Tic-Tac: A Translational Approach in Mechanisms Associated with Irregular Heartbeat and Sinus Rhythm Restoration in Atrial Fibrillation Patients
Abstract
1. Introduction
2. The Hallmarks of Atrial Fibrillation
2.1. At Risk for Atrial Fibrillation
2.1.1. Genetic Substrate
2.1.2. Age-Related Remodeling
2.1.3. Disease-Related Remodeling
2.2. Paroxysmal Atrial Fibrillation
2.3. Persistent Atrial Fibrillation
2.3.1. Structural Remodeling
2.3.2. Electrophysiological Remodeling
- Rotors: a re-entry mechanism that consists of a localized circular or spiral wavefront that rotates around an anatomical or functional obstacle, having heterogeneous conduction velocity and an unexcitable center that causes an irregular propagation of electrical activity.
- Ectopic foci: abnormal regions within the atria that initiate electrical impulses spontaneously or in response to triggers.
- Multiple wavelets: numerous re-entry circuits in the atria, that can interact with each other, merge, or divide.
2.3.3. Neurohormonal Remodeling
2.3.4. Inflammation and Oxidative Stress
2.4. Permanent Atrial Fibrillation
3. Clinical Implications of Atrial Fibrillation
3.1. Quality of Life
3.2. Hospitalizations
3.3. Increased Risk of Mortality
3.4. Stroke and Embolism
3.5. Heart Failure
3.6. Neuropsychiatric Disorders
4. Classic Therapeutic Approach for Rhythm Control in Atrial Fibrillation
4.1. Antiarrhythmic Drugs
4.1.1. Antiarrhythmic Arsenal
4.1.2. Drug Utilization Algorithm
4.1.3. Therapeutic Approach
4.2. Atrial Fibrillation Ablation
5. Rhythm Control in Atrial Fibrillation: Toward a Translational Approach
5.1. Modern Classification of Antiarrhythmics in Atrial Fibrillation
5.1.1. Class I
5.1.2. Class III
5.2. New Antiarrhythmics and Molecular Targets
5.2.1. RyR2 Channels
| Drug | Study | Primary Outcome Studied | Phase | NCT |
|---|---|---|---|---|
| Flecainide | To Evaluate the Impact of Oral Flecainide on Quality of Life in Patients with Paroxysmal Atrial Fibrillation | To assess the effect of Flecainide CR on patient-perceived health-related QoL (Quality of Life) | Phase 4 | NCT00189319 |
| Inhalation of Flecainide to Convert Recent Onset SympTomatic Atrial Fibrillation to siNus rhyThm (INSTANT) | Measure efficacy Objective evaluated using ECGs and telemetry to record heart rhythm | Phase 2 | NCT03539302 | |
| Predictive Factors to Effectively Terminate Paroxysmal Atrial Fibrillation by Blocking Atrial Selective Ionic Currents (SELECTCARFAP) | Electrocardiographic-based spectral parameters of atrial fibrillatory activity (Dominant frequency) associated with successful or unsuccessful cardioversion in both groups of patients | Phase 4 | NCT03005366 | |
| Flecainide Acetate Inhalation Solution for Cardioversion of Recent-Onset, Symptomatic Atrial Fibrillation (RESTORE-1) | Assessment of proportion of patients whose AF converts using continuous ECG monitoring | Phase 3 | NCT05039359 | |
| Comparative Study of Flecainide CR and Placebo in the Early Treatment of Atrial Fibrillation | Time to the first relapse after randomization, with or without symptoms documented on ECG, Holter or “Self ECG unit” recording | Phase 4 | NCT00408473 | |
| The Use of Flecainide for Treatment of Atrial Fibrillation | Arrythmia free health status | Phase 4 | NCT05084495 | |
| Flecainide Versus Amiodarone in the Cardioversion of Paroxysmal Atrial Fibrillation at the Emergency Department, in Patients With Coronary Artery Disease Without Residual Ischemia (FLECA-ED) | The frequency of successful cardioversion to sinus rhythm and The combined frequency of premature ventricular contractions (PVCs), non-sustained ventricular tachycardia (NSVT), sustained ventricular tachycardia (SVT), bradycardia < 50 bpm, and systolic blood pressure < 90 mmHg | Phase 3 | NCT05549752 | |
| Propafenone | Propafenone in the Treatment of Atrial Fibrillation | Proportion of patients with recurrent AF | Not applicable | NCT03674658 |
| Antazoline in Comparison to Propafenone in Pharmacological Cardioversion of Atrial Fibrillation (AnProAF) | Conversion of atrial fibrillation to sinus rhythm | Phase 4 | NCT05720572 | |
| Ranolazine | Ranolazine for the Prevention of Atrial Fibrillation After Electrical Cardioversion (GILEAD) | To determine whether ranolazine is effective in decreasing recurrences of AF in patients with persistent AF successfully treated with electrical cardioversion | Phase 3 | NCT01349491 |
| Study to Evaluate the Effect of Ranolazine and Dronedarone When Given Alone and in Combination in Patients with Paroxysmal Atrial Fibrillation (HARMONY) | Atrial Fibrillation Burden (AFB) at Baseline, Percent Change from Baseline in Atrial Fibrillation Burden (AFB) by Week 12 | Phase 2 | NCT01522651 | |
| Supression Of Atrial Fibrillation With Ranolazine After Cardiac Surgery | Freedom From Any Episode of Post-Operative Atrial Fibrillation Longer Than 6 h of Duration Occurring During the Study Period | Phase 3 | NCT01352416 | |
| Randomized Double Blind Control Trial on Effects of Ranolazine on New-Onset Atrial Fibrillation | Incidence of New-Onset Atrial Fibrillation Rate in Post-Operative Cardiac Surgery Patients | Not applicable | NCT01590979 |
5.2.2. NLRP3 Inflammasome
5.2.3. Atrial Selective Drugs
- Kv1.5 channel: ultra rapid delayed rectifier K current (IKur). These channels are expressed in greater quantities in human atria when compared to the ventricles. They even only contribute to the atrial repolarization since the functional current is detectable only in the atria [126].
- GIRK1/GIRK4 channels: also known as Kir3.1 and Kir3.4 (IKAch). They can be constitutively active or be activated by ACh, increasing K+ conductance in the heart. Constitutively active channels develop in the course of AF-related remodeling, predominantly in atrial cardiomyocytes [127]. IKAch hyperpolarizes the membrane and shortens the action potential and effective refractory period, supporting the maintenance of AF and facilitating the occurrence of re-entry [128]. This is why blocking these channels could prolong the APD and ERP, terminating the AF.
- Ca2+ activated Potassium Channels (SK Channels): SK channels (ISK) are small-conductance voltage-independent potassium channels that contribute to repolarization [116] and are activated by sub-micromolar concentrations of intracellular free Ca2+ ions. Chronic AF is associated with an elevation in the intracellular Ca2+ concentration during diastole [120] that may have an impact on SK channel function and its role in promoting AF. The blocking of these channels is expected to prolong the APD and reduce re-entry [117] producing also atrial-selective effects, since SK2 and SK3 channels are predominant in the human atria, and appear to have limited function in ventricles under physiological conditions [118].
- K2P 3.1 (K2P)/TWIK-1, TASK-1, and TASK-3 channels: these are voltage-independent background currents ITWIK-1, ITASK-1, ITASK-3, carried by two pore domain K channels. K2P 1.1, also known as the weak inward rectifying K+ channel (TWIK-1) and K2P 3.1, or the TWIK-related acid sensitive K+ channel (TASK-1), are the predominant atrial channel subunits, and K2P 3.1 (TASK-1) is preferentially expressed in the human atrium over the ventricle, which suggests atrial selectivity. Chronic AF is associated with an upregulation of the TASK-1 channel expression and function in the atria suggesting that the channels contribute to AF-induced action potential shortening, in order that targeting these could have a potential therapeutic role [129].
- Atrial-selective Na Channel (INa): The inactivation of the INa current initiates a refractory period dependent on the duration of action potential, it reduces the membrane excitability, slows down the conduction velocity and the propagation of refractory period, being able to suppress triggered activity and end the re-entry [130]. The possibility to selectively target the atrial tissue is due to the intrinsic functional differences between the atria and ventricular forms of fast sodium channel current INa. For example, the atrial current is inactivated at more negative voltages than the ventricular one, also having a faster onset and a slower recovery from inactivation [131].
- Vernakalant: This is an atrial selective class IIIa antiarrhythmic, that acts as a non-selective K+ channel blocker [105], targeting the Kv1.5 channel (IKur) and the GIRK channel (IKAch), both described above as currents that are present predominantly in atrial tissue, participating in atrial repolarization with little effect on ventricular repolarization. It also has a voltage and rate dependent (fast set, fast offset) INa-blocking effect and blocks the transient outward potassium current Ito, carried by the Kv4.3 channels [116,132]. The combined effect of the blockade of these channels allows for prolongation of the atrial action potential and refractory period duration, and theoretically minimizes the risk of Torsade de pointes as a result of perturbation of ventricular repolarization [133]. For treating new-onset AF, vernakalant proved its efficacy in three randomized, double-blind trials (ACT I, II, III). In these studies, it led to conversion to sinus rhythm in 51% of the patients (vs. 4% with placebo), and when the efficiency was tested against amiodarone infusion, the results showed a 51.7% cardioversion in vernakalant and only a 5.2% cardioversion with amiodarone was well tolerated, with minimal side effects, with transient hypotension and bradycardia in only 5–10% of patients [134]. According to most recent guidelines, vernakalant is considered one of the most effectively used drugs for cardioversion in patients with AF, even being more efficient and safer than amiodarone as a pharmacologic cardioversion agent as well as flecainide, IV amiodarone, ibutilide, and propafenone [1].
- Dronedarone: Class IIIa antiarrhythmic [105]. This drug is of particular interest since it is an analog of amiodarone, one of the most used drugs in the management of AF, but it lacks iodine molecules; therefore, it has less pulmonary and thyroid toxicity [135]. In vitro data show that dronedarone inhibits various potassium currents, including: Ikur, IKAch, and K2P channels, all with atrial selective properties. It also targets the transient outward potassium currents Ito in human atrial myocytes [136], and concentration-dependent inhibition of sodium currents has been demonstrated utilizing dronedarone in vitro in human atrial myocytes [137]. Addressing the drug security profile, the ATHENA multicenter trial concluded that dronedarone is associated with reduced cardiovascular events in patients with paroxysmal or persistent AF and HF [138]. Additionally, when compared with commonly used drugs, dronedarone was associated with significantly lowered risk of all-cause death than with the use of sotalol, with no differences in AF recurrence observed between the two therapies [139].
- SK blockers: Class IIIc antiarrhythmics [105]. The current leading SK channel blocker is AP30663l, which has proven to be safe for use in humans in phase 1 studies and has entered phase 2 clinical trials in a pig model AP30663, prolonging the effective refractory period in a dose-dependent manner [140,141].
- TASK-1 blockers: The respiratory stimulant doxapram acts as a selective TASK-1 blocker, showing antiarrhythmic class III properties. In a pig model, doxapram successfully cardioverted AF [142]. It is currently under study in the doxapram conversion to sinus rhythm (DOCTOS) trial, which will reveal whether doxapram, a potent TASK-1 inhibitor, can be used for acute cardioversion of persistent and paroxysmal AF in patients, potentially leading to a new treatment option for AF [143].
- Antazoline: First-generation antihistamine, H1 antagonist with a quinidine-like class Ia antiarrhythmic effect [144], targeting INa channels, which reduces ectopic ventricular/atrial automaticity, accessory pathway conduction and increases refractory period, decreasing re-entry tendency [105,144]. The CANT II study performed in Poland evaluated the efficacy and safety of antazoline, a first-generation antihistamine, for cardioversion of recent onset of AF in the setting of an emergency department. It showed a superior rhythm conversion rate with antazoline when compared to amiodarone and propafenone (78.3% vs. 66.9% and 72.7%) [145]. At the moment, there is a study being conducted also in Warsaw, Poland, comparing antazoline with propafenone in pharmacological cardioversion of AF (NCT05720572).
- Nifekalant: New class IIIa antiarrhythmic drug approved in Japan for the treatment of ventricular tachyarrhythmias. It is a selective Ikr blocker that prolongs effective atrial inactivity. It has no significant effect on myocardial cell conduction velocity or myocardial contractility, which translates in a low incidence of adverse events such as bradycardia and hypotension [146]. When compared with catheter ablation, nifekalant had a better success rate of conversion, with no difference in the incidence of adverse events between the two groups [147].
