Recent Progress of Spectroscopic Probes for Peroxynitrite and Their Potential Medical Diagnostic Applications
Abstract
1. Introduction
2. Fluorescent Probes
2.1. Xanthene as Fluorophore Core
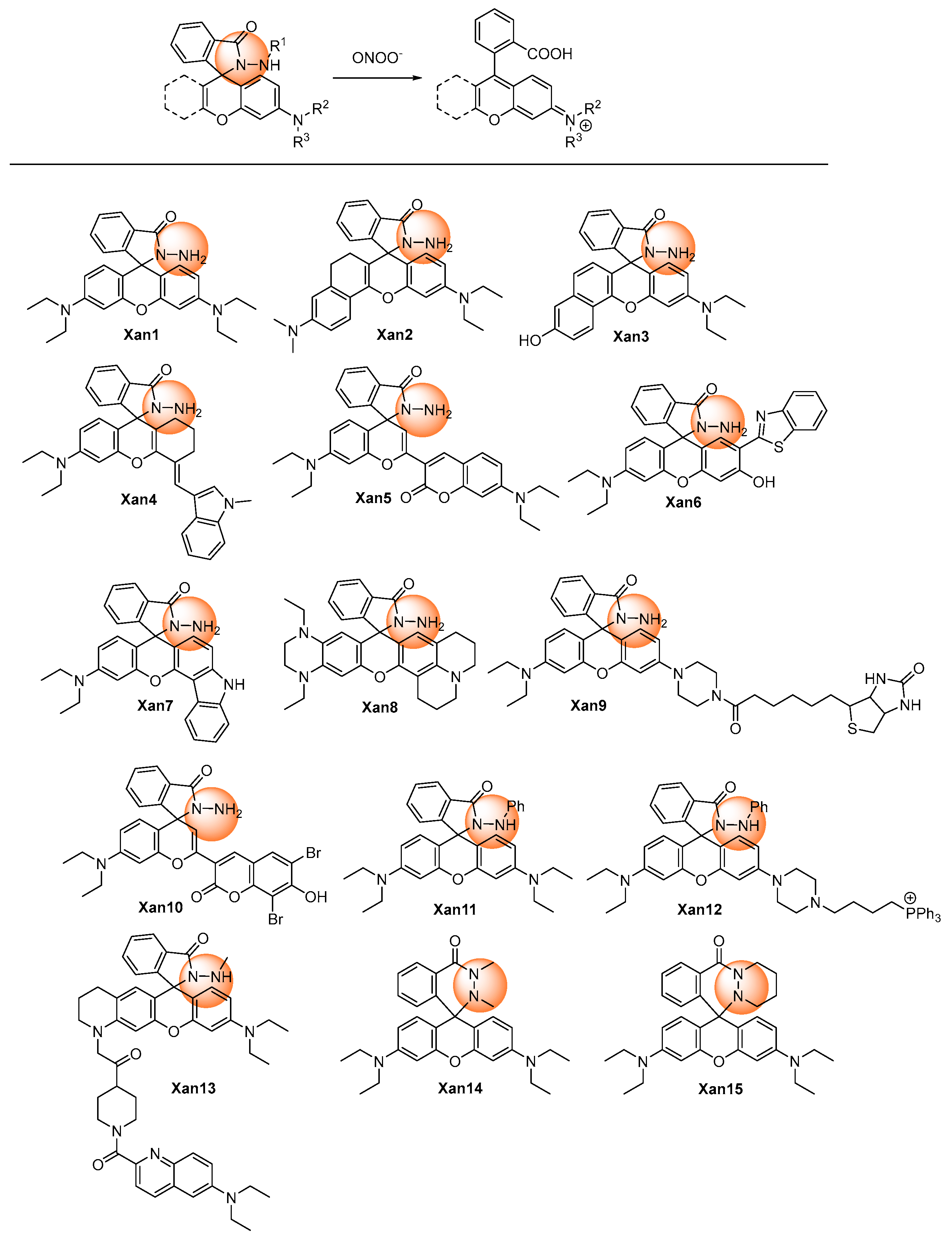
2.1.1. Hydrazide Oxidative Xanthene Probes
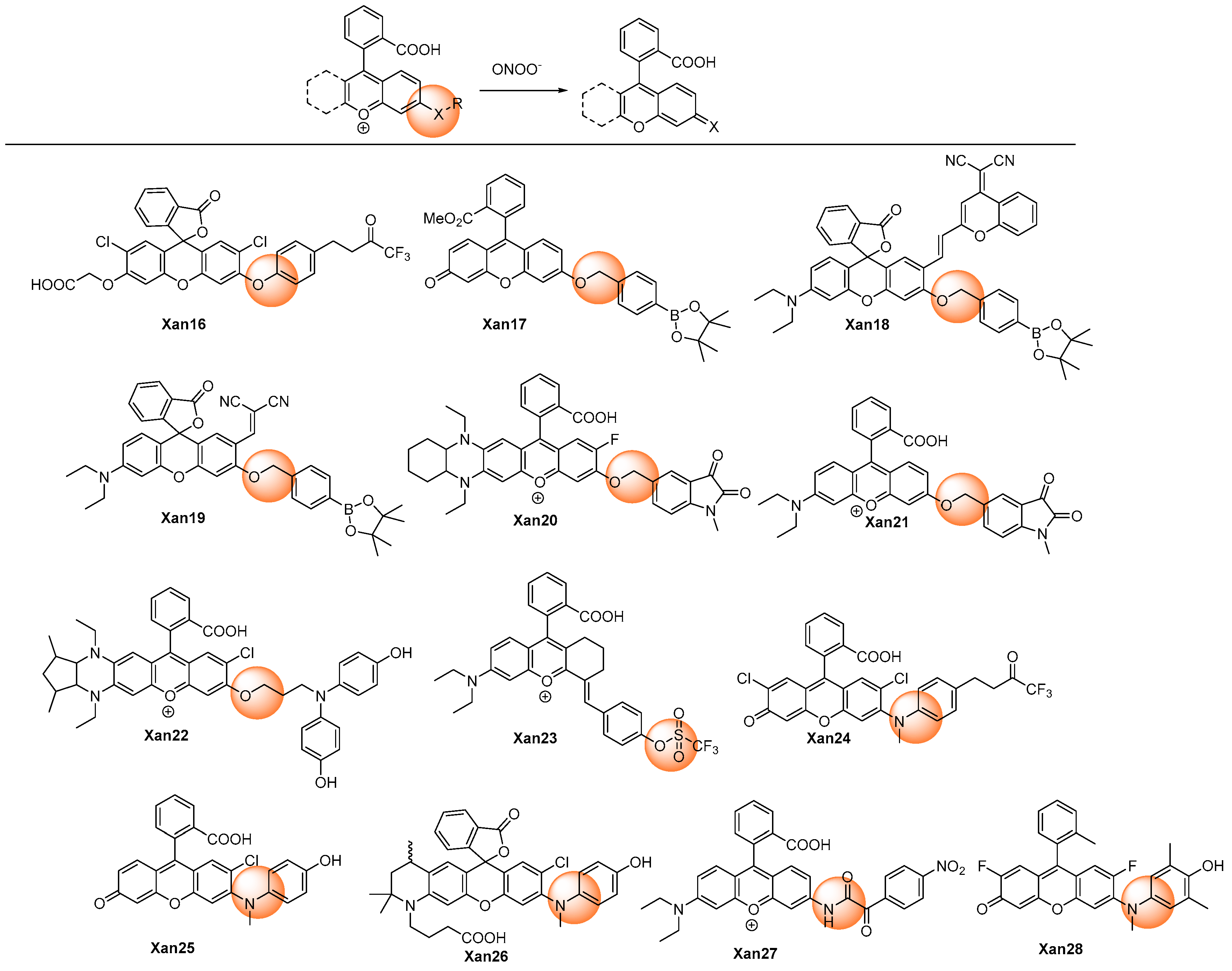
2.1.2. Oxidative Cleavage of the Recognition Groups to Release Xanthene Probes
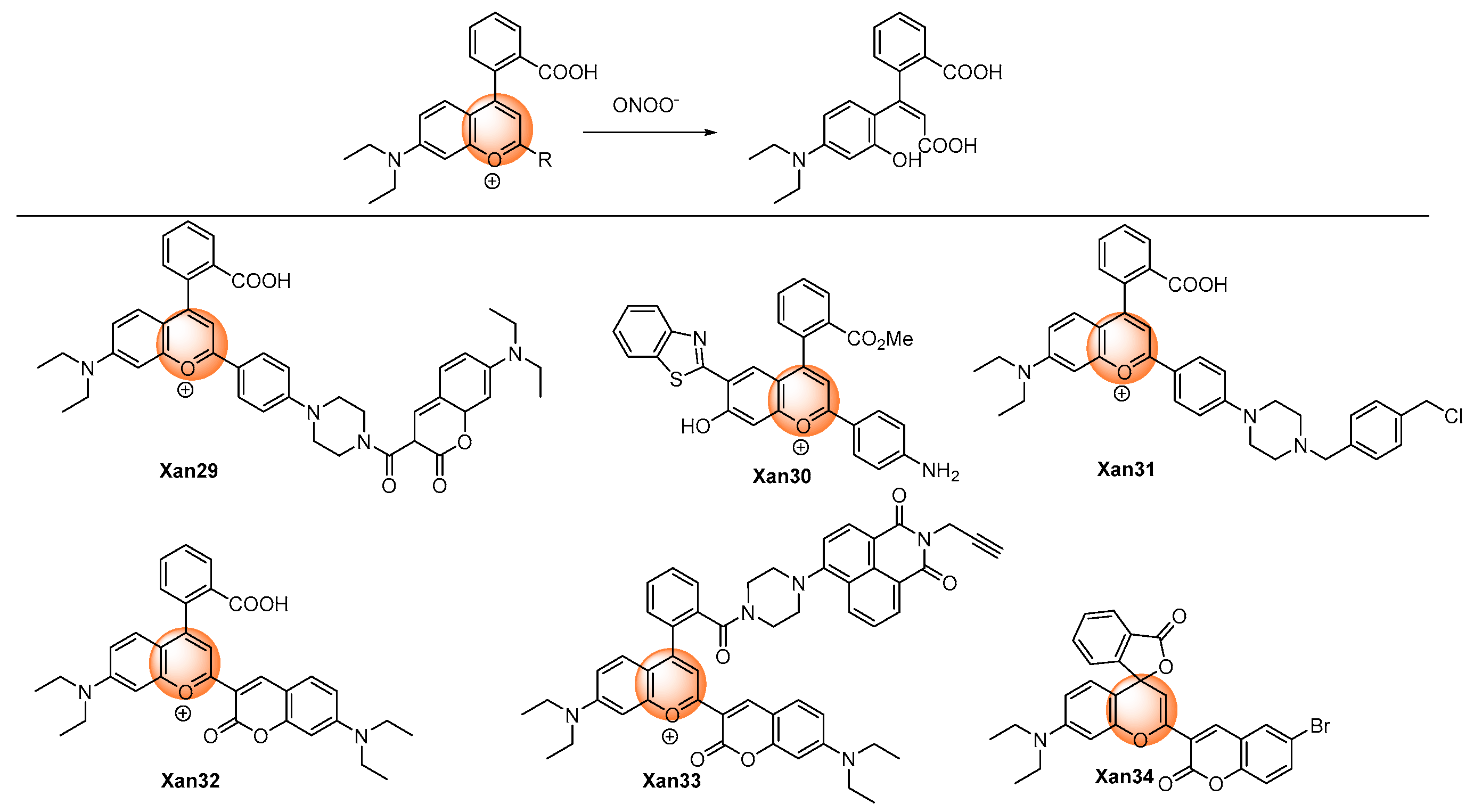
2.1.3. Oxidation of Pyrylium
2.1.4. Oxidation of Hydrogenated Xanthene
2.1.5. Others
2.2. Dicyano-Based Compounds as Fluorophore Core
2.3. Coumarin as Fluorophore Core
2.4. N-Substituted Coumarin as Fluorophore Core
2.5. 1,8-Naphthalimide as Fluorophore Core
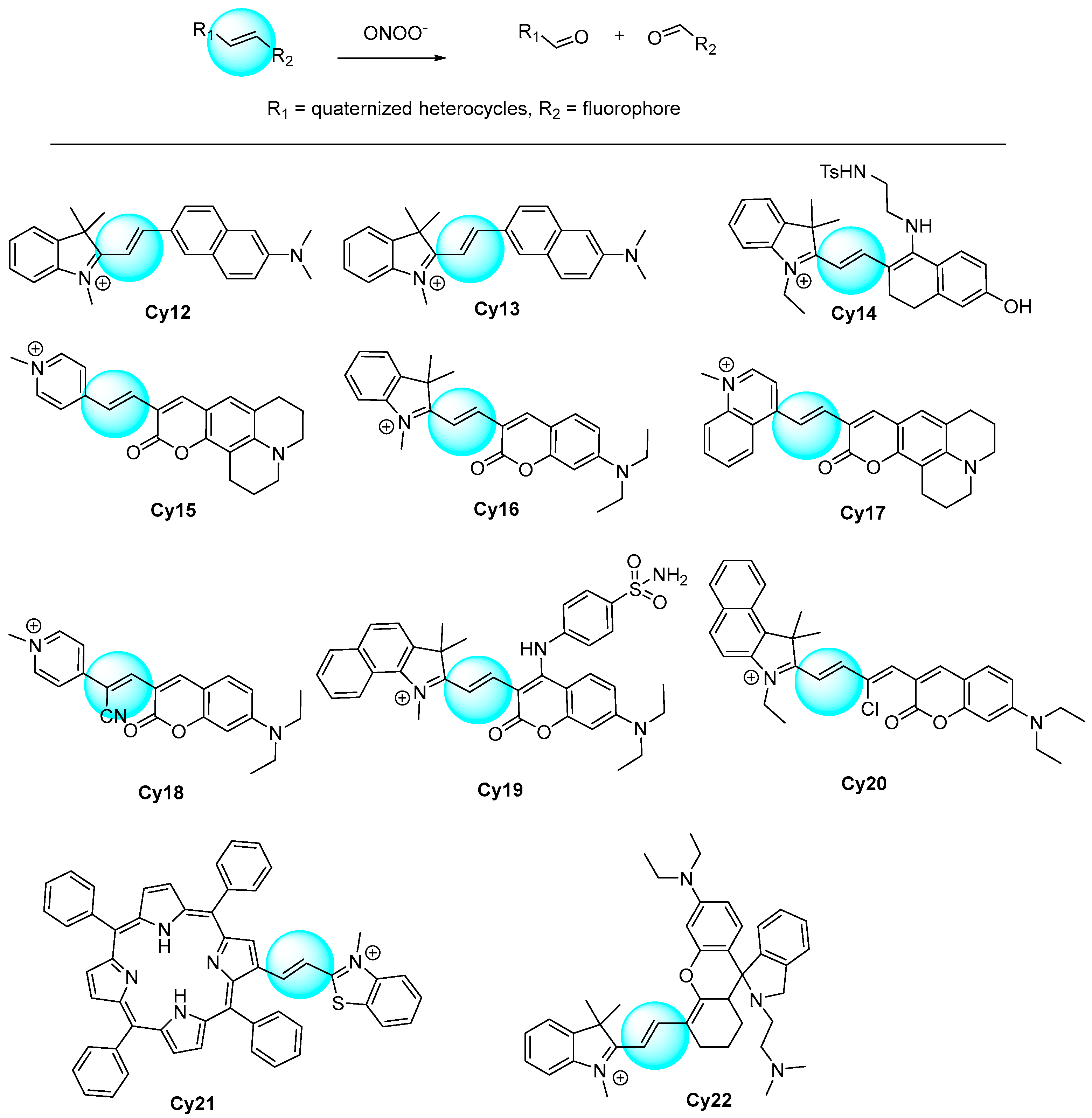
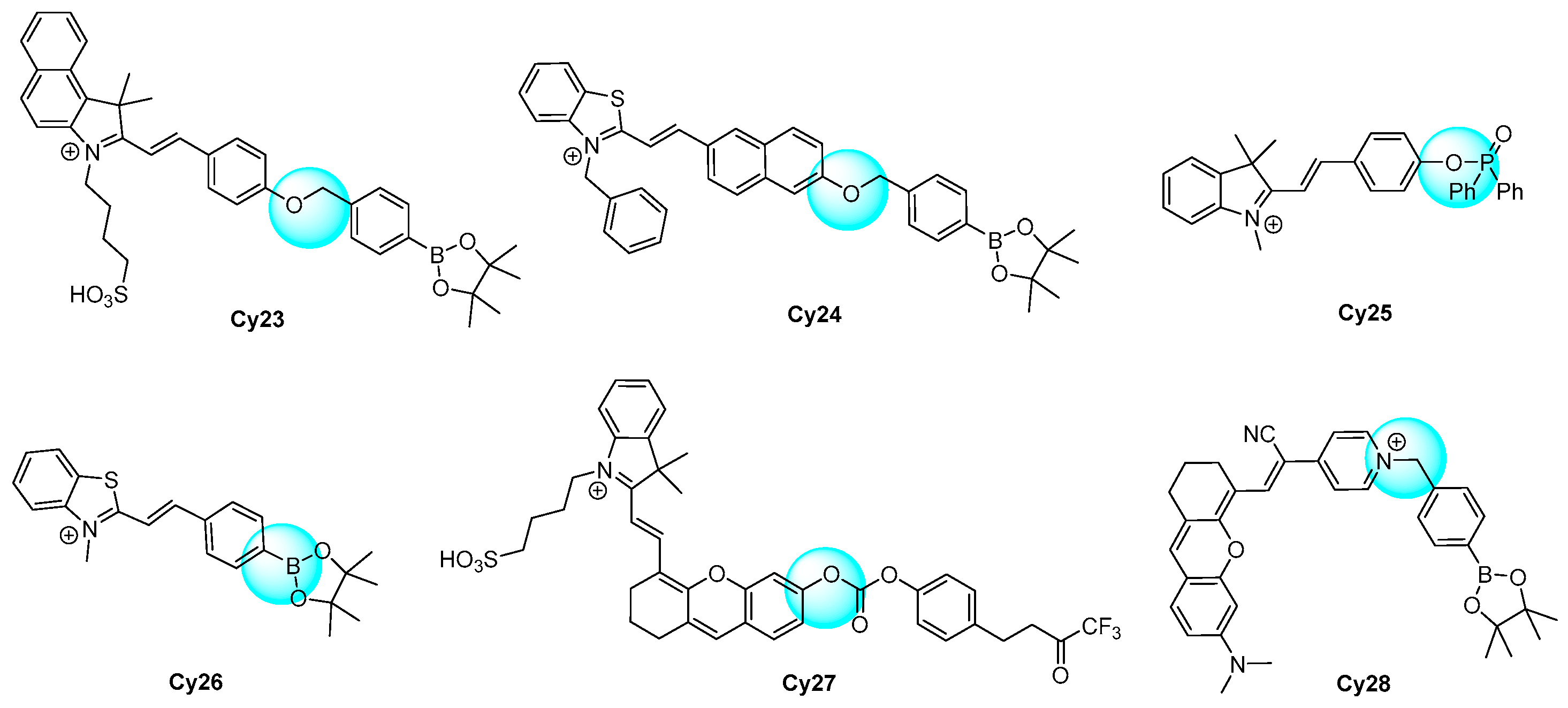
2.6. Cyanines as Fluorophore Core
2.7. Half-Cyanines as Fluorophore Core
| Reference Number | Dye | λem (nm) | Dynamic Range of Fluorescence Response (Fold) | Response Time | Range (μM) | Detection Limit (nM) | Interference Species (Reactive Species; Anion; Cation; Neutral Species) | ONOO—Production Pathways in the Biosystem | Fluorescent Bioimaging Objects |
|---|---|---|---|---|---|---|---|---|---|
| [18] | Xan1 | 556 | NR | <30 s | 0.075–3.0 | 24 | H2O2; NO2−, NO3−; Cu2+; Cys, Met, GSH, NHOH, Glucose, ascorbic acid, Epinephrine | Aqueous | NR |
| [19] | Xan2 | 638 | 80 | <180 | 0–34 | 45 | H2O2, ClO−, ·OH, ·O2−, 1O2, tBuOOH, tBuOO·, NO·; NO2−, NO3− | Pseudomonas aeruginosa (PAO1)-infected bone marrow-derived neutrophils | HeLa and RAW264.7 cells, mouse |
| [20] | Xan3 | 630 | 40 | <5 s | 0.5–8 | 17 | 1O2, H2O2, tBuOOH, ·O2−, ·OH, tBuOO·, OCl−; SO42−, NO3−, NO2−, Cl−; K+, Na+, Mg2+, Zn2+, Ca2+, Al3+; Cys, GSH | Cell endogenous | HeLa cells |
| [21] | Xan4 | 698 | 100 | <2 s | 0–100 | 25 | tBuOO·, tBuOOH, NO·, ClO−, ·OH, ·O2−, H2O2; NO2−, F−, Cl−, I−, SO42−, H2PO4−, SO32−, HCO3−, HS−, AcO−; Na+, Mg2+, K+, Ca2+, Cu2+; Cys, Hcy, GSH, Gly, Leu, Lys, Val, Glu | Cell exogenous and endogenous | RAW264.7 cells |
| [22] | Xan5 | 515/700 | NR | ~60 s | 0–50 | 59 | NO, ClO−, ·O2−, tBuOO·, tBuOOH, ·OH, H2O2; F−, Cl−, I−, H2PO4−, SO32−, HCO3−, AcO−, NO2−; Na+, Mg2+, K+, Ca2+; Gly, Leu, Glu, Val, Lys, Tyr, Cys, Hcy, GSH | Cell exogenous and endogenous | RAW264.7 cells and mouse |
| [23] | Xan6 | 581 | NR | <10 | 0–18 | 93 | tBuOO·, tBuOOH, ·OH, H2O2, ·O2−, 1O2, ClO−; NO3−, NO2−, Cl−, SO42−; Zn2+, Al3+, Na+, Mg2+, K+, Ca2+, Fe2+, Fe3+, Cu2+; Cys, Hcy, GSH | Cell exogenous and endogenous | RAW264.7 cells and zebrafish |
| [24] | Xan7 | 585 | NR | <15 | 0–8.0 | 10.9 | ·O2−, tBuOO·, tBuOOH,·OH, H2O2, 1O2; Zn2+, Na+, Mg2+, K+, Ca2+; Glu, Cys, GSH | Cell exogenous and endogenous | HeLa cells and zebrafish |
| [25] | Xan8 | 678 | 100 | <2 min | 0–70 | 30 | ClO−, H2O2, ·O2−, tBuOO·,·OH; SO32−, HSO3−, SCN−, CO32−, S2O32−, NO2−, HSO4−, S2O72−, AcO−, HCO3−, NO3−, F−, Br−, I−, Cl−, HS−; Zn2+, Na+, K+, Ca2+, Fe2+, Ba2+, Cu2+; Lys, Val, Asp, Phe, Asn, Ser, Ile, Arg, Tyr, His, Trp, Glu, Ala, Met, Thr, Leu | Cell exogenous and endogenous | RAW264.7 cells |
| [26] | Xan9 | 575 | NR | <1 min | 0–10 | 7 | ·1O2, tBuOO·,·OH, tBuOOH, H2O2, NO, HClO; NO3−, NO2−, F−, CO32−, S2−, SO32−; Zn2+, Na+, Mg2+, K+, Ca2+, Fe2+, Fe3+, Cu2+, Mn2+; Cys, Hcy, GSH | Cell exogenous and endogenous | HSC-2 and Cal-27 cells, 3D spheroid and mice |
| [27] | Xan10 | 631/669 | 10 | ~1 min | 0–20 | 8 | 1O2, H2O2, ·O2−, tBuOO·, tBuOOH, ·OH, HClO; Cl−, NO3−, NO2−, S2−; Cys, GSH, HSO3− | Cell exogenous and endogenous | HeLa and HepG2 cells, zebrafish. |
| [28] | Xan11 | 580 | NR | <10 min | 2–20 | 1.4 | H2O2; SO42−, NO3−, NO2−; Zn2+, Na+, Mg2+, K+, Ca2+, Fe2+, Fe3+, Cu2+, Mn2+, Hg2+; Cys, Met, Thr, Glu, Glucose, Urea, Ascorbic acid | Cell exogenous and endogenous | MCF-7 Cells |
| [29] | Xan12 | 578 | 80 | <30 min | 0–100 | 55 | ClO−, NO, H2O2, ·O2−, 1O2, tBuOOH, tBuOO·; NO2− | Cell exogenous and endogenous | HeLa and RAW264.7 cells |
| [30] | Xan13 | 574 | 200 | <2 min | 0–14 | NR | NR | Arginase 1 regulated | RAW264.7 cells and mouse |
| [31] | Xan14 | 585 | NR | <3 s | 0–10 | 0.68 | tBuOO·,·OH, 1O2, ·O2−, NO, H2O2, tBuOOH, ClO−; Br−, SO32−, CO32−, NO3−, NO2−; Zn2+, Na+, Mg2+, K+, Ca2+, Fe2+, Fe3+, Cu2+, Cu+; Cys, Hcy, GSH | Cell exogenous and endogenous | RAW264.7 cells and zebrafish |
| [32] | Xan15 | 585 | NR | <10 s | 0–5 | 61 | tBuOO·,·OH, 1O2, ·O2−, NO, H2O2, tBuOOH, ClO−; Br−, SO32−, CO32−, NO3−, NO2−; Zn2+, Na+, Mg2+, K+, Ca2+, Fe2+, Fe3+, Cu2+, Cu+; Cys, Hcy, GSH, | Cell exogenous and endogenous | RAW264.7 cells and zebrafish |
| [33] | Xan16 | 521 | 8 | <15 min | 0–20 | NR | ·OH, 1O2, ·O2−, NO, ClO−, tBuOO· | Cell exogenous and endogenous | Primary cultured neuronal cells |
| [34] | Xan17 | 518 | NR | <40 s | 0–20 | NR | H2O2, HClO, tBuOO·, GSH, ·O2−, ·NO | Doxorubicin introduced | EA.hy926 cells |
| [35] | Xan18 | 570 | NR | <15 min | 0–20 | 52 | HClO, H2O2, ·OH, 1O2, ·O2−, tBuOOH, tBuOO·; Cys, Hcy, GSH | APAP-induced liver injury | HepG2 cells, mice |
| [36] | Xan19 | 573 | 130 | <10 s | 0–20 | 34 | HClO, H2O2, ·OH, 1O2, ·O2−, tBuOOH, tBuOO·, NO2−, NO | Liver ischemia/reperfusion | HL-7702 cells, mice |
| [37] | Xan20 | 653 | NR | <4 min | 0–35 | 72 | ClO−, H2O2, ·OH, O2−, NO; NO2−; Zn2+, Na+, Mg2+, K+, Ca2+, Fe2+, Cu2+ | Cancer cell exogenous and endogenous | HeLa and RAW264.7 cells zebrafish, mice |
| [38] | Xan21 | 557 | 71 | <2 min | 0.1–10 | NR | ClO−, H2O2, ·OH, O2−, NO; NO2−; Zn2+, Na+, Mg2+, K+, Ca2+, Fe2+, Cu2+ | Brain stroke in mice, LPS induced kidney injury | RAW264.7 cells, zebrafish, mice |
| [39] | Xan22 | 698 | 50 | <30 min | 0–10 | 3.4 | ClO−, H2O2, ·OH, O2−, NO; NO2−, S2−; Zn2+, Na+, Mg2+, K+, Ca2+, Fe2+, Cu2+, Fe3+; Hcy, Cys, GSH, vitamin C, Aβ oligomer | Alzheimer’s disease | PC12 cells, mice |
| [40] | Xan23 | 725 | NR | <2 min | 0–10 | 85 | ClO−, H2O2, ·OH, ·NO; S2−, HS−, NO3−, SO32−, SO42−, NO2−; Na+, K+, Ca2+, Fe2+, Fe3+; Cys, GSH | Myocardium ischemia–reperfusion injury | H9c2 cells, mice |
| [41] | Xan24 | 535 | 140 | <30 min | 0–5 | 50 | tBuOO·, ClO−, H2O2, ·OH, O2−, ·NO, ClO− | Cell exogenous and endogenous | RAW264.7 cells |
| [42] | Xan25 | 535 | 290 | <5 s | 0–4 | 10 | tBuOO·, H2O2, ·OH, ·O2−, ·NO | Escherichia coli-challenged | RAW264.7 cells, mouse |
| [43] | Xan26 | 570 | 93 | <2 s | 0–8 | - | tBuOO·, H2O2, ·O2−, 1O2,·NO | Acute alcohol-induced liver injury and hepatic ischemic/reperfusion injury | SH-SY5Y cells and live tissues |
| [44] | Xan27 | 558 | 14 | <30 min | 0–16 | 43 | HClO, H2O2, ·O2−, HNO, NO, tBuOO·, ·OH; HSO3−, NO2−, NO3−, AcO−, SO42−; Na+, Mg2+, K+, Fe2+, Cu2+; H2S, H2S2, Cys, GSH | Drug-induced hepatotoxicity | HepG2 cells, |
| [45] | Xan28 | 536 | 1800 | <98 s | - | 40 | ClO−, tBuOO·,·OH, ·O2−, H2O2, NO | Cellular phagocytosis | RAW264.7 cells |
| [46] | Xan29 | 651 | 93 | <20 s | 0–7.5 | 11.3 | HClO, NO, tBuOO·, ·OH, ·O2−, H2O2, tBuOOH, HNO; SO32−, NO2−; H2S, H2S2 | Inflamed mouse | HepG2/RAW264.7 cells, hepatic tissue, mouse |
| [47] | Xan30 | 462 | 134 | <30 min | 0–6 | 1.8 | HClO, ·OH, ·O2−, H2O2, tBuOOH; SO32−, NO2−; H2S, H2S2, Cys, GSH | Drug-induced acute liver injury | RAW264.7 cells |
