Identification of Host PDZ-Based Interactions with the SARS-CoV-2 E Protein in Human Monocytes
Abstract
1. Introduction
2. Results
2.1. Construction and Expression of a Recombinant SARS-CoV2 E-Protein GFP Tagged at the Amino Terminal Region
2.2. Expression of the GFP-SARS-CoV-2 E Protein Affects the Viability of THP-1 Cells
2.3. Proteomic Analysis of the GFP-SARS-CoV-2 E Protein in THP-1 Cells
2.4. Identification of the PDZ Partners of the GFP-SARS-CoV-2 E Protein in Monocytes
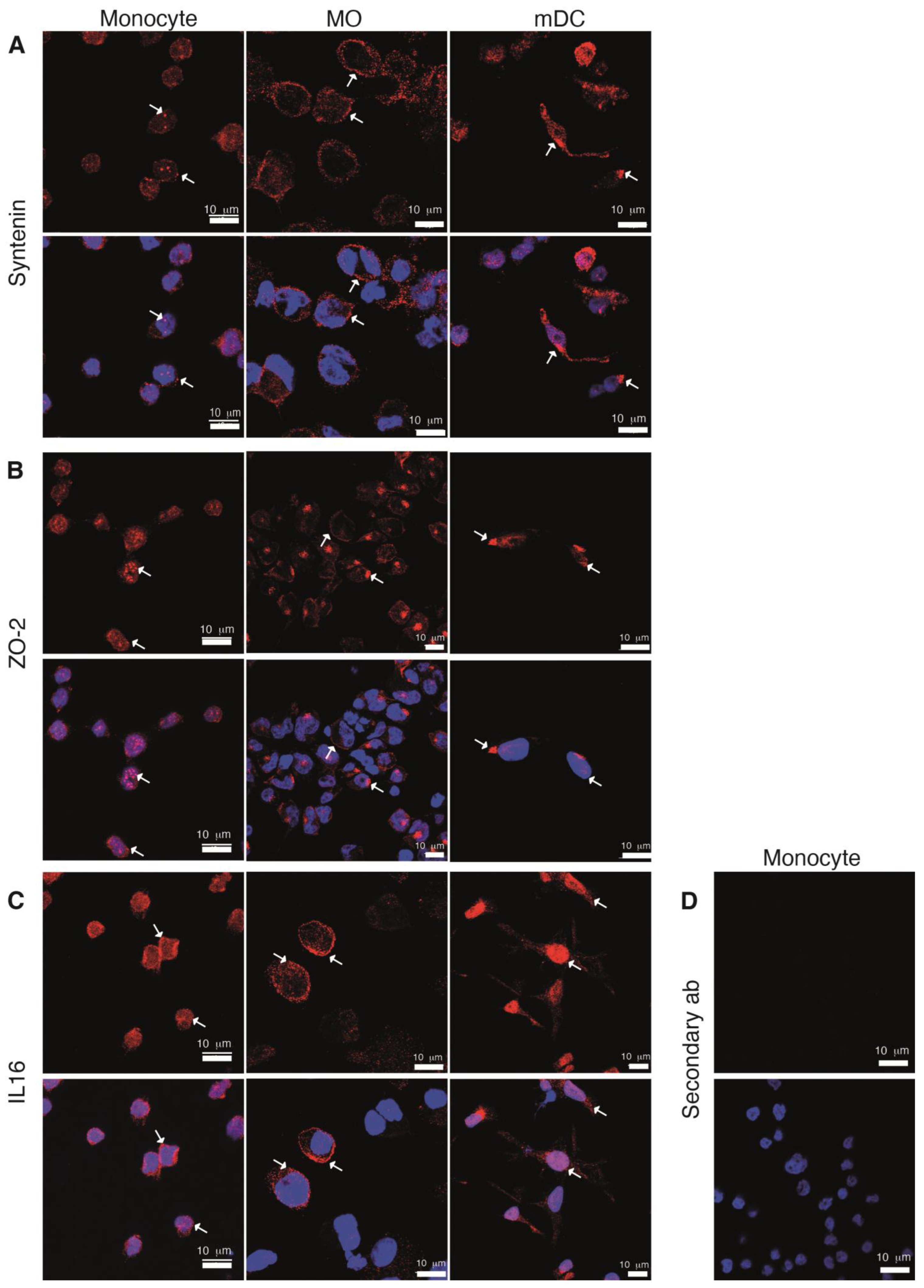
2.5. Expression along Cell Differentiation of the PDZ Proteins Partners of the SARS-CoV-2 E Protein
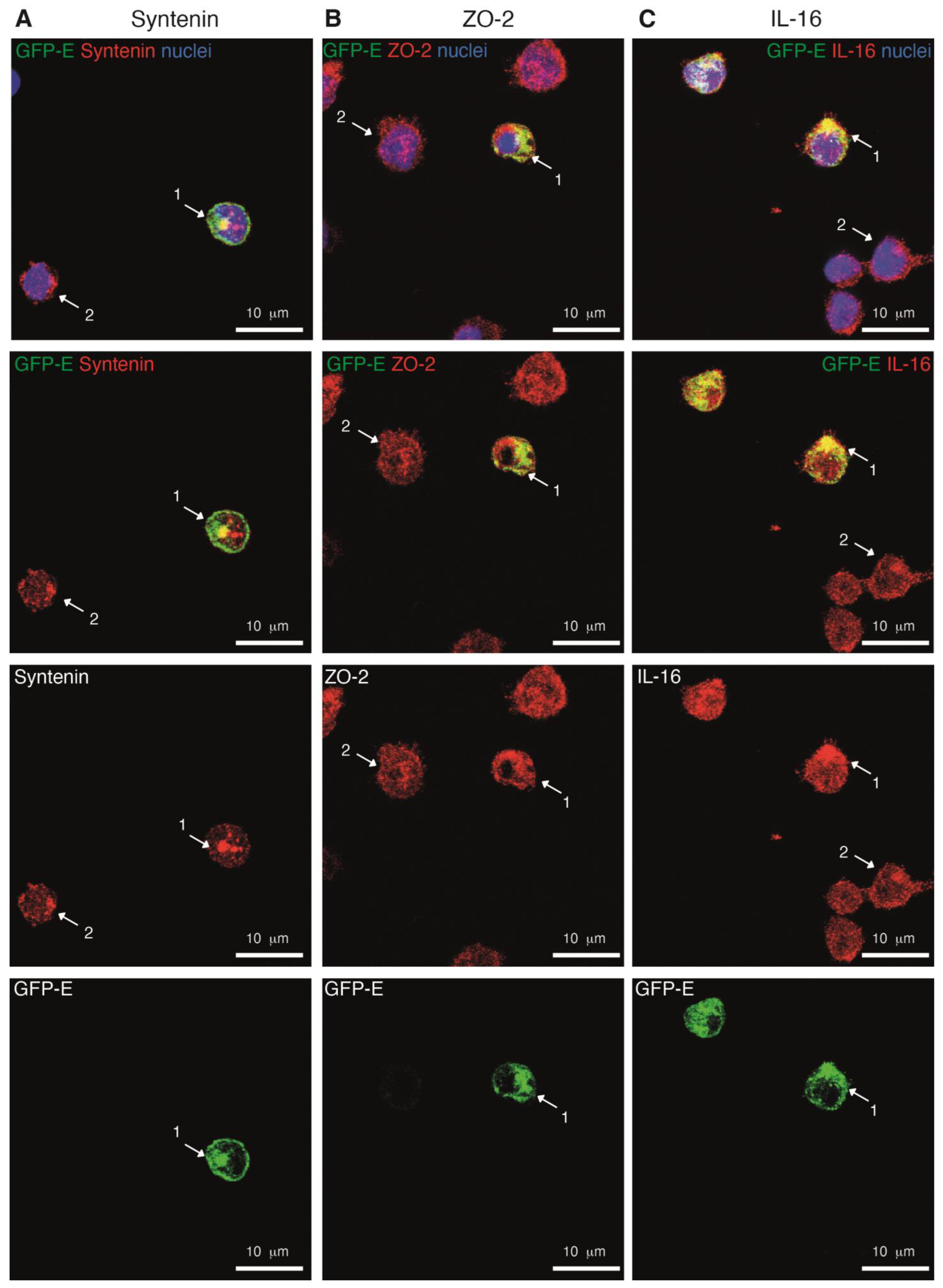
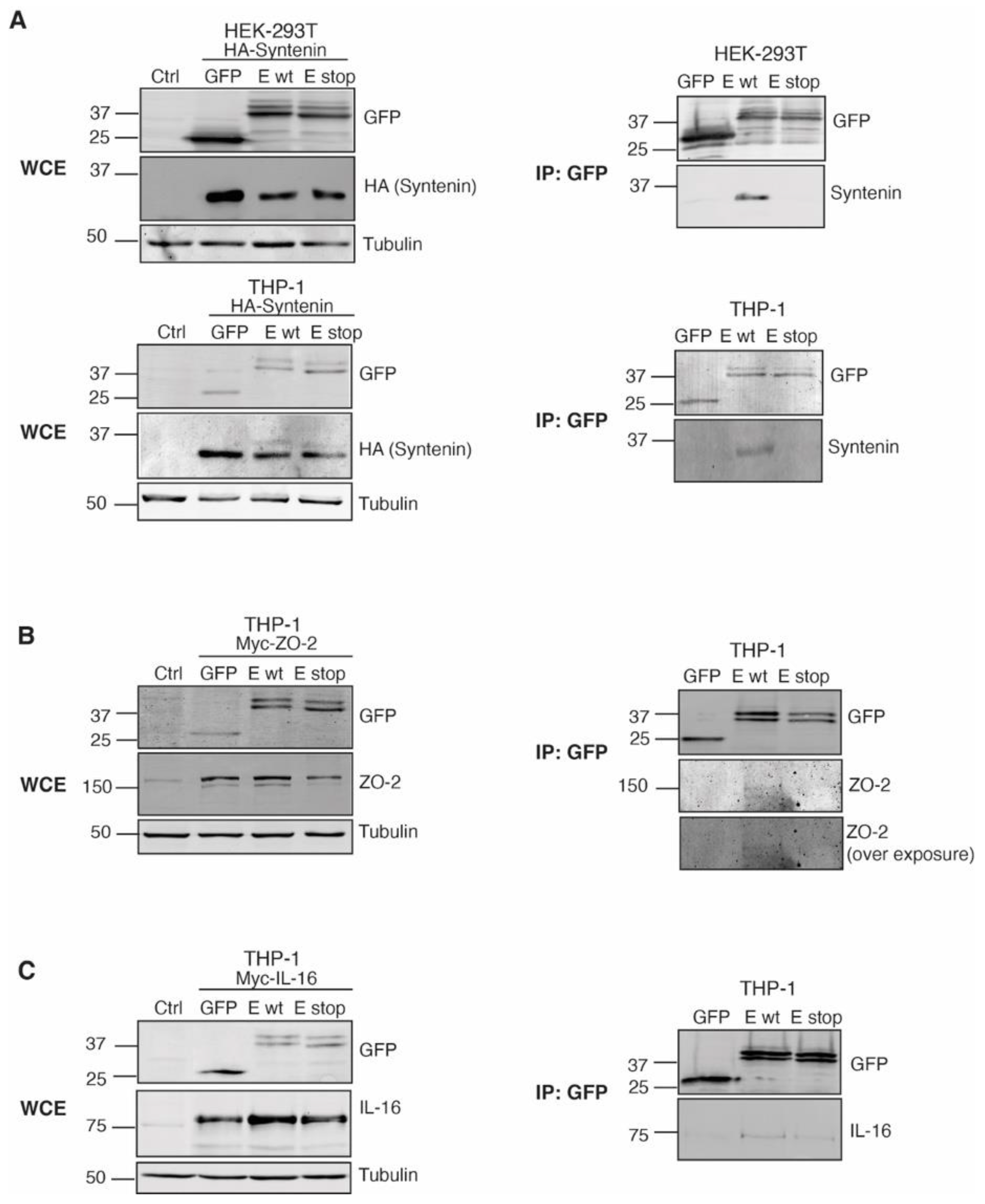
2.6. The GFP-SARS-CoV-2 E Protein Interacts with the PDZ Proteins Syntenin, ZO-2 and IL-16
3. Discussion
4. Materials and Methods
4.1. GFP-SARS-CoV-2 E Constructs
4.2. Cell Culture and Differentiation
4.3. GFP-SARS-CoV-2 E Protein Expression
4.4. Western Blot
4.5. Flow Cytometry
4.6. Immunofluorescence Analysis
4.7. Proteomic Analysis
4.8. GFP-SARS-CoV-2 E Protein-PDZ Protein Interaction Validation
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nourry, C.; Grant, S.G.; Borg, J.P. PDZ domain proteins: Plug and play! Sci. STKE 2003, 2003, RE7. [Google Scholar] [CrossRef]
- Tonikian, R.; Zhang, Y.; Sazinsky, S.L.; Currell, B.; Yeh, J.H.; Reva, B.; Held, H.A.; Appleton, B.A.; Evangelista, M.; Wu, Y.; et al. A specificity map for the PDZ domain family. PLoS Biol. 2008, 6, e239. [Google Scholar] [CrossRef]
- Christensen, N.R.; Calyseva, J.; Fernandes, E.F.A.; Luchow, S.; Clemmensen, L.S.; Haugaard-Kedstrom, L.M.; Stromgaard, K. PDZ Domains as Drug Targets. Adv. Ther. 2019, 2, 1800143. [Google Scholar] [CrossRef]
- Subbaiah, V.K.; Kranjec, C.; Thomas, M.; Banks, L. PDZ domains: The building blocks regulating tumorigenesis. Biochem. J. 2011, 439, 195–205. [Google Scholar] [CrossRef]
- Stessman, H.A.; Xiong, B.; Coe, B.P.; Wang, T.; Hoekzema, K.; Fenckova, M.; Kvarnung, M.; Gerdts, J.; Trinh, S.; Cosemans, N.; et al. Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat. Genet. 2017, 49, 515–526. [Google Scholar] [CrossRef]
- Barreda, D.; Sanchez-Galindo, M.; Lopez-Flores, J.; Nava-Castro, K.E.; Bobadilla, K.; Salgado-Aguayo, A.; Santos-Mendoza, T. PDZ proteins are expressed and regulated in antigen-presenting cells and are targets of influenza A virus. J. Leukoc. Biol. 2018, 103, 731–738. [Google Scholar] [CrossRef]
- Ludford-Menting, M.J.; Oliaro, J.; Sacirbegovic, F.; Cheah, E.T.; Pedersen, N.; Thomas, S.J.; Pasam, A.; Iazzolino, R.; Dow, L.E.; Waterhouse, N.J.; et al. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity 2005, 22, 737–748. [Google Scholar] [CrossRef]
