Influential Serum Kinases (Non-sFlt-1) and Phosphatases in Preeclampsia—Systemic Review and Metanalysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol Registration
2.2. Information Sources and Search Strategy
2.3. Eligibility Criteria
2.4. Study Selection
2.5. Assessment of Risk of Bias
2.6. Data Collection and Analysis
3. Results and Discussion
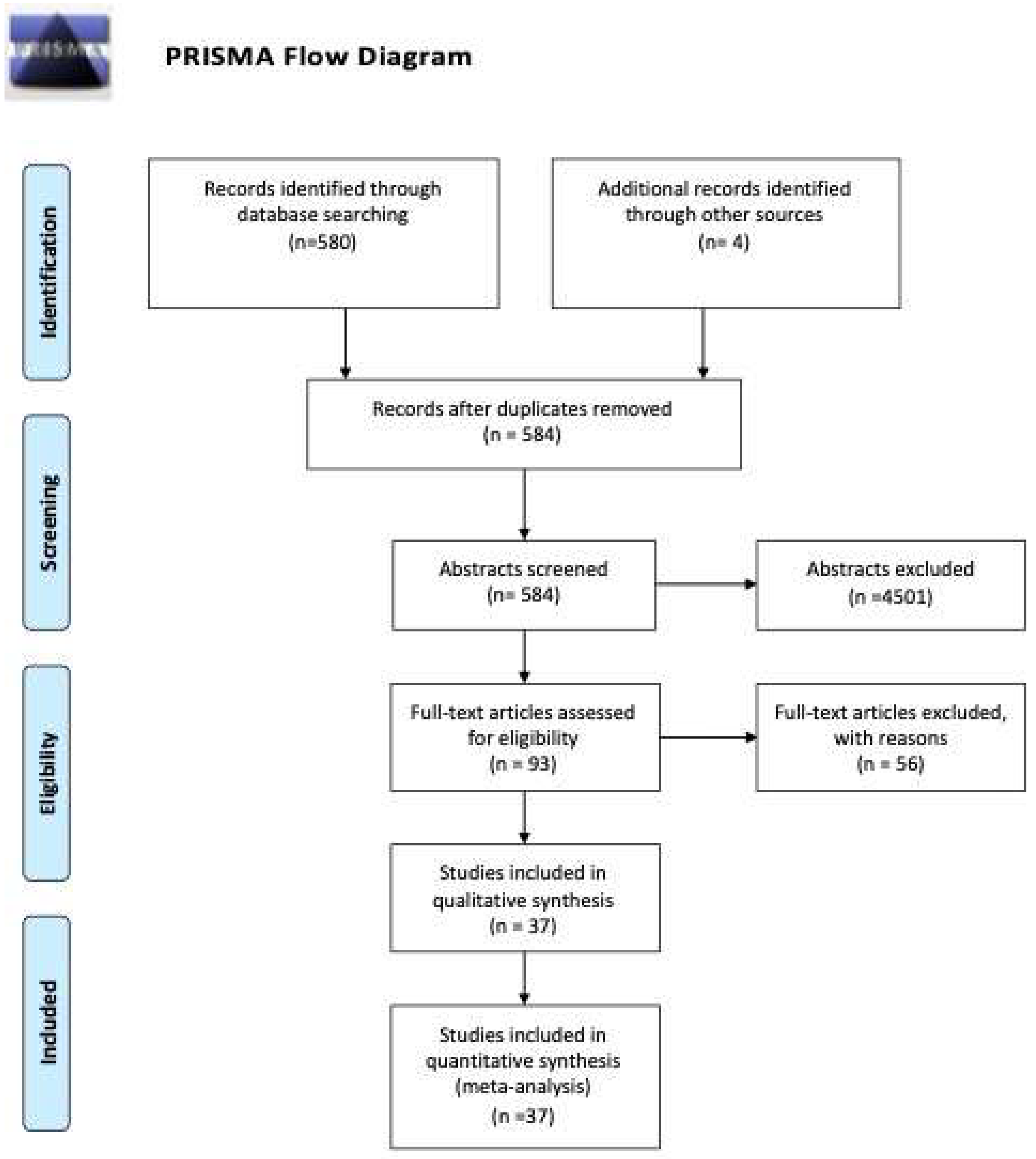
3.1. Study Selection and Study Characteristics
3.2. Risk of Bias in the Included Studies
3.3. Synthesis of Results
3.3.1. Kinases Significantly Related to Preeclampsia
3.3.2. Kinases Non-Significantly Related to Preeclampsia
3.3.3. Phosphatases and Preeclampsia
3.3.4. Meta-Regression and Publication Bias
3.4. Main Findings
3.5. Comparison with Existing Literature and Biological Plausibility of the Findings
3.6. Clinical Implications
3.7. Limitations of the Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Magee, L.A.; Nicolaides, K.H.; von Dadelszen, P. Preeclampsia. N. Engl. J. Med. 2022, 386, 1817–1832. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.; Vidaeff, A.; Pettker, C.M.; Simhan, H. Gestational Hypertension and Preeclampsia: ACOG Practice Bulletin, Number 222. Obstet. Gynecol. 2020, 135, e237–e260. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Romero, R.; Yeo, L.; Gomez-Lopez, N.; Chaemsaithong, P.; Jaovisidha, A.; Gotsch, F.; Erez, O. The etiology of preeclampsia. Am. J. Obstet. Gynecol. 2022, 226, S844–S866. [Google Scholar] [CrossRef] [PubMed]
- Phipps, E.; Prasanna, D.; Brima, W.; Jim, B. Preeclampsia: Updates in Pathogenesis, Definitions, and Guidelines. Clin. J. Am. Soc. Nephrol. 2016, 11, 1102–1113. [Google Scholar] [CrossRef]
- Burton, G.J.; Redman, C.W.; Roberts, J.M.; Moffett, A. Pre-eclampsia: Pathophysiology and clinical implications. BMJ 2019, 366, l2381. [Google Scholar] [CrossRef]
- Redman, C.W.; Staff, A.C.; Roberts, J.M. Syncytiotrophoblast stress in preeclampsia: The convergence point for multiple pathways. Am. J. Obstet. Gynecol. 2021, 226, S907–S927. [Google Scholar] [CrossRef]
- Dimitriadis, E.; Rolnik, D.L.; Zhou, W.; Estrada-Gutierrez, G.; Koga, K.; Francisco, R.P.V.; Whitehead, C.; Hyett, J.; Costa, F.d.S.; Nicolaides, K.; et al. Pre-eclampsia. Nat. Rev. Dis. Prim. 2023, 9, 1–22. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor receptor-1 (VEGFR-1/Flt-1): A dual regulator for angiogenesis. Angiogenesis 2006, 9, 225–230. [Google Scholar] [CrossRef]
- Tanner, M.S.; Davey, M.-A.; Mol, B.W.; Rolnik, D.L. The evolution of the diagnostic criteria of preeclampsia-eclampsia. Am. J. Obstet. Gynecol. 2022, 226, S835–S843. [Google Scholar] [CrossRef]
- Rana, S.; Burke, S.D.; Karumanchi, S.A. Imbalances in circulating angiogenic factors in the pathophysiology of preeclampsia and related disorders. Am. J. Obstet. Gynecol. 2022, 226, S1019–S1034. [Google Scholar] [CrossRef]
- Foidart, J.; Schaaps, J.; Chantraine, F.; Munaut, C.; Lorquet, S. Dysregulation of anti-angiogenic agents (sFlt-1, PLGF, and sEndoglin) in preeclampsia—a step forward but not the definitive answer. J. Reprod. Immunol. 2009, 82, 106–111. [Google Scholar] [CrossRef]
- Drexler, H.C.A.; Vockel, M.; Polaschegg, C.; Frye, M.; Peters, K.; Vestweber, D. Vascular Endothelial Receptor Tyrosine Phosphatase: Identification of Novel Substrates Related to Junctions and a Ternary Complex with EPHB4 and TIE2* [S]. Mol. Cell. Proteom. 2019, 18, 2058–2077. [Google Scholar] [CrossRef]
- Dobierzewska, A.; Palominos, M.; Sanchez, M.; Dyhr, M.; Helgert, K.; Venegas-Araneda, P.; Tong, S.; Illanes, S.E. Impairment of Angiogenic Sphingosine Kinase-1/Sphingosine-1-Phosphate Receptors Pathway in Preeclampsia. PLOS ONE 2016, 11, e0157221. [Google Scholar] [CrossRef]