- Refralon/Niferidil: New class III antiarrhythmic agent developed in Russia for pharmacological cardioversion. It blocks Ikur and prolongs atrial and ventricular action potential and refractory period [148]. In a study of this drug efficacy and safety, there was relief of paroxysmal AF in 95% of the cases, and while in 5% that had a prolongation of the QTc, none of the patients developed Torsade de pointes after administration of the drug [149].
| Drug | Study | Primary Outcome Studied | Phase | NCT |
|---|---|---|---|---|
| Vernakalant | RAFF4 Trial: Vernakalant vs. Procainamide for Acute Atrial Fibrillation in the Emergency Department | Conversion to sinus rhythm for a minimum duration of 30 min | Phase 4 | NCT04485195 |
| Predictive factors to effectively terminate paroxysmal atrial fibrillation by blocking atrial selective Ionic Currents (SELECTCARFAP) | Electrocardiographic-based spectral parameters of atrial fibrillatory activity (Dominant frequency) associated with successful or unsuccessful cardioversion in both groups of patients | Phase 4 | NCT03005366 | |
| Study of Normal Conditions of Use, Dosing, and Safety of Intravenous (IV) Administration of Vernakalant (MK-6621-049) | Number of Participants Experiencing Significant Hypotension, significant ventricular arrhythmia, atrial flutter and bradycardia | Phase 3 | NCT01370629 | |
| Dronedarone | Early Dronedarone versus usual care to improve Outcomes in Persons with Newly Diagnosed Atrial Fibrillation (CHANGE-AF) | Cardiovascular Hospitalization or Death | Phase 4 | NCT05130268 |
| Effect of Prolonged Use of Dronedarone on Recurrence in Patients with Non-paroxysmal Atrial Fibrillation After Radiofrequency Ablation | Cumulative non-recurrence rate | Phase 4 | NCT05655468 | |
| Study to Evaluate the Effect of Ranolazine and Dronedarone When Given Alone and in Combination in Patients with Paroxysmal Atrial Fibrillation (HARMONY) | Atrial Fibrillation Burden (AFB) at Baseline, Percent Change from Baseline in Atrial Fibrillation Burden (AFB) by Week 12 | Phase 2 | NCT01522651 | |
| Systematic Review and Meta-Analysis of Multaq® for Safety in Atrial Fibrillation | Number of participants with cardiovascular hospitalization, ventricular proarrhythmia, number of all-cause mortality events, number of participants with atrial fibrillation | Not applicable | NCT05279833 | |
| Early Aggressive Invasive Intervention for Atrial Fibrillation | Time to recurrence of symptomatic or asymptomatic Atrial Fibrillation, Atrial Flutter or Atrial Tachycardia | Not applicable | NCT02825979 | |
| Catheter Ablation vs. Anti-arrhythmic Drug Therapy for Atrial Fibrillation Trial (CABANA) | Number of Participants With Composite of Total Mortality, Disabling Stroke, Serious Bleeding, or Cardiac Arrest in Patients Warranting Therapy for AF | Not applicable | NCT00911508 | |
| Antazoline | Antazoline in Comparison to Propafenone in Pharmacological Cardioversion of Atrial Fibrillation (AnProAF) | Conversion of atrial fibrillation to sinus rhythm | Phase 4 | NCT05720572 |
| Antazoline in Rapid Cardioversion of Paroxysmal Atrial Fibrillation (AnPAF) | Conversion of AF to SN confirmed in standard 12-lead ECG during observation period after first iv bolus | Phase 4 | NCT01527279 | |
| Nifekalant | Comparison of Efficacy and Safety of Different Doses of Nifekalant Instant Cardioversion of Persistent Atrial Fibrillation During Radiofrequency Ablation | Comparison of the successful rates of different doses of nifekalant instant cardioversion of persistent atrial fibrillation after radiofrequency ablation, incidence of adverse effects | Phase 4 | NCT04209959 |
| Nifekalant Versus Amiodarone in New-Onset Atrial Fibrillation After Cardiac Surgery | Rate of cardioversion at 4 h | Phase 3 | NCT05169866 | |
| Refralon | Efficacy and Safety evaluation of Refralon, concentrate for Solution for intravenous injection in Patients With Paroxysmal and Persistent Atrial Fibrillation and Flutter | Incidence of sinus rhythm restoration | Phase 3 | NCT05773170 |
| Refralon Versus Amiodarone for Cardioversion of Paroxysmal Fibrillation and Atrial Flutter | Restoration of sinus rhythm, sinus rhythm recovery time, recurrent AF after successful cardioversion, ventricular arrhythmogenic effect | Phase 3 | NCT05445297 | |
| Refralon in Patients With Recurrence Paroxysmal and Persistent Forms of Atrial Fibrillation Who Underwent Catheter Ablation | Restoration of sinus rhythm, preservation of sinus rhythm, ventricular arrhythmogenic effect, increase QT interval | Phase 3 | NCT05456204 |
5.3. Mechanisms Associated with AF Ablation
5.3.1. Electrophysiological Disconnection and Trigger Reduction
5.3.2. Substrate Modification
5.3.3. Functional Ablation
5.3.4. Autonomic Modulation
5.3.5. Techniques for Obtaining Permanent PVI
5.4. Gene Therapy in Atrial Fibrillation
5.4.1. Targeting Atrial Conduction
- Ion channels: Ion channels have been a classic target for the management of AF. In the case of gene therapy, preclinical studies in pigs have shown that the use of an adenovirus with the mutant variant of the CERG-G627S long QT syndrome has been effective in postponing or suppressing the development of persistent AF, through the prolongation of the atrial action potential and its refractory periods [198]. In the same model, blockade of the tandem of P domains in a weak inward rectifying K+ channel-related acid-sensitive K+ channel-1 (TASK-1), which is an atrial-specific channel for action potential control, from viral vectors carrying anti-TASK-1-siRNA, showed a great decrease in AF burden in the animals studied [129]. The importance of this channel has subsequently been studied as a pharmacological target for AF [199]. Another potassium channel target has been the blockade of the KCNH2 channel through an adenovirus vector carrying KCNH2-G28S, a dominant negative mutation, was shown to prolong the action potential in the porcine model [200], also showing reduction in the same model in the incidence of post-operative AF [201].
- Ca2+ management: Abnormal Ca2+ handling is central to the pathogenesis of AF; therefore, efforts to target these mechanisms have been carried out. Wang et al. demonstrated that overexpression of SERCA2a has a suppressive effect on effective refractory period shortening, in addition to AF induced by rapid pacing atrium [202]. The post-transcriptional regulation of RyR2 mediated by the miR-106b-25 cluster could be a potential target for future gene therapy, since its loss would promote paroxysmal AF by the potentially arrhythmogenic Ca2+ leakage in the sarcoplasmic reticulum [203].
- Gap junctions: Modification of the expression of gap junctions is also a critical mechanism of the conduction impairment found in AF. These structures are critical for intracellular conduction by connecting neighboring cells. Downregulation of connexin 43 (Cx43) has been shown to be a contributing factor to AF persistence. In the porcine model, gene transfer of Cx43 has been shown to prevent AF persistence and improve LVEF [204]. Concordant with this finding, Igarashi et al. demonstrated that promoting overexpression of Cx40 and Cx43 connexins preserved ventricular conduction and prevented sustained AF, with no significant differences between the latter [205].
5.4.2. Parasympathetic Stimulus
5.4.3. Targeting Atrial Remodeling
Apoptosis
Atrial Fibrosis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Bax, J.J.; Boriani, G.; Dan, G.A.; Fauchier, L.; Kalman, J.M.; Lane, D.A.; Lettino, M.; et al. 2020 ESC Guidelines for the Diagnosis and Management of Atrial Fibrillation Developed in Collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the Diagnosis and Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Developed with the Special Contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef]
- Cheniti, G.; Vlachos, K.; Pambrun, T.; Hooks, D.; Frontera, A.; Takigawa, M.; Bourier, F.; Kitamura, T.; Lam, A.; Martin, C.; et al. Atrial Fibrillation Mechanisms and Implications for Catheter Ablation. Front. Physiol. 2018, 9, 1458. [Google Scholar] [CrossRef]
- Michaud, G.F.; Stevenson, W.G. Atrial Fibrillation. N. Engl. J. Med. 2021, 384, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Kornej, J.; Börschel, C.S.; Benjamin, E.J.; Schnabel, R.B. Epidemiology of Atrial Fibrillation in the 21st Century. Circ. Res. 2020, 127, 4–20. [Google Scholar] [CrossRef]
- Prystowsky, E.N. Rate Versus Rhythm Control for Atrial Fibrillation: Has the Debate Been Settled? Circulation 2022, 146, 1561–1563. [Google Scholar] [CrossRef] [PubMed]
- Wyse, D.G.; Waldo, A.L.; DiMarco, J.P.; Domanski, M.J.; Rosenberg, Y.; Schron, E.B.; Kellen, J.C.; Greene, H.L.; Mickel, M.C.; Dalquist, J.E.; et al. A Comparison of Rate Control and Rhythm Control in Patients with Atrial Fibrillation. N. Engl. J. Med. 2002, 347, 1825–1833. [Google Scholar] [CrossRef]
- Heijman, J.; Voigt, N.; Nattel, S.; Dobrev, D. Cellular and Molecular Electrophysiology of Atrial Fibrillation Initiation, Maintenance, and Progression. Circ. Res. 2014, 114, 1483–1499. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Camm, A.J.; Goette, A.; Brandes, A.; Eckardt, L.; Elvan, A.; Fetsch, T.; van Gelder, I.C.; Haase, D.; Haegeli, L.M.; et al. Early Rhythm-Control Therapy in Patients with Atrial Fibrillation. N. Engl. J. Med. 2020, 383, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Willems, S.; Borof, K.; Brandes, A.; Breithardt, G.; Camm, A.J.; Crijns, H.J.G.M.; Eckardt, L.; Gessler, N.; Goette, A.; Haegeli, L.M.; et al. Systematic, Early Rhythm Control Strategy for Atrial Fibrillation in Patients with or without Symptoms: The EAST-AFNET 4 Trial. Eur. Heart J. 2022, 43, 1219–1230. [Google Scholar] [CrossRef]
- Camm, A.J.; Naccarelli, G.V.; Mittal, S.; Crijns, H.J.G.M.; Hohnloser, S.H.; Ma, C.S.; Natale, A.; Turakhia, M.P.; Kirchhof, P. The Increasing Role of Rhythm Control in Patients With Atrial Fibrillation: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 1932–1948. [Google Scholar] [CrossRef]
- Nattel, S.; Heijman, J.; Zhou, L.; Dobrev, D. Molecular Basis of Atrial Fibrillation Pathophysiology and Therapy: A Translational Perspective. Circ. Res. 2020, 127, 51–72. [Google Scholar] [CrossRef]
- Brundel, B.J.J.M.; Ai, X.; Hills, M.T.; Kuipers, M.F.; Lip, G.Y.H.; de Groot, N.M.S. Atrial Fibrillation. Nat. Rev. Dis. Primers 2022, 8, 21. [Google Scholar] [CrossRef]
- Hansen, B.J.; Csepe, T.A.; Zhao, J.; Ignozzi, A.J.; Hummel, J.D.; Fedorov, V.V. Maintenance of Atrial Fibrillation: Are Reentrant Drivers with Spatial Stability the Key? Circ. Arrhythm. Electrophysiol. 2016, 9, e004398. [Google Scholar] [CrossRef]
- Chen, P.S.; Chen, L.S.; Fishbein, M.C.; Lin, S.F.; Nattel, S. Role of the Autonomic Nervous System in Atrial Fibrillation: Pathophysiology and Therapy. Circ. Res. 2014, 114, 1500–1515. [Google Scholar] [CrossRef]
- Zhou, X.; Dudley, S.C. Evidence for Inflammation as a Driver of Atrial Fibrillation. Front. Cardiovasc. Med. 2020, 7, 62. [Google Scholar] [CrossRef]
- Li, C.Y.; Zhang, J.R.; Hu, W.N.; Li, S.N. Atrial Fibrosis Underlying Atrial Fibrillation (Review). Int. J. Mol. Med. 2021, 47, 9. [Google Scholar] [CrossRef]
- Guo, Y.H.; Yang, Y.Q. Atrial Fibrillation: Focus on Myocardial Connexins and Gap Junctions. Biology 2022, 11, 489. [Google Scholar] [CrossRef]
- Sagris, M.; Vardas, E.P.; Theofilis, P.; Antonopoulos, A.S.; Oikonomou, E.; Tousoulis, D. Atrial Fibrillation: Pathogenesis, Predisposing Factors, and Genetics. Int. J. Mol. Sci. 2021, 23, 6. [Google Scholar] [CrossRef]
- Young, L.J.; Antwi-Boasiako, S.; Ferrall, J.; Wold, L.E.; Mohler, P.J.; El Refaey, M. Genetic and Non-Genetic Risk Factors Associated with Atrial Fibrillation. Life Sci. 2022, 299, 120529. [Google Scholar] [CrossRef]
- Lee, S.; Khrestian, C.M.; Sahadevan, J.; Waldo, A.L. Reconsidering the Multiple Wavelet Hypothesis of Atrial Fibrillation. Heart Rhythm. 2020, 17, 1976–1983. [Google Scholar] [CrossRef]
