Comparative Transcriptome Profiling Reveals Two WRKY Transcription Factors Positively Regulating Polysaccharide Biosynthesis in Polygonatum cyrtonema
Abstract
:1. Introduction
2. Results
2.1. Total PCP Content of Various P. cyrtonema Germplasms
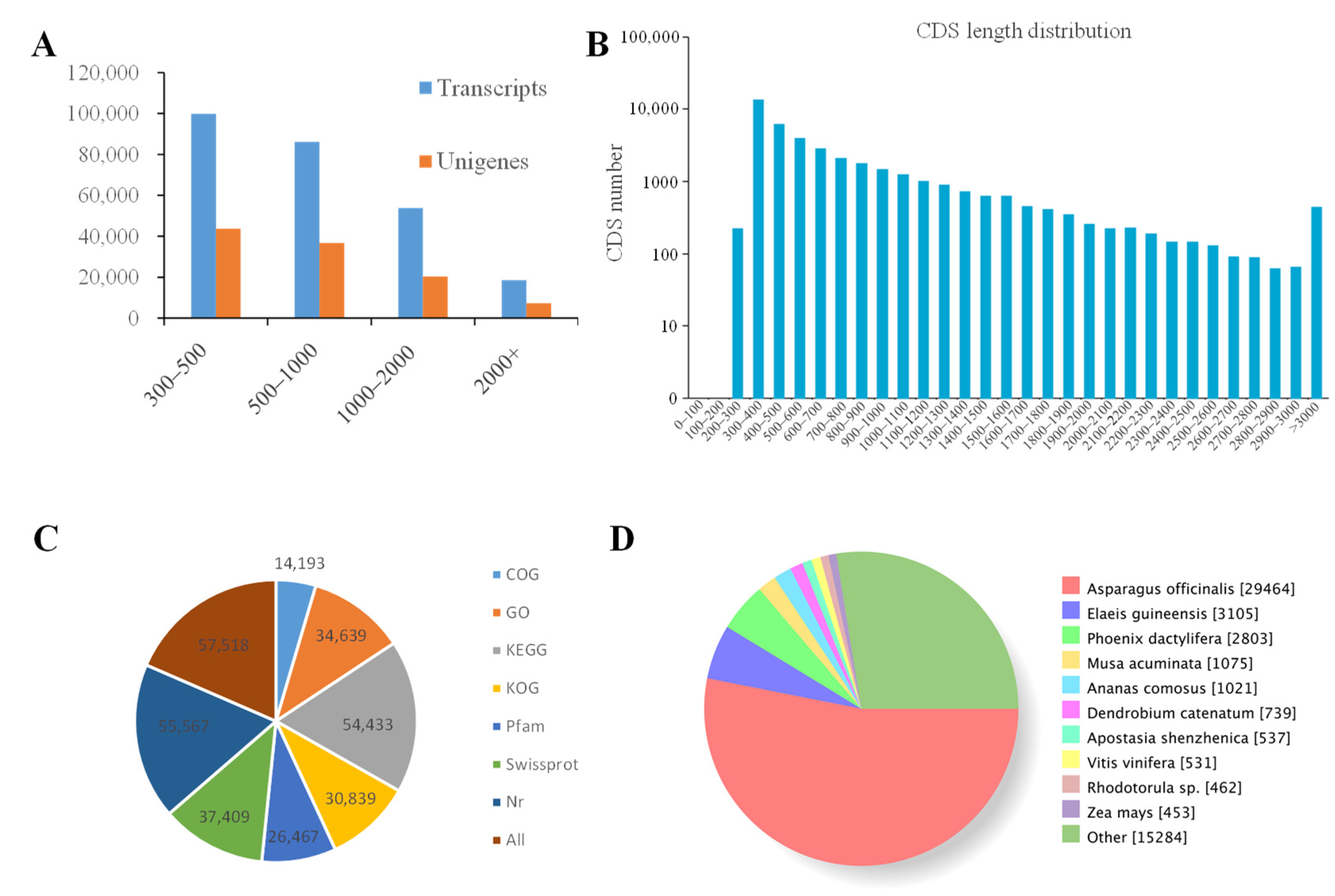
2.2. Illumina Sequencing, Assembly and Unigene Annotation
2.3. Classification of KOG, GO, and KEGG Terms
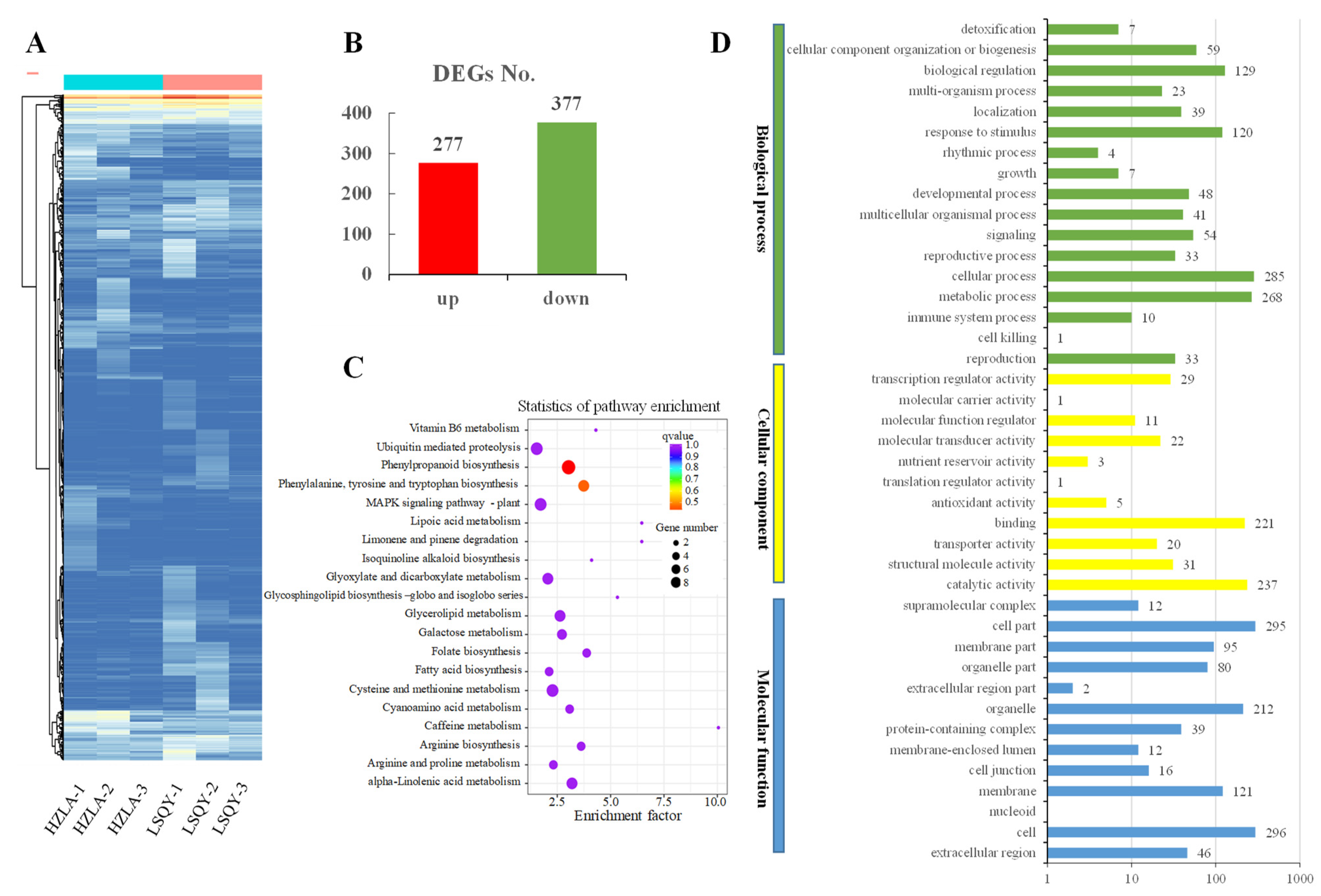
2.4. Identification of DEGs between Different Samples
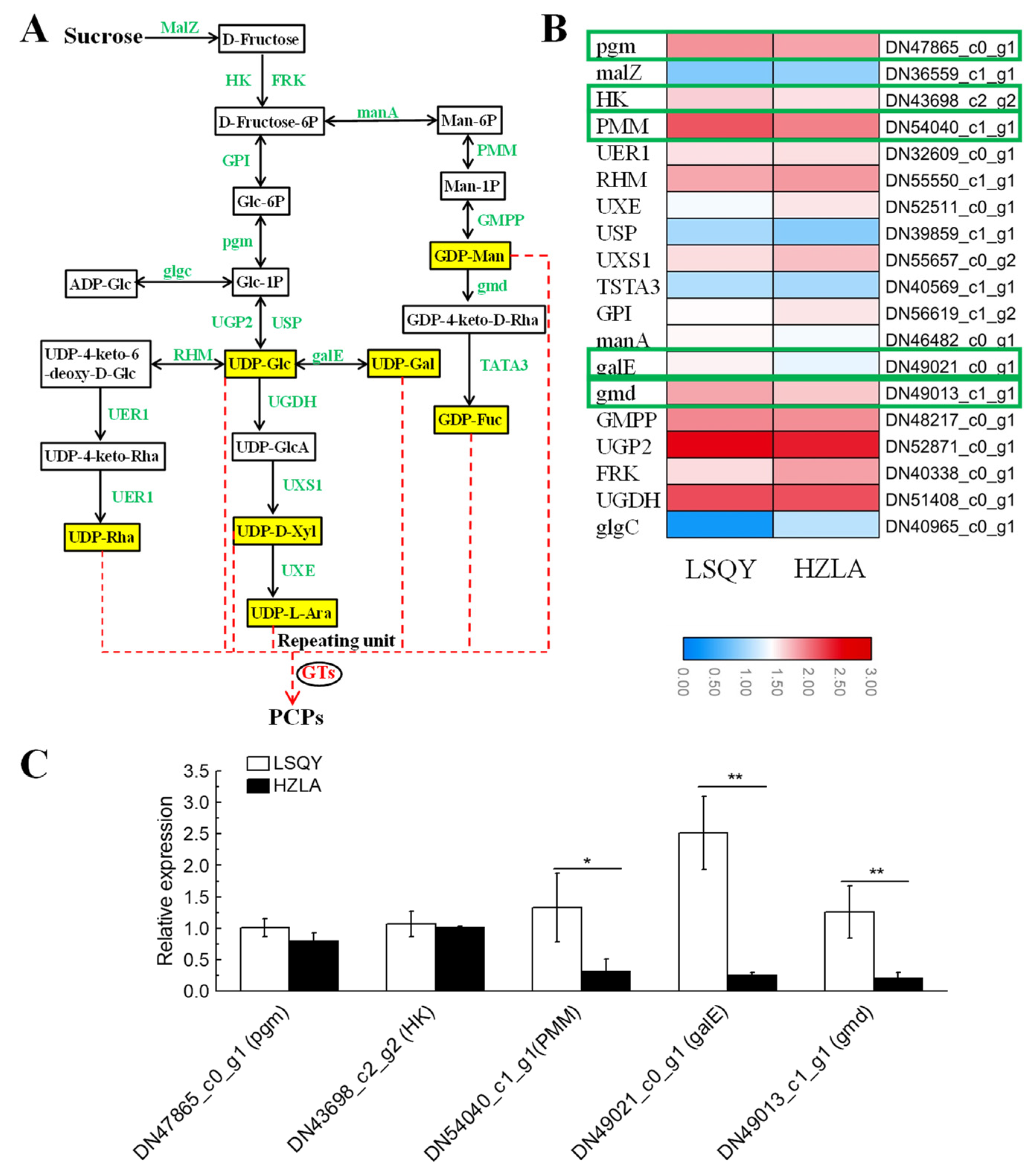
2.5. Identification of DEGs and Key Genes Involved in PCP Biosynthesis
2.6. Subcellular Localization and Functional Verification of PcgalE1
2.7. Screening and Identification of Transcriptional Modulators Involved in the PCP Biosynthesis Pathway
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Isolation and Detection of Total PCPs
4.3. Illumina Sequencing and Analysis
4.4. qRT-PCR Analysis
4.5. Promoter Sequence Cloning
4.6. Transient Expression of galE1 in Tobacco Leaves
4.7. Dual-Luciferase Assay
4.8. Yeast One-Hybrid Assay (Y1H)
4.9. Statistical and Sequence Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, X.Y.; Li, J. Chemical constituents of the genus polygonatum and their role in medicinal treatment. Nat. Prod. Commun. 2015, 10, 683–688. [Google Scholar] [CrossRef]
- Chen, J.; Zhu, J.Z.; Li, X.G.; He, A.G.; Xia, S.T.; Zhong, J. Botrytis cinerea causing gray mold of polygonatum sibiricum (Huang Jing) in China. Crop Prot. 2021, 140, 105424. [Google Scholar] [CrossRef]
- Gan, L.S.; Chen, J.J.; Shi, M.F.; Zhou, C.X. A new homoisoflavanone from the rhizomes of Polygonatum cyrtonema. Nat. Prod. Commun. 2013, 8, 597–598. [Google Scholar] [CrossRef]
- Wang, C.; Peng, D.; Zhu, J.; Zhao, D.; Huang, L. Transcriptome analysis of Polygonatum cyrtonema Hua: Identification of genes involved in polysaccharide biosynthesis. Plant Methods 2019, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure–activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Ray, B.; Ali, I.; Jana, S.; Mukherjee, S.; Pal, S.; Ray, S.; Schütz, M.; Marschall, M. Antiviral strategies using natural source-derived sulfated polysaccharides in the light