Management and Molecular Characterization of Intraventricular Glioblastoma: A Single-Institution Case Series
Abstract
1. Introduction
2. Materials and Methods
3. Case Presentation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Koshy, M.; Villano, J.L.; Dolecek, T.A.; Howard, A.; Mahmood, U.; Chmura, S.J.; Weichselbaum, R.R.; McCarthy, B.J. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J. Neuro Oncol. 2012, 107, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro Oncol. 2022, 24 (Suppl. S5), v1–v95. [Google Scholar] [CrossRef] [PubMed]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma Multiforme: A Review of its Epidemiology and Pathogenesis through Clinical Presentation and Treatment. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 3–9. [Google Scholar] [PubMed]
- Andoh, T.; Shinoda, J.; Miwa, Y.; Hirata, T.; Sakai, N.; Yamada, H.; Shimokawa, K. Tumors at the trigone of the lateral ventricle—Clinical analysis of eight cases. Neurol. Med. Chir. 1990, 30, 676–684. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dumont, A.S.; Farace, E.; Schiff, D.; Shaffrey, M.E. Intraventricular gliomas. Neurosurg. Clin. N. Am. 2003, 14, 571–591. [Google Scholar] [CrossRef]
- Guibaud, L.; Champion, F.; Buenerd, A.; Pelizzari, M.; Bourgeois, J.; Pracros, J.P. Fetal intraventricular glioblastoma: Ultraso-nographic, magnetic resonance imaging, and pathologic findings. J. Ultrasound Med. 1997, 16, 285–288. [Google Scholar] [CrossRef]
- Hambly, N.M.; Farrell, M.A.; Scanlon, T.G.; Mcerlean, A.; Kavanagh, E.C. Case report. Glioblastoma multiforme presenting as a haemorrhagic minimally enhancing mass of the trigone. Br. J. Radiol. 2009, 82, e204–e207. [Google Scholar] [CrossRef]
- Hariri, O.R.; Quadri, S.A.; Farr, S.; Gupta, R.; Bieber, A.J.; Dyurgerova, A.; Corsino, C.; Miulli, D.; Siddiqi, J. Third Ventricular Glioblastoma Multiforme: Case Report and Literature Review. J. Neurol. Surg. Rep. 2015, 76, e227–e232. [Google Scholar]
- Karmilov, V.O. Primary intraventricular brain tumor (glioblastoma multiforme) in a child 2 years and 7 months. Pediatr. Akusherstvo I Ginekol. 1978, 2, 30. [Google Scholar]
- Kim, Y.J.; Lee, S.K.; Cho, M.K.; Kim, Y.K. Intraventricular glioblastoma multiforme with previous history of intracerebral hem-orrhage: A case report. J. Korean Neurosurg. Soc. 2008, 44, 405–408. [Google Scholar] [CrossRef]