- Potpara, T.S.; Lip, G.Y.H.; Blomstrom-Lundqvist, C.; Boriani, G.; Van Gelder, I.C.; Heidbuchel, H.; Hindricks, G.; Camm, A.J. The 4S-AF Scheme (Stroke Risk; Symptoms; Severity of Burden; Substrate): A Novel Approach to In-Depth Characterization (Rather than Classification) of Atrial Fibrillation. Thromb. Haemost. 2021, 121, 270–278. [Google Scholar] [CrossRef]
- Nattel, S.; Dobrev, D. Electrophysiological and Molecular Mechanisms of Paroxysmal Atrial Fibrillation. Nat. Rev. Cardiol. 2016, 13, 575–590. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, E895–E1032. [Google Scholar] [CrossRef]
- Weng, L.C.; Preis, S.R.; Hulme, O.L.; Larson, M.G.; Choi, S.H.; Wang, B.; Trinquart, L.; McManus, D.D.; Staerk, L.; Lin, H.; et al. Genetic Predisposition, Clinical Risk Factor Burden, and Lifetime Risk of Atrial Fibrillation. Circulation 2018, 137, 1027–1038. [Google Scholar] [CrossRef]
- Wasmer, K.; Eckardt, L.; Breithardt, G. Predisposing Factors for Atrial Fibrillation in the Elderly. J. Geriatr. Cardiol. 2017, 14, 179. [Google Scholar] [CrossRef] [PubMed]
- Laredo, M.; Waldmann, V.; Khairy, P.; Nattel, S. Age as a Critical Determinant of Atrial Fibrillation: A Two-Sided Relationship. Can. J. Cardiol. 2018, 34, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.; Khairy, P.; Dobrev, D.; Nattel, S. The Clinical Profile and Pathophysiology of Atrial Fibrillation: Relationships among Clinical Features, Epidemiology, and Mechanisms. Circ. Res. 2014, 114, 1453–1468. [Google Scholar] [CrossRef] [PubMed]
- Vyas, V.; Lambiase, P. Obesity and Atrial Fibrillation: Epidemiology, Pathophysiology and Novel Therapeutic Opportunities. Arrhythm. Electrophysiol. Rev. 2019, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Verhaert, D.V.M.; Brunner-La Rocca, H.P.; Van Veldhuisen, D.J.; Vernooy, K. The Bidirectional Interaction between Atrial Fibrillation and Heart Failure: Consequences for the Management of Both Diseases. Europace 2021, 23, ii40. [Google Scholar] [CrossRef]
- Kashou, A.H.; Adedinsewo, D.A.; Noseworthy, P.A. Subclinical Atrial Fibrillation: A Silent Threat with Uncertain Implications. Annu. Rev. Med. 2022, 73, 355–362. [Google Scholar] [CrossRef]
- Nattel, S.; Harada, M. Atrial Remodeling and Atrial Fibrillation: Recent Advances and Translational Perspectives. J. Am. Coll. Cardiol. 2014, 63, 2335–2345. [Google Scholar] [CrossRef]
- Haïssaguerre, M.; Jaïs, P.; Shah, D.C.; Takahashi, A.; Hocini, M.; Quiniou, G.; Garrigue, S.; Le Mouroux, A.; Le Métayer, P.; Clémenty, J. Spontaneous Initiation of Atrial Fibrillation by Ectopic Beats Originating in the Pulmonary Veins. N. Engl. J. Med. 1998, 339, 659–666. [Google Scholar] [CrossRef]
- Teh, A.W.; Kistler, P.M.; Lee, G.; Medi, C.; Heck, P.M.; Spence, S.; Morton, J.B.; Sanders, P.; Kalman, J.M. Electroanatomic Properties of the Pulmonary Veins: Slowed Conduction, Low Voltage and Altered Refractoriness in AF Patients. J. Cardiovasc. Electrophysiol. 2011, 22, 1083–1091. [Google Scholar] [CrossRef]
- Benito, B.; Brugada, R.; Perich, R.M.; Lizotte, E.; Cinca, J.; Mont, L.; Berruezo, A.; Tolosana, J.M.; Freixa, X.; Brugada, P.; et al. A Mutation in the Sodium Channel Is Responsible for the Association of Long QT Syndrome and Familial Atrial Fibrillation. Heart Rhythm. 2008, 5, 1434–1440. [Google Scholar] [CrossRef]
- Kottkamp, H.; Tanner, H.; Kobza, R.; Schirdewahn, P.; Dorszewski, A.; Gerds-Li, J.H.; Carbucicchio, C.; Piorkowski, C.; Hindricks, G. Time Courses and Quantitative Analysis of Atrial Fibrillation Episode Number and Duration after Circular plus Linear Left Atrial Lesions: Trigger Elimination or Substrate Modification: Early or Delayed Cure? J. Am. Coll. Cardiol. 2004, 44, 869–877. [Google Scholar] [CrossRef]
- Wijffels, M.C.E.F.; Kirchhof, C.J.H.J.; Dorland, R.; Allessie, M.A. Atrial Fibrillation Begets Atrial Fibrillation: A Study in Awake Chronically Instrumented Goats. Circulation 1995, 92, 1954–1968. [Google Scholar] [CrossRef]
- Goette, A.; Honeycutt, C.; Langberg, J.J. Electrical Remodeling in Atrial Fibrillation. Time Course and Mechanisms. Circulation 1996, 94, 2968–2974. [Google Scholar] [CrossRef]
- Piccini, J.P.; Passman, R.; Turakhia, M.; Connolly, A.T.; Nabutovsky, Y.; Varma, N. Atrial Fibrillation Burden, Progression, and the Risk of Death: A Case-Crossover Analysis in Patients with Cardiac Implantable Electronic Devices. Europace 2019, 21, 404–413. [Google Scholar] [CrossRef]
- Lau, D.H.; Linz, D.; Schotten, U.; Mahajan, R.; Sanders, P.; Kalman, J.M. Pathophysiology of Paroxysmal and Persistent Atrial Fibrillation: Rotors, Foci and Fibrosis. Heart Lung Circ. 2017, 26, 887–893. [Google Scholar] [CrossRef]
- Beyer, C.; Tokarska, L.; Stühlinger, M.; Feuchtner, G.; Hintringer, F.; Honold, S.; Fiedler, L.; Schönbauer, M.S.; Schönbauer, R.; Plank, F. Structural Cardiac Remodeling in Atrial Fibrillation. JACC Cardiovasc. Imaging 2021, 14, 2199–2208. [Google Scholar] [CrossRef]
- Gu, J.; Liu, X.; Wang, Q.X.; Tan, H.W.; Guo, M.; Jiang, W.F.; Zhou, L. Angiotensin II Increases CTGF Expression via MAPKs/TGF-Β1/TRAF6 Pathway in Atrial Fibroblasts. Exp. Cell Res. 2012, 318, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Luo, X.; Qi, X.Y.; Tadevosyan, A.; Maguy, A.; Ordog, B.; Ledoux, J.; Kato, T.; Naud, P.; Voigt, N.; et al. Transient Receptor Potential Canonical-3 Channel-Dependent Fibroblast Regulation in Atrial Fibrillation. Circulation 2012, 126, 2051–2064. [Google Scholar] [CrossRef] [PubMed]
- Sakabe, M.; Fujiki, A.; Nishida, K.; Sugao, M.; Nagasawa, H.; Tsuneda, T.; Mizumaki, K.; Inoue, H. Enalapril Prevents Perpetuation of Atrial Fibrillation by Suppressing Atrial Fibrosis and Over-Expression of Connexin43 in a Canine Model of Atrial Pacing-Induced Left Ventricular Dysfunction. J. Cardiovasc. Pharmacol. 2004, 43, 851–859. [Google Scholar] [CrossRef]
- Tsai, C.F.; Yang, S.F.; Chu, H.J.; Ueng, K.C. Cross-Talk between Mineralocorticoid Receptor/Angiotensin II Type 1 Receptor and Mitogen-Activated Protein Kinase Pathways Underlies Aldosterone-Induced Atrial Fibrotic Responses in HL-1 Cardiomyocytes. Int. J. Cardiol. 2013, 169, 17–28. [Google Scholar] [CrossRef]
- Schneider, M.P.; Hua, T.A.; Böhm, M.; Wachtell, K.; Kjeldsen, S.E.; Schmieder, R.E. Prevention of Atrial Fibrillation by Renin-Angiotensin System Inhibition. J. Am. Coll. Cardiol. 2010, 55, 2299–2307. [Google Scholar] [CrossRef]
- Ma, H.; Jiang, H.; Feng, J.; Gan, Y. Angiotensin Receptor Blocker and Calcium Channel Blocker Preventing Atrial Fibrillation Recurrence in Patients with Hypertension and Atrial Fibrillation: A Meta-Analysis. Cardiovasc. Ther. 2021, 2021, 6628469. [Google Scholar] [CrossRef]
- Fogari, R.; Zoppi, A.; Maffioli, P.; Mugellini, A.; Preti, P.; Perrone, T.; Derosa, G. Effect of Telmisartan on Paroxysmal Atrial Fibrillation Recurrence in Hypertensive Patients with Normal or Increased Left Atrial Size. Clin. Cardiol. 2012, 35, 359–364. [Google Scholar] [CrossRef]
- Li, T.J.; Zang, W.D.; Chen, Y.L.; Geng, N.; Ma, S.M.; Li, X.D. Renin-Angiotensin System Inhibitors for Prevention of Recurrent Atrial Fibrillation: A Meta-Analysis. Int. J. Clin. Pract. 2013, 67, 536–543. [Google Scholar] [CrossRef]
- Chaugai, S.; Sherpa, L.Y.; Sepehry, A.A.; Arima, H.; Wang, D.W. Effect of RAAS Blockers on Adverse Clinical Outcomes in High CVD Risk Subjects with Atrial Fibrillation: A Meta-Analysis and Systematic Review of Randomized Controlled Trials. Medicine 2016, 95, e4059. [Google Scholar] [CrossRef]
- Voigt, N.; Trausch, A.; Knaut, M.; Matschke, K.; Varró, A.; Van Wagoner, D.R.; Nattel, S.; Ravens, U.; Dobrev, D. Left-to-Right Atrial Inward Rectifier Potassium Current Gradients in Patients with Paroxysmal versus Chronic Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2010, 3, 472–480. [Google Scholar] [CrossRef]
- Wijesurendra, R.S.; Casadei, B. Mechanisms of Atrial Fibrillation. Heart 2019, 105, 1860–1867. [Google Scholar] [CrossRef]
- Rebecchi, M.; Panattoni, G.; Edoardo, B.; Ruvo, E.; de Sciarra, L.; Politano, A.; Sgueglia, M.; Ricagni, C.; Verbena, S.; Crescenzi, C.; et al. Atrial Fibrillation and Autonomic Nervous System: A Translational Approach to Guide Therapeutic Goals. J. Arrhythm. 2021, 37, 320–330. [Google Scholar] [CrossRef]
- Goetze, J.P.; Bruneau, B.G.; Ramos, H.R.; Ogawa, T.; de Bold, M.K.; de Bold, A.J. Cardiac Natriuretic Peptides. Nat. Rev. Cardiol. 2020, 17, 698–717. [Google Scholar] [CrossRef]
- Tanase, D.M.; Radu, S.; Al Shurbaji, S.; Baroi, G.L.; Costea, C.F.; Turliuc, M.D.; Ouatu, A.; Floria, M. Natriuretic Peptides in Heart Failure with Preserved Left Ventricular Ejection Fraction: From Molecular Evidences to Clinical Implications. Int. J. Mol. Sci. 2019, 20, 2629. [Google Scholar] [CrossRef]
- Brady, P.F.; Chua, W.; Nehaj, F.; Connolly, D.L.; Khashaba, A.; Purmah, Y.J.V.; Ul-Qamar, M.J.; Thomas, M.R.; Varma, C.; Schnabel, R.B.; et al. Interactions Between Atrial Fibrillation and Natriuretic Peptide in Predicting Heart Failure Hospitalization or Cardiovascular Death. J. Am. Heart Assoc. 2022, 11, 22833. [Google Scholar] [CrossRef]
- Scott, L.; Li, N.; Dobrev, D. Role of Inflammatory Signaling in Atrial Fibrillation. Int. J. Cardiol. 2019, 287, 195–200. [Google Scholar] [CrossRef]
- Li, N.; Brundel, B.J.J.M. Inflammasomes and Proteostasis Novel Molecular Mechanisms Associated With Atrial Fibrillation. Circ. Res. 2020, 127, 73–90. [Google Scholar] [CrossRef]
- Mason, F.E.; Pronto, J.R.D.; Alhussini, K.; Maack, C.; Voigt, N. Cellular and Mitochondrial Mechanisms of Atrial Fibrillation. Basic. Res. Cardiol. 2020, 115, 72. [Google Scholar] [CrossRef]
- Abe, I.; Teshima, Y.; Kondo, H.; Kaku, H.; Kira, S.; Ikebe, Y.; Saito, S.; Fukui, A.; Shinohara, T.; Yufu, K.; et al. Association of Fibrotic Remodeling and Cytokines/Chemokines Content in Epicardial Adipose Tissue with Atrial Myocardial Fibrosis in Patients with Atrial Fibrillation. Heart Rhythm. 2018, 15, 1717–1727. [Google Scholar] [CrossRef]
- Marrouche, N.F.; Brachmann, J.; Andresen, D.; Siebels, J.; Boersma, L.; Jordaens, L.; Merkely, B.; Pokushalov, E.; Sanders, P.; Proff, J.; et al. Catheter Ablation for Atrial Fibrillation with Heart Failure. N. Engl. J. Med. 2018, 378, 417–427. [Google Scholar] [CrossRef]
- Prabhu, S.; Taylor, A.J.; Costello, B.T.; Kaye, D.M.; McLellan, A.J.A.; Voskoboinik, A.; Sugumar, H.; Lockwood, S.M.; Stokes, M.B.; Pathik, B.; et al. Catheter Ablation Versus Medical Rate Control in Atrial Fibrillation and Systolic Dysfunction: The CAMERA-MRI Study. J. Am. Coll. Cardiol. 2017, 70, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.V.; Simon, D.N.; Go, A.S.; Spertus, J.; Fonarow, G.C.; Gersh, B.J.; Hylek, E.M.; Kowey, P.R.; Mahaffey, K.W.; Thomas, L.E.; et al. Association between Atrial Fibrillation Symptoms, Quality of Life, and Patient Outcomes: Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Circ. Cardiovasc. Qual. Outcomes 2015, 8, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, B.A.; Kim, S.; Fonarow, G.C.; Thomas, L.; Ansell, J.; Kowey, P.R.; Mahaffey, K.W.; Gersh, B.J.; Hylek, E.; Naccarelli, G.; et al. Drivers of Hospitalization for Patients with Atrial Fibrillation: Results from the Outcomes Registry for Better Informed Treatment of Atrial Fibrillation (ORBIT-AF). Am. Heart J. 2014, 167, 735–742.e2. [Google Scholar] [CrossRef] [PubMed]
- Meyre, P.; Blum, S.; Berger, S.; Aeschbacher, S.; Schoepfer, H.; Briel, M.; Osswald, S.; Conen, D. Risk of Hospital Admissions in Patients With Atrial Fibrillation: A Systematic Review and Meta-Analysis. Can. J. Cardiol. 2019, 35, 1332–1343. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, C.; Niiranen, T.J.; Ojeda, F.M.; Gianfagna, F.; Blankenberg, S.; Njølstad, I.; Vartiainen, E.; Sans, S.; Pasterkamp, G.; Hughes, M.; et al. Sex Differences and Similarities in Atrial Fibrillation Epidemiology, Risk Factors and Mortality in Community Cohorts: Results from the BiomarCaRE. Circulation 2017, 136, 1588. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Wolf, P.A.; D’Agostino, R.B.; Silbershatz, H.; Kannel, W.B.; Levy, D. Impact of Atrial Fibrillation on the Risk of Death. Circulation 1998, 98, 946–952. [Google Scholar] [CrossRef]