| [48] | Xan31 | 640 | NR | <80 s | 0.2–1.5 | 23 | 1O2, ClO−, ·OH, H2O2, NO; HS−, HSO3− | Cell endogenous | HeLa and RAW264.7 cells |
| [49] | Xan32 | 469 | 130 | <25 s | 0–7 | 4.1 | tBuOO·, ·OH, tBuOOH, HClO, NO, ·O2−, H2O2; NO2−, SO32−; H2S, H2S2, Cys, GSH | Nonalcoholic fatty liver and drug-induced liver diseases | HepG2 and L02 cells, mouse |
| [50] | Xan33 | 468/526 | 116 | ~20 s | 0–20 | 11.6 | ClO−, ·OH, H2O2, NO, ·O2−, HNO; HSO3−, NO3−, SO32−, NO2−; H2S, Hcy, GSH, Cys | Arthritis | RAW264.7 cells, tissue, mouse |
| [51] | Xan34 | 520 | NR | NR | 0–40 | 1.2 | H2S, Hcy, Cys, GSH | Acrylamide-induced | PC-12 and HepG2 cells, mice |
| [52] | Xan35 | 496 | 100 | <5 s | 0–2.6 | 16 | 1O2, ClO−, H2O2, ·OH, NO, ·O2−; NO3−, NO2− | Cell endogenous | RAW264.7 cells |
| [53] | Xan36 | 648 | NR | <5 s | 0–10 | 30 | 1O2, ClO−, H2O2, ·OH, NO, ·O2−; Zn2+, Na+, Mn2+, Hg2+, Ca2+, Fe2+, Cu2+, Fe3+; Cys, GSH, Hcy, NADH | Cell endogenous | HeLa and RAW264.7 cells, mouse |
| [54] | Xan37 | 760 | 216 | <15 s | 0–2 | 5 | 1O2, ClO−, H2O2, ·OH, NO, ·O2− | Idiopathic pulmonary fibrosis | RAW264.7 cells, mice |
| [55] | Xan38 | 672 | 50 | <50 min | 0–60 | 80 | tBuOO·,·1O2, ClO−, tBuOOH, KO2, H2O2, ·OH; CO32−, SO32−, SO42−, S2−, HS−, HSO3−, NO3−, NO2−; Zn2+, Na+, Mg2+, Ca2+, K+, Cu2+, Fe3+; Hcy, Cys, GSH | Cell endogenous | RAW264.7 cells, mouse |
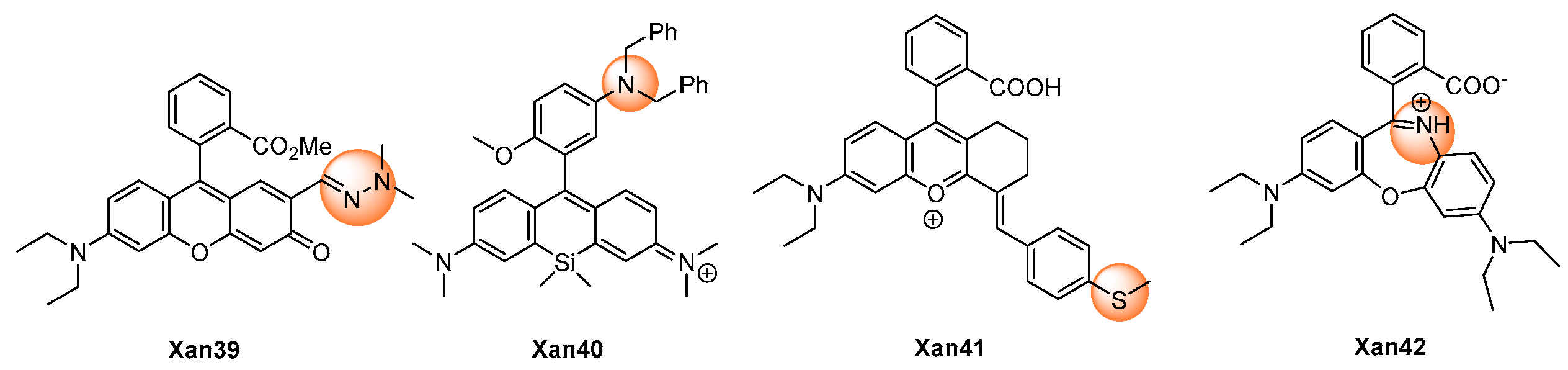
| [56] | Xan39 | 571 | 15 | <60 s | 0–15 | 57 | NO, tBuOOH, ClO−, ·O2−, ·OH; NO3−, NO2−, Cl−, S2O32−, Br−, HS−; K+,Fe3+; Cys, Hcy, Cys, GSH, Pro | Cell exogenous and endogenous | HeLa cells |
| [57] | Xan40 | 680 | NR | <30 s | 0–4 | 3 | 1O2, H2O2, ·OH, ·O2−, ClO−, NOC-9; NO2−; Zn2+, Al3+, Fe2+, Fe3+, Cu2+, Cu+, Na+, Mg2+, Ca2+, K+; GSH, DHA | Ischemia–reperfusion injury | RAW264.7, EA.hy926 and INS-1 cells, tissues, mouse |
| [58] | Xan41 | 548 | NR | <5 s | 0–26 | 47 | TEMPO, tBuOOH, ·OH, H2O2, ·O2−, 1O2,·NO, HOCl; NO3−, NO2−; Fe2+, Cu2+, Cu+; Hcy, Cys, GSH | Peritonitis | RAW264.7 cells, mouse |
| [59] | Xan42 | 672 | NR | <10 min | 0.05–2 | 6.3 | 1O2, ClO−, H2O2, ·OH, NO, ·O2−, tBuOOH; SO42−, Cl−, NO2−; Zn2+, Na+, K+, Mg2+, Ca2+, Cu2+; Glu, Cys, Glucose, BSA | Inflammatory | RAW264.7 and foam cells |
| [61] | Dic1 | 690 | 120 | <20 min | 0–180 | 4620 | KO2, NO, ClO−, tBuOO·,·tBuOOH, H2O2, ·OH | Cell exogenous | HeLa cells |
| [62] | Dic2 | 670 | NR | <20 min | 0–10 | 53 | HOCl, 1O2, ·O2−, tBuOOH, tBuOO·, H2O2, ·OH; NO2−, NO3−, F−, Cl−, Br−, CO32−, H2PO4−, AcO−; Zn2+, Al3+, Fe3+, Cu2+, K+;·Hcy, Cys, GSH, | Cell endogenous | HepG2 cells |
| [63] | Dic3 | 678 | NR | ~25 min | 10–200 | 78.7 | ·OH, NO, ClO−, ·O2−, tBuOO·, H2O2; S2O32−; H2S,Hcy, Cys, GSH, | Cell endogenous | HepG2 cells |
| [64] | Dic4 | 685 | NR | <10 min | 0–20 | 96 | ·O2−, tBuOO·, H2O2, ·OH, NO, ClO−; NO2−, NO3− | Kainate (KA)-induced rat epilepsy | RAW264.7, HT22 cells, brain tissue, mouse |
| [65] | Dic5 | 660 | 30 | <2 s | 0–100 | 81 | HNO, NO, ·OH, ·O2−, tBuOO·, H2O2, ClO−; F−, Cl−, I−, HCO3−, HSO3−, HS−, NO2−; Na+, K+, Fe3+, Cu2+; Tyr, Ala, Asp, Thr, Met, Ile, Phe, Hcy, Cys, GSH | Cell exogenous and endogenous | HeLa cells, zebrafish, mouse |
| [66] | Dic6 | 620 | 10 | <4 min | 1–6 | 27.5 | NO, HNO, ClO−, ·OH, tBuOOH, 1O2, ·O2−; F−, Cl−, Br−, I−, AcO−, CO32−, SO42−, NO2−, NO3−; Hcy, Cys, GSH | Cell exogenous and endogenous | EC1 cells |
| [67] | Dic7 | 678 | NR | <1 min | 0–15 | 212 | ·OH, tBuOOH, 1O2, H2O2, ClO−; Cl−, Br−, I−, S2−, NO3−, NO2−, CO32−, HSO3−, HCO3−, HSO4−; Fe2+, Cu2+, Fe3+, Na+, Ca2+, K+; Cys, GSH, | Parkinson’s disease | HeLa cells and zebrafish |
| [68] | Dic8 | 535 | NR | <5 min | 2–10 | 810 | ClO−, ·OH, 1O2, ·O2−, H2O2; Cl−, AcO−, SO42−, ClO4−, S2−, NO3−, NO2−,CO32−, HSO3−; Mg2+, Zn2+, Na+, Ca2+, K+; Hcy, Cys, GSH | Idiopathic pulmonary fibrosis | BEAS cells, mouse |
| [69] | Dic9 | 657 | NR | <100 s | 0–20 | 5300 | ClO−, ·OH, ·O2−, H2O2; SO32−, AcO−, SO42−, Cl−, NO2−; Al3+, Fe2+, Cu2+, Na+, Ca2+, K+ | Cell exogenous and endogenous | HeLa, Raw264.7 and HepG2 cells, zebrafish |
| [70] | Dic10 | 667 | 50 | <5 min | 0–270 | NR | tBuOO·, ·OH, ·O2−, 1O2, ClO−, H2O2 | Cell exogenous | HeLa cells |
| [71] | Dic11 | 660 | NR | <3 s | 0–15 | 5 | ·OH, tBuOOH, ClO−, H2O2, tBuOO·, NO, ·O2−, 1O2; NO3−, Cl−, NO2−, SO42−; Fe2+, Cu2+, Fe3+, Na+, Ca2+, K+, Mg2+, Zn2+, Cys, GSH, Hcy | Cell exogenous and endogenous | RAW264.7 cells and zebrafish |
| [72] | Dic12 | 650 | 30 | <5 s | 0–20 | 53 | tBuOOH, NO, ·OH, ·O2−, tBuOO·, 1O2, ClO−, H2O2; HSO3−, SO32−, NO2−; Cys | Inflammation | HepG2 cells and mouse |
| [73] | Dic13 | 560 | NR | <25 s | 0–10 | 130 | NO, ClO−, 1O2, ·O2−, H2O2, ·OH; Cl−, S2−, NO3−, NO2−, CO32−, AcO−, SO32−; Na+, Ca2+, K+, Mg2+, Al3+, Fe2+, Cu2+; Cys, GSH, Hcy | Acute liver injury | LX-2 cells and mouse |
| [74] | Dic14 | 670 | NR | <1 s | 0–20 | 4.59 | ·O2−, tBuOO·, ·OH, 1O2, ClO−, H2O2, NO, BrO−; HS−, SO42−, HSO3−, SO32−, S2−, CO32−, NO3−, NO2−; Zn2+, Na+, Ca2+, K+, Mg2+; Hcy, Cys, GSH | Parkinson’s disease | PC12 and SH-SY5Y cells, tissues, drosophila brains, mouse |
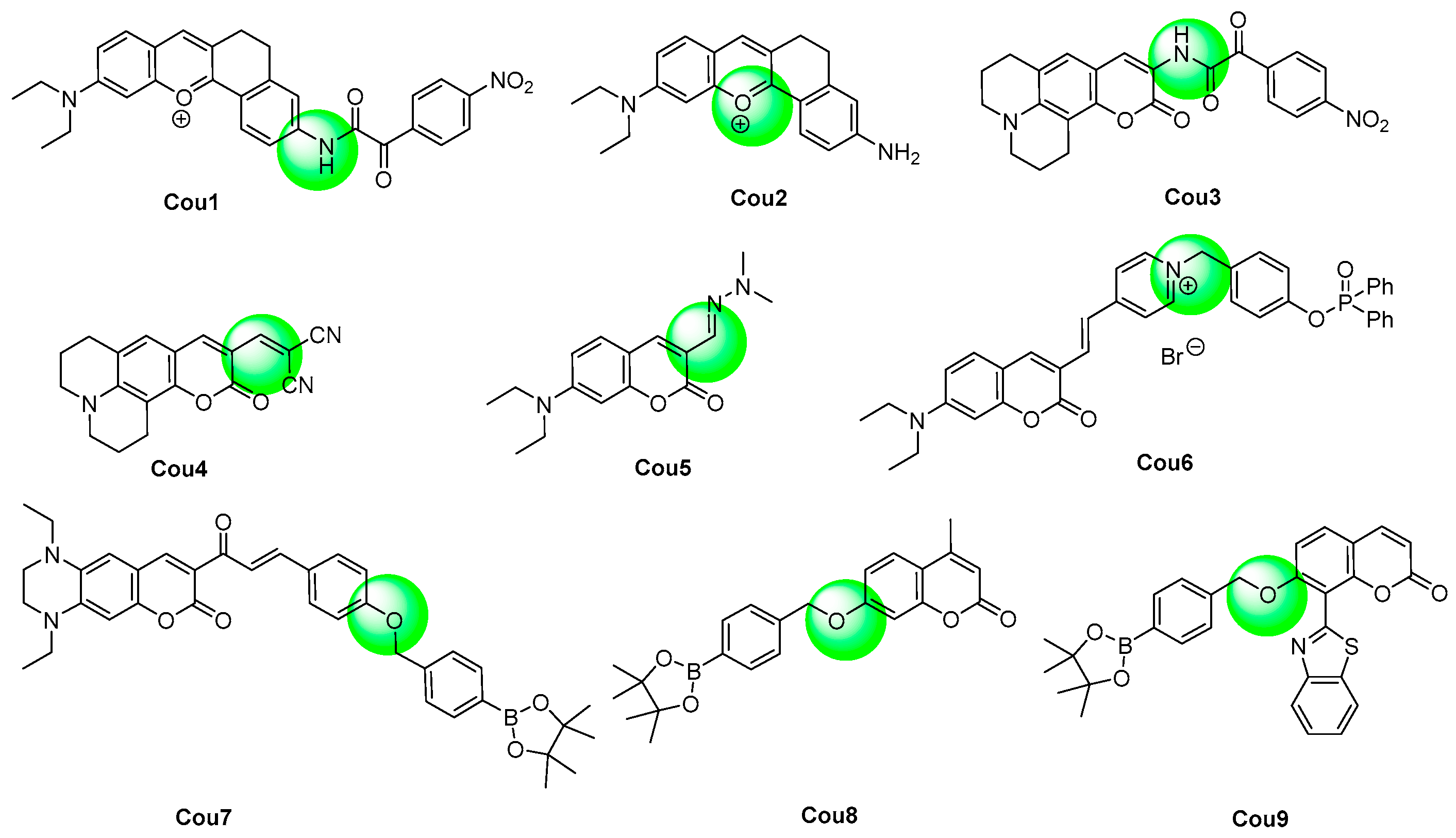
| [76] | Cou1 | 630 | 8 | <10 | 0–50 | 34 | tBuOOH, ·O2−, ·OH, 1O2, ClO−, H2O2, NO; NO2−, NO3−, CO32−, SO42−, SO32−, PO43−; Fe3+, Zn2+, Fe2+, Cu2+, Na+, Ca2+, K+; H2S, Hcy, Cys, GSH, vitamin C | Anthracycline-induced cardiotoxicity | H9c2 cardiomyocytes and mouse |
| [77] | Cou2 | 510 | 25 | <60 | 0–40 | 21.4 | ClO−, ·NO, ·O2−, 1O2, H2O2; F−, ClO4−, Cr2O72−, S2O32−, I−, S2−, CO32−, NO3−; Ca2+, K+, Cd2+, Mn2+, Cu2+, Ni2+, Ba2+, Al3+, Mg2+, Hg2+, Cr3+, Zn2+, Ag+ | γ-carrageenan-induced inflammation | RAW264.7 cells and mouse |
| [78] | Cou3 | 628 | 8 | <5 s | 0.064–0.64 | 3.7 | ClO−, H2O2, NO, ·OH, ·O2−, HNO; NO2−, SCN−, HSO3−, HS−; K+, Mg2+; Cys, GSH, FA | Cell exogenous and endogenous | SMMC-7721 and RAW264.7 cells |
| [79] | Cou4 | 520 | 111 | <5 min | 7–16 | 210 | HClO, tBuOO·, tBuOOH, ·OH, 1O2, NO, H2O2; NO2−, AcO−, SO42−, CO32−, S2O32−, S2−, SCN−; Zn2+, Ca2+, Mg2+, Fe2+, Cu2+, Na+, K+; Hydrazine hydrate, Cys, Hcy, GSH | Cell exogenous and endogenous | MCF cells and HepG2 cells |
| [80] | Cou5 | 480 | 76 | <1 min | 0–10 | 35 | ·O2−, tBuOO·, ·OH, 1O2, NO, HClO, tBuOOH, H2O2; NO2−, NO3−; Hcy, Cys, GSH | Cell exogenous and endogenous | RAW264.7 and H1299 cells |
| [81] | Cou6 | 538 | 153 | <3 min | 0–18 | 16 | ClO−, ·OH, 1O2, tBuOOH, ·O2−, H2O2, HNO; NO3−, NO2−; Ca2+, Mg2+, Fe2+, Cu2+; H2S2 | Cell endogenous | HepG2 cells |
| [82] | Cou7 | 650 | NR | <5 s | 0–15 | 53.8 | 1O2, ·O2−, HNO, NO, ClO−, H2O2, ·OH; SO32−, N3−, HSO4−, NO2−, Br−, CN−, F−, Cl−; Cys, Hcy, GSH | Cell exogenous and endogenous | Hela cells |
| [83] | Cou8 | 450 | NR | <4 min | 0–10 | 29.8 | ClO−, ·OH, ·O2−, NO, H2O2, tBuOOH, tBuOO·; NO3−, NO2−; IAA, Trp, Glu, BSA, HSA | High-fat diet-induced obese | RAW264.7 and EAhy926 cells, zebrafish and in live tissues |
| [84] | Cou9 | 500 | 1200 | <2 s | 0–2 | 70.8 | NO, ClO−, ·OH, tBuOOH; Fe3+, Fe2+, Ca2+, Cu2+, Al3+, Hg2+, Pb2+, Mg2+, Zn2+; HNO3, GSH, Cys, Hcy | Cell exogenous and endogenous | HepG2 and HL772 cells |
