Exploring the Impact of Endometrioma Aspiration and Dienogest Combination Therapy on Cyst Size, Inflammatory Cytokines in Follicular Fluid and Fertility Outcomes
Abstract
1. Introduction
2. Results
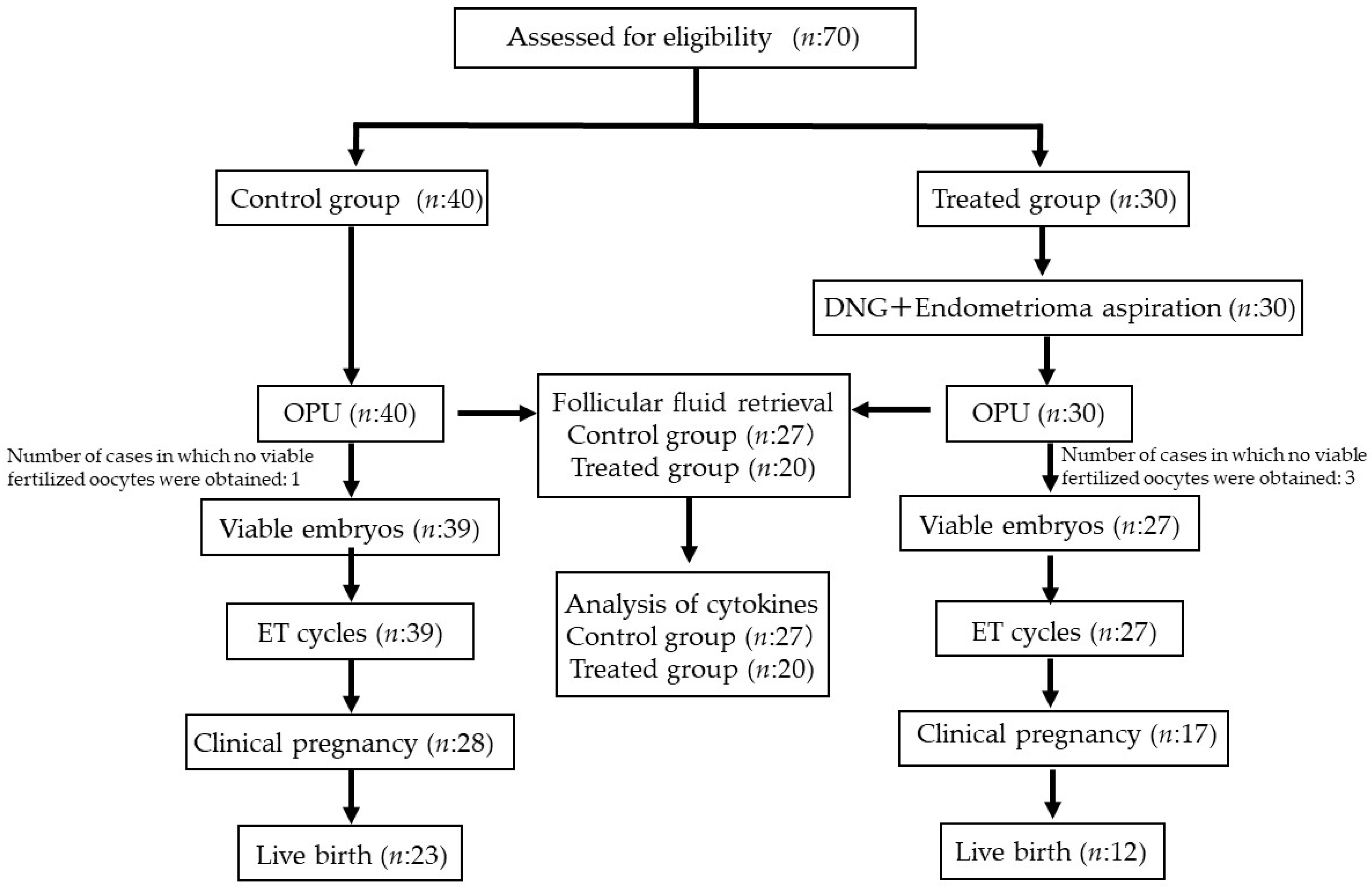
2.1. Patient Characteristics
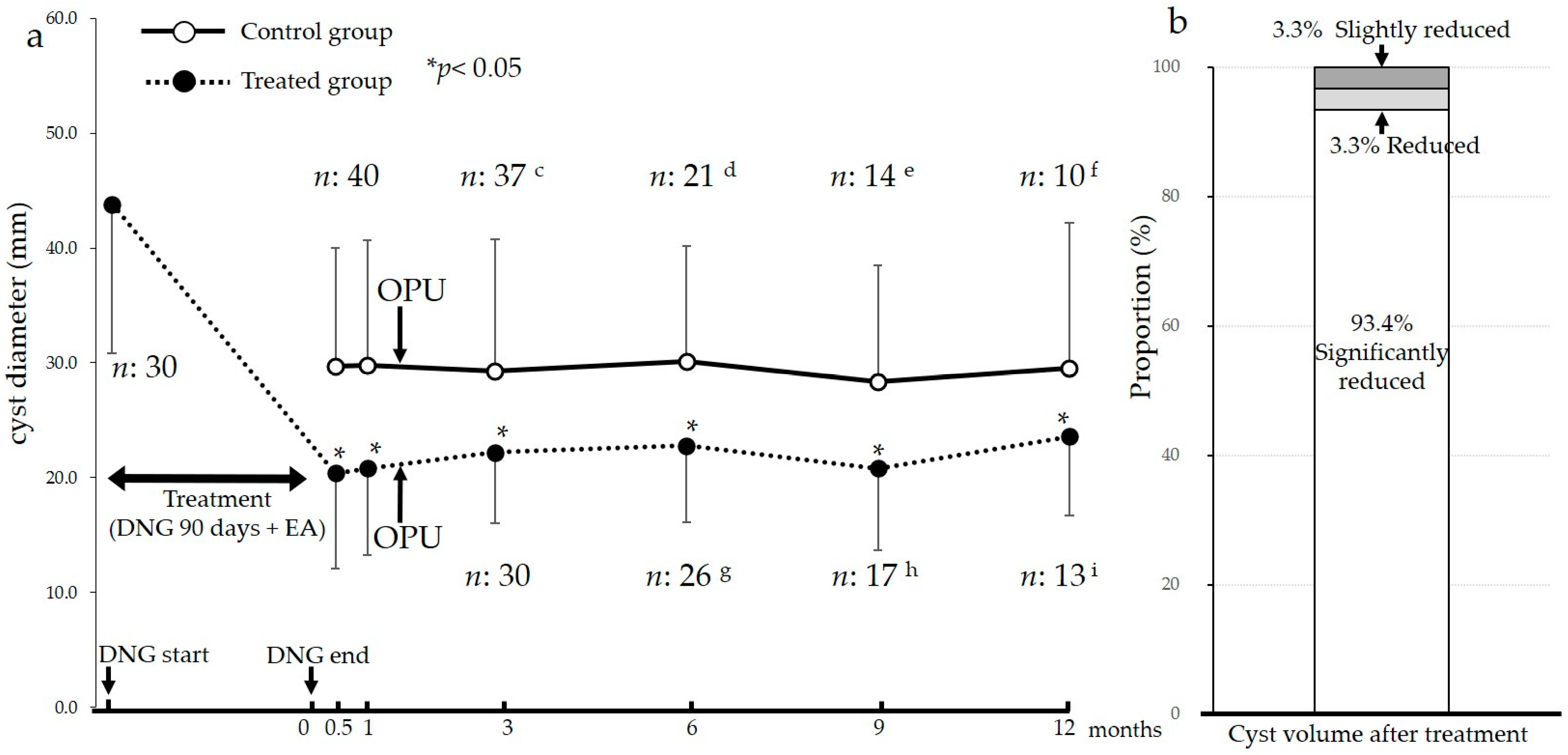
2.2. Effect on the Size of Endometriomas
2.3. Clinical Outcomes of IVF–ET in the Control and Treated Groups
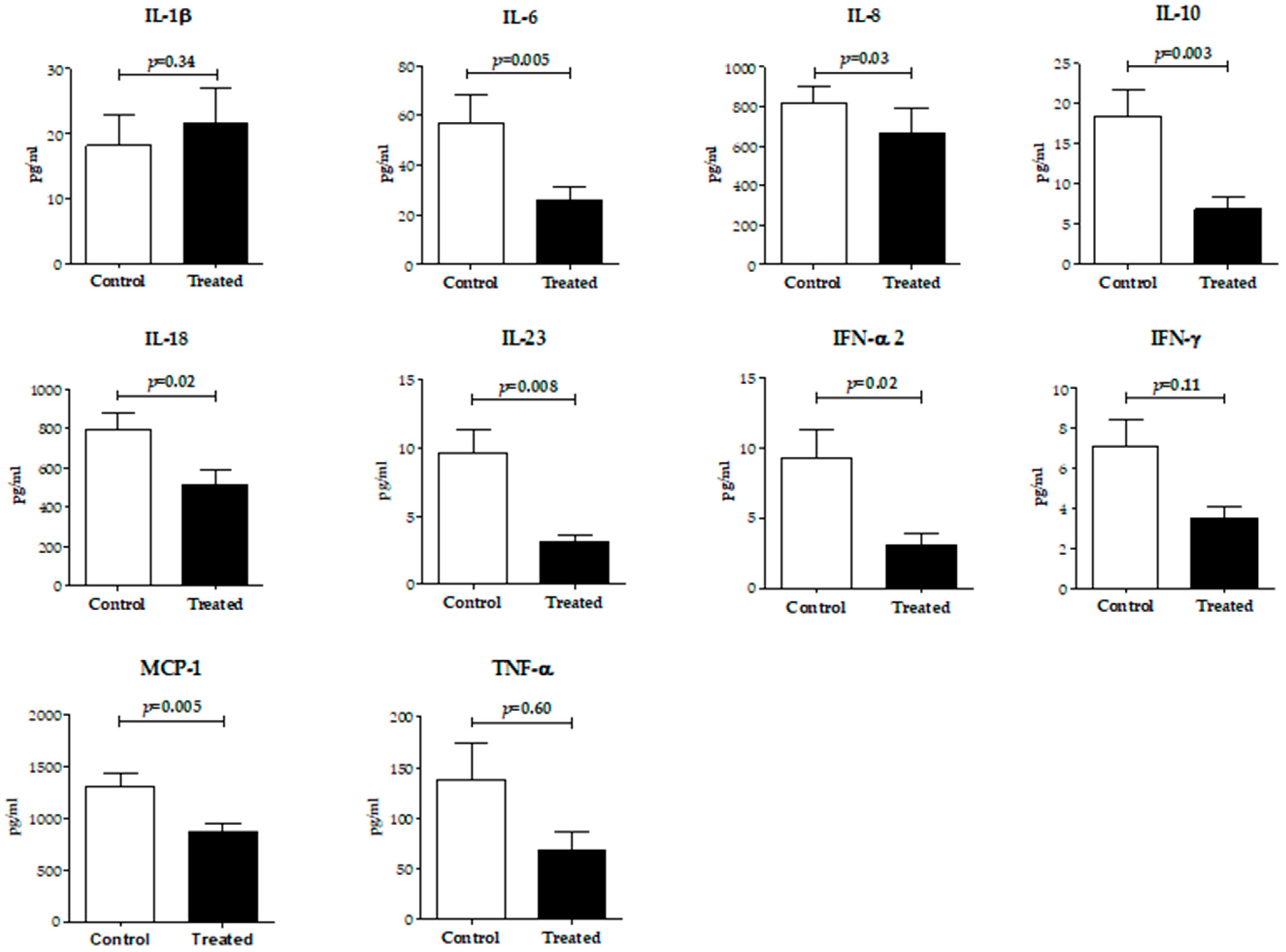
2.4. Inflammatory Cytokine Levels in Follicular Fluid
2.5. Effect of Time after Treatment on Clinical Outcomes
3. Discussion
4. Materials and Methods
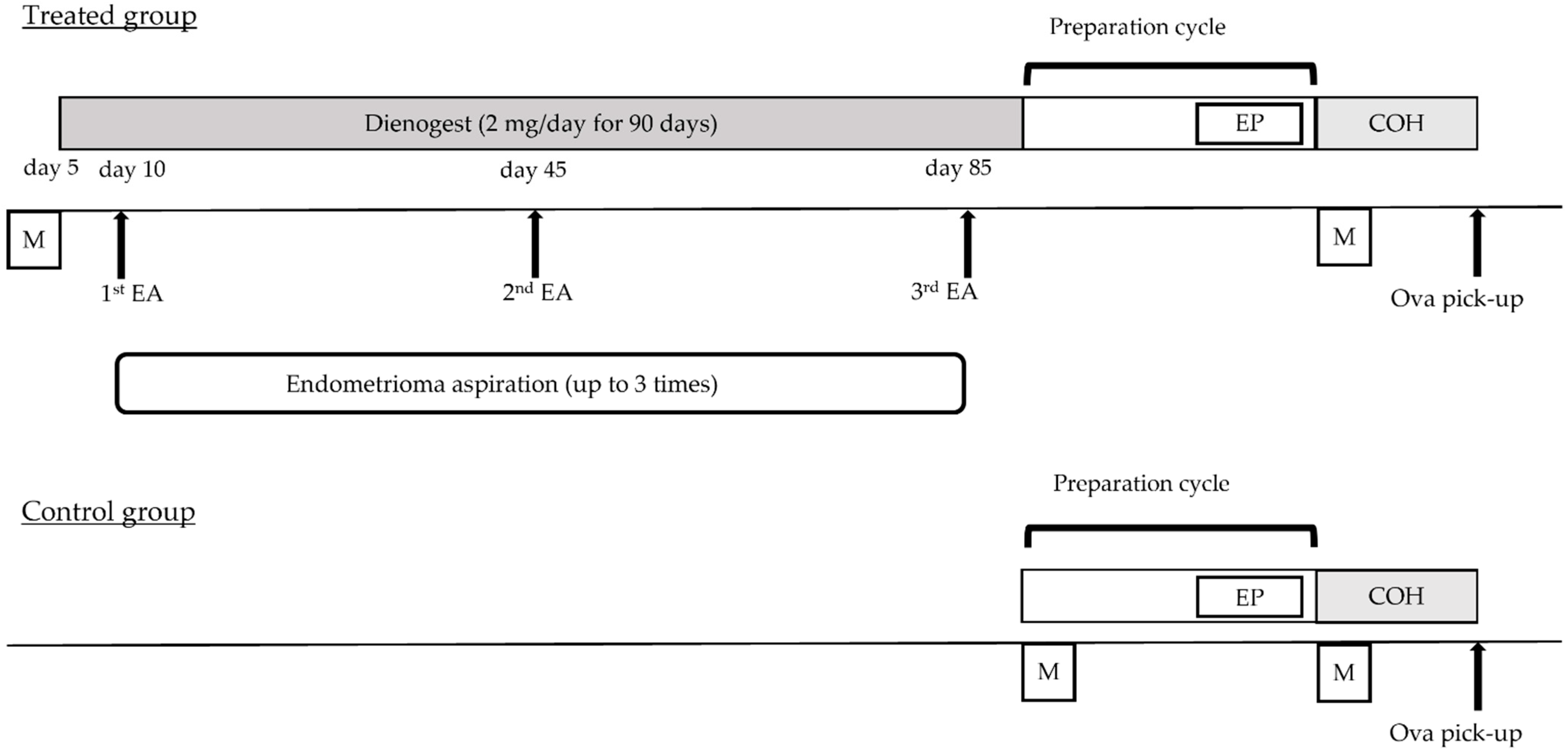
4.1. Study Design and Patient Selection
4.2. The Procedure of Endometrioma Aspiration Combined with DNG Therapy
4.3. COH, In Vitro Fertilization (IVF) and Embryo Transfer (ET)
4.4. Detection and Diagnosis of Endometriomas
4.5. Measurement of Endometrioma Size and Calculation of the Volume
4.6. Assessment Items
4.7. Collection of Follicular Fluid and Measurement of Cytokines and Chemokines
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giudice, L.C. Endometriosis. N. Engl. J. Med. 2010, 362, 2389–2398. [Google Scholar] [CrossRef] [PubMed]
- Benagiano, G.; Brosens, I. REVIEW. Hum. Reprod. 1991, 6, 963–968. [Google Scholar] [CrossRef] [PubMed]
- Redwine, D.B. Ovarian Endometriosis: A Marker for More Extensive Pelvic and Intestinal Disease. Fertil. Steril. 1999, 72, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, M.; Omar, S.Z.; Dunselman, G.; Cheong, Y. Influence of Endometriosis on Assisted Reproductive Technology Outcomes. Obstet. Gynecol. 2015, 125, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Viganò, P.; Somigliana, E.; Panina-Bordignon, P.; Vercellini, P.; Candiani, M. The Distinguishing Cellular and Molecular Features of the Endometriotic Ovarian Cyst: From Pathophysiology to the Potential Endometrioma-Mediated Damage to the Ovary. Hum. Reprod. Update 2014, 20, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Yland, J.; Carvalho, L.F.P.; Beste, M.; Bailey, A.; Thomas, C.; Abrão, M.S.; Racowsky, C.; Griffith, L.; Missmer, S.A. Endometrioma, the Follicular Fluid Inflammatory Network and Its Association with Oocyte and Embryo Characteristics. Reprod. Biomed. Online 2020, 40, 399–408. [Google Scholar] [CrossRef]