- Marijon, E.; Le Heuzey, J.Y.; Connolly, S.; Yang, S.; Pogue, J.; Brueckmann, M.; Eikelboom, J.; Themeles, E.; Ezekowitz, M.; Wallentin, L.; et al. Causes of Death and Influencing Factors in Patients with Atrial Fibrillation: A Competing-Risk Analysis from the Randomized Evaluation of Long-Term Anticoagulant Therapy Study. Circulation 2013, 128, 2192–2201. [Google Scholar] [CrossRef]
- Kishore, A.; Vail, A.; Majid, A.; Dawson, J.; Lees, K.R.; Tyrrell, P.J.; Smith, C.J. Detection of Atrial Fibrillation after Ischemic Stroke or Transient Ischemic Attack: A Systematic Review and Meta-Analysis. Stroke 2014, 45, 520–526. [Google Scholar] [CrossRef]
- Svennberg, E.; Engdahl, J.; Al-Khalili, F.; Friberg, L.; Frykman, V.; Rosenqvist, M. Mass Screening for Untreated Atrial Fibrillation: The STROKESTOP Study. Circulation 2015, 131, 2176–2184. [Google Scholar] [CrossRef]
- Essa, H.; Hill, A.M.; Lip, G.Y.H. Atrial Fibrillation and Stroke. Card. Electrophysiol. Clin. 2021, 13, 243–255. [Google Scholar] [CrossRef]
- Bekwelem, W.; Connolly, S.J.; Halperin, J.L.; Adabag, S.; Duval, S.; Chrolavicius, S.; Pogue, J.; Ezekowitz, M.D.; Eikelboom, J.W.; Wallentin, L.G.; et al. Extracranial Systemic Embolic Events in Patients with Nonvalvular Atrial Fibrillation: Incidence, Risk Factors, and Outcomes. Circulation 2015, 132, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.E.; Naditch-Brûlé, L.; Murin, J.; Goethals, M.; Inoue, H.; O-Neill, J.; Silva-Cardoso, J.; Zharinov, O.; Gamra, H.; Alam, S.; et al. Distribution and Risk Profile of Paroxysmal, Persistent, and Permanent Atrial Fibrillation in Routine Clinical Practice: Insight from the Real-Life Global Survey Evaluating Patients with Atrial Fibrillation International Registry. Circ. Arrhythm. Electrophysiol. 2012, 5, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, D.; Lam, C.S.P.; Van Veldhuisen, D.J.; Van Gelder, I.C.; Voors, A.A.; Rienstra, M. Heart Failure With Preserved Ejection Fraction and Atrial Fibrillation: Vicious Twins. J. Am. Coll. Cardiol. 2016, 68, 2217–2228. [Google Scholar] [CrossRef]
- Santhanakrishnan, R.; Wang, N.; Larson, M.G.; Magnani, J.W.; McManus, D.D.; Lubitz, S.A.; Ellinor, P.T.; Cheng, S.; Vasan, R.S.; Lee, D.S.; et al. Atrial Fibrillation Begets Heart Failure and Vice Versa: Temporal Associations and Differences in Preserved versus Reduced Ejection Fraction. Circulation 2016, 133, 484–492. [Google Scholar] [CrossRef]
- Sartipy, U.; Dahlström, U.; Fu, M.; Lund, L.H. Atrial Fibrillation in Heart Failure With Preserved, Mid-Range, and Reduced Ejection Fraction. JACC Heart Fail. 2017, 5, 565–574. [Google Scholar] [CrossRef]
- Kwok, C.S.; Loke, Y.K.; Hale, R.; Potter, J.F.; Myint, P.K. Atrial Fibrillation and Incidence of Dementia. Neurology 2011, 76, 914–922. [Google Scholar] [CrossRef]
- Gherasim, L. Subclinical Atrial Fibrillation, Embolic Risk, and Anticoagulant Treatment. Maedica 2018, 13, 261. [Google Scholar]
- Alturki, A.; Marafi, M.; Russo, V.; Proietti, R.; Essebag, V. Medicina Subclinical Atrial Fibrillation and Risk of Stroke: Past, Present and Future. Medicina 2019, 55, 611. [Google Scholar] [CrossRef]
- Rillig, A.; Magnussen, C.; Ozga, A.K.; Suling, A.; Brandes, A.; Breithardt, G.; Camm, A.J.; Crijns, H.J.G.M.; Eckardt, L.; Elvan, A.; et al. Early Rhythm Control Therapy in Patients with Atrial Fibrillation and Heart Failure. Circulation 2021, 144, 845–858. [Google Scholar] [CrossRef]
- Rillig, A.; Borof, K.; Breithardt, G.; Camm, A.J.; Crijns, H.J.G.M.; Goette, A.; Kuck, K.H.; Metzner, A.; Vardas, P.; Vettorazzi, E.; et al. Early Rhythm Control in Patients With Atrial Fibrillation and High Comorbidity Burden. Circulation 2022, 146, 836–847. [Google Scholar] [CrossRef]
- Deshpande, R.; Al Khadra, Y.; Al-Tamimi, R.; Albast, N.; Labedi, M. Atrial Fibrillation: Rate Control or Rhythm Control? Clevel. Clin. J. Med. 2022, 89, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yang, P.S.; Joung, B. Optimal Rhythm Control Strategy in Patients With Atrial Fibrillation. Korean Circ. J. 2022, 52, 496. [Google Scholar] [CrossRef]
- Kelly, J.P.; Devore, A.D.; Wu, J.J.; Hammill, B.G.; Sharma, A.; Cooper, L.B.; Felker, G.M.; Piccini, J.P.; Allen, L.A.; Heidenreich, P.A.; et al. Rhythm Control versus Rate Control in Patients with Atrial Fibrillation and Heart Failure with Preserved Ejection Fraction: Insights from Get with the Guidelines—Heart Failure. J. Am. Heart Assoc. 2019, 8, e011560. [Google Scholar] [CrossRef] [PubMed]
- Díez-Villanueva, P.; Alfonso, F. Atrial Fibrillation in the Elderly. J. Geriatr. Cardiol. 2019, 16, 49. [Google Scholar] [CrossRef]
- Karamichalakis, N.; Letsas, K.P.; Vlachos, K.; Georgopoulos, S.; Bakalakos, A.; Efremidis, M.; Sideris, A. Managing Atrial Fibrillation in the Very Elderly Patient: Challenges and Solutions. Vasc. Health Risk Manag. 2015, 11, 555. [Google Scholar] [CrossRef]
- Volgman, A.S.; Nair, G.; Lyubarova, R.; Merchant, F.M.; Mason, P.; Curtis, A.B.; Wenger, N.K.; Aggarwal, N.T.; Kirkpatrick, J.N.; Benjamin, E.J. Management of Atrial Fibrillation in Patients 75 Years and Older: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2022, 79, 166–179. [Google Scholar] [CrossRef]
- Pang, L.; Sager, P.; Yang, X.; Shi, H.; Sannajust, F.; Brock, M.; Wu, J.C.; Abi-Gerges, N.; Lyn-Cook, B.; Berridge, B.R.; et al. The FDA Workshop on Improving Cardiotoxicity Assessment with Human-Relevant Platforms. Circ. Res. 2019, 125, 855. [Google Scholar] [CrossRef]
- Dan, G.A.; Martinez-Rubio, A.; Agewall, S.; Boriani, G.; Borggrefe, M.; Gaita, F.; Van Gelder, I.; Gorenek, B.; Kaski, J.C.; Kjeldsen, K.; et al. Antiarrhythmic Drugs-Clinical Use and Clinical Decision Making: A Consensus Document from the European Heart Rhythm Association (EHRA) and European Society of Cardiology (ESC) Working Group on Cardiovascular Pharmacology, Endorsed by the Heart Rhythm Society (HRS), Asia-Pacific Heart Rhythm Society (APHRS) and International Society of Cardiovascular Pharmacotherapy (ISCP). Europace 2018, 20, 731–732. [Google Scholar] [CrossRef]
- Wang, F.; Zhou, B.; Sun, H.; Wu, X. Proarrhythmia Associated with Antiarrhythmic Drugs: A Comprehensive Disproportionality Analysis of the FDA Adverse Event Reporting System. Front. Pharmacol. 2023, 14, 1327. [Google Scholar] [CrossRef]
- Markman, T.M.; Geng, Z.; Epstein, A.E.; Nazarian, S.; Deo, R.; Marchlinski, F.E.; Groeneveld, P.W.; Frankel, D.S. Trends in Antiarrhythmic Drug Use among Patients in the United States between 2004 and 2016. Circulation 2020, 141, 937–939. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Alpert, J.S.; Calkins, H.; Cigarroa, J.E.; Cleveland, J.C.; Conti, J.B.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation 2014, 130, e199–e267. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J. Hopes and Disappointments with Antiarrhythmic Drugs. Int. J. Cardiol. 2017, 237, 71–74. [Google Scholar] [CrossRef]
- Calkins, H.; Hindricks, G.; Cappato, R.; Kim, Y.H.; Saad, E.B.; Aguinaga, L.; Akar, J.G.; Badhwar, V.; Brugada, J.; Camm, J.; et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE Expert Consensus Statement on Catheter and Surgical Ablation of Atrial Fibrillation. Heart Rhythm. 2017, 14, e275–e444. [Google Scholar] [CrossRef] [PubMed]
- Mujović, N.; Marinković, M.; Lenarczyk, R.; Tilz, R.; Potpara, T.S. Catheter Ablation of Atrial Fibrillation: An Overview for Clinicians. Adv. Ther. 2017, 34, 1897. [Google Scholar] [CrossRef]
- Katritsis, D.G.; Zografos, T.; Katritsis, G.D.; Giazitzoglou, E.; Vachliotis, V.; Paxinos, G.; Camm, A.J.; Josephson, M.E. Catheter Ablation vs. Antiarrhythmic Drug Therapy in Patients with Symptomatic Atrioventricular Nodal Re-Entrant Tachycardia: A Randomized, Controlled Trial. EP Europace 2017, 19, 602–606. [Google Scholar] [CrossRef]
- Hollanda Oliveira, L.; Viana, M.D.S.; Luize, C.M.; de Carvalho, R.S.; Cirenza, C.; de Oliveira Dietrich, C.; Correia, L.C.; Das Virgens, C.; Filgueiras, J.M.; Barreto, M.; et al. Underuse of Catheter Ablation as First-Line Therapy for Supraventricular Tachycardia. J. Am. Heart Assoc. 2022, 11, 22648. [Google Scholar] [CrossRef]
- Huang, K.; Bennett, R.G.; Campbell, T.; Lee, V.; Turnbull, S.; Chik, W.W.B.; El-Sokkari, I.; Hallani, H.; Dieleman, J.; Kruit, N.; et al. Early Catheter Ablation Versus Initial Medical Therapy for Ventricular Tachycardia Storm. Circ. Arrhythm. Electrophysiol. 2022, 15, E011129. [Google Scholar] [CrossRef]
- Asad, Z.U.A.; Yousif, A.; Khan, M.S.; Al-Khatib, S.M.; Stavrakis, S. Catheter Ablation Versus Medical Therapy for Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2019, 12, e011560. [Google Scholar] [CrossRef]
- Leung, L.W.M.; Imhoff, R.J.; Marshall, H.J.; Frame, D.; Mallow, P.J.; Goldstein, L.; Wei, T.; Velleca, M.; Taylor, H.; Gallagher, M.M. Cost-Effectiveness of Catheter Ablation versus Medical Therapy for the Treatment of Atrial Fibrillation in the United Kingdom. J. Cardiovasc. Electrophysiol. 2022, 33, 164–175. [Google Scholar] [CrossRef]
- Chew, D.S.; Li, Y.; Cowper, P.A.; Anstrom, K.J.; Piccini, J.P.; Poole, J.E.; Daniels, M.R.; Monahan, K.H.; Davidson-Ray, L.; Bahnson, T.D.; et al. Cost-Effectiveness of Catheter Ablation Versus Antiarrhythmic Drug Therapy in Atrial Fibrillation: The CABANA Randomized Clinical Trial. Circulation 2022, 146, 535–547. [Google Scholar] [CrossRef]
- Khurshid, R.; Awais, M.; Malik, J. Electrophysiology Practice in Low- and Middle-Income Countries: An Updated Review on Access to Care and Health Delivery. Heart Rhythm. O2 2023, 4, 69. [Google Scholar] [CrossRef]
- Namdar, M.; Shah, D. Staffing, Training, and Ongoing Volume Requirements. In Practical Guide to Catheter Ablation of Atrial Fibrillation, 2nd ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 17–22. [Google Scholar] [CrossRef]
- Calkins, H. Catheter Ablation to Maintain Sinus Rhythm. Circulation 2012, 125, 1439–1445. [Google Scholar] [CrossRef]
- Ioannidis, P.; Zografos, T.; Christoforatou, E.; Kouvelas, K.; Tsoumeleas, A.; Vassilopoulos, C. The Electrophysiology of Atrial Fibrillation: From Basic Mechanisms to Catheter Ablation. Cardiol. Res. Pract. 2021, 2021, 4109269. [Google Scholar] [CrossRef]
- Lei, M.; Wu, L.; Terrar, D.A.; Huang, C.L.-H. Modernized Classification of Cardiac Antiarrhythmic Drugs. Circulation 2018, 138, 1879–1896. [Google Scholar] [CrossRef]
- January, C.T.; Wann, L.S.; Calkins, H.; Chen, L.Y.; Cigarroa, J.E.; Cleveland, J.C.; Ellinor, P.T.; Ezekowitz, M.D.; Field, M.E.; Furie, K.L.; et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients with Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration with the Society of Thoracic Surgeons. Circulation 2019, 140, e125–e151. [Google Scholar] [CrossRef]
- Zimmer, T.; Haufe, V.; Blechschmidt, S. Voltage-Gated Sodium Channels in the Mammalian Heart. Glob. Cardiol. Sci. Pract. 2014, 2014, 449. [Google Scholar] [CrossRef]
- King, G.S.; Goyal, A.; Grigorova, Y.; Hashmi, M.F. Antiarrhythmic Medications. Semin. Dial. 2023, 3, 33–38. [Google Scholar] [CrossRef]
- Landstrom, A.P.; Dobrev, D.; Wehrens, X.H.T. Calcium Signaling and Cardiac Arrhythmias. Circ. Res. 2017, 120, 1969–1993. [Google Scholar] [CrossRef]
- Markman, T.M.; Jarrah, A.A.; Tian, Y.; Mustin, E.; Guandalini, G.S.; Lin, D.; Epstein, A.E.; Hyman, M.C.; Deo, R.; Supple, G.E.; et al. Safety of Pill-in-the-Pocket Class 1C Antiarrhythmic Drugs for Atrial Fibrillation. Clin. Electrophysiol. 2022, 8, 1515–1520. [Google Scholar] [CrossRef]
- Florek, J.B.; Girzadas, D. Amiodarone. In Encyclopedia of Toxicology, 3rd ed.; Lithuanian University of Health Sciences: Kaunas, Lithuania, 2023; pp. 197–199. [Google Scholar] [CrossRef]
- Merino, J.; Perez de Isla, L. Treatment with Amiodarone: How to Avoid Complications. Available online: https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-10/Treatment-with-amiodarone-How-to-avoid-complications (accessed on 27 May 2023).