- Czikk, M.; Drewlo, S.; Baczyk, D.; Adamson, S.; Kingdom, J. Dual specificity phosphatase 9 (DUSP9) expression is down-regulated in the severe pre-eclamptic placenta. Placenta 2013, 34, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Dathan-Stumpf, A.; Rieger, A.; Verlohren, S.; Wolf, C.; Stepan, H. sFlt-1/PlGF ratio for prediction of preeclampsia in clinical routine: A pragmatic real-world analysis of healthcare resource utilisation. PLOS ONE 2022, 17, e0263443. [Google Scholar] [CrossRef] [PubMed]
- Zeisler, H.; Llurba, E.; Chantraine, F.; Vatish, M.; Staff, A.C.; Sennström, M.; Olovsson, M.; Brennecke, S.P.; Stepan, H.; Allegranza, D.; et al. Predictive Value of the sFlt-1:PlGF Ratio in Women with Suspected Preeclampsia. N. Engl. J. Med. 2016, 374, 13–22. [Google Scholar] [CrossRef]
- Han, L.; Holland, O.J.; Costa, F.D.S.; Perkins, A.V. Potential biomarkers for late-onset and term preeclampsia: A scoping review. Front. Physiol. 2023, 14, 1143543. [Google Scholar] [CrossRef]
- Danielli, M.; Thomas, R.C.; Gillies, C.L.; Hu, J.; Khunti, K.; Tan, B.K. Blood biomarkers to predict the onset of pre-eclampsia: A systematic review and meta-analysis. Heliyon 2022, 8, e11226. [Google Scholar] [CrossRef]
- Mayeux, R. Biomarkers: Potential uses and limitations. NeuroRx 2004, 1, 182–188. [Google Scholar] [CrossRef]
- Shahid, R.; Bari, M.F.; Hussain, M. Serum biomarkers for the prediction and diagnosis of preeclampsia: A meta-analysis. J. Taibah Univ. Med Sci. 2022, 17, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Ahn, E.; Kang, H. Introduction to systematic review and meta-analysis. Korean J. Anesthesiol. 2018, 71, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Akolekar, R.; Casagrandi, D.; Skyfta, E.; Ahmed, A.A.; Nicolaides, K.H. Maternal serum angiopoietin-2 at 11 to 13 weeks of gestation in hypertensive disorders of pregnancy. Prenat. Diagn. 2009, 29, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Aoba, H.; Hariu, Y.; Yamaguchi, R. Serum Heat-stable Alkaline Phosphatase in Normal and Abnormal Pregnancy. Tohoku J. Exp. Med. 1967, 91, 201–207. [Google Scholar] [CrossRef]
- Bagga, O.; Mullick, V.; Madan, P.; Dewan, S. Total serum alkaline phosphate and its isoenzymes in normal and toxemic pregnancies. Am. J. Obstet. Gynecol. 1969, 104, 850–855. [Google Scholar] [CrossRef]
- Bolin, M.; Wiberg-Itzel, E.; Wikström, A.-K.; Goop, M.; Larsson, A.; Olovsson, M.; Åkerud, H. Angiopoietin-1/Angiopoietin-2 Ratio for Prediction of Preeclampsia. Am. J. Hypertens. 2009, 22, 891–895. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.; Ou, W.; Lin, D.; Lin, M.; Huang, X.; Ni, S.; Chen, S.; Yong, J.; O’Gara, M.C.; Tan, X.; et al. Increased Uric Acid, Gamma-Glutamyl Transpeptidase and Alkaline Phosphatase in Early-Pregnancy Associated With the Development of Gestational Hypertension and Preeclampsia. Front. Cardiovasc. Med. 2021, 8. [Google Scholar] [CrossRef]
- Gotsch, F.; Romero, R.; Kusanovic, J.P.; Chaiworapongsa, T.; Dombrowski, M.; Erez, O.; Than, N.G.; Mazaki-Tovi, S.; Mittal, P.; Espinoza, J.; et al. Preeclampsia and small-for-gestational age are associated with decreased concentrations of a factor involved in angiogenesis: Soluble Tie-2. J. Matern. Neonatal Med. 2008, 21, 389–402. [Google Scholar] [CrossRef]
- Han, S.Y.; Jun, J.K.; Lee, C.-H.; Park, J.S.; Syn, H.C. Angiopoietin-2: A Promising Indicator for the Occurrence of Severe Preeclampsia. Hypertens. Pregnancy 2010, 31, 189–199. [Google Scholar] [CrossRef]