- Klein, O.; Marchal, J.C. Intraventricular glioblastoma: A paediatric case report. Br. J. Neurosurg. 2007, 21, 411–413. [Google Scholar] [CrossRef]
- Koch, D. Spinal metastases of cerebral glioma. Case report. Neurosurg. Rev. 1996, 19, 201–203. [Google Scholar] [CrossRef]
- Park, P.; Choksi, V.R.; Gala, V.C.; Kaza, A.R.; Murphy, H.S.; Ramnath, S. Well-Circumscribed, Minimally Enhancing Glioblastoma Multiforme of the Trigone: A Case Report and Review of the Literature. Am. J. Neuroradiol. 2005, 26, 1475–1478. [Google Scholar]
- Prieto, R.; Pascual, J.M.; Roda, J.M. Third ventricle glioblastoma. Case report and review of literature. Clin. Neurol. Neurosurg. 2006, 108, 199–204. [Google Scholar] [CrossRef]
- Sarsilmaz, A.; Gelal, F.; Apaydin, M.; Varer, M.; Bezircioglu, H.; Rezanko, T. Intraventricular glioblastoma multiforme: A pediatric case report. J. Pediatr. Hematol. Oncol. 2010, 32, 519–522. [Google Scholar] [CrossRef]
- Secer, H.I.; Dinc, C.; Anik, I.; Duz, B.; Gonul, E. Glioblastoma multiforme of the lateral ventricle: Report of nine cases. Br. J. Neurosurg. 2008, 22, 398–401. [Google Scholar] [CrossRef]
- Shapiro, R. Intraventricular glioblastoma multiforme with the pneumographic characteristics of intraventricular epidermoids; a case report with a critical analysis. Radiology 1950, 55, 852–854. [Google Scholar] [CrossRef]
- Fenchel, M.; Beschorner, R.; Naegele, T.; Korn, A.; Ernemann, U.; Horger, M. Primarily solid intraventricular brain tumors. Eur. J. Radiol. 2012, 81, e688–e696. [Google Scholar] [CrossRef] [PubMed]
- Nsir, A.B.; Gdoura, Y.; Thai, Q.A.; Kassar, A.Z.; Hattab, N.; Jemel, H. Intraventricular Glioblastomas. World Neurosurg. 2016, 88, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Berger, T.R.; Wen, P.Y.; Lang-Orsini, M.; Chukwueke, U.N. World Health Organization 2021 Classification of Central Nervous System Tumors and Implications for Therapy for Adult-Type Gliomas: A Review. JAMA Oncol. 2022, 8, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Villa, C.; Miquel, C.; Mosses, D.; Bernier, M.; Di Stefano, A.L. The 2016 World Health Organization classification of tumours of the central nervous system. Presse Médicale 2018, 47, e187–e200. [Google Scholar] [CrossRef] [PubMed]