- Saljic, A.; Heijman, J.; Dobrev, D. Emerging Antiarrhythmic Drugs for Atrial Fibrillation. Int. J. Mol. Sci. 2022, 23, 4096. [Google Scholar] [CrossRef]
- Hall, A.J.M.; Mitchell, A.R.J. Introducing Vernakalant into Clinical Practice. Arrhythm. Electrophysiol. Rev. 2019, 8, 70. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.W.M. Management of Atrial Fibrillation: Focus on the Role of Dronedarone. Open Access Emerg. Med. 2011, 3, 55. [Google Scholar] [CrossRef][Green Version]
- Fedida, D.; Orth, P.; Chen, Y.; Lin, S.; Plouvier, B.; Jung, G.; Ezrin, A.M.; Beatch, G.N. The Mechanism of Atrial Antiarrhythmic Action of RSD1235. J. Cardiovasc. Electrophysiol. 2005, 16, 1227–1238. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.B.; de Lange, E.; Garfinkel, A.; Weiss, J.N.; Qu, Z. Delayed Afterdepolarizations Generate Both Triggers and a Vulnerable Substrate Promoting Reentry in Cardiac Tissue. Heart Rhythm. 2015, 12, 2115–2124. [Google Scholar] [CrossRef] [PubMed]
- Neef, S.; Dybkova, N.; Sossalla, S.; Ort, K.R.; Fluschnik, N.; Neumann, K.; Seipelt, R.; Schöndube, F.A.; Hasenfuss, G.; Maier, L.S. CaMKII-Dependent Diastolic SR Ca2+ Leak and Elevated Diastolic Ca2+ Levels in Right Atrial Myocardium of Patients with Atrial Fibrillation. Circ. Res. 2010, 106, 1134–1144. [Google Scholar] [CrossRef]
- Hwang, H.S.; Hasdemir, C.; Laver, D.; Mehra, D.; Turhan, K.; Faggioni, M.; Yin, H.; Knollmann, B.C. Inhibition of Cardiac Ca2+ Release Channels (RyR2) Determines Efficacy of Class I Antiarrhythmic Drugs in Catecholaminergic Polymorphic Ventricular Tachycardia. Circ. Arrhythm. Electrophysiol. 2011, 4, 128–135. [Google Scholar] [CrossRef]
- Ratte, A.; Wiedmann, F.; Kraft, M.; Katus, H.A.; Schmidt, C. Antiarrhythmic Properties of Ranolazine: Inhibition of Atrial Fibrillation Associated TASK-1 Potassium Channels. Front. Pharmacol. 2019, 10, 1367. [Google Scholar] [CrossRef]
- Hartmann, N.; Pabel, S.; Herting, J.; Schatter, F.; Renner, A.; Gummert, J.; Schotola, H.; Danner, B.C.; Maier, L.S.; Frey, N.; et al. Antiarrhythmic Effects of Dantrolene in Human Diseased Cardiomyocytes. Heart Rhythm. 2017, 14, 412–419. [Google Scholar] [CrossRef]
- Gungor, B.; Ekmekci, A.; Arman, A.; Ozcan, K.S.; Ucer, E.; Alper, A.T.; Calik, N.; Yilmaz, H.; Tezel, T.; Coker, A.; et al. Assessment of Interleukin-1 Gene Cluster Polymorphisms in Lone Atrial Fibrillation: New Insight into the Role of Inflammation in Atrial Fibrillation. Pacing Clin. Electrophysiol. 2013, 36, 1220–1227. [Google Scholar] [CrossRef]
- Yao, C.; Veleva, T.; Scott, L.; Cao, S.; Li, L.; Chen, G.; Jeyabal, P.; Pan, X.; Alsina, K.M.; Abu-Taha, I.; et al. Enhanced Cardiomyocyte NLRP3 Inflammasome Signaling Promotes Atrial Fibrillation. Circulation 2018, 138, 2227–2242. [Google Scholar] [CrossRef]
- Misawa, T.; Takahama, M.; Kozaki, T.; Lee, H.; Zou, J.; Saitoh, T.; Akira, S. Microtubule-Driven Spatial Arrangement of Mitochondria Promotes Activation of the NLRP3 Inflammasome. Nat. Immunol. 2013, 14, 454–460. [Google Scholar] [CrossRef]
- Ravens, U.; Odening, K.E. Atrial Fibrillation: Therapeutic Potential of Atrial K + Channel Blockers. Pharmacol. Ther. 2017, 176, 13–21. [Google Scholar] [CrossRef]
- Ravens, U.; Wettwer, E. Ultra-Rapid Delayed Rectifier Channels: Molecular Basis and Therapeutic Implications. Cardiovasc. Res. 2011, 89, 776–785. [Google Scholar] [CrossRef]
- Voigt, N.; Rozmaritsa, N.; Trausch, A.; Zimniak, T.; Christ, T.; Wettwer, E.; Matschke, K.; Dobrev, D.; Ravens, U. Inhibition of IK,ACh Current May Contribute to Clinical Efficacy of Class I and Class III Antiarrhythmic Drugs in Patients with Atrial Fibrillation. Naunyn Schmiedebergs Arch. Pharmacol. 2010, 381, 251–259. [Google Scholar] [CrossRef]
- Dobrev, D.; Nattel, S. New Antiarrhythmic Drugs for Treatment of Atrial Fibrillation. Lancet 2010, 375, 1212–1223. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Wiedmann, F.; Beyersdorf, C.; Zhao, Z.; El-Battrawy, I.; Lan, H.; Szabo, G.; Li, X.; Lang, S.; Korkmaz-Icöz, S.; et al. Genetic Ablation of TASK-1 (Tandem of P Domains in a Weak Inward Rectifying K+ Channel-Related Acid-Sensitive K+ Channel-1) (K2P3.1) K+ Channels Suppresses Atrial Fibrillation and Prevents Electrical Remodeling. Circ. Arrhythm. Electrophysiol. 2019, 12, e007465. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, M.; Qi, X.Y.; Huang, H.; Comtois, P.; Nattel, S. Fibroblast Electrical Remodeling in Heart Failure and Potential Effects on Atrial Fibrillation. Biophys. J. 2014, 107, 2444–2455. [Google Scholar] [CrossRef][Green Version]
- Chen, K.-H.; Xu, X.-H.; Sun, H.-Y.; Du, X.-L.; Liu, H.; Yang, L.; Xiao, G.-S.; Wang, Y.; Jin, M.-W.; Li, G.-R. Distinctive Property and Pharmacology of Voltage-Gated Sodium Current in Rat Atrial vs Ventricular Myocytes. Heart Rhythm. 2016, 13, 762–770. [Google Scholar] [CrossRef]
- Orth, P.; Hesketh, J.; Mak, C.; Yang, Y.; Lin, S.; Beatch, G.; Ezrin, A.; Fedida, D. RSD1235 Blocks Late INa and Suppresses Early Afterdepolarizations and Torsades de Pointes Induced by Class III Agents. Cardiovasc. Res. 2006, 70, 486–496. [Google Scholar] [CrossRef]
- Miller, J.M.; Ellenbogen, A. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine; Elsevier: Amsterdam, The Netherlands, 2023; Volume 64. [Google Scholar]
- Samuel, M.; Elsokkari, I.; Sapp, J.L. Ventricular Tachycardia Burden and Mortality: Association or Causality? Can. J. Cardiol. 2022, 38, 454–464. [Google Scholar] [CrossRef]
- Mankad, P.; Kalahasty, G. Antiarrhythmic Drugs. Med. Clin. N. Am. 2019, 103, 821–834. [Google Scholar] [CrossRef]
- Sun, W.; Chen, F.; Esmailian, F.; Sarma, J.S.; Wetzel, G.T.; Klitzner, T.S.; Singh, B.N. Acute Effects of Dronedarone on the Potassium Currents in Human Atrial Cells. J. Am. Coll. Cardiol. 2002, 39, 105. [Google Scholar] [CrossRef]
- Lalevée, N.; Barrère-lemaire, S.; Gautier, P.; Nargeot, J.; Richard, S. Effects of Amiodarone and Dronedarone on Voltage-Dependent Sodium Current in Human Cardiomyocytes. J. Cardiovasc. Electrophysiol. 2003, 14, 885–890. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Piccini, J.P.; Camm, A.J.; Crijns, H.J.G.M.; Anker, S.D.; Butler, J.; Stewart, J.; Braceras, R.; Albuquerque, A.P.A.; Wieloch, M.; et al. Dronedarone for the Treatment of Atrial Fibrillation with Concomitant Heart Failure with Preserved and Mildly Reduced Ejection Fraction: A post-hoc Analysis of the ATHENA Trial. Eur. J. Heart Fail. 2022, 24, 1094–1101. [Google Scholar] [CrossRef]
- Singh, J.P.; Blomström-Lundqvist, C.; Turakhia, M.P.; Camm, A.J.; Fazeli, M.S.; Kreidieh, B.; Crotty, C.; Kowey, P.R. Dronedarone versus Sotalol in Patients with Atrial Fibrillation: A Systematic Literature Review and Network Meta-analysis. Clin. Cardiol. 2023, 46, 589–597. [Google Scholar] [CrossRef]
- Diness, J.G.; Kirchhoff, J.E.; Speerschneider, T.; Abildgaard, L.; Edvardsson, N.; Sørensen, U.S.; Grunnet, M.; Bentzen, B.H. The KCa2 Channel Inhibitor AP30663 Selectively Increases Atrial Refractoriness, Converts Vernakalant-Resistant Atrial Fibrillation and Prevents Its Reinduction in Conscious Pigs. Front. Pharmacol. 2020, 11, 159. [Google Scholar] [CrossRef] [PubMed]
- Gal, P.; Klaassen, E.S.; Bergmann, K.R.; Saghari, M.; Burggraaf, J.; Kemme, M.J.B.; Sylvest, C.; Sørensen, U.; Bentzen, B.H.; Grunnet, M.; et al. First Clinical Study with AP30663—A KCa2 Channel Inhibitor in Development for Conversion of Atrial Fibrillation. Clin. Transl. Sci. 2020, 13, 1336–1344. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, F.; Beyersdorf, C.; Zhou, X.B.; Kraft, M.; Paasche, A.; Jávorszky, N.; Rinné, S.; Sutanto, H.; Büscher, A.; Foerster, K.I.; et al. Treatment of Atrial Fibrillation with Doxapram: TASK-1 Potassium Channel Inhibition as a Novel Pharmacological Strategy. Cardiovasc. Res. 2022, 118, 1728–1741. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Büscher, A.; Wiedmann, F.; L’hoste, Y.; Haefeli, W.E.; Frey, N.; Katus, H.A.; Schmidt, C. Current Drug Treatment Strategies for Atrial Fibrillation and TASK-1 Inhibition as an Emerging Novel Therapy Option. Front. Pharmacol. 2021, 12, 638445. [Google Scholar] [CrossRef] [PubMed]
