Possible Compensatory Role of ASICs in Glutamatergic Synapses
Abstract
:1. Introduction
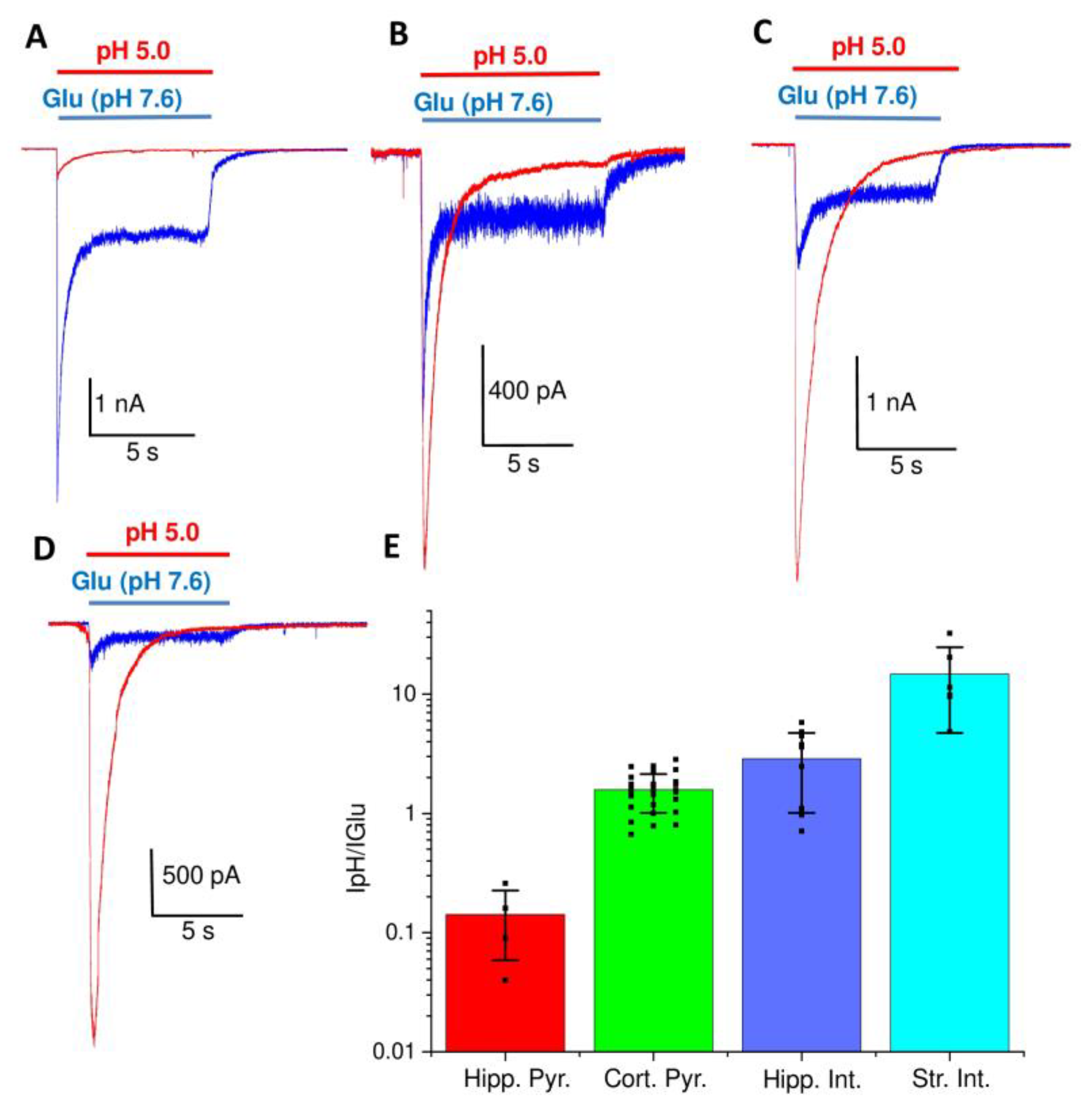
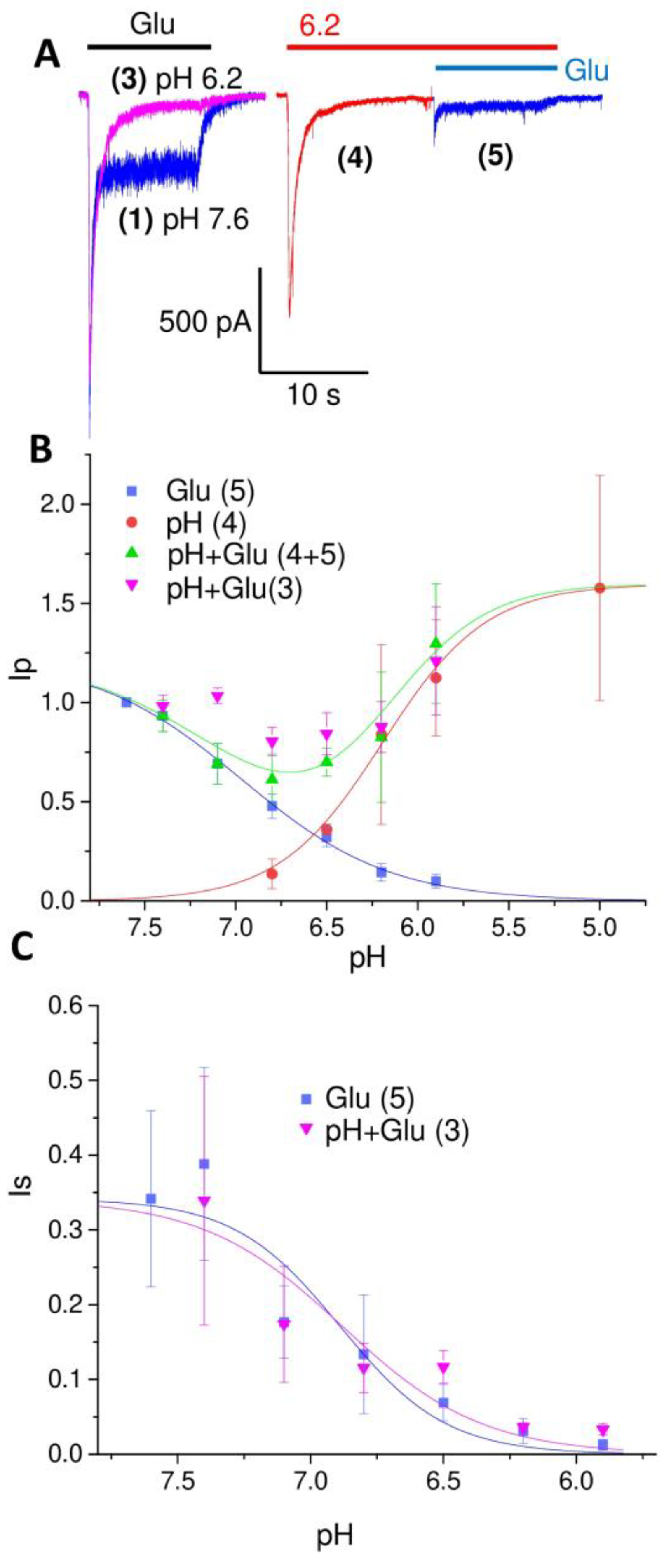
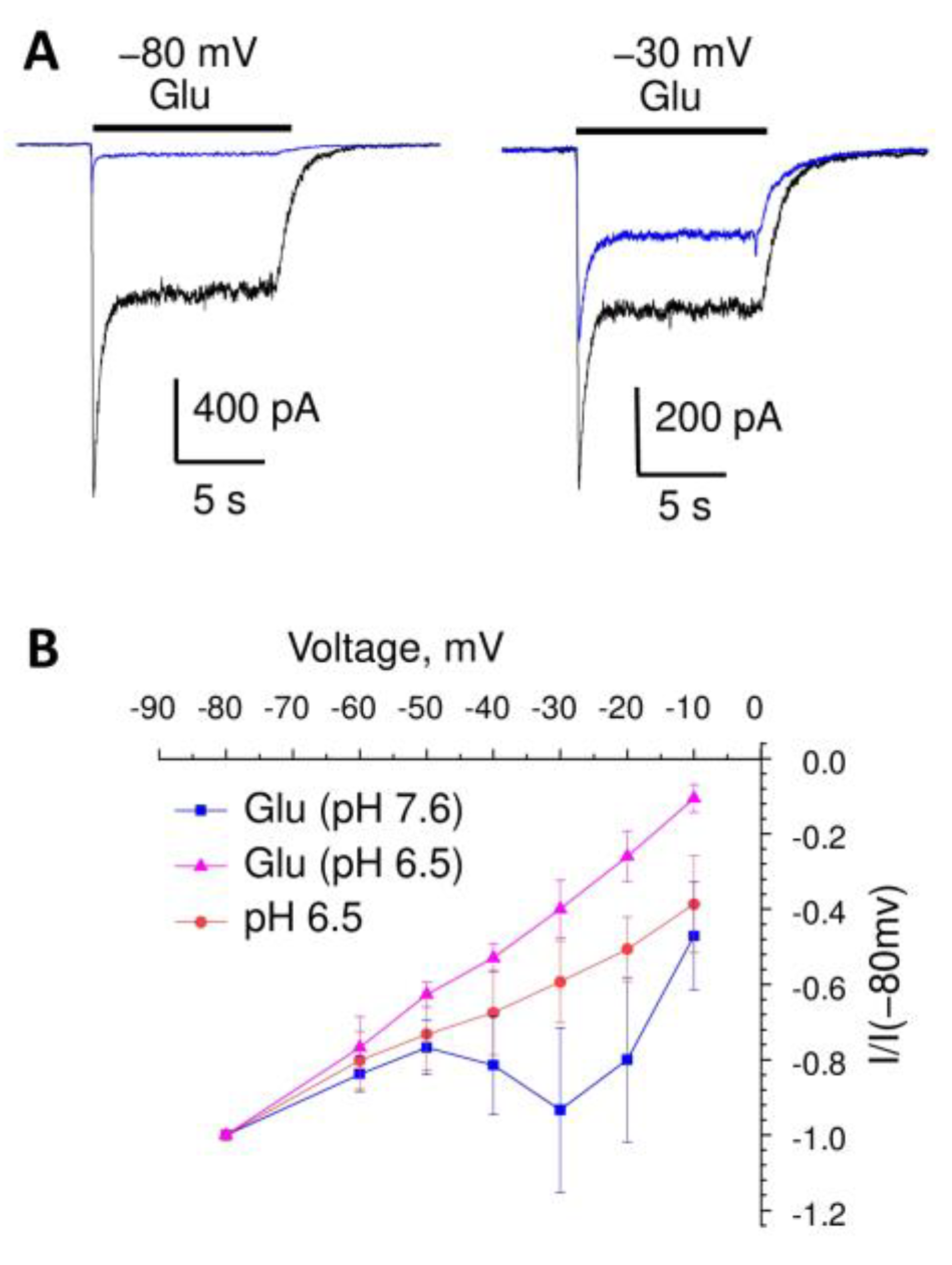
2. Results
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Storozhuk, M.; Cherninskyi, A.; Maximyuk, O.; Isaev, D.; Krishtal, O. Acid-Sensing Ion Channels: Focus on Physiological and Some Pathological Roles in the Brain. Curr. Neuropharmacol. 2021, 19, 1570–1589. [Google Scholar]
- Waldmann, R.; Champigny, G.; Bassilana, F.; Heurteaux, C.; Lazdunski, M. A proton-gated cation channel involved in acid-sensing. Nature 1997, 386, 173–177. [Google Scholar] [CrossRef]
- Kellenberger, S.; Schild, L. International Union of Basic and Clinical Pharmacology. XCI. structure. function, and pharmacology of acid-sensing ion channels and the epithelial Na+ channel. Pharmacol. Rev. 2015, 67, 1–35. [Google Scholar] [PubMed]
- Cegielski, V.; Chakrabarty, R.; Ding, S.; Wacker, M.J.; Monaghan-Nichols, P.; Chu, X.P. Acid-Sensing Ion Channels in Glial Cells. Membranes 2022, 12, 119. [Google Scholar] [CrossRef] [PubMed]
- Hesselager, M.; Timmermann, D.B.; Ahring, P.K. pH Dependency and desensitization kinetics of heterologously expressed combinations of acid-sensing ion channel subunits. J. Biol. Chem. 2004, 279, 11006–11015. [Google Scholar] [CrossRef] [PubMed]
- Bolshakov, K.V.; Essin, K.V.; Buldakova, S.L.; Dorofeeva, N.A.; Skatchkov, S.N.; Eaton, M.J.; Tikhonov, D.B.; Magazanik, L.G. Characterization of acid-sensitive ion channels in freshly isolated rat brain neurons. Neuroscience 2002, 110, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Alijevic, O.; Bignucolo, O.; Hichri, E.; Peng, Z.; Kucera, J.P.; Kellenberger, S. Slowing of the Time Course of Acidification Decreases the Acid-Sensing Ion Channel 1a Current Amplitude and Modulates Action Potential Firing in Neurons. Front. Cell. Neurosci. 2020, 14, 41. [Google Scholar] [CrossRef] [PubMed]
