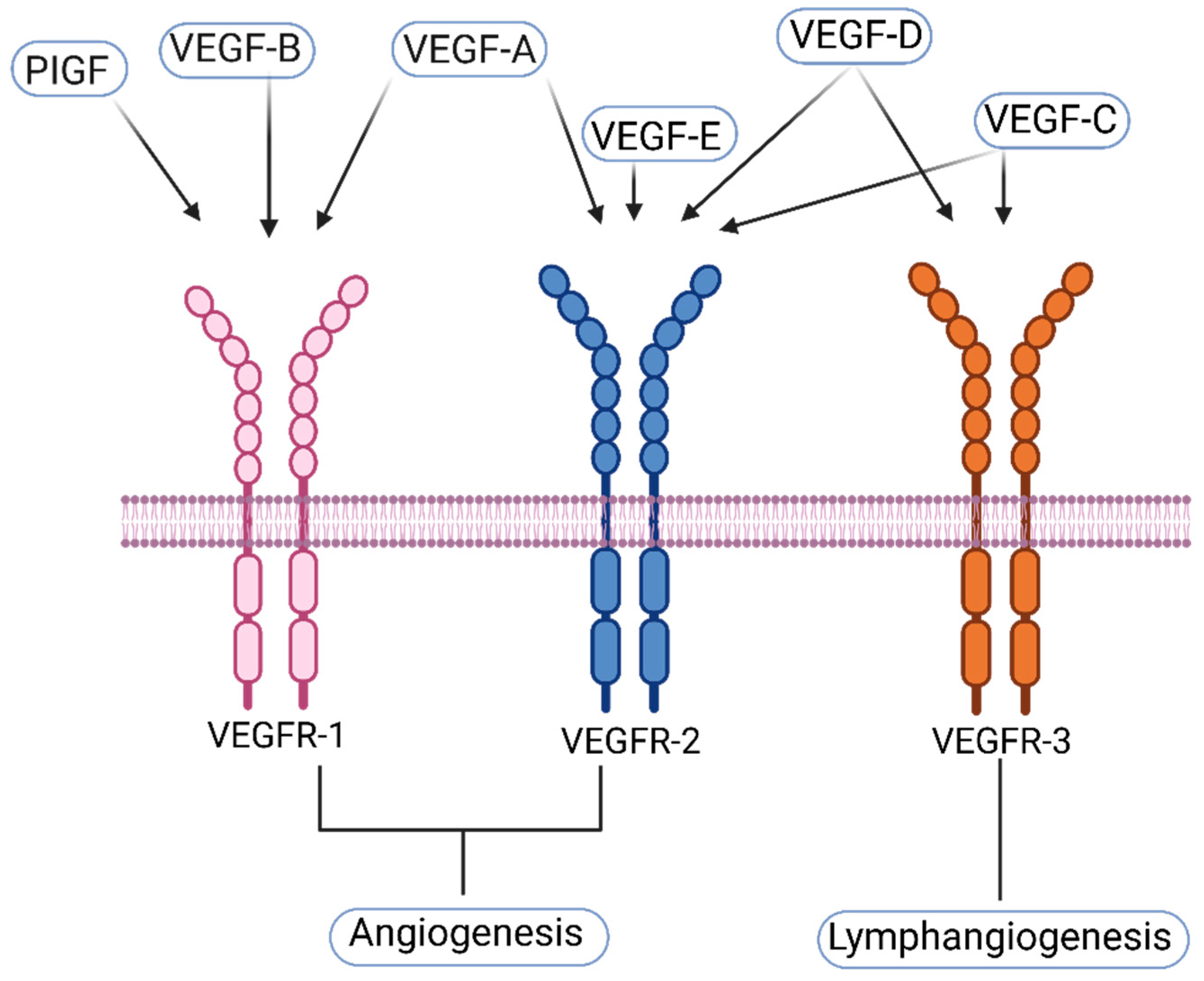
1.1. VEGF-A and Its Receptors
1.2. VEGF-C and Its Receptors
1.3. VEGF-D and Its Receptors
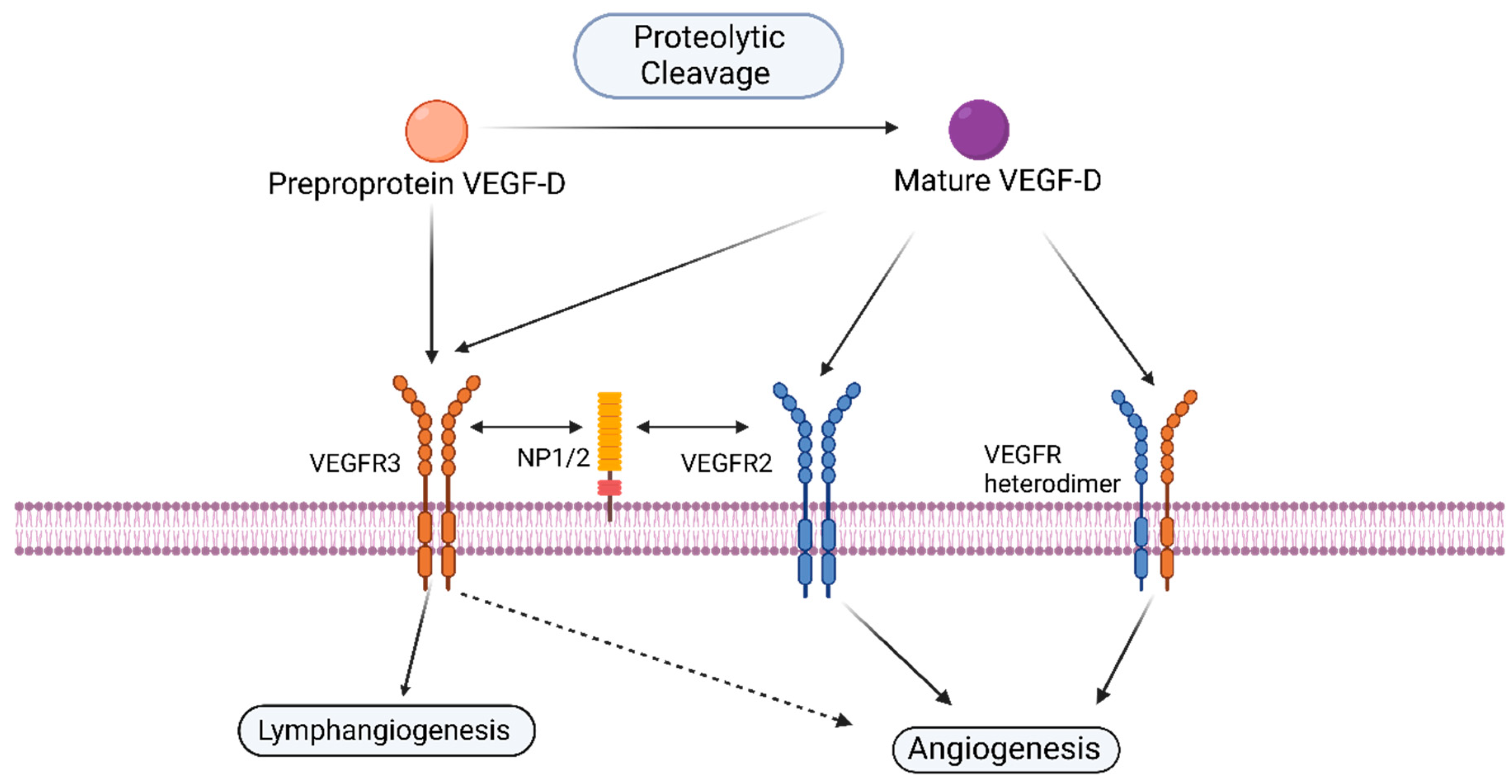
1.4. Other—Less Well Characterized—Members of the VEGF-Family
2. Lymphangiogenesis and Angiogenesis, the Paths Cross?
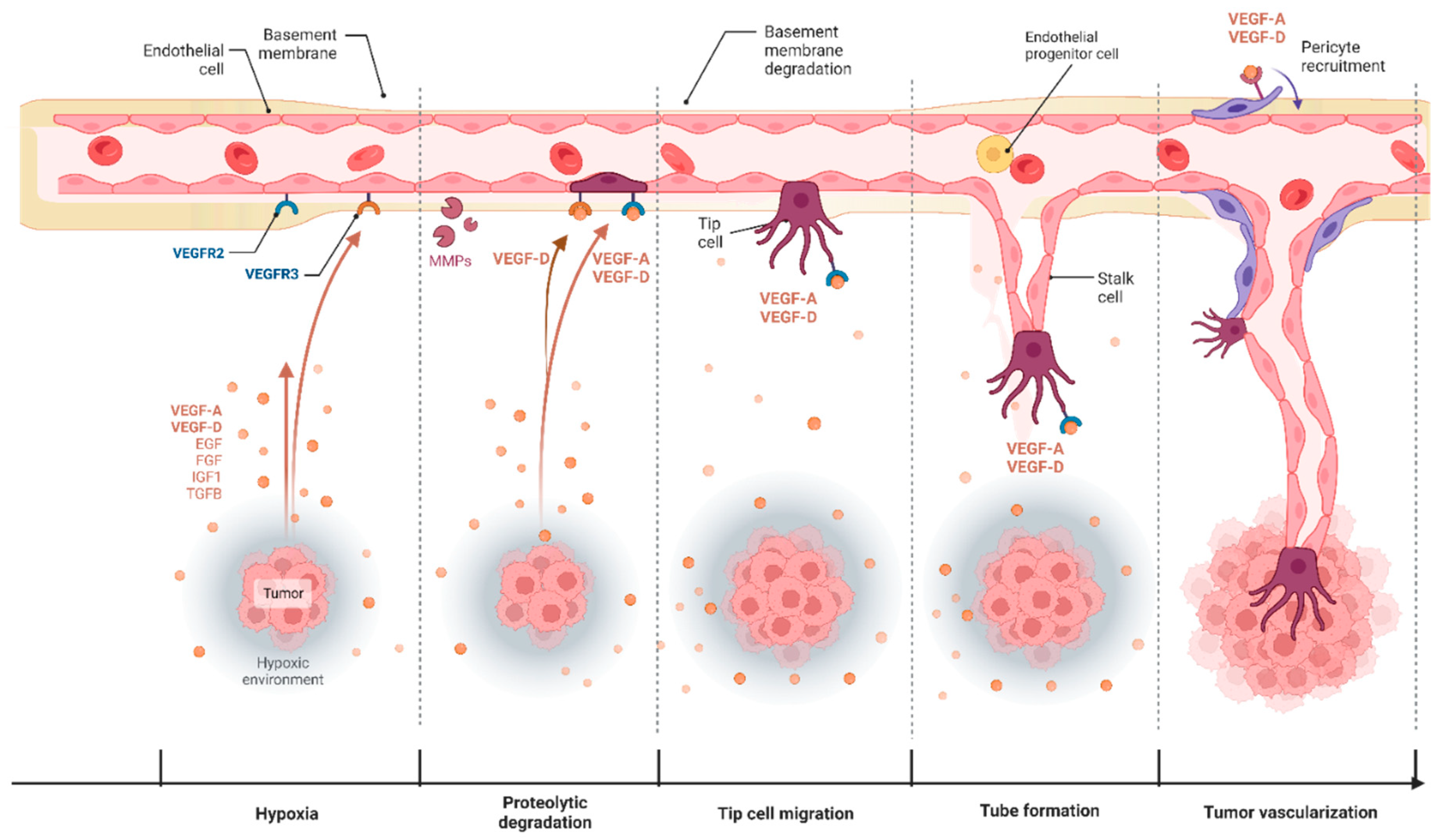
3. Angiogenesis in Cancer and Its Therapeutic Targeting
3.1. VEGF-D and Blood Capillary Angiogenesis
3.2. VEGF-D Mediated Angiogenic Signaling in Cancer
4. Clinical Significance of VEGF-D in Tumor Angiogenesis
5. Current VEGF-D Therapies
- (1)
- The structural similarity between VEGF-D, VEGF-C, and VEGF-A with similar receptor-binding domain (VHD);
- (2)
- The complex VEGF-D signaling through VEGFR-2, VEGFR-3, and NRP-1;
- (3)
- The multiplex proteolytic cleavage of VEGF-D by different proteases providing different isoforms with variable structures.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D.; Folkman, J. Patterns and Emerging Mechanisms of the Angiogenic Switch during Tumorigenesis. Cell 1996, 86, 353–364. [Google Scholar] [CrossRef]
- Ruoslahti, E. Specialization of tumour vasculature. Nat. Rev. Cancer 2002, 2, 83–90. [Google Scholar] [CrossRef]
- Melincovici, C.S.; Boşca, A.B.; Şuşman, S.; Mărginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Raza, A.; Franklin, M.J.; Dudek, A.Z. Pericytes and vessel maturation during tumor angiogenesis and metastasis. Am. J. Hematol. 2010, 85, 593–598. [Google Scholar] [CrossRef]
- Miron, L.; Gafton, B.; Marinca, M. Angiogeneza Tumorală-Implicaţii în Terapia Cancerelor. J. Chir. 2010, 6, 2. [Google Scholar]
- La Mendola, D.; Trincavell, M.L.; Martini, C. Angiogenesis in Disease. Int. J. Mol. Sci. 2022, 23, 10962. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor (VEGF) and its receptor (VEGFR) signaling in angiogenesis: A crucial target for anti- and pro-angiogenic therapies. Genes Cancer 2011, 2, 1097–1105. [Google Scholar] [CrossRef]
- Dakowicz, D.; Zajkowska, M.; Mroczko, B. Relationship between VEGF Family Members, Their Receptors and Cell Death in the Neoplastic Transformation of Colorectal Cancer. Int. J. Mol. Sci. 2022, 23, 3375. [Google Scholar] [CrossRef]
- Hamrah, P.; Chen, L.; Cursiefen, C.; Zhang, Q.; Joyce, N.C.; Dana, M.R. Expression of vascular endothelial growth factor receptor-3 (VEGFR-3) on monocytic bone marrow-derived cells in the conjunctiva. Exp. Eye Res. 2004, 79, 553–561. [Google Scholar] [CrossRef]
- Li, X.; Lee, C.; Tang, Z.; Zhang, F.; Arjunan, P.; Li, Y.; Hou, X.; Kumar, A.; Dong, L. VEGF-B: A survival, or an angiogenic factor? Cell Adhes. Migr. 2009, 3, 322–327. [Google Scholar] [CrossRef]
- Künnapuu, J.; Bokharaie, H.; Jeltsch, M. Proteolytic Cleavages in the VEGF Family: Generating Diversity among Angiogenic VEGFs, Essential for the Activation of Lymphangiogenic VEGFs. Biology 2021, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.C.; Paavonen, K.; Davydova, N.; Roufail, S.; Sato, T.; Karnezis, T.; Stacker, S.A.; Achen, M.G.; Zhang, Y.-F. Proteolytic processing of vascular endothelial growth factor-D is essential for its capacity to promote the growth and spread of cancer. FASEB J. 2011, 25, 2615–2625. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- De Smet, F.; Segura, I.; De Bock, K.; Hohensinner, P.J.; Carmeliet, P. Mechanisms of vessel branching: Filopodia on endothelial tip cells lead the way. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 639–649. [Google Scholar] [CrossRef] [PubMed]
- Thurston, G.; Kitajewski, J. VEGF and Delta-Notch: Interacting signalling pathways in tumour angiogenesis. Br. J. Cancer 2008, 99, 1204–1209. [Google Scholar] [CrossRef]
- Majidpoor, J.; Mortezaee, K. Angiogenesis as a hallmark of solid tumors—Clinical perspectives. Cell. Oncol. 2021, 44, 715–737. [Google Scholar] [CrossRef]
- Uemura, A.; Fruttiger, M.; D’Amore, P.A.; De Falco, S.; Joussen, A.M.; Sennlaub, F.; Brunck, L.R.; Johnson, K.T.; Lambrou, G.N.; Rittenhouse, K.D.; et al. VEGFR1 signaling in retinal angiogenesis and microinflammation. Prog. Retin. Eye Res. 2021, 84, 100954. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): A dual regulator for angiogenesis. Angiogenesis 2006, 9, 225–230. [Google Scholar] [CrossRef]
- Lacal, P.M.; Graziani, G. Therapeutic implication of vascular endothelial growth factor receptor-1 (VEGFR-1) targeting in cancer cells and tumor microenvironment by competitive and non-competitive inhibitors. Pharmacol. Res. 2018, 136, 97–107. [Google Scholar] [CrossRef]
- Ceci, C.; Atzori, M.G.; Lacal, P.M.; Graziani, G. Role of VEGFs/VEGFR-1 Signaling and Its Inhibition in Modulating Tumor Invasion: Experimental Evidence in Different Metastatic Cancer Models. Int. J. Mol. Sci. 2020, 21, 1388. [Google Scholar] [CrossRef]
- Mabeta, P.; Steenkamp, V. The VEGF/VEGFR Axis Revisited: Implications for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 15585. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Prabhu, K.; Krishnankutty, R.; Kuttikrishnan, S.; Tsakou, M.; Alali, F.Q.; Dermime, S.; Mohammad, R.M.; Uddin, S. Vascular Endothelial Growth Factor (VEGF) Signaling in Tumour Vascularization: Potential and Challenges. Curr. Vasc. Pharmacol. 2017, 15, 339–351. [Google Scholar] [CrossRef]
- Wang, X.; Bove, A.M.; Simone, G.; Ma, B. Molecular Bases of VEGFR-2-Mediated Physiological Function and Pathological Role. Front. Cell Dev. Biol. 2020, 8, 599281. [Google Scholar] [CrossRef] [PubMed]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell. Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kitsukawa, T.; Bekku, Y.; Matsuda, Y.; Sanbo, M.; Yagi, T.; Fujisawa, H. A requirement for neuropilin-1 in embryonic vessel formation. Development 1999, 126, 4895–4902. [Google Scholar] [CrossRef]
