Predictive Genetic Biomarkers for the Development of Peritoneal Metastases in Colorectal Cancer
Abstract
1. Introduction
2. Results
2.1. Study Cohort
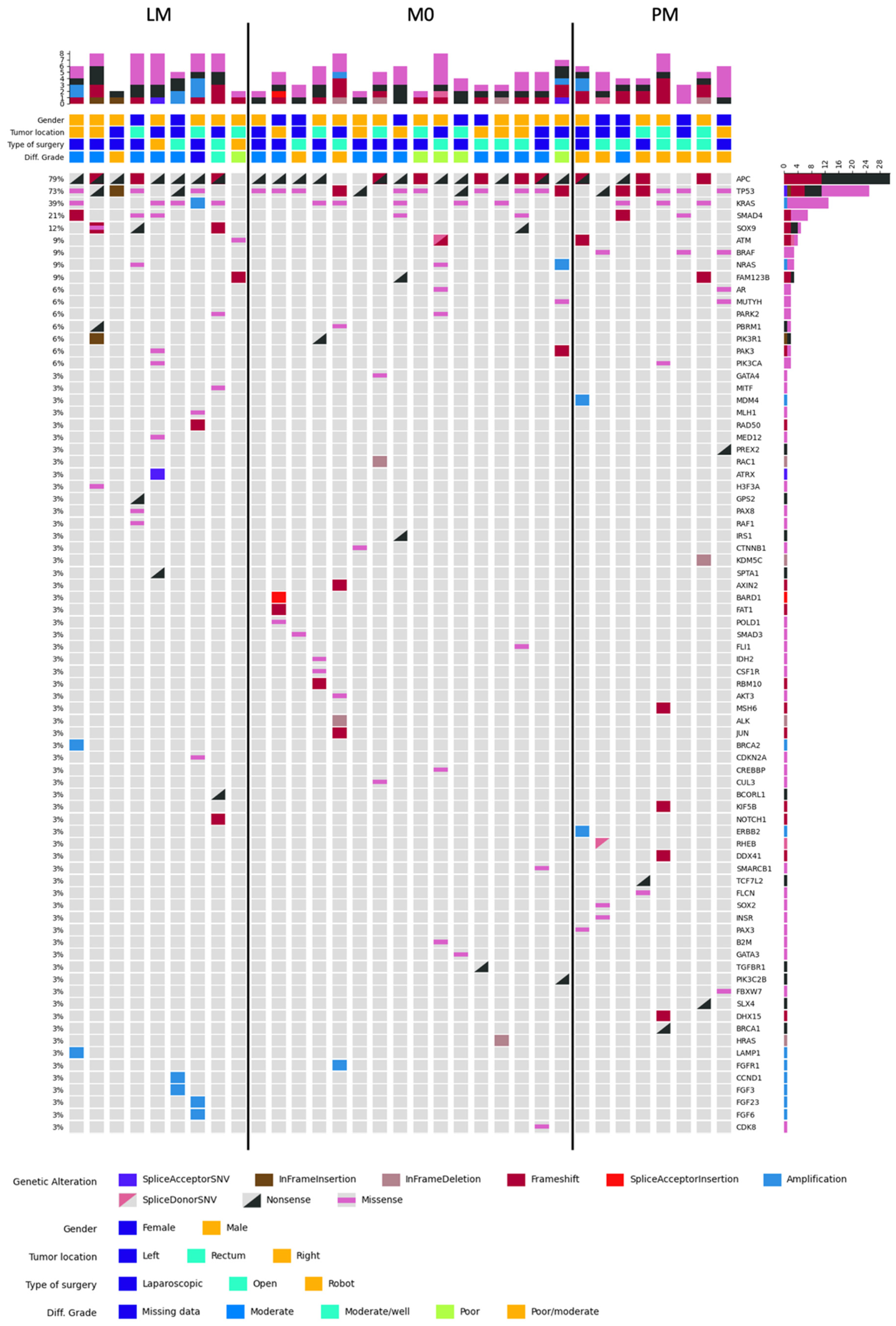
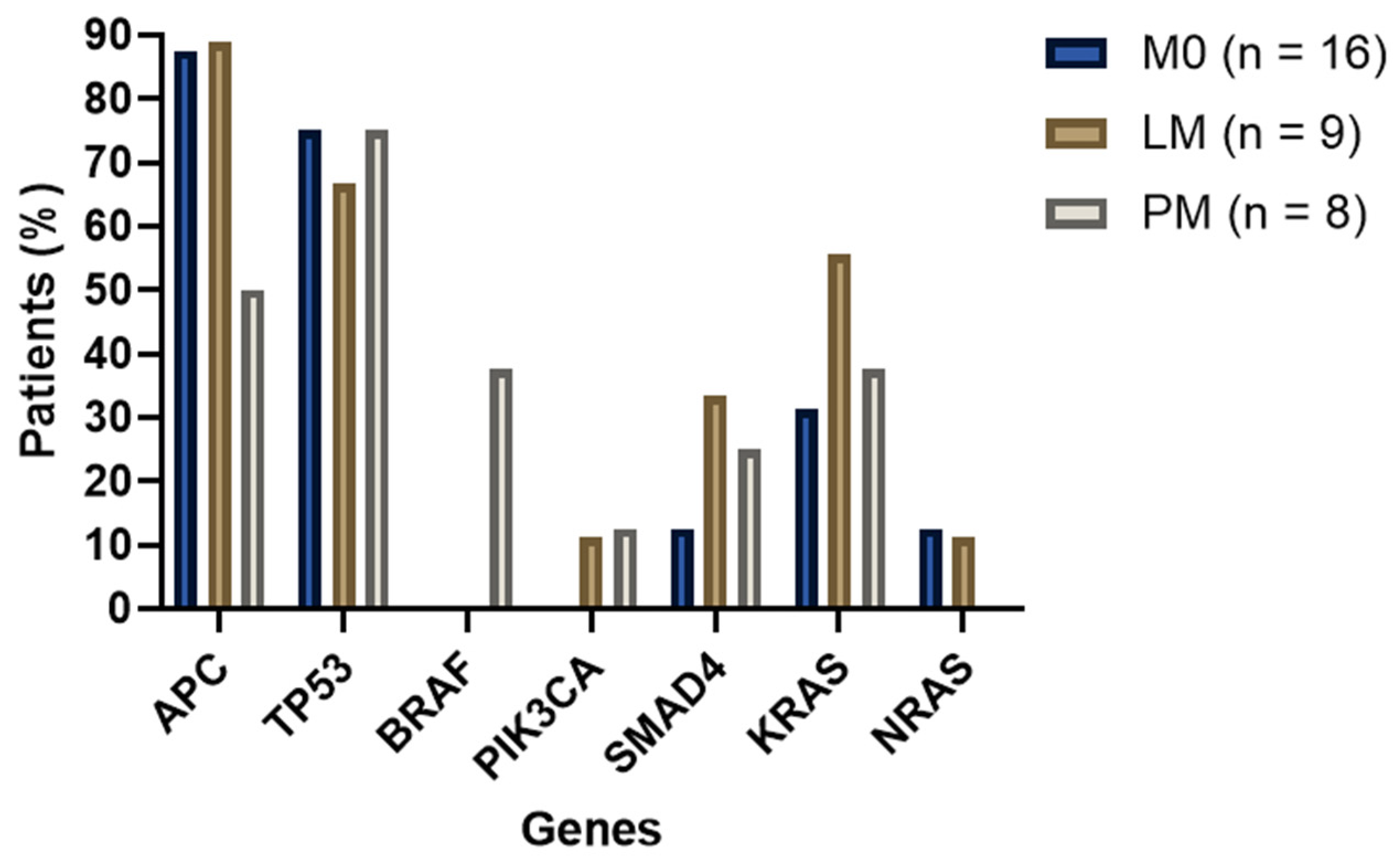
2.2. DNA Sequencing
2.2.1. MSS Samples Analysis
2.2.2. Additional Analyses
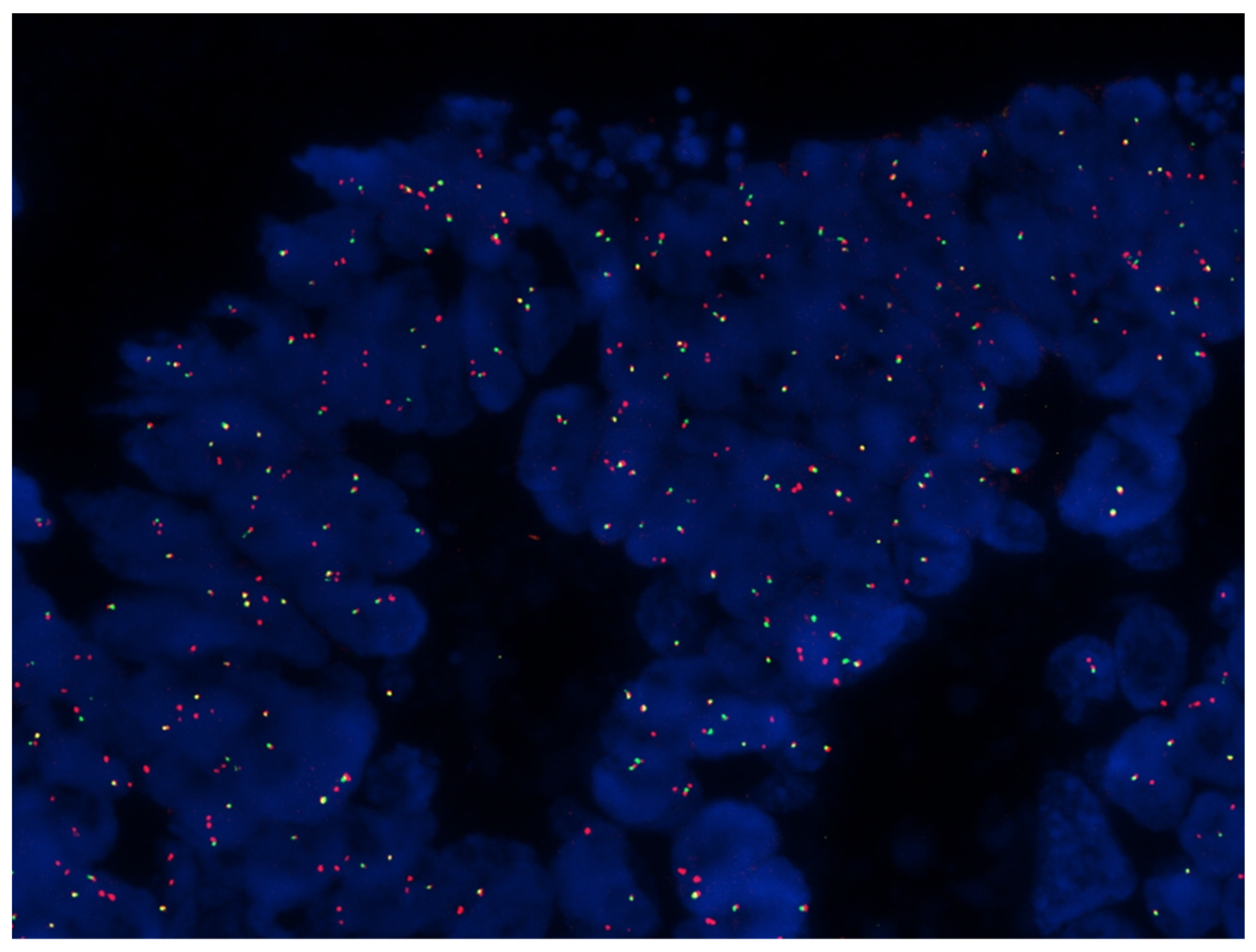
2.3. RNA Sequencing
3. Discussion
3.1. Patient Characteristics and Clinicopathological Variables
3.2. DNA and RNA Sequencing
3.3. Treatment Options and Future Perspectives
3.4. Strengths and Limitations
4. Materials and Methods
4.1. Patients
4.2. Tumor Samples
4.3. TruSight Oncology 500 Analysis
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van Gestel, Y.R.; Thomassen, I.; Lemmens, V.E.; Pruijt, J.F.; van Herk-Sukel, M.P.; Rutten, H.J.; Creemers, G.J.; de Hingh, I.H. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur. J. Surg. Oncol. 2014, 40, 963–969. [Google Scholar] [CrossRef]
- Simkens, G.A.; Wintjens, A.; Rovers, K.P.; Nienhuijs, S.W.; de Hingh, I.H. Effective Strategies to Predict Survival of Colorectal Peritoneal Metastases Patients Eligible for Cytoreductive Surgery and HIPEC. Cancer Manag. Res. 2021, 13, 5239–5249. [Google Scholar] [CrossRef]
- Lurvink, R.J.; Bakkers, C.; Rijken, A.; van Erning, F.N.; Nienhuijs, S.W.; Burger, J.W.; Creemers, G.J.; Verhoef, C.; Lemmens, V.E.; De Hingh, I.H. Increase in the incidence of synchronous and metachronous peritoneal metastases in patients with colorectal cancer: A nationwide study. Eur. J. Surg. Oncol. 2021, 47, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Koppe, M.J.; Boerman, O.C.; Oyen, W.J.; Bleichrodt, R.P. Peritoneal carcinomatosis of colorectal origin: Incidence and current treatment strategies. Ann. Surg. 2006, 243, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Klaver, Y.L.; Lemmens, V.E.; Nienhuijs, S.W.; Luyer, M.D.; de Hingh, I.H. Peritoneal carcinomatosis of colorectal origin: Incidence, prognosis and treatment options. World J. Gastroenterol. 2012, 18, 5489–5494. [Google Scholar] [CrossRef] [PubMed]
- Kranenburg, O.; van der Speeten, K.; de Hingh, I. Peritoneal Metastases from Colorectal Cancer: Defining and Addressing the Challenges. Front. Oncol. 2021, 11, 650098. [Google Scholar] [CrossRef]
- Kamiyama, H.; Noda, H.; Konishi, F.; Rikiyama, T. Molecular biomarkers for the detection of metastatic colorectal cancer cells. World J. Gastroenterol. 2014, 20, 8928–8938. [Google Scholar]
- Sadahiro, S.; Suzuki, T.; Ishikawa, K.; Nakamura, T.; Tanaka, Y.; Masuda, T.; Mukoyama, S.; Yasuda, S.; Tajima, T.; Makuuchi, H.; et al. Recurrence patterns after curative resection of colorectal cancer in patients followed for a minimum of ten years. Hepatogastroenterology 2003, 50, 1362–1366. [Google Scholar]
- Maggiori, L.; Elias, D. Curative treatment of colorectal peritoneal carcinomatosis: Current status and future trends. Eur. J. Surg. Oncol. 2010, 36, 599–603. [Google Scholar] [CrossRef][Green Version]