- Kitajima, M.; Defrère, S.; Dolmans, M.-M.; Colette, S.; Squifflet, J.; Van Langendonckt, A.; Donnez, J. Endometriomas as a Possible Cause of Reduced Ovarian Reserve in Women with Endometriosis. Fertil. Steril. 2011, 96, 685–691. [Google Scholar] [CrossRef]
- Ferrero, H.; Corachán, A.; Aguilar, A.; Quiñonero, A.; Carbajo-García, M.C.; Alamá, P.; Tejera, A.; Taboas, E.; Muñoz, E.; Pellicer, A.; et al. Single-Cell RNA Sequencing of Oocytes from Ovarian Endometriosis Patients Reveals a Differential Transcriptomic Profile Associated with Lower Quality. Hum. Reprod. 2019, 34, 1302–1312. [Google Scholar] [CrossRef]
- Bulun, S.E.; Cheng, Y.-H.; Yin, P.; Imir, G.; Utsunomiya, H.; Attar, E.; Innes, J.; Julie Kim, J. Progesterone Resistance in Endometriosis: Link to Failure to Metabolize Estradiol. Mol. Cell. Endocrinol. 2006, 248, 94–103. [Google Scholar] [CrossRef]
- Minici, F.; Tiberi, F.; Tropea, A.; Orlando, M.; Gangale, M.F.; Romani, F.; Campo, S.; Bompiani, A.; Lanzone, A.; Apa, R. Endometriosis and Human Infertility: A New Investigation into the Role of Eutopic Endometrium. Hum. Reprod. 2008, 23, 530–537. [Google Scholar] [CrossRef][Green Version]
- Benschop, L.; Farquhar, C.; van der Poel, N.; Heineman, M.J. Interventions for Women with Endometrioma Prior to Assisted Reproductive Technology. Cochrane Database Syst. Rev. 2010, 11, CD008571. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Sumimoto, K.; Moniwa, N.; Imai, M.; Takakura, K.; Kuromaki, T.; Morioka, E.; Arisawa, K.; Terao, T. Risk of Developing Ovarian Cancer among Women with Ovarian Endometrioma: A Cohort Study in Shizuoka, Japan. Int. J. Gynecol. Cancer 2007, 17, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Younis, J.S.; Shapso, N.; Fleming, R.; Ben-Shlomo, I.; Izhaki, I. Impact of Unilateral versus Bilateral Ovarian Endometriotic Cystectomy on Ovarian Reserve: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2019, 25, 375–391. [Google Scholar] [CrossRef]
