Abstract
The use of platelet-rich plasma (PRP) has gained increasing interest in recent decades. The platelet secretome contains a multitude of growth factors, cytokines, chemokines, and other biological biomolecules. In recent years, developments in the field of platelets have led to new insights, and attention has been focused on the platelets’ released extracellular vesicles (EVs) and their role in intercellular communication. In this context, the aim of this review was to compile the current evidence on PRP-derived extracellular vesicles to identify the advantages and limitations fortheir use in the upcoming clinical applications. A total of 172 articles were identified during the systematic literature search through two databases (PubMed and Web of Science). Twenty publications met the inclusion criteria and were included in this review. According to the results, the use of PRP-EVs in the clinic is an emerging field of great interest that represents a promising therapeutic option, as their efficacy has been demonstrated in the majority of fields of applications included in this review. However, the lack of standardization along the procedures in both the field of PRP and the EVs makes it extremely challenging to compare results among studies. Establishing standardized conditions to ensure optimized and detailed protocols and define parameters such as the dose or the EV origin is therefore urgent. Further studies to elucidate the real contribution of EVs to PRP in terms of composition and functionality should also be performed. Nevertheless, research on the field provides promising results and a novel basis to deal with the regenerative medicine and drug delivery fields in the future.
1. Introduction
Developing personalized strategies to support, enhance and accelerate functional recovery has been the focus of tissue regeneration in recent years. In this regard, the use of platelet-rich plasma (PRP) was introduced more than 20 years ago [1,2], and it has gained increasing interest in recent decades. Basically, this therapy is defined as a portion of autologous plasma with a platelet concentration above the blood baseline. Although its applications are highly widespread in diverse regenerative fields, including dentistry, orthopedic, ophthalmology, dermatology, and gynecology [3], there is still no standard for the best protocol for PRP preparation and administration. In fact, there is a long list of variables such as use and type of anticoagulant, conditions of centrifugation, inclusion of leukocytes, concentration of platelets, use and type of platelets’ activator, as well as the mode and type of application as a therapeutic. Despite many attempts to homogenize and classify PRP products [4,5,6,7,8], it has not been implemented in daily practice. This lack of suitable standardization has led to a large number of products with different biological and therapeutic outcomes. Nevertheless, the rationale for using PRP lies in the physiological role of platelets. They participate, beyond hemostasis, in a wide range of physiological processes, including the immune response, inflammation, and wound healing [3,9]. In particular, the platelet secretome contains a multitude of growth factors, cytokines, chemokines, and other biological biomolecules that are released upon platelet activation and bound to the corresponding receptors that finally initiate the signaling cascade to promote the process of regeneration [6,10]. The vast majority of studies have focused on the role of the plethora of growth factors present in the PRP that can orchestrate cell fate in terms of migration, proliferation, differentiation, apoptosis, and angiogenesis [11,12]. Additionally, PRP-based therapy also provides a biodegradable and provisional fibrin matrix that functions as both physical support and a controlled release system [3,13].
The scientific rationales by which PRP exerts its action are not yet fully understood; however, in recent years, developments in the field of platelets have led to new insights, and attention has been focused on the platelets’ released extracellular vesicles (EVs), including exosomes. The importance of these vesicles lies especially in their functionality [9,10,14], and their research has grown exponentially over the last past years. Extracellular vesicles (EVs) are nanosized structures presenting lipid bilayers with no replicate capacity [14,15]. Traditionally, different subtypes of EVs have been referred to based on their biogenesis, size, content, and function. However, there is some overlap which makes it difficult precisely classify them [16,17]. To address this issue, the International Society of Extracellular Vesicles (ISEV), according to the minimal information for studies of extracellular vesicles publication (MISEV2018), suggested authors consider the use of operational terms for EV subtypes that refer to (a) physical characteristics of EVs such as (a) size or density, (b) biochemical composition or (c) description of the cell of origin and state [18].
The history of EVs started in 1946 when Chargaff and West [19] discovered a subcellular factor that promoted blood clotting. Later, in 1967, Peter Wolf [20] described this factor more in detail as platelet-derived vesicles and referred to it as “platelet dust”. Despite initially relating to thrombosis and hemostasis, multiple biological functions have been subsequently described related to EVs, highlighting their role in cell-cell communication [9,10,14]. In fact, EVs may influence many relevant processes of physiological and pathological conditions, including inflammation, angiogenesis, immune response and wound healing [21,22,23]. Regarding platelet derivatives, it was not until 2014 that Torreggiani et al. [24] first successfully isolated exosomes from platelet lysates (PL), thus, providing evidence of exosomes as new additional effector of PL and as therapeutic potential for cell-free therapies in bone regeneration.
EVs are released by any cell type and found in almost all biological fluids, including blood, urine, saliva, etc. [25]. The content or cargo of EVs consists of lipids, proteins, and genetic material. Their composition is, to some extent, cell type dependent and reflects the physiological or pathological state of their parental cells, which has led to their study as potential biomarkers of disease [26,27,28,29].
Thus, the aim of this review is to gather the current evidence on PRP-derived extracellular vesicles in order to identify the advantages and limitations to favor translation into feasible upcoming clinical applications.
2. Material and Methods
2.1. Literature Search
For this narrative review, a systematic literature search was performed in PubMed and Web of Science database until 13 February 2023, using two search strategies: “((platelet rich plasma) AND (extracellular vesicles)) AND (exosomes)” and “(platelet rich plasma) AND (exosomes)”. The specific term “exosomes” was also included in the search strategies as this particular term has recently received considerable attention in the current research. All the articles that appeared in the first search strategy were included in the second one. As this occurred in both databases, the second search strategy, which already included the articles from the first one, was finally taken into account. Papers were excluded if: (1) the article was written in any language other than English or Spanish (2) duplicates (3) reviews, commentaries, thesis, book chapters (4) no full-text available (5) out of scope (6) did not include PRP (7) the terms exosomes or extracellular vesicles (EVs) did not appear (8) exosomes or EVs were not of PRP origin.
2.2. Data Extraction
In the first phase, the articles from both databases were screened based on the title and the abstracts to determine their suitability. Subsequently, all the full-text articles assessed for eligibility were examined to determine their inclusion. For data extraction, an evidence table was created with Microsoft Excel. The following data were included: author and year of publication, study type, the field of application, experimental system, PRP-related issues (origin, type of anticoagulant, presence or absence of leukocytes, type of activator, exosomes or EVs) isolation, exosomes or EVs-related issues (isolation, storage conditions, characterization methods, size, fractions), comparison groups, exosomes concentrations, methods, and summary results.
2.3. Assessment of Reporting Quality and Risk of Bias
The criteria reported by Golbach et al. [30] were followed to evaluate the quality and the risk of bias. The reporting quality was determined by the presence (“yes/partly”) or absence (“no”) of critical information. The risk of bias was divided into three categories: low, moderate, and high, depending on whether the answers were “yes”, “partly”, or “no”.
3. Results
The search strategy yielded a total of 172 articles from the two databases. After an exhaustive screening, 20 studies were finally included for the analysis in this review (Figure 1 and Table 1).
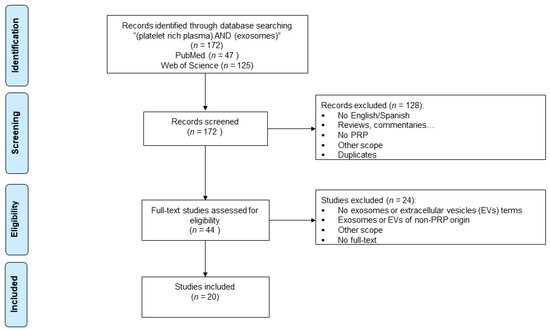
Figure 1.
Flowchart summarizing the selection process following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.

Table 1.
Summary information of the studies included in this review.
In recent years, PRP-derived extracellular vesicles have gained increasing attention in the regenerative biomedical field as potential therapeutic mediators. This is also reflected in the fact that 80% of the articles included in this review have been published in the last 4 years (Figure 2A). The role of EVs/exosomes from PRP was evaluated in many different fields of application, including osteoarthritis and tissue regeneration, which comprise 40% of the studies. Other medical fields, such as diabetic retinopathy, intervertebral disc degeneration, muscle injury, wound healing, hair loss, osteonecrosis, and dental pulp or cartilage regeneration, were also occasionally studied (Figure 2B). On the other hand, regarding the type of study, more than half of the articles performed in vitro assays (60%), and only 5% combined in vitro and in vivo tests (Figure 3A).
