Three-Dimensional Printing of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] Biodegradable Scaffolds: Properties, In Vitro and In Vivo Evaluation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Changing the Properties of P(3HB-co-3HV) in the Process of Obtaining Filaments and 3D Printing
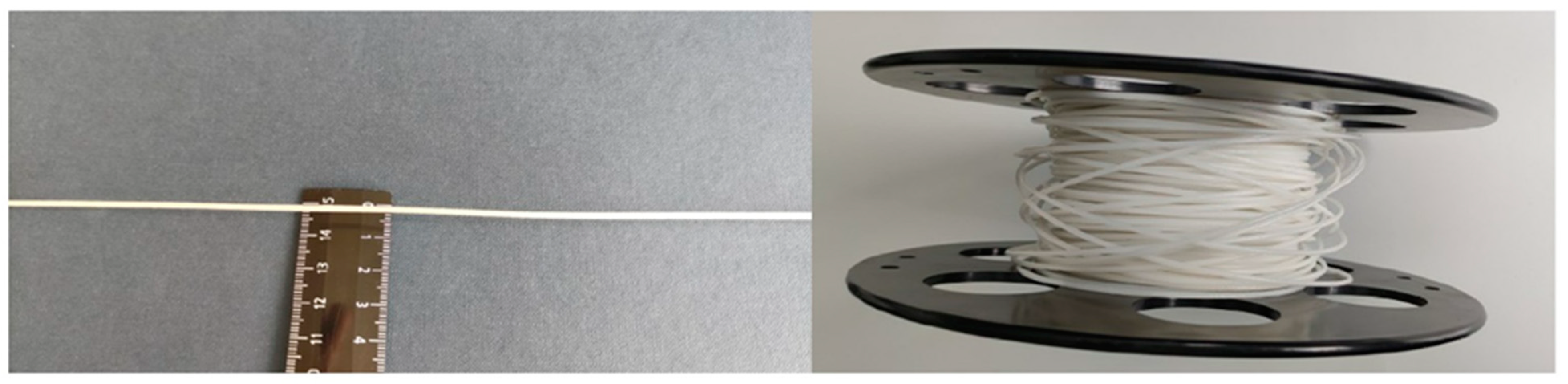
2.2. Characteristics of P(3HB-co-3HV) Filaments
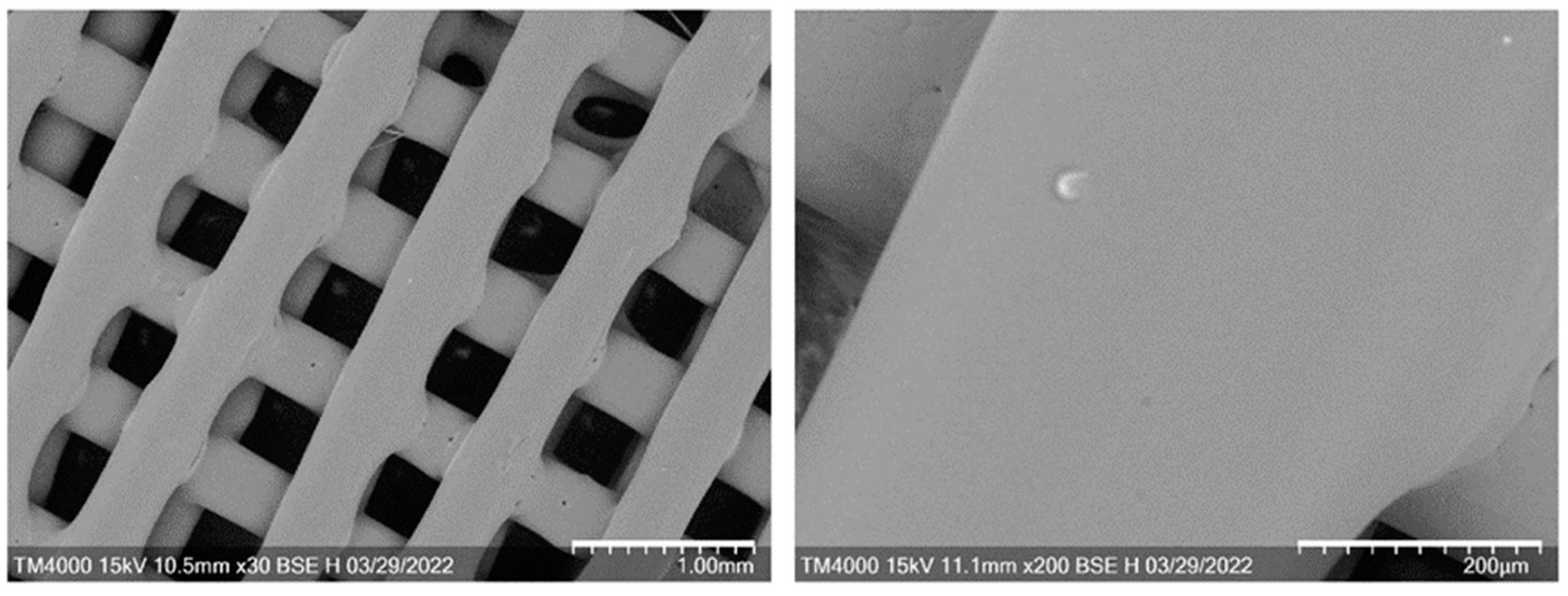
2.3. Characteristics of 3D Scaffolds
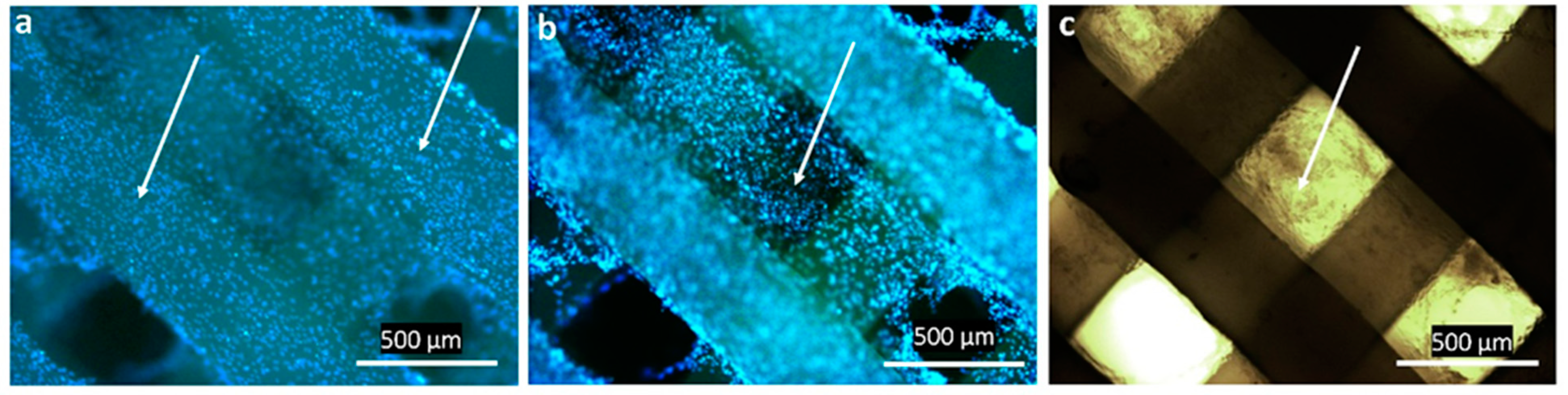
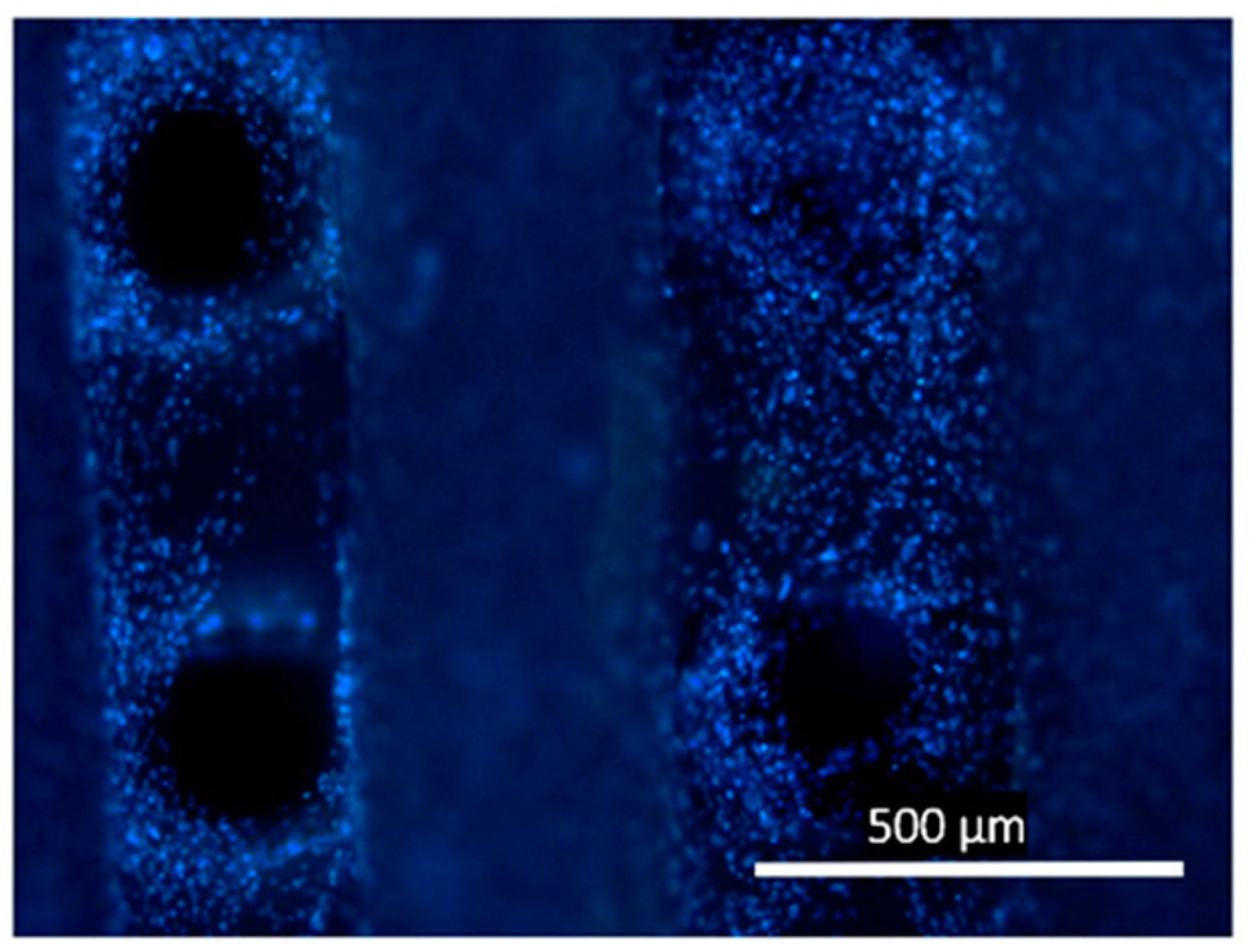
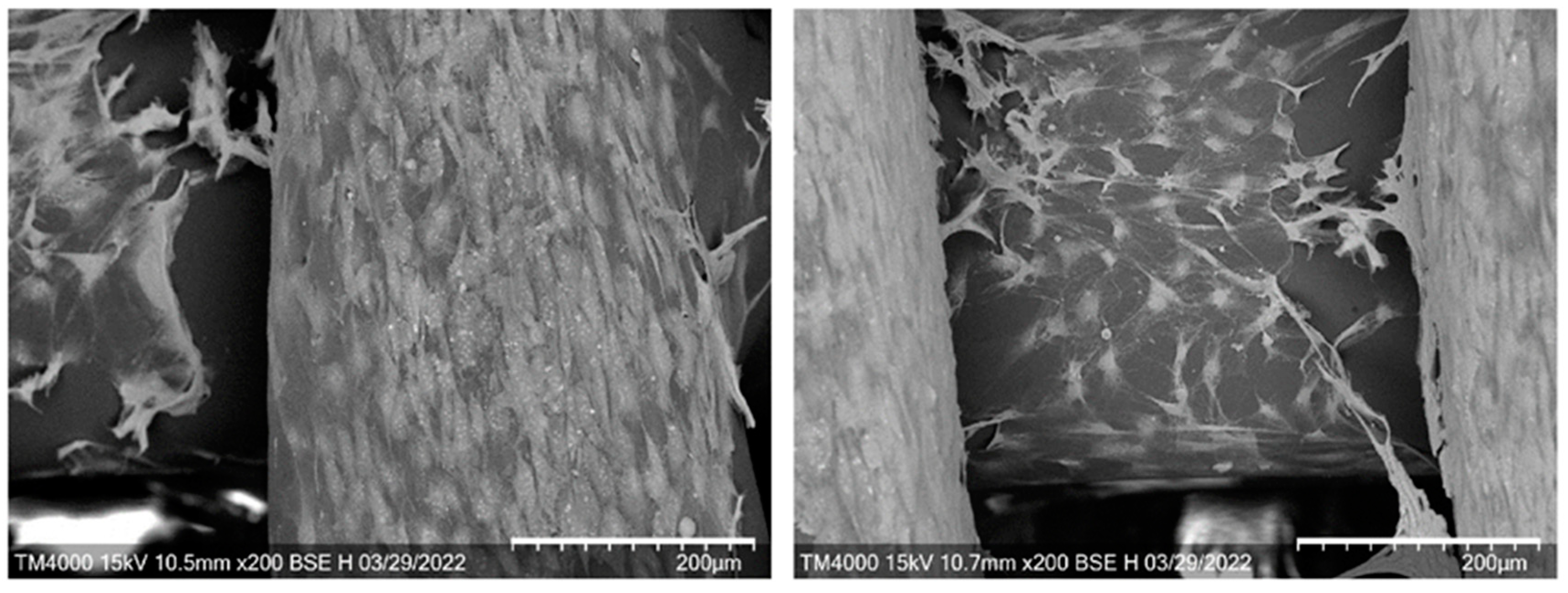
2.4. In Vitro Assays of 3D Scaffolds
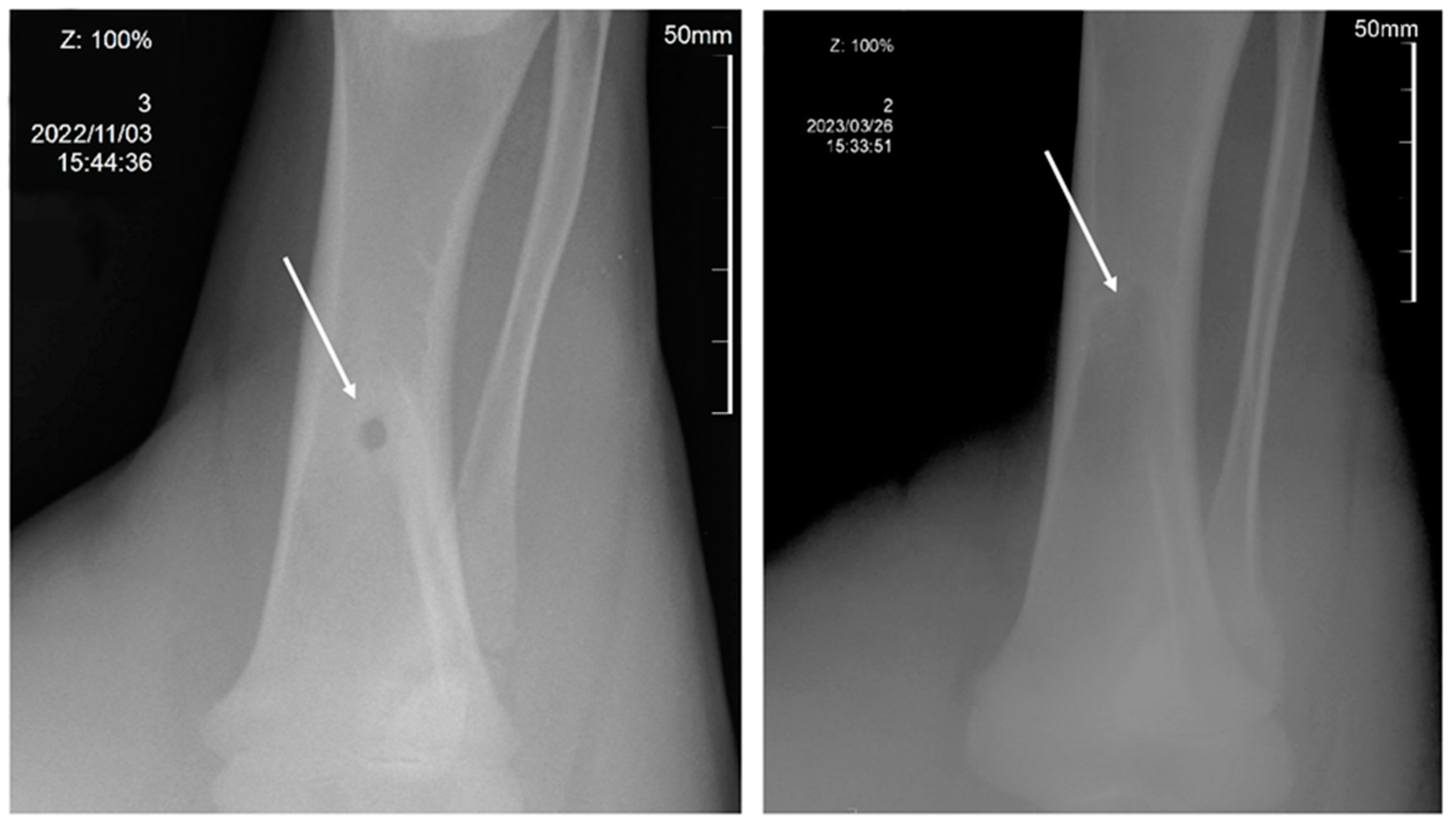
2.5. In Vivo Assays of 3D Scaffolds
3. Materials and Methods
3.1. Scaffold Material
3.2. PHA Recovery from Cell Biomass
3.3. PHA Chemical Composition
3.4. Obtaining Filaments for 3D Printing
3.5. Design and Fabrication of 3D Scaffolds
3.6. Morphology and Physicochemical Properties of Filaments and 3D Scaffolds
3.7. Tensile Testing of Filaments and 3D Scaffolds
3.8. In Vitro Assays
3.9. In Vivo Assays
3.10. Histology
3.11. Statistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Utela, B.; Storti, D.; Anderson, R.; Ganter, M. A Review of Process Development Steps for New Material Systems in Three Dimensional Printing (3DP). J. Manuf. Process 2008, 10, 96–104. [Google Scholar] [CrossRef]
- Ngo, T.D.; Kashani, A.; Imbalzano, G.; Nguyen, K.T.Q.; Hui, D. Additive Manufacturing (3D Printing): A Review of Materials, Methods, Applications and Challenges. Compos. B Eng. 2018, 143, 172–196. [Google Scholar] [CrossRef]
- Saleh Alghamdi, S.; John, S.; Roy Choudhury, N.; Dutta, N.K. Additive Manufacturing of Polymer Materials: Progress, Promise and Challenges. Polymers 2021, 13, 753. [Google Scholar] [CrossRef] [PubMed]
- Tümer, E.H.; Erbil, H.Y. Extrusion-Based 3D Printing Applications of PLA Composites: A Review. Coatings 2021, 11, 390. [Google Scholar] [CrossRef]
- Dip, T.M.; Emu, A.S.; Nafiz, M.N.H.; Kundu, P.; Rakhi, H.R.; Sayam, A.; Akhtarujjman, M.; Shoaib, M.; Ahmed, M.S.; Ushno, S.T.; et al. 3D Printing Technology for Textiles and Fashion. Text. Progress. 2020, 52, 167–260. [Google Scholar] [CrossRef]
- Rong, L.; Chen, X.; Shen, M.; Yang, J.; Qi, X.; Li, Y.; Xie, J. The Application of 3D Printing Technology on Starch-Based Product: A Review. Trends Food Sci. Technol. 2023, 134, 149–161. [Google Scholar] [CrossRef]