of the COVID-19 pandemic and major human pathogenic viruses. Viruses 2022, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.; Jana, S.; Mukherjee, S.; Bera, K.; Majee, S.K.; Ali, I.; Pal, S.; Ray, B.; Ray, S. The heteropolysaccharide of Mangifera indica fruit: Isolation, chemical profile, complexation with β-lactoglobulin and antioxidant activity. Int. J. Biol. Macromol. 2020, 165, 93–99. [Google Scholar] [CrossRef]
- Ghosh, D.; Ray, S.; Ghosh, K.; Micard, V.; Chatterjee, U.R.; Ghosal, P.K.; Ray, B. Antioxidative carbohydrate polymer from Enhydra fluctuans and its interaction with bovine serum albumin. Biomacromolecules 2013, 14, 1761–1768. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bera, K.; Jana, S.; Pal, S.; Anand, N.; Ray, B.; Ray, S. Conjugation reaction with ferulic acid boosts the antioxidant property of arabinogalactan-protein and enhances its ability to form complex with β-lactoglobulin. Int. J. Biol. Macromol. 2021, 167, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, M.S.; Bellini, M.F.; Angeli, J.P.; Oliveira, R.J.; Silva, A.F.; Ribeiro, L.R. β-glucans in promoting health: Prevention against mutation and cancer. Mutat. Res. 2008, 658, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Inngjerdingen, K.T.; Patel, T.R.; Chen, X.; Kenne, L.; Allen, S.; Morris, G.A.; Harding, S.E.; Matsumoto, T.; Diallo, D.; Yamada, H.; et al. Immunological and structural properties of a pectic polymer from Glinus oppositifolius. Glycobiology 2007, 17, 1299–1310. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.M.; Yue, G.G.; Chan, Y.Y.; Kwok, H.F.; Gao, S.; Wong, C.W.; Lau, C.B. A review on the immunomodulatory activity of Acanthopanax senticosus and its active components. Chin. Med. 2019, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Nergard, C.S.; Kiyohara, H.; Reynolds, J.C.; Thomas-Oates, J.E.; Matsumoto, T.; Yamada, H.; Patel, T.; Petersen, D.; Michaelsen, T.E.; Diallo, D.; et al. Structures and structure-activity relationships of three mitogenic and complement fixing Pectic Arabinogalactans from the Malian Antiulcer plants Cochlospermum tinctorium A. Rich and Vernonia kotschyana Sch. Bip. ex Walp. Biomacromolecules 2006, 7, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, B.S.; Barsett, H. Bioactive pectic polysaccharides. Adv. Polym. Sci. 2005, 186, 69–101. [Google Scholar] [CrossRef]