- Guo, H.-F.; Vander Kooi, C.W. Neuropilin Functions as an Essential Cell Surface Receptor. J. Biol. Chem. 2015, 290, 29120–29126. [Google Scholar] [CrossRef]
- Parker, M.W.; Guo, H.-F.; Li, X.; Linkugel, A.D.; Kooi, C.W.V. Function of Members of the Neuropilin Family as Essential Pleiotropic Cell Surface Receptors. Biochemistry 2012, 51, 9437–9446. [Google Scholar] [CrossRef]
- Peng, K.; Bai, Y.; Zhu, Q.; Hu, B.; Xu, Y. Targeting VEGF–neuropilin interactions: A promising antitumor strategy. Drug Discov. Today 2018, 24, 656–664. [Google Scholar] [CrossRef]
- Islam, R.; Mishra, J.; Bodas, S.; Bhattacharya, S.; Batra, S.K.; Dutta, S.; Datta, K. Role of Neuropilin-2-mediated signaling axis in cancer progression and therapy resistance. Cancer Metastasis Rev. 2022, 41, 771–787. [Google Scholar] [CrossRef]
- Tammela, T.; Zarkada, G.; Wallgard, E.; Murtomäki, A.; Suchting, S.; Wirzenius, M.; Waltari, M.; Hellström, M.; Schomber, T.; Peltonen, R.; et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 2008, 454, 656–660. [Google Scholar] [CrossRef]
- Xu, Z.; Goel, H.L.; Burkart, C.; Burman, L.; Chong, Y.E.; Barber, A.G.; Geng, Y.; Zhai, L.; Wang, M.; Kumar, A.; et al. Inhibition of VEGF binding to neuropilin-2 enhances chemosensitivity and inhibits metastasis in triple-negative breast cancer. Sci. Transl. Med. 2023, 15, eadf1128. [Google Scholar] [CrossRef]
- Goel, H.L.; Mercurio, A.M. VEGF targets the tumour cell. Nat. Rev. Cancer 2013, 13, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Moyon, D.; Pardanaud, L.; Bréant, C.; Karkkainen, M.J.; Alitalo, K.; Eichmann, A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development 2002, 129, 4797–4806. [Google Scholar] [CrossRef] [PubMed]
- Karpanen, T.; Heckman, C.A.; Keskitalo, S.; Jeltsch, M.; Ollila, H.; Neufeld, G.; Tamagnone, L.; Alitalo, K. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. FASEB J. 2006, 20, 1462–1472. [Google Scholar] [CrossRef]
- Chen, J.-C.; Chang, Y.-W.; Hong, C.-C.; Yu, Y.-H.; Su, J.-L. The Role of the VEGF-C/VEGFRs Axis in Tumor Progression and Therapy. Int. J. Mol. Sci. 2012, 14, 88–107. [Google Scholar] [CrossRef]
- Scavelli, C.; Vacca, A.; Di Pietro, G.; Dammacco, F.; Ribatti, D. Crosstalk between angiogenesis and lymphangiogenesis in tumor progression. Leukemia 2004, 18, 1054–1058. [Google Scholar] [CrossRef]
- McColl, B.K.; Baldwin, M.E.; Roufail, S.; Freeman, C.; Moritz, R.L.; Simpson, R.J.; Alitalo, K.; Stacker, S.A.; Achen, M.G. Plasmin Activates the Lymphangiogenic Growth Factors VEGF-C and VEGF-D. J. Exp. Med. 2003, 198, 863–868. [Google Scholar] [CrossRef]
- McColl, B.K.; Paavonen, K.; Karnezis, T.; Harris, N.C.; Davydova, N.; Rothacker, J.; Nice, E.C.; Harder, K.W.; Roufail, S.; Hibbs, M.L.; et al. Proprotein convertases promote processing of VEGF-D, a critical step for binding the angiogenic receptor VEGFR. FASEB J. 2007, 21, 1088–1098. [Google Scholar] [CrossRef]
- Jha, S.K.; Rauniyar, K.; Chronowska, E.; Mattonet, K.; Maina, E.W.; Koistinen, H.; Stenman, U.H.; Alitalo, K.; Jeltsch, M. KLK3/PSA and cathepsin D activate VEGF-C and VEGF-D. eLife 2019, 8, e44478. [Google Scholar] [CrossRef]
- Baldwin, M.E.; Halford, M.M.; Roufail, S.; Williams, R.A.; Hibbs, M.L.; Grail, D.; Kubo, H.; Stacker, S.A.; Achen, M.G. Vascular Endothelial Growth Factor D Is Dispensable for Development of the Lymphatic System. Mol. Cell Biol. 2005, 25, 2441–2449. [Google Scholar] [CrossRef]
- Astin, J.W.; Haggerty, M.J.L.; Okuda, K.S.; Le Guen, L.; Misa, J.P.; Tromp, A.; Hogan, B.M.; Crosier, K.E.; Crosier, P.S. Vegfd can compensate for loss of Vegfc in zebrafish facial lymphatic sprouting. Development 2014, 141, 2680–2690. [Google Scholar] [CrossRef] [PubMed]
- Karkkainen, M.J.; Haiko, P.; Sainio, K.; Partanen, J.; Taipale, J.; Petrova, T.V.; Jeltsch, M.; Jackson, D.G.; Talikka, M.; Rauvala, H.; et al. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from em-bryonic veins. Nat. Immunol. 2004, 5, 74–80. [Google Scholar] [CrossRef]
- Wang, C.-A.; Tsai, S.-J. The non-canonical role of vascular endothelial growth factor-C axis in cancer progression. Exp. Biol. Med. 2015, 240, 718–724. [Google Scholar] [CrossRef]
- Mattila, M.M.-T.; Ruohola, J.K.; Karpanen, T.; Jackson, D.G.; Alitalo, K.; Härkönen, P.L. VEGF-C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF-7 tumors. Int. J. Cancer 2002, 98, 946–951. [Google Scholar] [CrossRef]
- Saharinen, P.; Tammela, T.; Karkkainen, M.J.; Alitalo, K. Lymphatic vasculature: Development, molecular regulation and role in tumor metastasis and inflammation. Trends Immunol. 2004, 25, 387–395. [Google Scholar] [CrossRef]
- Hirakawa, S.; Brown, L.F.; Kodama, S.; Paavonen, K.; Alitalo, K.; Detmar, M. VEGF-C–induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood 2006, 109, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Achen, M.G.; Jeltsch, M.; Kukk, E.; Mäkinen, T.; Vitali, A.; Wilks, A.F.; Alitalo, K.; Stacker, S.A. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl. Acad. Sci. USA 1998, 95, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Orlandini, M.; Marconcini, L.; Ferruzzi, R.; Oliviero, S. Identification of a c-fos-induced gene that is related to the platelet-derived growth factor/vascular endothelial growth factor family. Proc. Natl. Acad. Sci. USA 1996, 93, 11675–11680. [Google Scholar] [CrossRef]
- Avantaggiato, V.; Orlandini, M.; Acampora, D.; Oliviero, S.; Simeone, A. Embryonic expression pattern of the murine figf gene, a growth factor belonging to platelet-derived growth factor/vascular endothelial growth factor family. Mech. Dev. 1998, 73, 221–224. [Google Scholar] [CrossRef]