- Babini, E.; Paukert, M.; Geisler, H.S.; Grunder, S. Alternative splicing and interaction with di- and polyvalent cations control the dynamic range of acid-sensing ion channel 1 (ASIC1). J. Biol. Chem. 2002, 277, 41597–41603. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Reznikov, L.R.; Price, M.P.; Zha, X.M.; Lu, Y.; Moninger, T.O.; Wemmie, J.A.; Welsh, M.J. Protons are a neurotransmitter that regulates synaptic plasticity in the lateral amygdala. Proc. Natl. Acad. Sci. USA 2014, 111, 8961–8966. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Inchauspe, C.; Urbano, F.J.; Di Guilmi, M.N.; Uchitel, O.D. Acid-Sensing Ion Channels Activated by Evoked Released Protons Modulate Synaptic Transmission at the Mouse Calyx of Held Synapse. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 2589–2599. [Google Scholar] [CrossRef]
- Li, H.S.; Su, X.Y.; Song, X.L.; Qi, X.; Li, Y.; Wang, R.Q.; Maximyuk, O.; Krishtal, O.; Wang, T.; Fang, H.; et al. Protein Kinase C Lambda Mediates Acid-Sensing Ion Channel 1a-Dependent Cortical Synaptic Plasticity and Pain Hypersensitivity. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 5773–5793. [Google Scholar] [CrossRef] [PubMed]
- Mango, D.; Nistico, R. Acid-Sensing Ion Channel 1a Is Involved in N-Methyl D-Aspartate Receptor-Dependent Long-Term Depression in the Hippocampus. Front. Pharmacol. 2019, 10, 555. [Google Scholar] [CrossRef] [PubMed]
- Kreple, C.J.; Lu, Y.; Taugher, R.J.; Schwager-Gutman, A.L.; Du, J.; Stump, M.; Wang, Y.; Ghobbeh, A.; Fan, R.; Cosme, C.V.; et al. Acid-sensing ion channels contribute to synaptic transmission and inhibit cocaine-evoked plasticity. Nat. Neurosci. 2014, 17, 1083–1091. [Google Scholar] [CrossRef]
- Gonzalez-Inchauspe, C.; Gobetto, M.N.; Uchitel, O.D. Modulation of acid sensing ion channel dependent protonergic neurotransmission at the mouse calyx of Held. Neuroscience 2020, 439, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Woodhull, A.M. Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 1973, 61, 687–708. [Google Scholar] [CrossRef] [PubMed]