| [85] | NCou1 | 540 | NR | <30 min | 3–10 | 2500 | ·O2−, ·NO, H2O2, tBuOO·, ClO−, ·OH, tBuOOH | Cell exogenous and endogenous | J774A.1cells |
| [86] | NCou2 | 530 | NR | <20 min | 0–10 | 15 | ·O2−, H2O2, tBuOO·,·OH, tBuOOH, ClO−; Fe3+, Ca2+, Cu2+, Zn2+; Cys, Glu | Drug-damaged liver | HepG2 cells and mouse |
| [87] | NCou3 | 525 | 24 | <50 min | 10–35 | 30 | 1O2, HNO, ·OH, ·O2−, tBuOOH, ClO−, H2O2; Zn2+, S2−, NO2−, NO3−; Ca2+, Mg2+, Na+, K+, Fe3+; GSH, Cys, Hcy | Cell exogenous and endogenous | HeLa cells and mouse |
| [88] | NCou4 | 522 | 155 | 50 s | 0–5 | 0.83 | ·OH, NO, ·O2−, H2O2, ClO−, 1O2; NO2−, SO42−, H2PO4−, I−, HCO3−, Br−, F−; Fe2+, Cu2+, Zn2+, Ca2+, Mg2+, Na+, K+ | Cell exogenous and endogenous | RAW264.7 cells |
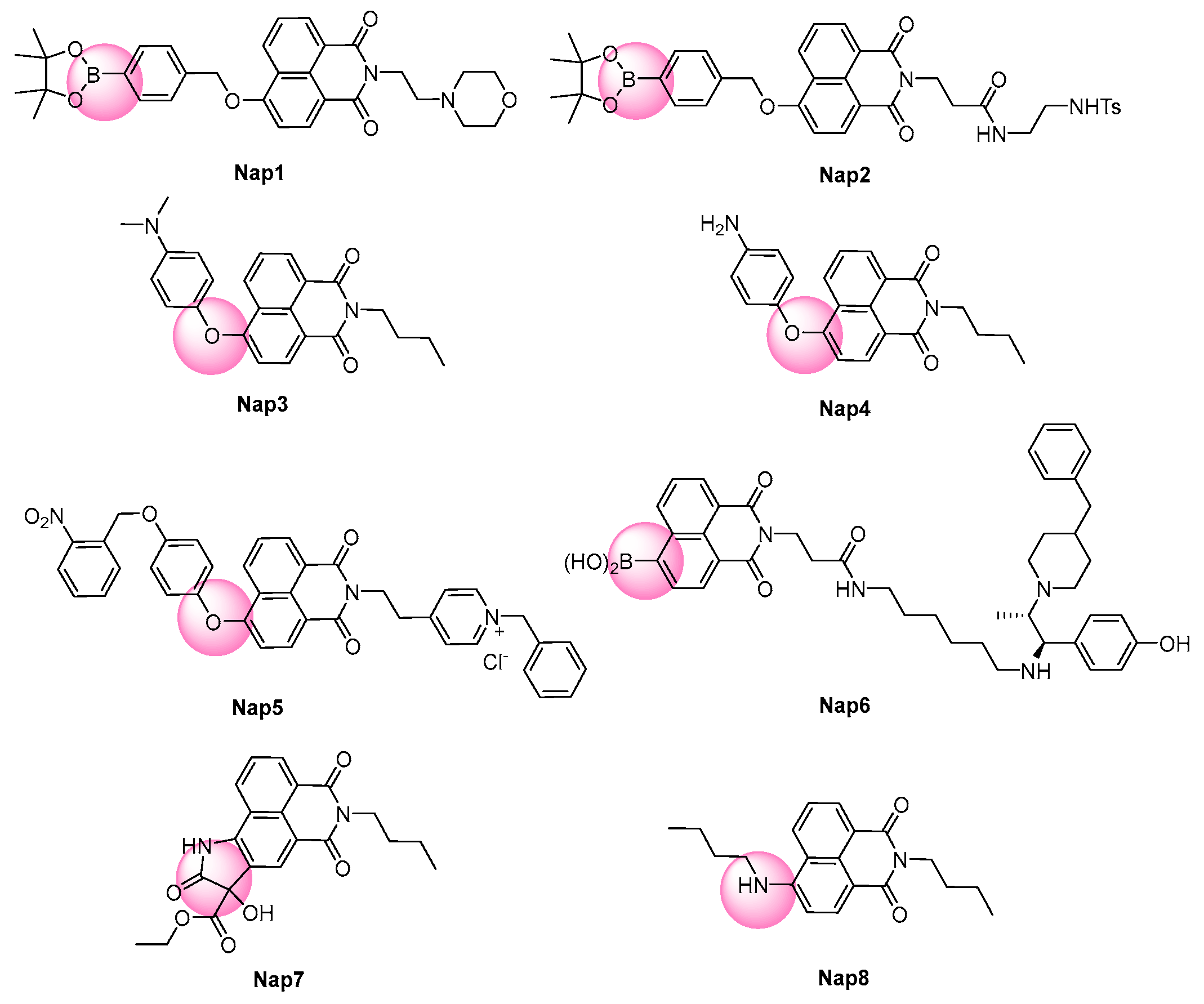
| [90] | Nap1 | 550 | NR | <70 s | 0–1000 | 130 | ClO−; SCN−, F−, Cl−, NO3−, I−, HPO42−, CO32−, HSO4−, SO42−; K+, Li+, Ba2+, Al3+, Fe2+, Pb2+, Cu2+, Ca2+, Mg2+; Asn, Arg, Leu, Trp | Acute liver injury | LX-2 cells, mouse |
| [91] | Nap2 | 558 | NR | <6 s | 2–15 | 69 | HNO, ·OH, NO, ·O2−, H2O2, ClO−; S2−, SO32−, I−; Zn2+, Ca2+, Fe2+; CO, vitamin C, Cys, Hcy, GSH | Cell exogenous and endogenous | Hela and HepG2 cells, mouse |
| [92] | Nap3 | 550 | NR | <100 s | 0–20 | 69 | HClO, tBuOO·,1O2, ·OH, ·O2−, tBuOOH, NO, H2O2; Cl−, SO42−, NO3−, NO2−, S2−; Fe3+, Cu2+, Fe2+, Zn2+, Mg2+, Na+, K+, Ca2+; Cys, Hcy, GSH | Cell exogenous and endogenous | RAW264.7 cells and zebrafish |
| [93] | Nap4 | 548 | NR | <12 min | 10–80 | 49.7 | 1O2, ClO−, ·OH, ·O2−, tBuOOH, NO, H2O2; Cl−, HSO3−, SO42−, S2O32−, NO2−, NO3−; Fe3+, Cu2+, Fe2+, Mg2+, Na+, K+, Ca2+; Cys, Hcy, GSH | Cell exogenous and endogenous | HepG2 cells and C. elegans |
| [94] | Nap5 | 553 | NR | <200 s | 0–44 | 48 | ClO−, ·OH, ·O2−, NO, H2O2; SO32−; Zn2+, Mg2+, Fe3+, Cu2+, Fe2+, Na+, K+; H2S, H2Sn, Cys, Hcy, GSH, BSA, DNA, erastin | Ferroptosis | HepG2 cells and zebrafish |
| [95] | Nap6 | 550 | 4 | <1 min | 0–10 | 184 | ClO−, ·OH, NO·, H2O2, tBuOO·, tBuOOH | Cell exogenous and endogenous | SH-SY5Y cells and mouse |
| [96] | Nap7 | 565 | NR | <120 s | 0–18 | NR | 1O2, ·OH, ·O2−, ClO−, tBuOOH, H2O2, NO; Cys, Hcy, GSH, H2S, Aβ42 peptide, BSA, DNA | Alzheimer’s disease | PC12 cells and mouse |
| [97] | Nap8 | 545 | 15 | <5 | 0–20 | 320 | ClO−, 1O2, ·OH, ·O2−, tBuOOH, tBuOO·, H2O2; HS−, ClO3−, HCO3−, SO42−, ClO−, SO32−, CO32−, NO3−, NO2−, Br−, H2PO4−, I−, F−, Cl−; Cys, Hcy, GSH | Cell exogenous and endogenous | RAW264.7 cells and zebrafish |
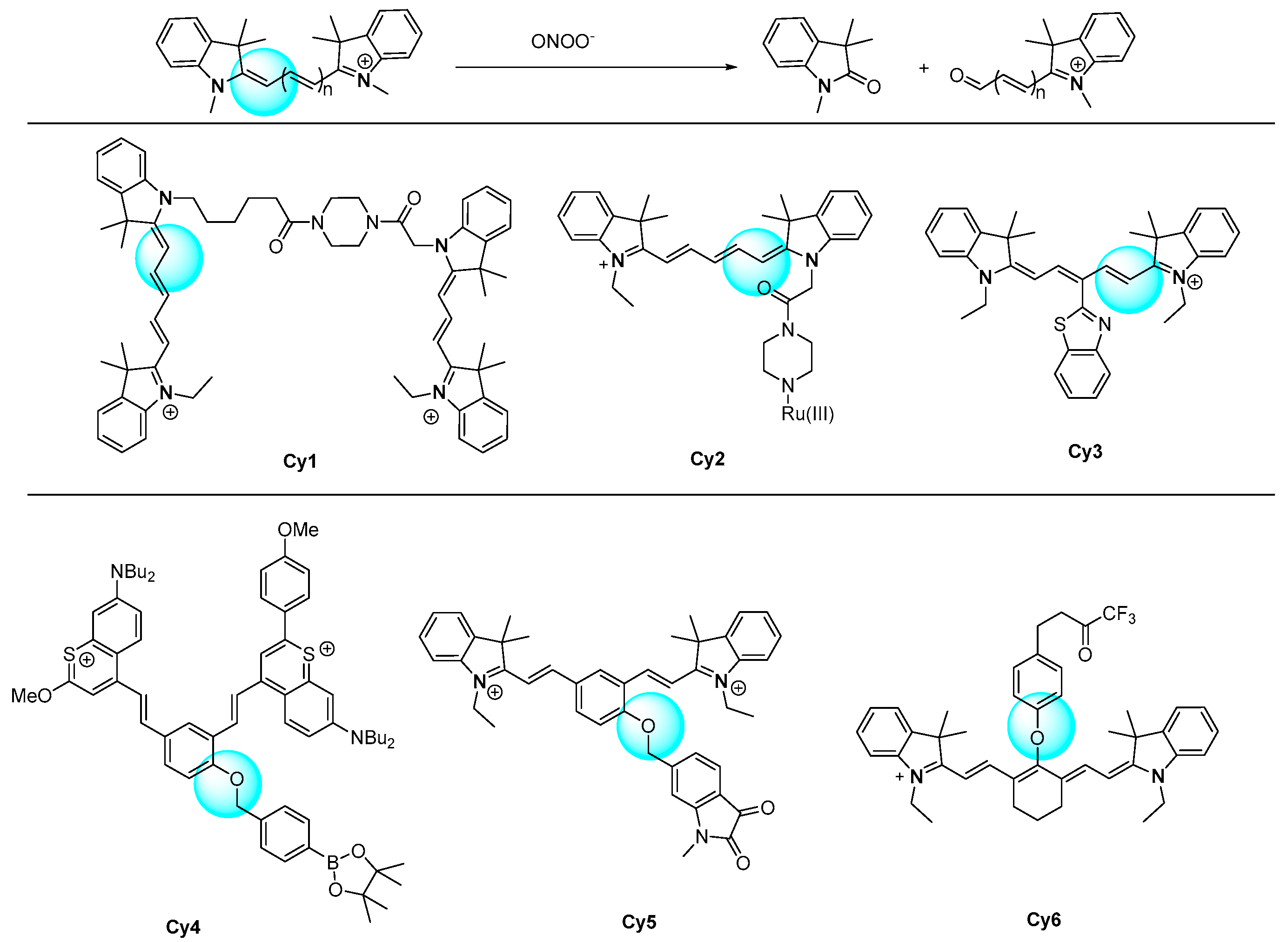
| [103] | Cy1 | 560 | 324 | <30 s | 0–0.7 | 0.65 | ClO−, 1O2, ·OH, ·O2−, tBuOOH, H2O2; HSO4−, SO32−; Cys, Hcy, GSH | Cell exogenous and endogenous | RAW264.7 cells, |
| [104] | Cy2 | 610 | 46 | <20 min | 0–30 | 280 | HClO, 1O2, ·OH, ·O2−, NO, H2O2; NO2−, HS−; Fe3+, Fe2+, Mg2+, Na+, K+, Ca2+, Zn2+; Cys, Hcy, GSH | Cell endogenous | HeLa cells |
| [105] | Cy3 | NR | NR | NR | 0–3.3 | 26 | ClO−, ·OH, ·O2−, H2O2; NO3−, NO2− | Cell exogenous and endogenous | RAW264.7 cells |
| [106] | Cy4 | 950 | NR | <3 min | 0–11 | 55.9 | 1O2, NO, ClO−, ·OH, ·O2−, H2O2; HS−, NO2−; Na+; Cys | APAP-induced hepatotoxicity | Mouse |
| [107] | Cy5 | 719 | 41 | <5 min | 0–35 | 25.4 | NO, ClO−, H2O2; NO3−, NO2−; Fe3+, Fe2+, Mg2+, Ca2+, Zn2+ Cu2+, Cd2+, Ag+; Cys, Hcy, GSH | Stroke-induced oxidative stress | PC12 cells and BV-2 cells, and mouse |
| [108] | Cy6 | 630 | NR | <15 min | 1–100 | 9.2 | 1O2, ·O2−, NO, ·OH, ClO−, H2O2; HS−, NO2−; Na+; S-nitrosoglutathione, methyl linoleate hydroperoxide | Hypoxic stress | LO2 cells, zebrafish, mice |
| [110] | Cy7 | 460 | 1728 | <60 s | 0.1–15 | 33 | tBuOO·, ·OH, 1O2, ClO−, H2O2, NO; SO42−, HSO3−, NO3−, NO2−; H2S, Hcy, Cys, GSH | Cell exogenous and endogenous | RAW264.7 cells |
| [111] | Cy8 | 484 | 448 | <10 min | 0.5–15 | 77 | tBuOOH, HClO, ·O2−, H2O2; N3−, NO3−, NO2−, HSO3−, SO32−; H2S, Hcy, Cys, GSH | Cell exogenous and endogenous | HepG2 cells |
| [112] | Cy9 | 456 | NR | <3 min | 0–30 | 326 | ·OH, 1O2, ClO−, H2O2; F−, Cl−, Br−, I−, AcO−, ClO4−, HPO42−, SO42−, S2O32−, NO2−, NO3−, HCO3−, CO32−, H2PO4−; Na+, K+; Hcy, Cys, GSH | Cyclophosphamide-induced oxidative stress | HeLa cells |
| [113] | Cy10 | 560 | NR | <15 min | 0–100 | 210 | NR | Cell exogenous and endogenous | HepG2 cells |
| [114] | Cy11 | 530 | NR | <4 min | 0–12 | 84 | ·OH, ·O2−, tBuOOH, tBuOO·, H2O2, ClO−; S2−, HS−, S2O32−, HSO3−, NO3−, NO2−; Na+, K+, Zn2+, Fe3+, Fe2+, Mg2+, Ca2+; Cys, Hcy, GSH | Cell exogenous and endogenous | HeLa cells |
| [115] | Cy12 | 535 | NR | <2 min | 5–50 | 85 | NO, ·OH, 1O2, tBuOOH, HClO, ·O2−, H2O2; Cys, GSH | Idiopathic pulmonary fibrosis | A549 and RAW264.7 cells, mouse |
| [116] | Cy13 | 444 | NR | <20 s | 0–20 | 40 | NO, ·OH, ·O2−, H2O2, ClO−, 1O2; S2−, NO3−, NO2−; Cys, Hcy, GSH | Cell exogenous and endogenous | HepG2 cells |
| [117] | Cy14 | 635 | NR | <250 s | 0–18 | 78 | NO, ·OH, ·O2−, H2O2, ClO−, 1O2; S2O32−, NO2−; Na+, Zn2+, Fe3+, Ca2+; Cys, GSH, citric acid | Tunicamycin -induced endoplasmic reticulum stress | HeLa cells and zebrafish |
| [118] | Cy15 | 493 | 25 | <4 min | 0–20 | 150 | ClO−, 1O2, ·OH, ·O2−, tBuOOH, H2O2, NO; NO3−, NO2− | Cell endogenous | RAW264.7 cells |
| [119] | Cy16 | 515 | 474 | NR | 0–20 | 49.7 | NO, ·OH, ·O2−, H2O2, ClO−, tBuOOH, tBuOO·; Cys, Hcy, GSH | Cell exogenous and endogenous | WI38 VA13 and RAW264.7 cells |
| [120] | Cy17 | 505 | 22 | <2 s | 0–40 | 67 | NO, ·OH, ·O2−, H2O2, ClO−, tBuOO·, HNO; NO2−, HSO3−, SO32−, Cl−, S2O32−, HS−; Na+, Fe2+, Mg2+, Ca2+, Zn2+ Cu2+; Cys, Hcy, GSH | Nonalcoholic fatty liver | Hela and RAW264.7 cells, mouse |
| [121] | Cy18 | 500 | 11 | <3 min | 0–10 | 16 | ClO−, 1O2, ·OH, ·O2−, tBuOOH, H2O2, NO; S2−, NO3−, NO2−, AcO−, HSO4−, Cl−, SO42−, HSO3−; Na+, K+, Zn2+, Fe3+, Fe2+, Mg2+, Ca2+, Cu2+; Cys, Hcy, GSH | Hepatotoxicity induced by acetaminophen | HepG2 cells and zebrafish |
| [122] | Cy19 | 477 | 125 | <10 s | 0–2 | 13 | tBuOO·, HNO, ·OH, NO, KO2, H2O2; HSO4−, F−, Cl−, Br−, I−, AcO−, S2O32−, HCO3−, CO32−, C2O42−, HS−, HSO3−, S2O7−;Na+, K+, Ca2+; Ser, Val, Lys, Trp, Gly, Ala, GSH, Hcy, Cys | Golgi oxidative stress and drug-induced liver injury | Hela cells and mouse |
| [123] | Cy20 | 484 | 52 | <5 min | 0–3 | 41.88 | tBuOOH, HClO, H2O2, 1O2, NO−; HSO3−, HPO42−, SO42−, S2O32−, NO3−; Fe2+, Na+; Cys, Hcy, GSH | Cell endogenous | HepG2 cells |
| [124] | Cy21 | 680 | NR | <90 min | 0–40 | 56 | ClO−, 1O2, ·OH, ·O2−, tBuOOH, H2O2; NO2−, CN−, HSO3−, NO3− | Cell exogenous and endogenous | RAW264.7 cells, zebrafish, live mouse tissues |
| [125] | Cy22 | 505 | 120 | <40 min | 0–80 | 13 | ClO−, 1O2, ·OH, ·O2−, tBuOO·, H2O2 | Rheumatoid arthritis | RAW264.7 cells and mouse |
| [126] | Cy23 | 576 | 32 | <120 s | 0–16 | 60.5 | ClO−, NO·, ·O2−, ·OH, H2O2, tBuOO·, tBuOOH; K+, Na+, Ca2+, Mg2+, Pb2+, Mn2+, Zn2+, Cu2+, Fe2+, Fe3+, Mn2+, Cd2+, Li+ | Inflammatory | RAW264.7 cells and zebrafish |
