A Lifelong Impact on Endometriosis: Pathophysiology and Pharmacological Treatment
Abstract
1. Introduction
2. The Pathophysiology of Endometriosis
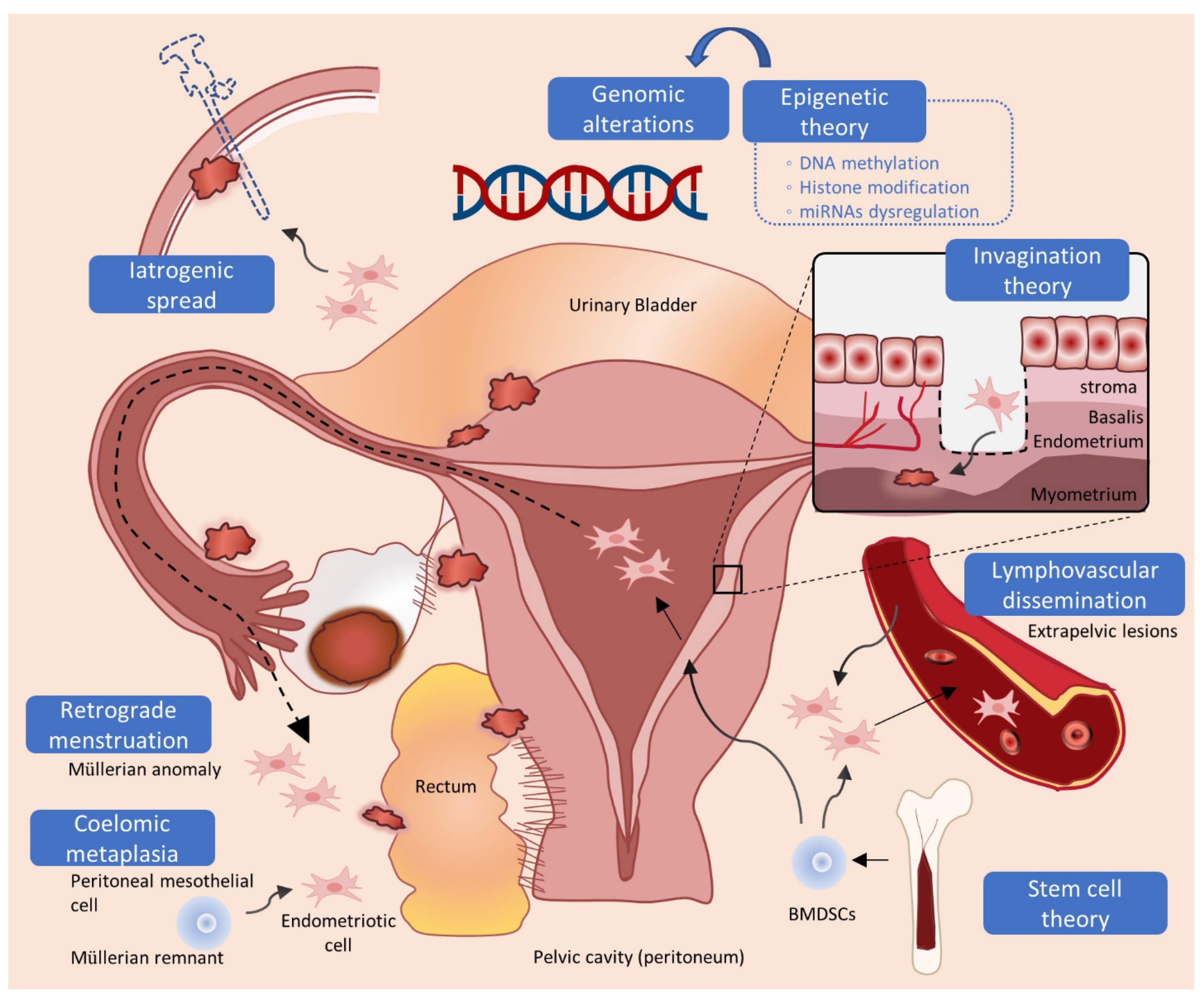
2.1. Retrograde Menstruation, Coelomic Metaplasia, and Müllerian Remnants Theory
2.2. Circular Dissemination and Stem Cell Theory
2.3. Invagination Theory in Adenomyosis
2.4. Epigenomic and Genomic Alterations
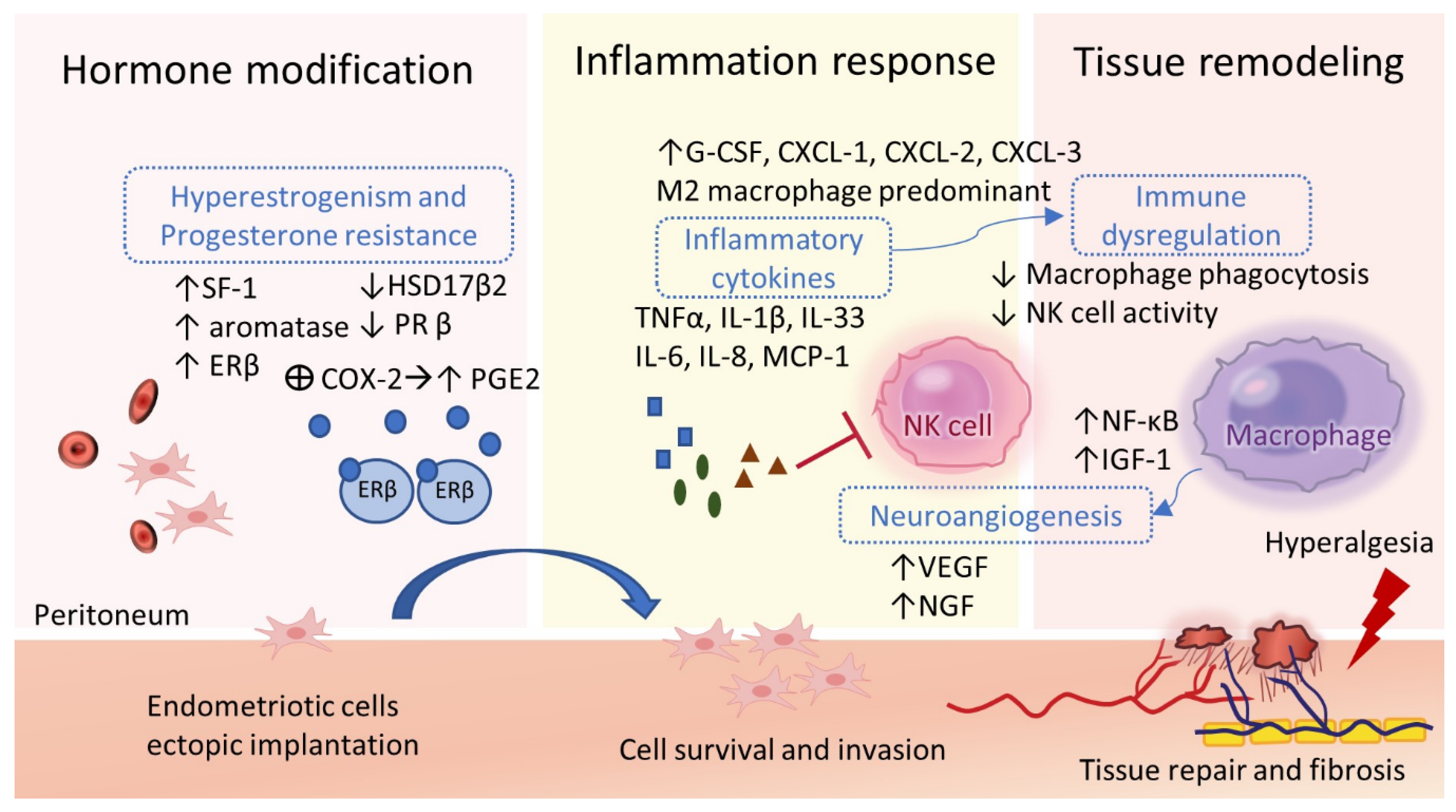
2.5. Estrogen and Progesterone Modulation
2.6. Inflammation, Angiogenesis, and Tissue Remodeling
2.7. Immune Dysregulations
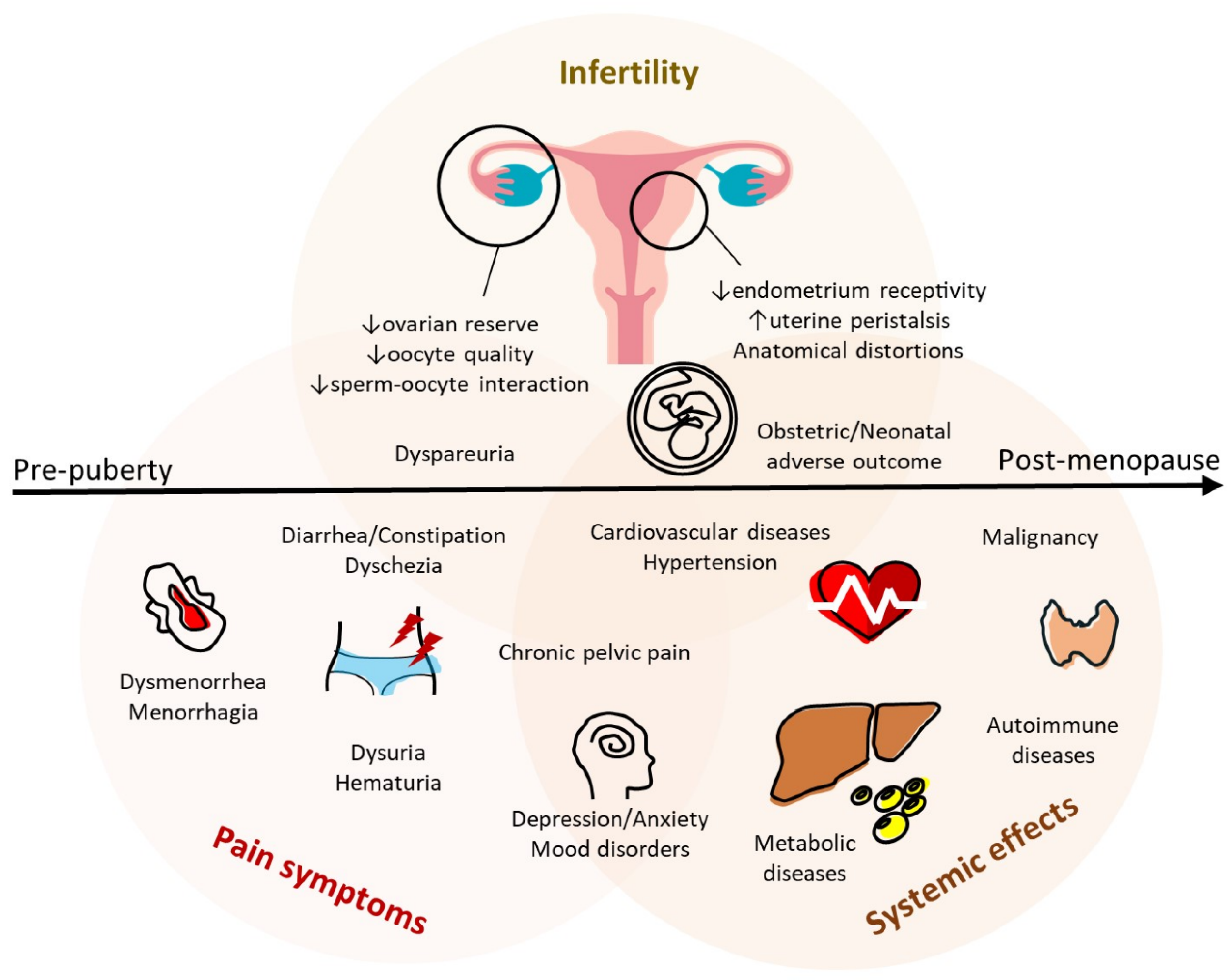
3. Clinical Features of Endometriosis and Its Lifelong Impacts
3.1. Menstrual Disorders in Adenomyosis
3.2. Endometriosis-Associated Symptoms
3.3. Endometriosis-Associated Infertility
3.4. Endometriosis-Associated Obstetric Complications
3.5. Malignancy Potential
3.6. Long-Term Systemic Diseases
4. Pharmacologic Therapies in Current Clinical Practice
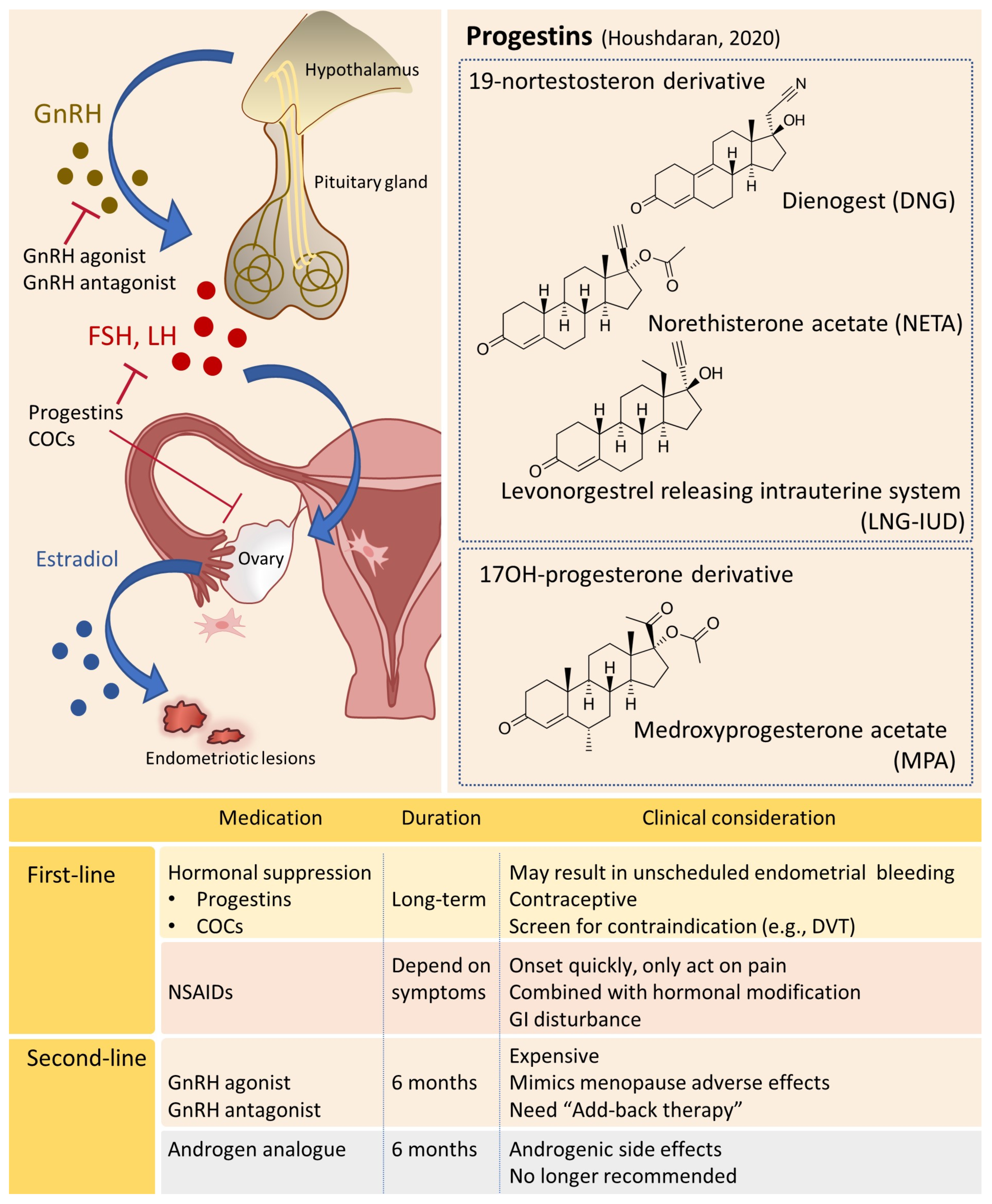
4.1. Hormonal Manipulation
4.1.1. Progestin-Based Therapies
4.1.2. GnRH Agonists
4.1.3. GnRH Antagonists
4.1.4. Other Potential Hormonal Drugs
4.2. Analgesics and Neuromodulators
4.3. Targeting on Inflammation, Angiogenesis, and Immunomodulators
4.4. Other Complementary Therapies
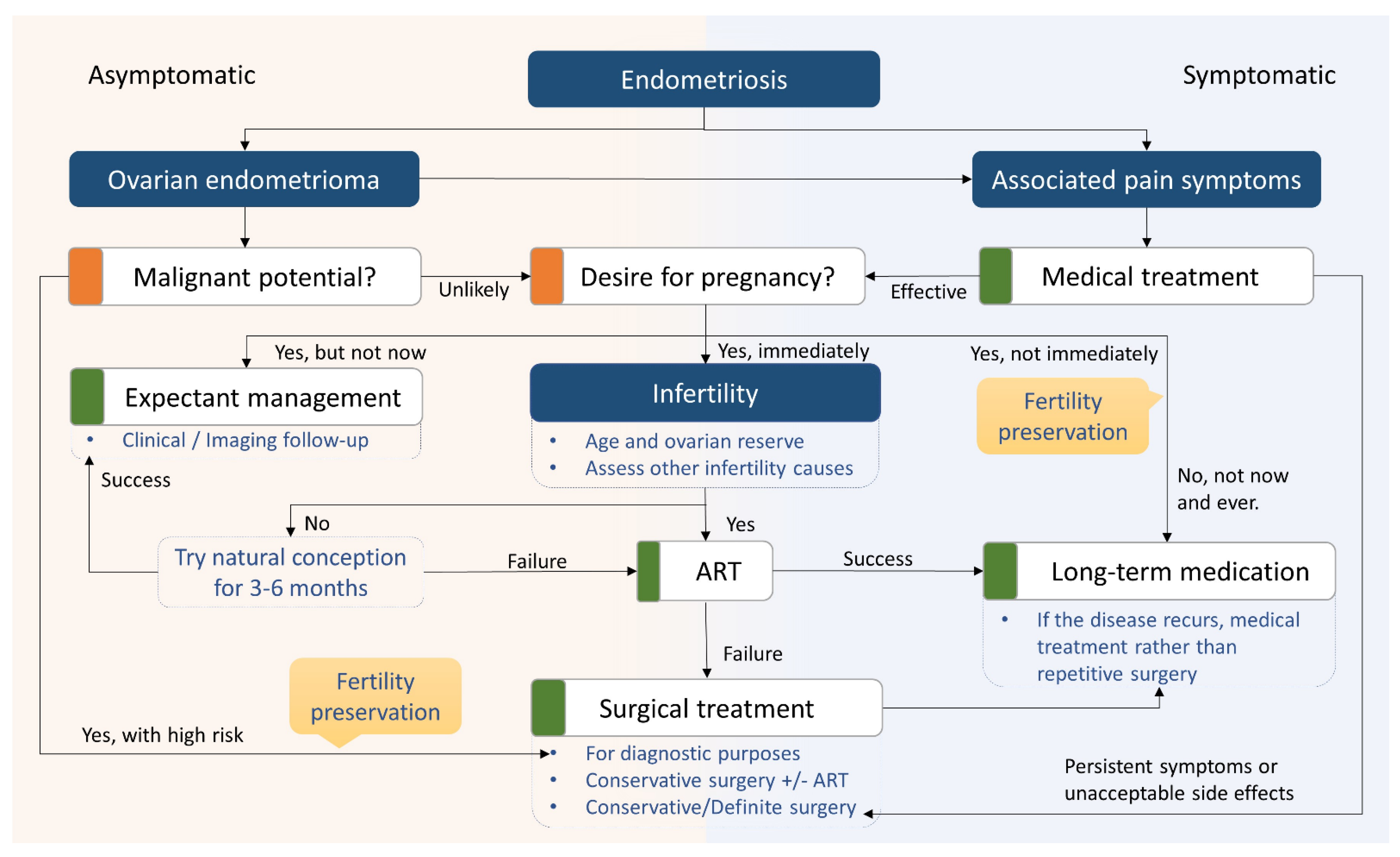
5. Surgical Considerations
6. Management of Endometriosis-Related Infertility
6.1. Fertility Treatments and Assisted Reproductive Technology (ART)
6.2. Fertility Preservation in Patients with Endometriosis
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 382, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Meuleman, C.; Vandenabeele, B.; Fieuws, S.; Spiessens, C.; Timmerman, D.; D’Hooghe, T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil. Steril. 2009, 92, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Kvaskoff, M.; Mu, F.; Terry, K.L.; Harris, H.R.; Poole, E.M.; Farland, L.; Missmer, S.A. Endometriosis: A high-risk population for major chronic diseases? Hum. Reprod. Update 2015, 21, 500–516. [Google Scholar] [CrossRef]
- Horne, A.W.; Saunders, P.T.K.; Abokhrais, I.M.; Hogg, L.; on behalf of theEndometriosis Priority Setting Partnership Steering Group. Top ten endometriosis research priorities in the UK and Ireland. Lancet 2017, 389, 2191–2192. [Google Scholar] [CrossRef]
- Rizner, T.L. Noninvasive biomarkers of endometriosis: Myth or reality? Expert Rev. Mol. Diagn. 2014, 14, 365–385. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, L.; Ayansina, D.; Cooper, K.G.; Bhattacharya, S.; Horne, A.W.; Bhattacharya, S. Impact of endometriosis on risk of further gynaecological surgery and cancer: A national cohort study. BJOG 2018, 125, 64–72. [Google Scholar] [CrossRef]
- Rogers, P.A.; Adamson, G.D.; Al-Jefout, M.; Becker, C.M.; D’Hooghe, T.M.; Dunselman, G.A.; Fazleabas, A.; Giudice, L.C.; Horne, A.W.; Hull, M.L.; et al. Research Priorities for Endometriosis. Reprod. Sci. 2017, 24, 202–226. [Google Scholar] [CrossRef]
- Sampson, J.A. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93–143. [Google Scholar]
- Gibson, D.A.; Simitsidellis, I.; Collins, F.; Saunders, P.T.K. Androgens, oestrogens and endometrium: A fine balance between perfection and pathology. J. Endocrinol. 2020, 246, R75–R93. [Google Scholar] [CrossRef]
- Wang, W.; Vilella, F.; Alama, P.; Moreno, I.; Mignardi, M.; Isakova, A.; Pan, W.; Simon, C.; Quake, S.R. Single-cell transcriptomic atlas of the human endometrium during the menstrual cycle. Nat. Med. 2020, 26, 1644–1653. [Google Scholar] [CrossRef]
- Signorile, P.G.; Baldi, F.; Bussani, R.; D’Armiento, M.; De Falco, M.; Baldi, A. Ectopic endometrium in human foetuses is a common event and sustains the theory of mullerianosis in the pathogenesis of endometriosis, a disease that predisposes to cancer. J. Exp. Clin. Cancer Res. 2009, 28, 49. [Google Scholar] [CrossRef]
- Batt, R.E.; Mitwally, M.F. Endometriosis from thelarche to midteens: Pathogenesis and prognosis, prevention and pedagogy. J. Pediatr. Adolesc. Gynecol. 2003, 16, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Tandoi, I.; Somigliana, E.; Riparini, J.; Ronzoni, S.; Vigano, P.; Candiani, M. High rate of endometriosis recurrence in young women. J. Pediatr. Adolesc. Gynecol. 2011, 24, 376–379. [Google Scholar] [CrossRef]
- Vinatier, D.; Orazi, G.; Cosson, M.; Dufour, P. Theories of endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 96, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Samani, E.N.; Mamillapalli, R.; Li, F.; Mutlu, L.; Hufnagel, D.; Krikun, G.; Taylor, H.S. Micrometastasis of endometriosis to distant organs in a murine model. Oncotarget 2019, 10, 2282–2291. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA 2004, 292, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Gargett, C.E.; Schwab, K.E.; Deane, J.A. Endometrial stem/progenitor cells: The first 10 years. Hum. Reprod. Update 2016, 22, 137–163. [Google Scholar] [CrossRef]
- Leyendecker, G.; Herbertz, M.; Kunz, G.; Mall, G. Endometriosis results from the dislocation of basal endometrium. Hum. Reprod. 2002, 17, 2725–2736. [Google Scholar] [CrossRef]
- Figueira, P.G.; Abrao, M.S.; Krikun, G.; Taylor, H.S. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann. N. Y. Acad. Sci. 2011, 1221, 10–17. [Google Scholar] [CrossRef]
- Du, H.; Taylor, H.S. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells 2007, 25, 2082–2086. [Google Scholar] [CrossRef]
- Bergeron, C.; Amant, F.; Ferenczy, A. Pathology and physiopathology of adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 511–521. [Google Scholar] [CrossRef]
- Zhai, J.; Vannuccini, S.; Petraglia, F.; Giudice, L.C. Adenomyosis: Mechanisms and Pathogenesis. Semin. Reprod. Med. 2020, 38, 129–143. [Google Scholar] [CrossRef]
- Curtis, K.M.; Hillis, S.D.; Marchbanks, P.A.; Peterson, H.B. Disruption of the endometrial-myometrial border during pregnancy as a risk factor for adenomyosis. Am. J. Obstet. Gynecol. 2002, 187, 543–544. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.G.; Chiantera, V.; Frangini, S.; Younes, S.; Kohler, C.; Taube, E.T.; Plendl, J.; Mechsner, S. Ultramicro-trauma in the endometrial-myometrial junctional zone and pale cell migration in adenomyosis. Fertil. Steril. 2015, 104, 1475–1483.e3. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Wildt, L.; Mall, G. The pathophysiology of endometriosis and adenomyosis: Tissue injury and repair. Arch. Gynecol. Obstet. 2009, 280, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Bilgicyildirim, A.; Inacker, M.; Stalf, T.; Huppert, P.; Mall, G.; Bottcher, B.; Wildt, L. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch. Gynecol. Obstet. 2015, 291, 917–932. [Google Scholar] [CrossRef]
- Simpson, J.L.; Bischoff, F.Z.; Kamat, A.; Buster, J.E.; Carson, S.A. Genetics of endometriosis. Obstet. Gynecol. Clin. N. Am. 2003, 30, 21–40, vii. [Google Scholar] [CrossRef]
- Deiana, D.; Gessa, S.; Anardu, M.; Daniilidis, A.; Nappi, L.; D’Alterio, M.N.; Pontis, A.; Angioni, S. Genetics of endometriosis: A comprehensive review. Gynecol. Endocrinol. 2019, 35, 553–558. [Google Scholar] [CrossRef]
- Nyholt, D.R.; Low, S.K.; Anderson, C.A.; Painter, J.N.; Uno, S.; Morris, A.P.; MacGregor, S.; Gordon, S.D.; Henders, A.K.; Martin, N.G.; et al. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat. Genet. 2012, 44, 1355–1359. [Google Scholar] [CrossRef]
- Rahmioglu, N.; Missmer, S.A.; Montgomery, G.W.; Zondervan, K.T. Insights into Assessing the Genetics of Endometriosis. Curr. Obstet. Gynecol. Rep. 2012, 1, 124–137. [Google Scholar] [CrossRef]