- Jayne, D.G.; Fook, S.; Loi, C.; Seow-Choen, F. Peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2002, 89, 1545–1550. [Google Scholar] [CrossRef]
- Quénet, F.; Elias, D.; Roca, L.; Goéré, D.; Ghouti, L.; Pocard, M.; Facy, O.; Arvieux, C.; Lorimier, G.; Pezet, D.; et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Bakkers, C.; Lurvink, R.J.; Rijken, A.; Nienhuijs, S.W.; Kok, N.F.; Creemers, G.J.; Verhoef, C.; Lemmens, V.E.; van Erning, F.N.; De Hingh, I.H. Treatment Strategies and Prognosis of Patients with Synchronous or Metachronous Colorectal Peritoneal Metastases: A Population-Based Study. Ann. Surg. Oncol. 2021, 28, 9073–9083. [Google Scholar] [CrossRef]
- Xue, L.; Hyman, N.H.; Turaga, K.K.; Eng, O.S. Peritoneal Metastases in Colorectal Cancer: Biology and Barriers. J. Gastrointest. Surg. 2020, 24, 720–727. [Google Scholar] [CrossRef]
- Sorich, M.J.; Wiese, M.D.; Rowland, A.; Kichenadasse, G.; McKinnon, R.A.; Karapetis, C.S. Extended RAS mutations and anti-EGFR monoclonal antibody survival benefit in metastatic colorectal cancer: A meta-analysis of randomized, controlled trials. Ann. Oncol. 2015, 26, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, H.; Uehara, K.; Nakayama, G.; Nakayama, H.; Aiba, T.; Hattori, N.; Kataoka, M.; Nakano, Y.; Kawase, Y.; Okochi, O.; et al. Tumor Location Is Associated with the Prevalence of Braf And Pik3ca Mutations in Patients with Wild-Type Ras Colorectal Cancer: A Prospective Multi-Center Cohort Study in Japan. Transl. Oncol. 2020, 13, 100786. [Google Scholar] [CrossRef]
- Graf, W.; Cashin, P.H.; Ghanipour, L.; Enblad, M.; Botling, J.; Terman, A.; Birgisson, H. Prognostic Impact of BRAF and KRAS Mutation in Patients with Colorectal and Appendiceal Peritoneal Metastases Scheduled for CRS and HIPEC. Ann. Surg. Oncol. 2020, 27, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Roth, L.; Russo, L.; Ulugoel, S.; Freire Dos Santos, R.; Breuer, E.; Gupta, A.; Lehmann, K. Peritoneal Metastasis: Current Status and Treatment Options. Cancers 2021, 14, 60. [Google Scholar] [CrossRef]
- Schneider, M.A.; Eden, J.; Pache, B.; Laminger, F.; Lopez-Lopez, V.; Steffen, T.; Hübner, M.; Kober, F.; Roka, S.; Campos, P.C.; et al. Mutations of RAS/RAF Proto-oncogenes Impair Survival after Cytoreductive Surgery and HIPEC for Peritoneal Metastasis of Colorectal Origin. Ann. Surg. 2018, 268, 845–853. [Google Scholar] [CrossRef]
- Heuvelings, D.J.I.; Wintjens, A.; Luyten, J.; Wilmink, G.; Moonen, L.; Speel, E.M.; de Hingh, I.; Bouvy, N.D.; Peeters, A. DNA and RNA Alterations Associated with Colorectal Peritoneal Metastases: A Systematic Review. Cancers 2023, 15, 549. [Google Scholar] [CrossRef]
- Zhang, Y.; Qin, X.; Chen, W.; Liu, D.; Luo, J.; Wang, H.; Wang, H. Risk factors for developing peritoneal metastases after curative surgery for colorectal cancer: A systematic review and meta-analysis. Color. Dis. 2021, 23, 2846–2858. [Google Scholar] [CrossRef]
- Segelman, J.; Granath, F.; Holm, T.; Machado, M.; Mahteme, H.; Martling, A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br. J. Surg. 2012, 99, 699–705. [Google Scholar] [CrossRef] [PubMed]