- Bianculli, R.H.; Mase, J.D.; Schulz, M.D. Antiviral polymers: Past approaches and future possibilities. Macromolecules 2020, 53, 9158–9186. [Google Scholar] [CrossRef]
- Fox, M.E.; Szoka, F.C.; Fréchet, J.M.J. Soluble polymer carriers for the treatment of cancer: The importance of molecular architecture. Acc. Chem. Res. 2009, 42, 1141–1151. [Google Scholar] [CrossRef]
- Nasongkla, N.; Chen, B.; Macaraeg, N.; Fox, M.E.; Fréchet, J.M.J.; Szoka, F.C. Dependence of pharmacokinetics and biodistribution on polymer architecture: Effect of cyclic versus linear polymers. J. Am. Chem. Soc. 2009, 131, 3842–3843. [Google Scholar] [CrossRef]
- Beaumont, M.; Tran, R.; Vera, G.; Niedrist, D.; Rousset, A.; Pierre, R.; Shastri, V.P.; Forget, A. Hydrogel-forming algae polysaccharides: From seaweed to biomedical applications. Biomacromolecules 2021, 22, 1027–1052. [Google Scholar] [CrossRef]
- Caputo, H.E.; Straub, J.E.; Grinstaff, M.W. Design, synthesis, and biomedical applications of synthetic sulphated polysaccharides. Chem. Soc. Rev. 2019, 48, 2338–2365. [Google Scholar] [CrossRef]
- El-Shafei, R.; Hegazy, H.; Achconzoarya, B. A review of antiviral and antioxidant activity of bioactive metabolite of macroalgae within an optimized extraction method. Energies 2021, 14, 3092. [Google Scholar] [CrossRef]
- Germershaus, O.; Lühmann, T.; Rybak, J.C.; Ritzer, J.; Meinel, L. Application of natural and semi-synthetic polymers for the delivery of sensitive drugs. Int. Mater. Rev. 2015, 60, 101–131. [Google Scholar] [CrossRef]
- Gogineni, V.; Schinazi, R.F.; Hamann, M.T. Role of marine natural products in the genesis of antiviral agents. Chem. Rev. 2015, 115, 9655–9706. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.W.; Nielsen, E. Targeting and regulation of cell wall synthesis during tip growth in plants. J. Integr. Plant Biol. 2013, 55, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Sampathkumar, A.; Peaucelle, A.; Fujita, M.; Schuster, C.; Persson, S.; Wasteneys, G.O.; Meyerowitz, E.M. Primary wall cellulose synthase regulates shoot apical meristem mechanics and growth. Development 2019, 146, dev179036. [Google Scholar] [CrossRef] [PubMed]