- Sapkota, Y.; Low, S.K.; Attia, J.; Gordon, S.D.; Henders, A.K.; Holliday, E.G.; MacGregor, S.; Martin, N.G.; McEvoy, M.; Morris, A.P.; et al. Association between endometriosis and the interleukin 1A (IL1A) locus. Hum. Reprod. 2015, 30, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Lagana, A.S.; Garzon, S.; Gotte, M.; Vigano, P.; Franchi, M.; Ghezzi, F.; Martin, D.C. The Pathogenesis of Endometriosis: Molecular and Cell Biology Insights. Int. J. Mol. Sci. 2019, 20, 5615. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Vigano, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Bulun, S.E.; Yilmaz, B.D.; Sison, C.; Miyazaki, K.; Bernardi, L.; Liu, S.; Kohlmeier, A.; Yin, P.; Milad, M.; Wei, J. Endometriosis. Endocr. Rev. 2019, 40, 1048–1079. [Google Scholar] [CrossRef]
- Barban, N.; Jansen, R.; de Vlaming, R.; Vaez, A.; Mandemakers, J.J.; Tropf, F.C.; Shen, X.; Wilson, J.F.; Chasman, D.I.; Nolte, I.M.; et al. Genome-wide analysis identifies 12 loci influencing human reproductive behavior. Nat. Genet. 2016, 48, 1462–1472. [Google Scholar] [CrossRef]
- Gallagher, C.S.; Makinen, N.; Harris, H.R.; Rahmioglu, N.; Uimari, O.; Cook, J.P.; Shigesi, N.; Ferreira, T.; Velez-Edwards, D.R.; Edwards, T.L.; et al. Genome-wide association and epidemiological analyses reveal common genetic origins between uterine leiomyomata and endometriosis. Nat. Commun. 2019, 10, 4857. [Google Scholar] [CrossRef]
- Painter, J.N.; O’Mara, T.A.; Morris, A.P.; Cheng, T.H.T.; Gorman, M.; Martin, L.; Hodson, S.; Jones, A.; Martin, N.G.; Gordon, S.; et al. Genetic overlap between endometriosis and endometrial cancer: Evidence from cross-disease genetic correlation and GWAS meta-analyses. Cancer Med. 2018, 7, 1978–1987. [Google Scholar] [CrossRef]
- Sapkota, Y.; Steinthorsdottir, V.; Morris, A.P.; Fassbender, A.; Rahmioglu, N.; De Vivo, I.; Buring, J.E.; Zhang, F.; Edwards, T.L.; Jones, S.; et al. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat. Commun. 2017, 8, 15539. [Google Scholar] [CrossRef]
- Uimari, O.; Rahmioglu, N.; Nyholt, D.R.; Vincent, K.; Missmer, S.A.; Becker, C.; Morris, A.P.; Montgomery, G.W.; Zondervan, K.T. Genome-wide genetic analyses highlight mitogen-activated protein kinase (MAPK) signaling in the pathogenesis of endometriosis. Hum. Reprod. 2017, 32, 780–793. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Signorile, P.G.; Severino, A.; Santoro, M.; Spyrou, M.; Viceconte, R.; Baldi, A. Methylation analysis of HOXA10 regulatory elements in patients with endometriosis. BMC Res. Notes 2018, 11, 722. [Google Scholar] [CrossRef]
- Wu, Y.; Halverson, G.; Basir, Z.; Strawn, E.; Yan, P.; Guo, S.W. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am. J. Obstet. Gynecol. 2005, 193, 371–380. [Google Scholar] [CrossRef]
- Wu, Y.; Strawn, E.; Basir, Z.; Halverson, G.; Guo, S.W. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics 2006, 1, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, B.D.; Bulun, S.E. Endometriosis and nuclear receptors. Hum. Reprod. Update 2019, 25, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Houshdaran, S.; Oke, A.B.; Fung, J.C.; Vo, K.C.; Nezhat, C.; Giudice, L.C. Steroid hormones regulate genome-wide epigenetic programming and gene transcription in human endometrial cells with marked aberrancies in endometriosis. PLoS Genet. 2020, 16, e1008601. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W. Epigenetics of endometriosis. Mol. Hum. Reprod. 2009, 15, 587–607. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, J.B.; Colon-Diaz, M.; Garcia, M.; Gutierrez, S.; Colon, M.; Seto, E.; Laboy, J.; Flores, I. Endometriosis is characterized by a distinct pattern of histone 3 and histone 4 lysine modifications. Reprod. Sci. 2014, 21, 305–318. [Google Scholar] [CrossRef]
- Xiaomeng, X.; Ming, Z.; Jiezhi, M.; Xiaoling, F. Aberrant histone acetylation and methylation levels in woman with endometriosis. Arch. Gynecol. Obstet. 2013, 287, 487–494. [Google Scholar] [CrossRef]
- Fehlmann, T.; Ludwig, N.; Backes, C.; Meese, E.; Keller, A. Distribution of microRNA biomarker candidates in solid tissues and body fluids. RNA Biol. 2016, 13, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Kuokkanen, S.; Chen, B.; Ojalvo, L.; Benard, L.; Santoro, N.; Pollard, J.W. Genomic profiling of microRNAs and messenger RNAs reveals hormonal regulation in microRNA expression in human endometrium. Biol. Reprod. 2010, 82, 791–801. [Google Scholar] [CrossRef]
- Sha, A.G.; Liu, J.L.; Jiang, X.M.; Ren, J.Z.; Ma, C.H.; Lei, W.; Su, R.W.; Yang, Z.M. Genome-wide identification of micro-ribonucleic acids associated with human endometrial receptivity in natural and stimulated cycles by deep sequencing. Fertil. Steril. 2011, 96, 150–155.e5. [Google Scholar] [CrossRef]
- Bjorkman, S.; Taylor, H.S. MicroRNAs in endometriosis: Biological function and emerging biomarker candidatesdagger. Biol. Reprod. 2019, 100, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Xu, H.; Kuang, Y. Systematic enrichment analysis of microRNA expression profiling studies in endometriosis. Iran. J. Basic Med. Sci. 2015, 18, 423–429. [Google Scholar] [PubMed]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The microRNA spectrum in 12 body fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef] [PubMed]
- Panir, K.; Schjenken, J.E.; Robertson, S.A.; Hull, M.L. Non-coding RNAs in endometriosis: A narrative review. Hum. Reprod. Update 2018, 24, 497–515. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.M.; Yang, W.X. Epithelial-to-mesenchymal transition in the development of endometriosis. Oncotarget 2017, 8, 41679–41689. [Google Scholar] [CrossRef]
- Vigano, P.; Ottolina, J.; Bartiromo, L.; Bonavina, G.; Schimberni, M.; Villanacci, R.; Candiani, M. Cellular Components Contributing to Fibrosis in Endometriosis: A Literature Review. J. Minim. Invasive Gynecol. 2020, 27, 287–295. [Google Scholar] [CrossRef]
- Saare, M.; Rekker, K.; Laisk-Podar, T.; Rahmioglu, N.; Zondervan, K.; Salumets, A.; Gotte, M.; Peters, M. Challenges in endometriosis miRNA studies—From tissue heterogeneity to disease specific miRNAs. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 2282–2292. [Google Scholar] [CrossRef]
- Gurates, B.; Bulun, S.E. Endometriosis: The ultimate hormonal disease. Semin. Reprod. Med. 2003, 21, 125–134. [Google Scholar] [CrossRef]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Vigano, P. Endometriosis. Nat. Rev. Dis. Primers 2018, 4, 9. [Google Scholar] [CrossRef]
- Burney, R.O.; Giudice, L.C. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril. 2012, 98, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.G.; Rudnicki, M.; Yu, J.; Shu, Y.; Taylor, R.N. Progesterone resistance in endometriosis: Origins, consequences and interventions. Acta Obstet. Gynecol. Scand. 2017, 96, 623–632. [Google Scholar] [CrossRef]
- Madjid, T.H.; Jumadi; Judistiani, R.T.D.; Hernowo, B.S.; Faried, A. Detection of endometriosis using immunocytochemistry of P450 Aromatase expressions in eutopic endometrial cells obtained from menstrual sloughing: A diagnostic study. BMC Res. Notes 2020, 13, 233. [Google Scholar] [CrossRef] [PubMed]
- Konrad, L.; Dietze, R.; Riaz, M.A.; Scheiner-Bobis, G.; Behnke, J.; Horne, F.; Hoerscher, A.; Reising, C.; Meinhold-Heerlein, I. Epithelial-Mesenchymal Transition in Endometriosis-When Does It Happen? J. Clin. Med. 2020, 9, 1915. [Google Scholar] [CrossRef] [PubMed]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Gomel, V.; Martin, D.C. Peritoneal fluid progesterone and progesterone resistance in superficial endometriosis lesions. Hum. Reprod. 2022, 37, 203–211. [Google Scholar] [CrossRef]
- Taylor, R.N.; Yu, J.; Torres, P.B.; Schickedanz, A.C.; Park, J.K.; Mueller, M.D.; Sidell, N. Mechanistic and therapeutic implications of angiogenesis in endometriosis. Reprod. Sci. 2009, 16, 140–146. [Google Scholar] [CrossRef]
- Cheong, Y.C.; Shelton, J.B.; Laird, S.M.; Richmond, M.; Kudesia, G.; Li, T.C.; Ledger, W.L. IL-1, IL-6 and TNF-alpha concentrations in the peritoneal fluid of women with pelvic adhesions. Hum. Reprod. 2002, 17, 69–75. [Google Scholar] [CrossRef]