- Yamada, Y.; Nezu, J.-I.; Shimane, M.; Hirata, Y. Molecular Cloning of a Novel Vascular Endothelial Growth Factor, VEGF-D. Genomics 1997, 42, 483–488. [Google Scholar] [CrossRef]
- Baldwin, M.E.; Roufail, S.; Halford, M.M.; Alitalo, K.; Stacker, S.A.; Achen, M.G. Multiple forms of mouse vascular endothelial growth factor-D are generated by RNA splicing and proteolysis. J. Biol. Chem. 2001, 276, 44307–44314. [Google Scholar] [CrossRef]
- Alitalo, K.; Tammela, T.; Petrova, T.V. Lymphangiogenesis in development and human disease. Nature 2005, 438, 946–953. [Google Scholar] [CrossRef]
- Stacker, S.A.; Stenvers, K.; Caesar, C.; Vitali, A.; Domagala, T.; Nice, E.; Roufail, S.; Simpson, R.J.; Moritz, R.; Karpanen, T.; et al. Biosynthesis of Vascular Endothelial Growth Factor-D Involves Proteolytic Processing Which Generates Non-covalent Homodimers. J. Biol. Chem. 1999, 274, 32127–32136. [Google Scholar] [CrossRef]
- Byzova, T.V.; Goldman, C.K.; Jankau, J.; Chen, J.; Cabrera, G.; Achen, M.G.; Stacker, S.A.; Carnevale, K.A.; Siemionow, M.; Deitcher, S.R.; et al. Adenovirus encoding vascular endothelial growth factor–D induces tissue-specific vascular patterns in vivo. Blood 2002, 99, 4434–4442. [Google Scholar] [CrossRef]
- Baldwin, M.E.; Catimel, B.; Nice, E.C.; Roufail, S.; Hall, N.E.; Stenvers, K.L.; Karkkainen, M.J.; Alitalo, K.; Stacker, S.A.; Achen, M.G. The Specificity of Receptor Binding by Vascular Endothelial Growth Factor-D Is Different in Mouse and Man. J. Biol. Chem. 2001, 276, 19166–19171. [Google Scholar] [CrossRef]
- Witmer, A.N.; van Blijswijk, B.C.; Dai, J.; Hofman, P.; Partanen, T.A.; Vrensen, G.F.J.M.; Schlingemann, R.O. VEGFR-3 in adult angiogenesis. J. Pathol. 2001, 195, 490–497. [Google Scholar] [CrossRef]
- Jia, H.; Bagherzadeh, A.; Bicknell, R.; Duchen, M.R.; Liu, D.; Zachary, I. Vascular Endothelial Growth Factor (VEGF)-D and VEGF-A Differentially Regulate KDR-mediated Signaling and Biological Function in Vascular Endothelial Cells. J. Biol. Chem. 2004, 279, 36148–36157. [Google Scholar] [CrossRef]
- Nilsson, I.; Bahram, F.; Li, X.; Gualandi, L.; Koch, S.; Jarvius, M.; Söderberg, O.; Anisimov, A.; Kholová, I.; Pytowski, B.; et al. VEGF receptor 2/-3 heterodimers detected in situ by proximity ligation on angiogenic sprouts. EMBO J. 2010, 29, 1377–1388. [Google Scholar] [CrossRef]
- Harris, N.C.; Davydova, N.; Roufail, S.; Paquet-Fifield, S.; Paavonen, K.; Karnezis, T.; Zhang, Y.-F.; Sato, T.; Rothacker, J.; Nice, E.C.; et al. The Propeptides of VEGF-D Determine Heparin Binding, Receptor Heterodimerization, and Effects on Tumor Biology. J. Biol. Chem. 2013, 288, 8176–8186. [Google Scholar] [CrossRef]
- Han, Z.; Jiang, G.; Zhang, Y.; Xu, J.; Chen, C.; Zhang, L.; Xu, Z.; DU, X. Effects of RNA interference-mediated NRP-1 silencing on the proliferation and apoptosis of breast cancer cells. Mol. Med. Rep. 2012, 12, 513–519. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, H.; Lu, L.; Wang, L.; Zhang, X.; Guo, X. New insights into the role of co-receptor neuropilins in tumour angiogenesis and lymphangiogenesis and targeted therapy strategies. J. Drug Target. 2021, 29, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Moessinger, C.; Nilsson, I.; Muhl, L.; Zeitelhofer, M.; Sahlgren, B.H.; Skogsberg, J.; Eriksson, U. VEGF-B signaling impairs endothelial glucose transcytosis by decreasing membrane cholesterol content. EMBO Rep. 2020, 21, e49343. [Google Scholar] [CrossRef] [PubMed]
- Ramjiawan, R.R.; Griffioen, A.W.; Duda, D.G. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 2017, 20, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Lee, C.; Lin, X.; Zhao, C.; Li, X. Novel function of VEGF-B as an antioxidant and therapeutic implications. Pharmacol. Res. 2019, 143, 33–39. [Google Scholar] [CrossRef]
- Robciuc, M.R.; Kivelä, R.; Williams, I.M.; de Boer, J.F.; van Dijk, T.H.; Elamaa, H.; Tigistu-Sahle, F.; Molotkov, D.; Leppänen, V.-M.; Käkelä, R.; et al. VEGFB/VEGFR1-Induced Expansion of Adipose Vasculature Counteracts Obesity and Related Metabolic Complications. Cell Metab. 2016, 23, 712–724. [Google Scholar] [CrossRef]
- Ogawa, S.; Oku, A.; Sawano, A.; Yamaguchi, S.; Yazaki, Y.; Shibuya, M. A Novel Type of Vascular Endothelial Growth Factor, VEGF-E (NZ-7 VEGF), Preferentially Utilizes KDR/Flk-1 Receptor and Carries a Potent Mitotic Activity without Heparin-binding Domain. J. Biol. Chem. 1998, 273, 31273–31282. [Google Scholar] [CrossRef]
- Brouillet, S.; Hoffmann, P.; Benharouga, M.; Salomon, A.; Schaal, J.-P.; Feige, J.-J.; Alfaidy, N.; Hellesøy, M.; Lorens, J.B.; Yap, M.E.A.; et al. Molecular Characterization of EG-VEGF-mediated Angiogenesis: Differential Effects on Microvascular and Macrovascular Endothelial Cells. Mol. Biol. Cell 2010, 21, 2832–2843. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor receptor-2: Its unique signaling and specific ligand, VEGF-E. Cancer Sci. 2003, 94, 751–756. [Google Scholar] [CrossRef]
- Komori, Y.; Nikai, T.; Taniguchi, K.; Masuda, K.; Sugihara, H. Vascular Endothelial Growth Factor VEGF-like Heparin-Binding Protein from the Venom of Vipera aspis aspis (Aspic Viper). Biochemistry 1999, 38, 11796–11803. [Google Scholar] [CrossRef]
- Ferreira, I.G.; Pucca, M.B.; de Oliveira, I.S.; Cerni, F.A.; Jacob, B.d.C.d.S.; Arantes, E.C. Snake venom vascular endothelial growth factors (svVEGFs): Unravelling their molecular structure, functions, and research potential. Cytokine Growth Factor Rev. 2021, 60, 133–143. [Google Scholar] [CrossRef]
- De Falco, S. The discovery of placenta growth factor and its biological activity. Exp. Mol. Med. 2012, 44, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Macarulla, T.; Montagut, C.; Sánchez-Martin, F.J.; Granja, M.; Verdaguer, H.; Sastre, J.; Tabernero, J. The role of PIGF blockade in the treatment of colorectal cancer: Overcoming the pitfalls. Expert Opin. Biol. Ther. 2019, 20, 15–22. [Google Scholar] [CrossRef]