- Gmyrek, G.B.; Graham, D.B.; Sandoval, G.J.; Blaufuss, G.S.; Akilesh, H.M.; Fujikawa, K.; Xavier, R.J.; Swat, W. Polarity gene discs large homolog 1 regulates the generation of memory T cells. Eur. J. Immunol. 2013, 43, 1185–1194. [Google Scholar] [CrossRef]
- Stephenson, L.M.; Sammut, B.; Graham, D.B.; Chan-Wang, J.; Brim, K.L.; Huett, A.S.; Miletic, A.V.; Kloeppel, T.; Landry, A.; Xavier, R.; et al. DLGH1 is a negative regulator of T-lymphocyte proliferation. Mol. Cell Biol. 2007, 27, 7574–7581. [Google Scholar] [CrossRef]
- Zheng, W.; Umitsu, M.; Jagan, I.; Tran, C.W.; Ishiyama, N.; BeGora, M.; Araki, K.; Ohashi, P.S.; Ikura, M.; Muthuswamy, S.K. An interaction between Scribble and the NADPH oxidase complex controls M1 macrophage polarization and function. Nat. Cell Biol. 2016, 18, 1244–1252. [Google Scholar] [CrossRef]
- Golebiewski, L.; Liu, H.; Javier, R.T.; Rice, A.P. The avian influenza virus NS1 ESEV PDZ binding motif associates with Dlg1 and Scribble to disrupt cellular tight junctions. J. Virol. 2011, 85, 10639–10648. [Google Scholar] [CrossRef]
- Mantovani, F.; Banks, L. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene 2001, 20, 7874–7887. [Google Scholar] [CrossRef] [PubMed]
- Zielecki, F.; Semmler, I.; Kalthoff, D.; Voss, D.; Mauel, S.; Gruber, A.D.; Beer, M.; Wolff, T. Virulence determinants of avian H5N1 influenza A virus in mammalian and avian hosts: Role of the C-terminal ESEV motif in the viral NS1 protein. J. Virol. 2010, 84, 10708–10718. [Google Scholar] [CrossRef] [PubMed]
- Castano-Rodriguez, C.; Honrubia, J.M.; Gutierrez-Alvarez, J.; Sola, I.; Enjuanes, L. Viral PDZ Binding Motifs Influence Cell Behavior Through the Interaction with Cellular Proteins Containing PDZ Domains. Methods Mol. Biol. 2021, 2256, 217–236. [Google Scholar] [CrossRef]
- Jimenez-Guardeño, J.M.; Nieto-Torres, J.L.; DeDiego, M.L.; Regla-Nava, J.A.; Fernandez-Delgado, R.; Castaño-Rodriguez, C.; Enjuanes, L. The PDZ-binding motif of severe acute respiratory syndrome coronavirus envelope protein is a determinant of viral pathogenesis. PLoS Pathog. 2014, 10, e1004320. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Boumaza, A.; Gay, L.; Mezouar, S.; Bestion, E.; Diallo, A.B.; Michel, M.; Desnues, B.; Raoult, D.; La Scola, B.; Halfon, P.; et al. Monocytes and Macrophages, Targets of Severe Acute Respiratory Syndrome Coronavirus 2: The Clue for Coronavirus Disease 2019 Immunoparalysis. J. Infect. Dis. 2021, 224, 395–406. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Y.; Li, K.; Meyerholz, D.K.; Allamargot, C.; Perlman, S. Severe Acute Respiratory Syndrome Coronavirus 2-Induced Immune Activation and Death of Monocyte-Derived Human Macrophages and Dendritic Cells. J. Infect. Dis. 2021, 223, 785–795. [Google Scholar] [CrossRef]
