Suppressing Src-Mediated EGFR Signaling by Sustained Calcium Supply Targeting Triple-Negative Breast Cancer
Abstract
:1. Introduction
2. Results
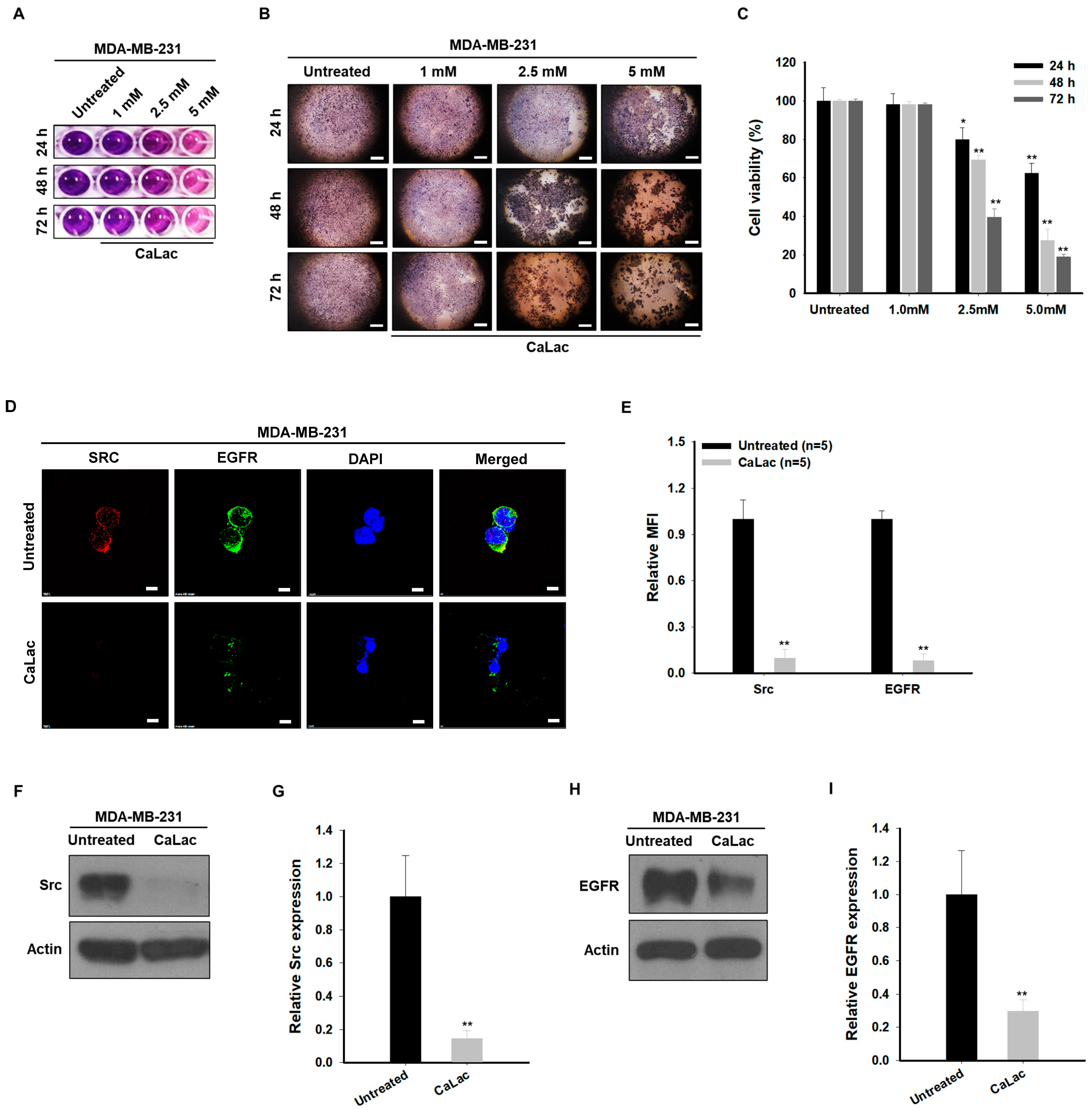
2.1. Sustained Calcium Supply Inhibits the Src-Dependent Trans-Activation of EGFR in TNBC Cells
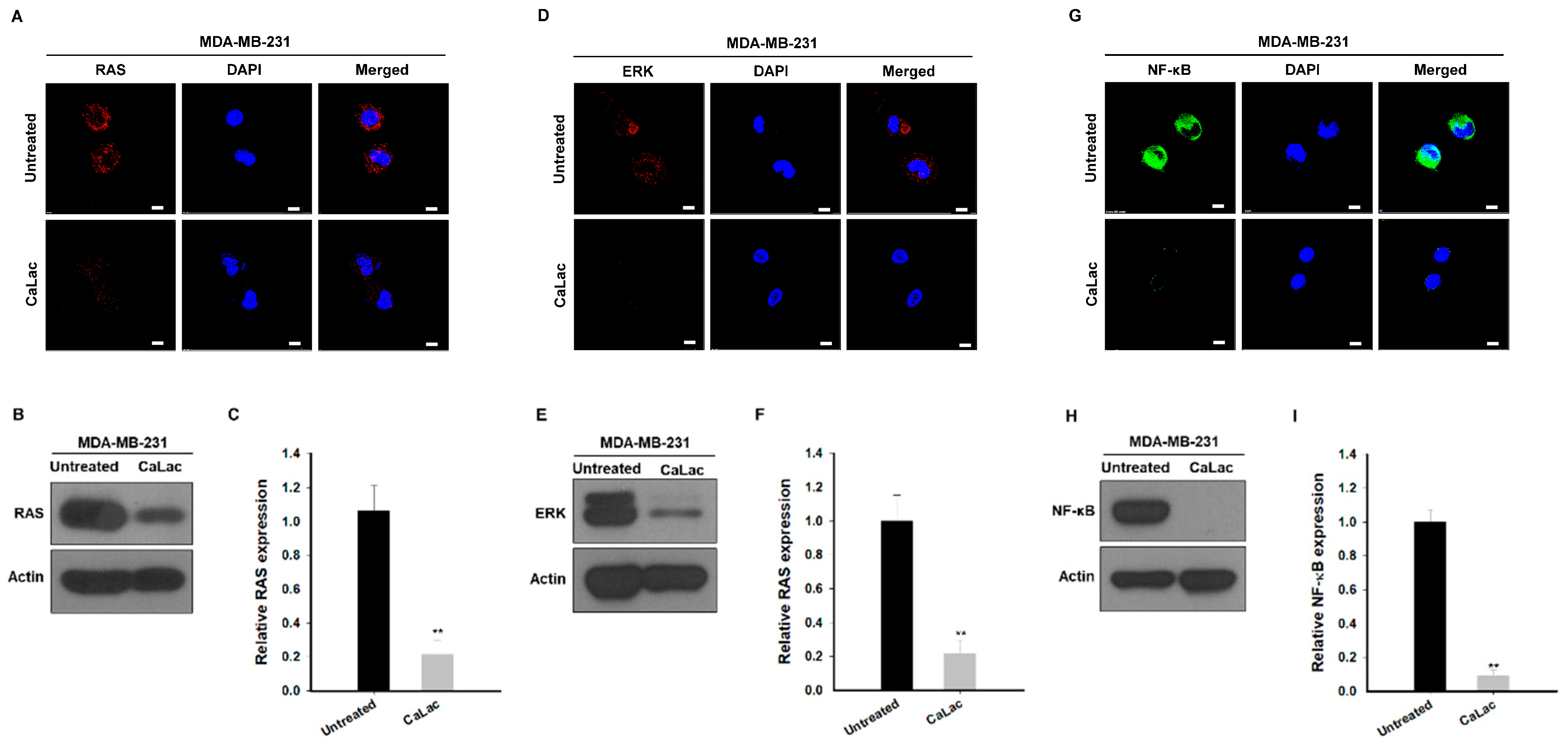
2.2. Sustained Calcium Supply Inhibits the Sub-Signals Stimulated from EGFR and Src in TNBC Cells
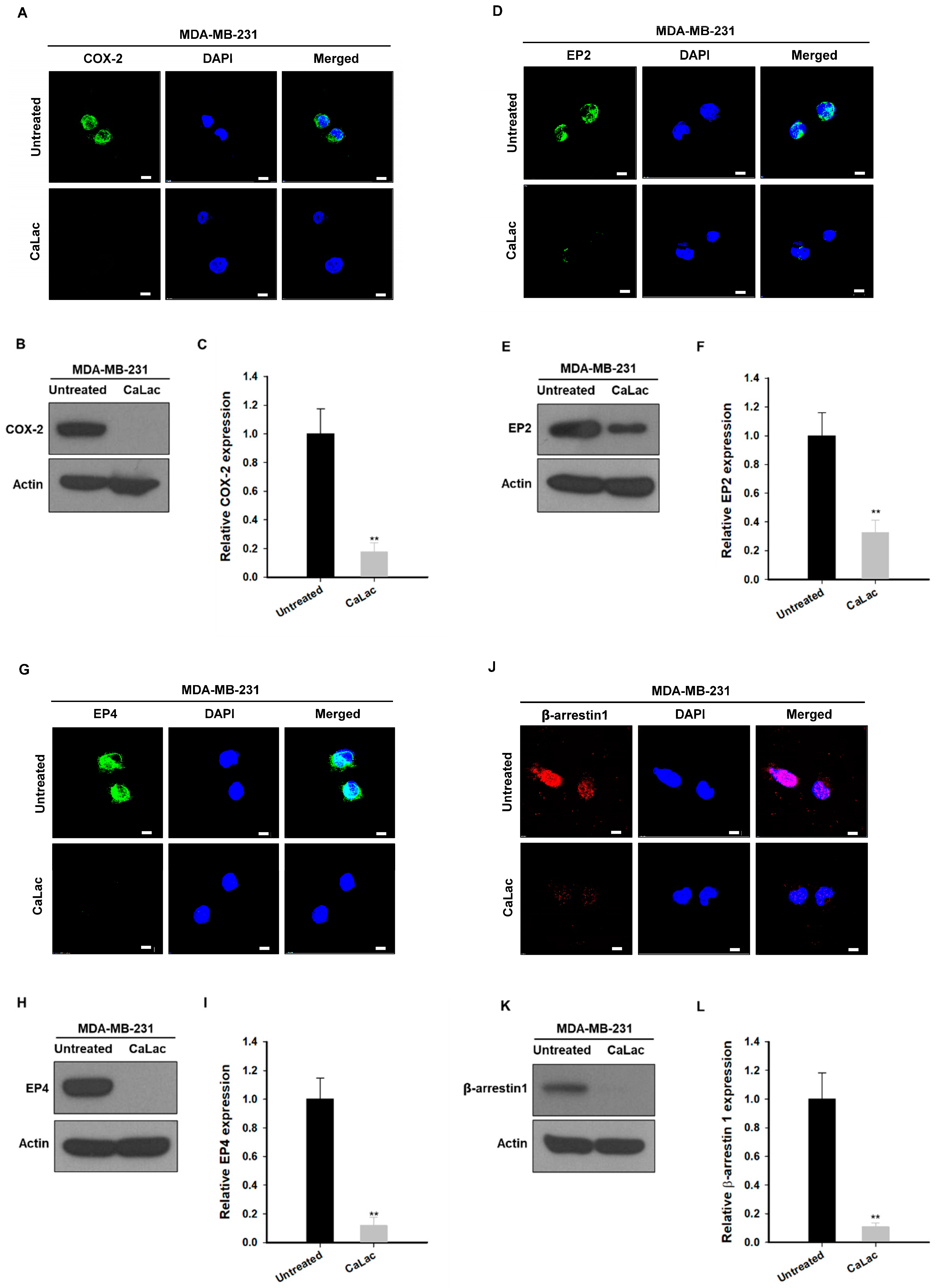
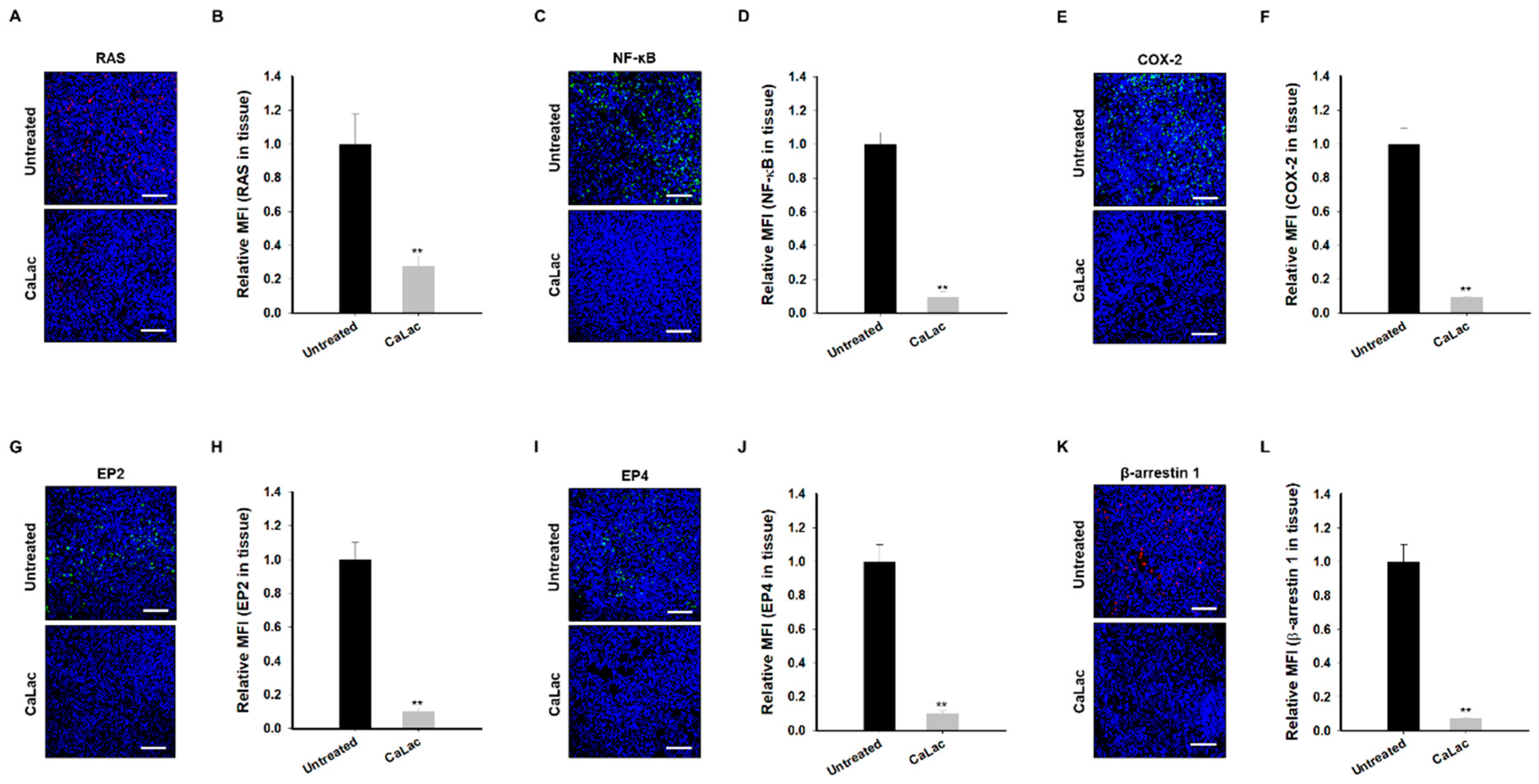
2.3. Calcium Supply Contributes to Inhibiting the Stimulation of Prostaglandin E2 Receptors (EP) Resulting from NF-κB Downregulation in TNBC Cells
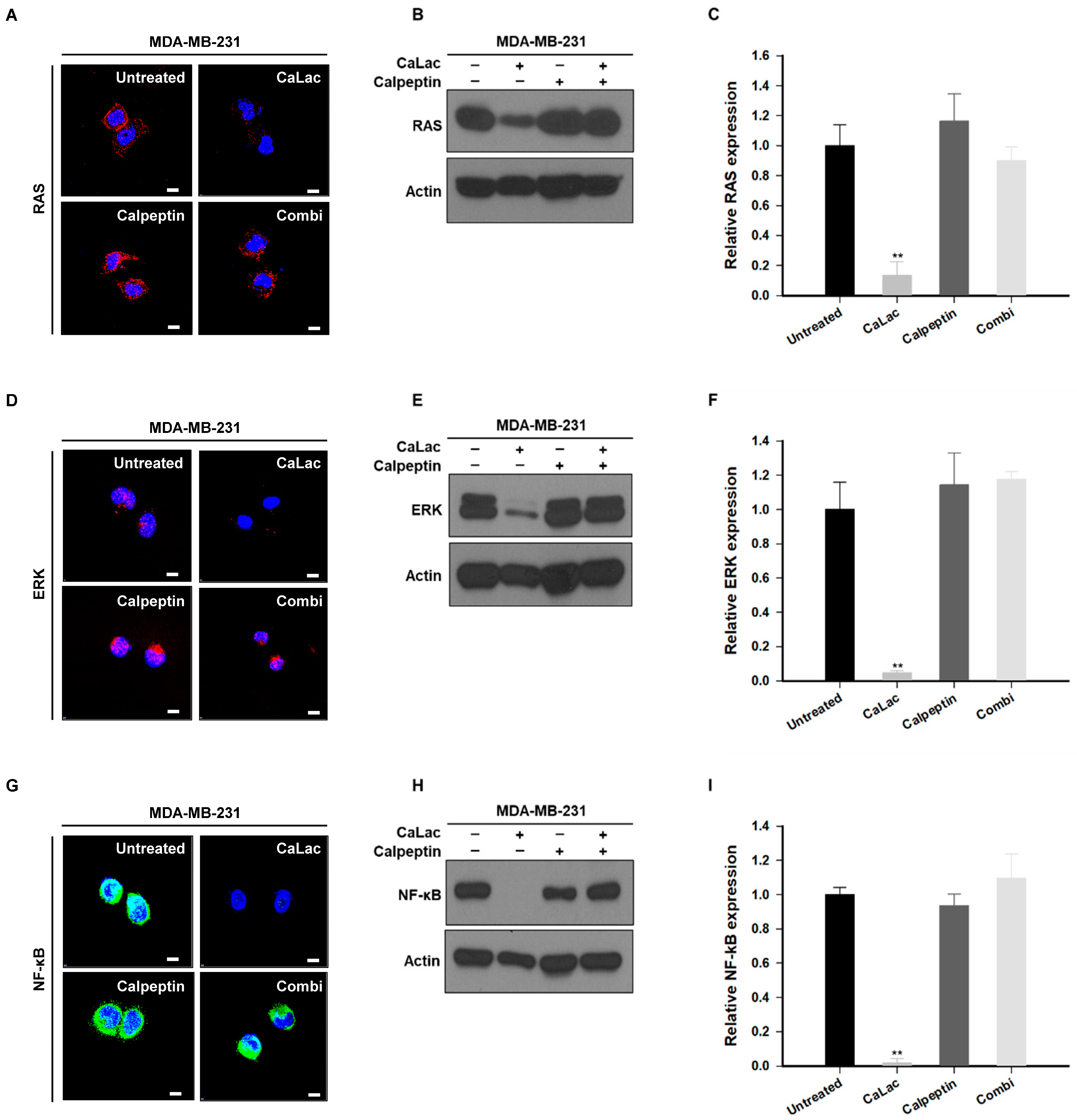
2.4. Calpeptin Reduces the Effect of Calcium Inhibiting Src-Dependent Trans-Activation of EGFR in TNBC Cells
2.5. Calpeptin Reduces the Effect of Calcium Inhibiting the Sub-Signals Stimulated by EGFR and Src in TNBC Cells
2.6. Calpeptin Reduces the Effect of Calcium Inhibiting the EP Stimulation Resulting from NF-κB Downregulation in TNBC Cells