- Mazurchevici, A.-D.; Nedelcu, D.; Popa, R.-I. Additive Manufacturing of Composite Materials by FDM Technology: A Review. Indian. J. Eng. Mater. Sci. 2020, 27, 179–192. [Google Scholar]
- Raees, S.; Ullah, F.; Javed, F.; Akil, H.M.; Jadoon Khan, M.; Safdar, M.; Din, I.U.; Alotaibi, M.A.; Alharthi, A.I.; Bakht, M.A.; et al. Classification, Processing, and Applications of Bioink and 3D Bioprinting: A Detailed Review. Int. J. Biol. Macromol. 2023, 232, 123476. [Google Scholar] [CrossRef]
- Li, Y.; Ren, X.; Zhu, L.; Li, C. Biomass 3D Printing: Principles, Materials, Post-Processing and Applications. Polymers 2023, 15, 2692. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, M.; Romero, L.; Domínguez, I.A.; del Espinosa, M.M.; Domínguez, M. Additive Manufacturing Technologies: An Overview about 3D Printing Methods and Future Prospects. Complexity 2019, 2019, 9656938. [Google Scholar] [CrossRef]
- Sachs, E.M.; Haggerty, J.S.; Michael, L.; Cima, J.; Lexington; Williams, P.A. Three-Dimensional Printing Techniques. U.S. Patent No 5,204,055, 20 April 1993. [Google Scholar]
- Bian, Y.; Zheng, Z.; Liu, Y.; Liu, J.; Zhu, J.; Zhou, T. Hybrid Wedge Plasmon Polariton Waveguide with Good Fabrication-Error-Tolerance for Ultra-Deep-Subwavelength Mode Confinement. Opt. Express 2011, 19, 22417. [Google Scholar] [CrossRef] [PubMed]
- Butscher, C.; Einstein, H.H.; Huggenberger, P. Effects of Tunneling on Groundwater Flow and Swelling of Clay-Sulfate Rocks. Water Resour. Res. 2011, 47. [Google Scholar] [CrossRef]
- Lam, S.S.K.; Chen, X.-P.; Schaubroeck, J. Participative decision making and employee performance in different cultures: The moderating effects of allocentrism/idiocentrism and efficacy. Acad. Manag. J. 2002, 45, 905–914. [Google Scholar] [CrossRef]
- Gleadall, A.; Visscher, D.; Yang, J.; Thomas, D.; Segal, J. Review of Additive Manufactured Tissue Engineering Scaffolds: Relationship between Geometry and Performance. Burns Trauma 2018, 6, 19. [Google Scholar] [CrossRef]
- Zein, I.; Hutmacher, D.W.; Tan, K.C.; Teoh, S.H. Fused Deposition Modeling of Novel Scaffold Architectures for Tissue Engineering Applications. Biomaterials 2002, 23, 1169–1185. [Google Scholar] [CrossRef]