- De Lorenzo, G.; Ferrari, S.; Giovannoni, M.; Mattei, B.; Cervone, F. Cell wall traits that influence plant development, immunity, and bioconversion. Plant J. 2019, 97, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Vaahtera, L.; Schulz, J.; Hamann, T. Cell wall integrity maintenance during plant development and interaction with the environment. Nat. Plants 2019, 5, 924–932. [Google Scholar] [CrossRef]
- Wolf, S.; Hematy, K.; Hofte, H. Growth control and cell wall signaling in plants. Annu. Rev. Plant Biol. 2012, 63, 381–407. [Google Scholar] [CrossRef]
- Wolf, S. Plant cell wall signalling and receptor-like kinases. Biochem. J. 2017, 474, 471–492. [Google Scholar] [CrossRef]
- Novakovic, L.; Guo, T.T.; Bacic, A.; Sampathkumar, A.; Johnson, K.L. Hitting the wall–sensing and signaling pathways involved in plant cell wall remodeling in response to abiotic stress. Plants 2018, 7, 89. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, H.; Liu, C.; Jing, Z.; Ning, L.; Zhao, Z.; Sun, G.; Zhong, Y. A molasses habitat-derived fungus Aspergillus tubingensis XG21 with high β-fructofuranosidase activity and its potential use for fructooligosaccharides production. AMB Express 2017, 7, 128. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhen, L.; Tan, X.; Li, L.; Wang, X. The involvement of hexokinase in the coordinated regulation of glucose and gibberellin on cell wall invertase and sucrose synthesis in grape berry. Mol. Biol. Rep. 2014, 41, 7899–7910. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, K.; Suzuki, N.; Iwamae, T.; Inui, M.; Yukawa, H. Expression of arabidopsis plastidial phosphoglucomutase in tobacco stimulates photosynthetic carbon flow into starch synthesis. J. Plant Physiol. 2012, 169, 1454–1462. [Google Scholar] [CrossRef] [PubMed]
- Perezcenci, M.; Salerno, G.L. Functional characterization of Synechococcus amylosucrase and fructokinase encoding genes discovers two novel actors on the stage of cyanobacterial sucrose metabolism. Plant Sci. 2014, 224, 95–102. [Google Scholar] [CrossRef]