- Forster, R.; Sarginson, A.; Velichkova, A.; Hogg, C.; Dorning, A.; Horne, A.W.; Saunders, P.T.K.; Greaves, E. Macrophage-derived insulin-like growth factor-1 is a key neurotrophic and nerve-sensitizing factor in pain associated with endometriosis. FASEB J. 2019, 33, 11210–11222. [Google Scholar] [CrossRef]
- Kato, T.; Yasuda, K.; Matsushita, K.; Ishii, K.J.; Hirota, S.; Yoshimoto, T.; Shibahara, H. Interleukin-1/-33 Signaling Pathways as Therapeutic Targets for Endometriosis. Front. Immunol. 2019, 10, 2021. [Google Scholar] [CrossRef]
- Othman Eel, D.; Hornung, D.; Salem, H.T.; Khalifa, E.A.; El-Metwally, T.H.; Al-Hendy, A. Serum cytokines as biomarkers for nonsurgical prediction of endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 137, 240–246. [Google Scholar] [CrossRef]
- Tseng, J.F.; Ryan, I.P.; Milam, T.D.; Murai, J.T.; Schriock, E.D.; Landers, D.V.; Taylor, R.N. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J. Clin. Endocrinol. Metab. 1996, 81, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Sawairi, M.; Nakagawa, M.; Itoh, N.; Wada, K.; Tamaya, T. Peritoneal fluid interleukin-1 beta and tumor necrosis factor in patients with benign gynecologic disease. Am. J. Reprod. Immunol. 1991, 26, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Akoum, A.; Jolicoeur, C.; Boucher, A. Estradiol amplifies interleukin-1-induced monocyte chemotactic protein-1 expression by ectopic endometrial cells of women with endometriosis. J. Clin. Endocrinol. Metab. 2000, 85, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Khorram, O.; Taylor, R.N.; Ryan, I.P.; Schall, T.J.; Landers, D.V. Peritoneal fluid concentrations of the cytokine RANTES correlate with the severity of endometriosis. Am. J. Obstet. Gynecol. 1993, 169, 1545–1549. [Google Scholar] [CrossRef]
- Arici, A.; Seli, E.; Zeyneloglu, H.B.; Senturk, L.M.; Oral, E.; Olive, D.L. Interleukin-8 induces proliferation of endometrial stromal cells: A potential autocrine growth factor. J. Clin. Endocrinol. Metab. 1998, 83, 1201–1205. [Google Scholar] [CrossRef]
- Arici, A.; Oral, E.; Attar, E.; Tazuke, S.I.; Olive, D.L. Monocyte chemotactic protein-1 concentration in peritoneal fluid of women with endometriosis and its modulation of expression in mesothelial cells. Fertil. Steril. 1997, 67, 1065–1072. [Google Scholar] [CrossRef]
- Gonzalez-Ramos, R.; Van Langendonckt, A.; Defrere, S.; Lousse, J.C.; Colette, S.; Devoto, L.; Donnez, J. Involvement of the nuclear factor-kappaB pathway in the pathogenesis of endometriosis. Fertil. Steril. 2010, 94, 1985–1994. [Google Scholar] [CrossRef]
- Mu, F.; Rich-Edwards, J.; Rimm, E.B.; Spiegelman, D.; Missmer, S.A. Endometriosis and Risk of Coronary Heart Disease. Circ. Cardiovasc. Qual. Outcomes 2016, 9, 257–264. [Google Scholar] [CrossRef]
- Alderman, M.H., 3rd; Yoder, N.; Taylor, H.S. The Systemic Effects of Endometriosis. Semin. Reprod. Med. 2017, 35, 263–270. [Google Scholar] [CrossRef]
- Asante, A.; Taylor, R.N. Endometriosis: The role of neuroangiogenesis. Annu. Rev. Physiol. 2011, 73, 163–182. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Basic mechanisms of vascularization in endometriosis and their clinical implications. Hum. Reprod. Update 2018, 24, 207–224. [Google Scholar] [CrossRef] [PubMed]
- Bozorgmehr, M.; Gurung, S.; Darzi, S.; Nikoo, S.; Kazemnejad, S.; Zarnani, A.H.; Gargett, C.E. Endometrial and Menstrual Blood Mesenchymal Stem/Stromal Cells: Biological Properties and Clinical Application. Front. Cell Dev. Biol. 2020, 8, 497. [Google Scholar] [CrossRef]
- Li, W.N.; Wu, M.H.; Tsai, S.J. HYPOXIA AND REPRODUCTIVE HEALTH: The role of hypoxia in the development and progression of endometriosis. Reproduction 2021, 161, F19–F31. [Google Scholar] [CrossRef] [PubMed]
- Ng, S.W.; Norwitz, S.G.; Taylor, H.S.; Norwitz, E.R. Endometriosis: The Role of Iron Overload and Ferroptosis. Reprod. Sci. 2020, 27, 1383–1390. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Liu, X.; Xu, H.; Guo, S.W. Mesothelial Cells Participate in Endometriosis Fibrogenesis Through Platelet-Induced Mesothelial-Mesenchymal Transition. J. Clin. Endocrinol. Metab. 2020, 105, e4124–e4147. [Google Scholar] [CrossRef]
- Riccio, L.; Santulli, P.; Marcellin, L.; Abrao, M.S.; Batteux, F.; Chapron, C. Immunology of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 39–49. [Google Scholar] [CrossRef]
- Guo, M.; Bafligil, C.; Tapmeier, T.; Hubbard, C.; Manek, S.; Shang, C.; Martinez, F.O.; Schmidt, N.; Obendorf, M.; Hess-Stumpp, H.; et al. Mass cytometry analysis reveals a distinct immune environment in peritoneal fluid in endometriosis: A characterisation study. BMC Med. 2020, 18, 3. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, X.; Guo, S.W. Platelets and Regulatory T Cells May Induce a Type 2 Immunity That Is Conducive to the Progression and Fibrogenesis of Endometriosis. Front. Immunol. 2020, 11, 610963. [Google Scholar] [CrossRef]
- Symons, L.K.; Miller, J.E.; Kay, V.R.; Marks, R.M.; Liblik, K.; Koti, M.; Tayade, C. The Immunopathophysiology of Endometriosis. Trends Mol. Med. 2018, 24, 748–762. [Google Scholar] [CrossRef]
- Kralickova, M.; Fiala, L.; Losan, P.; Tomes, P.; Vetvicka, V. Altered Immunity in Endometriosis: What Came First? Immunol. Investig. 2018, 47, 569–582. [Google Scholar] [CrossRef]
- Bacci, M.; Capobianco, A.; Monno, A.; Cottone, L.; Di Puppo, F.; Camisa, B.; Mariani, M.; Brignole, C.; Ponzoni, M.; Ferrari, S.; et al. Macrophages are alternatively activated in patients with endometriosis and required for growth and vascularization of lesions in a mouse model of disease. Am. J. Pathol. 2009, 175, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Hogg, C.; Panir, K.; Dhami, P.; Rosser, M.; Mack, M.; Soong, D.; Pollard, J.W.; Jenkins, S.J.; Horne, A.W.; Greaves, E. Macrophages inhibit and enhance endometriosis depending on their origin. Proc. Natl. Acad. Sci. USA 2021, 118, e2013776118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fu, Y.; Xue, S.; Ai, A.; Chen, H.; Lyu, Q.; Kuang, Y. The M2 polarization of macrophage induced by fractalkine in the endometriotic milieu enhances invasiveness of endometrial stromal cells. Int. J. Clin. Exp. Pathol. 2014, 7, 194–203. [Google Scholar] [PubMed]
- Miller, J.E.; Ahn, S.H.; Marks, R.M.; Monsanto, S.P.; Fazleabas, A.T.; Koti, M.; Tayade, C. IL-17A Modulates Peritoneal Macrophage Recruitment and M2 Polarization in Endometriosis. Front. Immunol. 2020, 11, 108. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, J.; Wang, W.; Xie, H.; Yao, S. Pro-endometriotic niche in endometriosis. Reprod. Biomed. Online 2019, 38, 549–559. [Google Scholar] [CrossRef]
- Sciezynska, A.; Komorowski, M.; Soszynska, M.; Malejczyk, J. NK Cells as Potential Targets for Immunotherapy in Endometriosis. J. Clin. Med. 2019, 8, 1468. [Google Scholar] [CrossRef]
- Xu, H.; Zhao, J.; Lu, J.; Sun, X. Ovarian endometrioma infiltrating neutrophils orchestrate immunosuppressive microenvironment. J. Ovarian Res. 2020, 13, 44. [Google Scholar] [CrossRef]
- Andres, M.P.; Arcoverde, F.V.L.; Souza, C.C.C.; Fernandes, L.F.C.; Abrao, M.S.; Kho, R.M. Extrapelvic Endometriosis: A Systematic Review. J. Minim. Invasive Gynecol. 2020, 27, 373–389. [Google Scholar] [CrossRef]
- Guo, S.W.; Mao, X.; Ma, Q.; Liu, X. Dysmenorrhea and its severity are associated with increased uterine contractility and overexpression of oxytocin receptor (OTR) in women with symptomatic adenomyosis. Fertil. Steril. 2013, 99, 231–240. [Google Scholar] [CrossRef]
- Nie, J.; Liu, X.; Guo, S.W. Immunoreactivity of oxytocin receptor and transient receptor potential vanilloid type 1 and its correlation with dysmenorrhea in adenomyosis. Am. J. Obstet. Gynecol. 2010, 202, 346.e1–346.e8. [Google Scholar] [CrossRef]
