SARS-CoV-2 Reinfections and Long COVID in the Post-Omicron Phase of the Pandemic
Abstract
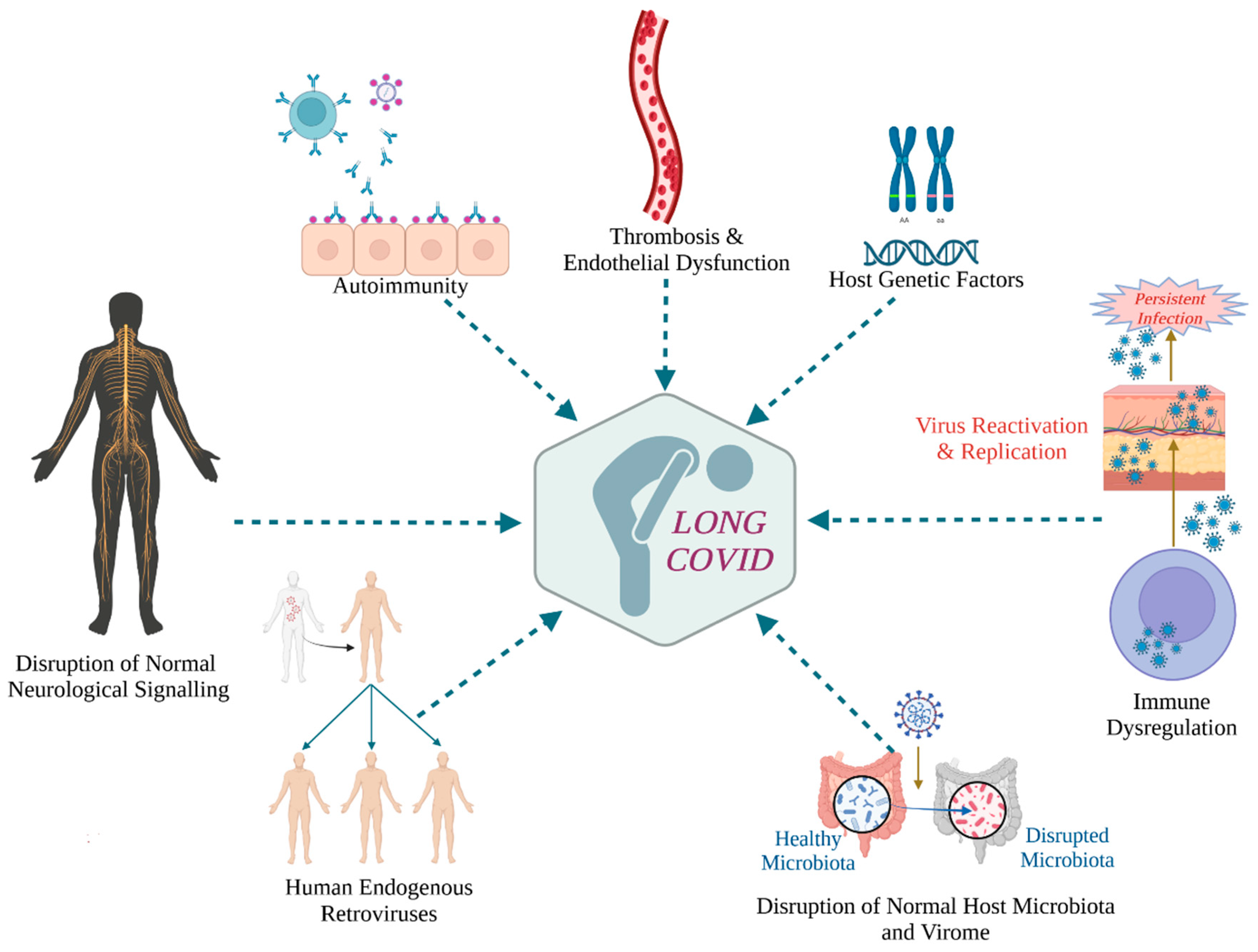
:1. Introduction
2. Literature Search Methodology
3. Virological Correlates of Long COVID
3.1. A Common Mechanism of Unexplained Post-Acute Infection Syndromes?
3.2. Viral Persistence and Long COVID
3.3. Viral Reactivation and Long COVID
4. Immunological Correlates of Long COVID
4.1. Innate Immune Cell Dysregulation
CCL11/Eotaxin1 and Neurological Long COVID Symptoms
4.2. Dysregulated Adaptive T Cell Responses
4.3. Aberrant B Cell and Antibody Responses
4.4. Autoreactive Antibodies
5. SARS-CoV-2 Reinfections and Long COVID
5.1. Do Repeated Infections with SARS-CoV-2 Increase the Likelihood of Long COVID?
5.2. Long COVID Following Reinfection with SARS-CoV-2 Variants Pre- and Post-Omicron
5.3. Future Directions on the Study of the Impact of Reinfections on Long COVID
5.4. Strategies to Prevent SARS-CoV-2 Reinfections
6. Vaccination and Long COVID
6.1. Does Vaccination against COVID-19 Protect from Long COVID?
6.2. The Benefits of Vaccination with Respect to Long COVID
6.3. The Potential Advantages of Vaccine-Induced vs. Hybrid Immunity in Long COVID
7. Long COVID in Children and Adolescents
7.1. Children May Also Suffer from Long COVID, but Less Frequently and Severely Than Adults
7.2. Clinical Manifestations of Long COVID in Children and Adolescents
7.3. Recommendations to Mitigate the Effects of Pediatric Long COVID
8. Conclusions
Prevention and Treatment Strategies of Long COVID
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- US Centers for Disease Control and Prevention. Long COVID or Post-COVID Conditions. Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 29 June 2023).