- Bachmann, P.; Zetsche, K. A close temporal and spatial correlation between cell growth, cell wall synthesis and the activity of enzymes of mannan synthesis in Acetabularia mediterranea. Planta 1979, 145, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Park, J.I.; Ishimizu, T.; Suwabe, K.; Sudo, K.; Masuko, H.; Hakozaki, H.; Nou, I.S.; Suzuki, G.; Watanabe, M. UDP-glucose pyrophosphorylase is rate limiting in vegetative and reproductive phases in Arabidopsis thaliana. Plant Cell Physiol. 2010, 51, 981–996. [Google Scholar] [CrossRef]
- Yin, Y.; Huang, J.; Gu, X.; Barpeled, M.; Xu, Y. Evolution of plant nucleotide-sugar interconversion enzymes. PLoS ONE 2011, 6, e27995. [Google Scholar] [CrossRef]
- Breton, C.; Snajdrová, L.; Jeanneau, C.; Koca, J.; Imberty, A. Structures and mechanism of glycosyltransferases. Glycobiology 2006, 16, 29R–37R. [Google Scholar] [CrossRef]
- Pauly, M.; Gille, S.; Liu, L.; Mansoori, N.; de Souza, A.; Schultink, A.; Xiong, G. Hemicellulose biosynthesis. Planta 2013, 238, 627–642. [Google Scholar] [CrossRef]
- Penfield, S.; Meissner, R.C.; Shoue, D.A.; Carpita, N.C.; Bevan, M.W. MYB61 is required for mucilage deposition and extrusion in the Arabidopsis seed coat. Plant Cell 2001, 13, 2777–2791. [Google Scholar] [CrossRef]
- Li, D.; Ye, G.; Li, J.; Lai, Z.; Ruan, S.; Qi, Q.; Wang, Z.; Duan, S.; Jin, H.L.; Wang, H.B. High light triggers flavonoid and polysaccharide synthesis through DoHY5-dependent signaling in Dendrobium officinale. Plant J. 2023. [Google Scholar] [CrossRef]
- Noda, S.; Koshiba, T.; Hattori, T.; Yamaguchi, M.; Suzuki, S.; Umezawa, T. The expression of a rice secondary wall-specific cellulose synthase gene, OsCesA7, is directly regulated by a rice transcription factor, OsMYB58/63. Planta 2015, 242, 589–600. [Google Scholar] [CrossRef]
- Rao, X.; Chen, X.; Shen, H.; Ma, Q.; Li, G.; Tang, Y.; Pena, M.; York, W.; Frazier, T.P.; Lenaghan, S.; et al. Gene regulatory networks for lignin biosynthesis in switchgrass (Panicum virgatum). Plant Biotechnol. J. 2019, 17, 580–593. [Google Scholar] [CrossRef]