- Craven, K.E.; Fischer, C.G.; Jiang, L.; Pallavajjala, A.; Lin, M.-T.; Eshleman, J.R. Optimizing Insertion and Deletion Detection Using Next-Generation Sequencing in the Clinical Laboratory. J. Mol. Diagn. 2022, 24, 1217–1231. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Lakke, J. Report on 16 Intraventricular Brain Tumors: A Clinical Study. Eur. Neurol. 1969, 2, 158–174. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Tramontin, A.D.; Quiñones-Hinojosa, A.; Barbaro, N.M.; Gupta, N.; Kunwar, S.; Lawton, M.T.; McDermott, M.W.; Parsa, A.T.; García-Verdugo, J.M.; et al. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 2004, 427, 740–744. [Google Scholar] [CrossRef]
- Wagner, J.A.; Frost, J.K.; Wisotzkey, H. Subarachnoid neoplasia: Incidence and problems of diagnosis. South. Med. J. 1960, 53, 1503–1508. [Google Scholar] [CrossRef]
- Rich, J.R. A survey of cerebrospinal fluid cytology. Bull. Los Angel. Neurol. Soc. 1969, 34, 115–131. [Google Scholar]
- Birzu, C.; Tran, S.; Bielle, F.; Touat, M.; Mokhtari, K.; Younan, N.; Psimaras, D.; Hoang-Xuan, K.; Sanson, M.; Delattre, J.; et al. Leptomeningeal Spread in Glioblastoma: Diagnostic and Therapeutic Challenges. Oncologist 2020, 25, e1763–e1776. [Google Scholar] [CrossRef]
- Alatakis, S.; Malham, G.M.; Thien, C. Spinal leptomeningeal metastasis from cerebral glioblastoma multiforme presenting with radicular pain: Case report and literature review. Surg. Neurol. 2001, 56, 33–37. [Google Scholar] [CrossRef]
- Lee, T.T.; Manzano, G.R. Third ventricular glioblastoma multiforme: Case report. Neurosurg. Rev. 1997, 20, 291–294. [Google Scholar] [CrossRef]
- Tan, A.P.; Mankad, K. Intraventricular Glioblastoma Multiforme in A Child with L2-Hydroxyglutaric Aciduria. World Neurosurg. 2018, 110, 288–290. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, C.; Cheng, D.; Tang, L.; Qi, Z. Effects of gross total resection and subtotal resection on survival outcomes of glioma patients: A meta-analysis. Biotechnol. Genet. Eng. Rev. 2023, 1, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.M.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [PubMed]