- Junqueira, C.; Crespo, Â.; Ranjbar, S.; de Lacerda, L.B.; Lewandrowski, M.; Ingber, J.; Parry, B.; Ravid, S.; Clark, S.; Schrimpf, M.R.; et al. FcγR-mediated SARS-CoV-2 infection of monocytes activates inflammation. Nature 2022, 606, 576–584. [Google Scholar] [CrossRef]
- Nieto-Torres, J.L.; Verdia-Baguena, C.; Jimenez-Guardeno, J.M.; Regla-Nava, J.A.; Castano-Rodriguez, C.; Fernandez-Delgado, R.; Torres, J.; Aguilella, V.M.; Enjuanes, L. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology 2015, 485, 330–339. [Google Scholar] [CrossRef]
- Zhou, S.; Lv, P.; Li, M.; Chen, Z.; Xin, H.; Reilly, S.; Zhang, X. SARS-CoV-2 E protein: Pathogenesis and potential therapeutic development. Biomed. Pharmacother. 2023, 159, 114242. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.F.; Kok, K.H.; Zhu, Z.; Chu, H.; To, K.K.; Yuan, S.; Yuen, K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Knapp, J.J.; Kuang, D.; Chawla, A.; Cassonnet, P.; Lee, H.; Sheykhkarimli, D.; Samavarchi-Tehrani, P.; Abdouni, H.; Rayhan, A.; et al. A Comprehensive, Flexible Collection of SARS-CoV-2 Coding Regions. G3 2020, 10, 3399–3402. [Google Scholar] [CrossRef]
- Guo, F.; Chiang, M.Y.; Wang, Y.; Zhang, Y.Z. An in vitro recombination method to convert restriction- and ligation-independent expression vectors. Biotechnol. J. 2008, 3, 370–377. [Google Scholar] [CrossRef]
- Liao, Y.; Yuan, Q.; Torres, J.; Tam, J.P.; Liu, D.X. Biochemical and functional characterization of the membrane association and membrane permeabilizing activity of the severe acute respiratory syndrome coronavirus envelope protein. Virology 2006, 349, 264–275. [Google Scholar] [CrossRef]
- Cohen, J.R.; Lin, L.D.; Machamer, C.E. Identification of a Golgi complex-targeting signal in the cytoplasmic tail of the severe acute respiratory syndrome coronavirus envelope protein. J. Virol. 2011, 85, 5794–5803. [Google Scholar] [CrossRef]
- Chai, J.; Cai, Y.; Pang, C.; Wang, L.; McSweeney, S.; Shanklin, J.; Liu, Q. Structural basis for SARS-CoV-2 envelope protein recognition of human cell junction protein PALS1. Nat. Commun. 2021, 12, 3433. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Amacher, J.F.; Brooks, L.; Hampton, T.H.; Madden, D.R. Specificity in PDZ-peptide interaction networks: Computational analysis and review. J. Struct. Biol. X 2020, 4, 100022. [Google Scholar] [CrossRef]