- Yatani, A.; Brown, A.M.; Akaike, N. Effect of extracellular pH on sodium current in isolated single rat ventricular cells. J. Membr. Biol. 1984, 78, 163–168. [Google Scholar]
- Baker, M.D.; Bostock, H. The pH dependence of late sodium current in large sensory neurons. Neuroscience 1999, 92, 1119–1130. [Google Scholar] [CrossRef]
- Landau, E.M.; Gavish, B.; Nachshen, D.A.; Lotan, I. pH dependence of the acetylcholine receptor channel: A species variation. J. Gen. Physiol. 1981, 77, 647–666. [Google Scholar] [CrossRef]
- Palma, A.; Li, L.; Chen, X.J.; Pappone, P.; McNamee, M. Effects of pH on acetylcholine receptor function. J. Membr. Biol. 1991, 120, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Orser, B.A.; Thatcher, G.R.; Reynolds, J.N.; MacDonald, J.F. Positive allosteric modulators of AMPA receptors reduce proton-induced receptor desensitization in rat hippocampal neurons. J. Neurophysiol. 2001, 85, 2030–2038. [Google Scholar] [CrossRef]
- Ihle, E.C.; Patneau, D.K. Modulation of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor desensitization by extracellular protons. Mol. Pharmacol. 2000, 58, 1204–1212. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Cull-Candy, S.G. Proton inhibition of N-methyl-D-aspartate receptors in cerebellar neurons. Nature 1990, 345, 347–350. [Google Scholar] [CrossRef]
- Magazanik, L.G.; Buldakova, S.L.; Samoilova, M.V.; Gmiro, V.E.; Mellor, I.R.; Usherwood, P.N. Block of open channels of recombinant AMPA receptors and native AMPA/kainate receptors by adamantane derivatives. J. Physiol. 1997, 505 Pt 3, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Buldakova, S.L.; Vorobjev, V.S.; Sharonova, I.N.; Samoilova, M.V.; Magazanik, L.G. Characterization of AMPA receptor populations in rat brain cells by the use of subunit-specific open channel blocking drug, IEM-1460. Brain Res. 1999, 846, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Samoilova, M.V.; Buldakova, S.L.; Vorobjev, V.S.; Sharonova, I.N.; Magazanik, L.G. The open channel blocking drug, IEM-1460, reveals functionally distinct alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate receptors in rat brain neurons. Neuroscience 1999, 94, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Zhigulin, A.S.; Tikhonov, D.B.; Barygin, O.I. Mechanisms of acid-sensing ion channels inhibition by nafamostat, sepimostat and diminazene. Eur. J. Pharmacol. 