- Hirokoshi, K.; Maeshima, Y.; Kobayashi, K.; Matsuura, E.; Sugiyama, H.; Yamasaki, Y.; Masuyama, H.; Hiramatsu, Y.; Makino, H. Increase of Serum Angiopoietin-2 During Pregnancy Is Suppressed in Women With Preeclampsia. Am. J. Hypertens. 2005, 18, 1181–1188. [Google Scholar] [CrossRef][Green Version]
- Hirokoshi, K.; Maeshima, Y.; Kobayashi, K.; Matsuura, E.; Sugiyama, H.; Yamasaki, Y.; Masuyama, H.; Hiramatsu, Y.; Makino, H. Elevated Serum sFlt-1/Ang-2 Ratio in Women with Preeclampsia. Nephron Clin. Pr. 2007, 106, c43–c50. [Google Scholar] [CrossRef] [PubMed]
- Horjus, D.L.; Bokslag, A.; Hooijberg, F.; Hutten, B.A.; Middeldorp, S.; De Groot, C.J. Creatine kinase and blood pressure in women with a history of early-onset preeclampsia. Pregnancy Hypertens. 2018, 15, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Kamal, M.; El-Khayat, W. Do Serum Angiopoietin-1, Angiopoietin-2, and Their Receptor Tie-2 and 4G/5G Variant of PAI-1 Gene Have a Role in the Pathogenesis of Preeclampsia? J. Investig. Med. 2011, 59, 1147–1150. [Google Scholar] [CrossRef]
- Karakus, S.; Akkar, O.B.; Yildiz, C.; Sancakdar, E.; Cetin, M.; Cetin, A. Serum levels of ET-1, M30, and angiopoietins-1 and -2 in HELLP syndrome and preeclampsia compared to controls. Arch. Gynecol. Obstet. 2015, 293, 351–359. [Google Scholar] [CrossRef]
- Khalil, A.; Maiz, N.; Garcia-Mandujano, R.; Elkhouli, M.; Nicolaides, K.H. Longitudinal changes in maternal soluble endoglin and angiopoietin-2 in women at risk for pre-eclampsia. Ultrasound Obstet. Gynecol. 2014, 44, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Koroglu, N.; Tola, E.; Yuksel, I.T.; Cetin, B.A.; Turhan, U.; Topcu, G.; Dag, I. Maternal serum AMP-activated protein kinase levels in mild and severe preeclampsia. J. Matern. Neonatal Med. 2018, 32, 2735–2740. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Devi, S.G.; Prasad, S.; Kapoor, S.; Sharma, S. Bone turnover in preeclampsia-complicated pregnancy in North Indian women. J. Obstet. Gynaecol. Res. 2011, 38, 172–179. [Google Scholar] [CrossRef]
- Leijnse, J.E.; de Heus, R.; de Jager, W.; Rodenburg, W.; Peeters, L.L.; Franx, A.; Eijkelkamp, N. First trimester placental vascularization and angiogenetic factors are associated with adverse pregnancy outcome. Pregnancy Hypertens. 2018, 13, 87–94. [Google Scholar] [CrossRef]
- Leinonen, E.; Wathén, K.-A.; Alfthan, H.; Ylikorkala, O.; Andersson, S.; Stenman, U.-H.; Vuorela, P. Maternal Serum Angiopoietin-1 and -2 and Tie-2 in Early Pregnancy Ending in Preeclampsia or Intrauterine Growth Retardation. J. Clin. Endocrinol. Metab. 2010, 95, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Machado, J.; Bertagnolli, T.; Martins, L.; Freitas, S.; Ovidio, P.; Sandrim, V.; Cardoso, V.; Bettiol, H.; Barbieri, M.; Cavalli, R. Role of plasma PlGF, PDGF-AA, ANG-1, ANG-2, and the ANG-1/ANG-2 ratio as predictors of preeclampsia in a cohort of pregnant women. Pregnancy Hypertens. 2019, 16, 105–111. [Google Scholar] [CrossRef]
- Martinez-Fierro, M.L.; Castruita-De La Rosa, C.; Garza-Veloz, I.; Cardiel-Hernandez, R.M.; Espinoza-Juarez, M.A.; Delgado-Enciso, I.; Castañeda-Lopez, M.E.; Cardenas-Vargas, E.; Trejo-Vázquez, F.; Sotelo-Ham, E.I.; et al. Early pregnancy protein multiplex screening reflects circulating and urinary divergences associated with the development of preeclampsia. Hypertens. Pregnancy 2018, 37, 37–50. [Google Scholar] [CrossRef]
- Mazibuko, M.; Moodley, J.; Naicker, T. Dysregulation of circulating sTie2 and sHER2 in HIV-infected women with preeclampsia. Hypertens. Pregnancy 2019, 38, 89–95. [Google Scholar] [CrossRef]