- Calvert, P.; Gupta, D.; Lip, G.Y.H. Antazoline: The Lazarus of Antiarrhythmic Drugs? Pol. Arch. Intern. Med. 2022, 132, 16264. [Google Scholar] [CrossRef]
- Wybraniec, M.T.; Maciąg, A.; Miśkowiec, D.; Ceynowa-Sielawko, B.; Balsam, P.; Wójcik, M.; Wróbel, W.; Farkowski, M.; Ćwiek-Rębowska, E.; Szołkiewicz, M.; et al. Efficacy and Safety of Antazoline for Cardioversion of Atrial Fibrillation: Propensity Score Matching Analysis of Multicenter Registry (CANT II Study). Pol. Arch. Intern. Med. 2022, 132, 16234. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Yu, L.; Chen, Z.; Zhang, M. Nifekalant. JACC Case Rep. 2020, 2, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, K.; Wang, S.; Liu, M.; Wang, L.; Su, G. Meta-analysis of the Efficacy and Safety of Nifekalant in the Conversion of Atrial Fibrillation. Exp. Ther. Med. 2022, 25, 56. [Google Scholar] [CrossRef]
- Maykov, E.B.; Yuricheva, Y.A.; Mironov, N.Y.; Sokolov, S.F.; Golitsyn, S.P.; Rozenshtraukh, L.V.; Chazov, E.I. Refralon (Niferidil) Is a New Class III Antiarrhythmic Agent for Pharmacological Cardioversion for Persistent Atrial Fibrillation and Atrial Flutter. Ter. Arkhiv 2015, 87, 38. [Google Scholar] [CrossRef] [PubMed]
- Dzaurova, K.M.; Mironov, N.Y.; Yuricheva, Y.A.; Vlodzyanovsky, V.V.; Mironova, N.A.; Laiovich, L.Y.; Malkina, T.A.; Zinchenko, L.V.; Sokolov, S.F.; Golitsyn, S.P. Efficiency and Safety of Using the Modified Protocol for the Administration of the Domestic Class III Antiarrhythmic Drug for the Relief of Paroxysmal Atrial Fibrillation. Ter. Arkhiv 2021, 93, 1052–1057. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Khan, K.; Kherallah, R.; Khan, S.; Kamat, I.; Ulhaq, O.; Marashly, Q.; Chelu, M.G. Innovations in Atrial Fibrillation Ablation. J. Interv. Card. Electrophysiol. 2022, 66, 737–756. [Google Scholar] [CrossRef]
- Buist, T.J.; Zipes, D.P.; Elvan, A. Atrial Fibrillation Ablation Strategies and Technologies: Past, Present, and Future. Clin. Res. Cardiol. 2021, 110, 775–788. [Google Scholar] [CrossRef]
- Haegeli, L.M.; Calkins, H. Catheter Ablation of Atrial Fibrillation: An Update. Eur. Heart J. 2014, 35, 2454–2459. [Google Scholar] [CrossRef]
- Sawhney, N.; Anousheh, R.; Chen, W.; Feld, G.K. Circumferential Pulmonary Vein Ablation With Additional Linear Ablation Results in an Increased Incidence of Left Atrial Flutter Compared With Segmental Pulmonary Vein Isolation as an Initial Approach to Ablation of Paroxysmal Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2010, 3, 243–248. [Google Scholar] [CrossRef]
- Sau, A.; Howard, J.P.; Al-Aidarous, S.; Ferreira-Martins, J.; Al-Khayatt, B.; Lim, P.B.; Kanagaratnam, P.; Whinnett, Z.I.; Peters, N.S.; Sikkel, M.B.; et al. Meta-Analysis of Randomized Controlled Trials of Atrial Fibrillation Ablation With Pulmonary Vein Isolation Versus Without. JACC Clin. Electrophysiol. 2019, 5, 968–976. [Google Scholar] [CrossRef]
- Quintanilla, J.G.; Pérez-Villacastín, J.; Pérez-Castellano, N.; Pandit, S.V.; Berenfeld, O.; Jalife, J.; Filgueiras-Rama, D. Mechanistic Approaches to Detect, Target, and Ablate the Drivers of Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2016, 9, e002481. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.A.; Tai, C.T. Catheter Ablation of Atrial Fibrillation Originating from the Non-Pulmonary Vein Foci. J. Cardiovasc. Electrophysiol. 2005, 16, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Van Campenhout, M.J.H.; Yaksh, A.; Kik, C.; De Jaegere, P.P.; Ho, S.Y.; Allessie, M.A.; De Groot, N.M.S. Bachmann’s Bundle a Key Player in the Development of Atrial Fibrillation? Circ. Arrhythm. Electrophysiol. 2013, 6, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Teuwen, C.P.; Yaksh, A.; Lanters, E.A.H.; Kik, C.; Van Der Does, L.J.M.E.; Knops, P.; Taverne, Y.J.H.J.; Van De Woestijne, P.C.; Oei, F.B.S.; Bekkers, J.A.; et al. Relevance of Conduction Disorders in Bachmann’s Bundle During Sinus Rhythm in Humans. Circ. Arrhythm. Electrophysiol. 2016, 9, e003972. [Google Scholar] [CrossRef]
- van Staveren, L.N.; van der Does, W.F.B.; Heida, A.; Taverne, Y.J.H.J.; Bogers, A.J.J.C.; de Groot, N.M.S. Af Inducibility Is Related to Conduction Abnormalities at Bachmann’s Bundle. J. Clin. Med. 2021, 10, 5536. [Google Scholar] [CrossRef]
- Hartley, A.; Shalhoub, J.; Ng, F.S.; Krahn, A.D.; Laksman, Z.; Andrade, J.G.; Deyell, M.W.; Kanagaratnam, P.; Sikkel, M.B. Size Matters in Atrial Fibrillation: The Underestimated Importance of Reduction of Contiguous Electrical Mass Underlying the Effectiveness of Catheter Ablation. EP Europace 2021, 23, 1698–1707. [Google Scholar] [CrossRef] [PubMed]
- Hassink, R.J.; Aretz, H.T.; Ruskin, J.; Keane, D. Morphology of Atrial Myocardium in Human Pulmonary Veins: A Postmortem Analysis in Patients with and without Atrial Fibrillation. J. Am. Coll. Cardiol. 2003, 42, 1108–1114. [Google Scholar] [CrossRef]
- Arentz, T.; Weber, R.; Bürkle, G.; Herrera, C.; Blum, T.; Stockinger, J.; Minners, J.; Neumann, F.J.; Kalusche, D. Small or Large Isolation Areas Around the Pulmonary Veins for the Treatment of Atrial Fibrillation? Circulation 2007, 115, 3057–3063. [Google Scholar] [CrossRef]
- Proietti, R.; Santangeli, P.; Di Biase, L.; Joza, J.; Bernier, M.L.; Wang, Y.; Sagone, A.; Viecca, M.; Essebag, V.; Natale, A. Comparative Effectiveness of Wide Antral versus Ostial Pulmonary Vein Isolation: A Systematic Review and Meta-Analysis. Circ. Arrhythm. Electrophysiol. 2014, 7, 39–45. [Google Scholar] [CrossRef]
- Kuck, K.H.; Hoffmann, B.A.; Ernst, S.; Wegscheider, K.; Treszl, A.; Metzner, A.; Eckardt, L.; Lewalter, T.; Breithardt, G.; Willems, S. Impact of Complete Versus Incomplete Circumferential Lines Around the Pulmonary Veins During Catheter Ablation of Paroxysmal Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2016, 9, e003337. [Google Scholar] [CrossRef]
- Liu, W.; Li, S.; Han, B. It Is Necessary to Re-Understand the Low-Voltage Area in Atrial Fibrillation Patients. Front. Cardiovasc. Med. 2022, 9, 919873. [Google Scholar] [CrossRef] [PubMed]
- Platonov, P.G.; Mitrofanova, L.B.; Orshanskaya, V.; Ho, S.Y. Structural Abnormalities in Atrial Walls Are Associated With Presence and Persistency of Atrial Fibrillation but Not with Age. J. Am. Coll. Cardiol. 2011, 58, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Oakes, R.S.; Badger, T.J.; Kholmovski, E.G.; Akoum, N.; Burgon, N.S.; Fish, E.N.; Blauer, J.J.E.; Rao, S.N.; Dibella, E.V.R.; Segerson, N.M.; et al. Detection and Quantification of Left Atrial Structural Remodeling with Delayed-Enhancement Magnetic Resonance Imaging in Patients with Atrial Fibrillation. Circulation 2009, 119, 1758–1767. [Google Scholar] [CrossRef] [PubMed]
- Marrouche, N.F.; Wilber, D.; Hindricks, G.; Jais, P.; Akoum, N.; Marchlinski, F.; Kholmovski, E.; Burgon, N.; Hu, N.; Mont, L.; et al. Association of Atrial Tissue Fibrosis Identified by Delayed Enhancement MRI and Atrial Fibrillation Catheter Ablation: The DECAAF Study. JAMA 2014, 311, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Gaspar, T.; Pohl, M.; Sitzy, J.; Richter, U.; Neudeck, S.; Mayer, J.; Kronborg, M.B.; Piorkowski, C. Prevalence and Predictors of Low Voltage Zones in the Left Atrium in Patients with Atrial Fibrillation. EP Europace 2018, 20, 956–962. [Google Scholar] [CrossRef]
- Moustafa, A.; Karim, S.; Kahaly, O.; Elzanaty, A.; Meenakshisundaram, C.; Abi-Saleh, B.; Eltahawy, E.; Chacko, P. Low Voltage Area Guided Substrate Modification in Nonparoxysmal Atrial Fibrillation: A Systematic Review and Meta-Analysis. J. Cardiovasc. Electrophysiol. 2023, 34, 455–464. [Google Scholar] [CrossRef]