- Chytla, A.; Gajdzik-Nowak, W.; Olszewska, P.; Biernatowska, A.; Sikorski, A.F.; Czogalla, A. Not Just Another Scaffolding Protein Family: The Multifaceted MPPs. Molecules 2020, 25, 4954. [Google Scholar] [CrossRef]
- Teoh, K.T.; Siu, Y.L.; Chan, W.L.; Schluter, M.A.; Liu, C.J.; Peiris, J.S.; Bruzzone, R.; Margolis, B.; Nal, B. The SARS coronavirus E protein interacts with PALS1 and alters tight junction formation and epithelial morphogenesis. Mol. Biol. Cell 2010, 21, 3838–3852. [Google Scholar] [CrossRef]
- Kaech, S.M.; Whitfield, C.W.; Kim, S.K. The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell 1998, 94, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Javorsky, A.; Humbert, P.O.; Kvansakul, M. Viral subversion of the cell polarity regulator Scribble. Biochem. Soc. Trans. 2023, 51, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Bilder, D.; Perrimon, N. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 2000, 03, 676–680. [Google Scholar] [CrossRef] [PubMed]
- Bonello, T.T.; Peifer, M. Scribble: A master scaffold in polarity, adhesion, synaptogenesis, and proliferation. J. Cell Biol. 2019, 218, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Barreda, D.; Ramon-Luing, L.A.; Duran-Luis, O.; Bobadilla, K.; Chacon-Salinas, R.; Santos-Mendoza, T. Scrib and Dlg1 polarity proteins regulate Ag presentation in human dendritic cells. J. Leukoc. Biol. 2020, 108, 883–893. [Google Scholar] [CrossRef]
- Van Itallie, C.M.; Fanning, A.S.; Bridges, A.; Anderson, J.M. ZO-1 stabilizes the tight junction solute barrier through coupling to the perijunctional cytoskeleton. Mol. Biol. Cell 2009, 20, 3930–3940. [Google Scholar] [CrossRef]
- Caillet-Saguy, C.; Durbesson, F.; Rezelj, V.V.; Gogl, G.; Tran, Q.D.; Twizere, J.C.; Vignuzzi, M.; Vincentelli, R.; Wolff, N. Host PDZ-containing proteins targeted by SARS-CoV-2. FEBS J. 2021, 288, 5148–5162. [Google Scholar] [CrossRef]
- Shepley-McTaggart, A.; Sagum, C.A.; Oliva, I.; Rybakovsky, E.; DiGuilio, K.; Liang, J.; Bedford, M.T.; Cassel, J.; Sudol, M.; Mullin, J.M.; et al. SARS-CoV-2 Envelope (E) Protein Interacts with PDZ-Domain-2 of Host Tight Junction Protein ZO1. bioRxiv 2020, 16, e0251955. [Google Scholar] [CrossRef]
- Pak, D.T.; Yang, S.; Rudolph-Correia, S.; Kim, E.; Sheng, M. Regulation of dendritic spine morphology by SPAR, a PSD-95-associated RapGAP. Neuron 2001, 31, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Miyagi, Y.; Yamashita, T.; Fukaya, M.; Sonoda, T.; Okuno, T.; Yamada, K.; Watanabe, M.; Nagashima, Y.; Aoki, I.; Okuda, K.; et al. Delphilin: A novel PDZ and formin homology domain-containing protein that synaptically colocalizes and interacts with glutamate receptor delta 2 subunit. J. Neurosci. 2002, 22, 803–814. [Google Scholar] [CrossRef]