- Morrison, J.C.; Whybrew, D.; Wiser, W.L.; Bucovaz, E.; Fish, S.A. Enzyme levels in the serum and cerebrospinal fluid in eclampsia. Am. J. Obstet. Gynecol. 1971, 110, 619–624. [Google Scholar] [CrossRef]
- Nadar, S.; Karalis, I.; Al Yemeni, E.; Blann, A.; Lip, G. Plasma markers of angiogenesis in pregnancy induced hypertension. Thromb. Haemost. 2005, 94, 1071–1076. [Google Scholar] [CrossRef]
- Naghshvar, F.; Torabizadeh, Z.; Zadeh, N.M.; Mirbaha, H.; Gheshlaghi, P. Investigating the Relationship between Serum Level of s-Met (Soluble Hepatic Growth Factor Receptor) and Preeclampsia in the First and Second Trimesters of Pregnancy. ISRN Obstet. Gynecol. 2013, 2013, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Nayel, S.A.; Enin, M.A.; El Hefni, S.E.; Khalil, S.A. Serum Alkaline Phosphatase in EPH Gestosis. Asia-Oceania J. Obstet. Gynaecol. 2010, 8, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Puttapitakpong, P.; Phupong, V. Combination of serum angiopoietin-2 and uterine artery Doppler for prediction of preeclampsia. Hypertens. Res. 2015, 39, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Aref, S.; Goda, H.; Abdelaal, E. Circulating Vascular Growth Factor (VEGF) Angiopoietin-1 (Angi-1) and Soluble Tie-2 Receptor in Pregnancy Complicated with Pre-eclampsia: A Prospective Study. J. Obstet. Gynecol. India 2013, 63, 316–320. [Google Scholar] [CrossRef][Green Version]
- Salgó, L.; Pál, A. Variation in Some Enzymes in Amniotic Fluid and Maternal Serum during Pregnancy. Enzyme 1989, 41, 101–107. [Google Scholar] [CrossRef]
- Sammour, M.B.; Fattah, M.M.; Ibrahim, F.K.; Ramadan, M.A. Creatine phosphokinase activity in maternal, cord blood and placenta of normal pregnancy, and in EPH-gestosis. Biochem. Med. 1974, 11, 205–209. [Google Scholar] [CrossRef]
- Sammour, M.B.; Ramadan, M.A.; Khalil, F.K.; Abd-El-Fattah, M.M. Serum and Placental Lactic Dehydrogenase and Alkaline Phosphatase Isoenzymes in Normal Pregnancy and in Pre-Eclampsia. Acta Obstet. et Gynecol. Scand. 1975, 54, 393–400. [Google Scholar] [CrossRef]
- Schneuer, F.J.; Roberts, C.L.; Ashton, A.W.; Guilbert, C.; Tasevski, V.; Morris, J.M.; Nassar, N. Angiopoietin 1 and 2 serum concentrations in first trimester of pregnancy as biomarkers of adverse pregnancy outcomes. Am. J. Obstet. Gynecol. 2014, 210, 345.e1–345.e9. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.-S.; Lee, C.H.; Jun, J.K. Midtrimester maternal plasma concentrations of angiopoietin 1, angiopoietin 2, and placental growth factor in pregnant women who subsequently develop preeclampsia. Obstet. Gynecol. Sci. 2015, 58, 10–16. [Google Scholar] [CrossRef]
- Kim, S.Y.; Park, S.Y.; Kim, M.J.; Lee, B.Y.; Han, J.Y.; Ryu, H.M. Preeclampsia is associated with an elevation of plasma sMet concentrations in the second trimester. J. Matern. Neonatal Med. 2013, 26, 860–865. [Google Scholar] [CrossRef]
- Sung, J.F.; Fan, X.; Dhal, S.; Dwyer, B.K.; Jafari, A.; El-Sayed, Y.Y.; Druzin, M.L.; Nayak, N.R. Decreased Circulating Soluble Tie2 Levels in Preeclampsia May Result from Inhibition of Vascular Endothelial Growth Factor (VEGF) Signaling. J. Clin. Endocrinol. Metab. 2011, 96, E1148–E1152. [Google Scholar] [CrossRef]
- Wang, L.-J.; Chen, W.-P.; Peng, W.; Xu, L.; Sui, A.-H.; Ye, Y.-H. Correlation of angiopoietin-2 and angiopoietin-2 receptor expressions in serum and placenta with preeclampsia. Zhonghua Fu Chan Ke Za Zhi 2011, 46. [Google Scholar]