- Klaver, C.E.L.; Wisselink, D.D.; Punt, C.J.A.; Snaebjornsson, P.; Crezee, J.; Aalbers, A.G.J.; Brandt, A.; Bremers, A.J.A.; Burger, J.W.A.; Fabry, H.F.J.; et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): A multicentre, open-label, randomised trial. Lancet Gastroenterol. Hepatol. 2019, 4, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Arjona-Sánchez, A.; Barrios, P.; Boldo-Roda, E.; Camps, B.; Carrasco-Campos, J.; Concepción Martín, V.; García-Fadrique, A.; Gutiérrez-Calvo, A.; Morales, R.; Ortega-Pérez, G.; et al. HIPECT4: Multicentre, randomized clinical trial to evaluate safety and efficacy of Hyperthermic intra-peritoneal chemotherapy (HIPEC) with Mitomycin C used during surgery for treatment of locally advanced colorectal carcinoma. BMC Cancer 2018, 18, 183. [Google Scholar] [CrossRef] [PubMed]
- Karunasena, E.; Sham, J.; McMahon, K.W.; Ahuja, N. Genomics of Peritoneal Malignancies. Surg. Oncol. Clin. N. Am. 2018, 27, 463–475. [Google Scholar] [CrossRef]
- Fodde, R. The APC gene in colorectal cancer. Eur. J. Cancer 2002, 38, 867–871. [Google Scholar] [CrossRef]
- Zhang, L.; Shay, J.W. Multiple Roles of APC and Its Therapeutic Implications in Colorectal Cancer. JNCI J. Natl. Cancer Inst. 2017, 109, djw332. [Google Scholar] [CrossRef]
- Lipsyc, M.; Yaeger, R. Impact of somatic mutations on patterns of metastasis in colorectal cancer. J. Gastrointest. Oncol. 2015, 6, 645–649. [Google Scholar] [CrossRef]
- Lan, Y.T.; Jen-Kou, L.; Lin, C.H.; Yang, S.H.; Lin, C.C.; Wang, H.S.; Chen, W.S.; Lin, T.C.; Jiang, J.K.; Chang, S.C. Mutations in the RAS and PI3K pathways are associated with metastatic location in colorectal cancers. J. Surg. Oncol. 2015, 111, 905–910. [Google Scholar] [CrossRef]
- He, K.; Wang, Y.; Zhong, Y.; Pan, X.; Si, L.; Lu, J. KRAS Codon 12 Mutation is Associated with More Aggressive Invasiveness in Synchronous Metastatic Colorectal Cancer (mCRC): Retrospective Research. OncoTargets Ther. 2020, 13, 12601–12613. [Google Scholar] [CrossRef]
- Zihui Yong, Z.; Ching, G.T.H.; Ching, M.T.C. Metastatic Profile of Colorectal Cancer: Interplay between Primary Tumor Location and KRAS Status. J. Surg. Res. 2020, 246, 325–334. [Google Scholar] [CrossRef]
- Oh, H.H.; Joo, Y.E. Novel biomarkers for the diagnosis and prognosis of colorectal cancer. Intest. Res. 2020, 18, 168–183. [Google Scholar] [CrossRef] [PubMed]
- Christensen, T.D.; Palshof, J.A.; Larsen, F.O.; Poulsen, T.S.; Høgdall, E.; Pfeiffer, P.; Jensen, B.V.; Yilmaz, M.K.; Nielsen, D. Associations between primary tumor RAS, BRAF and PIK3CA mutation status and metastatic site in patients with chemo-resistant metastatic colorectal cancer. Acta Oncol. 2018, 57, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.G.; Fisher, D.; Claes, B.; Maughan, T.S.; Idziaszczyk, S.; Peuteman, G.; Harris, R.; James, M.D.; Meade, A.; Jasani, B.; et al. Somatic profiling of the epidermal growth factor receptor pathway in tumors from patients with advanced colorectal cancer treated with chemotherapy ± cetuximab. Clin. Cancer Res. 2013, 19, 4104–4113. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, A.R.; Hamilton, S.R.; Allegra, C.J.; Grody, W.; Cushman-Vokoun, A.M.; Funkhouser, W.K.; Kopetz, S.E.; Lieu, C.; Lindor, N.M.; Minsky, B.D.; et al. Molecular Biomarkers for the Evaluation of Colorectal Cancer: Guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and American Society of Clinical Oncology. Arch. Pathol. Lab. Med. 2017, 141, 625–657. [Google Scholar] [CrossRef]
- Yokota, T.; Ura, T.; Shibata, N.; Takahari, D.; Shitara, K.; Nomura, M.; Kondo, C.; Mizota, A.; Utsunomiya, S.; Muro, K.; et al. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br. J. Cancer 2011, 104, 856–862. [Google Scholar] [CrossRef]
- Cheng, H.H.; Lin, J.K.; Chen, W.S.; Jiang, J.K.; Yang, S.H.; Chang, S.C. Clinical significance of the BRAFV600E mutation in Asian patients with colorectal cancer. Int. J. Color. Dis. 2018, 33, 1173–1181. [Google Scholar] [CrossRef]
- Sayagués, J.M.; Del Carmen, S.; Del Mar Abad, M.; Corchete, L.A.; Bengoechea, O.; Anduaga, M.F.; Baldeón, M.J.; Cruz, J.J.; Alcazar, J.A.; Angoso, M.; et al. Combined assessment of the TNM stage and BRAF mutational status at diagnosis in sporadic colorectal cancer patients. Oncotarget 2018, 9, 24081–24096. [Google Scholar] [CrossRef][Green Version]
- Sanz-Pamplona, R.; Lopez-Doriga, A.; Paré-Brunet, L.; Lázaro, K.; Bellido, F.; Alonso, M.H.; Aussó, S.; Guinó, E.; Beltrán, S.; Castro-Giner, F.; et al. Exome Sequencing Reveals AMER1 as a Frequently Mutated Gene in Colorectal Cancer. Clin. Cancer Res. 2015, 21, 4709–4718. [Google Scholar] [CrossRef]
- Fang, L.; Ford-Roshon, D.; Russo, M.; O’Brien, C.; Xiong, X.; Gurjao, C.; Grandclaudon, M.; Raghavan, S.; Corsello, S.M.; Carr, S.A.; et al. RNF43 G659fs is an oncogenic colorectal cancer mutation and sensitizes tumor cells to PI3K/mTOR inhibition. Nat. Commun. 2022, 13, 3181. [Google Scholar] [CrossRef]
- Giannakis, M.; Hodis, E.; Jasmine Mu, X.; Yamauchi, M.; Rosenbluh, J.; Cibulskis, K.; Saksena, G.; Lawrence, M.S.; Qian, Z.R.; Nishihara, R.; et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat. Genet. 2014, 46, 1264–1266. [Google Scholar] [CrossRef] [PubMed]
- Randon, G.; Fucà, G.; Rossini, D.; Raimondi, A.; Pagani, F.; Perrone, F.; Tamborini, E.; Busico, A.; Peverelli, G.; Morano, F.; et al. Prognostic impact of ATM mutations in patients with metastatic colorectal cancer. Sci. Rep. 2019, 9, 2858. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Oh, S.; Song, H.; Shin, S.; Zhang, B.; Freeman, W.M.; Janknecht, R. A potential common role of the Jumonji C domain-containing 1A histone demethylase and chromatin remodeler ATRX in promoting colon cancer. Oncol. Lett. 2018, 16, 6652–6662. [Google Scholar] [CrossRef] [PubMed]
- AACR Project GENIE Consortium. AACR Project GENIE: Powering Precision Medicine through an International Consortium. Cancer Discov. 2017, 7, 818–831. [Google Scholar] [CrossRef]