- Cruikshank, W.W.; Kornfeld, H.; Center, D.M. Interleukin-16. J. Leukoc. Biol. 2000, 67, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lu, Y.Q.; Wen, Y.; Yu, D.Y.; Ge, L.; Dong, W.R.; Xiang, L.X.; Shao, J.Z. IL-16 induces intestinal inflammation via PepT1 upregulation in a pufferfish model: New insights into the molecular mechanism of inflammatory bowel disease. J. Immunol. 2013, 191, 1413–1427. [Google Scholar] [CrossRef] [PubMed]
- Baier, M.; Bannert, N.; Werner, A.; Adler, H.S.; Otteken, A.; Beer, B.; Norley, S.; Kurth, R. Chemoattractant factors and the control of human immunodeficiency virus replication. Pathobiology 1998, 66, 128–130. [Google Scholar] [CrossRef] [PubMed]
- Cruikshank, W.W.; Long, A.; Tarpy, R.E.; Kornfeld, H.; Carroll, M.P.; Teran, L.; Holgate, S.T.; Center, D.M. Early identification of interleukin-16 (lymphocyte chemoattractant factor) and macrophage inflammatory protein 1 alpha (MIP1 alpha) in bronchoalveolar lavage fluid of antigen-challenged asthmatics. Am. J. Respir. Cell Mol. Biol. 1995, 13, 738–747. [Google Scholar] [CrossRef]
- Jia, R.; Jiang, C.; Li, L.; Huang, C.; Lu, L.; Xu, M.; Xu, J.; Liang, X. Interleukin 16 Enhances the Host Susceptibility to Influenza A Virus Infection. Front. Microbiol. 2021, 12, 736449. [Google Scholar] [CrossRef]
- Barreda, D.; Gutierrez-Gonzalez, L.H.; Martinez-Cordero, E.; Cabello-Gutierrez, C.; Chacon-Salinas, R.; Santos-Mendoza, T. The Scribble Complex PDZ Proteins in Immune Cell Polarities. J. Immunol. Res. 2020, 2020, 5649790. [Google Scholar] [CrossRef]
- González-Mariscal, L.; Gallego-Gutiérrez, H.; González-González, L.; Hernández-Guzmán, C. ZO-2 Is a Master Regulator of Gene Expression, Cell Proliferation, Cytoarchitecture, and Cell Size. Int. J. Mol. Sci. 2019, 20, 4128. [Google Scholar] [CrossRef]
- Huerta, M.; Muñoz, R.; Tapia, R.; Soto-Reyes, E.; Ramírez, L.; Recillas-Targa, F.; González-Mariscal, L.; López-Bayghen, E. Cyclin D1 is transcriptionally down-regulated by ZO-2 via an E box and the transcription factor c-Myc. Mol. Biol. Cell 2007, 18, 4826–4836. [Google Scholar] [CrossRef]
- Percivalle, E.; Sammartino, J.C.; Cassaniti, I.; Arbustini, E.; Urtis, M.; Smirnova, A.; Concardi, M.; Belgiovine, C.; Ferrari, A.; Lilleri, D.; et al. Macrophages and Monocytes: "Trojan Horses" in COVID-19. Viruses 2021, 13, 2178. [Google Scholar] [CrossRef]
- Schoeman, D.; Fielding, B.C. Is There a Link Between the Pathogenic Human Coronavirus Envelope Protein and Immunopathology? A Review of the Literature. Front. Microbiol. 2020, 11, 2086. [Google Scholar] [CrossRef]