- Scully, E.D.; Gries, T.; Palmer, N.A.; Sarath, G.; Funnell-Harris, D.L.; Baird, L.; Twigg, P.; Seravalli, J.; Clemente, T.E.; Sattler, S.E. Overexpression of SbMyb60 in Sorghum bicolor impacts both primary and secondary metabolism. New Phytol. 2018, 217, 82–104. [Google Scholar] [CrossRef] [PubMed]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [CrossRef]
- Che, Y.Y.; Qian, Y.; Wu, Y.; Qu, W.; Liang, J.Y. Two new homoisoflavanones from the rhizome of Polygonatum odoratum. Chem. Nat. Compd. 2015, 51, 54–56. [Google Scholar] [CrossRef]
- Li, L.; Thakur, K.; Liao, B.Y.; Zhang, J.G.; Wei, Z.J. Antioxidant and antimicrobial potential of polysaccharides sequentially extracted from Polygonatum cyrtonema Hua. Int. J. Biol. Macromol. 2018, 114, 317–323. [Google Scholar] [CrossRef]
- Liu, N.; Dong, Z.H.; Zhu, X.S.; Xu, H.Y.; Zhao, Z.X. Characterization and protective effect of Polygonatum sibiricum polysaccharide against cyclophosphamide-induced immunosuppression in Balb/c mice. Int. J. Biol. Macromol. 2018, 107, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, Q.; Bao, J.K.; Liu, B. Polygonatum cyrtonema lectin, a potential antineoplastic drug targeting programmed cell death pathways. Biochem. Bioph. Res. Commun. 2011, 406, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Q.; Wang, B.; Hua, W.P.; Niu, J.F.; Dang, K.K.; Qiang, Y.; Wang, Z. De novo assembly and analysis of Polygonatum sibiricum transcriptome and identification of genes involved in polysaccharide biosynthesis. Int. J. Mol. Sci. 2017, 18, 1950. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Dong, W.; Gen, W.; Xu, B.; Shen, C.; Yu, C. De novo transcriptome assembly and characterization of the synthesis genes of bioactive constituents in Abelmoschus esculentus (L.) Moench. Genes 2018, 9, 130. [Google Scholar] [CrossRef]
- Yuan, Y.; Song, L.; Li, M.; Liu, G.; Chu, Y.; Ma, L.; Zhou, Y.; Wang, X.; Gao, W.; Qin, S.; et al. Genetic variation and metabolic pathway intricacy govern the active compound content and quality of the Chinese medicinal plant Lonicera japonica thunb. BMC Genom. 2012, 13, 195. [Google Scholar] [CrossRef]