- Brainard, A.M.; Korovkina, V.P.; England, S.K. Potassium channels and uterine function. Semin. Cell Dev. Biol. 2007, 18, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bin, W.; Aksoy, M.O.; Kelsen, S.G. Regulation of interleukin-1beta and interleukin-1beta inhibitor release by human airway epithelial cells. Eur. Respir. J. 2004, 24, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Hayden, M.S.; Ghosh, S. Regulation of NF-kappaB by TNF family cytokines. Semin. Immunol. 2014, 26, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Aizawa, Y.; Yanai, Y.; Nagaoka, K.; Takeuchi, M.; Ohta, T.; Ikegami, H.; Ikeda, M.; Kurimoto, M. An essential role for NF-kappa B in IL-18-induced IFN-gamma expression in KG-1 cells. J. Immunol. 1999, 162, 5063–5069. [Google Scholar] [CrossRef]
- Chen, L.H.; Chan, S.H.; Li, C.J.; Wu, H.M.; Huang, H.Y. Altered Expression of Interleukin-18 System mRNA at the Level of Endometrial Myometrial Interface in Women with Adenomyosis. Curr. Issues Mol. Biol. 2022, 44, 5550–5561. [Google Scholar] [CrossRef]
- Nie, J.; Liu, X.; Zheng, Y.; Geng, J.G.; Guo, S.W. Increased immunoreactivity to SLIT/ROBO1 and its correlation with severity of dysmenorrhea in adenomyosis. Fertil. Steril. 2011, 95, 1164–1167. [Google Scholar] [CrossRef]
- Canis, M.; Donnez, J.G.; Guzick, D.S.; Halme, J.K.; Rock, J.A.; Schenken, R.S.; Vernon, M.W. Revised American Society for Reproductive Medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar] [CrossRef]
- Vercellini, P.; Buggio, L.; Somigliana, E. Role of medical therapy in the management of deep rectovaginal endometriosis. Fertil. Steril. 2017, 108, 913–930. [Google Scholar] [CrossRef]
- Chung, M.K.; Chung, R.R.; Gordon, D.; Jennings, C. The evil twins of chronic pelvic pain syndrome: Endometriosis and interstitial cystitis. JSLS 2002, 6, 311–314. [Google Scholar]
- Redwine, D.B. Diaphragmatic endometriosis: Diagnosis, surgical management, and long-term results of treatment. Fertil. Steril. 2002, 77, 288–296. [Google Scholar] [CrossRef]
- Li, T.; Mamillapalli, R.; Ding, S.; Chang, H.; Liu, Z.W.; Gao, X.B.; Taylor, H.S. Endometriosis alters brain electrophysiology, gene expression and increases pain sensitization, anxiety, and depression in female mice. Biol. Reprod. 2018, 99, 349–359. [Google Scholar] [CrossRef] [PubMed]
- As-Sanie, S.; Harris, R.E.; Napadow, V.; Kim, J.; Neshewat, G.; Kairys, A.; Williams, D.; Clauw, D.J.; Schmidt-Wilcke, T. Changes in regional gray matter volume in women with chronic pelvic pain: A voxel-based morphometry study. Pain 2012, 153, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, M.; Del Forno, S.; Spezzano, A.; Raimondo, D.; Arena, A.; Zanello, M.; Leonardi, D.; Paradisi, R.; Seracchioli, R. Painful Love: Superficial Dyspareunia and Three Dimensional Transperineal Ultrasound Evaluation of Pelvic Floor Muscle in Women with Endometriosis. J. Sex Marital Ther. 2020, 46, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Wahl, K.J.; Orr, N.L.; Lisonek, M.; Noga, H.; Bedaiwy, M.A.; Williams, C.; Allaire, C.; Albert, A.Y.; Smith, K.B.; Cox, S.; et al. Deep Dyspareunia, Superficial Dyspareunia, and Infertility Concerns Among Women with Endometriosis: A Cross-Sectional Study. Sex. Med. 2020, 8, 274–281. [Google Scholar] [CrossRef]
- Schenken, R.S.; Asch, R.H.; Williams, R.F.; Hodgen, G.D. Etiology of infertility in monkeys with endometriosis: Luteinized unruptured follicles, luteal phase defects, pelvic adhesions, and spontaneous abortions. Fertil. Steril. 1984, 41, 122–130. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Papaleo, E.; Corti, L.; Santambrogio, P.; Levi, S.; Vigano, P.; Candiani, M.; Panina-Bordignon, P. Iron availability is increased in individual human ovarian follicles in close proximity to an endometrioma compared with distal ones. Hum. Reprod. 2014, 29, 577–583. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Vigano, P.; Somigliana, E.; Panina-Bordignon, P.; Vercellini, P.; Candiani, M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: From pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum. Reprod. Update 2014, 20, 217–230. [Google Scholar] [CrossRef]
- Gomes, F.M.; Navarro, P.A.; de Abreu, L.G.; Ferriani, R.A.; dos Reis, R.M.; de Moura, M.D. Effect of peritoneal fluid from patients with minimal/mild endometriosis on progesterone release by human granulosa-lutein cells obtained from infertile patients without endometriosis: A pilot study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2008, 138, 60–65. [Google Scholar] [CrossRef]
- Skrzypczak, J. Morphology and steroidogenesis of cultured granulosa cells from endometrioidally changed ovaries. Exp. Clin. Endocrinol. Diabetes 1995, 103, 228–232. [Google Scholar] [CrossRef]
- Ferlita, A.; Battaglia, R.; Andronico, F.; Caruso, S.; Cianci, A.; Purrello, M.; Pietro, C.D. Non-Coding RNAs in Endometrial Physiopathology. Int. J. Mol. Sci. 2018, 19, 2120. [Google Scholar] [CrossRef]
- Wu, H.M.; Chen, L.H.; Hsu, L.T.; Lai, C.H. Immune Tolerance of Embryo Implantation and Pregnancy: The Role of Human Decidual Stromal Cell- and Embryonic-Derived Extracellular Vesicles. Int. J. Mol. Sci. 2022, 23, 13382. [Google Scholar] [CrossRef]
- Barrier, B.F.; Malinowski, M.J.; Dick, E.J., Jr.; Hubbard, G.B.; Bates, G.W. Adenomyosis in the baboon is associated with primary infertility. Fertil. Steril. 2004, 82 (Suppl. S3), 1091–1094. [Google Scholar] [CrossRef]
- Liang, T.; Zhang, W.; Pan, N.; Han, B.; Li, R.; Ma, C. Reproductive Outcomes of In Vitro Fertilization and Fresh Embryo Transfer in Infertile Women With Adenomyosis: A Retrospective Cohort Study. Front. Endocrinol. 2022, 13, 865358. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kawahara, N.; Ogawa, K.; Yoshimoto, C. A Relationship Between Endometriosis and Obstetric Complications. Reprod. Sci. 2020, 27, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Lalani, S.; Choudhry, A.J.; Firth, B.; Bacal, V.; Walker, M.; Wen, S.W.; Singh, S.; Amath, A.; Hodge, M.; Chen, I. Endometriosis and adverse maternal, fetal and neonatal outcomes, a systematic review and meta-analysis. Hum. Reprod. 2018, 33, 1854–1865. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Kawahara, N.; Ogawa, K.; Yoshimoto, C. Shared Molecular Features Linking Endometriosis and Obstetric Complications. Reprod. Sci. 2020, 27, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H. Imprinting genes associated with endometriosis. EXCLI J. 2014, 13, 252–264. [Google Scholar]
- Zadora, J.; Singh, M.; Herse, F.; Przybyl, L.; Haase, N.; Golic, M.; Yung, H.W.; Huppertz, B.; Cartwright, J.E.; Whitley, G.; et al. Disturbed Placental Imprinting in Preeclampsia Leads to Altered Expression of DLX5, a Human-Specific Early Trophoblast Marker. Circulation 2017, 136, 1824–1839. [Google Scholar] [CrossRef] [PubMed]
- Bellessort, B.; Le Cardinal, M.; Bachelot, A.; Narboux-Neme, N.; Garagnani, P.; Pirazzini, C.; Barbieri, O.; Mastracci, L.; Jonchere, V.; Duvernois-Berthet, E.; et al. Dlx5 and Dlx6 control uterine adenogenesis during post-natal maturation: Possible consequences for endometriosis. Hum. Mol. Genet. 2016, 25, 97–108. [Google Scholar] [CrossRef]
- Chen, P.; Wang, D.B.; Liang, Y.M. Evaluation of estrogen in endometriosis patients: Regulation of GATA-3 in endometrial cells and effects on Th2 cytokines. J. Obstet. Gynaecol. Res. 2016, 42, 669–677. [Google Scholar] [CrossRef]
- Cordeiro, A.; Neto, A.P.; Carvalho, F.; Ramalho, C.; Doria, S. Relevance of genomic imprinting in intrauterine human growth expression of CDKN1C, H19, IGF2, KCNQ1 and PHLDA2 imprinted genes. J. Assist. Reprod. Genet. 2014, 31, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Burris, H.H.; Baccarelli, A.A.; Motta, V.; Byun, H.M.; Just, A.C.; Mercado-Garcia, A.; Schwartz, J.; Svensson, K.; Tellez-Rojo, M.M.; Wright, R.O. Association between length of gestation and cervical DNA methylation of PTGER2 and LINE 1-HS. Epigenetics 2014, 9, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Paquette, A.G.; Lester, B.M.; Koestler, D.C.; Lesseur, C.; Armstrong, D.A.; Marsit, C.J. Placental FKBP5 genetic and epigenetic variation is associated with infant neurobehavioral outcomes in the RICHS cohort. PLoS ONE 2014, 9, e104913. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.A.; De Sutter, P.; Hamerlynck, T.; Imeraj, L.; Yao, Z.; Cloke, B.; Brosens, J.J.; Dhont, M. Endometriosis is associated with a decreased risk of pre-eclampsia. Hum. Reprod. 2007, 22, 1725–1729. [Google Scholar] [CrossRef]