- Busacca, M.; Riparini, J.; Somigliana, E.; Oggioni, G.; Izzo, S.; Vignali, M.; Candiani, M. Postsurgical Ovarian Failure after Laparoscopic Excision of Bilateral Endometriomas. Am. J. Obstet. Gynecol. 2006, 195, 421–425. [Google Scholar] [CrossRef]
- Kasapoglu, I.; Ata, B.; Uyaniklar, O.; Seyhan, A.; Orhan, A.; Yildiz Oguz, S.; Uncu, G. Endometrioma-Related Reduction in Ovarian Reserve (ERROR): A Prospective Longitudinal Study. Fertil. Steril. 2018, 110, 122–127. [Google Scholar] [CrossRef]
- Calagna, G.; Della Corte, L.; Giampaolino, P.; Maranto, M.; Perino, A. Endometriosis and Strategies of Fertility Preservation: A Systematic Review of the Literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 254, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Kumar, S.; Kumar, A.; Verma, A. Ultrasound Guided Aspiration of Endometrioma—A New Therapeutic Modality to Improve Reproductive Outcome. Int. J. Gynecol. Obstet. 1999, 65, 17–23. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, Y.; Xu, X.; Lu, S.; Yan, L. Effects of Ovarian Endometrioma Aspiration on in Vitro Fertilization-Intracytoplasmic Sperm Injection and Embryo Transfer Outcomes: A Systematic Review and Meta-Analysis. Arch. Gynecol. Obstet. 2022, 306, 17–28. [Google Scholar] [CrossRef]
- Guo, Y.; Lu, N.; Zhang, Y.; Su, Y.; Wang, Y.; Zhang, Y.; Sun, Y. Comparative Study on the Pregnancy Outcomes of in Vitro Fertilization–Embryo Transfer between Long-Acting Gonadotropin-Releasing Hormone Agonist Combined with Transvaginal Ultrasound-Guided Cyst Aspiration and Long-Acting Gonadotropin-Releasing Hormone Agonist Alone. Contemp. Clin. Trials 2012, 33, 1206–1210. [Google Scholar] [CrossRef]
- Zhu, W.; Tan, Z.; Fu, Z.; Li, X.; Chen, X.; Zhou, Y. Repeat Transvaginal Ultrasound-Guided Aspiration of Ovarian Endometrioma in Infertile Women with Endometriosis. Am. J. Obstet. Gynecol. 2011, 204, 61.e1–61.e6. [Google Scholar] [CrossRef]