- Ying, L.; Luo, H.M.; Chao, S.; Song, J.Y.; Sun, Y.Z.; Wu, Q.; Wang, N.; Yao, H.; Steinmetz, A.; Chen, S.L. EST analysis reveals putative genes involved in glycyrrhizin biosynthesis. BMC Genom. 2010, 11, 268. [Google Scholar] [CrossRef]
- Jung, S.C.; Kim, W.; Park, S.C.; Jeong, J.; Park, M.K.; Lim, S.; Lee, Y.; Im, W.T.; Lee, J.H.; Choi, G.; et al. Two ginseng UDP-glycosyltransferases synthesize ginsenoside Rg3 and Rd. Plant Cell Physiol. 2014, 55, 2177–2188. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.A.; Cota, J.; Alvarez, T.M.; Bruchli, F.; Bragato, J.; Pereira, B.M.P.; Pauletti, B.A.; Jackson, G.; Pimenta, M.T.B.; Murakami, M.T.; et al. The Penicillium echinulatum Secretome on Sugar Cane Bagasse. PLoS ONE 2012, 7, e50571. [Google Scholar] [CrossRef] [PubMed]
- Liepman, A.H.; Wightman, R.; Geshi, N.; Turner, S.R.; Scheller, H.V. Arabidopsis—A powerful model system for plant cell wall research. Plant J. 2010, 61, 1107–1121. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.P.; Wang, D.; Cao, L.Y.; Sun, H.F. Transcriptome sequencing of Codonopsis pilosula and identification of candidate genes involved in Polysaccharide biosynthesis. PLoS ONE 2015, 10, e0117342. [Google Scholar] [CrossRef]
- Li, N.; Qian, W.; Wang, L.; Cao, H.; Hao, X.; Yang, Y.; Wang, X. Isolation and expression features of hexose kinase genes under various abiotic stresses in the tea plant (Camellia sinensis). J. Plant Physiol. 2017, 209, 95–104. [Google Scholar] [CrossRef]
- He, C.; Teixeira da Silva, J.A.; Wang, H.; Si, C.; Zhang, M.; Zhang, X.; Li, M.; Tan, J.; Duan, J. Mining MYB transcription factors from the genomes of orchids (Phalaenopsis and Dendrobium) and characterization of an orchid R2R3-MYB gene involved in water-soluble polysaccharide biosynthesis. Sci. Rep. 2019, 9, 13818. [Google Scholar] [CrossRef]
- Kato, N.; Dubouzet, E.; Kokabu, Y.; Yoshida, S.; Taniguchi, Y.; Dubouzet, J.G.; Yazaki, K.; Sato, F. Identification of a WRKY protein as a transcriptional regulator of benzylisoquinoline alkaloid biosynthesis in Coptis japonica. Plant Cell Physiol. 2007, 48, 8–18. [Google Scholar] [CrossRef]


| No. | Germplasms | Longitude (E) | Latitude (N) | Altitude (m) | Polysaccharide Content/% |
|---|---|---|---|---|---|