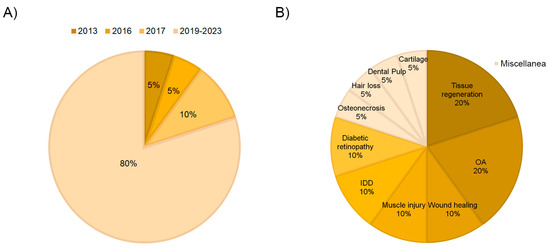
Figure 2.
Distribution of the selected articles according to year of publication (A) and field of application (B).
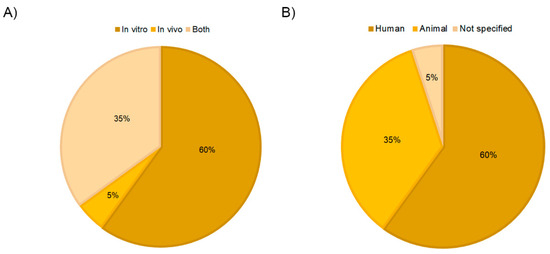
Figure 3.
Distribution of the selected publications according to study type (A) and PRP origin (B).
3.1. Reporting Quality and Risk of Bias
The reporting quality showed large differences (Figure 4). The experimental system and the PRP-EVs characterization were reported in all the articles. However, 30% of the studies did not specify the sample size, and in 25% of the articles, it was only partly done. That is, the sample size was not detailed for both the sample under study (PRP-EVs) and the experimental system. The lack of information regarding PRP composition had the greatest impact on the reporting quality since up to 45% of the studies did not mention any information on this matter. Additionally, 5% and 50% of the articles provided no information or only partial data on the process of obtaining PRP, respectively. However, in 90% of the studies, details were given on the PRP-EVs obtaining process. Concerning conflict of interest and ethical statements, most of the articles (90%) provided complete information. Regarding the performance bias, the EVs concentration was mentioned in all the articles, although 19% did so partially. Very low risk was associated with the detection bias, while that associated with selection increased, as animals were only randomly assigned in 75% of the studies.
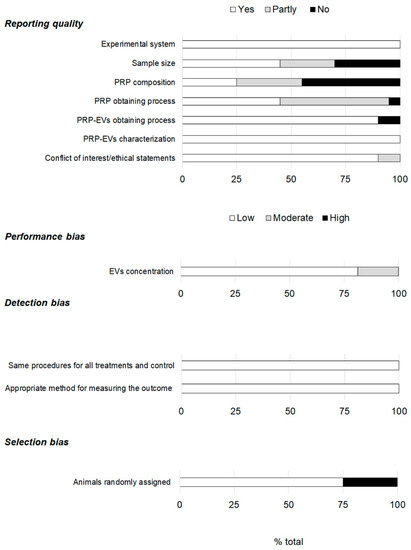
Figure 4.
Assessment of the reporting quality and risk of bias.
3.2. PRP Obtaining Process Affects Extracellular Vesicles Production
The major issue related to PRP is the extreme variability of preparation protocols that are often not well documented in the literature leading to biologically heterogeneous final products and making comparison or reproducibility of results challenging [4,51]. No exception was found in this review according to PRP-EVs, as Table 2 shows, considering the main PRP characteristics. Regarding PRP origin, sixty percent of the preparations had a human origin (Figure 3B). Rats were the most frequent non-human origin. Seventy percent of the studies referred to the term “exosome”, compared to 30% that referred to the generic term EVs.

Table 2.
Description of the PRP obtaining process in the reviewed articles.
All studies, except five, specified the use of an anticoagulant (acid citrate dextrose solution A (ACD-A, n = 7), trisodium or sodium citrate (n = 6), citrate glucose (n = 1) and sodium citrate and EDTA (n = 1)). The misinformation on PRP cellular composition was even greater. Nearly half of the studies (45%) did not provide information on the inclusion or not of leukocytes. Among those who did specify this concept (11/20), the vast majority did not include these white blood cells (8/11). PRP cell composition impacts, among other things, the secretory components, such as growth factors. In fact, the inclusion of leukocytes is one of the most important concerns about PRPs. These immune cells contain numerous proinflammatory interleukins and extracellular matrix-degrading enzymes that are mainly catabolic and promote pro-inflammatory conditions, thus influencing the clinical outcome of the PRP therapy [52,53]. Regarding EVs, the most abundant EVs in blood from healthy persons are the ones from platelets [54,55]. However, these vesicles also originate from other types of cells, such as red blood cells, leukocytes, and endothelial cells [56], that even may alter EV secretion in a cell-specific manner under different conditions (p.e. hypoxia or exercise) [57,58].
The type of agonist used for platelet stimulation influences PRP-EVs protein content [59]. In fact, several studies have already reported that the amount and proteome of EVs largely depend on the agonist triggering platelet activation [60,61]. In this review, sixty percent of the studies activated PRP compared to 20% that did not. No information concerning this issue was available for four studies. In 4 out of 12 studies that activated the PRP, the activator was not specified. Although, the article by Zhang et al. [39] did specify that high glucose was used for some assays to obtain PRP-Exos from stimulated platelets in vitro. When information was provided, different agonists were used for platelet activation, including thrombin (n = 3), calcium chloride (n = 1), calcium chloride and thrombin (n = 1), calcium gluconate (n = 1), thrombin and calcium gluconate (n = 1) and ionophore A23187 (n = 1). Physical methods such as sonication were also utilized for platelet activation in 2 articles. In fact, one of the articles included in this review [35] focused precisely on comparing the effects of different agonists (saline/thrombin/calcium gluconate/mixture of both) on the PRP-derived exosomes. PRP was obtained avoiding leukocyte contamination. They demonstrated that all the activation methods were suitable for PRP-exosomes (PRP-Exos) isolation, although the activation efficiency and cytokine content were different. Exosomes in the study measured 30–150 nm in diameter. The size of calcium-activated PRP-Exos was larger than the thrombin-activated group and the mixture-activated group. PRP activated by the mixture released the highest concentration of exosomes, followed by the calcium-activated group and then the thrombin-activated group. Calcium gluconate alone was found to be weaker than thrombin or thrombin and calcium gluconate together in PRP activation. PRP-Exos activated by thrombin and calcium gluconate together were found to contain more cytokines than the other groups. The effects of activating PRP with thrombin and calcium gluconate together were better than using thrombin or calcium alone, both in quality and quantity of exosomes. PRP-Exos activated by the mixture of agonists could more significantly promote HUVECs proliferation, migration, and the formation of vessel-like via the AKT ERK signal pathway, compared with other groups. Taken these results together showed that the number, content, and biological effect of the PRP-Exos was clearly depend on the stimulation conditions. In line with these results, Saumell-Esnaola et al. [36] isolated and characterized the exosomes released by human PRP platelets to evaluate the influence of CaCl2 on their secretion. They also specifically isolated exosomes from a single origin, exclusively released by PRP platelets, without contamination from other cellular components that could be present in PRP products. Their results showed that calcium activation caused protein amount to increase in both platelets and platelets-exosomes (PLT-Exos), but this was approximately 20 times higher in the platelet fraction than in the PLT-Exos fraction. They found that more than 65% of the PLT-Exos particles had a diameter ranging from 20 to 40 nm. The exosomes isolated from CaCl2-activated platelets exhibited high purity and met the most up-to-date biochemical criteria that characterize exosomes against other EVs and contaminants. In addition, calcium was proved to alter the cytokine cargo profile of exosomes, which differs markedly in relation to that from non-activated platelets´ EVs. The authors concluded that although PRP calcium activation promotes exosome release, its net contribution to the total PRP effect was minimal.
3.3. Extracellular Vesicles Obtaining Process and Storage Stability
Despite the EV source and the isolation method being especially relevant to determine their composition, recovery, and purity, no single method is available to date for all EV types and samples. In fact, complete isolation of EVs is an unrealistic goal; thus, the use of the “sample enrichment” term would be more precise [18,55]. Ultracentrifugation is the gold standard isolation method despite the low purity provided, and a large amount of sample is required [26,62,63]. In this review, in 70% of the studies, this size and density-based isolation method was used (Figure 5A and Table 3). Fifty percent used it as the sole method, while the remaining 20% combined it with other techniques (purification (15%) or extrusion (5%)). Other systems, such as centrifugation, fluorescence-activated cell sorting (FACS), and commercial kits, were also used for this purpose. In 10% of the articles, the isolation method was missing.