- Watson, D.; Weston, W.; Porter, R. Plasma Alkaline Phosphatases in Normal and Abnormal Terminal Pregnancy. Enzym. Biol. et Clin. 1965, 5, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Young, B.K.; Beller, F.K. Plasma acid phosphatase in normal and pre-eclamptic pregnancy. Am. J. Obstet. Gynecol. 1968, 101, 1068–1072. [Google Scholar] [CrossRef]
- Zeng, X.; Sun, Y.; Yang, H.-X.; Li, D.; Li, Y.-X.; Liao, Q.-P.; Wang, Y.-L. Plasma level of soluble c-Met is tightly associated with the clinical risk of preeclampsia. Am. J. Obstet. Gynecol. 2009, 201, 618.e1–618.e7. [Google Scholar] [CrossRef]
- Furuya, M.; Kurasawa, K.; Nagahama, K.; Kawachi, K.; Nozawa, A.; Takahashi, T.; Aoki, I. Disrupted Balance of Angiogenic and Antiangiogenic Signalings in Preeclampsia. J. Pregnancy 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Helske, S.; Vuorela, P.; Carpén, O.; Hornig, C.; Weich, H.; Halmesmäki, E. Expression of vascular endothelial growth factor receptors 1, 2 and 3 in placentas from normal and complicated pregnancies. Mol. Hum. Reprod. 2001, 7, 205–210. [Google Scholar] [CrossRef]
- Maynard, S.E.; Min, J.-Y.; Merchan, J.; Lim, K.-H.; Li, J.; Mondal, S.; Libermann, T.A.; Morgan, J.P.; Sellke, F.W.; Stillman, I.E.; et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Investig. 2003, 111, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Findley, C.M.; Cudmore, M.J.; Ahmed, A.; Kontos, C.D. VEGF Induces Tie2 Shedding via a Phosphoinositide 3-Kinase/Akt–Dependent Pathway to Modulate Tie2 Signaling. Arter. Thromb. Vasc. Biol. 2007, 27, 2619–2626. [Google Scholar] [CrossRef] [PubMed]
- Kauma, S.; Hayes, N.; Weatherford, S. The Differential Expression of Hepatocyte Growth Factor and Met in Human Placenta*. J. Clin. Endocrinol. Metab. 1997, 82, 949–954. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Somerset, D.A.; Li, X.-F.; Afford, S.; Strain, A.J.; Ahmed, A.; Sangha, R.K.; Whittle, M.J.; Kilby, M.D. Ontogeny of Hepatocyte Growth Factor (HGF) and Its Receptor (c-met) in Human Placenta: Reduced HGF Expression in Intrauterine Growth Restriction. Am. J. Pathol. 1998, 153, 1139–1147. [Google Scholar] [CrossRef] [PubMed]
- Elbanna, M.; Orillion, A.R.; Damayanti, N.P.; Adelaiye-Ogala, R.; Shen, L.; Miles, K.M.; Chintala, S.; Ciamporcero, E.; Ramakrishnan, S.; Ku, S.-Y.; et al. Dual Inhibition of Angiopoietin-TIE2 and MET Alters the Tumor Microenvironment and Prolongs Survival in a Metastatic Model of Renal Cell Carcinoma. Mol. Cancer Ther. 2020, 19, 147–156. [Google Scholar] [CrossRef]

| Author, Year | Country | Biomolecule | Total Number of Patients/PE Group/Control Group | Trimester of Measurement | Preeclampsia Type |
|---|---|---|---|---|---|
| Akolekar, 2009 [22] | UK | ANG-2 (angiopoietin-2) | 324/116/208 | First | Unspecified |
| Aoba, 1967 [23] | Japan | ALP (Alkaline Phosphatase) | 162/11/151 | Second | Severe preeclampsia |
| Aoba, 1967 [23] | Japan | ALP (Alkaline Phosphatase) | 162/11/151 | Third | Severe preeclampsia |
| Aoba, 1967 [23] | Japan | HSAP (Heat stable alkaline phos- phatase) | 162/11/151 | Second | Severe preeclampsia |
| Aoba, 1967 [23] | Japan | HSAP (Heat stable alkaline phos- phatase) | 162/11/151 | Third | Severe preeclampsia |
| Aoba, 1967 [23] | Japan | HLP (Heat-Labile Alkaline Phosphatase) | 162/11/151 | second | Severe preeclampsia |
| Aoba, 1967 [23] | Japan | HLP (Heat-Labile Alkaline Phosphatase) | 162/11/151 | Third | Severe preeclampsia |
| Bagga, 1969 [24] | India | ALP (Alkaline Phosphatase) | 100/45/55 | Third | Unspecified |
| Bolin, 2009 [25] | Sweden | Ang1/Ang2 ratio | 62/19/43 | First | Unspecified |
| Bolin, 2009 [25] | Sweden | Ang1/Ang2 ratio | 62/19/43 | Second | Unspecified |
| Bolin, 2009 [25] | Sweden | ANG-2 (angiopoietin-2) | 62/19/43 | First | Unspecified |