- Krebs, R.; Jeltsch, M. The lymphangiogenic growth factors VEGF-C and VEGF-D. LymphForsch 2013, 17, 30–37. [Google Scholar]
- Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 2005, 438, 932–936. [Google Scholar] [CrossRef]
- Oliver, G.; Kipnis, J.; Randolph, G.J.; Harvey, N.L. The Lymphatic Vasculature in the 21st Century: Novel Functional Roles in Homeostasis and Disease. Cell 2020, 182, 270–296. [Google Scholar] [CrossRef] [PubMed]
- Jeltsch, M.; Tammela, T.; Alitalo, K.; Wilting, J. Genesis and pathogenesis of lymphatic vessels. Cell Tissue Res. 2003, 314, 69–84. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, J.; Wu, F.; Wei, F.; Han, W.; Xu, X.; Zhang, Y. Current Status of Lymphangiogenesis: Molecular Mechanism, Immune Tolerance, and Application Prospect. Cancers 2023, 15, 1169. [Google Scholar] [CrossRef]
- Suarez, A.C.; Hammel, J.H.; Munson, J.M. Modeling lymphangiogenesis: Pairing in vitro and in vivo metrics. Microcirculation 2023, 30, e12802. [Google Scholar] [CrossRef]
- Patnam, M.; Dommaraju, S.R.; Masood, F.; Herbst, P.; Chang, J.-H.; Hu, W.-Y.; Rosenblatt, M.I.; Azar, D.T. Lymphangiogenesis Guidance Mechanisms and Therapeutic Implications in Pathological States of the Cornea. Cells 2023, 12, 319. [Google Scholar] [CrossRef]
- Wang, C.; Chu, M. Advances in Drugs Targeting Lymphangiogenesis for Preventing Tumor Progression and Metastasis. Front. Oncol. 2022, 11, 783309. [Google Scholar] [CrossRef]
- Cadamuro, M.; Romanzi, A.; Guido, M.; Sarcognato, S.; Cillo, U.; Gringeri, E.; Zanus, G.; Strazzabosco, M.; Simioni, P.; Villa, E.; et al. Translational Value of Tumor-Associated Lymphangiogenesis in Cholangiocarcinoma. J. Pers. Med. 2022, 12, 1086. [Google Scholar] [CrossRef] [PubMed]
- Schito, L. Hypoxia-Dependent Angiogenesis and Lymphangiogenesis in Cancer, in Hypoxia and Cancer Metastasis; Gilkes, D.M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 71–85. [Google Scholar]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular Mechanisms and Future Promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Suresh, R.; Diaz, R.J. The remodelling of actin composition as a hallmark of cancer. Transl. Oncol. 2021, 14, 101051. [Google Scholar] [CrossRef] [PubMed]
- Hida, K.; Maishi, N.; Annan, D.A.; Hida, Y. Contribution of Tumor Endothelial Cells in Cancer Progression. Int. J. Mol. Sci. 2018, 19, 1272. [Google Scholar] [CrossRef] [PubMed]
- Al-Ostoot, F.H.; Salah, S.; Khamees, H.A.; Khanum, S.A. Tumor Angiogenesis: Current Challenges and Therapeutic Opportunities (Review). Cancer Treat. Res. Commun. 2021, 28, 100422. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, R.; Dave, J.M.; Chandran, R.R.; Misra, A.; Sheikh, A.Q.; Greif, D.M. Vascular cells in blood vessel wall development and disease. Adv. Pharmacol. 2017, 78, 323–350. [Google Scholar]
- Cai, H.; Gong, L.; Liu, J.; Zhou, Q.; Zheng, Z. Diosgenin inhibits tumor angiogenesis through regulating GRP78-mediated HIF-1α and VEGF/VEGFR signaling pathways. Die Pharm. Int. J. Pharm. Sci. 2019, 74, 680–684. [Google Scholar] [CrossRef]
- Domigan, C.K.; Ziyad, S.; Iruela-Arispe, M.L. Canonical and noncanonical vascular endothelial growth factor pathways: New developments in biology and signal transduction. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 30–39. [Google Scholar] [CrossRef]
- Vimalraj, S. A concise review of VEGF, PDGF, FGF, Notch, angiopoietin, and HGF signalling in tumor angiogenesis with a focus on alternative approaches and future directions. Int. J. Biol. Macromol. 2022, 221, 1428–1438. [Google Scholar] [CrossRef]
- Qi, S.; Deng, S.; Lian, Z.; Yu, K. Novel Drugs with High Efficacy against Tumor Angiogenesis. Int. J. Mol. Sci. 2022, 23, 6934. [Google Scholar] [CrossRef] [PubMed]
- Iruela-Arispe, M.L.; Davis, G.E. Cellular and Molecular Mechanisms of Vascular Lumen Formation. Dev. Cell 2009, 16, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E.; Tournier-Lasserve, E.; Weinstein, B.M. The Control of Vascular Integrity by Endothelial Cell Junctions: Molecular Basis and Pathological Implications. Dev. Cell 2009, 16, 209–221. [Google Scholar] [CrossRef]
- Lees, D.M.; Reynolds, L.E.; Pedrosa, A.R.; Roy-Luzarraga, M.; Hodivala-Dilke, K.M. Phosphorylation of pericyte FAK-Y861 affects tumour cell apoptosis and tumour blood vessel regression. Angiogenesis 2021, 24, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Maishi, N.; Annan, D.A.; Kikuchi, H.; Hida, Y.; Hida, K. Tumor Endothelial Heterogeneity in Cancer Progression. Cancers 2019, 11, 1511. [Google Scholar] [CrossRef]
- Taleb, M.; Mohammadkhani, N.; Bahreini, F.; Ovais, M.; Nie, G. Modulation of Tumor Vasculature Network: Key Strategies. Small Struct. 2022, 3, 2100164. [Google Scholar] [CrossRef]
- Ozel, I.; Duerig, I.; Domnich, M.; Lang, S.; Pylaeva, E.; Jablonska, J. The Good, the Bad, and the Ugly: Neutrophils, Angiogenesis, and Cancer. Cancers 2022, 14, 536. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, L.; Nie, L.; Lin, H. Unraveling the molecular mechanisms between inflammation and tumor angiogenesis. Am. J. Cancer Res. 2021, 11, 301–317. [Google Scholar]
- Rajabi, M.; Mousa, S.A. The Role of Angiogenesis in Cancer Treatment. Biomedicines 2017, 5, 34. [Google Scholar] [CrossRef]
- Ferrara, N.; Hillan, K.J.; Gerber, H.-P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef]