2023, 938, 175394. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, M.V.; Komarova, M.S.; Tikhonova, T.B.; Korosteleva, A.S.; Potapjeva, N.N.; Tikhonov, D.B. Modulation of Proton-Gated Channels by Antidepressants. ACS Chem. Neurosci. 2019, 10, 1636–1648. [Google Scholar] [CrossRef]
- Barygin, O.I.; Komarova, M.S.; Tikhonova, T.B.; Korosteleva, A.S.; Nikolaev, M.V.; Magazanik, L.G.; Tikhonov, D.B. Complex action of tyramine. tryptamine and histamine on native and recombinant ASICs. Channels 2017, 11, 648–659. [Google Scholar]
- Tikhonova, T.B.; Nagaeva, E.I.; Barygin, O.I.; Potapieva, N.N.; Bolshakov, K.V.; Tikhonov, D.B. Monoamine NMDA receptor channel blockers inhibit and potentiate native and recombinant proton-gated ion channels. Neuropharmacology 2015, 89, 1–10. [Google Scholar] [CrossRef]
- Shteinikov, V.; Evlanenkov, K.; Bolshakov, K.; Tikhonov, D. Glutamate potentiates heterologously expressed homomeric acid-sensing ion channel 1a. Synapse 2022, 76, e33337. [Google Scholar] [CrossRef]
- Nagaeva, E.I.; Tikhonova, T.B.; Magazanik, L.G.; Tikhonov, D.B. Histamine selectively potentiates acid-sensing ion channel 1a. Neurosci. Lett. 2016, 632, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Nowak, L.; Bregestovski, P.; Ascher, P.; Herbet, A.; Prochiantz, A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature 1984, 307, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Farsi, Z.; Gowrisankaran, S.; Krunic, M.; Rammner, B.; Woehler, A.; Lafer, E.M.; Mim, C.; Jahn, R.; Milosevic, I. Clathrin coat controls synaptic vesicle acidification by blocking vacuolar ATPase activity. eLife 2018, 7, e32569. [Google Scholar] [CrossRef] [PubMed]