- Yang, G.; Zheng, L.; Jiang, C.; Fan, J.; Liu, X.; Zhan, X.; Li, J.; Wang, L.; Yang, H.; Zhu, W.; et al. Circumferential Pulmonary Vein Isolation Plus Low-Voltage Area Modification in Persistent Atrial Fibrillation: The STABLE-SR-II Trial. JACC Clin. Electrophysiol. 2022, 8, 882–891. [Google Scholar] [CrossRef]
- Nademanee, K.; McKenzie, J.; Kosar, E.; Schwab, M.; Sunsaneewitayakul, B.; Vasavakul, T.; Khunnawat, C.; Ngarmukos, T. A New Approach for Catheter Ablation of Atrial Fibrillation: Mapping of the Electrophysiologic Substrate. J. Am. Coll. Cardiol. 2004, 43, 2044–2053. [Google Scholar] [CrossRef]
- De Bakker, J.M.T.; Wittkampf, F.H.M. The Pathophysiologic Basis of Fractionated and Complex Electrograms and the Impact of Recording Techniques on Their Detection and Interpretation. Circ. Arrhythm. Electrophysiol. 2010, 3, 204–213. [Google Scholar] [CrossRef]
- Verma, A.; Mantovan, R.; MacLe, L.; De Martino, G.; Chen, J.; Morillo, C.A.; Novak, P.; Calzolari, V.; Guerra, P.G.; Nair, G.; et al. Substrate and Trigger Ablation for Reduction of Atrial Fibrillation (STAR AF): A Randomized, Multicentre, International Trial. Eur. Heart J. 2010, 31, 1344–1356. [Google Scholar] [CrossRef]
- Latchamsetty, R.; Kocheril, A.G. Review of Dominant Frequency Analysis in Atrial Fibrillation. J. Atr. Fibrill. 2009, 2, 204. [Google Scholar] [CrossRef][Green Version]
- Atienza, F.; Almendral, J.; Jalife, J.; Zlochiver, S.; Ploutz-Snyder, R.; Torrecilla, E.G.; Arenal, Á.; Kalifa, J.; Fernández-Avilés, F.; Berenfeld, O. Real-Time Dominant Frequency Mapping and Ablation of Dominant Frequency Sites in Atrial Fibrillation with Left-to-Right Frequency Gradients Predicts Long-Term Maintenance of Sinus Rhythm. Heart Rhythm. 2009, 6, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Lakkireddy, D.; Wulffhart, Z.; Pillarisetti, J.; Farina, D.; Beardsall, M.; Whaley, B.; Giewercer, D.; Tsang, B.; Khaykin, Y. Relationship between Complex Fractionated Electrograms (CFE) and Dominant Frequency (DF) Sites and Prospective Assessment of Adding DF-Guided Ablation to Pulmonary Vein Isolation in Persistent Atrial Fibrillation (AF). J. Cardiovasc. Electrophysiol. 2011, 22, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Atienza, F.; Almendral, J.; Ormaetxe, J.M.; Moya, Á.; Martínez-Alday, J.D.; Hernández-Madrid, A.; Castellanos, E.; Arribas, F.; Arias, M.Á.; Tercedor, L.; et al. Comparison of Radiofrequency Catheter Ablation of Drivers and Circumferential Pulmonary Vein Isolation in Atrial Fibrillation: A Noninferiority Randomized Multicenter RADAR-AF Trial. J. Am. Coll. Cardiol. 2014, 64, 2455–2467. [Google Scholar] [CrossRef] [PubMed]
- Vaquero, M.; Calvo, D.; Jalife, J. Cardiac Fibrillation: From Ion Channels to Rotors in the Human Heart. Heart Rhythm. 2008, 5, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.M.; Krummen, D.E.; Clopton, P.; Shivkumar, K.; Miller, J.M. Direct or Coincidental Elimination of Stable Rotors or Focal Sources May Explain Successful Atrial Fibrillation Ablation: On-Treatment Analysis of the CONFIRM Trial (Conventional Ablation for AF with or without Focal Impulse and Rotor Modulation). J. Am. Coll. Cardiol. 2013, 62, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Park, H.W.; Shen, M.J.; Lin, S.F.; Fishbein, M.C.; Chen, L.S.; Chen, P.S. Neural Mechanisms of Atrial Fibrillation. Curr. Opin. Cardiol. 2012, 27, 24. [Google Scholar] [CrossRef]
- Lemery, R.; Birnie, D.; Tang, A.S.L.; Green, M.; Gollob, M. Feasibility Study of Endocardial Mapping of Ganglionated Plexuses during Catheter Ablation of Atrial Fibrillation. Heart Rhythm. 2006, 3, 387–396. [Google Scholar] [CrossRef]
- Avazzadeh, S.; McBride, S.; O’brien, B.; Coffey, K.; Elahi, A.; O’Halloran, M.; Soo, A.; Quinlan, L.R. Ganglionated Plexi Ablation for the Treatment of Atrial Fibrillation. J. Clin. Med. 2020, 9, 3081. [Google Scholar] [CrossRef] [PubMed]
- Stavrakis, S.; Nakagawa, H.; Po, S.S.; Scherlag, B.J.; Lazzara, R.; Jackman, W.M. The Role of the Autonomic Ganglia in Atrial Fibrillation. JACC Clin. Electrophysiol. 2015, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Driessen, A.H.G.; Berger, W.R.; Krul, S.P.J.; van den Berg, N.W.E.; Neefs, J.; Piersma, F.R.; Chan Pin Yin, D.R.P.P.; de Jong, J.S.S.G.; van Boven, W.J.P.; de Groot, J.R. Ganglion Plexus Ablation in Advanced Atrial Fibrillation: The AFACT Study. J. Am. Coll. Cardiol. 2016, 68, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Ukena, C.; Becker, N.; Pavlicek, V.; Millenaar, D.; Ewen, S.; Linz, D.; Steinberg, J.S.; Böhm, M.; Mahfoud, F. Catheter-Based Renal Denervation as Adjunct to Pulmonary Vein Isolation for Treatment of Atrial Fibrillation: A Systematic Review and Meta-Analysis. J. Hypertens. 2020, 38, 783–790. [Google Scholar] [CrossRef]
- Younis, A.; Steinberg, J.S. Renal Denervation for Patients with Atrial Fibrillation. Curr. Cardiol. Rep. 2021, 23, 126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yu, S.; Zou, M.; Dai, Z.; Wang, X.; Xiao, J.; Huang, C. Effect of Renal Sympathetic Denervation on the Inducibility of Atrial Fibrillation during Rapid Atrial Pacing. J. Interv. Card. Electrophysiol. 2012, 35, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Yu, L.; Scherlag, B.J.; Wang, S.; He, B.; Yang, K.; Liao, K.; Lu, Z.; He, W.; Zhang, L.; et al. Left Renal Nerves Stimulation Facilitates Ischemia-Induced Ventricular Arrhythmia by Increasing Nerve Activity of Left Stellate Ganglion. J. Cardiovasc. Electrophysiol. 2014, 25, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Hering, D.; Lambert, E.A.; Marusic, P.; Walton, A.S.; Krum, H.; Lambert, G.W.; Esler, M.D.; Schlaich, M.P. Substantial Reduction in Single Sympathetic Nerve Firing after Renal Denervation in Patients with Resistant Hypertension. Hypertension 2013, 61, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, J.S.; Shabanov, V.; Ponomarev, D.; Losik, D.; Ivanickiy, E.; Kropotkin, E.; Polyakov, K.; Ptaszynski, P.; Keweloh, B.; Yao, C.J.; et al. Effect of Renal Denervation and Catheter Ablation vs. Catheter Ablation Alone on Atrial Fibrillation Recurrence Among Patients with Paroxysmal Atrial Fibrillation and Hypertension: The ERADICATE-AF Randomized Clinical Trial. JAMA 2020, 323, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Allessie, M.A.; De Groot, N.M.S.; Houben, R.P.M.; Schotten, U.; Boersma, E.; Smeets, J.L.; Crijns, H.J. Electropathological Substrate of Long-Standing Persistent Atrial Fibrillation in Patients With Structural Heart Disease. Circ. Arrhythm. Electrophysiol. 2010, 3, 606–615. [Google Scholar] [CrossRef] [PubMed]
- Kiełbasa, G.; Jastrzębski, M. Cryoballoon Pulmonary Vein Isolation as a Standard Approach for Interventional Treatment of Atrial Fibrillation. A Review and a Practical Guide to an Effective and Safe Procedure. Postep. Kardiol. Interwencyjnej 2020, 16, 359–375. [Google Scholar] [CrossRef]
- Su, W.W.; Reddy, V.Y.; Bhasin, K.; Champagne, J.; Sangrigoli, R.M.; Braegelmann, K.M.; Kueffer, F.J.; Novak, P.; Gupta, S.K.; Yamane, T.; et al. Cryoballoon Ablation of Pulmonary Veins for Persistent Atrial Fibrillation: Results from the Multicenter STOP Persistent AF Trial. Heart Rhythm. 2020, 17, 1841–1847. [Google Scholar] [CrossRef]
- Bourier, F.; Vlachos, K.; Lam, A.; Martin, C.A.; Takigawa, M.; Kitamura, T.; Massoullié, G.; Cheniti, G.; Frontera, A.; Duchateau, J.; et al. Three-Dimensional Image Integration Guidance for Cryoballoon Pulmonary Vein Isolation Procedures. J. Cardiovasc. Electrophysiol. 2019, 30, 2790–2796. [Google Scholar] [CrossRef]
- Trivedi, A.; Hoffman, J.; Arora, R. Gene Therapy for Atrial Fibrillation-How Close to Clinical Implementation? Int. J. Cardiol. 2019, 296, 177–183. [Google Scholar] [CrossRef]