- Garcia, J.; Hurwitz, H.I.; Sandler, A.B.; Miles, D.; Coleman, R.L.; Deurloo, R.; Chinot, O.L. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat. Rev. 2020, 86, 102017. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Lin, Y.; Wang, C.; Deng, L.; Chen, M.; Assaraf, Y.G.; Chen, Z.-S.; Ye, W.; Zhang, D. New insights into antiangiogenic therapy resistance in cancer: Mechanisms and therapeutic aspects. Drug Resist. Updat. 2022, 64, 100849. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-J.; Zhao, H.-C.; Hou, S.-J.; Zhang, H.-J.; Cheng, L.; Yuan, S.; Zhang, L.-R.; Song, J.; Zhang, S.-Y.; Chen, S.-W. Recent development of multi-target VEGFR-2 inhibitors for the cancer therapy. Bioorg. Chem. 2023, 133, 106425. [Google Scholar] [CrossRef] [PubMed]
- Welti, J.; Loges, S.; Dimmeler, S.; Carmeliet, P. Recent molecular discoveries in angiogenesis and antiangiogenic therapies in cancer. J. Clin. Investig. 2013, 123, 3190–3200. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K. Antiangiogenesis Strategies Revisited: From Starving Tumors to Alleviating Hypoxia. Cancer Cell 2014, 26, 605–622. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nat. Rev. Cancer 2008, 8, 592–603. [Google Scholar] [CrossRef]
- Huijbers, E.J.; van Beijnum, J.R.; Thijssen, V.L.; Sabrkhany, S.; Nowak-Sliwinska, P.; Griffioen, A.W. Role of the tumor stroma in resistance to anti-angiogenic therapy. Drug Resist. Updat. 2016, 25, 26–37. [Google Scholar] [CrossRef]
- Cascone, T.; Herynk, M.H.; Xu, L.; Du, Z.; Kadara, H.; Nilsson, M.B.; Oborn, C.J.; Park, Y.-Y.; Erez, B.; Jacoby, J.J.; et al. Upregulated stromal EGFR and vascular remodeling in mouse xenograft models of angiogenesis inhibitor–resistant human lung adenocarcinoma. J. Clin. Investig. 2011, 121, 1313–1328. [Google Scholar] [CrossRef]
- Mashima, T.; Wakatsuki, T.; Kawata, N.; Jang, M.-K.; Nagamori, A.; Yoshida, H.; Nakamura, K.; Migita, T.; Seimiya, H.; Yamaguchi, K. Neutralization of the induced VEGF-A potentiates the therapeutic effect of an anti-VEGFR2 antibody on gastric cancer in vivo. Sci. Rep. 2021, 11, 15125. [Google Scholar] [CrossRef]
- Moffat, B.A.; Chen, M.; Kariaapper, M.S.; Hamstra, D.A.; Hall, D.E.; Stojanovska, J.; Johnson, T.D.; Blaivas, M.; Kumar, M.; Chenevert, T.L.; et al. Inhibition of Vascular Endothelial Growth Factor (VEGF)-A Causes a Paradoxical Increase in Tumor Blood Flow and Up-Regulation of VEGF-D. Clin. Cancer Res. 2006, 12, 1525–1532. [Google Scholar] [CrossRef]
- Jain, R.K. Normalization of Tumor Vasculature: An Emerging Concept in Antiangiogenic Therapy. Science 2005, 307, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Suda, G.; Maehara, O.; Ohara, M.; Yoda, T.; Sasaki, T.; Kohya, R.; Yoshida, S.; Hosoda, S.; Tokuchi, Y.; et al. Changes in Serum Growth Factors during Resistance to Atezolizumab Plus Bevacizumab Treatment in Patients with Unresectable Hepatocellular Carcinoma. Cancers 2023, 15, 593. [Google Scholar] [CrossRef]
- Marconcini, L.; Marchiò, S.; Morbidelli, L.; Cartocci, E.; Albini, A.; Ziche, M.; Bussolino, F.; Oliviero, S. c-fos-induced growth factor/vascular endothelial growth factor D induces angiogenesis in vivo and in vitro. Proc. Natl. Acad. Sci. USA 1999, 96, 9671–9676. [Google Scholar] [CrossRef] [PubMed]
- Stacker, S.A.; Caesar, C.; Baldwin, M.E.; Thornton, G.E.; Williams, R.A.; Prevo, R.; Jackson, D.G.; Nishikawa, S.-I.; Kubo, H.; Achen, M.G. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat. Med. 2001, 7, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Rissanen, T.T.; Markkanen, J.E.; Gruchala, M.; Heikura, T.; Puranen, A.; Kettunen, M.I.; Kholová, I.; Kauppinen, R.A.; Achen, M.G.; Stacker, S.A.; et al. VEGF-D Is the Strongest Angiogenic and Lymphangiogenic Effector Among VEGFs Delivered into Skeletal Muscle via Adenoviruses. Circ. Res. 2003, 92, 1098–1106. [Google Scholar] [CrossRef]
- Rutanen, J.; Rissanen, T.T.; Markkanen, J.E.; Gruchala, M.; Silvennoinen, P.; Kivelä, A.; Hedman, A.; Hedman, M.; Heikura, T.; Ordén, M.-R.; et al. Adenoviral Catheter-Mediated Intramyocardial Gene Transfer Using the Mature Form of Vascular Endothelial Growth Factor-D Induces Transmural Angiogenesis in Porcine Heart. Circulation 2004, 109, 1029–1035. [Google Scholar] [CrossRef]
- Nag, S.; Manias, J.; Eubanks, J.H.; Stewart, D.J. Increased Expression of Vascular Endothelial Growth Factor-D Following Brain Injury. Int. J. Mol. Sci. 2019, 20, 1594. [Google Scholar] [CrossRef]
- Bower, N.I.; Vogrin, A.J.; Le Guen, L.; Chen, H.; Stacker, S.A.; Achen, M.G.; Hogan, B.M. Vegfd modulates both angiogenesis and lymphangiogenesis during zebrafish embryonic development. Development 2017, 144, 507–518. [Google Scholar] [CrossRef]
- Gangadaran, P.; Rajendran, R.L.; Oh, J.M.; Oh, E.J.; Hong, C.M.; Chung, H.Y.; Lee, J.; Ahn, B.C. Identification of Angiogenic Cargo in Extracellular Vesicles Secreted from Human Adipose Tis-sue-Derived Stem Cells and Induction of Angiogenesis In Vitro and In Vivo. Pharmaceutics 2021, 13, 495. [Google Scholar] [CrossRef]
- Hanrahan, V.; Currie, M.J.; Gunningham, S.P.; Morrin, H.R.; Scott, P.A.; Robinson, B.A.; Fox, S.B. The angiogenic switch for vascular endothelial growth factor (VEGF)-A, VEGF-B, VEGF-C, and VEGF-D in the adenoma–carcinoma sequence during colorectal cancer progression. J. Pathol. 2003, 200, 183–194. [Google Scholar] [CrossRef]
- Debinski, W.; Slagle-Webb, B.; Achen, M.G.; Stacker, S.A.; Tulchinsky, E.; Gillespie, G.Y.; Gibo, D.M. VEGF-D is an X-linked/AP-1 Regulated Putative Onco-angiogen in Human Glioblastoma Multiforme. Mol. Med. 2001, 7, 598–608. [Google Scholar] [CrossRef]
- Badodekar, N.; Sharma, A.; Patil, V.; Telang, G.; Sharma, R.; Patil, S.; Vyas, N.; Somasundaram, I. Angiogenesis induction in breast cancer: A paracrine paradigm. Cell Biochem. Funct. 2021, 39, 860–873. [Google Scholar] [CrossRef]