| [127] | Cy24 | 605 | NR | <10 min | 8–48 | 250 | ClO−, 1O2, ·OH, H2O2; NO3−, NO2−, ClO4−, AcO−, SO32−, HCO3−, CO32−, HSO3−, S2−; Na+, K+, Zn2+, Fe3+, Mg2+, Ca2+, Cu2+; Cys, Hcy, GSH | Cell exogenous and endogenous | RAW264.7 cells and zebrafish |
| [128] | Cy25 | 557 | NR | <10 min | 0–15 | 32 | ClO−, OH, ·O2−, H2O2; Na+, K+, Al3+, Zn2+, Fe3+, Ca2+, Cu2+; SO42−, Cl−, NO2−, CO32− | Drug-induced liver injury | RAW264.7 cells and zebrafish |
| [129] | Cy26 | 569 | NR | <1 min | 0–10 | 16 | tBuOO, ·tBuOOH, ClO−, OH, 1O2, H2O2; NO3−, NO2−, HSO4−, Cl−, Br−, I−, S2−, HCO3−, CO32−, HSO3−; Na+, K+, Fe2+, Fe3+, Ca2+, Cu2+; GSH, Cys, Ascorbic acid | Cell exogenous and endogenous | Hela cells |
| [130] | Cy27 | 712 | 59 | <2 min | 0–10 | 53 | ClO−, OH, ·O2−, H2O2, 1O2 | Tumor | RAW264.7 cells and mouse |
3. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Radi, R. Peroxynitrite, a Stealthy Biological Oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef] [PubMed]
- Radi, R.; Beckman, J.S.; Bush, K.M.; Freeman, B.A. Peroxynitrite oxidation of sulfhydryls. The cytotoxic potential of superoxide and nitric oxide. J. Biol. Chem. 1991, 266, 4244–4250. [Google Scholar] [CrossRef] [PubMed]
- Ducrocq, C.; Blanchard, B.; Pignatelli, B.; Ohshima, H. Peroxynitrite: An endogenous oxidizing and nitrating agent. Cell. Mol. Life Sci. 1999, 55, 1068–1077. [Google Scholar] [CrossRef]
- Masumoto, H.; Kissner, R.; Koppenol, W.H.; Sies, H. Kinetic study of the reaction of ebselen with peroxynitrite. FEBS Lett. 1996, 398, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Butler, A.R.; Feelisch, M. Therapeutic uses of inorganic nitrite and nitrate: From the past to the future. Circulation 2008, 117, 2151–2159. [Google Scholar]
- Singh, D.K.; Winocour, P.; Farrington, K. Oxidative stress in early diabetic nerphropathy: Fueling the fire. Nat. Rev. Endocrinol. 2011, 7, 176–184. [Google Scholar] [CrossRef]
- Migita, K.; Yamasaki, S.; Ida, H.; Kita, M.; Hida, A.; Shibatomi, K.; Kawakami, A.; Aoyagi, T.; Eguchi, K. The role of peroxynitrite in cyclooxygenase-2 expression of rheumatoid synovium. Clin. Exp. Rheumatol. 2002, 20, 59–62. [Google Scholar]
- Mahdi, A.; Tengbom, J.; Alvarsson, M.; Wernly, B.; Zhou, Z.; Pernow, J. Red Blood Cell Peroxynitrite Causes Endothelial Dysfunction in Type 2 Diabetes Mellitus via Arginase. Cells 2020, 9, 1712. [Google Scholar]
- Pandey, V.K.; Amin, P.J.; Shankar, B.S. G1-4A, a polysaccharide from Tinospora cordifolia induces peroxynitrite dependent killer dendritic cell (KDC) activity against tumor cells. Int. Immunopharmacol. 2014, 23, 480–488. [Google Scholar]
- Vana, L.; Kanaan, N.M.; Hakala, K.; Weintraub, S.T.; Binder, L.I. Peroxynitrite-induced nitrative and oxidative modifications alter tau filament formation. Biochemistry 2011, 50, 1203–1212. [Google Scholar] [CrossRef]
- Tarpey, M.M.; Fridovich, I. Methods of detection of vascular reactive species: Nitric oxide, superoxide, hydrogen peroxide, and peroxynitrite. Circ. Res. 2001, 89, 224–236. [Google Scholar] [PubMed]
- Ma, Q.; Xu, S.; Zhai, Z.; Wang, K.; Liu, X.; Xiao, H.; Zhuo, S.; Liu, Y. Recent Progress of Small-Molecule Ratiometric Fluorescent Probes for Peroxynitrite in Biological Systems. Chem. Eur. J. 2022, 28, e202200828. [Google Scholar] [PubMed]
- Wang, S.; Chen, L.; Jangili, P.; Sharma, A.; Li, W.; Hou, J.T.; Qin, C.; Yong, J.; Kim, J.S. Design and applications of fluorescent detectors for peroxynitrite. Coord. Chem. Rev. 2018, 374, 36–54. [Google Scholar] [CrossRef]
- Mao, Z.; Xiong, J.; Wang, P.; An, J.; Zhang, F.; Liu, Z.; Kim, J.S. Activity-based fluorescence probes for pathophysiological peroxynitrite fluxes. Coord. Chem. Rev. 2022, 454, 214356. [Google Scholar] [CrossRef]
- Cui, W.-L.; Wang, M.-H.; Yang, Y.-H.; Wang, J.-Y.; Zhu, X.; Zhang, H.; Ji, X. Recent advances and perspectives in reaction-based fluorescent probes for imaging peroxynitrite in Biological Systems. Coord. Chem. Rev. 2023, 474, 214848. [Google Scholar] [CrossRef]
- Khan, Z.; Sekar, N. Far-red to NIR emitting xanthene-based fluorophores. Dye. Pigment. 2022, 208, 110735. [Google Scholar] [CrossRef]
- Poronik, Y.M.; Vygranenko, K.V.; Gryko, D.; Gryko, D.T. Rhodols—Synthesis, photophysical properties and applications as fluorescent probes. Chem. Soc. Rev. 2019, 48, 5242–5265. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Guo, X.Q.; Zhao, Y.B. Development of a novel rhodamine-type fluorescent probe to determine peroxynitrite. Talanta 2002, 57, 883–890. [Google Scholar] [CrossRef]
- Wu, D.; Ryu, J.C.; Chung, Y.W.; Lee, D.; Ryu, J.H.; Yoon, J.H.; Yoon, J. A Far-Red-Emitting Fluorescence Probe for Sensitive and Selective Detection of Peroxynitrite in Live Cells and Tissues. Anal. Chem. 2017, 89, 10924–10931. [Google Scholar] [CrossRef]
- Zhu, B.C.; Zhang, M.; Wu, L.; Zhao, Z.Y.; Liu, C.Y.; Wang, Z.K.; Duan, Q.X.; Wang, Y.W.; Jia, P. A highly specific far-red fluorescent probe for imaging endogenous peroxynitrite in the mitochondria of living cells. Sens. Actuators B Chem. 2018, 257, 436–441. [Google Scholar] [CrossRef]
- Liu, D.; Feng, S.; Feng, G. A rapid responsive colorimetric and near-infrared fluorescent turn-on probe for imaging exogenous and endogenous peroxynitrite in living cells. Sens. Actuators B Chem. 2018, 269, 15–21. [Google Scholar] [CrossRef]
- Feng, S.M.; Liu, D.D.; Feng, G.Q. A dual-channel probe with green and near-infrared fluorescence changes for invitro and invivo detection of peroxynitrite. Anal. Chim. Acta 2019, 1054, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Zhuang, Z.H.; Liu, C.Y.; Wang, Z.K.; Duan, Q.X.; Li, Z.L.; Zhu, H.C.; Zhang, F.F.; Sheng, W.L.; Zhu, B.C. Development of a ratiometric fluorescent probe with a large emission shift for imaging ONOO− in live cells and zebrafish. Dye. Pigment. 2020, 173, 107942. [Google Scholar] [CrossRef]
- Ding, H.Y.; Peng, L.P.; Yuan, G.Q.; Zhou, L.Y. Design, synthesis and bioimaging application of a novel two-photon xanthene fluorescence probe for ratiometric visualization of endogenous peroxynitrite in living cells and zebrafish. Dye. Pigment. 2020, 176, 108232. [Google Scholar] [CrossRef]
- Xia, Q.F.; Feng, S.M.; Hong, J.X.; Feng, G.Q. One probe for multiple targets: A NIR fluorescent rhodamine-based probe for ONOO− and lysosomal pH detection in live cells. Sens. Actuators B Chem. 2021, 337, 129732. [Google Scholar]
- Wu, Y.; Zhang, X.; Lu, X.Y.; Chen, Y.; Ju, J.D.; Wu, H.W.; Zhu, B.C.; Huang, S.Y. An SMVT-targeting and peroxynitrite-activating fluorescent probe for head and neck cancer imaging and peroxynitrite detection. Sens. Actuators B Chem. 2021, 348, 130677. [Google Scholar] [CrossRef]
- Huang, W.M.; Du, X.M.; Zhang, C.J.; Zhang, S.R.; Zhang, J.J.; Yang, X.F. Rational Design of a Dual-Channel Fluorescent Probe for the Simultaneous Imaging of Hypochlorous Acid and Peroxynitrite in Living Organisms. Anal. Chem. 2022, 94, 17485–17493. [Google Scholar]
- Ambikapathi, G.; Kempahanumakkagari, S.K.; Lamani, B.R.; Shivanna, D.K.; Maregowda, H.B.; Gupta, A.; Malingappa, P. Bioimaging of Peroxynitrite in MCF-7 Cells by a New Fluorescent Probe Rhodamine B Phenyl Hydrazide. J. Fluoresc. 2013, 23, 705–712. [Google Scholar] [CrossRef]