- Rezai Rad, M.; Fahimipour, F.; Dashtimoghadam, E.; Nokhbatolfoghahaei, H.; Tayebi, L.; Khojasteh, A. Osteogenic Differentiation of Adipose-Derived Mesenchymal Stem Cells Using 3D-Printed PDLLA/β-TCP Nanocomposite Scaffolds. Bioprinting 2021, 21, e00117. [Google Scholar] [CrossRef]
- Lai, Y.; Li, Y.; Cao, H.; Long, J.; Wang, X.; Li, L.; Li, C.; Jia, Q.; Teng, B.; Tang, T.; et al. Osteogenic Magnesium Incorporated into PLGA/TCP Porous Scaffold by 3D Printing for Repairing Challenging Bone Defect. Biomaterials 2019, 197, 207–219. [Google Scholar] [CrossRef]
- Shim, J.-H.; Yoon, M.-C.; Jeong, C.-M.; Jang, J.; Jeong, S.-I.; Cho, D.-W.; Huh, J.-B. Efficacy of RhBMP-2 Loaded PCL/PLGA/β-TCP Guided Bone Regeneration Membrane Fabricated by 3D Printing Technology for Reconstruction of Calvaria Defects in Rabbit. Biomed. Mater. 2014, 9, 065006. [Google Scholar] [CrossRef]
- Li, H.; Yin, Y.; Xiang, Y.; Liu, H.; Guo, R. A Novel 3D Printing PCL/GelMA Scaffold Containing USPIO for MRI-Guided Bile Duct Repair. Biomed. Mater. 2020, 15, 045004. [Google Scholar] [CrossRef]
- Postiglione, G.; Natale, G.; Griffini, G.; Levi, M.; Turri, S. Conductive 3D Microstructures by Direct 3D Printing of Polymer/Carbon Nanotube Nanocomposites via Liquid Deposition Modeling. Compos. Part A Appl. Sci. Manuf. 2015, 76, 110–114. [Google Scholar] [CrossRef]
- Chen, G.-Q. Plastics Completely Synthesized by Bacteria: Polyhydroxyalkanoates. In Plastics from Bacteria; Springer: Berlin/Heidelberg, Germany, 2010; pp. 17–37. [Google Scholar]
- Laycock, B.; Halley, P.; Pratt, S.; Werker, A.; Lant, P. The Chemomechanical Properties of Microbial Polyhydroxyalkanoates. Prog. Polym. Sci. 2013, 38, 536–583. [Google Scholar] [CrossRef]
- Volova, T.G.; Shishatskaya, E.I.; Sinskey, A.J. Degradable Polymers: Production, Properties, Applications; Nova Science Pub. Inc.: Hauppauge, NY, USA, 2013; ISBN 9781622578320. [Google Scholar]
- Mitra, R.; Xu, T.; Chen, G.; Xiang, H.; Han, J. An Updated Overview on the Regulatory Circuits of Polyhydroxyalkanoates Synthesis. Microb. Biotechnol. 2022, 15, 1446–1470. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Mukherjee, A. A New Wave of Industrialization of PHA Biopolyesters. Bioengineering 2022, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Koller, M.; Mukherjee, A. Polyhydroxyalkanoates–Linking Properties, Applications and End-of-Life Options. Chem. Biochem. Eng. Q. 2020, 34, 115–129. [Google Scholar] [CrossRef]