- González-González, A.; González, A.; Rueda, N.; Alonso-González, C.; Menéndez, J.M.; Martínez-Campa, C.; Mitola, S.; Cos, S. Usefulness of melatonin as complementary to chemotherapeutic agents at different stages of the angiogenic process. Sci. Rep. 2020, 10, 4790. [Google Scholar] [CrossRef]
- Teng, X.; Li, D.; Johns, R.A. Hypoxia up-regulates mouse vascular endothelial growth factor D promoter activity in rat pulmonary microvascular smooth-muscle cells. Chest 2002, 121, 82S–83S. [Google Scholar] [CrossRef]
- Achen, M.G.; Williams, R.A.; Baldwin, M.E.; Lai, P.; Roufail, S.; Alitalo, K.; Stacker, S.A. The Angiogenic and Lymphangiogenic Factor Vascular Endothelial Growth Factor-D Exhibits a Paracrine Mode of Action in Cancer. Growth Factors 2002, 20, 99–107. [Google Scholar] [CrossRef]
- Achen, M.G.; Williams, R.A.; Minekus, M.P.; Thornton, G.E.; Stenvers, K.; Rogers, P.A.W.; Lederman, F.; Roufail, S.; Stacker, S.A. Localization of vascular endothelial growth factor-D in malignant melanoma suggests a role in tumour angiogenesis. J. Pathol. 2000, 193, 147–154. [Google Scholar] [CrossRef]
- Vacca, A.; Ria, R.; Ribatti, D.; Semeraro, F.; Djonov, V.; Di Raimondo, F.; Dammacco, F. A paracrine loop in the vascular endothelial growth factor pathway triggers tumor angiogenesis and growth in multiple myeloma. Haematologica 2003, 88, 176–185. [Google Scholar]
- Yokoyama, Y.; Charnock-Jones, D.S.; Licence, D.; Yanaihara, A.; Hastings, J.M.; Holland, C.M.; Emoto, M.; Sakamoto, A.; Sakamoto, T.; Maruyama, H.; et al. Expression of vascular endothelial growth factor (VEGF)-D and its receptor, VEGF receptor 3, as a prognostic factor in endometrial carcinoma. Clin. Cancer Res. 2003, 9, 1361–1369. [Google Scholar]
- Yu, H.; Zhang, S.; Zhang, R.; Zhang, L. The role of VEGF-C/D and Flt-4 in the lymphatic metastasis of early-stage invasive cervical carcinoma. J. Exp. Clin. Cancer Res. 2009, 28, 96–98. [Google Scholar] [CrossRef]
- Papiewska-Pajak, I.; Boncela, J.; Przygodzka, P.; Cierniewski, C.S. Autocrine effects of VEGF-D on endothelial cells after transduction with AD-VEGF-DΔNΔC. Exp. Cell Res. 2010, 316, 907–914. [Google Scholar] [CrossRef]
- Pan, T.; Jin, Z.; Yu, Z.; Wu, X.; Chang, X.; Fan, Z.; Li, F.; Wang, X.; Li, Z.; Zhou, Q.; et al. Cathepsin L promotes angiogenesis by regulating the CDP/Cux/VEGF-D pathway in human gastric cancer. Gastric Cancer 2020, 23, 974–987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Pang, X.; Lei, L.; Zhang, J.; Zhang, X.; Chen, Z.; Zhu, J.; Jiang, Y.; Chen, G.; Wu, Y.; et al. LncRNA CRART16/miR-122-5p/FOS axis promotes angiogenesis of gastric cancer by upregulating VEGFD expression. Aging 2022, 14, 4137–4157. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Partanen, T.A.; Alitalo, K.; Miettinen, M. Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor 3 in 185 vascular tumors. Cancer 1999, 86, 2406–2412. [Google Scholar] [CrossRef]
- Kubo, H.; Fujiwara, T.; Jussila, L.; Hashi, H.; Ogawa, M.; Shimizu, K.; Awane, M.; Sakai, Y.; Takabayashi, A.; Alitalo, K.; et al. Involvement of vascular endothelial growth factor receptor-3 in maintenance of integrity of endothelial cell lining during tumor angiogenesis. Blood 2000, 96, 546–553. [Google Scholar] [CrossRef]
- Valtola, R.; Salven, P.; Heikkilä, P.; Taipale, J.; Joensuu, H.; Rehn, M.; Pihlajaniemi, T.; Weich, H.; Dewaal, R.; Alitalo, K. VEGFR-3 and Its Ligand VEGF-C Are Associated with Angiogenesis in Breast Cancer. Am. J. Pathol. 1999, 154, 1381–1390. [Google Scholar] [CrossRef]
- Niki, T.; Iba, S.; Yamada, T.; Matsuno, Y.; Enholm, B.; Hirohashi, S. Expression of vascular endothelial growth factor receptor 3 in blood and lymphatic vessels of lung adenocar-cinoma. J. Pathol. 2001, 193, 450–457. [Google Scholar] [CrossRef]
- Uhlén, M.; Fagerberg, L.; Hallström, B.M.; Lindskog, C.; Oksvold, P.; Mardinoglu, A.; Sivertsson, Å.; Kampf, C.; Sjöstedt, E.; Asplund, A.; et al. Proteomics. Tissue-Based Map of the Human Proteome. Science 2015, 347, 1260419. [Google Scholar] [CrossRef]
- Hata, T.; Sugimoto, N.; Ohara, N.; Miyo, M.; Yoshioka, S.; Kagawa, Y.; Naito, A.; Tei, M.; Tamagawa, H.; Konishi, K.; et al. Clinical development and evaluation of plasma angiogenesis factors from phase II study of FOLFIRI plus ramucirumab with recurrent colorectal cancer refractory to adjuvant chemotherapy with oxaliplatin/fluoropyrimidine (RAINCLOUD): RAINCLOUD-TR. J. Clin. Oncol. 2023, 41, 173. [Google Scholar] [CrossRef]
- Nie, Z.; Zhang, K.; Li, Z.; Bing, X.; Jin, S.; Li, M. Human papillomavirus 16 E6 promotes angiogenesis of lung cancer via SNHG. Cell Biochem. Biophys. 2023, 81, 325–336. [Google Scholar] [CrossRef]
- Rusak, A.; Jablonska, K.; Piotrowska, A.; Grzegrzolka, J.; Nowak, A.; Wojnar, A.; Dziegiel, P. The Role of CHI3L1 Expression in Angiogenesis in Invasive Ductal Breast Carcinoma. Anticancer. Res. 2018, 38, 3357–3366. [Google Scholar] [CrossRef]
- Bussolati, B.; Deambrosis, I.; Russo, S.; Deregibus, M.C.; Camussi, G. Altered angiogenesis and survival in human tumor-derived endothelial cells. FASEB J. 2003, 17, 1159–1161. [Google Scholar] [CrossRef]
- Lee, S.; Zhou, P.; Gupta, A.; Shin, S. Reactive Ductules Are Associated with Angiogenesis and Tumor Cell Proliferation in Pediatric Liver Cancer. Hepatol. Commun. 2018, 2, 1199–1212. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Charnock-Jones, S.; Licence, D.; Yanaihara, A.; Hastings, J.; Holland, C.; Emoto, M.; Umemoto, M.; Sakamoto, T.; Sato, S.; et al. Vascular endothelial growth factor-D is an independent prognostic factor in epithelial ovarian carcinoma. Br. J. Cancer 2003, 88, 237–244. [Google Scholar] [CrossRef]