- Gowrisankaran, S.; Milosevic, I. Regulation of synaptic vesicle acidification at the neuronal synapse. IUBMB Life 2020, 72, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Fuldner, H.H.; Stadler, H. 31P-NMR analysis of synaptic vesicles. Status of ATP and internal pH. Eur. J. Biochem. 1982, 121, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Wang, Y.Z.; Yang, T.; Chu, X.P.; Yu, Y.; Huang, Y.; Cao, H.; Hansen, J.; Simon, R.P.; Zhu, M.X.; et al. Extracellular spermine exacerbates ischemic neuronal injury through sensitization of ASIC1a channels to extracellular acidosis. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Hansen, K.B.; Wollmuth, L.P.; Bowie, D.; Furukawa, H.; Menniti, F.S.; Sobolevsky, A.I.; Swanson, G.T.; Swanger, S.A.; Greger, I.H.; Nakagawa, T.; et al. Structure, Function, and Pharmacology of Glutamate Receptor Ion Channels. Pharmacol. Rev. 2021, 73, 298–487. [Google Scholar] [CrossRef]
- Wemmie, J.A.; Chen, J.; Askwith, C.C.; Hruska-Hageman, A.M.; Price, M.P.; Nolan, B.C.; Yoder, P.G.; Lamani, E.; Hoshi, T.; Freeman, J.H., Jr.; et al. The acid-activated ion channel ASIC contributes to synaptic plasticity, learning, and memory. Neuron 2002, 34, 463–477. [Google Scholar] [CrossRef]
- Liu, M.G.; Li, H.S.; Li, W.G.; Wu, Y.J.; Deng, S.N.; Huang, C.; Maximyuk, O.; Sukach, V.; Krishtal, O.; Zhu, M.X.; et al. Acid-sensing ion channel 1a contributes to hippocampal LTP inducibility through multiple mechanisms. Sci. Rep. 2016, 6, 23350. [Google Scholar] [CrossRef]
- Mango, D.; Braksator, E.; Battaglia, G.; Marcelli, S.; Mercuri, N.B.; Feligioni, M.; Nicoletti, F.; Bashir, Z.I.; Nistico, R. Acid-sensing ion channel 1a is required for mGlu receptor dependent long-term depression in the hippocampus. Pharmacol. Res. 2017, 119, 12–19. [Google Scholar] [CrossRef]
- Chiang, P.H.; Chien, T.C.; Chen, C.C.; Yanagawa, Y.; Lien, C.C. ASIC-dependent LTP at multiple glutamatergic synapses in amygdala network is required for fear memory. Sci. Rep. 2015, 5, 10143. [Google Scholar] [CrossRef] [PubMed]