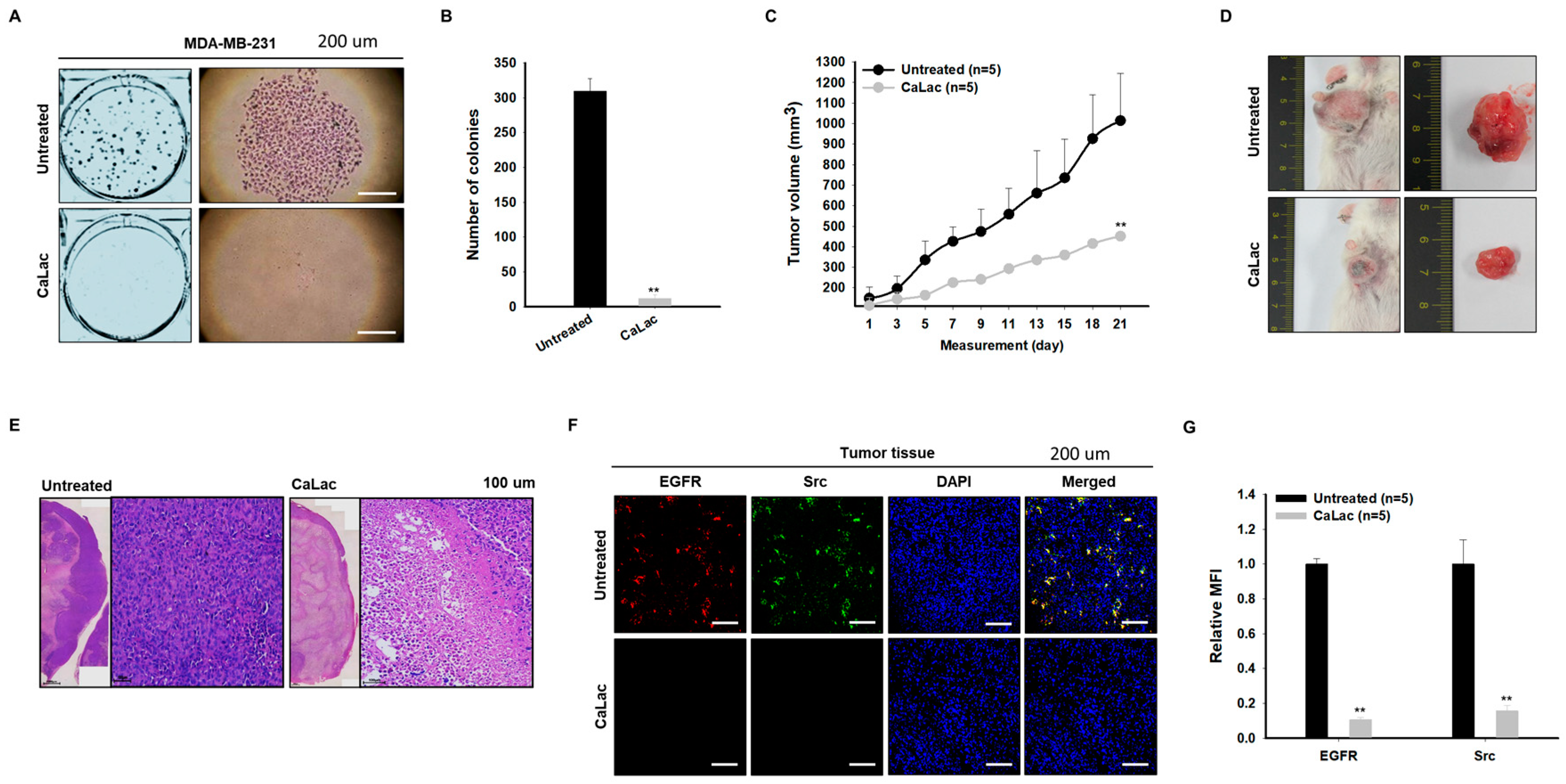
2.7. Sustained Calcium Supply Shows an Anticancer Effect on TNBC
2.8. Sustained Calcium Supply Inhibits the Sub-Signals to Be Stimulated from EGFR and Src in TNBC Tissue
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagent
4.2. Cell Viability Assay
4.3. Immunocytochemistry
4.4. Protein Extraction
4.5. Western Blot Analysis
4.6. Clonogenic Assay
4.7. Xenograft Animal Model
4.8. Immunofluorescence
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Nat. Rev. Dis. Primers 2019, 5, 66. [Google Scholar] [CrossRef]
- Kumar, P.; Aggarwal, R. An overview of triple-negative breast cancer. Arch. Gynecol. Obstet. 2016, 293, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Yost, S.E.; Yuan, Y. Neoadjuvant Treatment for Triple Negative Breast Cancer: Recent Progresses and Challenges. Cancers 2020, 12, 1404. [Google Scholar] [CrossRef] [PubMed]