- Boson, B.; Legros, V.; Zhou, B.; Siret, E.; Mathieu, C.; Cosset, F.L.; Lavillette, D.; Denolly, S. The SARS-CoV-2 envelope and membrane proteins modulate maturation and retention of the spike protein, allowing assembly of virus-like particles. J. Biol. Chem. 2021, 296, 100111. [Google Scholar] [CrossRef] [PubMed]
- Xia, B.; Shen, X.; He, Y.; Pan, X.; Liu, F.L.; Wang, Y.; Yang, F.; Fang, S.; Wu, Y.; Duan, Z.; et al. SARS-CoV-2 envelope protein causes acute respiratory distress syndrome (ARDS)-like pathological damages and constitutes an antiviral target. Cell Res. 2021, 31, 847–860. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xia, S.; Zhang, Z.; Wu, H.; Lieberman, J. Channelling inflammation: Gasdermins in physiology and disease. Nat. Rev. Drug Discov. 2021, 20, 384–405. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Gonzalez, L.H.; Santos-Mendoza, T. Viral targeting of PDZ polarity proteins in the immune system as a potential evasion mechanism. FASEB J. 2019, 33, 10607–10617. [Google Scholar] [CrossRef]
- Gräßel, L.; Fast, L.A.; Scheffer, K.D.; Boukhallouk, F.; Spoden, G.A.; Tenzer, S.; Boller, K.; Bago, R.; Rajesh, S.; Overduin, M.; et al. The CD63-Syntenin-1 Complex Controls Post-Endocytic Trafficking of Oncogenic Human Papillomaviruses. Sci. Rep. 2016, 6, 32337. [Google Scholar] [CrossRef]
- Kashyap, R.; Balzano, M.; Lechat, B.; Lambaerts, K.; Egea-Jimenez, A.L.; Lembo, F.; Fares, J.; Meeussen, S.; Kügler, S.; Roebroek, A.; et al. Syntenin-knock out reduces exosome turnover and viral transduction. Sci. Rep. 2021, 11, 4083. [Google Scholar] [CrossRef] [PubMed]
- Nkosi, D.; Sun, L.; Duke, L.C.; Patel, N.; Surapaneni, S.K.; Singh, M.; Meckes, D.G. Epstein-Barr Virus LMP1 Promotes Syntenin-1- and Hrs-Induced Extracellular Vesicle Formation for Its Own Secretion To Increase Cell Proliferation and Migration. mBio 2020, 11, e00589-20. [Google Scholar] [CrossRef]
- Ramírez-Medina, E.; Vuono, E.A.; Velazquez-Salinas, L.; Silva, E.; Rai, A.; Pruitt, S.; Berggren, K.A.; Zhu, J.; Borca, M.V.; Gladue, D.P. The MGF360-16R ORF of African Swine Fever Virus Strain Georgia Encodes for a Nonessential Gene That Interacts with Host Proteins SERTAD3 and SDCBP. Viruses 2020, 12, 60. [Google Scholar] [CrossRef]
- Sette, P.; O’Connor, S.K.; Yerramilli, V.S.; Dussupt, V.; Nagashima, K.; Chutiraka, K.; Lingappa, J.; Scarlata, S.; Bouamr, F. HIV-1 Nucleocapsid Mimics the Membrane Adaptor Syntenin PDZ to Gain Access to ESCRTs and Promote Virus Budding. Cell Host Microbe 2016, 19, 336–348. [Google Scholar] [CrossRef]