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef]
- Thaweethai, T.; Jolley, S.E.; Karlson, E.W.; Levitan, E.B.; Levy, B.; McComsey, G.A.; McCorkell, L.; Nadkarni, G.N.; Parthasarathy, S.; Singh, U.; et al. Development of a Definition of Postacute Sequelae of SARS-CoV-2 Infection. JAMA 2023, 329, 1934–1946. [Google Scholar] [CrossRef]
- Gottlieb, M.; Spatz, E.S.; Yu, H.; E Wisk, L.; Elmore, J.G.; Gentile, N.L.; Hill, M.; Huebinger, R.M.; Idris, A.H.; Kean, E.R.; et al. Long COVID Clinical Phenotypes up to 6 Months after Infection Identified by Latent Class Analysis of Self-Reported Symptoms. Open Forum Infect. Dis. 2023, 10, ofad277. [Google Scholar] [CrossRef]
- World Health Organization. A Clinical Case Definition of Post COVID-19 Condition by a Delphi Consensus, 6 October 2021. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (accessed on 29 June 2023).
- Maltezou, H.C.; Pavli, A.; Tsakris, A. Post-COVID Syndrome: An Insight on Its Pathogenesis. Vaccines 2021, 9, 497. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Julg, B.; Mohandas, S.; Bradfute, S.B.; Force, R.M.P.T.; Division of Infectious Diseases; Department of Medicine; Icahn School of Medicine at Mount Sinai; United States; Infectious Diseases Division; et al. Viral persistence, reactivation, and mechanisms of long COVID. eLife 2023, 12, e86015. [Google Scholar] [CrossRef]
- Choutka, J.; Jansari, V.; Hornig, M.; Iwasaki, A. Unexplained post-acute infection syndromes. Nat. Med. 2022, 28, 911–923. [Google Scholar] [CrossRef]
- Patterson, B.K.; Francisco, E.B.; Yogendra, R.; Long, E.; Pise, A.; Rodrigues, H.; Hall, E.; Herrera, M.; Parikh, P.; Guevara-Coto, J.; et al. Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection. Front. Immunol. 2022, 12, 746021. [Google Scholar] [CrossRef]
- Yao, X.H.; He, Z.C.; Li, T.Y.; Zhang, H.R.; Wang, Y.; Mou, H.; Guo, Q.; Yu, S.C.; Ding, Y.; Liu, X.; et al. Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res. 2020, 30, 541–543. [Google Scholar] [CrossRef]
- Nienhold, R.; Ciani, Y.; Koelzer, V.H.; Tzankov, A.; Haslbauer, J.D.; Menter, T.; Schwab, N.; Henkel, M.; Frank, A.; Zsikla, V.; et al. Two distinct immunopathological profiles in autopsy lungs of COVID-19. Nat. Commun. 2020, 11, 5086. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Puelles, V.G.; Lütgehetmann, M.; Lindenmeyer, M.T.; Sperhake, J.P.; Wong, M.N.; Allweiss, L.; Chilla, S.; Heinemann, A.; Wanner, N.; Liu, S.; et al. Multiorgan and Renal Tropism of SARS-CoV-2. N. Engl. J. Med. 2020, 383, 590–592. [Google Scholar] [CrossRef]
- Qi, F.; Qian, S.; Zhang, S.; Zhang, Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem. Biophys. Res. Commun. 2020, 526, 135–140. [Google Scholar] [CrossRef]
- Swank, Z.; Senussi, Y.; Manickas-Hill, Z.; Yu, X.G.; Li, J.Z.; Alter, G.; Walt, D.R. Persistent Circulating Severe Acute Respiratory Syndrome Coronavirus 2 Spike Is Associated with Post-acute Coronavirus Disease 2019 Sequelae. Clin. Infect. Dis. 2022, 76, e487–e490. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Guo, C.; Tang, L.; Hong, Z.; Zhou, J.; Dong, X.; Yin, H.; Xiao, Q.; Tang, Y.; Qu, X.; et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. [Google Scholar] [CrossRef]
- Viszlayová, D.; Sojka, M.; Dobrodenková, S.; Szabó, S.; Bilec, O.; Turzová, M.; Ďurina, J.; Baloghová, B.; Borbély, Z.; Kršák, M. SARS-CoV-2 RNA in the cerebrospinal fluid of a patient with long COVID. Ther. Adv. Infect Dis. 2021, 8, 20499361211048572. [Google Scholar] [CrossRef]
- Tejerina, F.; Catalan, P.; Rodriguez-Grande, C.; Adan, J.; Rodriguez-Gonzalez, C.; Aldamiz, T.; Diez, C.; Perez, L.; Fanciulli, C.; de Viedma, D.G.; et al. Post-COVID-19 syndrome. SARS-CoV-2 RNA detection in plasma, stool, and urine in patients with persistent symptoms after COVID-19. BMC Infect. Dis. 2022, 22, 211. [Google Scholar] [CrossRef]
- Goh, D.; Lim, J.C.T.; Fernaíndez, S.B.; Joseph, C.R.; Gil Edwards, S.; Neo, Z.W.; Lee, J.N.; Caballero, S.G.; Lau, M.C.; Yeong, J.P.S. Case report: Persistence of residual antigen and RNA of the SARS-CoV-2 virus in tissues of two patients with long COVID. Front. Immunol. 2022, 13, 939989. [Google Scholar] [CrossRef]
- Ceulemans, L.J.; Khan, M.; Yoo, S.J.; Zapiec, B.; Van Gerven, L.; Van Slambrouck, J.; Vanstapel, A.; Van Raemdonck, D.; Vos, R.; Wauters, E.; et al. Persistence of SARS-CoV-2 RNA in lung tissue after mild COVID-19. Lancet Respir. Med. 2021, 9, e78–e79. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.C.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef]