- Şuşman, S.; Leucuţa, D.-C.; Kacso, G.; Florian, L. High dose vs low dose irradiation of the subventricular zone in patients with glioblastoma—A systematic review and meta-analysis. Cancer Manag. Res. 2019, 11, 6741–6753. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Guerrero-Cazares, H.; Ye, X.; Ford, E.; McNutt, T.; Kleinberg, L.; Lim, M.; Chaichana, K.; Quinones-Hinojosa, A.; Redmond, K. Increased Subventricular Zone Radiation Dose Correlates With Survival in Glioblastoma Patients After Gross Total Resection. Int. J. Radiat. Oncol. 2013, 86, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chaichana, K.L.; Kleinberg, L.; Ye, X.; Quinones-Hinojosa, A.; Redmond, K. Glioblastoma recurrence patterns near neural stem cell regions. Radiother. Oncol. 2015, 116, 294–300. [Google Scholar] [CrossRef]
- Beiriger, J.; Habib, A.; Jovanovich, N.; Kodavali, C.V.; Edwards, L.; Amankulor, N.; Zinn, P.O. The Subventricular Zone in Glioblastoma: Genesis, Maintenance, and Modeling. Front. Oncol. 2022, 12, 790976. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Q.; Zhao, K.; Liu, M.; Sun, G.; Xu, B. MR Imaging, MGMT Promoter Methylation Features and Prognostic Analysis of Subventricular Zone Contacting IDH Wild-type Glioblastoma. Curr. Med. Imaging 2023, 19, 1378–1386. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, M.; Liu, X.; Wang, J.; Shou, Y.; Sun, H. Clinical features and prognostic significance of tumor involved with sub-ventricular zone in pediatric glioblastoma: A 10-year experience in a single hospital. Childs Nerv. Syst. 2022, 38, 1469–1477. [Google Scholar] [CrossRef]
- Comas, S.; Luguera, E.; Molero, J.; Balaña, C.; Estival, A.; Castañer, S.; Carrato, C.; Hostalot, C.; Teixidor, P.; Villà, S. Influence of glioblastoma contact with the subventricular zone on survival and recurrence patterns. Clin. Transl. Oncol. 2021, 23, 554–564. [Google Scholar] [CrossRef]
- van Dijken, B.R.J.; Schuuring, B.; Jeltema, H.-R.; van Laar, P.J.; Enting, R.H.; Dierckx, R.A.J.O.; Stormezand, G.N.; van der Hoorn, A. Ventricle contact may be associated with higher 11C methionine PET uptake in glioblastoma. Neuroradiology 2022, 64, 247–252. [Google Scholar] [CrossRef]
- Hallaert, G.; Pinson, H.; Broecke, C.V.D.; Vanhauwaert, D.; Van Roost, D.; Boterberg, T.; Kalala, J.P. Subventricular zone contacting glioblastoma: Tumor size, molecular biological factors and patient survival. Acta Oncol. 2020, 59, 1474–1479. [Google Scholar] [CrossRef] [PubMed]