- Lindqvist, R.; Benz, C.; Sereikaite, V.; Maassen, L.; Laursen, L.; Jemth, P.; Strømgaard, K.; Ivarsson, Y.; Överby, A.K. A Syntenin Inhibitor Blocks Endosomal Entry of SARS-CoV-2 and a Panel of RNA Viruses. Viruses 2022, 14, 2202. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Yasuda, S.; Sugiura, H.; Yamagata, K. Syntenin: PDZ Protein Regulating Signaling Pathways and Cellular Functions. Int. J. Mol. Sci. 2019, 20, 4171. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, D.; Farztdinov, V.; Yan, Y.; Meisel, C.; Sadlowski, H.; Kühn, J.; Perschel, F.H.; Endres, M.; Düzel, E.; Vielhaber, S.; et al. The brain reacting to COVID-19: Analysis of the cerebrospinal fluid proteome, RNA and inflammation. J. Neuroinflamm. 2023, 20, 30. [Google Scholar] [CrossRef]
- Hwang, N.; Huh, Y.; Bu, S.; Seo, K.J.; Kwon, S.H.; Kim, J.W.; Yoon, B.K.; Ahn, H.S.; Fang, S. Single-cell sequencing of PBMC characterizes the altered transcriptomic landscape of classical monocytes in BNT162b2-induced myocarditis. Front. Immunol. 2022, 13, 979188. [Google Scholar] [CrossRef] [PubMed]
- Rosas-Garcia, J.; Ramon-Luing, L.A.; Bobadilla, K.; Meraz-Rios, M.A.; Sevilla-Reyes, E.E.; Santos-Mendoza, T. Distinct Transcriptional Profile of PDZ Genes after Activation of Human Macrophages and Dendritic Cells. Int. J. Mol. Sci. 2022, 23, 7010. [Google Scholar] [CrossRef] [PubMed]
- Berges, C.; Naujokat, C.; Tinapp, S.; Wieczorek, H.; Höh, A.; Sadeghi, M.; Opelz, G.; Daniel, V. A cell line model for the differentiation of human dendritic cells. Biochem. Biophys. Res. Commun. 2005, 333, 896–907. [Google Scholar] [CrossRef]
- Chanput, W.; Mes, J.J.; Wichers, H.J. THP-1 cell line: An in vitro cell model for immune modulation approach. Int. Immunopharmacol. 2014, 23, 37–45. [Google Scholar] [CrossRef]
- Geijsen, N.; Uings, I.J.; Pals, C.; Armstrong, J.; McKinnon, M.; Raaijmakers, J.A.; Lammers, J.W.; Koenderman, L.; Coffer, P.J. Cytokine-specific transcriptional regulation through an IL-5Ralpha interacting protein. Science 2001, 293, 1136–1138. [Google Scholar] [CrossRef]




| Protein | Function | PDZ | Additional Domains | Viral Interactors | |
|---|---|---|---|---|---|
| 1 | syntenin-1 | Adaptor protein in cell trafficking, exosome biogenesis and tumorigenesis. | 2 | No. | SARS-CoV-1; VAC FII protein. |
| 2 | scribble human homolog; Scrib | Scaffold and cell polarity protein in epithelial and neuronal morphogenesis. | 4 | 16 LRR; 3CC. | HPV E6; HTLV-1 Tax; TBEV NS5; ESEV NS1. |
| 3 | zonula occludens protein 2; ZO-2; tight junction protein 2; TJP-2 | Role in tight and adherens junctions. | 3 | 1 SH3; 1 guanylate kinase-like; scribble interacting region. | Adenovirus E4; TBEV NSE5; WNVNS5. |