| Bolin, 2009 [25] | Sweden | ANG-2 (angiopoietin-2) | 62/19/43 | Second | Unspecified |
| Bolin, 2009 [25] | Sweden | ANG-2 (angiopoietin-2) | 62/19/43 | Third | Unspecified |
| Chen, 2021 [26] | China | ALP (Alkaline Phosphatase) | 1012/31/981 | First, Second and Third | Unspecified |
| Gotsch, 2008 [27] | USA | (sTie-2) | 247/112/135 | Second and Third | Mild, severe, early and late preeclampsia |
| Han, 2012 [28] | Korea | ANG-2 (angiopoietin-2) | 45/16/29 | Third | Severe preeclampsia |
| Hirokoshi, 2005 [29] | Japan | ANG-2 (angiopoietin-2) | 55/26/29 | Second and Third | Mild and severe preeclampsia |
| Hirokoshi, 2007 [30] | Japan | ANG-2 (angiopoietin-2) | 65/36/29 | Second and Third | Mild and severe preeclampsia |
| Horjus, 2019 [31] | Netherlands | Creatine kinase (CK) | 3215/127/3088 | First and second | Early preeclampsia |
| Kamal, 2011 [32] | Egypt | ANG-2 (angiopoietin-2) | 103/68/35 | Not specified | Unspecified |
| Karakus, 2015 [33] | Germany | Ang1/Ang2 ratio | 62/25/37 | Third | Unspecified |
| Karakus, 2015 [33] | Germany | ANG-2 (angiopoietin-2) | 51/17/34 | Second and Third | Unspecified |
| Khalil, 2014 [34] | UK | ANG-2 (angiopoietin-2) | 106/22/84 | First, Second and Third | Preterm preeclampsia and term preeclampsia |
| Koroglu, 2018 [35] | Finland | Adenosine AMP-activated protein kinase (AMPK) | 80/50/30 | Third | Mild and severe preeclampsia |
| Kumar, 2011 [36] | India | sBAP (serum bone alkaline phosphatase) | 120/22/98 | Second | Unspecified |
| Leijnse, 2018 [37] | Netherlands | Ang1/Ang2 ratio | 57/6/51 | First | Late onset preeclampsia |
| Leijnse, 2018 [37] | Netherlands | ANG-2 (angiopoietin-2) | 57/6/51 | First | Late onset preeclampsia |
| Leinonen, 2009 [38] | Finland | Ang1/Ang2 ratio | 91/50/41 | Second | Mild and severe preeclampsia |
| Leinonen, 2009 [38] | Finland | Specific tyrosine kinase receptor (sTie2) | 108/49/59 | Second | Mild and severe preeclampsia |
| Leinonen, 2009 [38] | Finland | ANG-2 (angiopoietin-2) | 108/49/59 | Second | Mild and severe preeclampsia |
| Machado, 2019 [39] | Brazil | Ang1/Ang2 ratio | 120/30/90 | Second | Unspecified |
| Machado, 2019 [39] | Brazil | ANG-2 (angiopoietin-2) | 120/30/90 | Second | Unspecified |
| Martinez, 2018 [40] | Mexico | ANG-2 (angiopoietin-2) | 36/16/20 | Second | Early, late and severe preeclampsia |
| Mazibuko, 2019 [41] | South Africa | Specific tyrosine kinase receptor (sTie2) | 40/20/20 | Not specified | Unspecified |
| Morrison, 1971 [42] | USA | Creatine phosphokinase | 65/35/30 | Third | Severe preeclampsia |
| Nadar, 2005 [43] | UK | Ang1/Ang2 ratio | 99/35/64 | Third | Unspecified |
| Nadar, 2005 [43] | UK | ANG-2 (angiopoietin-2) | 99/35/64 | Third | Unspecified |
| Naghshvar, 2013 [44] | Iran | s-Met (soluble mesenchymal-epithelial transition factor) | 95/44/51 | First and second | Mild, severe, early and late preeclampsia |
| Nayel, 1982 [45] | Egypt | ALP (Alkaline Phosphatase) | 30/20/10 | Third | Severe preeclampsia |
| Puttapitakpong, 2015 [46] | Japan | ANG-2 (angiopoietin-2) | 366/25/341 | Second | Early preeclampsia |
| Aref, 2013 [47] | India | ANG-1 (angiopoietin-1) and Soluble Tie-2 receptor (sTie2) | 238/150/88 | Not specified | Mild, severe, early and late preeclampsia |
| Salgó, 1989 [48] | Hungary | Alkaline phosphatase, acid phosphatase and creatine kinase | 184/172/12 | Second and Third | Unspecified |
| Sammour, 1974 [49] | Egypt | Creatine phospho-kinase | 30/20/10 | Third | Mild and severe preeclampsia |
| Sammour, 1975 [50] | Egypt | HSP (Heat-stable alkaline phosphatase) | 30/20/10 | Third | Unspecified |