- Menuchin-Lasowski, Y.; Schreiber, A.; Lecanda, A.; Mecate-Zambrano, A.; Brunotte, L.; Psathaki, O.E.; Ludwig, S.; Rauen, T.; Schöler, H.R. SARS-CoV-2 infects and replicates in photoreceptor and retinal ganglion cells of human retinal organoids. Stem. Cell Rep. 2022, 17, 789–803. [Google Scholar] [CrossRef]
- Cheung, C.C.L.; Goh, D.; Lim, X.; Tien, T.Z.; Lim, J.C.T.; Lee, J.N.; Tan, B.; Tay, Z.E.A.; Wan, W.Y.; Chen, E.X.; et al. Residual SARS-CoV-2 viral antigens detected in GI and hepatic tissues from five recovered patients with COVID-19. Gut 2022, 71, 226–229. [Google Scholar] [CrossRef]
- Kumata, R.; Ito, J.; Takahashi, K.; Suzuki, T.; Sato, K. A tissue level atlas of the healthy human virome. BMC Biol. 2020, 18, 55. [Google Scholar] [CrossRef]
- Xu, R.; Zhou, Y.; Cai, L.; Wang, L.; Han, J.; Yang, X.; Chen, J.; Ma, C.; Shen, L. Co-reactivation of the human herpesvirus alpha subfamily (herpes simplex virus-1 and varicella zoster virus) in a critically ill patient with COVID-19. Br. J. Dermatol. 2020, 183, 1145–1147. [Google Scholar] [CrossRef]
- Simula, E.R.; Manca, M.A.; Noli, M.; Jasemi, S.; Ruberto, S.; Uzzau, S.; Rubino, S.; Manca, P.; Sechi, L.A. Increased Presence of Antibodies against Type I Interferons and Human Endogenous Retrovirus W in Intensive Care Unit COVID-19 Patients. Microbiol. Spectr. 2022, 10, e0128022. [Google Scholar] [CrossRef]
- Temerozo, J.R.; Fintelman-Rodrigues, N.; dos Santos, M.C.; Hottz, E.D.; Sacramento, C.Q.; Silva, A.d.P.D.d.; Mandacaru, S.C.; Moraes, E.C.d.S.; Trugilho, M.R.O.; Gesto, J.S.M.; et al. Human endogenous retrovirus K in the respiratory tract is associated with COVID-19 physiopathology. Microbiome 2022, 10, 65. [Google Scholar] [CrossRef]
- Klein, J.; Wood, J.; Jaycox, J.; Lu, P.; Dhodapkar, R.M.; Gehlhausen, J.R.; Tabachnikova, A.; Tabacof, L.; Malik, A.A.; Kamath, K.; et al. Distinguishing features of Long COVID identified through immune profiling. medRxiv 2022. [CrossRef]
- Schultheiß, C.; Willscher, E.; Paschold, L.; Gottschick, C.; Klee, B.; Henkes, S.-S.; Bosurgi, L.; Dutzmann, J.; Sedding, D.; Frese, T.; et al. The IL-1β, IL-6, and TNF cytokine triad is associated with post-acute sequelae of COVID-19. Cell Rep. Med. 2022, 3, 100663. [Google Scholar] [CrossRef]
- Peluso, M.J.; Lu, S.; Tang, A.F.; Durstenfeld, M.S.; Ho, H.-E.; A Goldberg, S.; A Forman, C.; E Munter, S.; Hoh, R.; Tai, V.; et al. Markers of Immune Activation and Inflammation in Individuals with Postacute Sequelae of Severe Acute Respiratory Syndrome Coronavirus 2 Infection. J. Infect. Dis. 2021, 224, 1839–1848. [Google Scholar] [CrossRef]
- Talla, A.; Vasaikar, S.V.; Lemos, M.P.; Moodie, Z.; Lee Pebworth, M.P.; Henderson, K.E.; Cohen, K.W.; Czartoski, J.L.; Lai, L.; Suthar, M.S.; et al. Longitudinal immune dynamics of mild COVID-19 define signatures of recovery and persistence. bioRxiv 2021. [Google Scholar] [CrossRef]
- Littlefield, K.M.; Watson, R.O.; Schneider, J.M.; Neff, C.P.; Yamada, E.; Zhang, M.; Campbell, T.B.; Falta, M.T.; Jolley, S.E.; Fontenot, A.P.; et al. SARS-CoV-2-specific T cells associate with inflammation and reduced lung function in pulmonary post-acute sequalae of SARS-CoV-2. PLoS Pathog. 2022, 18, e1010359. [Google Scholar] [CrossRef] [PubMed]
- Phetsouphanh, C.; Darley, D.R.; Wilson, D.B.; Howe, A.; Munier, C.M.L.; Patel, S.K.; Juno, J.A.; Burrell, L.M.; Kent, S.J.; Dore, G.J.; et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 210–216. [Google Scholar] [CrossRef]
- Fernández-Castañeda, A.; Lu, P.; Geraghty, A.C.; Song, E.; Lee, M.-H.; Wood, J.; O’dea, M.R.; Dutton, S.; Shamardani, K.; Nwangwu, K.; et al. Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 2022, 185, 2452–2468.e16. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.K.; Giles, D.A.; Segal, B.M.; Irani, D.N. An emerging role for eotaxins in neurodegenerative disease. Clin. Immunol. 2018, 189, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.L.; Gama, C.S.; Rocha, N.P.; Teixeira, M.M. Revisiting the Role of Eotaxin-1/CCL11 in Psychiatric Disorders. Front. Psychiatry 2018, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Glynne, P.; Tahmasebi, N.; Gant, V.; Gupta, R. Long COVID following Mild SARS-CoV-2 Infection: Characteristic T Cell Alterations and Response to Antihistamines. J. Investig. Med. 2022, 70, 61–67. [Google Scholar] [CrossRef]
- Peluso, M.J.; Deitchman, A.N.; Torres, L.; Iyer, N.S.; Munter, S.E.; Nixon, C.C.; Donatelli, J.; Thanh, C.; Takahashi, S.; Hakim, J.; et al. Long-term SARS-CoV-2-specific immune and inflammatory responses in individuals recovering from COVID-19 with and without post-acute symptoms. Cell Rep. 2021, 36, 109518. [Google Scholar] [CrossRef]