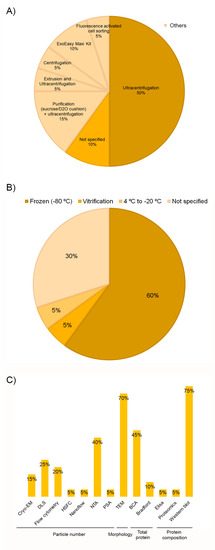
Figure 5.
Distribution of the selected publications according to EVs isolation methods (A), storage temperature (B), and characterization methods (C).

Table 3.
EVs isolation methods and storage conditions.
Storage conditions of both the matrix and the isolated EVs have an impact on their properties, including concentration and functionality [18,64]. Although −80 °C storage is a widespread approach, the optimal preservation conditions remain to be established, with inconsistencies among research. Some studies have found that both 4 °C and −80 °C are suitable for short-term storage [65] and up to one month [66]. However, Gelibter et al. [64] recommended working with fresh samples as none of the storage conditions studied was able to prevent or mitigate the impact of storage on EVs. Nevertheless, when storage is strictly needed, they suggested a short-term −80 °C preservation and the storage of biological matrix instead of the isolated EVs. Sivanantham and Jin [67] determined that according to the current evidence, EVs can be stored at 4 °C for short times (a day or a few weeks), and a temperature of −80 °C was recommended for long-term (months or years) storage. Despite storage conditions are also crucial, as many as 30% of the studies included in this review (n = 6) did not describe the storage process (Figure 5B). For those who did detail, there was a large consensus on temperature; EVs were frozen at −80 °C in 12 out of 14 articles; however, only in 2 articles [41,48] the storage time was specified. Bagio et al. [48] stored the thrombin-activated platelet-derived exosomes (T-aPDE) obtained from a PRP at 4 °C for 7 days prior to the assays’ beginning. Dai et al. [41] also demonstrated the in vitro stability of platelet-derived EVs (PEV) at 1, 3, 5, and 7 days, but storage temperature was not stated (Table 3). Determining storage conditions does not only involve temperature; other issues such as buffers, storage tubes, and protective excipients should also be optimized [65]. The articles included in this review have provided little information about the material used for storing EVs. Ultra-clearTM tubes from Beckman Coulter were the most commonly used [34,45,50]. On the other hand, ultra-carbon fiber tubes were used by Iyer et al. [44] for EV storage. In the remaining papers, the type of tube where EVs were stored was not specifically indicated. In some cases, details were given about the kind of tube where the PRP was transferred or the isolation process was performed. However, it was not specified if the storage was completed in the same device (Table 3). It has been reported that nonspecific adhesion to the surface of storage containers can influence EV yields, but no consensus has been reached regarding the optimal materials for EV storage [64,65]. In fact, van de Wakker et al. [65] reported polypropylene tubes to be superior for EVs storage compared to glass tubes. However, Evtushenko et al. [68] recommended to use low protein binding tubes or ordinary tubes treated with bovine serum albumin (BSA) in order to reduce particle loss. As far as the choice of buffer is concerned, a large consensus has been found in this review. In 75% of the articles, the phosphate-buffered saline (PBS) was the option of choice (Table 3). Despite PBS being so far the most commonly used buffer for EV storage, a recent study [69] has demonstrated that using PBS results in a drastic reduction in EV recovery. They have identified the usage of PBS supplemented with human serum albumin and trehalose (PBSHAT) as an alternative to both short-term and long-term preservation.
Regarding EVs characterization, it is important to use multiple complementary techniques, as no current method can fulfill the entire EVs information. Several procedures have been routinely used to determine some of these features, such as size, concentration, morphology, and molecular composition [18,70,71]. All the studies included in this review did use several complementary techniques. Nanoparticle tracking analysis (NTA), transmission electron microscopy (TEM), bicinchoninic acid protein assay (BCA), and Western-blotting were the most prevalent methods among studies to determine the particle number, morphology, total protein, and composition, respectively (Figure 5C). There is no universally proposed specific marker for identifying EVs, given the different origins and types [72]. Nevertheless, according to MISEV2018 [18], three categories of markers must be analyzed in all bulk EV preparations to demonstrate their presence and assess their purity from common contaminants. However, only seven studies [32,33,35,36,43,50] met this strong recommendation (Table 4). As for the first category, the tetraspanins CD9, CD63, and CD81 were the most analyzed proteins as membrane EVs markers, and CD41 was quite often measured as a platelet source marker. On the opposite, the absence of apolipoproteins (category 3) is considered a good marker to assess the degree of purity [32,33,36,43], mainly in plasma and serum, that contain numerous non-EV lipidic structures. Proteins associated with membranous intracellular compartments (p.e. calnexin, category 4) were also used as purity control [35,50]. Proteins associated with other intracellular compartments or functional components (secreted proteins) were also measured in some of the studies as part of the objective of the article itself. However, they will be addressed in the next section. Assessment of particle size revealed small EVs below 200 nm in all but two studies [37,38], although there was heterogeneity among articles (Table 4). These discrepancies could be related to methodological differences in terms of PRP collection, activation, and composition, as well as to EVs isolation and quantification techniques. As with other parameters, there were studies in which these data were not provided [39,42,43].

Table 4.
Analysis of different markers in PRP-EVs. Markers from the corresponding categories were included (✓) or not (✕) in the articles.
Finally, it should be noted that only 2 out 20 studies [32,33] reported having submitted the methodological details to the EV-TRACK knowledgebase as strongly recommended by the MISEV2018.
3.4. Fields of Application
Table 1 summarizes the information on the studies included in this review.
3.5. Osteoarthritis
Four articles [31,32,33,34] investigated the potential of EVs derived from PRP as a therapeutic approach for osteoarthritis (OA). The efficacy was assessed in vitro in all cases. Additionally, the in vivo effect was also examined by two studies [31,34]. Although the experimental systems were different, the isolation methods and the EVs concentration were not the same, and they were obtained from both activated and non-activated PRP, the conclusion reached by all these authors was the same: PRP-derived EVs present a novel therapy for OA.
One major driver of OA is inflammation; therefore, any tool that aims at tackling this disease must be directed at relieving this inflammatory context. In this sense, Liu et al. [31] reported in a model consisting of IL-1β-treated chondrocytes resembling OA, PRP-EVs (PRP-Exo) significantly decreased the apoptotic rate of OA chondrocytes and presented the ability to reverse the typical increase in β-catenin, RUNX2 and Wnt5a associated to IL-1β-treated chondrocytes. In agreement with these authors, Otahal et al. [32] reported PRP-Exo anti-inflammatories effects in acute OA patient-derived chondrocytes. Another study by the same group [33] used a co-culture model involving primary chondrocytes exposed to activated macrophages to mimic the inflammatory environment in an OA joint. A lower proinflammatory cytokine profile was associated with plasma-derived EVs treatment in comparison with the complete blood products. Zhang et al. [34] also showed that PRP-Exo inhibited inflammation-induced chondrocyte degeneration. Concerning the in vivo models, Liu et al. [31] described the Wnt/β-catenin signaling pathway as a potential mechanism of action of platelet-rich plasma-derived exosomes (PRP-Exos) for OA therapy. In addition to reporting a protective role against IL-1β-induced apoptosis and degeneration of chondrocytes by PRP-Exo in vivo, Zhang et al. [34] confirmed that incorporating PRP-Exo into a thermosensitive hydrogel increased the local retention of exosomes thus delaying the development of subtalar osteoarthritis (STOA).
Additionally, the effect of PRP-derived EVs was also compared to other types of blood products. In this regard, Liu et al. [31] did so with respect to an activated PRP (PRP-As), concluding that the effect of PRP on alleviating OA may take place through PRP-Exos. On the other hand, Otahal et al. [32,33], under the assumption that cell composition differs among different blood products, investigated the role of EVs isolated from PRP and from hyperacute serum (hypACT), a serum-based blood product free of platelets, leukocytes, and fibrin. They reported differences in EVs populations between plasma (PRP) and serum (hypACT) derivatives regarding concentration and origin. The total EV concentration was significantly higher in the citrate-anticoagulated platelet-rich plasma (CPRP) [32,33], but only 44% were of platelet origin [32]. The authors concluded that CPRP EVs might provide beneficial anti-inflammatory effects in acute OA, whereas hypACT EVs would be more suitable for promoting regeneration in chronic OA [32].