- Lizarraga-Valderrama, L.R.; Nigmatullin, R.; Taylor, C.; Haycock, J.W.; Claeyssens, F.; Knowles, J.C.; Roy, I. Nerve Tissue Engineering Using Blends of Poly(3-Hydroxyalkanoates) for Peripheral Nerve Regeneration. Eng. Life Sci. 2015, 15, 612–621. [Google Scholar] [CrossRef]
- Shumilova, A.A.; Myltygashev, M.P.; Kirichenko, A.K.; Nikolaeva, E.D.; Volova, T.G.; Shishatskaya, E.I. Porous 3D Implants of Degradable Poly-3-Hydroxybutyrate Used to Enhance Regeneration of Rat Cranial Defect. J. Biomed. Mater. Res. A 2017, 105, 566–577. [Google Scholar] [CrossRef]
- Volova, T.G.; Vinnik, Y.S.; Shishatskaya, E.I.; Markelova, N.M.; Zaikov, G.E. Natural-Based Polymers for Biomedical Applications; Woodhead Publishing: Sawston, UK, 2017; ISBN 9781771884365. [Google Scholar]
- Koller, M. Biodegradable and Biocompatible Polyhydroxy-Alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef]
- Zhang, J.; Shishatskaya, E.I.; Volova, T.G.; da Silva, L.F.; Chen, G.-Q. Polyhydroxyalkanoates (PHA) for Therapeutic Applications. Mater. Sci. Eng. C 2018, 86, 144–150. [Google Scholar] [CrossRef]
- Asare, E.; Gregory, D.A.; Fricker, A.; Marcello, E.; Paxinou, A.; Taylor, C.S.; Haycock, J.W.; Roy, I. Polyhydroxyalkanoates, Their Processing and Biomedical Applications. In The Handbook of Polyhydroxyalkanoates; CRC Press: Boca Raton, FL, USA, 2020; pp. 255–284. [Google Scholar]
- Williams, S.F.; Martin, D.P. Applications of Polyhydroxyalkanoates (PHA) in Medicine and Pharmacy. In Biopolymers Online; Doi, Y., Steinbüchel, A., Eds.; Wiley: Hoboken, NJ, USA, 2002. [Google Scholar]
- Chen, G.-Q.; Wu, Q. The Application of Polyhydroxyalkanoates as Tissue Engineering Materials. Biomaterials 2005, 26, 6565–6578. [Google Scholar] [CrossRef]
- Rentsch, C.; Rentsch, B.; Breier, A.; Hofmann, A.; Manthey, S.; Scharnweber, D.; Biewener, A.; Zwipp, H. Evaluation of the Osteogenic Potential and Vascularization of 3D Poly(3)Hydroxybutyrate Scaffolds Subcutaneously Implanted in Nude Rats. J. Biomed. Mater. Res. A 2010, 92A, 185–195. [Google Scholar] [CrossRef]
- Volova, T.G.; Goncharov, D.B.; Nikolaeva, E.D.; Shishatskaya, E.I. Electrospinning of Degradable Phas: Process, Properties, Applications. In Electrospinning: Fundamentals, Methods and Applications; Nova Science Pub. Inc.: Hauppauge, NY, USA, 2017; ISBN 9781536123890. [Google Scholar]
