Aging Triggers Mitochondrial Dysfunction in Mice
Abstract
1. Introduction
2. Results
2.1. Mitochondrial Respiration
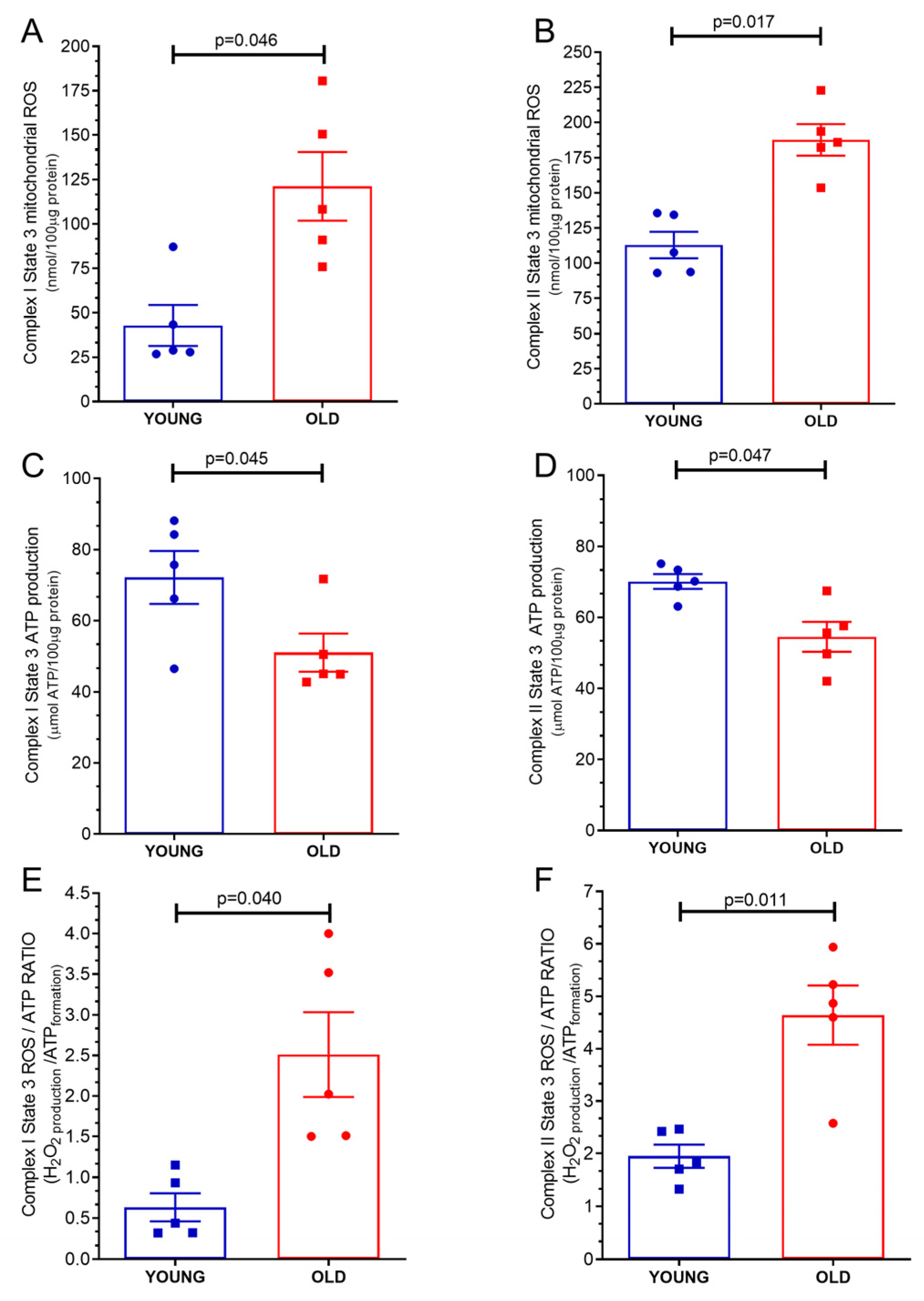
2.2. Mitochondrial ROS Production
2.3. Mitochondrial Adenosine Triphosphate (ATP) Production
2.4. Mitochondrial ATP/ROS Ratio
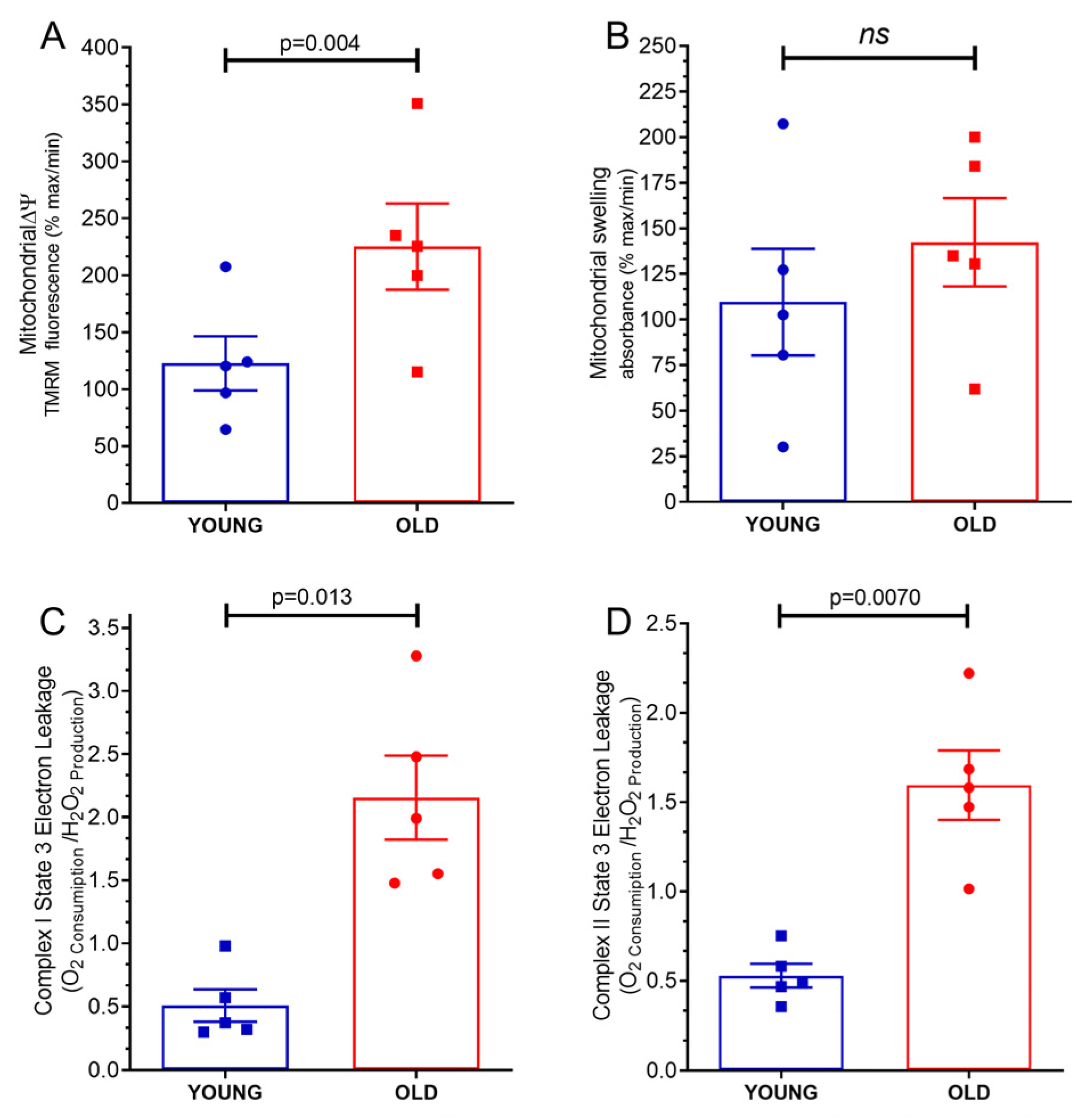
2.5. Mitochondrial Transmembrane Potential Δψ
2.6. Mitochondrial Swelling
2.7. Mitochondrial Proton Leakage
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Mitochondria Isolation and Measurement of Mitochondrial Function
4.4. Mitochondrial Oxygen Consumption
4.5. Mitochondrial ATP Production
4.6. Mitochondrial Swelling and Transmembrane Potential
4.7. Mitochondrial ROS Concentration
4.8. Electron Leakage and ATP/ROS Production Ratio
4.9. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Csekes, E.; Račková, L. Skin Aging, Cellular Senescence and Natural Polyphenols. Int. J. Mol. Sci. 2021, 22, 12641. [Google Scholar] [CrossRef]
- Kudlova, N.; De Sanctis, J.B.; Hajduch, M. Cellular Senescence: Molecular Targets, Biomarkers, and Senolytic Drugs. Int. J. Mol. Sci. 2022, 23, 4168. [Google Scholar] [CrossRef]
- Lee, Y.I.; Choi, S.; Roh, W.S.; Lee, J.H.; Kim, T.-G. Cellular Senescence and Inflammaging in the Skin Microenvironment. Int. J. Mol. Sci. 2021, 22, 3849. [Google Scholar] [CrossRef]
- Roger, L.; Tomas, F.; Gire, V. Mechanisms and Regulation of Cellular Senescence. Int. J. Mol. Sci. 2021, 22, 13173. [Google Scholar] [CrossRef]
- Sun, N.; Youle, R.J.; Finkel, T. The Mitochondrial Basis of Aging. Mol. Cell 2016, 61, 654–666. [Google Scholar] [CrossRef]
- Lane, R.K.; Hilsabeck, T.; Rea, S.L. The role of mitochondrial dysfunction in age-related diseases. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 1387–1400. [Google Scholar] [CrossRef]
- Zhunina, O.A.; Yabbarov, N.G.; Grechko, A.V.; Starodubova, A.V.; Ivanova, E.; Nikiforov, N.G.; Orekhov, A.N. The Role of Mitochondrial Dysfunction in Vascular Disease, Tumorigenesis, and Diabetes. Front. Mol. Biosci. 2021, 8, 671908. [Google Scholar] [CrossRef]