- White, J.D.; Hewett, P.W.; Kosuge, D.; McCulloch, T.; Enholm, B.C.; Carmichael, J.; Murray, J.C. Vascular endothelial growth factor-D expression is an independent prognostic marker for survival in colo-rectal carcinoma. Cancer Res. 2002, 62, 1669–1675. [Google Scholar]
- Nakamura, Y.; Sato, S.; Futagami, M.; Fukushi, Y.; Sakamoto, T.; Umemoto, M.; Saito, Y. Prognostic significance of vascular endothelial growth factor D in breast carcinoma with long-term fol-low-up. Clin Cancer Res. 2003, 9, 716–721. [Google Scholar]
- Sopo, M.; Anttila, M.; Hämäläinen, K.; Kivelä, A.; Ylä-Herttuala, S.; Kosma, V.-M.; Keski-Nisula, L.; Sallinen, H. Expression profiles of VEGF-A, VEGF-D and VEGFR1 are higher in distant metastases than in matched primary high grade epithelial ovarian cancer. BMC Cancer 2019, 19, 584. [Google Scholar] [CrossRef]
- Nienhüser, H.; Crnovrsanin, N.; Nerz, D.; Heckler, M.; Sisic, L.; Lasitschka, F.; Schneider, M.; Schmidt, T. Expression of Angiogenic Proteins in Tumor and Stroma Affects Survival in Patients with Gastric Cancer. J. Surg. Res. 2020, 255, 172–180. [Google Scholar] [CrossRef]
- Oplawski, M.; Dziobek, K.; Zmarzły, N.; Grabarek, B.; Halski, T.; Januszyk, P.; Kuś-Kierach, A.; Adwent, I.; Dąbruś, D.; Kiełbasiński, K.; et al. Expression Profile of VEGF-C, VEGF-D, and VEGFR-3 in Different Grades of Endometrial Cancer. Curr. Pharm. Biotechnol. 2019, 20, 1004–1010. [Google Scholar] [CrossRef]
- Nalezinska, M. EP1288 The role of angiogenesis in early detection of epithelial ovarian carcinoma (EOC)—VEGF D (vascular endothelial growth factor D) as a potential novel biomarker. ePoster 2019, 29, A648. [Google Scholar] [CrossRef]
- Lieu, C.H.; Tran, H.; Jiang, Z.-Q.; Mao, M.; Overman, M.J.; Lin, E.; Eng, C.; Morris, J.; Ellis, L.; Heymach, J.V.; et al. The Association of Alternate VEGF Ligands with Resistance to Anti-VEGF Therapy in Metastatic Colorectal Cancer. PLoS ONE 2013, 8, e77117. [Google Scholar] [CrossRef]
- Weickhardt, A.J.; Williams, D.S.; Lee, C.K.; Chionh, F.; Simes, J.; Murone, C.; Wilson, K.; Parry, M.M.; Asadi, K.; Scott, A.M.; et al. Vascular endothelial growth factor D expression is a potential biomarker of bevacizumab benefit in colorectal cancer. Br. J. Cancer 2015, 113, 37–45. [Google Scholar] [CrossRef]
- Tabernero, J.; Hozak, R.; Yoshino, T.; Cohn, A.; Obermannova, R.; Bodoky, G.; Garcia-Carbonero, R.; Ciuleanu, T.-E.; Portnoy, D.; Prausová, J.; et al. Analysis of angiogenesis biomarkers for ramucirumab efficacy in patients with metastatic colorectal cancer from RAISE, a global, randomized, double-blind, phase III study. Ann. Oncol. 2017, 29, 602–609. [Google Scholar] [CrossRef]
- Carmeliet, P.; Li, X.; Treps, L.; Conradi, L.-C.; Loges, S. RAISEing VEGF-D’s importance as predictive biomarker for ramucirumab in metastatic colorectal cancer patients. Ann. Oncol. 2018, 29, 529–532. [Google Scholar] [CrossRef]
- Ose, J.; Gigic, B.; Hardikar, S.; Lin, T.; Himbert, C.; Warby, C.A.; Peoples, A.R.; Lindley, C.L.; Boehm, J.; Schrotz-King, P.; et al. Presurgery Adhesion Molecules and Angiogenesis Biomarkers Are Differently Associated with Outcomes in Colon and Rectal Cancer: Results from the ColoCare Study. Cancer Epidemiol. Biom. Prev. 2022, 31, 1650–1660. [Google Scholar] [CrossRef]
- Himbert, C.; Ose, J.; Lin, T.; Warby, C.A.; Gigic, B.; Steindorf, K.; Schrotz-King, P.; Abbenhardt-Martin, C.; Zielske, L.; Boehm, J.; et al. Inflammation- and angiogenesis-related biomarkers are correlated with cancer-related fatigue in colorectal cancer patients: Results from the ColoCare Study. Eur. J. Cancer Care 2019, 28, e13055. [Google Scholar] [CrossRef]
- Mazeda, I.; Martins, S.F.; Garcia, E.A.; Rodrigues, M.; Longatto, A. VEGF Expression in Colorectal Cancer Metastatic Lymph Nodes: Clinicopathological Correlation and Prognostic Significance. Gastrointest. Disord. 2020, 2, 267–280. [Google Scholar] [CrossRef]
- Izawa, N.; Shitara, K.; Masuishi, T.; Denda, T.; Yamazaki, K.; Moriwaki, T.; Okuda, H.; Kondoh, C.; Nishina, T.; Makiyama, A.; et al. Vascular endothelial growth factor (VEGF)-D and clinical outcomes in metastatic colorectal cancer (mCRC) patients (pts) treated with second-line FOLFIRI plus bevacizumab (Bev): A biomarker study of the WJOG 6210G trial. J. Clin. Oncol. 2020, 38, 226. [Google Scholar] [CrossRef]
- Woei-A-Jin, F.S.H.; Weijl, N.I.; Burgmans, M.C.; Fariña Sarasqueta, A.; Tom van Wezel, J.; Wasser, M.N.; Coenraad, M.J.; Burggraaf, J.; Osanto, S. Neoadjuvant Treatment with Angiogenesis-Inhibitor Dovitinib Prior to Local Therapy in Hepato-cellular Carcinoma: A Phase II Study. Oncologist 2021, 26, 854–864. [Google Scholar] [CrossRef]
- Miao, Y.-D.; Tang, X.-L.; Wang, J.-T.; Mi, D.-H. Prognostic role of expression of angiogenesis markers in hepatocellular carcinoma: A bioinformatics analysis. World J. Gastroenterol. 2022, 28, 4221–4226. [Google Scholar] [CrossRef]
- Gkika, E.; Adebahr, S.; Brenner, A.; Schimek-Jasch, T.; Radicioni, G.; Exner, J.-P.; Rühle, A.; Spohn, S.K.B.; Popp, I.; Zamboglou, C.; et al. Changes in Blood Biomarkers of Angiogenesis and Immune Modulation after Radiation Therapy and Their Association with Outcomes in Thoracic Malignancies. Cancers 2021, 13, 5725. [Google Scholar] [CrossRef]
- Taniguchi, H.; Yuki, S.; Shiozawa, M.; Masuishi, T.; Nishina, T.; Kagawa, Y.; Takahashi, N.; Yasui, H.; Denda, T.; Sunakawa, Y.; et al. Plasma VEGF-D and PlGF levels according to prior use of biologics among metastatic colorectal cancer: Preliminary results from GI-SCREEN CRC-Ukit study. J. Clin. Oncol. 2020, 38, 178. [Google Scholar] [CrossRef]
- Pisa, U.O. Predictive Role of Circulating Angiogenic Factors for Second-line Paclitaxel and Ramucirumab. 2021. Available online: https://ClinicalTrials.gov/show/NCT05301465 (accessed on 24 July 2023).