- Li, H.Y.; Li, X.H.; Wu, X.F.; Shi, W.; Ma, H.M. Observation of the Generation of ONOO– in Mitochondria under Various Stimuli with a Sensitive Fluorescence Probe. Anal. Chem. 2017, 89, 5519–5525. [Google Scholar] [CrossRef]
- Chen, S.Y.; Vurusaner, B.; Pena, S.; Thu, C.T.; Mahal, L.K.; Fisher, E.A.; Canary, J.W. Two-Photon, Ratiometric, Quantitative Fluorescent Probe Reveals Fluctuation of Peroxynitrite Regulated by Arginase 1. Anal. Chem. 2021, 93, 10090–10098. [Google Scholar] [CrossRef]
- Liu, C.Y.; Zhang, X.; Li, Z.L.; Chen, Y.N.; Zhuang, Z.H.; Jia, P.; Zhu, H.C.; Yu, Y.M.; Zhu, B.C.; Sheng, W.L. Novel Dimethylhydrazine-Derived Spirolactam Fluorescent Chemodosimeter for Tracing Basal Peroxynitrite in Live Cells and Zebrafish. J. Agric. Food Chem. 2019, 67, 6407–6413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.N.; Liu, C.Y.; Zhuang, Z.H.; Li, Z.; Jia, P.; Zhu, H.C.; Yu, Y.M.; Zhu, B.C.; Sheng, W. A novel hexahydropyridazin-modified rhodamine fluorescent probe for tracing endogenous/exogenous peroxynitrite in live cells and zebrafish. Dye. Pigment. 2019, 170, 107573. [Google Scholar] [CrossRef]
- Yang, D.; Wang, H.L.; Sun, Z.N.; Chung, N.W.; Shen, J.G. A Highly Selective Fluorescent Probe for the Detection and Imaging of Peroxynitrite in Living Cells. J. Am. Chem. Soc. 2006, 128, 6004–6005. [Google Scholar] [CrossRef] [PubMed]
- Dębowska, K.; Dębski, D.; Michałowski, B.; Dybala-Defratyka, A.; Wójcik, T.; Michalski, R.; Jakubowska, M.; Selmi, A.; Smulik, R.; Piotrowski, Ł.; et al. Characterization of Fluorescein-Based Monoboronate Probe and Its Application to the Detection of Peroxynitrite in Endothelial Cells Treated with Doxorubicin. Chem. Res. Toxicol. 2016, 29, 735–746. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Ma, X.Y.; Yang, J.; Jiang, M.M.; Peng, C.; Ma, Z.Y.; Yu, H.; Li, Y.H. A New Ratiometric Design Strategy Based on Modulation of π-Conjugation Unit for Developing Fluorescent Probe and Imaging of Cellular Peroxynitrite. Anal. Chem. 2022, 94, 4763–4769. [Google Scholar] [CrossRef]
- Peng, C.; Yang, J.F.; Li, W.; Lin, D.; Fei, Y.X.; Chen, X.L.; Yuan, L.; Li, Y.H. Development of Probes with High Signal-to-Noise Ratios Based on the Facile Modification of Xanthene Dyes for Imaging Peroxynitrite during the Liver Ischemia/Reperfusion Process. Anal. Chem. 2022, 94, 10773–10780. [Google Scholar] [CrossRef]
- Wang, W.W.; Xiong, J.H.; Song, X.J.; Wang, Z.; Zhang, F.; Mao, Z.Q. Activatable Two-Photon Near-Infrared Fluorescent Probe Tailored toward Peroxynitrite In Vivo Imaging in Tumors. Anal. Chem. 2020, 92, 13305–13312. [Google Scholar] [CrossRef]
- Xiong, J.H.; Wang, W.W.; Wang, C.X.; Zhong, C.; Ruan, R.Q.; Mao, Z.Q.; Liu, Z.H. Visualizing Peroxynitrite in Microvessels of the Brain with Stroke Using an Engineered Highly Specific Fluorescent Probe. ACS Sens. 2020, 5, 3237–3245. [Google Scholar] [CrossRef]
- Wang, P.Z.; Yu, L.; Gong, J.K.; Xiong, J.H.; Zi, S.Y.; Xie, H.; Zhang, F.; Mao, Z.Q.; Liu, Z.H.; Kim, J.S. An Activity-Based Fluorescent Probe for Imaging Fluctuations of Peroxynitrite (ONOO−) in the Alzheimer’s Disease Brain. Angew. Chem. 2022, 61, e202206894. [Google Scholar]
- Shi, A.; Zeng, Y.L.; Xin, D.X.; Zhou, Y.Y.; Zhao, L.Z.; Peng, J.J. Real-Time Visualization of the Antioxidative Potency of Drugs for the Prevention of Myocardium Ischemia-Reperfusion Injury by a NIR Fluorescent Nanoprobe. ACS Sens. 2022, 7, 3867–3875. [Google Scholar]
- Peng, T.; Yang, D. HKGreen-3: A Rhodol-Based Fluorescent Probe for Peroxynitrite. Org. Lett. 2010, 12, 4932–4935. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Wong, N.K.; Chen, X.; Chan, Y.K.; Ho, D.H.H.; Sun, Z.N.; Hu, J.J.; Shen, J.Q.; El-Nezami, H.; Yang, D. Molecular Imaging of Peroxynitrite with HKGreen-4 in Live Cells and Tissues. J. Am. Chem. Soc. 2014, 136, 11728–11734. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Chen, X.M.; Gao, L.; Zhang, T.; Wang, W.; Shen, J.G.; Yang, D. A rationally designed rhodamine-based fluorescent probe for molecular imaging of peroxynitrite in live cells and tissues. Chem. Sci. 2016, 7, 5407–5413. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Xu, W.; Yuan, L.; Zhang, X.B. Investigation of Drug-Induced Hepatotoxicity and Its Remediation Pathway with Reaction-Based Fluorescent Probes. Anal. Chem. 2017, 89, 7693–7700. [Google Scholar] [CrossRef] [PubMed]
- Knewtson, K.E.; Rane, D.; Peterson, B.R. Targeting Fluorescent Sensors to Endoplasmic Reticulum Membranes Enables Detection of Peroxynitrite During Cellular Phagocytosis. ACS Chem. Biol. 2018, 13, 2595–2602. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Pan, Y.; Wang, L.; Zeng, Z.B.; Yuan, L.; Zhang, X.B.; Chang, Y.T. Selective Visualization of the Endogenous Peroxynitrite in an Inflamed Mouse Model by a Mitochondria-Targetable Two-Photon Ratiometric Fluorescent Probe. J. Am. Chem. Soc. 2017, 139, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.Y.; Cheng, D.; Li, W.; Shen, Y.; Peng, R.; Shi, L.W.; He, L.; Yuan, L. A highly selective ratiometric molecular probe for imaging peroxynitrite during drug-induced acute liver injury. J. Mater. Chem. B 2021, 9, 8246–8252. [Google Scholar] [CrossRef]
- Li, M.L.; Huang, Y.; Song, S.M.; Shuang, S.M.; Dong, C. Piperazine-Based Mitochondria-Immobilized pH Fluorescent Probe for Imaging Endogenous ONOO– and Real-Time Tracking of Mitophagy. ACS Appl. Bio Mater. 2022, 5, 2777–2785. [Google Scholar] [CrossRef]
- Cheng, D.; Gong, X.Y.; Wu, Q.; Yuan, J.; Lv, Y.; Yuan, L.; Zhang, X.B. High-Selectivity Fluorescent Reporter toward Peroxynitrite in a Coexisting Nonalcoholic Fatty Liver and Drug-Induced Liver Diseases Model. Anal. Chem. 2020, 92, 11396–11404. [Google Scholar] [CrossRef]
- Xu, W.Z.; Yang, Q.M.; Zeng, J.Q.; Tan, L.B.; Zhou, L.Y.; Peng, L.P.; Zhou, Y.Z.; Xie, C.; Luo, K.; Zhang, Z. A biomarker (ONOO−)-activated multicolor fluorescent probe for early detection and assessment of arthritis. Sens. Actuators B Chem. 2022, 359, 131565. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Z.W.; Xiao, Y.S.; Wang, X.; Jiao, X.Y.; Xie, X.L.; Zhang, J.; Tang, B. Reactivity Modulation of Benzopyran-Coumarin Platform by Introducing Electron-Withdrawing Groups: Specific Detection of Biothiols and Peroxynitrite. Anal. Chem. 2019, 91, 6097–6102. [Google Scholar]
- Li, Z.H.; Liu, R.; Tan, Z.L.; He, L.; Lu, Z.L.; Gong, B. Aromatization of 9,10-Dihydroacridine Derivatives: Discovering a Highly Selective and Rapid-Responding Fluorescent Probe for Peroxynitrite. ACS Sens. 2017, 28, 501–505. [Google Scholar]
- Ren, M.H.; Wang, L.F.; Lv, X.; Liu, J.; Chen, H.; Wang, J.J.; Guo, W. Development of a benzothiazole-functionalized red-emission pyronin dye and its dihydro derivative for imaging lysosomal viscosity and tracking endogenous peroxynitrite. J. Mater. Chem. B 2019, 7, 6181–6186. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.F.; Liu, J.; Zhao, S.W.; Zhang, H.X.; Sun, Y.Q.; Wei, A.; Guo, W. Fluorescence imaging of hypochlorous acid and peroxynitrite in vitro and in vivo with emission wavelength beyond 750 nm. Chem. Commun. 2020, 56, 7718–7721. [Google Scholar] [CrossRef]