- Iacoangeli, M.; Rienzo, D.; Colasanti, R.; Zizzi, A.; Gladi, M.; Alvaro, L.; Nocchi, N.; Di Somma, L.G.M.; Scarpelli, M.; Scerrati, M. Endoscopy-verified occult subependymal dissemination of glioblastoma and brain metastasis undetected by MRI: Prognostic significance. OncoTargets Ther. 2012, 5, 449–456. [Google Scholar] [CrossRef]
- Cohen, A.L.; Colman, H. Glioma Biology and Molecular Markers. In Current Understanding and Treatment of Gliomas; Springer: Cham, Switzerland, 2015; Volume 163, pp. 15–30. [Google Scholar] [CrossRef]
- Brennan, C.W.; Verhaak, R.G.W.; McKenna, A.; Campos, B.; Noushmehr, H.; Salama, S.R.; Zheng, S.; Chakravarty, D.; Sanborn, J.Z.; Berman, S.H.; et al. The Somatic Genomic Landscape of Glioblastoma. Cell 2013, 155, 462–477. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Simbolo, M.; Mafficini, A.; Martini, M.; Calicchia, M.; Piredda, M.L.; Ciaparrone, C.; Bonizzato, G.; Ammendola, S.; Caffo, M.; et al. IDH-wild type glioblastomas featuring at least 30% giant cells are characterized by frequent RB1 and NF1 alterations and hypermutation. Acta Neuropathol. Commun. 2021, 9, 200. [Google Scholar] [CrossRef] [PubMed]
- Jankowska, S.; Lewandowska, M.; Masztalewicz, M.; Sagan, L.; Nowacki, P.; Urasińska, E. Molecular classification of glioblastoma based on immunohistochemical expression of EGFR, PDGFRA, NF1, IDH1, p53 and PTEN proteins. Pol. J. Pathol. 2021, 72, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Reifenberger, G.; Wirsching, H.-G.; Knobbe-Thomsen, C.B.; Weller, M. Advances in the molecular genetics of gliomas—Implications for classification and therapy. Nat. Rev. Clin. Oncol. 2017, 14, 434–452. [Google Scholar] [CrossRef]
- Zhu, Y.; Guignard, F.; Zhao, D.; Liu, L.; Burns, D.K.; Mason, R.P.; Messing, A.; Parada, L.F. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces ma-lignant astrocytoma. Cancer Cell 2005, 8, 119–130. [Google Scholar] [CrossRef]
- Pisapia, D.J.; Ohara, K.; Bareja, R.; Wilkes, D.C.; Hissong, E.; Croyle, J.A.; Kim, J.-H.; Saab, J.; MacDonald, T.Y.; Beg, S.; et al. Fusions involving BCOR and CREBBP are rare events in infiltrating glioma. Acta Neuropathol. Commun. 2020, 8, 80. [Google Scholar] [CrossRef]