- Visvabharathy, L.; Hanson, B.A.; Orban, Z.S.; Lim, P.H.; Palacio, N.M.; Jimenez, M.; Clark, J.R.; Graham, E.L.; Liotta, E.M.; Tachas, G.; et al. T cell responses to SARS-CoV-2 in people with and without neurologic symptoms of long COVID. medRxiv 2022. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef] [PubMed]
- Bergamaschi, L.; Mescia, F.; Turner, L.; Hanson, A.L.; Kotagiri, P.; Dunmore, B.J.; Ruffieux, H.; De Sa, A.; Huhn, O.; Morgan, M.D.; et al. Longitudinal analysis reveals that delayed bystander CD8+ T cell activation and early immune pathology distinguish severe COVID-19 from mild disease. Immunity 2021, 54, 1257–1275.e8. [Google Scholar] [CrossRef]
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Di Cristanziano, V.; Osebold, L.; et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: A longitudinal prospective cohort study. Lancet Reg. Health-Eur. 2021, 6, 100122. [Google Scholar] [CrossRef] [PubMed]
- García-Abellán, J.; Padilla, S.; Fernández-González, M.; García, J.A.; Agulló, V.; Andreo, M.; Ruiz, S.; Galiana, A.; Gutiérrez, F.; Masiá, M. Antibody Response to SARS-CoV-2 is Associated with Long-term Clinical Outcome in Patients with COVID-19: A Longitudinal Study. J. Clin. Immunol. 2021, 41, 1490–1501. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Harris, B.H.L.; Di Giovannantonio, M.; Rosadas, C.; Short, C.-E.; Quinlan, R.; Sureda-Vives, M.; Fernandez, N.; Day-Weber, I.; Khan, M.; et al. The Association Between Antibody Response to Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Post–COVID-19 Syndrome in Healthcare Workers. J. Infect. Dis. 2021, 223, 1671–1676. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse Functional Autoantibodies in Patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef]
- Chang, S.E.; Feng, A.; Meng, W.; Apostolidis, S.A.; Mack, E.; Artandi, M.; Barman, L.; Bennett, K.; Chakraborty, S.; Chang, I.; et al. New-onset IgG autoantibodies in hospitalized patients with COVID-19. Nat. Commun. 2021, 12, 5417. [Google Scholar] [CrossRef] [PubMed]
- Woodruff, M.C.; Ramonell, R.P.; Haddad, N.S.; Anam, F.A.; Rudolph, M.E.; Walker, T.A.; Truong, A.D.; Dixit, A.N.; Han, J.E.; Cabrera-Mora, M.; et al. Dysregulated naive B cells and de novo autoreactivity in severe COVID-19. Nature 2022, 611, 139–147. [Google Scholar] [CrossRef]
- Arthur, J.M.; Forrest, J.C.; Boehme, K.W.; Kennedy, J.L.; Owens, S.; Herzog, C.; Liu, J.; Harville, T.O. Development of ACE2 autoantibodies after SARS-CoV-2 infection. PLoS ONE 2021, 16, e0257016. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G.; Hohberger, B.; Wenzel, K.; Fürst, J.; Schulze-Rothe, S.; Wallukat, A.; Hönicke, A.-S.; Müller, J. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J. Transl. Autoimmun. 2021, 4, 100100. [Google Scholar] [CrossRef]
- Skiba, M.A.; Kruse, A.C. Autoantibodies as Endogenous Modulators of GPCR Signaling. Trends Pharmacol. Sci. 2021, 42, 135–150. [Google Scholar] [CrossRef]
- Getts, D.R.; Alanna Spiteri Nicholas, J.C.; King Stephen, D. Miller, Chapter 21—Microbial Infection as a Trigger of T-Cell Autoimmunity, The Autoimmune Diseases, 6th ed.; Noel, R., Ian, R.M., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 363–374. [Google Scholar]
- Medić, S.; Anastassopoulou, C.; Lozanov-Crvenković, Z.; Vuković, V.; Dragnić, N.; Petrović, V.; Ristić, M.; Pustahija, T.; Gojković, Z.; Tsakris, A.; et al. Risk and severity of SARS-CoV-2 reinfections during 2020–2022 in Vojvodina, Serbia: A population-level observational study. Lancet Reg. Health-Eur. 2022, 20, 100453. [Google Scholar] [CrossRef] [PubMed]
- Bowe, B.; Xie, Y.; Al-Aly, Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat. Med. 2022, 28, 2398–2405. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Deng, J.; Liu, Q.; Du, M.; Liu, M.; Liu, J. Long-Term Consequences of Asymptomatic SARS-CoV-2 Infection: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 1613. [Google Scholar] [CrossRef] [PubMed]
- Hadley, E.; Yoo, Y.J.; Patel, S.; Zhou, A.; Laraway, B.; Wong, R.; Preiss, A.; Chew, R.; Davis, H.; Chute, C.G.; et al. N3C and RECOVER consortia. SARS-CoV-2 Reinfection is Preceded by Unique Biomarkers and Related to Initial Infection Timing and Severity: An N3C RECOVER EHR-Based Cohort Study. medRxiv 2023. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Notarte, K.I.; Peligro, P.J.; Velasco, J.V.; Ocampo, M.J.; Henry, B.M.; Arendt-Nielsen, L.; Torres-Macho, J.; Plaza-Manzano, G. Long-COVID Symptoms in Individuals Infected with Different SARS-CoV-2 Variants of Concern: A Systematic Review of the Literature. Viruses 2022, 14, 2629. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, H.; Shannon, C.P.; Tebbutt, S.J. Omicron variants of SARS-CoV-2 and long COVID. Front. Immunol. 2022, 13, 1061686. [Google Scholar] [CrossRef]