- Harada, T.; Taniguchi, F.; Onishi, K.; Kurozawa, Y.; Hayashi, K.; Harada, T.; Japan, E.; Children’s Study, G. Obstetrical Complications in Women with Endometriosis: A Cohort Study in Japan. PLoS ONE 2016, 11, e0168476. [Google Scholar] [CrossRef]
- Leone Roberti Maggiore, U.; Ferrero, S.; Mangili, G.; Bergamini, A.; Inversetti, A.; Giorgione, V.; Vigano, P.; Candiani, M. A systematic review on endometriosis during pregnancy: Diagnosis, misdiagnosis, complications and outcomes. Hum. Reprod. Update 2016, 22, 70–103. [Google Scholar] [CrossRef]
- Kvaskoff, M.; Mahamat-Saleh, Y.; Farland, L.V.; Shigesi, N.; Terry, K.L.; Harris, H.R.; Roman, H.; Becker, C.M.; As-Sanie, S.; Zondervan, K.T.; et al. Endometriosis and cancer: A systematic review and meta-analysis. Hum. Reprod. Update 2021, 27, 393–420. [Google Scholar] [CrossRef]
- Anglesio, M.S.; Papadopoulos, N.; Ayhan, A.; Nazeran, T.M.; Noe, M.; Horlings, H.M.; Lum, A.; Jones, S.; Senz, J.; Seckin, T.; et al. Cancer-Associated Mutations in Endometriosis without Cancer. N. Engl. J. Med. 2017, 376, 1835–1848. [Google Scholar] [CrossRef]
- Akahane, T.; Sekizawa, A.; Purwosunu, Y.; Nagatsuka, M.; Okai, T. The role of p53 mutation in the carcinomas arising from endometriosis. Int. J. Gynecol. Pathol. 2007, 26, 345–351. [Google Scholar] [CrossRef]
- Amemiya, S.; Sekizawa, A.; Otsuka, J.; Tachikawa, T.; Saito, H.; Okai, T. Malignant transformation of endometriosis and genetic alterations of K-ras and microsatellite instability. Int. J. Gynaecol. Obstet. 2004, 86, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, G.M.; Abrao, M.S.; Taube, E.T.; Darb-Esfahani, S.; Kohler, C.; Chiantera, V.; Mechsner, S. (Partial) Loss of BAF250a (ARID1A) in rectovaginal deep-infiltrating endometriosis, endometriomas and involved pelvic sentinel lymph nodes. Mol. Hum. Reprod. 2016, 22, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Er, T.K.; Su, Y.F.; Wu, C.C.; Chen, C.C.; Wang, J.; Hsieh, T.H.; Herreros-Villanueva, M.; Chen, W.T.; Chen, Y.T.; Liu, T.C.; et al. Targeted next-generation sequencing for molecular diagnosis of endometriosis-associated ovarian cancer. J. Mol. Med. 2016, 94, 835–847. [Google Scholar] [CrossRef]
- Siufi Neto, J.; Kho, R.M.; Siufi, D.F.; Baracat, E.C.; Anderson, K.S.; Abrao, M.S. Cellular, histologic, and molecular changes associated with endometriosis and ovarian cancer. J. Minim. Invasive Gynecol. 2014, 21, 55–63. [Google Scholar] [CrossRef]
- Soyama, H.; Miyamoto, M.; Takano, M.; Iwahashi, H.; Kato, K.; Sakamoto, T.; Kuwahara, M.; Ishibashi, H.; Matuura, H.; Yoshikawa, T.; et al. A Pathological Study Using 2014 WHO Criteria Reveals Poor Prognosis of Grade 3 Ovarian Endometrioid Carcinomas. In Vivo 2018, 32, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Murali, R.; Murray, M.P.; Veras, E.; Park, K.J.; Soslow, R.A. Morphological and Immunohistochemical Reevaluation of Tumors Initially Diagnosed as Ovarian Endometrioid Carcinoma With Emphasis on High-grade Tumors. Am. J. Surg. Pathol. 2016, 40, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Vigano, P.; Buggio, L.; Makieva, S.; Scarfone, G.; Cribiu, F.M.; Parazzini, F.; Somigliana, E. Perimenopausal management of ovarian endometriosis and associated cancer risk: When is medical or surgical treatment indicated? Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 51, 151–168. [Google Scholar] [CrossRef]
- Huang, K.J.; Li, Y.X.; Wu, C.J.; Chang, W.C.; Wei, L.H.; Sheu, B.C. Sonographic features differentiating early-stage ovarian clear cell carcinoma from endometrioma with atypical features. J. Ovarian Res. 2022, 15, 84. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Ferrero, S.; Anserini, P.; Remorgida, V.; Ragni, N. Body mass index in endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 121, 94–98. [Google Scholar] [CrossRef]
- Goetz, T.G.; Mamillapalli, R.; Taylor, H.S. Low Body Mass Index in Endometriosis Is Promoted by Hepatic Metabolic Gene Dysregulation in Mice. Biol. Reprod. 2016, 95, 115. [Google Scholar] [CrossRef]
- Zolbin, M.M.; Mamillapalli, R.; Nematian, S.E.; Goetz, T.G.; Taylor, H.S. Adipocyte alterations in endometriosis: Reduced numbers of stem cells and microRNA induced alterations in adipocyte metabolic gene expression. Reprod. Biol. Endocrinol. 2019, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Bajaj, P.; Madsen, H.; Arendt-Nielsen, L. Endometriosis is associated with central sensitization: A psychophysical controlled study. J. Pain 2003, 4, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Lagana, A.S.; Condemi, I.; Retto, G.; Muscatello, M.R.; Bruno, A.; Zoccali, R.A.; Triolo, O.; Cedro, C. Analysis of psychopathological comorbidity behind the common symptoms and signs of endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 194, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Capezzuoli, T.; Rossi, M.; La Torre, F.; Vannuccini, S.; Petraglia, F. Hormonal drugs for the treatment of endometriosis. Curr. Opin. Pharmacol. 2022, 67, 102311. [Google Scholar] [CrossRef]
- Houshdaran, S.; Chen, J.C.; Vallve-Juanico, J.; Balayan, S.; Vo, K.C.; Smith-McCune, K.; Greenblatt, R.M.; Irwin, J.C.; Giudice, L.C. Progestins Related to Progesterone and Testosterone Elicit Divergent Human Endometrial Transcriptomes and Biofunctions. Int. J. Mol. Sci. 2020, 21, 2625. [Google Scholar] [CrossRef] [PubMed]
- Dunselman, G.A.; Vermeulen, N.; Becker, C.; Calhaz-Jorge, C.; D’Hooghe, T.; De Bie, B.; Heikinheimo, O.; Horne, A.W.; Kiesel, L.; Nap, A.; et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014, 29, 400–412. [Google Scholar] [CrossRef]
- Brown, J.; Crawford, T.J.; Datta, S.; Prentice, A. Oral contraceptives for pain associated with endometriosis. Cochrane Database Syst. Rev. 2018, 5, CD001019. [Google Scholar] [CrossRef]
- Meresman, G.F.; Auge, L.; Baranao, R.I.; Lombardi, E.; Tesone, M.; Sueldo, C. Oral contraceptives suppress cell proliferation and enhance apoptosis of eutopic endometrial tissue from patients with endometriosis. Fertil. Steril. 2002, 77, 1141–1147. [Google Scholar] [CrossRef]
- Caruso, S.; Cianci, A.; Iraci, M.; Fava, V.; Di Pasqua, S.; Cianci, S. Does Nomegestrol Acetate Plus 17beta-Estradiol Oral Contraceptive Improve Endometriosis-Associated Chronic Pelvic Pain in Women? J. Womens Health 2020, 29, 1184–1191. [Google Scholar] [CrossRef]
- Caruso, S.; Cianci, A.; Iraci Sareri, M.; Panella, M.; Caruso, G.; Cianci, S. Randomized study on the effectiveness of nomegestrol acetate plus 17beta-estradiol oral contraceptive versus dienogest oral pill in women with suspected endometriosis-associated chronic pelvic pain. BMC Womens Health 2022, 22, 146. [Google Scholar] [CrossRef]
- Reis, F.M.; Coutinho, L.M.; Vannuccini, S.; Batteux, F.; Chapron, C.; Petraglia, F. Progesterone receptor ligands for the treatment of endometriosis: The mechanisms behind therapeutic success and failure. Hum. Reprod. Update 2020, 26, 565–585. [Google Scholar] [CrossRef] [PubMed]
- Buggio, L.; Somigliana, E.; Barbara, G.; Frattaruolo, M.P.; Vercellini, P. Oral and depot progestin therapy for endometriosis: Towards a personalized medicine. Expert Opin. Pharmacother. 2017, 18, 1569–1581. [Google Scholar] [CrossRef] [PubMed]
- Casper, R.F. Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil. Steril. 2017, 107, 533–536. [Google Scholar] [CrossRef]