- Yoo, S.; Geist, G.E.; Pfenniger, A.; Rottmann, M.; Arora, R. Recent Advances in Gene Therapy for Atrial Fibrillation. J. Cardiovasc. Electrophysiol. 2021, 32, 2854–2864. [Google Scholar] [CrossRef]
- Soucek, R.; Thomas, D.; Kelemen, K.; Bikou, O.; Seyler, C.; Voss, F.; Becker, R.; Koenen, M.; Katus, H.A.; Bauer, A. Genetic Suppression of Atrial Fibrillation Using a Dominant-Negative Ether-a-Go-Go-Related Gene Mutant. Heart Rhythm. Soc. 2012, 9, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Wiedmann, F.; Beyersdorf, C.; Zhou, X.-B.; Kraft, M.; Foerster, K.I.; El-Battrawy, I.; Lang, S.; Borggrefe, M.; Haefeli, W.E.; Frey, N.; et al. The Experimental TASK-1 Potassium Channel Inhibitor A293 Can Be Employed for Rhythm Control of Persistent Atrial Fibrillation in a Translational Large Animal Model. Front. Phisiol. 2021, 11, 629421. [Google Scholar] [CrossRef] [PubMed]
- Guy, A.; Kikuchi, K.; Greener, I.D.; Yang, L.; Novack, V.; Donahue, J.K. Selective Molecular Potassium Channel Blockade Prevents Atrial Fibrillation. Circulation 2010, 121, 2263–2270. [Google Scholar] [CrossRef]
- Liu, Z.; Hutt, J.A.; Rajeshkumar, B.; Azuma, Y.; Duan, K.L.; Donahue, J.K. Preclinical Efficacy and Safety of KCNH2-G628S Gene Therapy for Postoperative Atrial Fibrillation. J. Thorac. Cardiovasc. Surg. 2017, 154, 1644–1651.e8. [Google Scholar] [CrossRef]
- Wang, H.L.; Zhou, X.H.; Li, Z.Q.; Fan, P.; Na Zhou, Q.; Li, Y.D.; Hou, Y.M.; Tang, B.P. Prevention of Atrial Fibrillation by Using Sarcoplasmic Reticulum Calcium ATPase Pump Overexpression in a Rabbit Model of Rapid Atrial Pacing. Med. Sci. Monit. 2017, 23, 3952–3960. [Google Scholar] [CrossRef]
- Chiang, D.Y.; Kongchan, N.; Beavers, D.L.; Alsina, K.M.; Voigt, N.; Neilson, J.R.; Jakob, H.; Martin, J.F.; Dobrev, D.; Wehrens, X.H.; et al. Loss of MicroRNA-106b-25 Cluster Promotes Atrial Fibrillation by Enhancing Ryanodine Receptor Type-2 Expression and Calcium Release. Circ. Arrhythm. Electrophysiol. 2014, 7, 1214–1222. [Google Scholar] [CrossRef]
- Bikou, O.; Thomas, D.; Trappe, K.; Lugenbiel, P.; Kelemen, K.; Koch, M.; Soucek, R.; Voss, F.; Diger Becker, R.; Katus, H.A.; et al. Connexin 43 Gene Therapy Prevents Persistent Atrial Fibrillation in a Porcine Model. Cardiovasc. Res. 2011, 92, 218–225. [Google Scholar] [CrossRef]
- Igarashi, T.; Finet, J.E.; Takeuchi, A.; Fujino, Y.; Strom, M.; Greener, I.D.; Rosenbaum, D.S.; Donahue, J.K. Connexin Gene Transfer Preserves Conduction Velocity and Prevents Atrial Fibrillation. Circulation 2012, 125, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Aistrup, G.L.; Cokic, I.; Ng, J.; Gordon, D.; Koduri, H.; Browne, S.; Arapi, D.; Segon, Y.; Goldstein, J.; Angulo, A.; et al. Targeted Nonviral Gene-Based Inhibition of Gα i/o-Mediated Vagal Signaling in the Posterior Left Atrium Decreases Vagal-Induced Atrial Fibrillation. Heart Rythm. 2011, 8, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Donahue, J.K. Biological Therapies for Atrial Fibrillation: Ready for Prime Time? J. Cardiovasc. Pharmacol. 2016, 67, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Trappe, K.; Thomas, D.; Bikou, O.; Kelemen, K.; Lugenbiel, P.; Voss, F.; Diger Becker, R.; Katus, H.A.; Bauer, A. Suppression of Persistent Atrial Fibrillation by Genetic Knockdown of Caspase 3: A Pre-Clinical Pilot Study. Eur. Heart J. 2013, 34, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zheng, S.; Geng, Y.; Xue, J.; Wang, Z.; Xie, X.; Wang, J.; Zhang, S.; Hou, Y. MicroRNA Profiling of Atrial Fibrillation in Canines: MiR-206 Modulates Intrinsic Cardiac Autonomic Nerve Remodeling by Regulating SOD1. PLoS ONE 2015, 10, e0122674. [Google Scholar] [CrossRef]
- Kim, Y.M.; Guzik, T.J.; Zhang, Y.H.; Zhang, M.H.; Kattach, H.; Ratnatunga, C.; Pillai, R.; Channon, K.M.; Casadei, B. A Myocardial Nox2 Containing NAD(P)H Oxidase Contributes to Oxidative Stress in Human Atrial Fibrillation. Circ. Res. 2005, 97, 629–636. [Google Scholar] [CrossRef]
- Kunamalla, A.; Ng, J.; Parini, V.; Yoo, S.; Mcgee, K.A.; Tomson, T.T.; Gordon, D.; Thorp, E.B.; Lomasney, J.; Zhang, Q.; et al. Constitutive Expression of a Dominant Negative TGF-β Type II Receptor in the Posterior Left Atrium Leads to Beneficial Remodeling of Atrial Fibrillation Substrate. Circ. Res. 2016, 119, 69–82. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, Z.; Fan, J.; Chen, S.; Tan, Z.; Yang, H.; Yin, Y. Angiotensin-Converting Enzyme-2 Overexpression Improves Atrial Remodeling and Function in a Canine Model of Atrial Fibrillation. J. Am. Heart Assoc. 2015, 4, e001530. [Google Scholar] [CrossRef]
- Fan, J.; Zou, L.; Cui, K.; Woo, K.; Du, H.; Chen, S.; Ling, Z.; Zhang, Q.; Zhang, B.; Lan, X.; et al. Atrial Overexpression of Angiotensin-Converting Enzyme 2 Improves the Canine Rapid Atrial Pacing-Induced Structural and Electrical Remodeling Fan, ACE2 Improves Atrial Substrate Remodeling. Basic. Res. Cardiol. 2015, 110, 45. [Google Scholar] [CrossRef]
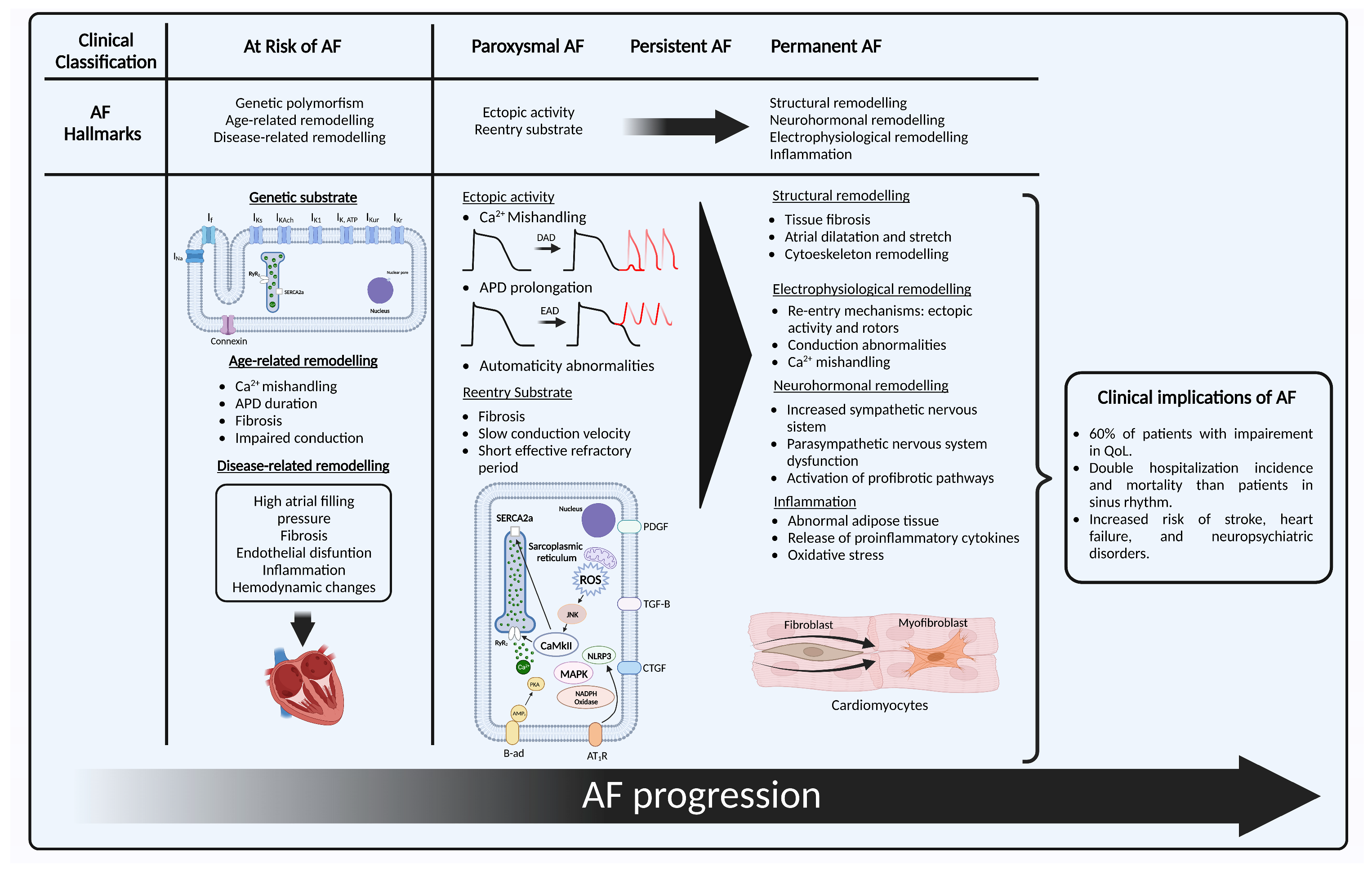
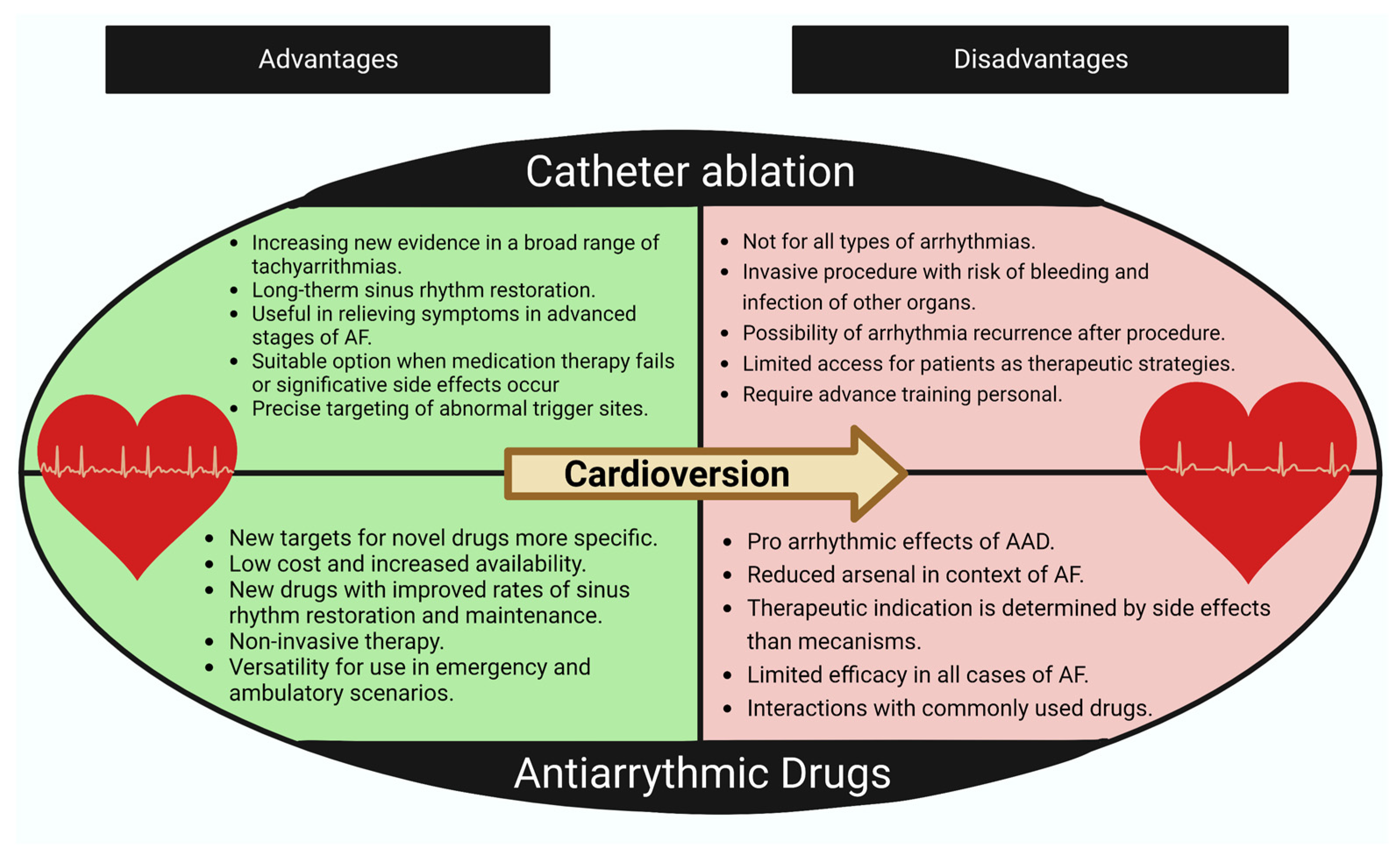



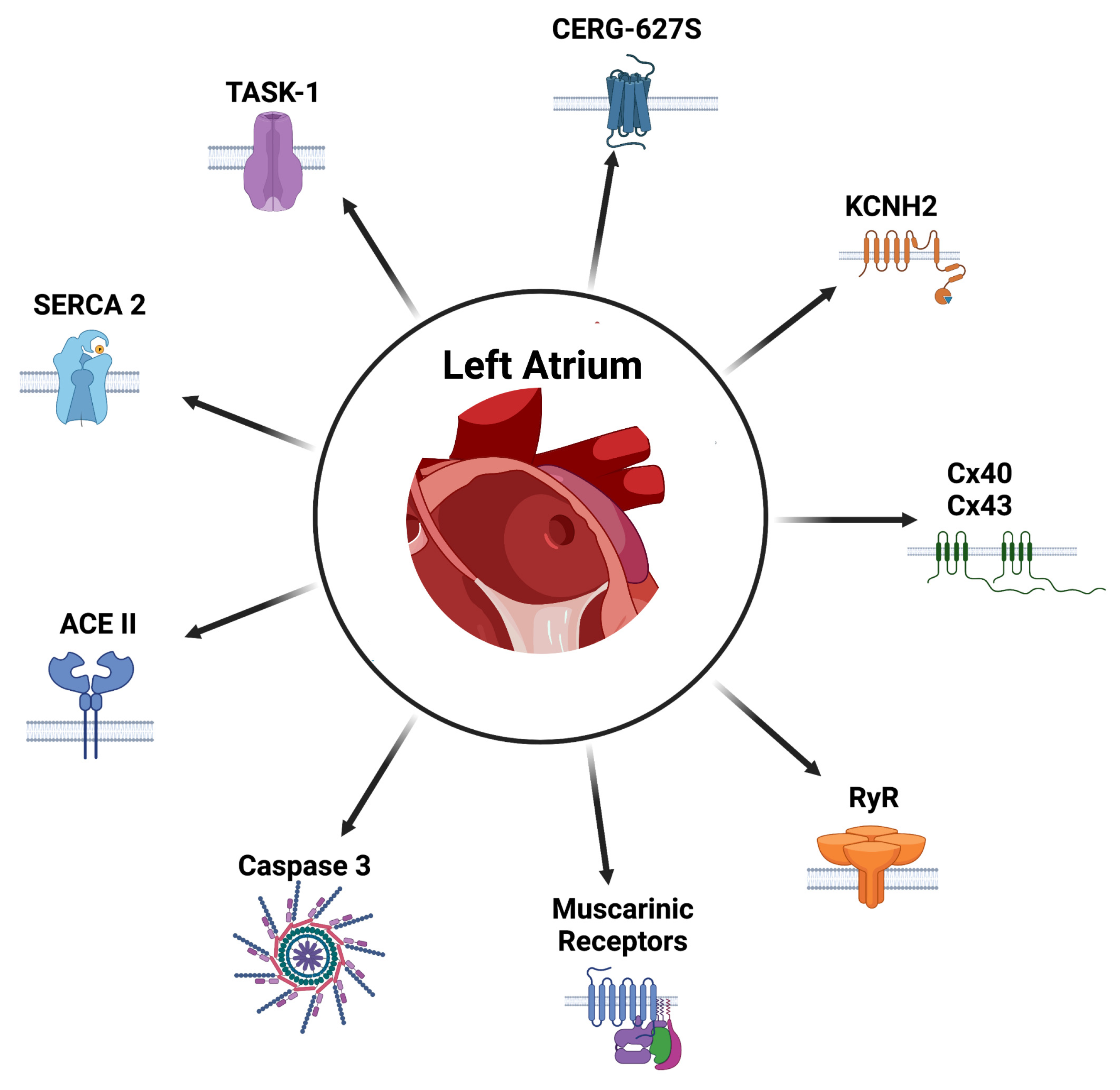
| Drug | Study | Primary Outcome Studied | Phase | NCT |
|---|---|---|---|---|
| Colchicine | Colchicine and CRP in Atrial Fibrillation and AF Ablation | NCT01755949 | Phase 2 | NCT01755949 |
| Use of Colchicine to Decrease Atrial Fibrillation Recurrence After Ablation | Compare atrial fibrillation recurrence and post-ablation quality of life | Phase 3 | NCT05459974 | |
| Colchicine in Atrial Fibrillation to Prevent Stroke (CIAFS-1) | Investigate the efficacy of an anti-inflammatory drug, colchicine, at reducing well validated markers of thrombosis (D-dimer) and inflammation (hs-CRP) | Phase 3 | NCT02282098 | |
| Colchicine For Prevention of Perioperative Atrial Fibrillation in Patients Undergoing Thoracic Surgery Pilot Study (COP-AF Pilot) | Clinically Significant Atrial Fibrillation | Phase 3 | NCT01985425 | |
| Effect of Colchicine on the Incidence of Atrial Fibrillation in Open Heart Surgery Patients (END-AF) | Number of participants with Atrial fibrillation and adverse effects of colchicine | Phase 3 | NCT03021343 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra-Lucares, A.; Villa, E.; Romero-Hernández, E.; Méndez-Valdés, G.; Retamal, C.; Vizcarra, G.; Henríquez, I.; Maldonado-Morales, E.A.J.; Grant-Palza, J.H.; Ruíz-Tagle, S.; et al. Tic-Tac: A Translational Approach in Mechanisms Associated with Irregular Heartbeat and Sinus Rhythm Restoration in Atrial Fibrillation Patients. Int. J. Mol. Sci. 2023, 24, 12859. https://doi.org/10.3390/ijms241612859
Parra-Lucares A, Villa E, Romero-Hernández E, Méndez-Valdés G, Retamal C, Vizcarra G, Henríquez I, Maldonado-Morales EAJ, Grant-Palza JH, Ruíz-Tagle S, et al. Tic-Tac: A Translational Approach in Mechanisms Associated with Irregular Heartbeat and Sinus Rhythm Restoration in Atrial Fibrillation Patients. International Journal of Molecular Sciences. 2023; 24(16):12859. https://doi.org/10.3390/ijms241612859
Chicago/Turabian StyleParra-Lucares, Alfredo, Eduardo Villa, Esteban Romero-Hernández, Gabriel Méndez-Valdés, Catalina Retamal, Geovana Vizcarra, Ignacio Henríquez, Esteban A. J. Maldonado-Morales, Juan H. Grant-Palza, Sofía Ruíz-Tagle, and et al. 2023. "Tic-Tac: A Translational Approach in Mechanisms Associated with Irregular Heartbeat and Sinus Rhythm Restoration in Atrial Fibrillation Patients" International Journal of Molecular Sciences 24, no. 16: 12859. https://doi.org/10.3390/ijms241612859
APA StyleParra-Lucares, A., Villa, E., Romero-Hernández, E., Méndez-Valdés, G., Retamal, C., Vizcarra, G., Henríquez, I., Maldonado-Morales, E. A. J., Grant-Palza, J. H., Ruíz-Tagle, S., Estrada-Bobadilla, V., & Toro, L. (2023). Tic-Tac: A Translational Approach in Mechanisms Associated with Irregular Heartbeat and Sinus Rhythm Restoration in Atrial Fibrillation Patients. International Journal of Molecular Sciences, 24(16), 12859. https://doi.org/10.3390/ijms241612859