- Antonelli, M.; Pujol, J.C.; Spector, T.D.; Ourselin, S.; Steves, C.J. Risk of long COVID associated with delta versus omicron variants of SARS-CoV-2. Lancet 2022, 399, 2263–2264. [Google Scholar] [CrossRef]
- Stein, C.; Nassereldine, H.; Sorensen, R.J.D.; O Amlag, J.; Bisignano, C.; Byrne, S.; Castro, E.; Coberly, K.; Collins, J.K.; Dalos, J.; et al. Past SARS-CoV-2 infection protection against re-infection: A systematic review and meta-analysis. Lancet 2023, 401, 833–842. [Google Scholar] [CrossRef]
- Orendáčová, M.; Kvašňák, E. Effects of vaccination, new SARS-CoV-2 variants and reinfections on post-COVID-19 complications. Front. Public Health 2022, 10, 903568. [Google Scholar] [CrossRef]
- Byambasuren, O.; Stehlik, P.; Clark, J.; Alcorn, K.; Glasziou, P. Effect of covid-19 vaccination on long covid: Systematic review. BMJ Med. 2023, 2, e000385. [Google Scholar] [CrossRef]
- Tofarides, A.G.; Christaki, E.; Milionis, H.; Nikolopoulos, G.K. Effect of Vaccination against SARS-CoV-2 on Long COVID-19: A Narrative Review. Life 2022, 12, 2057. [Google Scholar] [CrossRef] [PubMed]
- Taquet, M.; Dercon, Q.; Harrison, P.J. Six-month sequelae of post-vaccination SARS-CoV-2 infection: A retrospective cohort study of 10,024 breakthrough infections. Brain Behav. Immun. 2022, 103, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Al-Aly, Z.; Bowe, B.; Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat. Med. 2022, 28, 1461–1467. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Bosworth, M.L.; King, S.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; A Alwan, N.; Walker, A.S. Risk of Long COVID in People Infected with Severe Acute Respiratory Syndrome Coronavirus 2 After 2 Doses of a Coronavirus Disease 2019 Vaccine: Community-Based, Matched Cohort Study. Open Forum Infect. Dis. 2022, 9, ofac464. [Google Scholar] [CrossRef] [PubMed]
- Notarte, K.I.; Catahay, J.A.; Velasco, J.V.; Pastrana, A.; Ver, A.T.; Pangilinan, F.C.; Peligro, P.J.; Casimiro, M.; Guerrero, J.J.; Gellaco, M.M.L.; et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: A systematic review. eClinicalMedicine 2022, 53, 101624. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Liu, J.; Liu, M. Effect of COVID-19 Vaccines on Reducing the Risk of Long COVID in the Real World: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 12422. [Google Scholar] [CrossRef]
- Mumtaz, A.; Sheikh, A.A.E.; Khan, A.M.; Khalid, S.N.; Khan, J.; Nasrullah, A.; Sagheer, S.; Sheikh, A.B. COVID-19 Vaccine and Long COVID: A Scoping Review. Life 2022, 12, 1066. [Google Scholar] [CrossRef] [PubMed]
- Ayoubkhani, D.; Bermingham, C.; Pouwels, K.B.; Glickman, M.; Nafilyan, V.; Zaccardi, F.; Khunti, K.; A Alwan, N.; Walker, A.S. Trajectory of long covid symptoms after covid-19 vaccination: Community based cohort study. BMJ 2022, 377, e069676. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, E.; Levi, R.; Sarti, R.; Pozzi, C.; Mollura, M.; Mantovani, A.; Rescigno, M. Association Between BNT162b2 Vaccination and Long COVID After Infections Not Requiring Hospitalization in Health Care Workers. JAMA 2022, 328, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, M.; Penfold, R.S.; Merino, J.; Sudre, C.H.; Molteni, E.; Berry, S.; Canas, L.S.; Graham, M.S.; Klaser, K.; Modat, M.; et al. Risk factors and disease profile of post-vaccination SARS-CoV-2 infection in UK users of the COVID Symptom Study app: A prospective, community-based, nested, case-control study. Lancet Infect Dis. 2022, 22, 43–55. [Google Scholar] [CrossRef]
- Mizrahi, B.; Sudry, T.; Flaks-Manov, N.; Yehezkelli, Y.; Kalkstein, N.; Akiva, P.; Ekka-Zohar, A.; Ben David, S.S.; Lerner, U.; Bivas-Benita, M.; et al. Long covid outcomes at one year after mild SARS-CoV-2 infection: Nationwide cohort study. BMJ 2023, 380, e072529. [Google Scholar] [CrossRef]
- Watanabe, A.; Iwagami, M.; Yasuhara, J.; Takagi, H.; Kuno, T. Protective effect of COVID-19 vaccination against long COVID syndrome: A systematic review and meta-analysis. Vaccine 2023, 41, 1783–1790. [Google Scholar] [CrossRef]
- Slawson, D.C. Likelihood of Long COVID Varies by Variant, Sex, and Vaccination Status. Am. Fam. Physic. 2023, 107, 199. [Google Scholar]