3.6. Tissue Regeneration
Four in vitro studies [35,36,37,38] have been gathered in this general group. Two of them performed a biochemical and morphological characterization of PRP-derived exosomes after activation by different agonists [35] or by calcium chloride (CaCl2) [36] that have been already addressed in the previous section: “PRP obtaining process affects extracellular vesicles production”. The remaining two studies evaluated two different techniques, high-sensitivity flow cytometry [37] and cryo-electron microscopy [38] for EV characterization. In order to enhance the sensitivity of the conventional bench top flow cytometers, Stoner et al. [37] developed a custom device. They stimulated PRP with the ionophore A21387 to induce platelet EV release. The use of this calcium ionophore led to an increase in the fraction of annexin V and CD61-positive EVs. They reported that this high-sensitivity flow cytometry was able to detect individual EVs in plasma and EV sub-populations expressing cell surface markers, thus representing an alternative to light scatter-based EV detection devices. Yuana et al. [38] stated that morphological information on EV in fresh plasma is limited due to the limitations of the current analytical methods. Therefore, they studied the cryo-EM technique to support the EV analysis by other technologies. The authors concluded that cryo-EM allowed the visualization and characterization of individual EV in their native state in fresh human plasma and that most of the particles identified were lipoproteins thus recommending fasting to limit this non-EV lipidic structures contamination in the collection of blood samples.
3.7. Diabetic Retinopathy
Two studies from the same group fall into this category. Firstly, Zhang et al. [39] investigated the effects of PRP-Exos on retinal endothelial injury in diabetic rats and human retinal endothelial cells (HRECs) in vitro. Exosomes were obtained from activated PRP. The authors did not specify the type of activator they used for the normal PRP-Exos, whereas high glucose (HG) was employed for platelet activation in the in vitro culture system used for HG-PRP-Exos obtention. The authors reported that high glucose increased PRP-Exo levels in early diabetic retinopathy (DR). According to the results, the activation of the TLR4 pathway was involved in the role that PRP-Exos plays in this disease. The authors demonstrated that blocking this signaling pathway with TAK-242 could alleviate the PRP-Exo-induced negative effects, such as the decreased superoxide dismutase (SOD) activity, the increase production of malonyldialdehyde (MDA) and reactive oxygen species (ROS) and the blood-retinal barrier (BRB) dysfunction. They also suggested that the blockade of CXCL10 led to the TLR4 signaling pathway downregulation, thereby reducing PRP-Exo-induced adhesion molecules expression and preserving PRB function, thus it can be used as a therapeutic target for DR. Regarding this disease, Zhang et al. [40] conducted another in vitro study. They studied the effects of PRP-Exos on the fibrogenic activity of human retinal Müller cells (hMCs) by isolating PRP-Exos from the plasma of diabetic rats (DM-PRP-Exos) and normal control rats (Nor-PRP-Exos). As in the previous study, the authors also found an increase concentration of exosomes in the DM-PRP-Exos compared with that of the Nor-PRP-Exos. The analysis of the exosome cargo revealed enrichment of PDGF, bFGF, and TGF-β in the DM-PRP-Exos group. Additionally, they reported that DM-PRP-Exos significantly increased the proliferative and migratory ability of hMCs and the expression of CTGF and fibronectin. The authors also investigated the underlaying molecular mechanism, and they concluded that under hyperglycaemic conditions, the profibrogenic activity of PRP-Exos is mediated by YAP activation. They also demonstrated that DM-PRP-Exos activated this signaling pathway and enhanced both proliferative and fibrogenic activity of hMCs via PI3K/Akt pathway.
3.8. Intervertebral Disc Degeneration
The effect of PRP-derived EVs in intervertebral disc degeneration (IVD) has also been examined in two of the articles. Both studies concluded that PRP-EVs were effective in ameliorating IVD, albeit through different mechanisms of action. H2O2 was used to mimic the IVD conditions in vitro. Firstly, Xu et al. [42] compared the effects of PRP-Exo and reactive oxygen species scavenger (NAC) to revert the detrimental consequences of H2O2 addition on nucleus pulposus (NP) cells. Both treatments suppressed ROS and pro-inflammatory cytokines generation in NP cells treated with H2O2. The authors also identified the Keap1-Nrf2 pathway as the underlying mechanism by which PRP-Exo exerted its protective effects. PRP-Exo was enriched in miR-141-3p that activates the Keap1-Nrf2 pathway by degrading Keap1 and leading to the release of Nrf2 from the Keap1-Nrf2 complex, which finally results in a translocation from cytoplasm to the nucleus to trigger its antioxidant role. Dai et al. [41] compared platelet-derived EVs (PEV) vs. platelets (PLTs) from PRP using the same previous in vitro model (H2O2 addition), but with the double EVs concentration (100 µg/mL) and in cells of non-human origin. They also observed that PEV reduced oxidative stress and the inflammatory response. In fact, activation of PEV did not modify inflammatory factor levels associated with these two responses. Additionally, the results confirmed that PEVs could inhibit apoptosis and senescence and restore the metabolism of NP cells by increasing anabolic protein levels (Col2α, Sox9, and ACAN) and reducing the catabolic (MMP3, MMP13, and ADAMTS5). Moreover, they demonstrated that the SIRT1-PGC1α-TFAM pathway was involved in the PEV’s ability to maintain mitochondrial function. These authors also evaluated the therapeutic effects of PEV in a rat IVD model. Overall, the results demonstrated that PEVs could retard the progression of IVD in vivo. The data indicated that PEVs could restore the water content of NP, suppress matrix degradation, attenuate ROS levels and promote mitochondrial function, whereas platelets did not exert such a significant effect.
3.9. Muscle Injury
Two articles were included in this category. Catitti et al. [43] performed an in vitro study to analyze the cargo of PRP-EVs from athletes recovering from injuries by label-free proteomics. Despite the authors reporting to remove the leukocyte fraction from PRP, they claimed that leukocyte-derived EVs were the most represented EV subpopulation, followed by platelet derived-EVs and EVs from endothelium. The shotgun proteomic analysis revealed that the cargo of PRP-EVs was related to the regeneration process. One hundred and five proteins were identified, of which 32% were associated with “defense and immunity” biological function. These proteins were also involved in vesicle-mediated transport and wound-healing events. The authors suggested that platelet-derived EVs might be behind the regenerative potential of PRP. In turn, Iyer et al. [44] evaluated the effect of activated PRP-derived exosomes on the recovery of muscle function after injury. An in vivo rat model of muscle strain injury was used. They also compared the effect with MSC-derived exosomes. According to the published data, similar recovery of contractile function was reported for both groups compared to the control. Centrally nucleated fibers (CNFs) (a muscle regeneration marker) were also significantly increased after treatment with exosomes from both origins. However, genes involved in skeletal muscle regeneration were differentially affected. The Myogenin gene was significantly upregulated in muscles treated with PRP-exos, whereas MSC-exos treatment significantly reduced TGF-β expression. The authors concluded that exosomes derived from both origins could enable recovery after a muscle strain injury.
3.10. Wound Healing
Regarding cutaneous wound healing, two articles were included. Both in vitro and in vivo experiments were conducted. Despite the differences between these two studies (such as the experimental system or origin and composition of PRP), both reached the same conclusion that PRP-Exos could promote wound healing. Guo et al. [45] utilized the supernatant of activated PRP (PRP-AS) in order to compare the effect of PRP-Exos with PRP. Human microvascular endothelial cell line (HMEC-1) and primary dermal fibroblasts were established as the experimental system for the in vitro assays, while a diabetic rat model was used for the in vivo. The authors reported that PRP-Exos stimulated the proliferation and migration of HMEC-1 cells and fibroblasts to a greater extent than PRP. PRP-Exos also promoted a more significant tube formation in vitro. Similar effects were found in vivo. Moreover, the results showed that activation of Erk and Akt signaling pathways might underlie the PRP-Exos-induced angiogenesis. They also suggested that the effect of PRP-Exos on reepithelization could be mediated by increasing collagen synthesis through YAP activation. All these data stated that a substantial part of the PRP effects were mediated by exosomes. Despite Xu et al. [46] using immortalized keratinocytes (HaCaT cells) to evaluate the effects of PRP-Exos in vitro, they found a similar effect; that is, they also reported that these EVs effectively enhanced the proliferation, migration, and wound healing of these cells. This treatment was also superior to PRP treatment. However, the authors suggested a different underlying mechanism involved in this PRP-Exos-mediated wound healing. The authors demonstrated that USP15, a key mediator of protein deubiquitination, was detected at significantly higher levels in PRP-Exos and enhanced HaCaT cell functionality by promoting EIF4A1 deubiquitination. Moreover, according to the published data, this mechanism was also behind the in vivo promotion of cutaneous wound healing by PRP-Exos, as demonstrated by USP15 knockdown.