- Passos, J.F.; Saretzki, G.; Ahmed, S.; Nelson, G.; Richter, T.; Peters, H.; Wappler, I.; Birket, M.J.; Harold, G.; Schaeuble, K.; et al. Mitochondrial dysfunction accounts for the stochastic heterogeneity in telomere-dependent senescence. PLoS Biol. 2007, 5, e110. [Google Scholar] [CrossRef]
- Nelson, G.; Kucheryavenko, O.; Wordsworth, J.; Von Zglinicki, T. The senescent bystander effect is caused by ROS-activated NF-κB signalling. Mech. Ageing Dev. 2018, 170, 30–36. [Google Scholar] [CrossRef]
- Di Micco, R.; Fumagalli, M.; Cicalese, A.; Piccinin, S.; Gasparini, P.; Luise, C.; Schurra, C.; Garre’, M.; Nuciforo, P.G.; Bensimon, A.; et al. Oncogene-induced senescence is a DNA damage response triggered by DNA hyper-replication. Nature 2006, 444, 638–642. [Google Scholar] [CrossRef]
- Passos, J.F.; Nelson, G.; Wang, C.; Richter, T.; Simillion, C.; Proctor, C.J.; Miwa, S.; Olijslagers, S.; Hallinan, J.; Wipat, A.; et al. Feedback between p21 and reactive oxygen production is necessary for cell senescence. Mol. Syst. Biol. 2010, 6, 347. [Google Scholar] [CrossRef]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’arca, D. ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 3565127. [Google Scholar] [CrossRef]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef]
- Dai, D.-F.; Rabinovitch, P.S.; Ungvari, Z. Mitochondria and cardiovascular aging. Circ. Res. 2012, 110, 1109–1124. [Google Scholar] [CrossRef]
- Hiona, A.; Sanz, A.; Kujoth, G.C.; Pamplona, R.; Seo, A.Y.; Hofer, T.; Someya, S.; Miyakawa, T.; Nakayama, C.; Samhan-Arias, A.K.; et al. Mitochondrial DNA mutations induce mitochondrial dysfunction, apoptosis and sarcopenia in skeletal muscle of mitochondrial DNA mutator mice. PLoS ONE 2010, 5, e11468. [Google Scholar] [CrossRef]
- Kumaran, S.; Subathra, M.; Balu, M.; Panneerselvam, C. Age-associated decreased activities of mitochondrial electron transport chain complexes in heart and skeletal muscle: Role of l-carnitine. Chem. Biol. Interact. 2004, 148, 11–18. [Google Scholar] [CrossRef]