- Poulogiannis, G.; McIntyre, R.E.; Dimitriadi, M.; Apps, J.R.; Wilson, C.H.; Ichimura, K.; Luo, F.; Cantley, L.C.; Wyllie, A.H.; Adams, D.J.; et al. PARK2 deletions occur frequently in sporadic colorectal cancer and accelerate adenoma development in Apc mutant mice. Proc. Natl. Acad. Sci. USA 2010, 107, 15145–15150. [Google Scholar] [CrossRef]
- Bhat, Z.I.; Kumar, B.; Bansal, S.; Naseem, A.; Tiwari, R.R.; Wahabi, K.; Sharma, G.D.; Alam Rizvi, M.M. Association of PARK2 promoter polymorphisms and methylation with colorectal cancer in North Indian population. Gene 2019, 682, 25–32. [Google Scholar] [CrossRef]
- Gonzalez-Pons, M.; Cruz-Correa, M. Colorectal Cancer Biomarkers: Where Are We Now? Biomed. Res. Int. 2015, 2015, 149014. [Google Scholar] [CrossRef] [PubMed]
- Baran, B.; Mert Ozupek, N.; Yerli Tetik, N.; Acar, E.; Bekcioglu, O.; Baskin, Y. Difference between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterol. Res. 2018, 11, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Akce, M.; Zakka, K.; Jiang, R.; Williamson, S.; Alese, O.; Shaib, W.; Wu, C.; Behera, M.; El-Rayes, B. Impact of Tumor Side on Clinical Outcomes in Stage II and III Colon Cancer with Known Microsatellite Instability Status. Front. Oncol. 2021, 11, 592351. [Google Scholar] [CrossRef] [PubMed]
- Caputo, F.; Santini, C.; Bardasi, C.; Cerma, K.; Casadei-Gardini, A.; Spallanzani, A.; Andrikou, K.; Cascinu, S.; Gelsomino, F. BRAF-Mutated Colorectal Cancer: Clinical and Molecular Insights. Int. J. Mol. Sci. 2019, 20, 5369. [Google Scholar] [CrossRef]
- Cantwell-Dorris, E.R.; O’Leary, J.J.; Sheils, O.M. BRAFV600E: Implications for carcinogenesis and molecular therapy. Mol. Cancer Ther. 2011, 10, 385–394. [Google Scholar] [CrossRef]
- Tran, B.; Kopetz, S.; Tie, J.; Gibbs, P.; Jiang, Z.Q.; Lieu, C.H.; Agarwal, A.; Maru, D.M.; Sieber, O.; Desai, J. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer 2011, 117, 4623–4632. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ahn, B.K.; Baik, S.S.; Lee, K.H. Comprehensive Analysis of Somatic Mutations in Colorectal Cancer with Peritoneal Metastasis. In Vivo 2019, 33, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Astrosini, C.; Roeefzaad, C.; Dai, Y.Y.; Dieckgraefe, B.K.; Jöns, T.; Kemmner, W. REG1A expression is a prognostic marker in colorectal cancer and associated with peritoneal carcinomatosis. Int. J. Cancer 2008, 123, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Iwaya, T.; Sawada, G.; Kurashige, J.; Matsumura, T.; Uchi, R.; Ueo, H.; Takano, Y.; Eguchi, H.; Sudo, T.; et al. Up-regulation of NEK2 by microRNA-128 methylation is associated with poor prognosis in colorectal cancer. Ann. Surg. Oncol. 2014, 21, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Shirahata, A.; Shinmura, K.; Kitamura, Y.; Sakuraba, K.; Yokomizo, K.; Goto, T.; Mizukami, H.; Saito, M.; Ishibashi, K.; Kigawa, G.; et al. MACC1 as a marker for advanced colorectal carcinoma. Anticancer Res. 2010, 30, 2689–2692. [Google Scholar] [PubMed]