- Luo, Z.; Wu, Y.; Li, Z.; Loh, X.J. Recent Progress in Polyhydroxyalkanoates-Based Copolymers for Biomedical Applications. Biotechnol. J. 2019, 14, 1900283. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Srivastava, J.K.; Chandel, A.K.; Sharma, L.; Mallick, N.; Singh, S.P. Biomedical Applications of Microbially Engineered Polyhydroxyalkanoates: An Insight into Recent Advances, Bottlenecks, and Solutions. Appl. Microbiol. Biotechnol. 2019, 103, 2007–2032. [Google Scholar] [CrossRef]
- Shiny, P.J.; Vimala Devi, M.; Felciya, S.J.G.; Ramanathan, G.; Fardim, P.; Sivagnanam, U.T. In Vitro and in Vivo Evaluation of Poly-3-Hydroxybutyric Acid-Sodium Alginate as a Core-Shell Nanofibrous Matrix with Arginine and Bacitracin-Nanoclay Complex for Dermal Reconstruction of Excision Wound. Int. J. Biol. Macromol. 2021, 168, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, R.; Pandey, R.; Kumar, S.; Mehrotra, D. Poly Hydroxyalkanoates (PHA): Role in Bone Scaffolds. J. Oral Biol. Craniofac. Res. 2020, 10, 389–392. [Google Scholar] [CrossRef]
- Shrivastav, A.; Kim, H.-Y.; Kim, Y.-R. Advances in the Applications of Polyhydroxyalkanoate Nanoparticles for Novel Drug Delivery System. Biomed Res. Int. 2013, 2013, 581684. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Martin, D. Poly-4-Hydroxybutyrate (P4HB) in Biomedical Applications and Tissue Engineering. In Biodegradable Polymers; Chu, C.-C., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2015; Volume 2, pp. 199–231. ISBN 1634836332. [Google Scholar]
- Nair, L.S.; Laurencin, C.T. Biodegradable Polymers as Biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable Polymers with a Range of Applications. J. Chem. Technol. Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Kovalcik, A.; Meixner, K.; Mihalic, M.; Zeilinger, W.; Fritz, I.; Fuchs, W.; Kucharczyk, P.; Stelzer, F.; Drosg, B. Characterization of Polyhydroxyalkanoates Produced by Synechocystis Salina from Digestate Supernatant. Int. J. Biol. Macromol. 2017, 102, 497–504. [Google Scholar] [CrossRef]
- Barnard, G.N.; Sanders, J.K.M. Observation of Mobile Poly(β-Hydroxybutyrate) in the Storage Granules of Methylobacterium AM1 by in Vivo 13C-NMR Spectroscopy. FEBS Lett. 1988, 231, 16–18. [Google Scholar] [CrossRef]