- Lin, X.F.; Fan, M.T.; Li, N.; Yang, J.J.; Zhu, H.D.; Chen, B.; Zhu, J.R.; Zhang, D.Z.; Wang, T.; Cui, X.Y. Phosphorus-substituted rhodamines for bioimaging of the lysosomal peroxynitrite in vivo. Dye. Pigment. 2022, 201, 110201. [Google Scholar] [CrossRef]
- Wu, J.C.; Lin, Y.F.; Yu, Y.T.; Li, Y.Q.; Ye, T.Q.; Zhou, H.W.; Li, L.; Wang, J.B. A highly selective and sensitive fluorescence probe based on Rhodol for imaging of endogenous peroxynitrite in living cells. Dye. Pigment. 2022, 206, 110597. [Google Scholar] [CrossRef]
- Miao, J.F.; Huo, Y.Y.; Shi, H.; Fang, J.R.; Wang, J.J.; Guo, W. A Si-rhodamine-based near-infrared fluorescent probe for visualizing endogenous peroxynitrite in living cells, tissues, and animals. J. Mater. Chem. B 2018, 6, 4466–4473. [Google Scholar] [CrossRef]
- Li, Z.; Lu, J.; Pang, Q.; You, J. Construction of a Near-Infrared Fluorescent Probe for Ratiometric Imaging of Peroxynitrite during Tumor Progression. Analyst 2021, 146, 5204–5211. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Li, H.; Shi, W.; Li, X.; Ma, H. New Rhodamines with Changeable π-Conjugation for Lengthening Fluorescence Wavelengths and Imaging Peroxynitrite. Chem 2022, 8, 287–295. [Google Scholar] [CrossRef]
- Zhang, W.; Huo, F.; Yin, C. Recent advances of dicyano-based materials in biology and medicine. J. Mater. Chem. B 2018, 6, 6919–6929. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.V.; Kim, Y.; Lee, K.J.; Yudhistira, T.; Park, H.S.; Churchill, D.G. A fluorogenic and red-shifted diphenyl phosphinate-based probe for selective peroxynitrite detection as demonstrated in fixed cells. New J. Chem. 2017, 41, 11934–11940. [Google Scholar] [CrossRef]
- Gu, B.; Liu, C.F.; Wu, Y.; Zhang, C.X.; Shen, Y.M.; Liu, M.Q. Application of a Colorimetric and Near-Infrared Fluorescent Probe in Peroxynitrite Detection and Imaging in Living Cells. ACS Omega 2020, 5, 27530–27535. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.B.; Ma, D.G. Selective detection of peroxynitrite in living cells by a near-infrared diphenyl phosphinate-based dicyanoisophorone probe. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 244, 118890. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.Z.; Cheng, Z.Y.; Wang, R.; Yu, F.B. Indication of Dynamic Peroxynitrite Fluctuations in the Rat Epilepsy Model with a Near-Infrared Two-Photon Fluorescent Probe. Anal. Chem. 2021, 93, 2490–2499. [Google Scholar] [CrossRef]
- Yin, X.Y.; Feng, W.Y.; Gong, S.Y.; Feng, G.Q. Near-infrared fluorescent probe with rapid response and large Stokes shift for imaging peroxynitrite in living cells, zebrafish and mice. Dye. Pigment. 2020, 172, 107820. [Google Scholar] [CrossRef]
- Han, X.J.; Yang, X.P.; Zhang, Y.R.; Li, Z.P.; Cao, W.B.; Zhang, D.; Ye, Y. A novel activatable AIEgen fluorescent probe for peroxynitrite detection and its application in EC1 cells. Sens. Actuators B Chem. 2020, 321, 128510. [Google Scholar] [CrossRef]
- Kang, H.; Shu, W.; Yu, J.; Gao, M.X.; Han, R.B.; Jing, J.; Zhang, R.B.; Zhang, X.L. A near-infrared fluorescent probe for ratiometric imaging peroxynitrite in Parkinson’s disease model. Sens. Actuators B Chem. 2022, 359, 131393. [Google Scholar] [CrossRef]
- Chen, C.Y.; Yang, Y.S.; Chen, H.; Fan, X.J.; Zhu, H.L.; Li, Z. Imaging pulmonary fibrosis with a practical probe for the detection of peroxynitrite in living cells and mice. Dye. Pigment. 2022, 204, 110443. [Google Scholar] [CrossRef]
- Xu, J.Q.; Gao, M.J.; Guo, J.S.; Wang, Y.H.; Wei, R.; Meng, Y.L.; Kang, Y.F. A highly selective probe for ratiometric imaging peroxynitrite in living cells and in vivo. Bioorg. Chem. 2022, 128, 106055. [Google Scholar] [CrossRef]
- Wu, L.L.; Tian, X.; Han, H.H.; Wang, J.; Groleau, R.R.; Tosuwan, P.; Wannalerse, B.; Sedgwick, A.C.; Bull, S.D.; He, X.P.; et al. A Simple Near-Infrared Fluorescent Probe for the Detection of Peroxynitrite. ChemistryOpen 2019, 8, 1407–1409. [Google Scholar] [CrossRef]
- Jia, P.; Liu, D.M.; Zhuang, Z.H.; Liu, C.Y.; Li, Z.L.; Yu, C.; Chen, Y.N.; Zhu, H.C.; Zhang, X.; Yu, Y.M.; et al. Dicyanoisophorone-Derived Near-Infrared Fluorescent Probe for Ultrasensitive Detection of Peroxynitrite in Living Cells and Zebrafish. Ind. Eng. Chem. Res. 2019, 58, 19778–19784. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Zhou, H.; Ji, D.D.; Li, G.; Wang, F.; Song, D.Y.; Deng, B.; Li, C.; Qiao, R.Z. A near-infrared fluorescence probe for ultrafast and selective detection of peroxynitrite with large Stokes shift in inflamed mouse models. Dye. Pigment. 2019, 168, 77–83. [Google Scholar] [CrossRef]
- Jin, C.; Wu, P.F.; Yang, Y.S.; He, Z.X.; Zhu, H.L.; Li, Z. A novel fluorescent probe for the detection of peroxynitrite and its application in acute liver injury model. Redox Biol. 2021, 46, 102068. [Google Scholar] [CrossRef]
- Sun, Q.; Xu, J.J.; Ji, C.L.; Shaibani, M.S.S.; Li, Z.; Lim, K.; Zhang, C.W.; Li, L.; Liu, Z.P. Ultrafast Detection of Peroxynitrite in Parkinson’s Disease Models Using a Near-Infrared Fluorescent Probe. Anal. Chem. 2020, 92, 4038–4045. [Google Scholar] [CrossRef]
- Cao, D.X.; Liu, Z.Q.; Verwilst, P.; Koo, S.; Jangjili, P.; Kim, J.S.; Lin, W.Y. Coumarin-Based Small-Molecule Fluorescent Chemosensors. Chem. Rev. 2019, 119, 10403–10519. [Google Scholar] [CrossRef]
- Xie, X.L.; Tang, F.Y.; Liu, G.Z.; Li, Y.; Su, X.X.; Jiao, X.Y.; Wang, X.; Tang, B. Mitochondrial Peroxynitrite Mediation of Anthracycline-Induced Cardiotoxicity as Visualized by a Two-Photon Near-Infrared Fluorescent Probe. Anal. Chem. 2018, 90, 11629–11635. [Google Scholar] [CrossRef]
- Li, M.L.; Huang, Y.; Song, S.M.; Shuang, S.M.; Wang, R.B.; Dong, C. Sensitive monitoring mitochondrial peroxynitrite based on a new reaction site and cell imaging by anthracycline-based red emitting fluorescence probe. Dye. Pigment. 2021, 195, 109727. [Google Scholar] [CrossRef]
- Wei, W.P.; Li, R.; Zhu, M.; Zhao, L.L.; Ran, H.Y.; Pang, M.L.; Zhu, G.H. Coumarin-based fluorescence turn-on probes for high selectivity peroxynitrite detection and imaging in living cells and γ-carrageenan-induced inflammatory tissue and mice. Microchem. J. 2022, 183, 108003. [Google Scholar]
- Fang, Y.; Chen, R.X.; Qin, H.F.; Wang, J.J.; Zhang, Q.; Chen, S.J.; Wen, Y.H.; Wang, K.P.; Hu, Z.Q. A chromene based fluorescence probe: Accurate detection of peroxynitrite in mitochondria, not elsewhere. Sens. Actuators B Chem. 2021, 334, 129603. [Google Scholar]
- Kim, S.; Ko, C.W.; Lim, T.; Yoo, S.; Ham, H.J.; Kang, S.Y.; Kang, S.; Cho, S.K.; Han, M.S. A hydrazone-based turn-on fluorescent probe for peroxynitrite detection and live-cell imaging. Dye. Pigment. 2019, 171, 107762. [Google Scholar] [CrossRef]