- Vercellini, P.; Pietropaolo, G.; De Giorgi, O.; Pasin, R.; Chiodini, A.; Crosignani, P.G. Treatment of symptomatic rectovaginal endometriosis with an estrogen-progestogen combination versus low-dose norethindrone acetate. Fertil. Steril. 2005, 84, 1375–1387. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Momoeda, M.; Taketani, Y.; Aso, T.; Fukunaga, M.; Hagino, H.; Terakawa, N. Dienogest is as effective as intranasal buserelin acetate for the relief of pain symptoms associated with endometriosis—A randomized, double-blind, multicenter, controlled trial. Fertil. Steril. 2009, 91, 675–681. [Google Scholar] [CrossRef]
- Abou-Setta, A.M.; Houston, B.; Al-Inany, H.G.; Farquhar, C. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst. Rev. 2013, 1, CD005072. [Google Scholar] [CrossRef]
- Hayashi, A.; Tanabe, A.; Kawabe, S.; Hayashi, M.; Yuguchi, H.; Yamashita, Y.; Okuda, K.; Ohmichi, M. Dienogest increases the progesterone receptor isoform B/A ratio in patients with ovarian endometriosis. J. Ovarian Res. 2012, 5, 31. [Google Scholar] [CrossRef]
- Strowitzki, T.; Marr, J.; Gerlinger, C.; Faustmann, T.; Seitz, C. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: A 24-week, randomized, multicentre, open-label trial. Hum. Reprod. 2010, 25, 633–641. [Google Scholar] [CrossRef]
- Strowitzki, T.; Marr, J.; Gerlinger, C.; Faustmann, T.; Seitz, C. Detailed analysis of a randomized, multicenter, comparative trial of dienogest versus leuprolide acetate in endometriosis. Int. J. Gynaecol. Obstet. 2012, 117, 228–233. [Google Scholar] [CrossRef]
- Strowitzki, T.; Faustmann, T.; Gerlinger, C.; Seitz, C. Dienogest in the treatment of endometriosis-associated pelvic pain: A 12-week, randomized, double-blind, placebo-controlled study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 151, 193–198. [Google Scholar] [CrossRef]
- Muzii, L.; Galati, G.; Di Tucci, C.; Di Feliciantonio, M.; Perniola, G.; Di Donato, V.; Benedetti Panici, P.; Vignali, M. Medical treatment of ovarian endometriomas: A prospective evaluation of the effect of dienogest on ovarian reserve, cyst diameter, and associated pain. Gynecol. Endocrinol. 2020, 36, 81–83. [Google Scholar] [CrossRef] [PubMed]
- Del Forno, S.; Mabrouk, M.; Arena, A.; Mattioli, G.; Giaquinto, I.; Paradisi, R.; Seracchioli, R. Dienogest or Norethindrone acetate for the treatment of ovarian endometriomas: Can we avoid surgery? Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 238, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Angioni, S.; Pontis, A.; Malune, M.E.; Cela, V.; Luisi, S.; Litta, P.; Vignali, M.; Nappi, L. Is dienogest the best medical treatment for ovarian endometriomas? Results of a multicentric case control study. Gynecol. Endocrinol. 2020, 36, 84–86. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Bracco, B.; Mosconi, P.; Roberto, A.; Alberico, D.; Dhouha, D.; Somigliana, E. Norethindrone acetate or dienogest for the treatment of symptomatic endometriosis: A before and after study. Fertil. Steril. 2016, 105, 734–743.e3. [Google Scholar] [CrossRef]
- Morotti, M.; Venturini, P.L.; Biscaldi, E.; Racca, A.; Calanni, L.; Vellone, V.G.; Stabilini, C.; Ferrero, S. Efficacy and acceptability of long-term norethindrone acetate for the treatment of rectovaginal endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 213, 4–10. [Google Scholar] [CrossRef]
- Brown, J.; Kives, S.; Akhtar, M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst. Rev. 2012, 2012, CD002122. [Google Scholar] [CrossRef]
- Committee on Adolescent Health Care Committee. Committee Opinion No. 602: Depot medroxyprogesterone acetate and bone effects. Obstet. Gynecol. 2014, 123, 1398–1402. [Google Scholar] [CrossRef]
- Vigano, P.; Somigliana, E.; Vercellini, P. Levonorgestrel-releasing intrauterine system for the treatment of endometriosis: Biological and clinical evidence. Womens Health 2007, 3, 207–214. [Google Scholar] [CrossRef]
- Gibbons, T.; Georgiou, E.X.; Cheong, Y.C.; Wise, M.R. Levonorgestrel-releasing intrauterine device (LNG-IUD) for symptomatic endometriosis following surgery. Cochrane Database Syst. Rev. 2021, 12, CD005072. [Google Scholar] [CrossRef]
- Chen, Y.J.; Hsu, T.F.; Huang, B.S.; Tsai, H.W.; Chang, Y.H.; Wang, P.H. Postoperative maintenance levonorgestrel-releasing intrauterine system and endometrioma recurrence: A randomized controlled study. Am. J. Obstet. Gynecol. 2017, 216, 582.e1–582.e9. [Google Scholar] [CrossRef]
- Romer, T. Long-term treatment of endometriosis with dienogest: Retrospective analysis of efficacy and safety in clinical practice. Arch. Gynecol. Obstet. 2018, 298, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.; Pan, A.; Hart, R.J. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst. Rev. 2010, 2010, CD008475. [Google Scholar] [CrossRef] [PubMed]
- Sagsveen, M.; Farmer, J.E.; Prentice, A.; Breeze, A. Gonadotrophin-releasing hormone analogues for endometriosis: Bone mineral density. Cochrane Database Syst. Rev. 2003, 2003, CD001297. [Google Scholar] [CrossRef] [PubMed]
- Tosti, C.; Biscione, A.; Morgante, G.; Bifulco, G.; Luisi, S.; Petraglia, F. Hormonal therapy for endometriosis: From molecular research to bedside. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 61–66. [Google Scholar] [CrossRef]
- Sauerbrun-Cutler, M.T.; Alvero, R. Short- and long-term impact of gonadotropin-releasing hormone analogue treatment on bone loss and fracture. Fertil. Steril. 2019, 112, 799–803. [Google Scholar] [CrossRef]
- Bedaiwy, M.A.; Alfaraj, S.; Yong, P.; Casper, R. New developments in the medical treatment of endometriosis. Fertil. Steril. 2017, 107, 555–565. [Google Scholar] [CrossRef]
- Donnez, J.; Taylor, R.N.; Taylor, H.S. Partial suppression of estradiol: A new strategy in endometriosis management? Fertil. Steril. 2017, 107, 568–570. [Google Scholar] [CrossRef]
- Tukun, F.L.; Olberg, D.E.; Riss, P.J.; Haraldsen, I.; Kaass, A.; Klaveness, J. Recent Development of Non-Peptide GnRH Antagonists. Molecules 2017, 22, 2188. [Google Scholar] [CrossRef]
- Taylor, H.S.; Giudice, L.C.; Lessey, B.A.; Abrao, M.S.; Kotarski, J.; Archer, D.F.; Diamond, M.P.; Surrey, E.; Johnson, N.P.; Watts, N.B.; et al. Treatment of Endometriosis-Associated Pain with Elagolix, an Oral GnRH Antagonist. N. Engl. J. Med. 2017, 377, 28–40. [Google Scholar] [CrossRef]
- Hornstein, M.D. An Oral GnRH Antagonist for Endometriosis—A New Drug for an Old Disease. N. Engl. J. Med. 2017, 377, 81–83. [Google Scholar] [CrossRef]
- Surrey, E.; Taylor, H.S.; Giudice, L.; Lessey, B.A.; Abrao, M.S.; Archer, D.F.; Diamond, M.P.; Johnson, N.P.; Watts, N.B.; Gallagher, J.C.; et al. Long-Term Outcomes of Elagolix in Women With Endometriosis: Results From Two Extension Studies. Obstet. Gynecol. 2018, 132, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.S.; Soliman, A.M.; Johns, B.; Pokrzywinski, R.M.; Snabes, M.; Coyne, K.S. Health-Related Quality of Life Improvements in Patients With Endometriosis Treated With Elagolix. Obstet. Gynecol. 2020, 136, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Osuga, Y.; Seki, Y.; Tanimoto, M.; Kusumoto, T.; Kudou, K.; Terakawa, N. Relugolix, an oral gonadotropin-releasing hormone receptor antagonist, reduces endometriosis-associated pain in a dose-response manner: A randomized, double-blind, placebo-controlled study. Fertil. Steril. 2021, 115, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Osuga, Y.; Suzuki, Y.; Fujisawa, M.; Fukui, M.; Kitawaki, J. Relugolix, an oral gonadotropin-releasing hormone receptor antagonist, reduces endometriosis-associated pain compared with leuprorelin in Japanese women: A phase 3, randomized, double-blind, noninferiority study. Fertil. Steril. 2022, 117, 583–592. [Google Scholar] [CrossRef]
- Giudice, L.C.; As-Sanie, S.; Arjona Ferreira, J.C.; Becker, C.M.; Abrao, M.S.; Lessey, B.A.; Brown, E.; Dynowski, K.; Wilk, K.; Li, Y.; et al. Once daily oral relugolix combination therapy versus placebo in patients with endometriosis-associated pain: Two replicate phase 3, randomised, double-blind, studies (SPIRIT 1 and 2). Lancet 2022, 399, 2267–2279. [Google Scholar] [CrossRef]