- Gazzano, M.; Focarete, M.L.; Riekel, C.; Ripamonti, A.; Scandola, M. Structural Investigation of Poly(3-Hydroxybutyrate) Spherulites by Microfocus X-Ray Diffraction. Macromol. Chem. Phys. 2001, 202, 1405–1409. [Google Scholar] [CrossRef]
- Kovalcik, A.; Sangroniz, L.; Kalina, M.; Skopalova, K.; Humpolíček, P.; Omastova, M.; Mundigler, N.; Müller, A.J. Properties of Scaffolds Prepared by Fused Deposition Modeling of Poly(Hydroxyalkanoates). Int. J. Biol. Macromol. 2020, 161, 364–376. [Google Scholar] [CrossRef]
- Rydz, J.; Sikorska, W.; Musioł, M.; Janeczek, H.; Włodarczyk, J.; Misiurska-Marczak, M.; Łęczycka, J.; Kowalczuk, M. 3D-Printed Polyester-Based Prototypes for Cosmetic Applications—Future Directions at the Forensic Engineering of Advanced Polymeric Materials. Materials 2019, 12, 994. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Liao, H.T. Interface Design of Environmentally Friendly Carbon Nanotube-Filled Polyester Composites: Fabrication, Characterisation, Functionality and Application. Express Polym. Lett. 2017, 11, 187–198. [Google Scholar] [CrossRef]
- Wu, C.-S.; Liao, H.-T.; Cai, Y.-X. Characterisation, Biodegradability and Application of Palm Fibre-Reinforced Polyhydroxyalkanoate Composites. Polym. Degrad. Stab. 2017, 140, 55–63. [Google Scholar] [CrossRef]
- Duan, B.; Cheung, W.L.; Wang, M. Optimized Fabrication of Ca–P/PHBV Nanocomposite Scaffolds via Selective Laser Sintering for Bone Tissue Engineering. Biofabrication 2011, 3, 015001. [Google Scholar] [CrossRef]
- Puppi, D.; Pirosa, A.; Morelli, A.; Chiellini, F. Design, Fabrication and Characterization of Tailored Poly[(R)-3-Hydroxybutyrate-Co-(R)-3-Hydroxyexanoate] Scaffolds by Computer-Aided Wet-Spinning. Rapid Prototyp. J. 2018, 24, 1–8. [Google Scholar] [CrossRef]
- Puppi, D.; Chiellini, F. Additive Manufacturing of PHA. In The Handbook of Polyhydroxyalkanoates; CRC Press: Boca Raton, FL, USA, 2020; pp. 119–136. [Google Scholar]
- Gonzalez Ausejo, J.; Rydz, J.; Musioł, M.; Sikorska, W.; Sobota, M.; Włodarczyk, J.; Adamus, G.; Janeczek, H.; Kwiecień, I.; Hercog, A.; et al. A Comparative Study of Three-Dimensional Printing Directions: The Degradation and Toxicological Profile of a PLA/PHA Blend. Polym. Degrad. Stab. 2018, 152, 191–207. [Google Scholar] [CrossRef]
- ColorFabb, Filaments PLA/PHA Value Pack. Available online: https://colorfabb.com/pla-pha-value-pack (accessed on 29 July 2023).