- Tatarková, Z.; Kuka, S.; Račay, P.; Lehotský, J.; Dobrota, D.; Mištuna, D.; Kaplán, P. Effects of aging on activities of mitochondrial electron transport chain complexes and oxidative damage in rat heart. Physiol. Res. 2011, 60, 281–289. [Google Scholar] [CrossRef]
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef]
- Rizvi, F.; Preston, C.C.; Emelyanova, L.; Yousufuddin, M.; Viqar, M.; Dakwar, O.; Ross, G.R.; Faustino, R.S.; Holmuhamedov, E.L.; Jahangir, A. Effects of Aging on Cardiac Oxidative Stress and Transcriptional Changes in Pathways of Reactive Oxygen Species Generation and Clearance. J. Am. Heart Assoc. 2021, 10, e019948. [Google Scholar] [CrossRef]
- Nguyen, B.Y.; Ruiz-Velasco, A.; Bui, T.; Collins, L.; Wang, X.; Liu, W. Mitochondrial function in the heart: The insight into mechanisms and therapeutic potentials. Br. J. Pharmacol. 2018, 176, 4302–4318. [Google Scholar] [CrossRef]
- Barja, G. Mitochondrial oxygen consumption and reactive oxygen species production are independently modulated: Implications for aging studies. Rejuvenation Res. 2007, 10, 215–224. [Google Scholar] [CrossRef]
- Halestrap, A.P.; Pasdois, P. The role of the mitochondrial permeability transition pore in heart disease. Biochim. Biophys. Acta Bioenerg. 2009, 1787, 1402–1415. [Google Scholar] [CrossRef]
- Maciel, L.; De Oliveira, D.F.; Monnerat, G.; De Carvalho, A.C.C.; Nascimento, J.H.M. Exogenous 10 kDa-Heat Shock Protein Preserves Mitochondrial Function After Hypoxia/Reoxygenation. Front. Pharmacol. 2020, 11, 545. [Google Scholar] [CrossRef]
- Sandhu, S.K.; Kaur, G. Mitochondrial electron transport chain complexes in aging rat brain and lymphocytes. Biogerontology 2003, 4, 19–29. [Google Scholar] [CrossRef]
- Serviddio, G.; Bellanti, F.; Romano, A.; Tamborra, R.; Rollo, T.; Altomare, E.; Vendemiale, G. Bioenergetics in aging: Mitochondrial proton leak in aging rat liver, kidney and heart. Redox Rep. 2007, 12, 91–95. [Google Scholar] [CrossRef]