| 1 | Wenzhou City, Taishun country (WZTS) | 119.69 | 27.65 | 773 | 9.08 ± 0.84 bcd |
| 2 | Wenzhou City, yongjia country (WZYJ) | 120.76 | 28.51 | 724 | 8.14 ± 0.28 de |
| 3 | Wenzhou City, Yueqing country (WZYQ) | 121.06 | 28.39 | 88 | 8.33 ± 0.77 cde |
| 4 | Wenzhou City, Rui’an country (WZRA) | 120.34 | 27.83 | 268 | 7.35 ± 0.46 e |
| 5 | Lishui City, Longquan country (LSLQ) | 119.24 | 28.04 | 589 | 9.74 ± 0.46 b |
| 6 | Lishui City, Qingyuan country (LSQY) | 118.99 | 27.60 | 352 | 11.84 ± 1.18 a |
| 7 | Jinhua City, Pan’an country (JHPA) | 120.55 | 28.99 | 574 | 9.58 ± 0.56 b |
| 8 | Taizhou City, Tiantai country (TZTT) | 121.01 | 29.20 | 177 | 9.51 ± 0.46 bc |
| 9 | Hangzhou City, Chun’an country (HZCA) | 118.95 | 29.49 | 117 | 9.18 ± 0.52 bcd |
| 10 | Hangzhou City, Lin’an country (HZLA) | 119.45 | 30.32 | 436 | 7.18 ± 0.25 e |
| 11 | Quzhou City, Jiangshan country (QZJS) | 118.73 | 28.83 | 89 | 8.68 ± 0.80 bcd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, W.; Chen, J.; Duan, X.; Li, Y.; Tao, Z. Comparative Transcriptome Profiling Reveals Two WRKY Transcription Factors Positively Regulating Polysaccharide Biosynthesis in Polygonatum cyrtonema. Int. J. Mol. Sci. 2023, 24, 12943. https://doi.org/10.3390/ijms241612943
Jiang W, Chen J, Duan X, Li Y, Tao Z. Comparative Transcriptome Profiling Reveals Two WRKY Transcription Factors Positively Regulating Polysaccharide Biosynthesis in Polygonatum cyrtonema. International Journal of Molecular Sciences. 2023; 24(16):12943. https://doi.org/10.3390/ijms241612943
Chicago/Turabian StyleJiang, Wu, Jiadong Chen, Xiaojing Duan, Yaping Li, and Zhengming Tao. 2023. "Comparative Transcriptome Profiling Reveals Two WRKY Transcription Factors Positively Regulating Polysaccharide Biosynthesis in Polygonatum cyrtonema" International Journal of Molecular Sciences 24, no. 16: 12943. https://doi.org/10.3390/ijms241612943
APA StyleJiang, W., Chen, J., Duan, X., Li, Y., & Tao, Z. (2023). Comparative Transcriptome Profiling Reveals Two WRKY Transcription Factors Positively Regulating Polysaccharide Biosynthesis in Polygonatum cyrtonema. International Journal of Molecular Sciences, 24(16), 12943. https://doi.org/10.3390/ijms241612943