- Brannock, M.D.; Chew, R.F.; Preiss, A.J.; Hadley, E.C.; McMurry, J.A.; Leese, P.J.; Girvin, A.T.; Crosskey, M.; Zhou, A.G.; Moffitt, R.A.; et al. The N3C and RECOVER Consortia. Long COVID Risk and Pre-COVID Vaccination: An EHR-Based Cohort Study from the RECOVER Program. medRxiv 2022. [Google Scholar] [CrossRef]
- Nascimento, T.C.D.C.; Costa, L.D.V.; Ruiz, A.D.; Ledo, C.B.; Fernandes, V.P.L.; Cardoso, L.F.; Junior, J.M.V.; Saretta, R.; Kalil-Filho, R.; Drager, L.F. Vaccination status and long COVID symptoms in patients discharged from hospital. Sci. Rep. 2023, 13, 2481. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.M.; Nugawela, M.D.; Rojas, N.K.; Shafran, R.; McOwat, K.; Simmons, R.; Ford, T.; Heyman, I.; Ladhani, S.N.; Cheung, E.Y.; et al. Post-COVID-19 condition at 6 months and COVID-19 vaccination in non-hospitalised children and young people. Arch. Dis. Child 2023, 108, 289–295. [Google Scholar]
- Tran, V.-T.; Perrodeau, E.; Saldanha, J.; Pane, I.; Ravaud, P. Efficacy of first dose of covid-19 vaccine versus no vaccination on symptoms ofpatientswith long covid: Target trial emulation based on ComPaRe e-cohort. BMJ Med. 2023, 2, e000229. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, C.R.; Strahm, C.; Güsewell, S.; Cusini, A.; Brucher, A.; Goppel, S.; Möller, E.; Möller, J.C.; Ortner, M.; Ruetti, M.; et al. Post-Acute Sequelae After Severe Acute Respiratory Syndrome Coronavirus 2 Infection by Viral Variant and Vaccination Status: A Multicenter Cross-Sectional Study. Clin. Infect. Dis. 2023, 77, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Català, M.; Mercadé-Besora, N.; Kolde, R.; Trinh, N.T.; Roel, E.; Burn, E.; Rathod-Mistry, T.; Kostka, K.; Man, W.Y.; Delmestri, A.; et al. The Effectiveness of COVID-19 Vaccines to Prevent Long COVID Symptoms: Staggered Cohort Analyses of Data from the UK, Spain, and Estonia. Available online: https://ssrn.com/abstract=4474215 (accessed on 14 June 2023).
- Anastassopoulou, C.; Gkizarioti, Z.; Patrinos, G.P.; Tsakris, A. Human genetic factors associated with susceptibility to SARS-CoV-2 infection and COVID-19 disease severity. Hum. Genom. 2020, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Anastassopoulou, C.; Spanakis, N.; Tsakris, A. SARS-CoV-2 transmission, the ambiguous role of children and considerations for the reopening of schools in the fall. Futur. Microbiol. 2020, 15, 1201–1206. [Google Scholar] [CrossRef]
- Chou, J.; Thomas, P.G.; Randolph, A.G. Immunology of SARS-CoV-2 infection in children. Nat. Immunol. 2022, 23, 177–185. [Google Scholar] [CrossRef]
- Medić, S.; Anastassopoulou, C.; Lozanov-Crvenković, Z.; Dragnić, N.; Petrović, V.; Ristić, M.; Pustahija, T.; Tsakris, A.; Ioannidis, J.P.A. Incidence, Risk, and Severity of SARS-CoV-2 Reinfections in Children and Adolescents between March 2020 and July 2022 in Serbia. JAMA Netw. Open 2023, 6, e2255779. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; del Valle, N.C.A.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. Long-COVID in children and adolescents: A systematic review and meta-analyses. Sci. Rep. 2022, 12, 9950. [Google Scholar] [CrossRef] [PubMed]
- Fainardi, V.; Meoli, A.; Chiopris, G.; Motta, M.; Skenderaj, K.; Grandinetti, R.; Bergomi, A.; Antodaro, F.; Zona, S.; Esposito, S. Long COVID in Children and Adolescents. Life 2022, 12, 285. [Google Scholar] [CrossRef] [PubMed]
- Morello, R.; Martino, L.; Buonsenso, D. Diagnosis and management of post-COVID (Long COVID) in children: A moving target. Curr. Opin. Pediatr. 2023, 35, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Pujol, J.; Moron-Lopez, S.; Dalmau, J.; Gonzalez-Aumatell, A.; Carreras-Abad, C.; Mendez, M.; Rodrigo, C.; Martinez-Picado, J. Post COVID-19 Condition in Children and Adolescents: An Emerging Problem. Front. Pediatr. 2022, 10, 894204. [Google Scholar] [CrossRef] [PubMed]
- Funk, A.L.; Kuppermann, N.; Florin, T.A.; Tancredi, D.J.; Xie, J.; Kim, K.; Finkelstein, Y.; Neuman, M.I.; Salvadori, M.I.; Yock-Corrales, A.; et al. Post–COVID-19 Conditions Among Children 90 Days After SARS-CoV-2 Infection. JAMA Netw. Open 2022, 5, e2223253. [Google Scholar] [CrossRef] [PubMed]
- Berg, S.K.; Palm, P.; Nygaard, U.; Bundgaard, H.; Petersen, M.N.S.; Rosenkilde, S.; Thorsted, A.B.; Ersbøll, A.K.; Thygesen, L.C.; Nielsen, S.D.; et al. Long COVID symptoms in SARS-CoV-2-positive children aged 0–14 years and matched controls in Denmark (LongCOVIDKidsDK): A national, cross-sectional study. Lancet Child Adolesc. Health 2022, 6, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Buonsenso, D.; Munblit, D.; Pazukhina, E.; Ricchiuto, A.; Sinatti, D.; Zona, M.; De Matteis, A.; D’ilario, F.; Gentili, C.; Lanni, R.; et al. Post-COVID Condition in Adults and Children Living in the Same Household in Italy: A Prospective Cohort Study Using the ISARIC Global Follow-Up Protocol. Front. Pediatr. 2022, 10, 834875. [Google Scholar] [CrossRef] [PubMed]