3.11. Miscellanea
3.11.1. Cartilage Regeneration
Another in vitro study evaluated the effects of PRP-exos in the chondrogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) from rats. They also assessed the combination with berberine (Exos-Ber), an isoquinoline alkaloid with anti-inflammatory properties. Three different concentrations were studied (5, 25, and 50 µg/mL). Their findings indicated that both PRP-Exos and Exos-Ber significantly promoted the proliferation, migration, and chondrogenic differentiation of BMSCs. They also found an increase in the chondrogenesis-related proteins Collagen II, SOX9, and ACAN. The greatest effect on all these biological processes was observed with the combination of exosomes and berberine. The results also suggested that Exos-Ber treatment exerted its role via the Wnt/β-Catenin pathway.
3.11.2. Dental Pulp Regeneration
Bagio et al. [48] performed an in vitro analysis to determine the effect of different concentrations (0.5, 1, and 5%) of the thrombin-activated platelet-derived exosomes (T-aPDE) obtained from a PRP in the dental pulp regeneration. According to the authors, the 5% T-aDPE group promoted a greater human dental pulp stem cells (hDPSCs) viability rate and migration activity than the rest of the studied groups. The results also showed that mitochondria function improved after cells were cultured with 5% T-aDPE compared to the control groups. Finally, and regarding the angiogenesis process, this study demonstrated that hDPSCs treated with 5% T-aDPE showed a significantly increased in VEGF-A compared to the rest of the control groups and treatments after 24 h and 72 h.
3.11.3. Hair Loss
Nilforoushzadeh et al. [49] compared the in vitro effects of different concentrations of exosomes derived from two different origins: adipose stem cells (hASCs) and platelet-rich plasma (PRP) for hair loss treatment. Preserving hair follicle inductivity of dermal papilla cells (DPCs) is the main concern in hair follicle regeneration. The authors reported that ASC-Exo significantly increased migration, proliferation, and amount of alkaline phosphatase (ALP), Versican, and alpha-smooth muscle actin (α-SMA) compared to other experimental groups. The 100 ug/mL concentration had the best biological effect.
3.11.4. Osteonecrosis
Tao et al. [50] focused on the effect of PRP-Exos on the osteonecrosis of the femoral head (ONFH), a significant side effect of glucocorticoid (GC) use. Dexamethasone (DEX)-treated in vitro cell model and methylprednisolone (MPS)-treated in vivo rat model were used. The results showed that PRP-Exos treatment reverted the GC-induced apoptosis and antiproliferative effect both in vivo and in vitro. The authors concluded that the activation of the Akt/Bad/Bcl-2 signal pathway was responsible for this antiapoptotic role. Moreover, PRP-Exos could also rescue angiogenesis during DEX treatment and the co-administration with MPS-protected blood vessels in femoral heads in vivo. Similar results were found regarding osteogenesis, as the authors claimed that PRP-Exos could prevent the inhibition of osteogenesis mediated by DEX and promote the overregulation of osteogenesis-related proteins in vitro. This was further confirmed in vivo, where the cotreatment with PRP-Exos drastically reduced the GC-induced ONFH in rats. The authors concluded that PRP-Exos maintained the osteogenic differentiation and osteogenesis through the Wnt/β-catenin signaling pathway.
4. Discussion and Future Perspectives
According to all reported works compiled in this review, PRP-EVs represent a promising therapeutic approach in the field of tissue repair and regeneration. PRP-EVs promoted cell proliferation and migration [31,34,35,40,41,42,45,46,47,48,49,50] and angiogenesis [35,45,50] while reducing the inflammatory response [31,32,33,41,42], apoptosis [31,34,41,42,50] the oxidative stress [41] and senescence [41], among others. PRP-EVs seem to exert their function through different signaling pathways such as Wnt/β-catenin [31,47,50], AKT/ERK [35,45], TLR4 [39], PI3K/Akt [40], PGC1α-TFAM [41], Keap1-Nrf2 [42], YAP [45] and Akt/Bad/Bcl-2 [50]. All these effects may be due to their unique features, such as their lower immunogenicity, their ability to protect from degradation, their involvement in intercellular communication thanks to their composition, their ability to cross biological barriers, and the lack of need for expansion associated with other cellular sources [14,21]. However, from a scientific point of view, the lack of standardization in both the field of PRP and the EVs obtaining process makes it extremely challenging to compare results among studies. In this sense, the great variability in the PRP composition and obtaining procedures, the need for platelet activation or not and, if applicable, the type of agonist, the different EVs’ isolation and storage protocols, the absence of specific and universal markers, and the lack of consensus on the dose, among others, may have a direct impact on the yield, purity and cargo composition [21,64] (Figure 6). In addition, a large amount of information is omitted or not specified in the articles, which not only makes it impossible to compare but also to reproduce. Moreover, the International Society for Extracellular Vesicles, in its 2018 updated guideline [18], suggests a number of mandatory points concerning nomenclature, collection, characterization, functional studies, and reporting, which have not been followed in all the articles, notably the quantitative comparison of functional activity of EVs vs. EV-depleted fluid which none of the studies have conducted. Therefore, the resolution of all these issues is an essential requirement for performing translational studies.
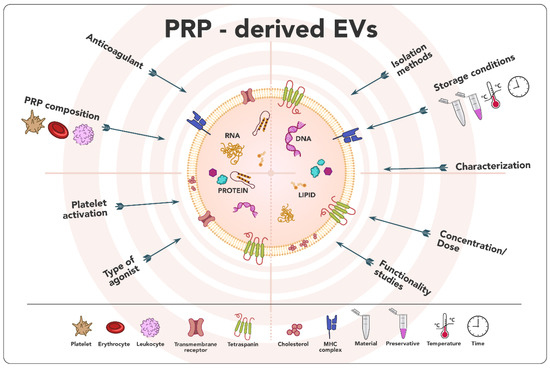
Figure 6.
Issues needed to be elucidated in order to promote the clinical translation of PRP-derived EVs.
The effect of PRP-EVs compared to that of PRP was conducted in 9 out of 20 articles included in this review [31,32,33,35,36,38,45,46,48]. In general, better performance was obtained with the former, which might suggest it could be a plausible alternative to replace the application of PRP for regenerative purposes. However, as previously mentioned, functionality studies depleting EVs should be carried out to confirm the contribution of these EVs in the PRP role. Furthermore, we should keep in mind that PRP is not only a source of proteins and growth factors, but it can also provide an autologous 3D fibrin matrix, which is necessary, in many cases, to support tissue regeneration. In this context, this naturally occurring scaffold provides physical support and mediates cell behavior [73]. Therefore, its critical biological role in tissue engineering cannot be ignored.
Their stability, extensive cargo-cell type and state-dependent, and their unique ability to cross the blood-brain barrier confer EVs as a promising powerful non-invasive tool for drug delivery and use as disease biomarkers [74,75]. Several review articles have indeed deeply addressed their role in several disease diagnoses, including autoimmune diseases [76,77], cardiovascular diseases [78,79,80], and neurodegenerative diseases [81,82,83]. Moreover, two clinical trials have been registered to evaluate the potential of PRP-EVs, both in chronic otitis media, one of which is still recruiting (NCT04761562), while the other is completed (NCT04281901) and with results already published [84]. In the latter, the authors concluded that autologous platelet—and extracellular vesicles—rich plasma represents a novel and successful treatment for a chronically radical mastoid cavity when the standard methods have been exhausted. In this case, the authors further specified that the inclusion of leukocytes was avoided in the plasma preparation. Additional controlled clinical studies should also be performed to evaluate the real clinical relevance of this emerging approach.
In summary, the use of PRP-EVs is an emerging field of great interest that represents a promising therapeutic option, as their efficacy has been confirmed in the majority of fields of applications included in this review. Nevertheless, there is an urgent need to establish standardized conditions to ensure optimized and detailed protocols to define, among others, the obtaining process, the dose, or the origin. Further studies should also be performed to elucidate the real contribution of EVs to PRP. Additionally, the evaluation of an integrated strategy that takes advantage of the synergistic effects of combining PRP-derived fibrin and EVs can entail a leap forward in the development of new applications. Nevertheless, these promising results provide a novel basis to deal with the regenerative medicine and drug delivery fields in the future.