- Monnerat, G.; Kasai-Brunswick, T.H.; Asensi, K.D.; Silva Dos Santos, D.; Barbosa, R.A.Q.; Cristina Paccola Mesquita, F.; Calvancanti Albuquerque, J.P.; Raphaela, P.F.; Wendt, C.; Miranda, K.; et al. Modelling premature cardiac aging with induced pluripotent stem cells from a hutchinson-gilford Progeria Syndrome patient. Front. Physiol. 2022, 13, 1007418. [Google Scholar] [CrossRef]
- Caldeira, D.A.F.; de Oliveira, D.F.; Cavalcanti-de-Albuquerque, J.P.; Nascimento, J.H.M.; Zin, W.A.; Maciel, L. Isolation of Mitochondria From Fresh Mice Lung Tissue. Front. Physiol. 2021, 12, 748261. [Google Scholar] [CrossRef]
- Gedik, N.; Maciel, L.; Schulte, C.; Skyschally, A.; Heusch, G.; Kleinbongard, P. Cardiomyocyte mitochondria as targets of humoral factors released by remote ischemic preconditioning. Arch. Med. Sci. 2017, 2, 448–458. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosa, F.L.L.; de Souza, I.I.A.; Monnerat, G.; Campos de Carvalho, A.C.; Maciel, L. Aging Triggers Mitochondrial Dysfunction in Mice. Int. J. Mol. Sci. 2023, 24, 10591. https://doi.org/10.3390/ijms241310591
Rosa FLL, de Souza IIA, Monnerat G, Campos de Carvalho AC, Maciel L. Aging Triggers Mitochondrial Dysfunction in Mice. International Journal of Molecular Sciences. 2023; 24(13):10591. https://doi.org/10.3390/ijms241310591
Chicago/Turabian StyleRosa, Frederico Luis Lima, Itanna Isis Araujo de Souza, Gustavo Monnerat, Antonio Carlos Campos de Carvalho, and Leonardo Maciel. 2023. "Aging Triggers Mitochondrial Dysfunction in Mice" International Journal of Molecular Sciences 24, no. 13: 10591. https://doi.org/10.3390/ijms241310591
APA StyleRosa, F. L. L., de Souza, I. I. A., Monnerat, G., Campos de Carvalho, A. C., & Maciel, L. (2023). Aging Triggers Mitochondrial Dysfunction in Mice. International Journal of Molecular Sciences, 24(13), 10591. https://doi.org/10.3390/ijms241310591