- Nagahara, M.; Nishida, N.; Iwatsuki, M.; Ishimaru, S.; Mimori, K.; Tanaka, F.; Nakagawa, T.; Sato, T.; Sugihara, K.; Hoon, D.S.; et al. Kinesin 18A expression: Clinical relevance to colorectal cancer progression. Int. J. Cancer 2011, 129, 2543–2552. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Hu, W.M.; Xia, L.P.; He, W.Z. Association between somatic RET mutations and clinical and genetic characteristics in patients with metastatic colorectal cancer. Cancer Med. 2021, 10, 8876–8882. [Google Scholar] [CrossRef]
- Sakuraba, K.; Yasuda, T.; Sakata, M.; Kitamura, Y.H.; Shirahata, A.; Goto, T.; Mizukami, H.; Saito, M.; Ishibashi, K.; Kigawa, G.; et al. Down-regulation of Tip60 gene as a potential marker for the malignancy of colorectal cancer. Anticancer Res. 2009, 29, 3953–3955. [Google Scholar]
- Stein, M.K.; Williard, F.W.; Xiu, J.; Tsao, M.W.; Martin, M.G.; Deschner, B.W.; Dickson, P.V.; Glazer, E.S.; Yakoub, D.; Shibata, D.; et al. Comprehensive tumor profiling reveals unique molecular differences between peritoneal metastases and primary colorectal adenocarcinoma. J. Surg. Oncol. 2020, 121, 1320–1328. [Google Scholar] [CrossRef]
- Brannon, A.R.; Vakiani, E.; Sylvester, B.E.; Scott, S.N.; McDermott, G.; Shah, R.H.; Kania, K.; Viale, A.; Oschwald, D.M.; Vacic, V.; et al. Comparative sequencing analysis reveals high genomic concordance between matched primary and metastatic colorectal cancer lesions. Genome Biol. 2014, 15, 454. [Google Scholar] [CrossRef]
- Lenos, K.J.; Bach, S.; Ferreira Moreno, L.; Ten Hoorn, S.; Sluiter, N.R.; Bootsma, S.; Vieira Braga, F.A.; Nijman, L.E.; van den Bosch, T.; Miedema, D.M.; et al. Molecular characterization of colorectal cancer related peritoneal metastatic disease. Nat. Commun. 2022, 13, 4443. [Google Scholar] [CrossRef] [PubMed]
- Priestley, P.; Baber, J.; Lolkema, M.P.; Steeghs, N.; de Bruijn, E.; Shale, C.; Duyvesteyn, K.; Haidari, S.; van Hoeck, A.; Onstenk, W.; et al. Pan-cancer whole-genome analyses of metastatic solid tumours. Nature 2019, 575, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Thanki, K.; Nicholls, M.E.; Gajjar, A.; Senagore, A.J.; Qiu, S.; Szabo, C.; Hellmich, M.R.; Chao, C. Consensus Molecular Subtypes of Colorectal Cancer and their Clinical Implications. Int. Biol. Biomed. J. 2017, 3, 105–111. [Google Scholar]
- Ten Hoorn, S.; de Back, T.R.; Sommeijer, D.W.; Vermeulen, L. Clinical Value of Consensus Molecular Subtypes in Colorectal Cancer: A Systematic Review and Meta-Analysis. JNCI J. Natl. Cancer Inst. 2021, 114, 503–516. [Google Scholar] [CrossRef]
- Valenzuela, G.; Canepa, J.; Simonetti, C.; Solo de Zaldívar, L.; Marcelain, K.; González-Montero, J. Consensus molecular subtypes of colorectal cancer in clinical practice: A translational approach. World J. Clin. Oncol. 2021, 12, 1000–1008. [Google Scholar] [CrossRef]
- Rebersek, M. Consensus molecular subtypes (CMS) in metastatic colorectal cancer—Personalized medicine decision. Radiol. Oncol. 2020, 54, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Ubink, I.; van Eden, W.J.; Snaebjornsson, P.; Kok, N.F.M.; van Kuik, J.; van Grevenstein, W.M.U.; Laclé, M.M.; Sanders, J.; Fijneman, R.J.A.; Elias, S.G.; et al. Histopathological and molecular classification of colorectal cancer and corresponding peritoneal metastases. Br. J. Surg. 2018, 105, e204–e211. [Google Scholar] [CrossRef]