- Woodrow, M.; Carey, C.; Ziauddeen, N.; Thomas, R.; Akrami, A.; Lutje, V.; Greenwood, D.C.; Alwan, N.A. Systematic review of the prevalence of Long COVID. medRxiv 2022. [Google Scholar] [CrossRef]
- Zheng, Y.-B.; Zeng, N.; Yuan, K.; Tian, S.-S.; Yang, Y.-B.; Gao, N.; Chen, X.; Zhang, A.-Y.; Kondratiuk, A.L.; Shi, P.-P.; et al. Prevalence and risk factor for long COVID in children and adolescents: A meta-analysis and systematic review. J. Infect. Public Health 2023, 16, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Warren-Gash, C.; Lacey, A.; Cook, S.; Stocker, D.; Toon, S.; Lelii, F.; Ford, B.; Ireland, G.; Ladhani, S.N.; Stephenson, T.; et al. Post-COVID-19 condition and persisting symptoms in English schoolchildren: Repeated surveys to March 2022. BMC Infect. Dis. 2023, 23, 201. [Google Scholar] [CrossRef] [PubMed]
- Borch, L.; Holm, M.; Knudsen, M.; Ellermann-Eriksen, S.; Hagstroem, S. Long COVID symptoms and duration in SARS-CoV-2 positive children—A nationwide cohort study. Eur. J. Pediatr. 2022, 181, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, T.; Shafran, R.; Ladhani, S.N. Long COVID in children and adolescents. Curr. Opin. Infect. Dis. 2022, 35, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Nesa, F.; Das, J.; Aggad, R.; Tasnim, S.; Bairwa, M.; Ma, P.; Ramirez, G. Global burden of mental health problems among children and adolescents during COVID-19 pandemic: An umbrella review. Psychiatry Res. 2022, 317, 114814. [Google Scholar] [CrossRef] [PubMed]
- Shachar-Lavie, I.; Shorer, M.; Segal, H.; Fennig, S.; Ashkenazi-Hoffnung, L. Mental health among children with long COVID during the COVID-19 pandemic. Eur. J. Pediatr. 2023, 182, 1793–1801. [Google Scholar] [CrossRef]
- Miller, F.; Nguyen, V.; Navaratnam, A.M.; Shrotri, M.; Kovar, J.; Hayward, A.C.; Fragaszy, E.; Aldridge, R.W.; Hardelid, P. Prevalence and Characteristics of Persistent Symptoms in Children During the COVID-19 Pandemic: Evidence from a Household Cohort Study in England and Wales. Pediatr. Infect. Dis. J. 2022, 41, 979–984. [Google Scholar] [CrossRef]
- Dumont, R.; Richard, V.; Lorthe, E.; Loizeau, A.; Pennacchio, F.; Zaballa, M.-E.; Baysson, H.; Nehme, M.; Perrin, A.; L’huillier, A.G.; et al. A population-based serological study of post-COVID syndrome prevalence and risk factors in children and adolescents. Nat. Commun. 2022, 13, 7086. [Google Scholar] [CrossRef]
- Adler, L.; Israel, M.; Yehoshua, I.; Azuri, J.; Hoffman, R.; Shahar, A.; Reuveni, M.M.; Grossman, Z. Long COVID symptoms in Israeli children with and without a history of SARS-CoV-2 infection: A cross-sectional study. BMJ Open 2023, 13, e064155. [Google Scholar] [CrossRef] [PubMed]
- Haddad, A.; Janda, A.; Renk, H.; Stich, M.; Frieh, P.; Kaier, K.; Lohrmann, F.; Nieters, A.; Willems, A.; Huzly, D.; et al. Long COVID symptoms in exposed and infected children, adolescents and their parents one year after SARS-CoV-2 infection: A prospective observational cohort study. Ebiomedicine 2022, 84, 104245. [Google Scholar] [CrossRef]
- Bechmann, N.; Barthel, A.; Schedl, A.; Herzig, S.; Varga, Z.; Gebhard, C.; Mayr, M.; Hantel, C.; Beuschlein, F.; Wolfrum, C.; et al. Sexual dimorphism in COVID-19: Potential clinical and public health implications. Lancet Diabetes Endocrinol. 2022, 10, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Seery, V.; Raiden, S.; Penedo, J.M.G.; Borda, M.; Herrera, L.; Uranga, M.; del Pont, M.M.; Chirino, C.; Erramuspe, C.; Alvarez, L.S.; et al. Persistent symptoms after COVID-19 in children and adolescents from Argentina. Int. J. Infect. Dis. 2023, 129, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Jat, K.R. Post-COVID-19 Sequelae in Children. Indian J. Pediatr. 2023, 90, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Jarnig, G.; Kerbl, R.; van Poppel, M.N.M. Changes in Children’s Fitness and Health Status Over the Course of the COVID-19 Pandemic: A 34-month Longitudinal Study of 331 Primary School Children (September 2019 to June 2022). medRxiv 2022. [Google Scholar] [CrossRef]
- Balderas, L.M.d.C.J.; Fernández, A.N.; Garza, S.A.D.; Jerves, M.I.O.; Figueroa, W.E.S.; Koretzky, S.G.; González, H.M.; Klünder, M.K.; Espinosa, J.G.; Zermeño, J.N.; et al. Long COVID in children and adolescents: COVID-19 follow-up results in third-level pediatric hospital. Front. Pediatr. 2023, 11, 1016394. [Google Scholar] [CrossRef]