- Garutti, M.; Pelizzari, G.; Bartoletti, M.; Malfatti, M.C.; Gerratana, L.; Tell, G.; Puglisi, F. Platinum Salts in Patients with Breast Cancer: A Focus on Predictive Factors. Int. J. Mol. Sci. 2019, 10, 3390. [Google Scholar] [CrossRef]
- Liu, M.; Mo, Q.G.; Wei, C.Y.; Qin, Q.H.; Huang, Z.; He, J. Platinum-based chemotherapy in triple-negative breast cancer: A meta-analysis. Oncol. Lett. 2013, 5, 983–991. [Google Scholar] [CrossRef]
- Miglietta, F.; Fabi, A.; Generali, D.; Dieci, M.V.; Arpino, G.; Bianchini, G.; Cinieri, S.; Conte, P.F.; Curigliano, G.; Laurentiis, M.D.; et al. Optimizing choices and sequences in the diagnostic-therapeutic landscape of advanced triple-negative breast cancer: An Italian consensus paper and critical review. Cancer Treat. Rev. 2023, 114, 102511, Erratum in Cancer Treat. Rev. 2023, 117, 102570. Erratum in Cancer Treat. Rev. 2023, 119, 102594. [Google Scholar] [CrossRef]
- Masuda, H.; Zhang, D.; Bartholomeusz, C.; Doihara, H.; Hortobagyi, G.N.; Ueno, N.T. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res. Treat. 2012, 136, 331–345. [Google Scholar] [CrossRef]