- Surrey, E.S.; Silverberg, K.M.; Surrey, M.W.; Schoolcraft, W.B. Effect of Prolonged Gonadotropin-Releasing Hormone Agonist Therapy on the Outcome of in Vitro Fertilization-Embryo Transfer in Patients with Endometriosis. Fertil. Steril. 2002, 78, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.M.; Rashwan, H.; Mahmoud, M.; El-Mazny, A.; Farouk, M.; Belal, D.S.; Marie, H.M. Effect of Prolonged GnRH Agonist Downregulation on ICSI Outcome in Patients With Endometriomas of Less Than 5 Cm: A Randomized Controlled Trial. Reprod. Sci. 2018, 25, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Barra, F.; Laganà, A.S.; Scala, C.; Garzon, S.; Ghezzi, F.; Ferrero, S. Pretreatment with Dienogest in Women with Endometriosis Undergoing IVF after a Previous Failed Cycle. Reprod. Biomed. Online 2020, 41, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, E.X.; Melo, P.; Baker, P.E.; Sallam, H.N.; Arici, A.; Garcia-Velasco, J.A.; Abou-Setta, A.M.; Becker, C.; Granne, I.E. Long-Term GnRH Agonist Therapy before in Vitro Fertilisation (IVF) for Improving Fertility Outcomes in Women with Endometriosis. Cochrane Database Syst. Rev. 2019, 2019, CD013240. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, J.; Zhang, L.; Liu, Y. Pretreatment of Dienogest for Women with Endometriosis in in Vitro Fertilization: A Systematic Review and Meta-Analysis. Gynecol. Obstet. Investig. 2023, 88, 135–142. [Google Scholar] [CrossRef]
- Hayashi, A.; Tanabe, A.; Kawabe, S.; Hayashi, M.; Yuguchi, H.; Yamashita, Y.; Okuda, K.; Ohmichi, M. Dienogest Increases the Progesterone Receptor Isoform B/A Ratio in Patients with Ovarian Endometriosis. J. Ovarian Res. 2012, 5, 31. [Google Scholar] [CrossRef]
- Alviggi, C.; Andersen, C.Y.; Buehler, K.; Conforti, A.; De Placido, G.; Esteves, S.C.; Fischer, R.; Galliano, D.; Polyzos, N.P.; Sunkara, S.K.; et al. A New More Detailed Stratification of Low Responders to Ovarian Stimulation: From a Poor Ovarian Response to a Low Prognosis Concept. Fertil. Steril. 2016, 105, 1452–1453. [Google Scholar] [CrossRef]
- Momoeda, M.; Harada, T.; Terakawa, N.; Aso, T.; Fukunaga, M.; Hagino, H.; Taketani, Y. Long-Term Use of Dienogest for the Treatment of Endometriosis. J. Obstet. Gynaecol. Res. 2009, 35, 1069–1076. [Google Scholar] [CrossRef]
- Fisch, J.D.; Sher, G. Sclerotherapy with 5% Tetracycline Is a Simple Alternative to Potentially Complex Surgical Treatment of Ovarian Endometriomas before in Vitro Fertilization. Fertil. Steril. 2004, 82, 437–441. [Google Scholar] [CrossRef]