- Garazzino, S.; Denina, M.; Pruccoli, G.; Funiciello, E.; Ramenghi, U.; Fagioli, F. Long COVID-19/post-COVID condition in children: Do we all speak the same language? Ital. J. Pediatr. 2023, 49, 12. [Google Scholar] [CrossRef]
- Marshall, M. Long COVID: Answers emerge on how many people get better. Nature 2023, 619, 20. [Google Scholar] [CrossRef]
- Xie, Y.; Choi, T.; Al-Aly, Z. Association of Treatment with Nirmatrelvir and the Risk of Post–COVID-19 Condition. JAMA Intern. Med. 2023, 183, 554–564. [Google Scholar] [CrossRef]
- Bramante, C.T.; Buse, J.B.; Liebovitz, D.M.; Nicklas, J.M.; A Puskarich, M.; Cohen, K.; Belani, H.K.; Anderson, B.J.; Huling, J.D.; Tignanelli, C.J.; et al. Outpatient treatment of COVID-19 and incidence of post-COVID-19 condition over 10 months (COVID-OUT): A multicentre, randomised, quadruple-blind, parallel-group, phase 3 trial. Lancet Infect. Dis. 2023, S1473-3099(23)00299-2. [Google Scholar] [CrossRef]
- Anastassopoulou, C.; Hatziantoniou, S.; Boufidou, F.; Patrinos, G.P.; Tsakris, A. The Role of Oral Antivirals for COVID-19 Treatment in Shaping the Pandemic Landscape. J. Pers. Med. 2022, 12, 439. [Google Scholar] [CrossRef]
- Stergiou, K.D.; Minopoulos, G.M.; Memos, V.A.; Stergiou, C.L.; Koidou, M.P.; Psannis, K.E. A Machine Learning-Based Model for Epidemic Forecasting and Faster Drug Discovery. Appl. Sci. 2022, 12, 10766. [Google Scholar] [CrossRef]
- Pfaff, E.R.; Girvin, A.T.; Bennett, T.D.; Bhatia, A.; Brooks, I.M.; Deer, R.R.; Dekermanjian, J.P.; Jolley, S.E.; Kahn, M.G.; Kostka, K.; et al. Identifying who has long COVID in the USA: A machine learning approach using N3C data. Lancet Digit. Health 2022, 4, e532–e541. [Google Scholar] [CrossRef] [PubMed]
| Factor Type | Risk Factor for Long COVID upon Reinfection | |
|---|---|---|
| Host | Biological | Female sex |
| Older age 1 | ||
| Certain comorbidities (e.g., type 2 diabetes) | ||
| Having had severe COVID-19 (particularly during the first few weeks of illness) 2 | ||
| Lifestyle | Obesity | |
| Smoking | ||
| Viral | SARS-CoV-2 variant | Omicron 3 |
| Key Findings Regarding Long COVID | Main Reference(s) | |
|---|---|---|
| Reinfection | Reinfections increase the likelihood of long COVID (and additional complications, e.g., cardiac, pulmonary, neurological, in older subjects). | [55] |
| The risk of developing long COVID symptoms is significantly lower after asymptomatic (compared to symptomatic) reinfection. | [56] | |
| Long COVID cases have been increasing upon reinfection with Omicron subvariants. | [57,58,60] | |
| Vaccination | Vaccination against (severe) COVID-19 seems to also protect from long COVID (reduced risk by 15–41%). | [63,66,67] |
| Two vaccine doses (of the primary scheme) are more effective than one dose. | [64,68,69,70] | |
| No difference in relation to the type of received vaccine. | [70] | |
| Affected population | Children may also suffer from long COVID, but less frequently and less severely than adults. | [87,88,93] |
| Chronic fatigue is one of the most common symptoms of long COVID present in up to 87% of children and adolescents with long COVID. | [90,91,97] | |
| Older age, comorbidities, and symptomatic infection are risk factors for long COVID in children. | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boufidou, F.; Medić, S.; Lampropoulou, V.; Siafakas, N.; Tsakris, A.; Anastassopoulou, C. SARS-CoV-2 Reinfections and Long COVID in the Post-Omicron Phase of the Pandemic. Int. J. Mol. Sci. 2023, 24, 12962. https://doi.org/10.3390/ijms241612962
Boufidou F, Medić S, Lampropoulou V, Siafakas N, Tsakris A, Anastassopoulou C. SARS-CoV-2 Reinfections and Long COVID in the Post-Omicron Phase of the Pandemic. International Journal of Molecular Sciences. 2023; 24(16):12962. https://doi.org/10.3390/ijms241612962
Chicago/Turabian StyleBoufidou, Fotini, Snežana Medić, Vicky Lampropoulou, Nikolaos Siafakas, Athanasios Tsakris, and Cleo Anastassopoulou. 2023. "SARS-CoV-2 Reinfections and Long COVID in the Post-Omicron Phase of the Pandemic" International Journal of Molecular Sciences 24, no. 16: 12962. https://doi.org/10.3390/ijms241612962
APA StyleBoufidou, F., Medić, S., Lampropoulou, V., Siafakas, N., Tsakris, A., & Anastassopoulou, C. (2023). SARS-CoV-2 Reinfections and Long COVID in the Post-Omicron Phase of the Pandemic. International Journal of Molecular Sciences, 24(16), 12962. https://doi.org/10.3390/ijms241612962