- Sato, K. Cellular functions regulated by phosphorylation of EGFR on Tyr845. Int. J. Mol. Sci. 2013, 14, 10761–10790. [Google Scholar] [CrossRef]
- El-Hashim, A.Z.; Khajah, M.A.; Renno, W.M.; Babyson, R.S.; Uddin, M.; Benter, I.F.; Ezeamuzie, C.; Akhtar, S. Src-dependent EGFR transactivation regulates lung inflammation via downstream signaling involving ERK1/2, PI3Kdelta/Akt and NFkappaB induction in a murine asthma model. Sci. Rep. 2017, 7, 9919. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Nag, S.A.; Zhang, R. Targeting the NFkappaB signaling pathways for breast cancer prevention and therapy. Curr. Med. Chem. 2015, 22, 264–289. [Google Scholar] [CrossRef]
- Tryfonopoulos, D.; Walsh, S.; Collins, D.M.; Flanagan, L.; Quinn, C.; Corkery, B.; McDermott, E.W.; Evoy, D.; Pierce, A.; O’Donovan, N.; et al. Src: A potential target for the treatment of triple-negative breast cancer. Ann. Oncol. 2011, 22, 2234–2240. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.; Logothetis, C. Dasatinib: A potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer Treat. Rev. 2010, 36, 492–500. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, J.K.; Park, M.H.; Jeong, K.Y.; Kim, H.M. Potential Anticancer Effect of Calcium-mediated Src Degradation on Hormone-dependent Breast Cancer. Anticancer. Res. 2020, 40, 1989–1996. [Google Scholar] [CrossRef]
- Luo, J.; Zou, H.; Guo, Y.; Tong, T.; Ye, L.; Zhu, C.; Deng, L.; Wang, B.; Pan, Y.; Li, P. SRC kinase-mediated signaling pathways and targeted therapies in breast cancer. Breast Cancer Res. 2022, 24, 99. [Google Scholar] [CrossRef] [PubMed]
- Canonici, A.; Browne, A.L.; Ibrahim, M.F.K.; Fanning, K.P.; Roche, S.; Conlon, N.T.; O’Neill, F.; Meiller, J.; Cremona, M.; Morgan, C.; et al. Combined targeting EGFR and SRC as a potential novel therapeutic approach for the treatment of triple negative breast cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835919897546. [Google Scholar] [CrossRef]