- Gregory, D.A.; Taylor, C.S.; Fricker, A.T.R.; Asare, E.; Tetali, S.S.V.; Haycock, J.W.; Roy, I. Polyhydroxyalkanoates and Their Advances for Biomedical Applications. Trends Mol. Med. 2022, 28, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Gregory, D.A.; Fricker, A.T.R.; Mitrev, P.; Ray, M.; Asare, E.; Sim, D.; Larpnimitchai, S.; Zhang, Z.; Ma, J.; Tetali, S.S.V.; et al. Additive Manufacturing of Polyhydroxyalkanoate-Based Blends Using Fused Deposition Modelling for the Development of Biomedical Devices. J. Funct. Biomater. 2023, 14, 40. [Google Scholar] [CrossRef]
- Saska, S.; Pires, L.C.; Cominotte, M.A.; Mendes, L.S.; de Oliveira, M.F.; Maia, I.A.; da Silva, J.V.L.; Ribeiro, S.J.L.; Cirelli, J.A. Three-Dimensional Printing and in Vitro Evaluation of Poly(3-Hydroxybutyrate) Scaffolds Functionalized with Osteogenic Growth Peptide for Tissue Engineering. Mater. Sci. Eng. C 2018, 89, 265–273. [Google Scholar] [CrossRef]
- Culenova, M.; Birova, I.; Alexy, P.; Galfiova, P.; Nicodemou, A.; Moncmanova, B.; Plavec, R.; Tomanova, K.; Mencik, P.; Ziaran, S.; et al. In Vitro Characterization of Poly(Lactic Acid)/Poly(Hydroxybutyrate)/Thermoplastic Starch Blends for Tissue Engineering Application. Cell Transplant. 2021, 30, 096368972110210. [Google Scholar] [CrossRef] [PubMed]
- Pryadko, A.; Surmeneva, M.A.; Surmenev, R.A. Review of Hybrid Materials Based on Polyhydroxyalkanoates for Tissue Engineering Applications. Polymers 2021, 13, 1738. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, T.; Mohideen, M.M.; Hu, P.; Gupta, R.; Ramakrishna, S.; Liu, Y. Realization of Circular Economy of 3D Printed Plastics: A Review. Polymers 2021, 13, 744. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Wang, X.; Yang, C.; Huang, R.; Wang, H.; Zhao, Y. Microfluidic 3D Printing Polyhydroxyalkanoates-Based Bionic Skin for Wound Healing. Mater. Futures 2022, 1, 015401. [Google Scholar] [CrossRef]
- Pecorini, G.; Braccini, S.; Parrini, G.; Chiellini, F.; Puppi, D. Additive Manufacturing of Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate)/Poly(D,L-Lactide-Co-Glycolide) Biphasic Scaffolds for Bone Tissue Regeneration. Int. J. Mol. Sci. 2022, 23, 3895. [Google Scholar] [CrossRef]
- Thaxton, R.D. A Thesis: Extrusion of Polyhydroxyalkanoate Filament For Use in 3D Printers. 22 April 2016; 11 p. Available online: https://www.wm.edu/as/physics/documents/seniorstheses/class2016theses/thaxton,-richard.pdf (accessed on 29 July 2023).
- Batchelor, W.M. A Thesis: PHA Biopolymer Filament for 3D Printing. 22 April 2016; 14 p. Available online: https://www.wm.edu/as/physics/documents/seniorstheses/class2016theses/batchelor,-wyndham.pdf (accessed on 29 July 2023).
- Morgan, E.F.; Unnikrisnan, G.U.; Hussein, A.I. Bone Mechanical Properties in Healthy and Diseased States. Annu. Rev. Biomed. Eng. 2018, 20, 119–143. [Google Scholar] [CrossRef] [PubMed]
- Mandal, B.B.; Grinberg, A.; Seok Gil, E.; Panilaitis, B.; Kaplan, D.L. High-Strength Silk Protein Scaffolds for Bone Repair. Proc. Natl. Acad. Sci. USA 2012, 109, 7699–7704. [Google Scholar] [CrossRef] [PubMed]
- Pereira, T.F.; Oliveira, M.F.; Maia, I.A.; Silva, J.V.L.; Costa, M.F.; Thiré, R.M.S.M. 3D Printing of Poly(3-Hydroxybutyrate) Porous Structures Using Selective Laser Sintering. Macromol. Symp. 2012, 319, 64–73. [Google Scholar] [CrossRef]
- Ronca, D.; Langella, F.; Chierchia, M.; D’Amora, U.; Russo, T.; Domingos, M.; Gloria, A.; Bartolo, P.; Ambrosio, L. Bone Tissue Engineering: 3D PCL-Based Nanocomposite Scaffolds with Tailored Properties. Procedia CIRP 2016, 49, 51–54. [Google Scholar] [CrossRef]
- Goranov, V.; Shelyakova, T.; De Santis, R.; Haranava, Y.; Makhaniok, A.; Gloria, A.; Tampieri, A.; Russo, A.; Kon, E.; Marcacci, M.; et al. 3D Patterning of Cells in Magnetic Scaffolds for Tissue Engineering. Sci. Rep. 2020, 10, 2289. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, K.; Chen, G.-Q. Effect of Surface Treatment on the Biocompatibility of Microbial Polyhydroxyalkanoates. Biomaterials 2002, 23, 1391–1397. [Google Scholar] [CrossRef]
- Sombatmankhong, K.; Sanchavanakit, N.; Pavasant, P.; Supaphol, P. Bone Scaffolds from Electrospun Fiber Mats of Poly(3-Hydroxybutyrate), Poly(3-Hydroxybutyrate-Co-3-Hydroxyvalerate) and Their Blend. Polymers 2007, 48, 1419–1427. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, M.; Zhang, J.; Zhang, Y.; Liu, Z.; Zhu, Y.; Zhang, C. Three Dimensionally Printed Mesoporous Bioactive Glass and Poly(3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) Composite Scaffolds for Bone Regeneration. J. Mater. Chem. B 2014, 2, 6106. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, J.; Tang, L.; Ao, H.; Tan, H.; Tang, T.; Liu, C. Mesoporous Bioactive Glass Doped-Poly (3-Hydroxybutyrate-Co-3-Hydroxyhexanoate) Composite Scaffolds with 3-Dimensionally Hierarchical Pore Networks for Bone Regeneration. Colloids Surf. B Biointerfaces 2014, 116, 72–80. [Google Scholar] [CrossRef]
- Volova, T.G.; Shishatskaya, E.I. Bacterial Strain VKPM B-10646—A Producer of Polyhydroxyalkanoates and a Method of Their Production). RF Patent No. 2439143, 10 January 2012. [Google Scholar]