- Torre, M.; Meredith, D.M.; Dubuc, A.; Solomon, D.A.; Perry, A.; Vasudevaraja, V.; Serrano, J.; Snuderl, M.; Ligon, K.L.; Alexandrescu, S. Recurrent EP300-BCOR Fusions in Pediatric Gliomas with Distinct Clinicopathologic Features. J. Neuropathol. Exp. Neurol. 2019, 78, 305–314. [Google Scholar] [CrossRef]
- Lobbous, M.; Bernstock, J.D.; Coffee, E.; Friedman, G.K.; Metrock, L.K.; Chagoya, G.; Elsayed, G.; Nakano, I.; Hackney, J.R.; Korf, B.R.; et al. An Update on Neurofibromatosis Type 1-Associated Gliomas. Cancers 2020, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Zureick, A.H.; McFadden, K.A.; Mody, R.; Koschmann, C. Successful treatment of a TSC2-mutant glioblastoma with everolimus. BMJ Case Rep. 2019, 12, e227734. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Miki, Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004, 95, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Dono, A.; Ramesh, A.V.; Wang, E.; Shah, M.; Tandon, N.; Ballester, L.Y.; Esquenazi, Y. The role of RB1 alteration and 4q12 amplification in IDH-WT glioblastoma. Neuro Oncol. Adv. 2021, 3, vdab050. [Google Scholar] [CrossRef]
- Goldstein, M.; Gabriel, N.; Inkman, M.; Zhang, J.; Dahiya, S. DDRE-32. SETD2 histone methyltransferase mutation status predicts treatment response in glioblastoma: Strategies to overcome chemoresistance. Neuro Oncol. 2021, 23 (Suppl. S6). [Google Scholar] [CrossRef]
- Takigawa, K.; Hata, N.; Sangatsuda, Y.; Suzuki, S.O.; Sirozu, N.; Hatae, R.; Akagi, Y.; Iwaki, T.; Nagata, S.; Mizoguchi, M. Intraventricular mucin-producing glioblastoma arising in the septum pellucidum at the frontal horn of the lateral ventricle: A case report. Neuropathology 2021, 41, 381–386. [Google Scholar] [CrossRef]
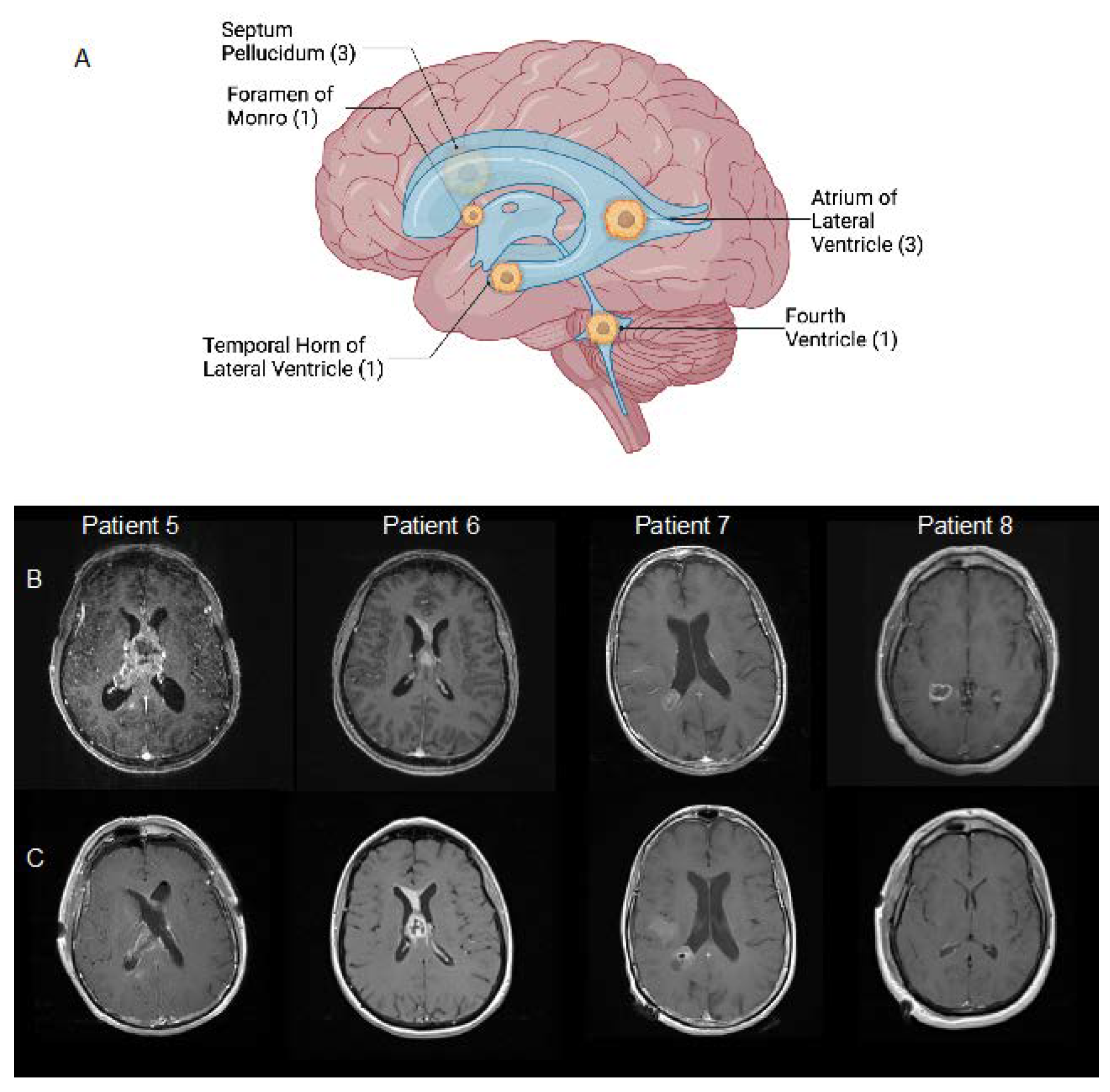
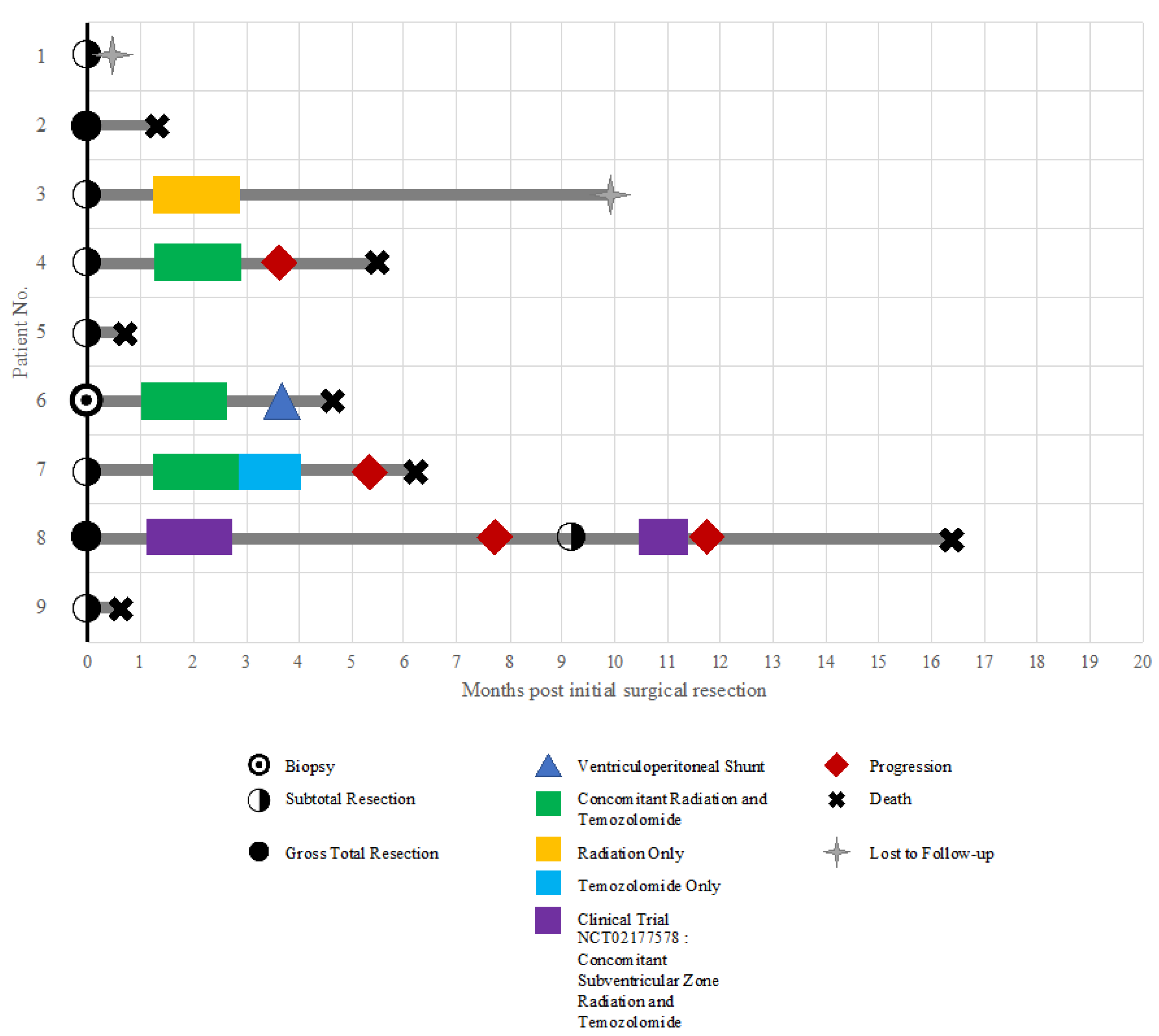
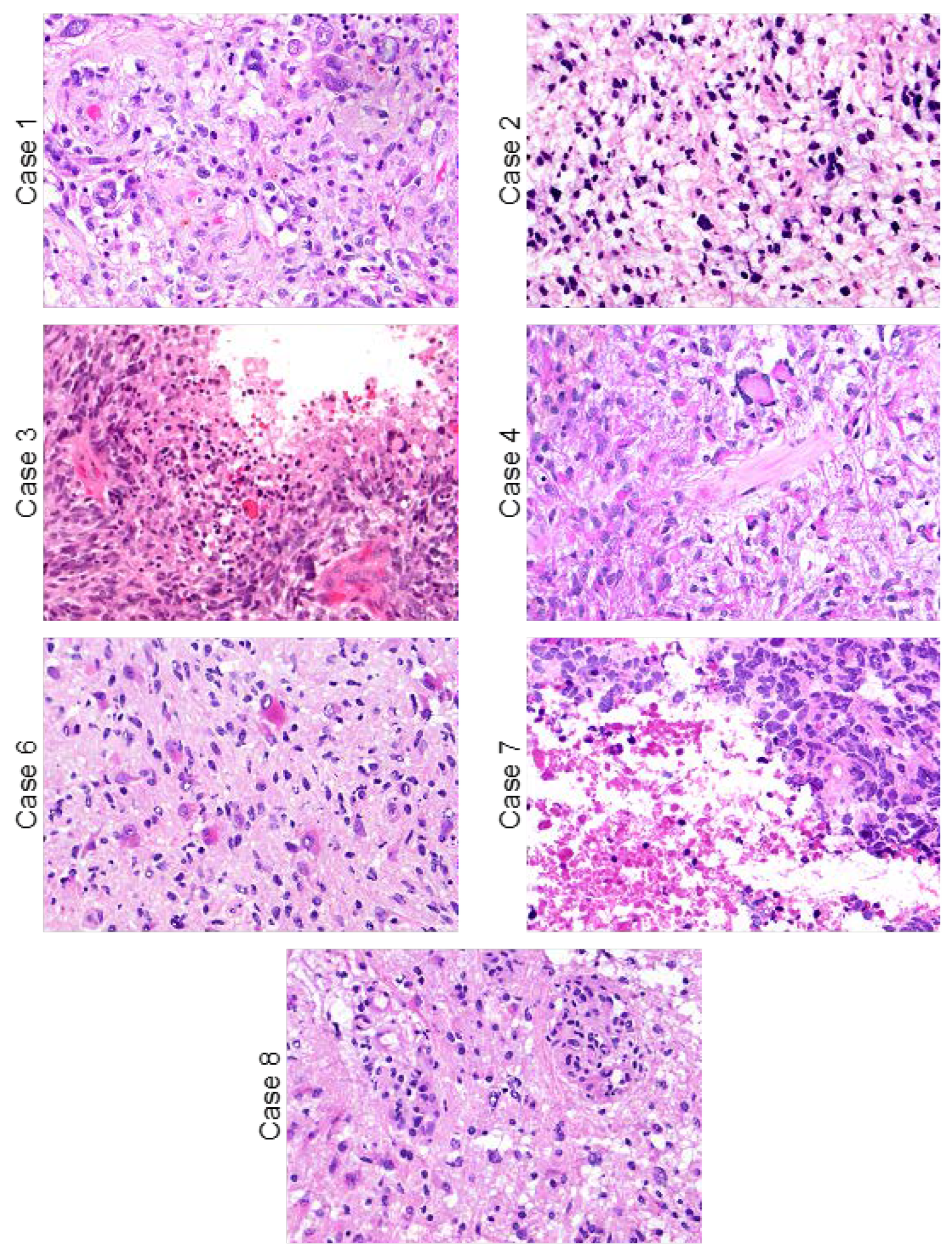
| Patient | Age, y | Sex | Presenting Symptoms | Location | Surgical Approach | Extent of Resection | Shunt/Drain | Postoperative Complications | Postop LOS (Days) | Adjuvant Therapy | Overall Survival [1] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 57 | M | Seizures, headaches | Temporal horn of right lateral ventricle | Right temporal transcortical | STR | None | Unknown | 3 | Unknown | Lost to follow-up |
| 2 | 65 | M | Confusion, memory deficit | Septum pellucidum | Right anterior transcallosal | GTR | EVD | Prolonged intubation, seizure, pulmonary embolism, sepsis, renal insufficiency, atelectasis | 36 | None | 36 days |