- Ros, J.; Baraibar, I.; Sardo, E.; Mulet, N.; Salvà, F.; Argilés, G.; Martini, G.; Ciardiello, D.; Cuadra, J.L.; Tabernero, J.; et al. BRAF, MEK and EGFR inhibition as treatment strategies in BRAF V600E metastatic colorectal cancer. Ther. Adv. Med. Oncol. 2021, 13, 1758835921992974. [Google Scholar] [CrossRef]
- Korphaisarn, K.; Kopetz, S. BRAF-Directed Therapy in Metastatic Colorectal Cancer. Cancer J. 2016, 22, 175–178. [Google Scholar] [CrossRef][Green Version]
- Argilés, G.; Tabernero, J.; Labianca, R.; Hochhauser, D.; Salazar, R.; Iveson, T.; Laurent-Puig, P.; Quirke, P.; Yoshino, T.; Taieb, J.; et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1291–1305. [Google Scholar] [CrossRef]
- Riley, R.D.; Ensor, J.; Snell, K.I.E.; Harrell, F.E.; Martin, G.P.; Reitsma, J.B.; Moons, K.G.M.; Collins, G.; van Smeden, M. Calculating the sample size required for developing a clinical prediction model. BMJ 2020, 368, m441. [Google Scholar] [CrossRef]
- Verkouteren, B.J.; Roemen, G.M.; Schuurs-Hoeijmakers, J.H.; Abdul Hamid, M.; van Geel, M.; Speel, E.M.; Mosterd, K. Molecular mechanism of extracutaneous tumours in patients with basal cell nevus syndrome. J. Clin. Pathol. 2022, 76, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Ebert, D.; Muruganujan, A.; Mills, C.; Albou, L.-P.; Mushayamaha, T.; Thomas, P.D. PANTHER version 16: A revised family classification, tree-based classification tool, enhancer regions and extensive API. Nucleic Acids Res. 2020, 49, D394–D403. [Google Scholar] [CrossRef]
- Gene Ontology Consortium. The Gene Ontology resource: Enriching a GOld mine. Nucleic Acids Res. 2021, 49, D325–D334. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Jassal, B.; Matthews, L.; Viteri, G.; Gong, C.; Lorente, P.; Fabregat, A.; Sidiropoulos, K.; Cook, J.; Gillespie, M.; Haw, R. The reactome pathway knowledgebase. Nucleic Acids Res. 2020, 48, D498–D503. [Google Scholar] [CrossRef] [PubMed]
| Variable | M0 (N = 20) | LM (N = 10) | PM (N = 9) | p Value |
|---|---|---|---|---|
| Age at time of diagnosis (years)—median (Q1–Q3) | 69.00 | 69.00 | 68.00 | 0.801 a |
| (62.00–74.90) | (63.75–74.25) | (58.00–74.00) | ||
| Gender–n (%) | 0.514 b | |||
| Male | 12 (60) | 8 (80) | 5 (55.6) | |
| Female | 8 (40) | 2 (20) | 4 (44.4) | |
| Primary tumor location †—n (%) | 0.433 b | |||
| Right colon | 10 (50) | 2 (20) | 2 (22.2) | |
| Left colon | 7 (35) | 5 (50) | 4 (44.4) | |
| Rectum | 3 (15) | 3 (30) | 3 (33.3) | |
| Tumor size (cm) —median (Q1–Q3) | 4.10 | 2.25 | 3.00 | 0.061 a |
| (3.28–5.38) | (1.80–5.43) | (2.40–3.50) | ||
| Differentiation grade—n (%) * | <0.001 b | |||
| Poor | 4 (20) | 0 (0) | 0 (0) | |
| Poor/moderate | 2 (10) | 2 (20) | 8 (88.9) | |
| Moderate | 14 (70) | 6 (60) | 1 (11.1) | |
| Moderate/well | 0 (0) | 1 (10) | 0 (0) | |
| Type of surgery—n (%) | 0.153 b | |||
| Open | 10 (50) | 2 (20) | 5 (55.6) | |