- Li, W.G.; Liu, M.G.; Deng, S.; Liu, Y.M.; Shang, L.; Ding, J.; Hsu, T.T.; Jiang, Q.; Li, Y.; Li, F.; et al. ASIC1a regulates insular long-term depression and is required for the extinction of conditioned taste aversion. Nat. Commun. 2016, 7, 13770. [Google Scholar] [CrossRef] [PubMed]
- Wemmie, J.A.; Taugher, R.J.; Kreple, C.J. Acid-sensing ion channels in pain and disease. Nat. Rev. Neurosci. 2013, 14, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.G.; Chu, X.P.; Simon, R.P. Acid sensing ion channels—Novel therapeutic targets for ischemic brain injury. Front. Biosci. 2007, 12, 1376–1386. [Google Scholar] [CrossRef]
- O’Bryant, Z.; Vann, K.T.; Xiong, Z.G. Translational strategies for neuroprotection in ischemic stroke--focusing on acid-sensing ion channel 1a. Transl. Stroke Res. 2014, 5, 59–68. [Google Scholar] [CrossRef]
- Xiong, Z.G.; Pignataro, G.; Li, M.; Chang, S.Y.; Simon, R.P. Acid-sensing ion channels (ASICs) as pharmacological targets for neurodegenerative diseases. Curr. Opin. Pharmacol. 2008, 8, 25–32. [Google Scholar] [CrossRef]
- Vorobjev, V.S. Vibrodissociation of sliced mammalian nervous tissue. J. Neurosci. Methods 1991, 38, 145–150. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evlanenkov, K.K.; Zhigulin, A.S.; Tikhonov, D.B. Possible Compensatory Role of ASICs in Glutamatergic Synapses. Int. J. Mol. Sci. 2023, 24, 12974. https://doi.org/10.3390/ijms241612974
Evlanenkov KK, Zhigulin AS, Tikhonov DB. Possible Compensatory Role of ASICs in Glutamatergic Synapses. International Journal of Molecular Sciences. 2023; 24(16):12974. https://doi.org/10.3390/ijms241612974
Chicago/Turabian StyleEvlanenkov, Konstantin K., Arseniy S. Zhigulin, and Denis B. Tikhonov. 2023. "Possible Compensatory Role of ASICs in Glutamatergic Synapses" International Journal of Molecular Sciences 24, no. 16: 12974. https://doi.org/10.3390/ijms241612974
APA StyleEvlanenkov, K. K., Zhigulin, A. S., & Tikhonov, D. B. (2023). Possible Compensatory Role of ASICs in Glutamatergic Synapses. International Journal of Molecular Sciences, 24(16), 12974. https://doi.org/10.3390/ijms241612974