- Achen, M.G.; Roufail, S.; Domagala, T.; Catimel, B.; Nice, E.C.; Geleick, D.M.; Murphy, R.; Scott, A.M.; Caesar, C.; Makinen, T.; et al. Monoclonal antibodies to vascular endothelial growth factor-D block its interactions with both VEGF receptor-2 and VEGF receptor-3. JBIC J. Biol. Inorg. Chem. 2000, 267, 2505–2515. [Google Scholar] [CrossRef]
- Davydova, N.; Roufail, S.; Streltsov, V.A.; Stacker, S.A.; Achen, M.G. The VD1 Neutralizing Antibody to Vascular Endothelial Growth Factor-D: Binding Epitope and Rela-tionship to Receptor Binding. J. Mol. Biol. 2011, 407, 581–593. [Google Scholar] [CrossRef]
- Davydova, N.; Harris, N.C.; Roufail, S.; Paquet-Fifield, S.; Ishaq, M.; Streltsov, V.A.; Williams, S.P.; Karnezis, T.; Stacker, S.A.; Achen, M.G. Differential receptor binding and regulatory mechanisms for the lymphangiogenic growth factors vas-cular endothelial growth factor (VEGF)-C and-D. J. Biol. Chem. 2016, 291, 27265–27278. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Zheng, Z.; Shi, L.; Huang, Y.; Lu, B.; Wang, Z. Andrographolide decreased VEGFD expression in hepatoma cancer cells by inducing ubiquitin/proteasome-mediated cFos protein degradation. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2015, 1850, 750–758. [Google Scholar] [CrossRef]
- Turunen, T.; Hua, A.; Shatos, M.; Teague, G.C.; Baldwin, M.; Lashkari, K. VEGF-C and VEGF-D Inhibition by VGX-300 Effectively Reduces Leukocyte Adhesion and Vascular Leakage in the STZ-Rat Model of Diabetic Retinal Edema. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3667. [Google Scholar]
- Lashkari, K.; Ma, J.; Sun, Y.; Teague, G.C.; Baldwin, M.E. VGX-300, a ‘Trap’for VEGF-C and VEGF-D, Inhibits Choroidal Neovascularization and Vascular Leakage in a Mouse Model of Wet AMD. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4802. [Google Scholar]
- Pytowski, B.; Goldman, J.; Persaud, K.; Wu, Y.; Witte, L.; Hicklin, D.J.; Skobe, M.; Boardman, K.C.; Swartz, M.A. Complete and Specific Inhibition of Adult Lymphatic Regeneration by a Novel VEGFR-3 Neutralizing Antibody. Gynecol. Oncol. 2005, 97, 14–21. [Google Scholar] [CrossRef]
- Persaud, K.; Tille, J.-C.; Liu, M.; Zhu, Z.; Jimenez, X.; Pereira, D.S.; Miao, H.-Q.; Brennan, L.A.; Witte, L.; Pepper, M.S.; et al. Involvement of the VEGF receptor 3 in tubular morphogenesis demonstrated with a human anti-human VEGFR-3 monoclonal antibody that antagonizes receptor activation by VEGF-C. J. Cell Sci. 2004, 117, 2745–2756. [Google Scholar] [CrossRef]
- Jackson, T.L.; Slakter, J.; Buyse, M.; Wang, K.; Dugel, P.U.; Wykoff, C.C.; Boyer, D.S.; Gerometta, M.; Baldwin, M.E.; Price, C.F.; et al. A Randomized Controlled Trial of OPT-302, a VEGF-C/D Inhibitor for Neovascular Age-Related Macular Degeneration. Ophthalmology 2023, 130, 588–597. [Google Scholar] [CrossRef]
- Limited, O. Study Evaluating the Safety, Pharmacokinetics and Pharmacodynamics of OPT-302 with or Without Lucentis™ in Patients with Wet AMD. 2015. Available online: https://ClinicalTrials.gov/show/NCT02543229 (accessed on 24 July 2023).
- Lee, W.S.; Pyun, B.-J.; Kim, S.-W.; Shim, S.R.; Nam, J.R.; Yoo, J.Y.; Jin, Y.; Jin, J.; Kwon, Y.-G.; Yun, C.-O.; et al. TTAC-0001, a human monoclonal antibody targeting VEGFR-2/KDR, blocks tumor angiogenesis. mAbs 2015, 7, 957–968. [Google Scholar] [CrossRef]
- PharmAbcine. Phase I Trial of Tanibirumab in Advanced or Metastatic Cancer. 2011. Available online: https://ClinicalTrials.gov/show/NCT01660360 (accessed on 24 July 2023).
- PharmAbcine. Trial to Evaluate the Safety of TTAC-0001(Tanibirumab) in Recurrent Glioblastoma. 2016. Available online: https://ClinicalTrials.gov/show/NCT03033524 (accessed on 24 July 2023).
- Kras, P.; Talkowski, K.; Grabarek, B.O.; Skalska-Dziobek, N.; Boroń, D.; Oplawski, M. Evaluation of Variances in VEGF-AD and VEGFR-1-3 Expression in the Ishikawa Endometrial Cancer Cell Line Treated with Salinomycin and Anti-Angiogenic/Lymphangiogenic Effect. Curr. Pharm. Biotechnol. 2021, 22, 697–705. [Google Scholar] [CrossRef]
- Pan, M.-S.; Cao, J.; Fan, Y.-Z. Insight into norcantharidin, a small-molecule synthetic compound with potential multi-target anticancer activities. Chin. Med. 2020, 15, 55. [Google Scholar] [CrossRef]
- Laakkonen, P.; Waltari, M.; Holopainen, T.; Takahashi, T.; Pytowski, B.; Steiner, P.; Hicklin, D.; Persaud, K.; Tonra, J.R.; Witte, L.; et al. Vascular Endothelial Growth Factor Receptor 3 Is Involved in Tumor Angiogenesis and Growth. Cancer Res. 2007, 67, 593–599. [Google Scholar] [CrossRef]
- Hwang, S.D.; Song, J.H.; Kim, Y.; Lim, J.H.; Kim, M.Y.; Kim, E.N.; Hong, Y.A.; Chung, S.; Choi, B.S.; Kim, Y.-S.; et al. Inhibition of lymphatic proliferation by the selective VEGFR-3 inhibitor SAR131675 ameliorates diabetic nephropathy in db/db mice. Cell Death Dis. 2019, 10, 219. [Google Scholar] [CrossRef]
- Tempfer, H.; Spitzer, G.; Lehner, C.; Wagner, A.; Gehwolf, R.; Fierlbeck, J.; Weissenbacher, N.; Jessen, M.; Heindl, L.M.; Traweger, A. VEGF-D-mediated signaling in tendon cells is involved in degenerative processes. FASEB J. 2022, 36, e22126. [Google Scholar] [CrossRef]
- Oholendt, A.L.; Zadlo, J.L. Ramucirumab: A New Therapy for Advanced Gastric Cancer. J. Adv. Pract. Oncol. 2015, 6, 71–75. [Google Scholar] [CrossRef]
- Lee, S.H. Tanibirumab (TTAC-0001): A fully human monoclonal antibody targets vascular endothelial growth factor receptor 2 (VEGFR-2). Arch. Pharmacal Res. 2011, 34, 1223–1226. [Google Scholar] [CrossRef]
- Hao, Z.; Sadek, I. Sunitinib: The antiangiogenic effects and beyond. OncoTargets Ther. 2016, 9, 5495–5505. [Google Scholar] [CrossRef]
- Taghour, M.S.; Mahdy, H.A.; Gomaa, M.H.; Aglan, A.; Eldeib, M.G.; Elwan, A.; Dahab, M.A.; Elkaeed, E.B.; Alsfouk, A.A.; Khalifa, M.M.; et al. Benzoxazole derivatives as new VEGFR-2 inhibitors and apoptosis inducers: Design, synthesis, in silico studies, and antiproliferative evaluation. J. Enzym. Inhib. Med. Chem. 2022, 37, 2063–2077. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Liu, M.; Huang, W.; Wang, Z.; He, Y.; Wu, J.; Ren, S.; Ju, Y.; Geng, R.; Li, Z. Recombinant Human Endostatin Endostar Inhibits Tumor Growth and Metastasis in a Mouse Xenograft Model of Colon Cancer. Pathol. Oncol. Res. 2011, 18, 315–323. [Google Scholar] [CrossRef] [PubMed]
| Drug | Mechanism of Action | Selectivity, Mechanism of Action | Reference |
|---|---|---|---|
| Andrographolide | Targets the VEGF-D inducer: c-fos | Non-selective | [168] |
| VGX-300 | Soluble VEGFR-3 | Bind and inhibit the activation of VEGFR-3 by all of its ligands (VEGF-D/C/A) | [169,170,171,172,173,180,181,182] |
| SAR131675 | VEGFR-3 tyrosine kinase inhibitor (it does not say about its property. Inhibits phosphorylation and hence activation of the receptor) | ||
| mF4-31C1, hF4-3C5 | mABs against VEGFR-3 | ||
| OPT-302 | VEGFR-3 decoy | ||
| mAB 286 | mAb | Selective: Binds mature VEGF-D, prevents its binding to VEGFR-2 and VEGFR-3 | [165,167] |
| VD1 | mAb | Competes with VEGF-D in activation of VEGFR-2 and VEGFR-3 | [166] |
| Tanibitumab, Ramucirumab, SU5614, Sunitinib, Benzoxazole and its derivatives | VEGFR-2 inhibitors | VEGFR-2 inhibition prevents blood angiogenic signaling from (VEGF-A/C/D) | [57,183,184,185,186] |
| Endostatin | VEGF-D (and VEGF-A, VEGF-C) inhibitor | Non-selective | [187] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokhari, S.M.Z.; Hamar, P. Vascular Endothelial Growth Factor-D (VEGF-D): An Angiogenesis Bypass in Malignant Tumors. Int. J. Mol. Sci. 2023, 24, 13317. https://doi.org/10.3390/ijms241713317
Bokhari SMZ, Hamar P. Vascular Endothelial Growth Factor-D (VEGF-D): An Angiogenesis Bypass in Malignant Tumors. International Journal of Molecular Sciences. 2023; 24(17):13317. https://doi.org/10.3390/ijms241713317
Chicago/Turabian StyleBokhari, Syeda Mahak Zahra, and Peter Hamar. 2023. "Vascular Endothelial Growth Factor-D (VEGF-D): An Angiogenesis Bypass in Malignant Tumors" International Journal of Molecular Sciences 24, no. 17: 13317. https://doi.org/10.3390/ijms241713317
APA StyleBokhari, S. M. Z., & Hamar, P. (2023). Vascular Endothelial Growth Factor-D (VEGF-D): An Angiogenesis Bypass in Malignant Tumors. International Journal of Molecular Sciences, 24(17), 13317. https://doi.org/10.3390/ijms241713317