- Hsieh, C.-L.; Shiau, C.-S.; Lo, L.-M.; Hsieh, T.-T.; Chang, M.-Y. Effectiveness of Ultrasound-Guided Aspiration and Sclerotherapy with 95% Ethanol for Treatment of Recurrent Ovarian Endometriomas. Fertil. Steril. 2009, 91, 2709–2713. [Google Scholar] [CrossRef]
- Stern, R.C.; Dash, R.; Bentley, R.C.; Snyder, M.J.; Haney, A.F.; Robboy, S.J. Malignancy in Endometriosis: Frequency and Comparison of Ovarian and Extraovarian Types. Int. J. Gynecol. Pathol. 2001, 20, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Iwabe, T.; Terakawa, N. Role of Cytokines in Endometriosis. Fertil. Steril. 2001, 76, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Goldberg, J.M.; Aziz, N.; Goldberg, E.; Krajcir, N.; Agarwal, A. Pathogenic Mechanisms in Endometriosis-Associated Infertility. Fertil. Steril. 2008, 90, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, C.G.; Genro, V.K.; Souza, C.A.; Michelon, T.; Bilibio, J.P.; Scheffel, C.; Cunha-Filho, J.S. T Helper (Th)1, Th2, and Th17 Interleukin Pathways in Infertile Patients with Minimal/Mild Endometriosis. Fertil. Steril. 2011, 95, 2477–2480. [Google Scholar] [CrossRef] [PubMed]
- Malutan, A.M.; Drugan, T.; Costin, N.; Ciortea, R.; Bucuri, C.; Rada, M.P.; Mihu, D. Clinical Immunology Pro-Inflammatory Cytokines for Evaluation of Inflammatory Status in Endometriosis. Cent. Eur. J. Immunol. 2015, 1, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Bersinger, N.A.; Mueller, M.D.; von Wolff, M. Intrafollicular Inflammatory Cytokines but Not Steroid Hormone Concentrations Are Increased in Naturally Matured Follicles of Women with Proven Endometriosis. J. Assist. Reprod. Genet. 2017, 34, 357–364. [Google Scholar] [CrossRef]
- Tamura, H.; Yoshida, H.; Kikuchi, H.; Josaki, M.; Mihara, Y.; Shirafuta, Y.; Shinagawa, M.; Tamura, I.; Taketani, T.; Takasaki, A.; et al. The Clinical Outcome of Dienogest Treatment Followed by in Vitro Fertilization and Embryo Transfer in Infertile Women with Endometriosis. J. Ovarian Res. 2019, 12, 123. [Google Scholar] [CrossRef]