Author Contributions
Conceptualization, E.A., J.M.F.-P. and M.H.A.; methodology, E.A., M.T. and M.H.A.; investigation, E.A. and M.T.; writing—original draft preparation, E.A., M.T. and E.G.; writing—review and editing, M.T., J.M.F.-P., S.L.-S., E.G. and M.H.A.; supervision, E.A. and M.H.A. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Basque Country Government (Spain) under the research and development project Elkartek (reference KK-2022/00038).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated in this research was reported in the manuscript.
Conflicts of Interest
The authors declare the following competing financial interests: E.A. is the Scientific Director of and M.T. and M.H.A. are scientists at BTI Biotechnology Institute, a dental implant company that investigates the fields of oral implantology and PRGF-Endoret technology. J.M.F.-P., S.L.-S. and E.G. at CIC bioGUNE disclose no conflict of interest for this study.
References
- Anitua, E. Plasma rich in growth factors: Preliminary results of use in the preparation of future sites for implants. Int. J. Oral Maxillofac. Implant. 1999, 14, 529–535. [Google Scholar]
- Marx, R.E.; Carlson, E.R.; Eichstaedt, R.M.; Schimmele, S.R.; Strauss, J.E.; Georgeff, K.R. Platelet-rich plasma: Growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1998, 85, 638–646. [Google Scholar] [CrossRef]
- Anitua, E.; Troya, M.; Zalduendo, M.; Orive, G. Personalized plasma-based medicine to treat age-related diseases. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 459–464. [Google Scholar] [CrossRef] [PubMed]
- DeLong, J.M.; Russell, R.P.; Mazzocca, A.D. Platelet-rich plasma: The PAW classification system. Arthroscopy 2012, 28, 998–1009. [Google Scholar] [CrossRef]
- Magalon, J.; Brandin, T.; Francois, P.; Degioanni, C.; De Maria, L.; Grimaud, F.; Veran, J.; Dignat-George, F.; Sabatier, F. Technical and biological review of authorized medical devices for platelets-rich plasma preparation in the field of regenerative medicine. Platelets 2021, 32, 200–208. [Google Scholar] [CrossRef]
- Acebes-Huerta, A.; Arias-Fernandez, T.; Bernardo, A.; Munoz-Turrillas, M.C.; Fernandez-Fuertes, J.; Seghatchian, J.; Gutierrez, L. Platelet-derived bio-products: Classification update, applications, concerns and new perspectives. Transfus. Apher. Sci. 2020, 59, 102716. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Padilla, S.; Prado, R.; Alkhraisat, M.H. Platelet-rich plasma: Are the obtaining methods, classification and clinical outcome always connected? Regen. Med. 2022, 17, 887–890. [Google Scholar] [CrossRef]
- Kon, E.; Di Matteo, B.; Delgado, D.; Cole, B.J.; Dorotei, A.; Dragoo, J.L.; Filardo, G.; Fortier, L.A.; Giuffrida, A.; Jo, C.H.; et al. Platelet-rich plasma for the treatment of knee osteoarthritis: An expert opinion and proposal for a novel classification and coding system. Expert. Opin. Biol. Ther. 2020, 20, 1447–1460. [Google Scholar] [CrossRef]
- van der Meijden, P.E.J.; Heemskerk, J.W.M. Platelet biology and functions: New concepts and clinical perspectives. Nat. Rev. Cardiol. 2019, 16, 166–179. [Google Scholar] [CrossRef]
- Sun, J.; Hu, Y.; Fu, Y.; Zou, D.; Lu, J.; Lyu, C. Emerging roles of platelet concentrates and platelet-derived extracellular vesicles in regenerative periodontology and implant dentistry. APL Bioeng. 2022, 6, 031503. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Troya, M.; Zalduendo, M.; Tejero, R.; Orive, G. Progress in the Use of Autologous Regenerative Platelet-based Therapies in Implant Dentistry. Curr. Pharm. Biotechnol. 2016, 17, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Alkhraisat, M.H.; Orive, G. Perspectives and challenges in regenerative medicine using plasma rich in growth factors. J. Control. Release 2012, 157, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Troya, M.; Tierno, R.; Zalduendo, M.; Alkhraisat, M.H. The Effectiveness of Platelet-Rich Plasma as a Carrier of Stem Cells in Tissue Regeneration: A Systematic Review of Pre-Clinical Research. Cells Tissues Organs 2021, 210, 339–350. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Piao, Y.; Liu, Q.; Yang, X. Platelet-rich plasma-derived extracellular vesicles: A superior alternative in regenerative medicine? Cell Prolif. 2021, 54, e13123. [Google Scholar] [CrossRef]
- Antich-Rossello, M.; Forteza-Genestra, M.A.; Monjo, M.; Ramis, J.M. Platelet-Derived Extracellular Vesicles for Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8580. [Google Scholar] [CrossRef] [PubMed]
- Eustes, A.S.; Dayal, S. The Role of Platelet-Derived Extracellular Vesicles in Immune-Mediated Thrombosis. Int. J. Mol. Sci. 2022, 23, 7837. [Google Scholar] [CrossRef]
- Abels, E.R.; Breakefield, X.O. Introduction to Extracellular Vesicles: Biogenesis, RNA Cargo Selection, Content, Release, and Uptake. Cell. Mol. Neurobiol. 2016, 36, 301–312. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Chargaff, E.; West, R. The biological significance of the thromboplastic protein of blood. J. Biol. Chem. 1946, 166, 189–197. [Google Scholar] [CrossRef]
- Wolf, P. The nature and significance of platelet products in human plasma. Br. J. Haematol. 1967, 13, 269–288. [Google Scholar] [CrossRef]
- Bahmani, L.; Ullah, M. Different Sourced Extracellular Vesicles and Their Potential Applications in Clinical Treatments. Cells 2022, 11, 1989. [Google Scholar] [CrossRef]
- Gasecka, A.; Nieuwland, R.; Siljander, P.R.M. Platelet-Derived Extracellular Vesicles. In Platelets; Academic Press: Cambridge, MA, USA, 2019; pp. 401–416. [Google Scholar]
- Palazzolo, S.; Canzonieri, V.; Rizzolio, F. The history of small extracellular vesicles and their implication in cancer drug resistance. Front. Oncol. 2022, 12, 948843. [Google Scholar] [CrossRef]
- Torreggiani, E.; Perut, F.; Roncuzzi, L.; Zini, N.; Baglio, S.R.; Baldini, N. Exosomes: Novel effectors of human platelet lysate activity. Eur. Cells Mater. 2014, 28, 137–151, discussion 151. [Google Scholar] [CrossRef] [PubMed]
- Momen-Heravi, F.; Getting, S.J.; Moschos, S.A. Extracellular vesicles and their nucleic acids for biomarker discovery. Pharmacol. Ther. 2018, 192, 170–187. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.M.; Wang, M.Z. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Hessvik, N.P.; Llorente, A. Current knowledge on exosome biogenesis and release. Cell. Mol. Life Sci. 2018, 75, 193–208. [Google Scholar] [CrossRef]
- Ramirez-Garrastacho, M.; Bajo-Santos, C.; Line, A.; Martens-Uzunova, E.S.; de la Fuente, J.M.; Moros, M.; Soekmadji, C.; Tasken, K.A.; Llorente, A. Extracellular vesicles as a source of prostate cancer biomarkers in liquid biopsies: A decade of research. Br. J. Cancer 2022, 126, 331–350. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Li, S.; Zhang, G.; Xu, H.H.K.; Zhang, K.; Bai, Y. New frontiers of oral sciences: Focus on the source and biomedical application of extracellular vesicles. Front. Bioeng. Biotechnol. 2022, 10, 1023700. [Google Scholar] [CrossRef]