| 3 | 37 | M | Headache, ophthalmoplegia, facial droop, ataxia | Fourth ventricle | Suboccipital transvermian | STR | None | Temporary swallowing deficit with PEG, hemiplegia, new cognitive deficit | 18 | RT only (5840 cGy) | Lost to follow-up |
| 4 | 71 | M | Confusion, memory deficit | Septum pellucidum | Right frontal transcortical | STR | EVD | Pulmonary emboli, NSTEMI | 17 | RT (6000 cGy)/TMZ | 5 months |
| 5 | 77 | M | Confusion, memory deficit | Septum pellucidum | Right frontal transcortical | STR | EVD | Prolonged coma | 12 | None | 19 days |
| 6 | 61 | F | Headache, confusion, memory deficit, sleepiness | Foramen of Monro | Stereotactic needle biopsy | Biopsy | VP shunt | Pulmonary embolism; intracranial hemorrhage | 4 | RT (6000 cGy)/TMZ | 4 months |
| 7 | 68 | F | Transient ischemic attack | Atrium of right lateral ventricle | Right parietal transcortical | STR | None | Hearing loss | 2 | RT (6000 cGy)/TMZ + TMZ x2 cycles | 5 months |
| 8 | 56 | M | Confusion | Atrium of right lateral ventricle | Right temporal transcortical | GTR | None | None | 2 | :SVZ RT (6000 cGy)/TMZ [2] | 16 months |
| 9 | 69 | M | Headaches, nausea/vomiting, confusion, memory deficit | Atrium of right lateral ventricle | Right anterior transcallosal | STR | None | New sensory deficit | 15 | None | 18 days |
| Patient | Year of Surgery | Initial Diagnosis | WHO 2016 Grade | MVP | Necrosis | Mitotic Rate | IDH-1 R132H IHC | ATRX IHC | P53 IHC | 1p/19q |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1995 | Glioblastoma | IV | + | + | Increased | NP | NP | NP | NP |
| 2 | 1998 | Glioblastoma [1] | III | − | − | Increased | NP | NP | NP | NP |
| 3 | 2002 | Glioblastoma | IV | + | + | Increased | NP | NP | NP | NP |
| 4 | 2009 | Glioblastoma | IV | + | + | Increased | NP | NP | NP | NP |
| 5 | 2006 | Glioblastoma | IV | + | + | Increased | NP | NP | NP | NP |
| 6 | 2019 | Glioblastoma | IV | + | + | Increased | Negative | Retained | Wildtype | Intact on NGS |
| 7 | 2017 | Glioblastoma | IV | − | + | Increased | Negative | Retained | Wildtype | NP |
| 8 | 2021 | Glioblastoma | IV | + | − | Increased | Negative | Retained | Wildtype | Intact on NGS |