| 4 | membrane palmitoylated protein 1; MPP1; 55 kDa erythrocyte membrane protein | Essential regulator of neutrophil polarity. | 1 | 1 SH3, 1 guanylate kinase-like; MPP5 interaction region. | - |
| 5 | protein lin-7 homolog A; Lin-7A | Establishment and maintenance of the asymmetric distribution of channels and receptors in polarized cells. | 1 | 1 L27; 1 kinase interacting site. | - |
| 6 | pro-interleukin 16; pro-IL-16 | Migratory response in lymphocytes, monocytes and eosinophilsPriming of CD4+ T-cells for responsiveness to cytokines. Ligand for CD4. Transcription. | 4 | Interaction regions with GRIN2A, HTLV-1 Tax; PPP1R12A; PPP1R12B; PPP1R12C. | HTLV-1 Tax; TBEV NS5; WNV NS6. |
| 7 | signal-induced proliferation-associated 1-like protein 1; SIPA1-like protein 1; SIPA1L1 | Reorganization of actin cytoskeleton. Stimulation of GTPase activity. | 1 | 1 RapGAP; 1 potential CC region. | HPV E6 |
| 8 | delphilin; glutamate receptor, ionotropic, delta 2 interacting protein (GRID2IP) | Post-synaptic scaffolding protein. Linking of GRID2 with actin cytoskeleton. | 2 | 1 FH2. | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila-Flores, A.; Sánchez-Cabezón, J.J.; Ochoa-Echeverría, A.; Checa, A.I.; Rosas-García, J.; Téllez-Araiza, M.; Casado, S.; Liébana, R.; Santos-Mendoza, T.; Mérida, I. Identification of Host PDZ-Based Interactions with the SARS-CoV-2 E Protein in Human Monocytes. Int. J. Mol. Sci. 2023, 24, 12793. https://doi.org/10.3390/ijms241612793
Ávila-Flores A, Sánchez-Cabezón JJ, Ochoa-Echeverría A, Checa AI, Rosas-García J, Téllez-Araiza M, Casado S, Liébana R, Santos-Mendoza T, Mérida I. Identification of Host PDZ-Based Interactions with the SARS-CoV-2 E Protein in Human Monocytes. International Journal of Molecular Sciences. 2023; 24(16):12793. https://doi.org/10.3390/ijms241612793
Chicago/Turabian StyleÁvila-Flores, Antonia, Juan José Sánchez-Cabezón, Ane Ochoa-Echeverría, Ana I. Checa, Jorge Rosas-García, Mariana Téllez-Araiza, Sara Casado, Rosa Liébana, Teresa Santos-Mendoza, and Isabel Mérida. 2023. "Identification of Host PDZ-Based Interactions with the SARS-CoV-2 E Protein in Human Monocytes" International Journal of Molecular Sciences 24, no. 16: 12793. https://doi.org/10.3390/ijms241612793
APA StyleÁvila-Flores, A., Sánchez-Cabezón, J. J., Ochoa-Echeverría, A., Checa, A. I., Rosas-García, J., Téllez-Araiza, M., Casado, S., Liébana, R., Santos-Mendoza, T., & Mérida, I. (2023). Identification of Host PDZ-Based Interactions with the SARS-CoV-2 E Protein in Human Monocytes. International Journal of Molecular Sciences, 24(16), 12793. https://doi.org/10.3390/ijms241612793