- Shen, Y.M.; Dai, L.C.; Zhang, Y.Y.; Li, H.T.; Chen, Y.D.; Zhang, C.X. A novel pyridinium-based fluorescent probe for ratiometric detection of peroxynitrite in mitochondria. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 228, 117762. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, C.; Manivannan, R.; Son, Y.A. A novel near-infrared fluorescent probe for rapid detection of peroxynitrite with large stokes shift and imaging in living cells. J. Photochem. Photobiol. A 2022, 423, 113579. [Google Scholar] [CrossRef]
- Palanisamy, S.; Wu, P.Y.; Wu, S.C.; Chen, Y.J.; Tzou, S.C.; Wang, C.H.; Chen, C.Y.; Wang, Y.M. In vitro and in vivo imaging of peroxynitrite by a ratiometric boronate-based fluorescent probe. Biosens. Bioelectron. 2017, 91, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Wang, C.; Song, W.W.; Zhong, W.T.; Sun, T.M.; Zhu, J.L.; Wang, J. A novel borate fluorescent probe for rapid selective intracellular peroxynitrite imaging. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 251, 119398. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Lee, H.; Choi, Y.; Kim, Y. A boronate-based fluorescent probe for the selective detection of cellular peroxynitrite. Chem. Commun. 2014, 50, 9353–9356. [Google Scholar] [CrossRef]
- Xia, L.L.; Tong, Y.; Li, L.S.; Cui, M.Y.; Gu, Y.Q.; Wang, P. A selective fluorescent turn-on probe for imaging peroxynitrite in living cells and drug-damaged liver tissues. Talanta 2019, 204, 431–437. [Google Scholar] [CrossRef]
- Jiang, G.Y.; Li, C.B.; Lai, Q.F.; Liu, X.; Chen, Q.Q.; Zhang, P.F.; Wang, J.G.; Tang, B.Z. An easily available ratiometric AIE probe for peroxynitrite in vitro and in vivo imaging. Sens. Actuators B Chem. 2021, 329, 129223. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Y.P.; Zhao, J.J.; Guo, W. An arylboronate-based fluorescent probe for selective and sensitive detection of peroxynitrite and its applications for fluorescence imaging in living cells. Sens. Actuators B Chem. 2016, 237, 67–74. [Google Scholar] [CrossRef]
- Jain, N.; Kaur, N. A comprehensive compendium of literature of 1,8-Naphthalimide based chemosensors from 2017 to 2021. Coord. Chem. Rev. 2022, 459, 214454. [Google Scholar] [CrossRef]
- Wang, K.; Guo, R.; Chen, X.Y.; Yang, Y.S.; Qiao, L.Q.; Wang, M.L. Multifunctional lysosome-targetable fluorescent probe for imaging peroxynitrite in acute liver injury model. Chem. Eng. J. 2023, 455, 140491. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, R.F.; Wang, J.X.; Wei, H.; Chen, Q.X.; Wang, Y.; Dong, B. Construction of a ratiometric two-photon ER-targeting fluorescent probe for the imaging of peroxynitrite in living systems. Sens. Actuators B Chem. 2022, 370, 132439. [Google Scholar] [CrossRef]
- Sheng, W.L.; Wang, K.; Gao, N.; Wang, L.Z.; Wang, R.C.; Zhang, X.M.; Chen, X.Q.; Zhang, Y.; Zhu, B.C.; Liu, K.C. A novel p-dimethylaminophenylether-based fluorescent probe for the detection of native ONOO− in cells and zebrafish. Analyst 2021, 146, 5264–5270. [Google Scholar] [CrossRef]
- Liu, X.L.; Gu, F.Y.; Zhou, X.Y.; Zhou, W.; Zhang, S.P.; Cui, L.; Guo, T. A naphthalimide-based turn-on fluorescence probe for peroxynitrite detection and imaging in living cells. RSC Adv. 2020, 10, 38281–38286. [Google Scholar] [CrossRef]
- Xie, X.L.; Liu, Y.W.; Liu, G.Z.; Zhao, Y.Y.; Bian, J.; Li, Y.; Zhang, J.; Wang, X.; Tang, B. Photocontrollable Fluorescence Imaging of Mitochondrial Peroxynitrite during Ferroptosis with High Fidelity. Anal. Chem. 2022, 94, 10213–10220. [Google Scholar] [CrossRef]
- Lee, D.; Lim, C.S.; Ko, G.; Kim, D.; Cho, M.K.; Nam, S.J.; Kim, H.M.; Yoon, J. A Two-Photon Fluorescent Probe for Imaging Endogenous ONOO– near NMDA Receptors in Neuronal Cells and Hippocampal Tissues. Anal. Chem. 2018, 90, 9347–9352. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.L.; Liu, Y.W.; Liu, G.Z.; Zhao, Y.Y.; Liu, J.Y.; Li, Y.; Zhang, J.; Jiao, X.Y.; Wang, X.; Tang, B. Two-photon fluorescence imaging of the cerebral peroxynitrite stress in Alzheimer’s disease. Chem. Commun. 2022, 58, 6300–6303. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.D.; Chen, X.; Chen, J.; Ma, M.S.; Jin, H.; Yu, S.H.; Liu, Z.G. A simple highly selective ratiometric fluorescent probe for detection of peroxynitrite and its bioimaging applications. Dye. Pigment. 2023, 210, 110993. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, Y.; Yue, X.; Dai, Z. Cyanine Conjugate-Based Biomedical Imaging Probes. Adv. Healthc. Mater. 2020, 9, 2001327. [Google Scholar] [CrossRef]
- Mo, S.; Zhang, X.; Hameed, S.; Zhou, Y.; Dai, Z. Glutathione-responsive disassembly of disulfide dicyanine for tumor imaging with reduction in background signal intensity. Theranostics 2020, 10, 2130–2140. [Google Scholar] [CrossRef]
- Hao, Z.M.; Hu, L.M.; Wang, X.N.; Liu, Y.J.; Mo, S.Y. Synthesis of Heptamethine Cyanines from Furfural Derivatives. Org. Lett. 2023, 25, 1078–1082. [Google Scholar] [CrossRef]
- Gorka, A.P.; Nani, R.R.; Schnermann, M.J. Harnessing Cyanine Reactivity for Optical Imaging and Drug Delivery. Acc. Chem. Res. 2018, 51, 3226–3235. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, H.; Tano, H.; Toyota, K.; Tajima, R.; Miyake, H.; Takahashi, I.; Hirota, S.; Itoh, S. Reduction of Bis(dithiolene)oxo(disulfido)tungsten(VI) Complex with Dihydrogen Related to the Chemical Function of the Fourth Tungsten-Containing Enzyme (WOR4) from Pyrococcus furiosus. J. Am. Chem. Soc. 2010, 132, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.T.; Chen, Q.Q.; Yang, Y.F.; Tang, Y.; Wang, R.; Xu, Y.F.; Zhu, W.P.; Qian, X.H. FRET-Based Mito-Specific Fluorescent Probe for Ratiometric Detection and Imaging of Endogenous Peroxynitrite: Dyad of Cy3 and Cy5. J. Am. Chem. Soc. 2016, 138, 10778–10781. [Google Scholar]
- Zhang, W.Z.; Liu, Y.; Gao, Q.K.; Liu, C.L.; Song, B.; Zhang, R.; Yuan, J.L. A ruthenium(II) complex-cyanine energy transfer scaffold based luminescence probe for ratiometric detection and imaging of mitochondrial peroxynitrite. Chem. Commun. 2018, 54, 13698–13701. [Google Scholar] [CrossRef]
- Hou, T.X.; Zhang, K.; Kang, X.X.; Guo, X.L.; Du, L.B.; Chen, X.F.; Yu, L.; Yue, J.; Ge, H.W.; Liu, Y.; et al. Sensitive detection and imaging of endogenous peroxynitrite using a benzo[d]thiazole derived cyanine probe. Talanta 2019, 196, 345–351. [Google Scholar] [CrossRef]
- Li, D.D.; Wang, S.F.; Lei, Z.H.; Sun, C.X.; Ahmed, M.E.; Mansour, S.A.; Fan, Y.; Zhang, F. Peroxynitrite Activatable NIR-II Fluorescent Molecular Probe for Drug-Induced Hepatotoxicity Monitoring. Anal. Chem. 2019, 91, 4771–4779. [Google Scholar] [CrossRef]
- An, Q.; Su, S.Z.; Chai, L.; Wang, Y.Y.; Wang, X.M.; Li, X.C.; Liang, T.; Hu, W.; Song, X.J.; Li, C.Y. Imaging of peroxynitrite in mitochondria by a near-infrared fluorescent probe with a large Stokes shift. Talanta 2023, 253, 124073. [Google Scholar] [CrossRef]
- Huang, Y.; Yu, L.; Fu, L.L.; Hou, J.J.; Wang, L.X.; Sun, M.Z.; Wang, X.Y.; Chen, L.X. Molecular fluorescent probes for imaging and evaluation of peroxynitrite fluctuations in living cells and in vivo under hypoxic stress. Sens. Actuators B Chem. 2022, 370, 132410. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zhao, S.; Gao, W.; Chen, B.; He, L.; Zhu, S. A Unique Approach to Development of Near-Infrared Fluorescent Sensors for in Vivo Imaging. J. Am. Chem. Soc. 2012, 134, 13510–13523. [Google Scholar] [CrossRef]