- Sasagawa, S.; Shimizu, Y.; Nagaoka, T.; Tokado, H.; Imada, K.; Mizuguchi, K. Dienogest, a Selective Progestin, Reduces Plasma Estradiol Level through Induction of Apoptosis of Granulosa Cells in the Ovarian Dominant Follicle without Follicle-Stimulating Hormone Suppression in Monkeys. J. Endocrinol. Investig. 2008, 31, 636–641. [Google Scholar] [CrossRef]
- Zheng, L.; Kimura, F.; Wu, D.; Morimune, A.; Niwa, Y.; Mita, S.; Takahashi, K.; Murakami, T. Dienogest Suppresses the Activation of Primordial Follicles and Preserves the Primordial Follicle Stockpile for Fertility in Mice. Reprod. Biomed. Online 2018, 36, 371–379. [Google Scholar] [CrossRef]
- VEECK, L.L. Oocyte Assessment and Biological Performance. Ann. N. Y. Acad. Sci. 1988, 541, 259–274. [Google Scholar] [CrossRef]
- Gardner, D.K.; Lane, M.; Stevens, J.; Schlenker, T.; Schoolcraft, W.B. Blastocyst Score Affects Implantation and Pregnancy Outcome: Towards a Single Blastocyst Transfer. Fertil. Steril. 2000, 73, 1155–1158. [Google Scholar] [CrossRef] [PubMed]
| Control (n = 40) | Treated (n = 30) | p Value | |
|---|---|---|---|
| Age (years) | 33.7 ± 4.0 | 33.0 ± 4.0 | 0.519 |
| BMI (kg/m2) | 21.1 ± 2.7 | 20.4 ± 2.3 | 0.256 |
| AMH (ng/mL) | 3.1 ± 2.1 | 2.9 ± 1.9 | 0.565 |
| Unilateral endometriomas (%) | 70.0 (28/40) | 56.7 (17/30) | 0.261 |
| Bilateral endometriomas (%) | 30.0 (12/40) | 43.3 (13/30) | 0.261 |
| Largest endometrioma diameter b (mm) | 29.8 ± 10.3 | 43.8 ± 13.0 | <0.001 |
| Antagonist protocol (n) | 13 | 6 | |
| Short protocol (n) | 8 | 12 | |
| Long protocol (n) | 18 | 12 | |
| Primary infertility (%) | 70.0 (28/40) | 86.7 (26/30) | 0.100 |
| Duration of infertility (months) | 22.7 ± 23.0 | 29.0 ± 28.1 | 0.142 |
| History of ovarian operation c (%) | 7.5 (3/40) | 10.0 (3/30) | 0.712 |
| Duration from the surgery to COH (months) | 39.0 ± 12.8 | 44.6 ± 22.2 | 0.775 |
| Control (n = 40) | Treated (n = 30) | p | |
|---|---|---|---|
| Gonadotropin (FSH) dose (IU) | 2124 ± 706.2 | 1982 ± 480.1 | 0.332 |
| Gonadotropin (FSH) duration (day) | 9.5 ± 1.7 | 9.0 ± 1.9 | 0.217 |
| Ovarian follicle ≥ 15 mm (n) | 10.5 ± 6.4 (420) | 6.6 ± 3.7 (197) | 0.002 |
| E2 on the trigger day (ng/mL) | 2564 ± 1232 | 1948 ± 1072 | 0.027 |