- Irwin, M.E.; Bohin, N.; Boerner, J.L. Src family kinases mediate epidermal growth factor receptor signaling from lipid rafts in breast cancer cells. Cancer Biol. Ther. 2011, 12, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, D.; Braun, M.; Mieszkowska, M.; Kowalczyk, L.; Kopczynski, J.; Kordek, R.; Sadej, R.; Romanska, H.M. Upregulation of HIF1-alpha via an NF-kappaB/COX2 pathway confers proliferative dominance of HER2-negative ductal carcinoma in situ cells in response to inflammatory stimuli. Neoplasia 2020, 22, 576–589. [Google Scholar] [CrossRef]
- Jin, K.; Qian, C.; Lin, J.; Liu, B. Cyclooxygenase-2-Prostaglandin E2 pathway: A key player in tumor-associated immune cells. Front. Oncol. 2023, 13, 1099811. [Google Scholar] [CrossRef]
- Krishnamachary, B.; Stasinopoulos, I.; Kakkad, S.; Penet, M.F.; Jacob, D.; Wildes, F.; Mironchik, Y.; Pathak, A.P.; Solaiyappan, M.; Bhujwalla, Z.M. Breast cancer cell cyclooxygenase-2 expression alters extracellular matrix structure and function and numbers of cancer associated fibroblasts. Oncotarget 2017, 8, 17981–17994. [Google Scholar] [CrossRef] [PubMed]