| Laparoscopic | 10 (50) | 6 (60) | 4 (44.4) | |
| Robot assisted | 0 (0) | 2 (20) | 0 (0) | |
| Positive lymph nodes—n (%) | 0.389 b | |||
| No | 11 (55) | 8 (80) | 5 (55.6) | |
| Yes | 9 (45) | 2 (20) | 4 (44.4) | |
| Neoadjuvant treatment—n (%) | 0.039 b | |||
| No | 17 (85) | 4 (40) | 7 (77.8) | |
| Yes | 3 (15) | 6 (60) | 2 (22.2) | |
| Adjuvant treatment—n (%) * | 0.247 b | |||
| No | 9 (45) | 7 (70) | 4 (44.4) | |
| Yes | 11 (55) | 2 (20) | 5 (55.6) | |
| Oncological history—n (%) | 0.882 b | |||
| No | 18 (90) | 8 (80) | 8 (88.9) | |
| Yes | 2 (10) | 2 (20) | 1 (11.1) | |
| Oncological family history—n (%) * | 1.000 b | |||
| No | 6 (30) | 3 (30) | 0 (0) | |
| Yes | 12 (60) | 5 (50) | 1 (11.1) | |
| Time between surgery and metastases (months)—median (Q1–Q3) | N/A | 18.09 | 16.42 | 0.744 c |
| (7.77–28.95) | (9.71–25.05) | |||
| PCI score—median (Q1–Q3) | N/A | N/A | 3.50 | N/A |
| (3.00–4.00) |
| M Group | Gene Pair | Breakpoint 1 | Breakpoint 2 | Fusion Supporting Reads |
|---|---|---|---|---|
| M0 | TARSL2-NTRK3 | Exon 18 chr15:102197123 | Exon 14 chr15:88576274 | 19 |
| PM | FAM198A-RAF1 | Exon not found chr3:43101459 | Exon 3 chr3:12653448 | 85 |
| PM | RPS6KB1-HSF5 | Exon 1 chr17:57970685 | Exon 3 chr17:56544340 | 21 |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heuvelings, D.J.I.; Wintjens, A.G.W.E.; Moonen, L.; Engelen, S.M.E.; de Hingh, I.H.J.T.; Valkenburg-van Iersel, L.B.; den Dulk, M.; Beckervordersandforth, J.; Thijssen, S.G.M.; Leunissen, D.J.G.; et al. Predictive Genetic Biomarkers for the Development of Peritoneal Metastases in Colorectal Cancer. Int. J. Mol. Sci. 2023, 24, 12830. https://doi.org/10.3390/ijms241612830
Heuvelings DJI, Wintjens AGWE, Moonen L, Engelen SME, de Hingh IHJT, Valkenburg-van Iersel LB, den Dulk M, Beckervordersandforth J, Thijssen SGM, Leunissen DJG, et al. Predictive Genetic Biomarkers for the Development of Peritoneal Metastases in Colorectal Cancer. International Journal of Molecular Sciences. 2023; 24(16):12830. https://doi.org/10.3390/ijms241612830
Chicago/Turabian StyleHeuvelings, Danique J. I., Anne G. W. E. Wintjens, Laura Moonen, Sanne M. E. Engelen, Ignace H. J. T. de Hingh, Liselot B. Valkenburg-van Iersel, Marcel den Dulk, Jan Beckervordersandforth, Sharon G. M. Thijssen, Daphne J. G. Leunissen, and et al. 2023. "Predictive Genetic Biomarkers for the Development of Peritoneal Metastases in Colorectal Cancer" International Journal of Molecular Sciences 24, no. 16: 12830. https://doi.org/10.3390/ijms241612830
APA StyleHeuvelings, D. J. I., Wintjens, A. G. W. E., Moonen, L., Engelen, S. M. E., de Hingh, I. H. J. T., Valkenburg-van Iersel, L. B., den Dulk, M., Beckervordersandforth, J., Thijssen, S. G. M., Leunissen, D. J. G., Stassen, L. P. S., Keszthelyi, D., Mujagic, Z., Speel, E.-J. M., & Bouvy, N. D. (2023). Predictive Genetic Biomarkers for the Development of Peritoneal Metastases in Colorectal Cancer. International Journal of Molecular Sciences, 24(16), 12830. https://doi.org/10.3390/ijms241612830