- Donnez, J.; Taylor, H.S.; Taylor, R.N.; Akin, M.D.; Tatarchuk, T.F.; Wilk, K.; Gotteland, J.P.; Lecomte, V.; Bestel, E. Treatment of endometriosis-associated pain with linzagolix, an oral gonadotropin-releasing hormone-antagonist: A randomized clinical trial. Fertil. Steril. 2020, 114, 44–55. [Google Scholar] [CrossRef]
- Taylor, R.N.; Bestel, E.; Gotteland, J.P.; Lecomte, V.; Dubouloz, R.; Terrill, P.; Humberstone, A.; Loumaye, E. Long term treatment of endometriosis associated pain (EAP) with linzagolix: Efficacy and safety after 12 months of treatment. Fertil. Steril. 2019, 112, e323. [Google Scholar] [CrossRef]
- Laux-Biehlmann, A.; d’Hooghe, T.; Zollner, T.M. Menstruation pulls the trigger for inflammation and pain in endometriosis. Trends Pharmacol. Sci. 2015, 36, 270–276. [Google Scholar] [CrossRef]
- Donnez, J.; Nisolle, M.; Casanas-Roux, F.; Brion, P.; Da Costa Ferreira, N. Stereometric evaluation of peritoneal endometriosis and endometriotic nodules of the rectovaginal septum. Hum. Reprod. 1996, 11, 224–228. [Google Scholar] [CrossRef]
- Dolmans, M.M.; Donnez, J. Emerging Drug Targets for Endometriosis. Biomolecules 2022, 12, 1654. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Kennedy, S.H.; Barlow, D.H. Endometriotic disease: The role of peritoneal fluid. Hum. Reprod. Update 1998, 4, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, L.; Dworzynski, K.; Davies, M.; Overton, C.; Guideline, C. Diagnosis and management of endometriosis: Summary of NICE guidance. BMJ 2017, 358, j3935. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.; Barcena de Arellano, M.L.; Ruster, C.; Vercellino, G.F.; Chiantera, V.; Schneider, A.; Mechsner, S. Imbalance between sympathetic and sensory innervation in peritoneal endometriosis. Brain Behav. Immun. 2012, 26, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Tokushige, N.; Markham, R.; Russell, P.; Fraser, I.S. Nerve fibres in peritoneal endometriosis. Hum. Reprod. 2006, 21, 3001–3007. [Google Scholar] [CrossRef] [PubMed]
- Greaves, E.; Grieve, K.; Horne, A.W.; Saunders, P.T. Elevated peritoneal expression and estrogen regulation of nociceptive ion channels in endometriosis. J. Clin. Endocrinol. Metab. 2014, 99, E1738–E1743. [Google Scholar] [CrossRef] [PubMed]
- Trapero, C.; Martin-Satue, M. Purinergic Signaling in Endometriosis-Associated Pain. Int. J. Mol. Sci. 2020, 21, 8512. [Google Scholar] [CrossRef]
- Chan, B.C.L.; Lam, C.W.K.; Tam, L.S.; Wong, C.K. IL33: Roles in Allergic Inflammation and Therapeutic Perspectives. Front. Immunol. 2019, 10, 364. [Google Scholar] [CrossRef]
- Lu, D.; Song, H.; Shi, G. Anti-TNF-alpha treatment for pelvic pain associated with endometriosis. Cochrane Database Syst. Rev. 2013, 3, CD008088. [Google Scholar] [CrossRef]
- Abokhrais, I.M.; Denison, F.C.; Whitaker, L.H.R.; Saunders, P.T.K.; Doust, A.; Williams, L.J.; Horne, A.W. A two-arm parallel double-blind randomised controlled pilot trial of the efficacy of Omega-3 polyunsaturated fatty acids for the treatment of women with endometriosis-associated pain (PurFECT1). PLoS ONE 2020, 15, e0227695. [Google Scholar] [CrossRef]
- Bruner-Tran, K.L.; Osteen, K.G.; Taylor, H.S.; Sokalska, A.; Haines, K.; Duleba, A.J. Resveratrol inhibits development of experimental endometriosis in vivo and reduces endometrial stromal cell invasiveness in vitro. Biol. Reprod. 2011, 84, 106–112. [Google Scholar] [CrossRef]
- Vallee, A.; Lecarpentier, Y. Curcumin and Endometriosis. Int. J. Mol. Sci. 2020, 21, 2440. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Becker, C.M.; Lui, W.T.; Chu, C.Y.; Davis, T.N.; Kung, A.L.; Birsner, A.E.; D’Amato, R.J.; Wai Man, G.C.; Wang, C.C. Green tea epigallocatechin-3-gallate inhibits angiogenesis and suppresses vascular endothelial growth factor C/vascular endothelial growth factor receptor 2 expression and signaling in experimental endometriosis in vivo. Fertil. Steril. 2011, 96, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Harlev, A.; Gupta, S.; Agarwal, A. Targeting oxidative stress to treat endometriosis. Expert Opin. Ther. Targets 2015, 19, 1447–1464. [Google Scholar] [CrossRef]
- Saunders, P.T.K.; Horne, A.W. Endometriosis: Etiology, pathobiology, and therapeutic prospects. Cell 2021, 184, 2807–2824. [Google Scholar] [CrossRef] [PubMed]
- Langebrekke, A.; Johannessen, H.O.; Qvigstad, E. Surgical treatment of endometriosis. Tidsskr. Nor. Laegeforen. 2008, 128, 1515–1518. [Google Scholar]
- Zanelotti, A.; Decherney, A.H. Surgery and Endometriosis. Clin. Obstet. Gynecol. 2017, 60, 477–484. [Google Scholar] [CrossRef]
- Donnez, J.; Squifflet, J. Complications, pregnancy and recurrence in a prospective series of 500 patients operated on by the shaving technique for deep rectovaginal endometriotic nodules. Hum. Reprod. 2010, 25, 1949–1958. [Google Scholar] [CrossRef]
- De Cicco, C.; Corona, R.; Schonman, R.; Mailova, K.; Ussia, A.; Koninckx, P. Bowel resection for deep endometriosis: A systematic review. BJOG 2011, 118, 285–291. [Google Scholar] [CrossRef]
- Meuleman, C.; Tomassetti, C.; D’Hoore, A.; Van Cleynenbreugel, B.; Penninckx, F.; Vergote, I.; D’Hooghe, T. Surgical treatment of deeply infiltrating endometriosis with colorectal involvement. Hum. Reprod. Update 2011, 17, 311–326. [Google Scholar] [CrossRef]
- Kent, A.; Shakir, F.; Rockall, T.; Haines, P.; Pearson, C.; Rae-Mitchell, W.; Jan, H. Laparoscopic Surgery for Severe Rectovaginal Endometriosis Compromising the Bowel: A Prospective Cohort Study. J. Minim. Invasive Gynecol. 2016, 23, 526–534. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Treatment of pelvic pain associated with endometriosis. Fertil. Steril. 2008, 90, S260–S269. [Google Scholar] [CrossRef] [PubMed]
- Koga, K.; Takamura, M.; Fujii, T.; Osuga, Y. Prevention of the recurrence of symptom and lesions after conservative surgery for endometriosis. Fertil. Steril. 2015, 104, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Capezzuoli, T.; Vannuccini, S.; Mautone, D.; Sorbi, F.; Chen, H.; Reis, F.M.; Ceccaroni, M.; Petraglia, F. Long-term hormonal treatment reduces repetitive surgery for endometriosis recurrence. Reprod. Biomed. Online 2021, 42, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Ceccaroni, M.; Clarizia, R.; Liverani, S.; Donati, A.; Ceccarello, M.; Manzone, M.; Roviglione, G.; Ferrero, S. Dienogest vs. GnRH agonists as postoperative therapy after laparoscopic eradication of deep infiltrating endometriosis with bowel and parametrial surgery: A randomized controlled trial. Gynecol. Endocrinol. 2021, 37, 930–933. [Google Scholar] [CrossRef]
- Guo, S.W. Recurrence of endometriosis and its control. Hum. Reprod. Update 2009, 15, 441–461. [Google Scholar] [CrossRef] [PubMed]
- Simanski, C.J.; Althaus, A.; Hoederath, S.; Kreutz, K.W.; Hoederath, P.; Lefering, R.; Pape-Kohler, C.; Neugebauer, E.A. Incidence of chronic postsurgical pain (CPSP) after general surgery. Pain Med. 2014, 15, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Althaus, A.; Hinrichs-Rocker, A.; Chapman, R.; Arranz Becker, O.; Lefering, R.; Simanski, C.; Weber, F.; Moser, K.H.; Joppich, R.; Trojan, S.; et al. Development of a risk index for the prediction of chronic post-surgical pain. Eur. J. Pain 2012, 16, 901–910. [Google Scholar] [CrossRef]
- Hughes, E.; Brown, J.; Collins, J.J.; Farquhar, C.; Fedorkow, D.M.; Vandekerckhove, P. Ovulation suppression for endometriosis. Cochrane Database Syst. Rev. 2007, 2007, CD000155. [Google Scholar] [CrossRef]
- Horton, J.; Sterrenburg, M.; Lane, S.; Maheshwari, A.; Li, T.C.; Cheong, Y. Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 592–632. [Google Scholar] [CrossRef]
- Bonavina, G.; Taylor, H.S. Endometriosis-associated infertility: From pathophysiology to tailored treatment. Front. Endocrinol. 2022, 13, 1020827. [Google Scholar] [CrossRef]