| 9 | 2014 | Glioblastoma | IV | − | + | Increased | Negative | NP | NP | NP |
| Variant | RefSeq Transcript | Chromosome | Genomic Position | Reference Allele | Alternate Allele | Function | Sequencing Depth | Mutant Allele Frequency |
|---|---|---|---|---|---|---|---|---|
| Patient 6 | ||||||||
| PTEN p.N292fs | NM_000314 | chr10 | g.89,720,720 | GA | G | frameshift | 262 | 62% |
| NF1 p.Y1659fs | NM_000267 | chr17 | g.29,653,035 | ATATC | A | frameshift | 1169 | 33% |
| EGFR focal amplification | NM_005228 | chr7 | whole gene amplification | |||||
| Chromozome (7+/10−) | ||||||||
| Patient 8 | ||||||||
| TERT c.-146C>T | NM_198253 | chr5 | g.1,295,250 | G | A | upstream | 620 | 35% |
| PTEN p.C124Y | NM_000314.7 | chr10 | g.89,692,887 | G | A | missense | 220 | 46% |
| Chromosome (7+/10−) | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, M.; Kalluri, A.; Materi, J.; Gujar, S.K.; Schreck, K.; Mukherjee, D.; Weingart, J.; Brem, H.; Redmond, K.J.; Lucas, C.-H.G.; et al. Management and Molecular Characterization of Intraventricular Glioblastoma: A Single-Institution Case Series. Int. J. Mol. Sci. 2023, 24, 13285. https://doi.org/10.3390/ijms241713285
Parker M, Kalluri A, Materi J, Gujar SK, Schreck K, Mukherjee D, Weingart J, Brem H, Redmond KJ, Lucas C-HG, et al. Management and Molecular Characterization of Intraventricular Glioblastoma: A Single-Institution Case Series. International Journal of Molecular Sciences. 2023; 24(17):13285. https://doi.org/10.3390/ijms241713285
Chicago/Turabian StyleParker, Megan, Anita Kalluri, Joshua Materi, Sachin K. Gujar, Karisa Schreck, Debraj Mukherjee, Jon Weingart, Henry Brem, Kristin J. Redmond, Calixto-Hope G. Lucas, and et al. 2023. "Management and Molecular Characterization of Intraventricular Glioblastoma: A Single-Institution Case Series" International Journal of Molecular Sciences 24, no. 17: 13285. https://doi.org/10.3390/ijms241713285
APA StyleParker, M., Kalluri, A., Materi, J., Gujar, S. K., Schreck, K., Mukherjee, D., Weingart, J., Brem, H., Redmond, K. J., Lucas, C.-H. G., Bettegowda, C., & Rincon-Torroella, J. (2023). Management and Molecular Characterization of Intraventricular Glioblastoma: A Single-Institution Case Series. International Journal of Molecular Sciences, 24(17), 13285. https://doi.org/10.3390/ijms241713285