- Golbach, L.A.; Portelli, L.A.; Savelkoul, H.F.; Terwel, S.R.; Kuster, N.; de Vries, R.B.; Verburg-van Kemenade, B.M. Calcium homeostasis and low-frequency magnetic and electric field exposure: A systematic review and meta-analysis of in vitro studies. Environ. Int. 2016, 92–93, 695–706. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Ma, C.; Wang, G.; Zhang, Y.; Sun, S. Exosomes derived from platelet-rich plasma present a novel potential in alleviating knee osteoarthritis by promoting proliferation and inhibiting apoptosis of chondrocyte via Wnt/beta-catenin signaling pathway. J. Orthop. Surg. Res. 2019, 14, 470. [Google Scholar] [CrossRef]
- Otahal, A.; Kramer, K.; Kuten-Pella, O.; Weiss, R.; Stotter, C.; Lacza, Z.; Weber, V.; Nehrer, S.; De Luna, A. Characterization and Chondroprotective Effects of Extracellular Vesicles from Plasma- and Serum-Based Autologous Blood-Derived Products for Osteoarthritis Therapy. Front. Bioeng. Biotechnol. 2020, 8, 584050. [Google Scholar] [CrossRef] [PubMed]
- Otahal, A.; Kramer, K.; Kuten-Pella, O.; Moser, L.B.; Neubauer, M.; Lacza, Z.; Nehrer, S.; De Luna, A. Effects of Extracellular Vesicles from Blood-Derived Products on Osteoarthritic Chondrocytes within an Inflammation Model. Int. J. Mol. Sci. 2021, 22, 7224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.; Chen, J.; Qian, D.; Gao, P.; Qin, T.; Jiang, T.; Yi, J.; Xu, T.; Huang, Y.; et al. Exosomes derived from platelet-rich plasma administration in site mediate cartilage protection in subtalar osteoarthritis. J. Nanobiotechnology 2022, 20, 56. [Google Scholar] [CrossRef] [PubMed]
- Rui, S.; Yuan, Y.; Du, C.; Song, P.; Chen, Y.; Wang, H.; Fan, Y.; Armstrong, D.G.; Deng, W.; Li, L. Comparison and Investigation of Exosomes Derived from Platelet-Rich Plasma Activated by Different Agonists. Cell Transplant. 2021, 30, 9636897211017833. [Google Scholar] [CrossRef]
- Saumell-Esnaola, M.; Delgado, D.; Garcia Del Cano, G.; Beitia, M.; Salles, J.; Gonzalez-Burguera, I.; Sanchez, P.; Lopez de Jesus, M.; Barrondo, S.; Sanchez, M. Isolation of Platelet-Derived Exosomes from Human Platelet-Rich Plasma: Biochemical and Morphological Characterization. Int. J. Mol. Sci. 2022, 23, 2861. [Google Scholar] [CrossRef]
- Stoner, S.A.; Duggan, E.; Condello, D.; Guerrero, A.; Turk, J.R.; Narayanan, P.K.; Nolan, J.P. High sensitivity flow cytometry of membrane vesicles. Cytom. Part A 2016, 89, 196–206. [Google Scholar] [CrossRef]
- Yuana, Y.; Koning, R.I.; Kuil, M.E.; Rensen, P.C.; Koster, A.J.; Bertina, R.M.; Osanto, S. Cryo-electron microscopy of extracellular vesicles in fresh plasma. J. Extracell. Vesicles 2013, 2, 21494. [Google Scholar] [CrossRef]
- Zhang, W.; Dong, X.; Wang, T.; Kong, Y. Exosomes derived from platelet-rich plasma mediate hyperglycemia-induced retinal endothelial injury via targeting the TLR4 signaling pathway. Exp. Eye Res. 2019, 189, 107813. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Kong, Y. Exosomes derived from platelet-rich plasma activate YAP and promote the fibrogenic activity of Muller cells via the PI3K/Akt pathway. Exp. Eye Res. 2020, 193, 107973. [Google Scholar] [CrossRef]
- Dai, Z.; Xia, C.; Zhao, T.; Wang, H.; Tian, H.; Xu, O.; Zhu, X.; Zhang, J.; Chen, P. Platelet-derived extracellular vesicles ameliorate intervertebral disc degeneration by alleviating mitochondrial dysfunction. Mater. Today Bio 2023, 18, 100512. [Google Scholar] [CrossRef]
- Xu, J.; Xie, G.; Yang, W.; Wang, W.; Zuo, Z.; Wang, W. Platelet-rich plasma attenuates intervertebral disc degeneration via delivering miR-141-3p-containing exosomes. Cell Cycle 2021, 20, 1487–1499. [Google Scholar] [CrossRef] [PubMed]
- Catitti, G.; Cufaro, M.C.; De Bellis, D.; Cicalini, I.; Vespa, S.; Tonelli, F.; Miscia, G.; Secondi, L.; Simeone, P.; De Laurenzi, V.; et al. Extracellular Vesicles in Regenerative Processes Associated with Muscle Injury Recovery of Professional Athletes Undergoing Sub Maximal Strength Rehabilitation. Int. J. Mol. Sci. 2022, 23, 14913. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.R.; Scheiber, A.L.; Yarowsky, P.; Henn, R.F. 3rd.; Otsuru, S.; Lovering, R.M. Exosomes Isolated from Platelet-Rich Plasma and Mesenchymal Stem Cells Promote Recovery of Function After Muscle Injury. Am. J. Sports Med. 2020, 48, 2277–2286. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.C.; Tao, S.C.; Yin, W.J.; Qi, X.; Yuan, T.; Zhang, C.Q. Exosomes derived from platelet-rich plasma promote the re-epithelization of chronic cutaneous wounds via activation of YAP in a diabetic rat model. Theranostics 2017, 7, 81–96. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Z.; He, L.; Qu, Y.; Ouyang, L.; Han, Y.; Xu, C.; Duan, D. Platelet-Rich Plasma-Derived Exosomal USP15 Promotes Cutaneous Wound Healing via Deubiquitinating EIF4A1. Oxidative Med. Cell. Longev. 2021, 2021, 9674809. [Google Scholar] [CrossRef]
- Dong, B.; Liu, X.; Li, J.; Wang, B.; Yin, J.; Zhang, H.; Liu, W. Berberine Encapsulated in Exosomes Derived from Platelet-Rich Plasma Promotes Chondrogenic Differentiation of the Bone Marrow Mesenchymal Stem Cells via the Wnt/β-Catenin Pathway. Biol. Pharm. Bull. 2022, 45, 1444–1451. [Google Scholar] [CrossRef]
- Bagio, D.A.; Julianto, I.; Margono, A.; Suprastiwi, E. Analysis of Thrombin-Activated Platelet-Derived Exosome (T-aPDE) Potential for Dental Pulp Regeneration: In-Vitro Study. Eur. J. Dent. 2023, 17, 173–182. [Google Scholar] [CrossRef]
- Nilforoushzadeh, M.A.; Aghdami, N.; Taghiabadi, E. Effects of Adipose-Derived Stem Cells and Platelet-Rich Plasma Exosomes on The Inductivity of Hair Dermal Papilla Cells. Cell J. 2021, 23, 576–583. [Google Scholar]
- Tao, S.C.; Yuan, T.; Rui, B.Y.; Zhu, Z.Z.; Guo, S.C.; Zhang, C.Q. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics 2017, 7, 733–750. [Google Scholar] [CrossRef]
- Chahla, J.; Cinque, M.E.; Piuzzi, N.S.; Mannava, S.; Geeslin, A.G.; Murray, I.R.; Dornan, G.J.; Muschler, G.F.; LaPrade, R.F. A Call for Standardization in Platelet-Rich Plasma Preparation Protocols and Composition Reporting: A Systematic Review of the Clinical Orthopaedic Literature. J. Bone Jt. Surg. 2017, 99, 1769–1779. [Google Scholar] [CrossRef]
- Sundman, E.A.; Cole, B.J.; Fortier, L.A. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am. J. Sports Med. 2011, 39, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Zalduendo, M.; Troya, M.; Padilla, S.; Orive, G. Leukocyte inclusion within a platelet rich plasma-derived fibrin scaffold stimulates a more pro-inflammatory environment and alters fibrin properties. PLoS ONE 2015, 10, e0121713. [Google Scholar] [CrossRef] [PubMed]
- Auber, M.; Svenningsen, P. An estimate of extracellular vesicle secretion rates of human blood cells. J. Extracell. Biol. 2022, 1, e46. [Google Scholar] [CrossRef]
- Alberro, A.; Iparraguirre, L.; Fernandes, A.; Otaegui, D. Extracellular Vesicles in Blood: Sources, Effects, and Applications. Int. J. Mol. Sci. 2021, 22, 8163. [Google Scholar] [CrossRef] [PubMed]
- Tissot, J.-D.; Canellini, G.; Rubin, O.; Angelillo-Scherrer, A.; Delobel, J.; Prudent, M.; Lion, N. Blood microvesicles: From proteomics to physiology. Transl. Proteom. 2013, 1, 38–52. [Google Scholar] [CrossRef][Green Version]