| Oocytes retrieved (n) | 14.0 ± 9.6 (561) | 10.5 ± 7.2 (316) | 0.091 |
| Mature oocytes (n) | 11.5 ± 7.4 (459) | 8.1 ± 6.2 (244) | 0.046 |
| Maturation rate (%) | 81.8 (459/561) | 78.0 (244/316) | 0.055 |
| Fertilized oocytes (n) | 9.0 ± 6.6 (360) | 6.5 ± 5.3 (196) | 0.093 |
| Fertilization rate (%) | 78.4 (360/459) | 80.3 (196/244) | 0.930 |
| Fertilization rate (IVF) (%) | 71.6 (101/141) | 79.4 (77/97) | 0.081 |
| Fertilization rate (ICSI) (%) | 81.4 (259/318) | 80.9 (119/147) | 0.381 |
| Blastocysts (n) | 5.2 ± 4.7 | 3.6 ± 4.1 | 0.112 |
| Blastulation rate (%) | 57.8 (208/360) | 54.6 (107/196) | 0.751 |
| Morphologically good blastocysts (n) | 2.9 ± 3.1 | 2.3 ± 3.3 | 0.409 |
| Morphologically good blastocyst rate (%) | 32.5 (117/360) | 34.7 (68/196) | 0.620 |
| ET cancellation rate (%) | 2.5 (1/40) | 10.0 (3/30) | 0.182 |
| Embryos transferred (n) | 80 | 51 | |
| Fresh embryo (n) | 30.0 (24/80) | 31.4 (16/51) | 0.868 |
| Frozen thawed embryo (n) | 70.0 (56/80) | 68.6 (35/51) | |
| Gestational sacs (n) | 30 | 19 | |
| Fresh embryo (n) | 20.0 (6/30) | 42.1 (8/19) | 0.095 |
| Frozen thawed embryo (n) | 80.0 (24/30) | 57.8 (11/19) | |
| Implantation rate (%) | 37.5 (30/80) | 37.3 (19/51) | 0.978 |
| Pregnancy rate (%) | 70.0 (28/40) | 56.7 (17/30) | 0.250 |
| Live birth rate (%) | 57.5 (23/40) | 40.0 (12/30) | 0.147 |
| Abortion rate (%) | 20.0 (6/30) | 31.6 (6/19) | 0.358 |
| Ectopic pregnancy (%) | 3.3(1/30) | 0 (0/19) | 0.421 |
| Treated Group (n = 9) | |||
|---|---|---|---|
| 1st OPU b | 2nd OPU c | p | |
| Antral follicle count (n) | 4.8 ± 2.8 (43) | 8.7 ± 4.7 (78) | 0.014 |
| Gonadotropin (FSH) dose (IU) | 2161 ± 510.6 | 2433 ± 571.3 | 0.135 |
| Gonadotropin (FSH) duration (day) | 9.0 ± 2.2 | 10.4 ± 2.4 | 0.056 |
| Ovarian follicle ≥ 15 mm (n) | 3.6 ± 1.3 (32) | 7.8 ± 3.4 (70) | 0.018 |
| E2 on the trigger day (ng/mL) | 1333 ± 552.8 | 2289 ± 803.0 | 0.034 |
| Oocytes retrieved (n) | 4.7 ± 2.2 (42) | 9.3 ± 3.0 (84) | 0.005 |
| Mature oocytes (n) | 3.7 ± 2.4 (33) | 7.0 ± 3.9 (63) | 0.038 |
| Maturation rate (%) | 78.6 (33/42) | 75.0 (63/84) | 0.582 |
| Fertilized oocytes (n) | 1.8 ± 1.7 (16) | 5.6 ± 4.1 (50) | 0.021 |