| Schneuer, 2013 [51] | Australia | ANG-2 (angiopoietin-2) | 3893/163/3730 | First | Early preeclampsia |
| Shim, 2015 [52] | Korea | Ang1/Ang2 ratio | 74/37/37 | Second | Mild and severe preeclampsia |
| Shim, 2015 [52] | Korea | ANG-2 (angiopoietin-2) | 74/37/37 | Second | Mild and severe preeclampsia |
| Shin, 2013 [53] | Seoul | sMet | 331/115/216 | Second and Third | Unspecified |
| Sung, 2011 [54] | USA | Specific tyrosine kinase receptor (sTie2) | 55/24/31 | First, Second and Third | Unspecified |
| Wang, 2011 [55] | China | ANG-2 (Angiopoietin-2) | 92/62/30 | Not specified | Moderate and severe preeclampsia |
| Watson, 1965 [56] | Australia | ALP (Alkaline Phosphatase) | 28/3/25 | Third | Unspecified |
| Watson, 1965 [56] | Australia | HSP (Heat-stable alkaline phosphatase) | 28/3/25 | Third | Unspecified |
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | TOTAL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Akolekar et al., 2009 [22] | YES | YES | YES | YES | YES | YES | UNC | UNC | YES | YES | 8 |
| Aoba et al., 1967 [23] | NO | NO | UNC | NO | NO | NO | NO | NO | YES | NO | 1 |
| Bagga et al., 1969 [24] | YES | NO | NO | NO | NO | NO | NO | NO | UNC | UNC | 1 |
| Bolin et al., 2009 [25] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Chen et al., 2021 [26] | UNC | YES | YES | YES | YES | YES | YES | YES | YES | YES | 9 |
| Gotsch et al., 2008 [27] | YES | YES | YES | YES | YES | UNC | YES | YES | YES | YES | 9 |
| Han et al., 2012 [28] | YES | YES | YES | YES | YES | UNC | YES | YES | NO | YES | 8 |
| Hirokoshi et al., 2005 [29] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Hirokoshi et al., 2007 [30] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Horjus et al., 2019 [31] | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | 10 |
| Kamal et al., 2011 [32] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Karakus et al., 2015 [33] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Khalil et al., 2014 [34] | YES | YES | YES | YES | YES | YES | YES | YES | UNC | YES | 9 |
| Koroglu et al., 2018 [35] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Kumar et al., 2011 [36] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Leinonen et al., 2009 [38] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Leijnse et al., 2018 [37] | YES | YES | YES | YES | YES | YES | NO | YES | YES | YES | 9 |
| Machado et al., 2019 [39] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Martínez et al., 2018 [40] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Mazibuko et al., 2019 [41] | YES | UNC | YES | YES | YES | NO | NO | YES | YES | UNC | 6 |
| Morrison et al., 1971 [42] | NO | NO | UNC | YES | UNC | NO | NO | UNC | YES | NO | 2 |
| Nadar et al., 2005 [43] | YES | YES | YES | YES | YES | UNC | UNC | YES | YES | YES | 8 |
| Naghshvar et al., 2013 [44] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Nayel et al., 1982 [45] | YES | UNC | UNC | NO | UNC | NO | NO | NO | YES | NO | 2 |
| Puttapitakpong et al., 2015 [46] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Aref et al., 2013 [47] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Salgo et al., 1989 [48] | NO | NO | NO | NO | UNC | NO | NO | NO | UNC | UNC | 0 |
| Sammour et al., 1974 [49] | YES | YES | UNC | YES | YES | NO | NO | YES | UNC | YES | 6 |
| Sammour et al., 1975 [50] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Schneuer et al., 2013 [51] | YES | NO | YES | UNC | UNC | NO | NO | YES | YES | YES | 5 |