- Bister, N.; Pistono, C.; Huremagic, B.; Jolkkonen, J.; Giugno, R.; Malm, T. Hypoxia and extracellular vesicles: A review on methods, vesicular cargo and functions. J. Extracell. Vesicles 2020, 10, e12002. [Google Scholar] [CrossRef]
- Brahmer, A.; Neuberger, E.; Esch-Heisser, L.; Haller, N.; Jorgensen, M.M.; Baek, R.; Mobius, W.; Simon, P.; Kramer-Albers, E.M. Platelets, endothelial cells and leukocytes contribute to the exercise-triggered release of extracellular vesicles into the circulation. J. Extracell. Vesicles 2019, 8, 1615820. [Google Scholar] [CrossRef]
- Milioli, M.; Ibáñez-Vea, M.; Sidoli, S.; Palmisano, G.; Careri, M.; Larsen, M.R. Quantitative proteomics analysis of platelet-derived microparticles reveals distinct protein signatures when stimulated by different physiological agonists. J. Proteom. 2015, 121, 56–66. [Google Scholar] [CrossRef]
- Shai, E.; Rosa, I.; Parguina, A.F.; Motahedeh, S.; Varon, D.; Garcia, A. Comparative analysis of platelet-derived microparticles reveals differences in their amount and proteome depending on the platelet stimulus. J. Proteom. 2012, 76, 287–296. [Google Scholar] [CrossRef]
- Suades, R.; Padro, T.; Vilahur, G.; Badimon, L. Platelet-released extracellular vesicles: The effects of thrombin activation. Cell. Mol. Life Sci. 2022, 79, 190. [Google Scholar] [CrossRef]
- Janouskova, O.; Herma, R.; Semeradtova, A.; Poustka, D.; Liegertova, M.; Hana Auer, M.; Maly, J. Conventional and Nonconventional Sources of Exosomes-Isolation Methods and Influence on Their Downstream Biomedical Application. Front. Mol. Biosci. 2022, 9, 846650. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Im, H.; Castro, C.M.; Breakefield, X.; Weissleder, R.; Lee, H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018, 118, 1917–1950. [Google Scholar] [CrossRef] [PubMed]
- Gelibter, S.; Marostica, G.; Mandelli, A.; Siciliani, S.; Podini, P.; Finardi, A.; Furlan, R. The impact of storage on extracellular vesicles: A systematic study. J. Extracell. Vesicles 2022, 11, e12162. [Google Scholar] [CrossRef]
- van de Wakker, S.I.; van Oudheusden, J.; Mol, E.A.; Roefs, M.T.; Zheng, W.; Gorgens, A.; El Andaloussi, S.; Sluijter, J.P.G.; Vader, P. Influence of short term storage conditions, concentration methodsand excipients on extracellular vesicle recovery and function. Eur. J. Pharm. Biopharm. 2022, 170, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Deville, S.; Berckmans, P.; Van Hoof, R.; Lambrichts, I.; Salvati, A.; Nelissen, I. Comparison of extracellular vesicle isolation and storage methods using high-sensitivity flow cytometry. PLoS ONE 2021, 16, e0245835. [Google Scholar] [CrossRef]
- Sivanantham, A.; Jin, Y. Impact of Storage Conditions on EV Integrity/Surface Markers and Cargos. Life 2022, 12, 697. [Google Scholar] [CrossRef]
- Evtushenko, E.G.; Bagrov, D.V.; Lazarev, V.N.; Livshits, M.A.; Khomyakova, E. Adsorption of extracellular vesicles onto the tube walls during storage in solution. PLoS ONE 2020, 15, e0243738. [Google Scholar] [CrossRef]
- Gorgens, A.; Corso, G.; Hagey, D.W.; Jawad Wiklander, R.; Gustafsson, M.O.; Felldin, U.; Lee, Y.; Bostancioglu, R.B.; Sork, H.; Liang, X.; et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J. Extracell. Vesicles 2022, 11, e12238. [Google Scholar] [CrossRef]
- Szatanek, R.; Baj-Krzyworzeka, M.; Zimoch, J.; Lekka, M.; Siedlar, M.; Baran, J. The Methods of Choice for Extracellular Vesicles (EVs) Characterization. Int. J. Mol. Sci. 2017, 18, 1153. [Google Scholar] [CrossRef]
- Imanbekova, M.; Suarasan, S.; Lu, Y.; Jurchuk, S.; Wachsmann-Hogiu, S. Recent advances in optical label-free characterization of extracellular vesicles. Nanophotonics 2022, 11, 2827–2863. [Google Scholar] [CrossRef]
- Campos-Silva, C.; Suarez, H.; Jara-Acevedo, R.; Linares-Espinos, E.; Martinez-Pineiro, L.; Yanez-Mo, M.; Vales-Gomez, M. High sensitivity detection of extracellular vesicles immune-captured from urine by conventional flow cytometry. Sci. Rep. 2019, 9, 2042. [Google Scholar] [CrossRef]
- Anitua, E.; Zalduendo, M.; Troya, M.; Tierno, R.; Alkhraisat, M.H. The inclusion of leukocytes into platelet rich plasma reduces scaffold stability and hinders extracellular matrix remodelling. Ann. Anat.-Anat. Anz. 2022, 240, 151853. [Google Scholar] [CrossRef] [PubMed]
- Amiri, A.; Bagherifar, R.; Ansari Dezfouli, E.; Kiaie, S.H.; Jafari, R.; Ramezani, R. Exosomes as bio-inspired nanocarriers for RNA delivery: Preparation and applications. J. Transl. Med. 2022, 20, 125. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Zhao, T.; Song, N.; Pan, K.; Yang, Y.; Zhu, X.; Chen, P.; Zhang, J.; Xia, C. Platelets and platelet extracellular vesicles in drug delivery therapy: A review of the current status and future prospects. Front. Pharmacol. 2022, 13, 1026386. [Google Scholar] [CrossRef]
- Xu, K.; Liu, Q.; Wu, K.; Liu, L.; Zhao, M.; Yang, H.; Wang, X.; Wang, W. Extracellular vesicles as potential biomarkers and therapeutic approaches in autoimmune diseases. J. Transl. Med. 2020, 18, 432. [Google Scholar] [CrossRef]
- Wu, W.C.; Song, S.J.; Zhang, Y.; Li, X. Role of Extracellular Vesicles in Autoimmune Pathogenesis. Front. Immunol. 2020, 11, 579043. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.; Nickenig, G.; Werner, N. Extracellular Vesicles in Cardiovascular Disease: Potential Applications in Diagnosis, Prognosis, and Epidemiology. Circ. Res. 2017, 120, 1649–1657. [Google Scholar] [CrossRef]
- Mallia, A.; Gianazza, E.; Zoanni, B.; Brioschi, M.; Barbieri, S.S.; Banfi, C. Proteomics of Extracellular Vesicles: Update on Their Composition, Biological Roles and Potential Use as Diagnostic Tools in Atherosclerotic Cardiovascular Diseases. Diagnostics 2020, 10, 843. [Google Scholar] [CrossRef]
- Patel, S.; Guo, M.K.; Abdul Samad, M.; Howe, K.L. Extracellular vesicles as biomarkers and modulators of atherosclerosis pathogenesis. Front. Cardiovasc. Med. 2023, 10, 1202187. [Google Scholar] [CrossRef]
- Valencia, J.; Ferreira, M.; Merino-Torres, J.F.; Marcilla, A.; Soriano, J.M. The Potential Roles of Extracellular Vesicles as Biomarkers for Parkinson’s Disease: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 11508. [Google Scholar] [CrossRef]
- Vassileff, N.; Cheng, L.; Hill, A.F. Extracellular vesicles—Propagators of neuropathology and sources of potential biomarkers and therapeutics for neurodegenerative diseases. J. Cell. Sci. 2020, 133, jcs243139. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wang, Y.; Zheng, J.C. Extracellular vesicles, from the pathogenesis to the therapy of neurodegenerative diseases. Transl. Neurodegener. 2022, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Vozel, D.; Bozic, D.; Jeran, M.; Jan, Z.; Pajnic, M.; Paden, L.; Steiner, N.; Kralj-Iglic, V.; Battelino, S. Autologous Platelet- and Extracellular Vesicle-Rich Plasma Is an Effective Treatment Modality for Chronic Postoperative Temporal Bone Cavity Inflammation: Randomized Controlled Clinical Trial. Front. Bioeng. Biotechnol. 2021, 9, 677541. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).