- Vesali, S.; Razavi, M.; Rezaeinejad, M.; Maleki-Hajiagha, A.; Maroufizadeh, S.; Sepidarkish, M. Endometriosis fertility index for predicting non-assisted reproductive technology pregnancy after endometriosis surgery: A systematic review and meta-analysis. BJOG 2020, 127, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Tomassetti, C.; Bafort, C.; Meuleman, C.; Welkenhuysen, M.; Fieuws, S.; D’Hooghe, T. Reproducibility of the Endometriosis Fertility Index: A prospective inter-/intra-rater agreement study. BJOG 2020, 127, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.; Sammel, M.D.; Morse, C.; Barnhart, K.T. Impact of endometriosis on in vitro fertilization outcomes: An evaluation of the Society for Assisted Reproductive Technologies Database. Fertil. Steril. 2016, 106, 164–171.e1. [Google Scholar] [CrossRef] [PubMed]
- Practice Committee of the American Society for Reproductive Medicine. Fertility evaluation of infertile women: A committee opinion. Fertil. Steril. 2021, 116, 1255–1265. [Google Scholar] [CrossRef]
- Surrey, E.S.; Silverberg, K.M.; Surrey, M.W.; Schoolcraft, W.B. Effect of prolonged gonadotropin-releasing hormone agonist therapy on the outcome of in vitro fertilization-embryo transfer in patients with endometriosis. Fertil. Steril. 2002, 78, 699–704. [Google Scholar] [CrossRef]
- Sallam, H.N.; Garcia-Velasco, J.A.; Dias, S.; Arici, A. Long-term pituitary down-regulation before in vitro fertilization (IVF) for women with endometriosis. Cochrane Database Syst. Rev. 2006, 2006, CD004635. [Google Scholar] [CrossRef]
- de Ziegler, D.; Gayet, V.; Aubriot, F.X.; Fauque, P.; Streuli, I.; Wolf, J.P.; de Mouzon, J.; Chapron, C. Use of oral contraceptives in women with endometriosis before assisted reproduction treatment improves outcomes. Fertil. Steril. 2010, 94, 2796–2799. [Google Scholar] [CrossRef]
- Tamura, H.; Yoshida, H.; Kikuchi, H.; Josaki, M.; Mihara, Y.; Shirafuta, Y.; Shinagawa, M.; Tamura, I.; Taketani, T.; Takasaki, A.; et al. The clinical outcome of Dienogest treatment followed by in vitro fertilization and embryo transfer in infertile women with endometriosis. J. Ovarian Res. 2019, 12, 123. [Google Scholar] [CrossRef]
- Khalifa, E.; Mohammad, H.; Abdullah, A.; Abdel-Rasheed, M.; Khairy, M.; Hosni, M. Role of suppression of endometriosis with progestins before IVF-ET: A non-inferiority randomized controlled trial. BMC Pregnancy Childbirth 2021, 21, 264. [Google Scholar] [CrossRef]
- Cao, X.; Chang, H.Y.; Xu, J.Y.; Zheng, Y.; Xiang, Y.G.; Xiao, B.; Geng, X.J.; Ni, L.L.; Chu, X.Y.; Tao, S.B.; et al. The effectiveness of different down-regulating protocols on in vitro fertilization-embryo transfer in endometriosis: A meta-analysis. Reprod. Biol. Endocrinol. 2020, 18, 16. [Google Scholar] [CrossRef]
- Barra, F.; Lagana, A.S.; Scala, C.; Garzon, S.; Ghezzi, F.; Ferrero, S. Pretreatment with dienogest in women with endometriosis undergoing IVF after a previous failed cycle. Reprod. Biomed. Online 2020, 41, 859–868. [Google Scholar] [CrossRef] [PubMed]
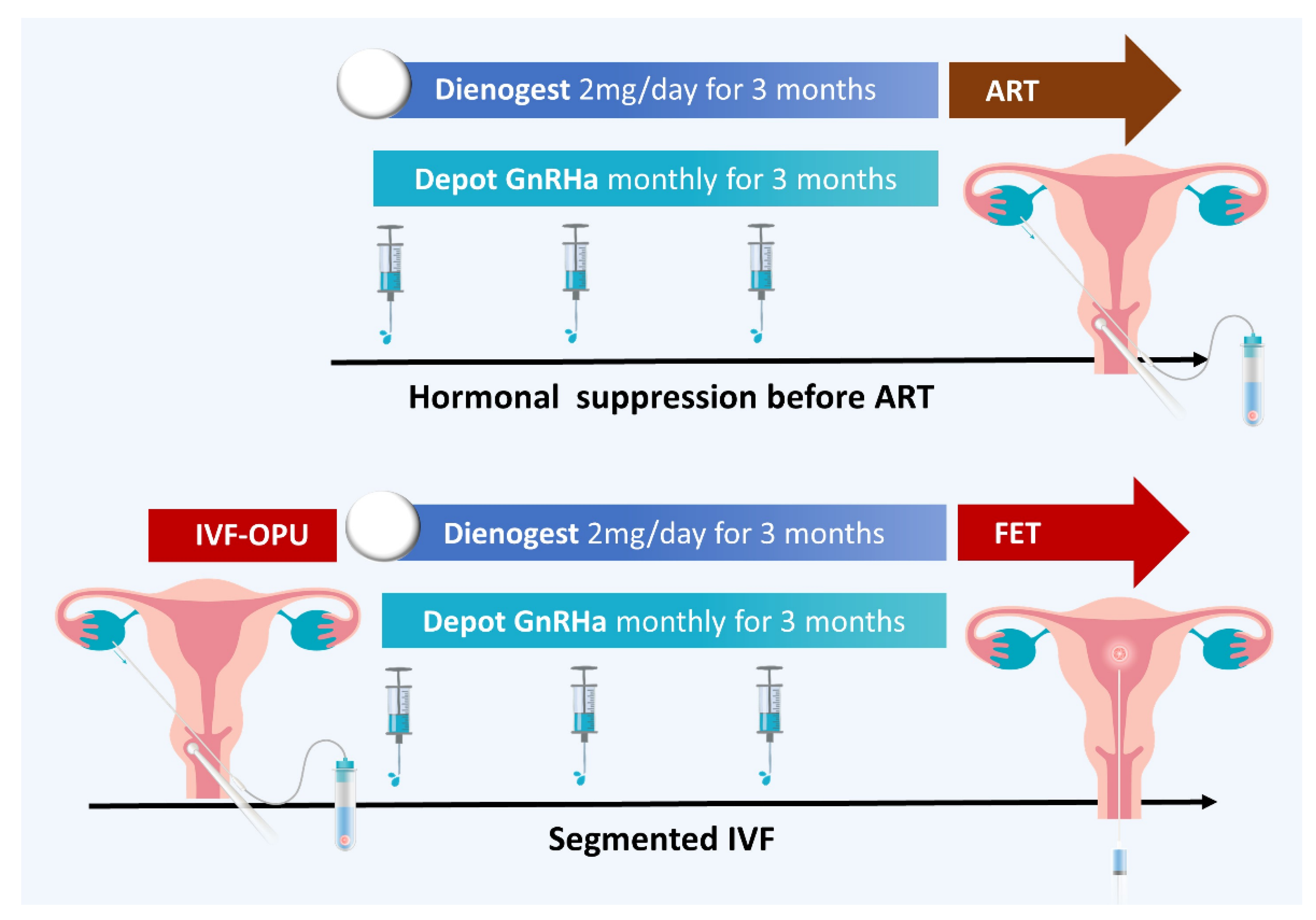
- Ozgur, K.; Humaidan, P.; Coetzee, K. Segmented ART—The new era in ART? Reprod. Biol. 2016, 16, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Feng, M.; Chen, L.H.; Hsu, L.T.; Wu, H.M. Segmented in vitro fertilization and frozen embryo transfer in dienogest-treated patient with adenomyosis: A case report and lierature review. Taiwan. J. Obstet. Gynecol. 2022, 61, 906–908. [Google Scholar] [CrossRef] [PubMed]
- Khine, Y.M.; Taniguchi, F.; Harada, T. Clinical management of endometriosis-associated infertility. Reprod. Med. Biol. 2016, 15, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Somigliana, E.; Vigano, P.; Abbiati, A.; Barbara, G.; Crosignani, P.G. Surgery for endometriosis-associated infertility: A pragmatic approach. Hum. Reprod. 2009, 24, 254–269. [Google Scholar] [CrossRef]
- Kuroda, K.; Ikemoto, Y.; Ochiai, A.; Ozaki, R.; Matsumura, Y.; Nojiri, S.; Nakagawa, K.; Sugiyama, R. Combination Treatment of Preoperative Embryo Cryopreservation and Endoscopic Surgery (Surgery-ART Hybrid Therapy) in Infertile Women with Diminished Ovarian Reserve and Uterine Myomas or Ovarian Endometriomas. J. Minim. Invasive Gynecol. 2019, 26, 1369–1375. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil. Steril. 2019, 112, 1022–1033. [Google Scholar] [CrossRef]
- Cobo, A.; Giles, J.; Paolelli, S.; Pellicer, A.; Remohi, J.; Garcia-Velasco, J.A. Oocyte vitrification for fertility preservation in women with endometriosis: An observational study. Fertil. Steril. 2020, 113, 836–844. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, S.K.; Lee, J.R.; Suh, C.S.; Kim, S.H. Oocyte cryopreservation for fertility preservation in women with ovarian endometriosis. Reprod. Biomed. Online 2020, 40, 827–834. [Google Scholar] [CrossRef]
- Calagna, G.; Della Corte, L.; Giampaolino, P.; Maranto, M.; Perino, A. Endometriosis and strategies of fertility preservation: A systematic review of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 254, 218–225. [Google Scholar] [CrossRef]
- Somigliana, E.; Vigano, P.; Filippi, F.; Papaleo, E.; Benaglia, L.; Candiani, M.; Vercellini, P. Fertility preservation in women with endometriosis: For all, for some, for none? Hum. Reprod. 2015, 30, 1280–1286. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.; Becker, C.; Davies, M. AGAINST: Fertility preservation for women with ovarian endometriosis: It is time to adopt this as routine practice. BJOG 2022, 129, 1937–1938. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Saridogan, E.; Yasmin, E. FOR: Fertility preservation for women with ovarian endometriosis: It is time to adopt it as routine practice. BJOG 2022, 129, 1935–1936. [Google Scholar] [CrossRef] [PubMed]
- Streuli, I.; Benard, J.; Hugon-Rodin, J.; Chapron, C.; Santulli, P.; Pluchino, N. Shedding light on the fertility preservation debate in women with endometriosis: A swot analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 229, 172–178. [Google Scholar] [CrossRef]
| Mechanism | Targets | Investigational Drugs | Clinical Trials | Status |
|---|---|---|---|---|
| Anti-angiogenic drugs | Selective dopamine D2-receptor agonist | Quinagolide (vaginal rings) Cabergoline (oral) | NCT03749109 NCT03692403 NCT03928288 | Phase II completed Phase II completed Phase II recruiting |
| Immunomodulators | Suppression NF-ᴋB and COX-2 | DLBS1442 (oral) | NCT01942122 | Phase II completed |
| Antioxidants | Downregulates inflammatory cytokines (e.g., IL-6, IL-1β, MCP-1) | Resveratrol (oral) | NCT02475564 | Phase IV completed |
| Anti-neurogenic and Anti-inflammatory drugs | Purinergic P2X3 receptors antagonist | BAY1817080 (oral) | NCT04487431 NCT04471337 NCT04454424 NCT04423744 | Phase I competed |
| Monoclonal antibodies of IL-33 | MT-2990 (intravenous) | NCT03840993 | Phase II completed | |
| An IL-1 receptor antagonist | Anakinra (subcutaneous) | NCT03991520 | Phase II recruiting |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-H.; Lo, W.-C.; Huang, H.-Y.; Wu, H.-M. A Lifelong Impact on Endometriosis: Pathophysiology and Pharmacological Treatment. Int. J. Mol. Sci. 2023, 24, 7503. https://doi.org/10.3390/ijms24087503
Chen L-H, Lo W-C, Huang H-Y, Wu H-M. A Lifelong Impact on Endometriosis: Pathophysiology and Pharmacological Treatment. International Journal of Molecular Sciences. 2023; 24(8):7503. https://doi.org/10.3390/ijms24087503
Chicago/Turabian StyleChen, Liang-Hsuan, Wei-Che Lo, Hong-Yuan Huang, and Hsien-Ming Wu. 2023. "A Lifelong Impact on Endometriosis: Pathophysiology and Pharmacological Treatment" International Journal of Molecular Sciences 24, no. 8: 7503. https://doi.org/10.3390/ijms24087503
APA StyleChen, L.-H., Lo, W.-C., Huang, H.-Y., & Wu, H.-M. (2023). A Lifelong Impact on Endometriosis: Pathophysiology and Pharmacological Treatment. International Journal of Molecular Sciences, 24(8), 7503. https://doi.org/10.3390/ijms24087503