| Shim et al., 2015 [52] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Sung et al., 2011 [54] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Wang et al., 2011 [55] | YES | YES | YES | YES | YES | NO | NO | YES | UNC | YES | 7 |
| Watson et al., 1965 [56] | NO | NO | YES | UNC | UNC | NO | NO | UNC | YES | UNC | 2 |
| Young et al., 1968 [57] | NO | YES | YES | YES | YES | NO | NO | YES | YES | YES | 7 |
| Kim et al., 2013 [53] | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | 10 |
| Zeng et al., 2009 [58] | YES | YES | YES | YES | YES | NO | NO | YES | YES | YES | 8 |
| Q1. Were the groups comparable other than the presence of disease in cases or the absence of disease in controls? Q2. Were cases and controls matched appropriately? Q3. Were the same criteria used for the identification of cases and controls? Q4. Was exposure measured in a standard, valid, and reliable way? Q5. Was exposure measured in the same way for cases and controls? Q6. Were confounding factors identified? Q7. Were strategies to deal with confounding factors stated? Q8. Were outcomes assessed in a standard, valid, and reliable way for cases and controls? Q9. Was the exposure period of interest long enough to be meaningful? Q10. Was appropriate statistical analysis used? | |||||||||||
| Covariate/Modulator | Estimate | 95% CI | p-Value | R2 (%) | |
|---|---|---|---|---|---|
| Gestational Age | 0.01794 | −0.0316 | 0.3903 | 0.0956 | 26.59 |
| Maternal Age | −1.4117 | −2.6379 | −0.1856 | 0.0240 | 71.70 |
| Pregestational BMI | - | - | - | - | - |
| Covariate/Modulator | Estimate | 95% CI | p-Value | R2 (%) | |
|---|---|---|---|---|---|
| Gestational Age | −0.2335 | −0.4834 | 0.0163 | 0.0670 | 32.63 |
| Maternal Age | 0.1996 | −0.5839 | 0.9831 | 0.6176 | 0.00 |
| Pregestational BMI | −24.0521 | −35.7118 | −12.3924 | <0.001 | 84.72 |
| Covariate/Modulator | Estimate | 95% CI | p-Value | R2 (%) | |
|---|---|---|---|---|---|
| Gestational Age | −0.0078 | −0.0226 | 0.0069 | 0.2964 | 0.000 |
| Maternal Age | −0.0214 | −0.0646 | 0.0217 | 0.3295 | 0.000 |
| Pregestational BMI | −0.1046 | −0.3795 | 0.1703 | 0.9042 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marrufo-Gallegos, K.C.; Villafán-Bernal, J.R.; Espino-y-Sosa, S.; Estrada-Gutierrez, G.; Guzmán-Guzmán, I.P.; Martinez-Portilla, R.J.; Torres-Torres, J. Influential Serum Kinases (Non-sFlt-1) and Phosphatases in Preeclampsia—Systemic Review and Metanalysis. Int. J. Mol. Sci. 2023, 24, 12842. https://doi.org/10.3390/ijms241612842
Marrufo-Gallegos KC, Villafán-Bernal JR, Espino-y-Sosa S, Estrada-Gutierrez G, Guzmán-Guzmán IP, Martinez-Portilla RJ, Torres-Torres J. Influential Serum Kinases (Non-sFlt-1) and Phosphatases in Preeclampsia—Systemic Review and Metanalysis. International Journal of Molecular Sciences. 2023; 24(16):12842. https://doi.org/10.3390/ijms241612842
Chicago/Turabian StyleMarrufo-Gallegos, Karla Cecilia, Jose Rafael Villafán-Bernal, Salvador Espino-y-Sosa, Guadalupe Estrada-Gutierrez, Iris Paola Guzmán-Guzmán, Raigam Jafet Martinez-Portilla, and Johnatan Torres-Torres. 2023. "Influential Serum Kinases (Non-sFlt-1) and Phosphatases in Preeclampsia—Systemic Review and Metanalysis" International Journal of Molecular Sciences 24, no. 16: 12842. https://doi.org/10.3390/ijms241612842
APA StyleMarrufo-Gallegos, K. C., Villafán-Bernal, J. R., Espino-y-Sosa, S., Estrada-Gutierrez, G., Guzmán-Guzmán, I. P., Martinez-Portilla, R. J., & Torres-Torres, J. (2023). Influential Serum Kinases (Non-sFlt-1) and Phosphatases in Preeclampsia—Systemic Review and Metanalysis. International Journal of Molecular Sciences, 24(16), 12842. https://doi.org/10.3390/ijms241612842