| Fertilization rate (%) | 48.5 (16/33) | 79.4 (50/63) | 0.054 |
| Cleavage rate (%) | 100 (16/16) | 100 (50/50) | |
| Good embryos (n) d | 0 | 20 | |
| Blastocysts (n) | 7 | 8 | |
| ET cancellation rate (%) | 33.3 (3/9) | 11.1 (1/9) | 0.576 |
| Embryos transferred (n) | 8 | 15 | |
| Gestational sacs (n) | 2 | 6 | |
| Implantation rate (%) | 25.0 (2/8) | 40.0 (6/15) | 0.657 |
| Pregnancy rate (%) | 22.2 (2/9) | 55.6 (5/9) | 0.335 |
| Live birth rate (%) | 0 (0/9) | 33.3 (3/9) | 0.206 |
| Abortion rate (%) | 100 (2/2) | 50.0 (3/6) | 0.464 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shigeta, M.; Tsuji, I.; Hashimoto, S.; Kankanam Gamage, U.S.; Yamanaka, M.; Fukuda, A.; Morimoto, Y.; Tachibana, D. Exploring the Impact of Endometrioma Aspiration and Dienogest Combination Therapy on Cyst Size, Inflammatory Cytokines in Follicular Fluid and Fertility Outcomes. Int. J. Mol. Sci. 2023, 24, 12891. https://doi.org/10.3390/ijms241612891
Shigeta M, Tsuji I, Hashimoto S, Kankanam Gamage US, Yamanaka M, Fukuda A, Morimoto Y, Tachibana D. Exploring the Impact of Endometrioma Aspiration and Dienogest Combination Therapy on Cyst Size, Inflammatory Cytokines in Follicular Fluid and Fertility Outcomes. International Journal of Molecular Sciences. 2023; 24(16):12891. https://doi.org/10.3390/ijms241612891
Chicago/Turabian StyleShigeta, Mamoru, Isao Tsuji, Shu Hashimoto, Udayanga Sanath Kankanam Gamage, Masaya Yamanaka, Aisaku Fukuda, Yoshiharu Morimoto, and Daisuke Tachibana. 2023. "Exploring the Impact of Endometrioma Aspiration and Dienogest Combination Therapy on Cyst Size, Inflammatory Cytokines in Follicular Fluid and Fertility Outcomes" International Journal of Molecular Sciences 24, no. 16: 12891. https://doi.org/10.3390/ijms241612891
APA StyleShigeta, M., Tsuji, I., Hashimoto, S., Kankanam Gamage, U. S., Yamanaka, M., Fukuda, A., Morimoto, Y., & Tachibana, D. (2023). Exploring the Impact of Endometrioma Aspiration and Dienogest Combination Therapy on Cyst Size, Inflammatory Cytokines in Follicular Fluid and Fertility Outcomes. International Journal of Molecular Sciences, 24(16), 12891. https://doi.org/10.3390/ijms241612891