- Volova, T.; Kiselev, E.; Vinogradova, O.; Nikolaeva, E.; Chistyakov, A.; Sukovatiy, A.; Shishatskaya, E. A Glucose-Utilizing Strain, Cupriavidus Euthrophus B-10646: Growth Kinetics, Characterization and Synthesis of Multicomponent PHAs. PLoS ONE 2014, 9, e87551. [Google Scholar] [CrossRef]
- Schlegel, H.G.; Kaltwasser, H.; Gottschalk, G. A Submersion Method for Culture of Hydrogen-Oxidizing Bacteria: Growth Physiological Studies. Arch. Mikrobiol. 1961, 38, 209–222. [Google Scholar] [CrossRef]
- Braunegg, G.; Sonnleitner, B.; Lafferty, R.M. A Rapid Gas Chromatographic Method for the Determination of Poly-β-Hydroxybutyric Acid in Microbial Biomass. Eur. J. Appl. Microbiol. Biotechnol. 1978, 6, 29–37. [Google Scholar] [CrossRef]
- Kisterskij, K. Air Flow Swirler for Extruded Mixtures Cooling. RF Patent No. 2769197, 29 March 2022. [Google Scholar]
- Volova, T.; Kiselev, E.; Nemtsev, I.; Lukyanenko, A.; Sukovatyi, A.; Kuzmin, A.; Ryltseva, G.; Shishatskaya, E. Properties of Degradable Polyhydroxyalkanoates with Different Monomer Compositions. Int. J. Biol. Macromol. 2021, 182, 98–114. [Google Scholar] [CrossRef] [PubMed]
- GOST R ISO 10993.6-99; Medical Devices. Biological Evaluation of Medical Devices. Part 6. Tests for Local Effects after Implantation. GOSSTANDART of Russia: Moscow, Russia, 2010.
- GOST R 53434-2009; Principles of Good Laboratory Practice. Federal Agency for Technical Regulation and Metrology: Moscow, Russia, 2002.
- ASTM F981-04; Standard Practice for Assessment of Compatibility of Biomaterials for Surgical Implants with Respect to Effect of Materials on Muscle and Bone. ASTM International: West Conshohocken, PA, USA, 2010.
- ASTM F1983-99; Standard Practice for Assessment of Compatibility of Absorbable/Resorbable Biomaterials for Implant Applications. ASTM International: West Conshohocken, PA, USA, 2016.
- Wancket, L.M. Animal Models for Evaluation of Bone Implants and Devices. Vet Pathol. 2015, 52, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Quarto, R. Experimental Animal Models for Tissue-Engineered Bone Regeneration. In Engineered Bone; Petite, H., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 103–118. [Google Scholar]
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal Models for Bone Tissue Engineering and Modelling Disease. Dis. Model Mech. 2018, 11, dmm033084. [Google Scholar] [CrossRef] [PubMed]
- ETS 123; Protection of Vertebrate Animals. European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes. Council of Europe: Strasbourg, France, 1986.
- Directive 2010/63/EU; Protection of Animals Used for Scientific Purposes. European Parliament and the Council: Brussels, Belgium, 2010.
- The Federation of European Laboratory Animal Science Associations. Available online: https://felasa.eu/ (accessed on 29 July 2023).



| Sample/Processing Stage | Average Molecular Weight Mw, кDa | Polydispersity Đ | Melting Temperature Tmelt, °C | Thermal Degradation Temperature Tdegr, °C | Enthalpy of Melting H, J/g | Glass Transition Temperature Tg, °C | Crystallization Temperature Tcryst, °C |
|---|---|---|---|---|---|---|---|
| Original P(3HB-co-3HV) | 530 | 2.3 | 168 | 280 | 98.3 | 1.1 | 59/67 |
| Granulate (after the first heating–melting) | 480 | 3.03 | 149/168/185 | 279 | 65.2 | 1.1 | 59/62 |
| Filament (after the second heating–melting) | 425 | 2.72 | 147/168/183 | 274 | 73,1 | 0.3 | 50/59 |
| 3D Scaffold (after the third heating–melting) | 390 | 3.96 | 161/172/184 | 275 | 80,3 | 0.2 | 50/54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishatskaya, E.I.; Demidenko, A.V.; Sukovatyi, A.G.; Dudaev, A.E.; Mylnikov, A.V.; Kisterskij, K.A.; Volova, T.G. Three-Dimensional Printing of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] Biodegradable Scaffolds: Properties, In Vitro and In Vivo Evaluation. Int. J. Mol. Sci. 2023, 24, 12969. https://doi.org/10.3390/ijms241612969
Shishatskaya EI, Demidenko AV, Sukovatyi AG, Dudaev AE, Mylnikov AV, Kisterskij KA, Volova TG. Three-Dimensional Printing of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] Biodegradable Scaffolds: Properties, In Vitro and In Vivo Evaluation. International Journal of Molecular Sciences. 2023; 24(16):12969. https://doi.org/10.3390/ijms241612969
Chicago/Turabian StyleShishatskaya, Ekaterina I., Aleksey V. Demidenko, Aleksey G. Sukovatyi, Alexey E. Dudaev, Aleksey V. Mylnikov, Konstantin A. Kisterskij, and Tatiana G. Volova. 2023. "Three-Dimensional Printing of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] Biodegradable Scaffolds: Properties, In Vitro and In Vivo Evaluation" International Journal of Molecular Sciences 24, no. 16: 12969. https://doi.org/10.3390/ijms241612969
APA StyleShishatskaya, E. I., Demidenko, A. V., Sukovatyi, A. G., Dudaev, A. E., Mylnikov, A. V., Kisterskij, K. A., & Volova, T. G. (2023). Three-Dimensional Printing of Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) [P(3HB-co-3HV)] Biodegradable Scaffolds: Properties, In Vitro and In Vivo Evaluation. International Journal of Molecular Sciences, 24(16), 12969. https://doi.org/10.3390/ijms241612969