- Walker, O.L.; Dahn, M.L.; Power Coombs, M.R.; Marcato, P. The Prostaglandin E2 Pathway and Breast Cancer Stem Cells: Evidence of Increased Signaling and Potential Targeting. Front. Oncol. 2021, 11, 791696. [Google Scholar] [CrossRef]
- Belli, S.; Esposito, D.; Servetto, A.; Pesapane, A.; Formisano, L.; Bianco, R. c-Src and EGFR Inhibition in Molecular Cancer Therapy: What Else Can We Improve? Cancers 2020, 12, 1489. [Google Scholar] [CrossRef] [PubMed]
- Shapovalov, I.; Harper, D.; Greer, P.A. Calpain as a therapeutic target in cancer. Expert Opin. Ther. Targets 2022, 26, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Wang, X.; Zhao, D.; Liu, H.; Hu, Y. Calcium homeostasis and cancer: Insights from endoplasmic reticulum-centered organelle communications. Trends Cell Biol. 2023, 33, 312–323. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, K.-Y.; Park, S.Y.; Park, M.H.; Kim, H.M. Suppressing Src-Mediated EGFR Signaling by Sustained Calcium Supply Targeting Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2023, 24, 13291. https://doi.org/10.3390/ijms241713291
Jeong K-Y, Park SY, Park MH, Kim HM. Suppressing Src-Mediated EGFR Signaling by Sustained Calcium Supply Targeting Triple-Negative Breast Cancer. International Journal of Molecular Sciences. 2023; 24(17):13291. https://doi.org/10.3390/ijms241713291
Chicago/Turabian StyleJeong, Keun-Yeong, Seon Young Park, Min Hee Park, and Hwan Mook Kim. 2023. "Suppressing Src-Mediated EGFR Signaling by Sustained Calcium Supply Targeting Triple-Negative Breast Cancer" International Journal of Molecular Sciences 24, no. 17: 13291. https://doi.org/10.3390/ijms241713291
APA StyleJeong, K.-Y., Park, S. Y., Park, M. H., & Kim, H. M. (2023). Suppressing Src-Mediated EGFR Signaling by Sustained Calcium Supply Targeting Triple-Negative Breast Cancer. International Journal of Molecular Sciences, 24(17), 13291. https://doi.org/10.3390/ijms241713291