- Zhou, D.Y.; Ou-Yang, J.; Li, Y.; Jiang, W.L.; Tian, Y.; Yi, Z.M.; Li, C.Y. A ratiometric fluorescent probe for the detection of peroxynitrite with simple synthesis and large emission shift and its application in cells image. Dye. Pigment. 2019, 161, 288–295. [Google Scholar] [CrossRef]
- Gu, J.; Liu, Y.N.; Shen, J.W.; Cao, Y.Y.; Zhang, L.; Lu, Y.D.; Wang, B.Z.; Zhu, H.L. A three-channel fluorescent probe for selective detection of ONOO− and its application to cell imaging. Talanta 2022, 244, 123401. [Google Scholar] [CrossRef]
- Li, J.S.; Peng, S.X.; Li, Z.P.; Zhao, F.F.; Han, X.J.; Liu, J.F.; Cao, W.B.; Ye, Y. Visualization of peroxynitrite in cyclophosphamide-induced oxidative stress by an activatable probe. Talanta 2022, 238, 123007. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Q.; Dong, C.; Zhang, J.; Zhao, B.; Zhou, Y.Q.; Fan, C.H.; Lu, Z.L. A mitochondria-targeted ratiometric NIR fluorescent probe for simultaneously monitoring viscosity and ONOO− based on two different channels in living HepG2 cells. Dye. Pigment. 2023, 210, 111045. [Google Scholar] [CrossRef]
- Han, R.B.; Shu, W.; Kang, H.; Duan, Q.X.; Zhang, X.L.; Liang, C.L.; Gao, M.X.; Xu, L.R.; Jing, J.; Zhang, X.L. A deep red ratiometric fluorescent probe for accurate detection of peroxynitrite in mitochondria. Anal. Chim. Acta 2022, 1203, 339652. [Google Scholar] [CrossRef]
- Zhan, Z.X.; Liu, R.; Chai, L.; Dai, Y.C.; Lv, Y. Visualization of Lung Inflammation to Pulmonary Fibrosis via Peroxynitrite Fluctuation. Anal. Chem. 2019, 91, 11461–11466. [Google Scholar] [CrossRef]
- Du, Y.T.; Wang, H.L.; Zhang, T.; Wei, W.; Guo, M.M. ICT-based fluorescent ratiometric probe for monitoring mitochondrial peroxynitrite in living cells. New J. Chem. 2021, 45, 12915–12921. [Google Scholar] [CrossRef]
- He, L.C.; Liu, H.; Wu, J.S.; Cheng, Z.Y.; Yu, F.B. Construction of a Mitochondria-Endoplasmic Reticulum Dual-Targeted Red-Emitting Fluorescent Probe for Imaging Peroxynitrite in Living Cells and Zebrafish. Chem. Asian J. 2022, 17, e202200388. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.T.; Yang, J.; Li, K.; Liao, Y.X.; Yu, K.K.; Xie, Y.M.; Yu, X.Q. A highly selective water-soluble optical probe for endogenous peroxynitrite. Chem. Commun. 2014, 50, 9947–9950. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Kwon, Y.; Kim, G.; Ryu, J.H.; Yoon, J. A ratiometric fluorescent probe based on a coumarin–hemicyanine scaffold for sensitive and selective detection of endogenous peroxynitrite. Biosens. Bioelectron. 2015, 64, 285–291. [Google Scholar] [CrossRef]
- Liu, Y.J.; Feng, S.M.; Gong, S.Y.; Feng, G.Q. Dual-Channel Fluorescent Probe for Detecting Viscosity and ONOO– without Signal Crosstalk in Nonalcoholic Fatty Liver. Anal. Chem. 2022, 94, 17439–17447. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Li, X.C.; Tang, W.; Xie, L.; Dai, F.; Zhou, B. A coumarin-based fluorescent probe: Small but multi-signal. Sens. Actuators B Chem. 2022, 368, 132169. [Google Scholar]
- Feng, S.M.; Zheng, Z.P.; Gong, S.Y.; Feng, G.Q. A unique probe enables labeling cell membrane and Golgi apparatus and tracking peroxynitrite in Golgi oxidative stress and drug-induced liver injury. Sens. Actuators B Chem. 2022, 361, 131751. [Google Scholar] [CrossRef]
- Xue, X.L.; Zhang, H.; Chen, G.H.; Yu, G.H.; Hu, H.R.; Niu, S.Y.; Wang, K.P.; Hu, Z.Q. Coumarin-cyanine hybrid: A ratiometric fluorescent probe for accurate detection of peroxynitrite in mitochondria. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 292, 122443. [Google Scholar] [PubMed]
- Li, K.Y.; Lu, P.H.; Wu, B.H.; Wu, S.P. A mitochondria-targeting near-infrared fluorescent probe for the in vivo detection of peroxynitrite. Dye. Pigment. 2022, 205, 110521. [Google Scholar] [CrossRef]
- Chen, W.J.; Liu, H.H.; Song, F.X.; Xin, L.T.; Zhang, Q.; Zhang, P.; Ding, C.F. pH-Switched Near-Infrared Fluorescent Strategy for Ratiometric Detection of ONOO– in Lysosomes and Precise Imaging of Oxidative Stress in Rheumatoid Arthritis. Anal. Chem. 2023, 95, 1301–1308. [Google Scholar]
- Sonawane, P.M.; Yudhistira, T.; Halle, M.B.; Roychaudhury, A.; Kim, Y.; Surwase, S.S.; Bhosale, V.K.; Kim, J.; Park, H.S.; Kim, Y.C.; et al. A water-soluble boronate masked benzoindocyanin fluorescent probe for the detection of endogenous mitochondrial peroxynitrite in live cells and zebrafish as inflammation models. Dye. Pigment. 2021, 191, 109371. [Google Scholar] [CrossRef]
- Wang, J.H.; Liu, Y.M.; Dong, C.; Wang, Y.; Shuang, S.M. Ratiometric imaging of peroxynitrite in live cells, Locusta Malpighian tubes and zebrafish by a benzothiazole-based mitochondria-targetable fluorescent probe. J. Lumin. 2023, 254, 119504. [Google Scholar] [CrossRef]
- Wang, B.D.; Wei, R.; Gao, M.J.; Wang, Y.H.; Zhang, C.F.; Guo, X.H.; Liang, Z.S.; Zhou, J.T.; Sun, J.X.; Xu, J.Q.; et al. Development of peroxynitrite-responsive fluorescence probe for recognition of drug-induced liver injury. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 283, 121755. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Wu, Y.L.; Zang, S.P.; Su, S.; Kang, H.; Jing, J.; Zhang, X.L. A mitochondria-targeting highly specific fluorescent probe for fast sensing of endogenous peroxynitrite in living cells. Sens. Actuators B Chem. 2020, 303, 127284. [Google Scholar] [CrossRef]
- Zhang, J.J.; Zhen, X.; Zeng, J.F.; Pu, K.Y. A Dual-Modal Molecular Probe for Near-Infrared Fluorescence and Photoacoustic Imaging of Peroxynitrite. Anal. Chem. 2018, 90, 9301–9307. [Google Scholar] [CrossRef]
- Xu, C.; Li, Y.; Wu, X.; Li, X.; Li, L.; Kong, F.P.; Tang, B. A dual-responsive probe for the simultaneous monitoring of viscosity and peroxynitrite with different fluorescence signals in living cells. Chem. Commun. 2022, 58, 5976–5979. [Google Scholar] [CrossRef] [PubMed]
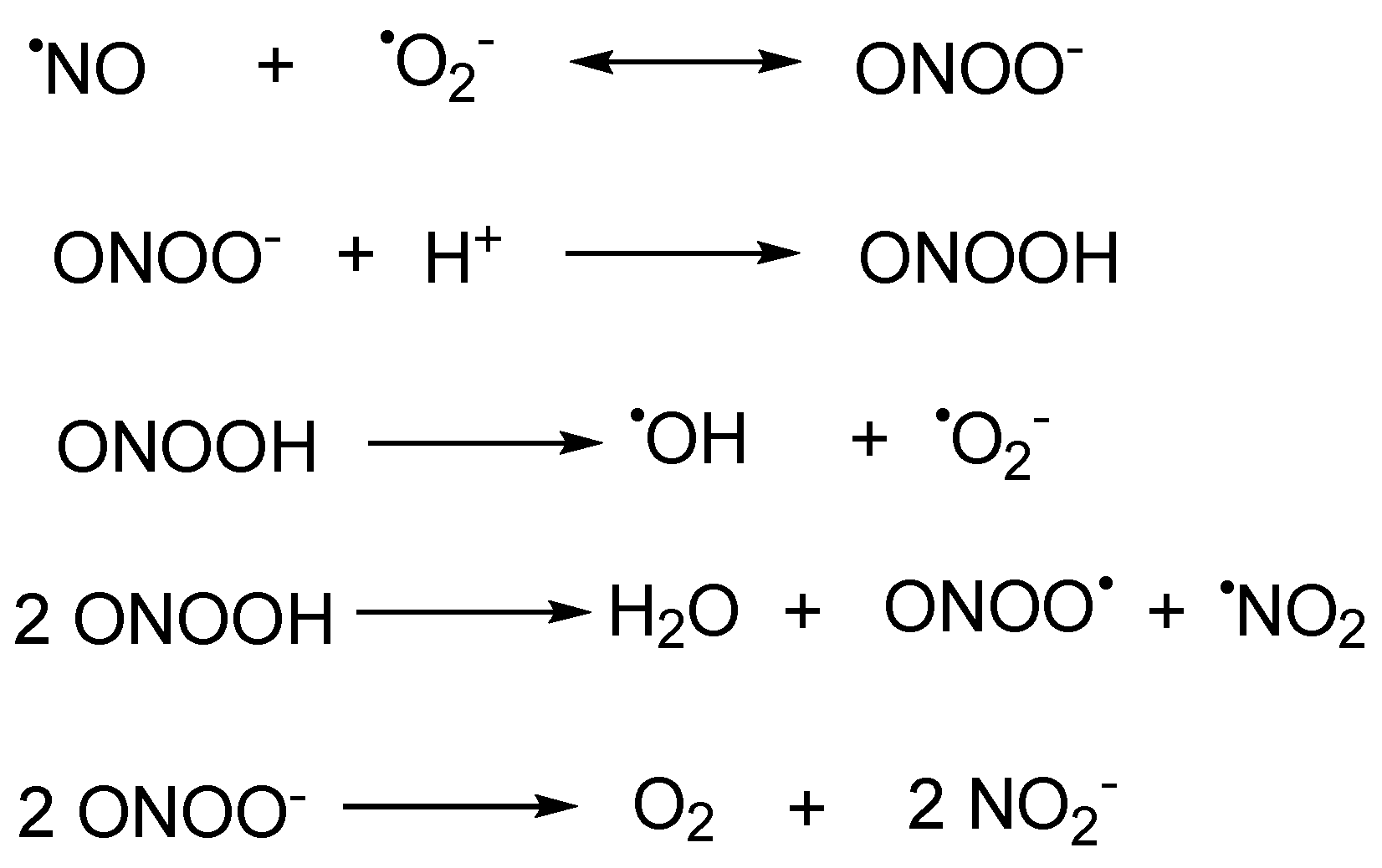




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Mo, S.; Hao, Z.; Hu, L. Recent Progress of Spectroscopic Probes for Peroxynitrite and Their Potential Medical Diagnostic Applications. Int. J. Mol. Sci. 2023, 24, 12821. https://doi.org/10.3390/ijms241612821
Liu Z, Mo S, Hao Z, Hu L. Recent Progress of Spectroscopic Probes for Peroxynitrite and Their Potential Medical Diagnostic Applications. International Journal of Molecular Sciences. 2023; 24(16):12821. https://doi.org/10.3390/ijms241612821
Chicago/Turabian StyleLiu, Zixin, Shanyan Mo, Zhenming Hao, and Liming Hu. 2023. "Recent Progress of Spectroscopic Probes for Peroxynitrite and Their Potential Medical Diagnostic Applications" International Journal of Molecular Sciences 24, no. 16: 12821. https://doi.org/10.3390/ijms241612821
APA StyleLiu, Z., Mo, S., Hao, Z., & Hu, L. (2023). Recent Progress of Spectroscopic Probes for Peroxynitrite and Their Potential Medical Diagnostic Applications. International Journal of Molecular Sciences, 24(16), 12821. https://doi.org/10.3390/ijms241612821






